Abstract
Background
The common cold is a spontaneously remitting infection of the upper respiratory tract, characterised by a runny nose, nasal congestion, sneezing, cough, malaise, sore throat, and fever (usually < 37.8 ºC). Whilst the common cold is generally not harmful, it is a cause of economic burden due to school and work absenteeism. In the United States, economic loss due to the common cold is estimated at more than USD 40 billion per year, including an estimate of 70 million workdays missed by employees, 189 million school days missed by children, and 126 million workdays missed by parents caring for children with a cold. Additionally, data from Europe show that the total cost per episode may be up to EUR 1102. There is also a large expenditure due to inappropriate antimicrobial prescription. Vaccine development for the common cold has been difficult due to antigenic variability of the common cold viruses; even bacteria can act as infective agents. Uncertainty remains regarding the efficacy and safety of interventions for preventing the common cold in healthy people, thus we performed an update of this Cochrane Review, which was first published in 2011 and updated in 2013 and 2017.
Objectives
To assess the clinical effectiveness and safety of vaccines for preventing the common cold in healthy people.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (April 2022), MEDLINE (1948 to April 2022), Embase (1974 to April 2022), CINAHL (1981 to April 2022), and LILACS (1982 to April 2022). We also searched three trials registers for ongoing studies, and four websites for additional trials (April 2022). We did not impose any language or date restrictions.
Selection criteria
Randomised controlled trials (RCTs) of any virus vaccine compared with placebo to prevent the common cold in healthy people.
Data collection and analysis
We used Cochrane’s Screen4Me workflow to assess the initial search results. Four review authors independently performed title and abstract screening to identify potentially relevant studies. We retrieved the full‐text articles for those studies deemed potentially relevant, and the review authors independently screened the full‐text reports for inclusion in the review, recording reasons for exclusion of the excluded studies. Any disagreements were resolved by discussion or by consulting a third review author when needed. Two review authors independently collected data on a data extraction form, resolving any disagreements by consensus or by involving a third review author. We double‐checked data transferred into Review Manager 5 software. Three review authors independently assessed risk of bias using RoB 1 tool as outlined in the Cochrane Handbook for Systematic Reviews of Interventions. We carried out statistical analysis using Review Manager 5. We did not conduct a meta‐analysis, and we did not assess publication bias. We used GRADEpro GDT software to assess the certainty of the evidence and to create a summary of findings table.
Main results
We did not identify any new RCTs for inclusion in this update. This review includes one RCT conducted in 1965 with an overall high risk of bias. The RCT included 2307 healthy young men in a military facility, all of whom were included in the analyses, and compared the effect of three adenovirus vaccines (live, inactivated type 4, and inactivated type 4 and 7) against a placebo (injection of physiological saline or gelatin capsule). There were 13 (1.14%) events in 1139 participants in the vaccine group, and 14 (1.19%) events in 1168 participants in the placebo group. Overall, we do not know if there is a difference between the adenovirus vaccine and placebo in reducing the incidence of the common cold (risk ratio 0.95, 95% confidence interval 0.45 to 2.02; very low‐certainty evidence). Furthermore, no difference in adverse events when comparing live vaccine preparation with placebo was reported. We downgraded the certainty of the evidence to very low due to unclear risk of bias, indirectness because the population of this study was only young men, and imprecision because confidence intervals were wide and the number of events was low. The included study did not assess vaccine‐related or all‐cause mortality.
Authors' conclusions
This Cochrane Review was based on one study with very low‐certainty evidence, which showed that there may be no difference between the adenovirus vaccine and placebo in reducing the incidence of the common cold. We identified a need for well‐designed, adequately powered RCTs to investigate vaccines for the common cold in healthy people. Future trials on interventions for preventing the common cold should assess a variety of virus vaccines for this condition, and should measure such outcomes as common cold incidence, vaccine safety, and mortality (all‐cause and related to the vaccine).
Keywords: Child; Humans; Male; Adenovirus Vaccines; Adenovirus Vaccines/adverse effects; Common Cold; Common Cold/prevention & control; Incidence; Randomized Controlled Trials as Topic; Systematic Reviews as Topic; Vaccines, Attenuated; Vaccines, Attenuated/adverse effects
Plain language summary
Vaccines for preventing the common cold
Review question
Can vaccines help prevent the common cold?
Background
The common cold is mainly caused by a viral infection of the upper respiratory tract. People with the common cold feel unwell, have a runny nose, nasal congestion, sneezing, cough with or without sore throat, and have slightly elevated temperatures. However, people usually recover when their immune system controls the impact of the viral infection. Treatment for this condition is aimed at relieving symptoms. Globally, the common cold causes widespread illness and large economic loss. In the United States, economic loss due to the common cold is estimated at more than USD 40 billion per year, including millions of workdays and school days missed. In Europe, the total cost per episode may be up to EUR 1102. There is also a large expenditure on inappropriate antimicrobial prescriptions. It has been difficult to manufacture vaccines to prevent the common cold because it is caused by several viruses. The effect of vaccines for preventing the common cold in healthy people is still unknown.
Search date
The evidence is current to 26 April 2022.
Study characteristics
We did not identify any new trials for inclusion in this update. This review includes one previously identified randomised controlled trial (a type of study where participants are randomly assigned to one of two or more treatment groups) performed in 1965. This study involved 2307 young, healthy military men at a training facility in the United States Navy, and evaluated the effects of a live attenuated (weakened) adenovirus vaccine, an inactivated type 4, and an inactivated type 4 and 7 vaccines compared to a placebo (fake vaccine).
Study funding sources
The included trial was funded by a government institution.
Key results
There were no differences in the frequency of occurrence of the common cold between those who received a live attenuated adenovirus vaccine compared to those who received a placebo. There were no differences between groups in adverse events. However, as the trial participants were not representative of the general population and there were flaws in the study design, our confidence in the results is very low. Further research is needed to find out if vaccines can prevent the common cold, as the current evidence does not support the use of the adenovirus vaccine to prevent the common cold in healthy people.
Certainty of the evidence
We assessed the certainty of the evidence as very low due to high risk of bias; because the study population was only young men; and due to the small number of people included in the study and low numbers of colds.
Summary of findings
Summary of findings 1. Virus vaccines compared to placebo for preventing the common cold in healthy people.
| Virus vaccines compared to placebo for preventing the common cold in healthy people | ||||||
| Patient or population: young, healthy men in a military facility Settings: navy training centre Intervention: adenovirus vaccines (live, inactivated type 4, and inactivated type 4 and 7) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Virus vaccines for preventing the common cold | |||||
|
Incidence of the common cold Number of participants with common cold by group Follow‐up: mean 9 weeks |
Study population | RR 0.95 (0.45 to 2.02) | 2307 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c | ||
| 12 per 1000 | 11 per 1000 (5 to 24) | |||||
|
Vaccine safety Follow‐up: mean 9 weeks |
The study reported that there were no differences between groups in vaccine‐related adverse events. | 2307 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c | |||
|
Mortality: vaccine related and all cause ‐ not reported Follow‐up: mean 9 weeks |
See comments | The included study did not report this outcome. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to unclear risk of bias. bDowngraded one level due to indirectness as the study population is only young men. cDowngraded one level due to imprecision as confidence intervals are wide, and the number of events is low.
Background
Description of the condition
Although there is no standard definition for the common cold (see Appendix 1), it is generally defined as a spontaneously remitting infection of the upper respiratory tract (URT), characterised by a runny nose, nasal congestion, and sneezing. Other symptoms associated with the common cold include cough, malaise, sore throat, and fever (usually < 100 ºF/37.8 ºC) (Eccles 2009). A temperature of 100 ºF/37.8 ºC or higher for three to four days is typically associated with influenza and other respiratory diseases (see Appendix 2) (DDCP 2010). Despite the fact that the common cold is not considered to be a deadly disease, bacterial complications can lead to high morbidity and mortality (Giraud‐Gatineau 2020; Veiga 2021).
The common cold is a disease of diverse aetiology (see Appendix 3) (Heikkinen 2003). Premature babies, children, the elderly, and other populations with comorbidities such as chronic lung diseases (chronic obstructive pulmonary disease), congenital heart disease, and asthma are more prone to viral infections that cause the common cold, including respiratory syncytial virus (RSV), human rhinovirus (HRV), parainfluenza, coronavirus, and adenovirus (non‐polio) (Johnston 2017; Lu 2020; Rubner 2017; Shibata 2018). Additionally, in humans, coronavirus such as the HCoV‐229E, HCoV‐OC43, HCoV‐NL63, and HCoV‐HKU1 strains are responsible for URT infections (URTI) associated with the common cold (Zumla 2016). It has been shown that 1% to 10% of the population is infected with the HCoV‐NL63 coronavirus annually (Szelazek 2017). Even the infectious agent SARS‐CoV‐2 can be associated with the common cold (Zimmermann 2020). The transmission of the common cold occurs through aerosols, direct contact with infected nasal secretions, or fomites (DeGeorge 2019; L'Huillier 2015; Reynolds 2016); the primary factors that contribute to the spread of this disease include poor hand hygiene, overcrowding, and interactions in places such as schools, day‐care centres, and between households (Adler 2018; Alexandrino 2016).
The common cold is one of the most frequent illnesses experienced by humans. Children may experience up to 11 URTIs annually (Lambert 2007; Tang 2019), whilst adults experience an average of two episodes per year (Tomita 2012; Visseaux 2017). For instance, HRV is responsible for 50% to 80% of common colds, and it is an important cause of morbidity, reduced productivity, and inappropriate use of antibiotics (Kardos 2017; Warner 2019). Although there are few studies on the socioeconomic costs of the common cold, and most existing studies are problematic in terms of methodology (e.g. recall bias) for mainly being cross‐sectional studies on self‐reported surveys performed in a small or specific population, the impact on school and work absenteeism caused by common cold and the economic burden it causes cannot be overlooked (Dicpinigaitis 2015; Jaume 2020; Jaume 2021). In the United States, economic loss due to the common cold is estimated at more than USD 40 billion per year, including an estimate of 70 million workdays missed by employees suffering from a cold, 189 million school days missed by children, and 126 million work days missed by parents caring for children with a cold (Kardos 2017), all of which have an impact on productivity (Dicpinigaitis 2015). In Europe, the total cost per episode may be up to EUR 1102, of which 75% are indirect costs (Stjärne 2012). Additionally, there is a large expenditure due to inappropriate antimicrobial prescription and use for URTI (Tsuzuki 2020).
Description of the intervention
There is no specific treatment for the common cold. Treatment is focused on easing the symptoms. Prevention is therefore essential to stop the spread of viruses that cause the common cold. In terms of preventive measures, studies have shown that simple measures such as handwashing and maintaining physical distance are relevant to all respiratory infections, but difficult to apply or enforce because, as time passes, motivation and compliance may decrease (Allan 2014; Jefferson 2020). Consequently, another method of prevention is vaccination. The development of vaccines for the common cold has been challenging because of the aetiological diversity of the disease and the antigenic variability of the common cold viruses (Glanville 2013; To 2017). The case of HRV remains a challenge for the public health and scientific communities due to technical, logistical, and fundamental biological difficulties (Stobart 2017). Unlike several human viruses, HRV vaccine must be able to elicit protective neutralising antibodies to potentially over 150 serologically distinct types spanning three different species (Ren 2017; Stobart 2017). For this reason, it is difficult to develop a vaccine that provides full protection (Stepanova 2019). Despite these challenges, recent attempts using rhinovirus‐derived VP1, a surface protein that is critically involved in the infection of respiratory cells, has demonstrated that with enough exposure and recombinant VP1 as an immunogen, cross‐serotype reactive antibodies can be generated (Edlmayr 2011; McLean 2012). The future of vaccine development for the common cold seems promising, considering that studies on virus genotyping such as HRV genotype are being published more frequently (Luka 2020; Ren 2017), and there is a rapid technological development of vaccines that include micro‐/nanoparticle material and recombinant technologies (Papadopoulos 2017).
Adenovirus is a recognised pathogen of the URT (Biserni 2020). Adenovirus serotype 4 (Ad4) and serotype 7 (Ad7) vaccines were used during immunisation programmes beginning in 1971. Unfortunately, their interruption triggered the re‐emergence of adenovirus‐produced diseases in crowded locations. An example of this reappearance was documented in US military training sites, where Ad4 accounted for 98% of all diagnoses (Russell 2006). The development and deployment of AdV‐4 and AdV‐7 vaccines is essential in controlling AdV‐4‐ and AdV‐7‐related URTI (Collins 2020). Adenoviral vaccines delivered orally have been used for decades to prevent respiratory illnesses. New studies have concluded that these vaccines are safe and have brought about a large immune response in the studied populations. For instance, a study examining the duration of the neutralising antibody response generated from a live oral AdV‐4 and AdV‐7 vaccine showed that, regardless of pre‐vaccination serostatus, participants developed a significant antibody response which persisted for at least six years after vaccination (Collins 2020).
RSV is an important cause of respiratory infection in older adults, and almost all children have been infected with RSV by the age of two; due to incomplete and short‐lived natural immunity, repeated RSV infections occur throughout life (Williams 2020). The development of an RSV vaccine has been difficult due to antigenic variability, especially in proteins F and G. However, a first‐in‐human phase 1 trial has been reported to evaluate the safety and immunogenicity of an experimental RSV vaccine of Ad26.RSV.preF (a replication‐incompetent adenovirus‐26 vector encoding the F protein), which is administered intramuscularly 12 months apart in healthy adults aged ≥ 60 years old (Williams 2020). There have also been concerns of enhanced respiratory disease (ERD) after vaccination with the formalin‐inactivated RSV vaccines in the 1960s, in which patients showed X‐ray evidence of severe pneumonia and bronchiolitis, in addition to immunopotentiation induced by a T helper (Th)‐2 and Th17 T cell responses with the enrolment of T cells, neutrophils, and eosinophils causing inflammation and tissue damage (Rey‐Jurado 2017). Current vaccine candidates, especially those designed for infants and children, whose immune systems are still immature, must therefore be safe and avoid these immunologic features of ERD (Mazur 2018).
One RSV immunisation approach is the development of a vaccine for pregnant women, since it has been demonstrated that RSV‐neutralising antibodies are transferred from the pregnant woman to the foetus through the placenta (Chu 2014). A clinical trial in which healthy pregnant women received either an intramuscular dose of RSV fusion (F) protein nanoparticle vaccine or placebo, showed that during the first 90 days of life, infants with RSV had a significantly lower number of respiratory tract infections (1.5%) compared to the placebo group (2.4%). However, to tag the vaccine as successful in terms of efficacy, its possible benefits with respect to other endpoint events have to be demonstrated (Madhi 2020). Another phase II clinical trial evaluated the tolerability and safety of RSV fusion (F) protein nanoparticle vaccine compared to placebo in 50 healthy third‐trimester pregnant women and their infants (Muňoz 2019). The trial demonstrated that the vaccine was well tolerated with no differences on safety outcomes other than expected short‐term reactogenicity in women who received the active vaccine. In addition, transplacental antibody transfer ranged between 90% and 120% across assays for infants of the vaccinated group of women (Muňoz 2019).
Regarding parainfluenza, there is still no approved vaccine available. However, the intranasal administration of two doses of a live‐attenuated human parainfluenza virus type 3 HPIV3‐cp45 vaccine seems promising, as it has been shown to be well tolerated and immunogenic in seronegative children over six months of age (Karron 2011).
How the intervention might work
Vaccines work by inducing an immune response, such as an antibody response that interferes with a pathogenic invasion and prevents their adherence to epithelial cells through opsonisation, phagocytosis, and other mechanisms (Pichichero 2009; Wooden 2018). A correlate of protection to a pathogen is a measurable sign that a person is immune after vaccination, and can either be absolute or relative (Plotkin 2020). There may also be more than one correlate of protection for a disease, known as 'co‐correlates' (Plotkin 2020). The immune memory is a critical correlate (effector memory for short‐incubation and central memory for long‐incubation diseases), and cell‐mediated immunity can operate as a correlate or co‐correlate of protection against a disease (Plotkin 2010). However, some vaccines only have surrogates for an unknown protective response, which are easy measurements but not functional (Plotkin 2010). For instance, as there is no correlate of protection for the development of HRV vaccines, serum IgA is used as a surrogate marker for studies on all live attenuated HRV vaccines to demonstrate vaccine take (Armah 2016).
Studies suggest that vaccines that mimic natural infection and take into account the structure of pathogens seem to be effective in inducing long‐term protective immunity (Kang 2009). Different types of vaccines against respiratory viruses already exist, but all have been focused on decreasing the incidence of lower respiratory infections. Traditionally inactivated or live attenuated viruses are used. However, there are promising approaches that use micro‐/nanoparticles material and recombinant technologies that produce a broad immunogenic, reproducible, safe, and often self‐adjuvating response (Gomes 2017; Papadopoulos 2017).
Why it is important to do this review
Although the common cold is self‐limiting with symptoms often lasting up to 10 days, it is the most common acute illness in industrialised countries with a very high incidence, presenting several episodes per person a year, as well as being one of the main causes of primary care consultations (DeGeorge 2019; Jaume 2020). Despite being generally mild, the common cold can occur with other respiratory illnesses, which can predispose susceptible individuals to potentially serious complications. Vaccines could therefore be used to reduce the prevalence of the disease around the world and decrease primary care consultations that might saturate healthcare systems.
The socioeconomic burden from the common cold has not been fully studied, and the few studies published in this area mainly have methodological limitations. However, it has been demonstrated that the common cold can lead to work and school absenteeism as well as having an impact on productivity and healthcare costs (Dicpinigaitis 2015; Jaume 2021; Kardos 2017), which could also be reduced by preventing the illness through vaccine use, if effective.
Furthermore, if randomised controlled trials demonstrate that there is an effective and safe vaccine to prevent the common cold, scientists could continue researching this field.
Objectives
To assess the clinical effectiveness and safety of vaccines for preventing the common cold in healthy people.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We did not apply limits with respect to follow‐up periods. In future updates, we will consider at least 60 days of follow‐up. We only included RCTs that reported data about the incidence of the common cold.
Types of participants
Healthy people aged between 6 months and 90 years.
Types of interventions
Any vaccine that prevents the common cold, which protects against RSV, rhinovirus, parainfluenza, or adenovirus (non‐polio), irrespective of dose, schedule, or administration route, versus placebo. We excluded trials on the prevention of influenza A and B because influenza and the common cold are two different diseases (Jefferson 2012). See Appendix 3 for details.
Types of outcome measures
Primary outcomes
Incidence of the common cold after vaccination, regardless of the causal agent determined by laboratory or clinical examination. In future updates, we will consider the incidence of the common cold as one of the primary outcomes, measured 60 days after the last dose of the vaccine.
Vaccine safety, i.e. adverse events ("any untoward medical occurrence that may present during treatment with a pharmaceutical product but which does not necessarily have a causal relationship with this treatment") and adverse drug reactions ("a response to a drug which is noxious, uninitiated and which occurs at doses normally used in men for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiologic functions") (Nebeker 2004).
Mortality: vaccine related and all cause.
Secondary outcomes
We did not consider any secondary outcomes. In future updates, we will consider mortality as a secondary outcome.
Search methods for identification of studies
Electronic searches
We updated the search strategies in the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 4, 2022), which contains the Cochrane Acute Respiratory Infections Specialised Register, searched 26 April 2022, in the Cochrane Library (Appendix 4);
MEDLINE via Ovid (September 2016 to 22 April 2022) (Appendix 5);
Embase via Elsevier (September 2016 to 22 April 2022) (Appendix 6);
CINAHL via EBSCO (Cumulative Index to Nursing and Allied Health Literature) (September 2016 to 22 April 2022) (Appendix 7); and
LILACS via BIREME (Latin American and Caribbean Health Science Information database) (September 2016 to 22 April 2022) (Appendix 8).
We used the Cochrane Highly Sensitive Search Strategy to identify randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2021). We adapted the search strategy to search Embase, CINAHL, and LILACS.
We searched the following trial registries on 26 April 2022:
ISRCTN registry (www.isrctn.com);
ClinicalTrials.gov (clinicaltrials.gov/); and
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/search/en/).
We did not restrict the results by language, search dates, or publication status (published, unpublished, in press, or in progress).
Searching other resources
We checked the reference lists of all relevant trials and identified reviews. We searched the following websites for trials on 29 April 2022:
US Food and Drug Administration (www.fda.gov);
European Medicines Agency (www.emea.europa.eu);
Medicines & Healthcare Products Regulatory Agency (www.mhra.gov.uk/index.htm); and
Evidence in Health and Social Care (www.evidence.nhs.uk/).
Data collection and analysis
Selection of studies
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labelled as an RCT or as non‐RCT; the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs; and if appropriate, Cochrane Crowd – Cochrane’s citizen science platform where the Crowd help to identify and describe health evidence. For more information about Screen4Me and the evaluations that have been done, visit the Screen4Me web page on the Cochrane Information Specialist’s portal. More detailed information regarding evaluations of the Screen4Me components can be found in the following publications: Marshall 2018, Noel‐Storr 2020, Noel‐Storr 2021, Thomas 2021.
Following Screen4Me assessment, four review authors (CM, DB, MLF, MJMZ) independently screened the titles and abstracts of studies identified as a result of the search for potential relevance. We retrieved the full‐text reports of those studies deemed potentially relevant, and four review authors (MJMZ, CM, DB, MLF) independently screened the full texts to identify studies for inclusion in the review, and identified and recorded the reasons for exclusion of excluded studies. Any disagreements were resolved through discussion or by consulting a third review author (DSR) when needed. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and Characteristics of included studies table (Moher 2009). We imposed no language restrictions.
1.
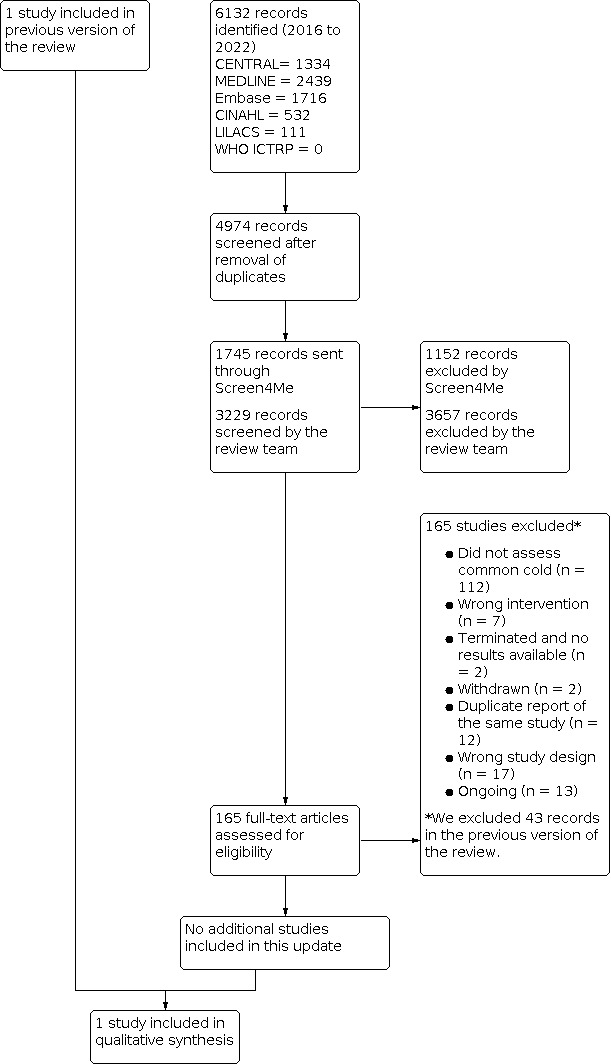
PRISMA flowchart
Data extraction and management
We used a data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. Two review authors (DSR, CVG) extracted the following study characteristics from the included studies.
Methods: study design, total duration of the study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of the study.
Participants: N, mean age, age range, gender, severity of the condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for the trial, and notable conflicts of interest of trial authors.
Two review authors (DSR, CVG) independently extracted outcome data from the included studies. We described in the Characteristics of included studies table if outcome data were not reported in a usable way. Any disagreements were resolved by consensus or by involving a third review author (RH). One review author (DSR) transferred data into the Review Manager 5 file (Review Manager 2020). We double‐checked that the data had been entered correctly by comparing the data presented in the systematic review with the study report. A second review author (MJMZ) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Three review authors (DSR, CVG, RH) independently assessed risk of bias of the included studies using Cochrane's risk of bias tool RoB 1, according to the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). Any disagreements were resolved by discussion or by involving another review author (MJMZ). We assessed the risk of bias based on the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as low, high, or unclear and provided quotes from the study report together with a justification for our judgement in the risk of bias table. Where necessary, we considered blinding separately for different key outcomes.
Measures of treatment effect
We calculated the risk ratio (RR) with 95% confidence intervals (CIs) for incidence of the common cold. We added outcome data for the included study into a data table in Review Manager 5 to calculate treatment effects (Review Manager 2020). We used RR for dichotomous outcomes.
Unit of analysis issues
The unit of analysis was the participant. We collected and analysed a single measurement for each outcome from each participant.
Dealing with missing data
We had planned to contact investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified only as an abstract); however, this was not needed.
Assessment of heterogeneity
We had planned to use the I² statistic to measure heterogeneity amongst trials in each analysis; however, we did not conduct a meta‐analysis because only one trial satisfied the inclusion criteria.
Assessment of reporting biases
We did not assess publication bias using a funnel plot because we included only one trial. In future updates, we will attempt to assess whether the review is subject to publication bias by using a funnel plot if 10 or more trials are included.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (Review Manager 2020). In future updates, we will summarise findings using a fixed‐effect model following the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Subgroup analysis and investigation of heterogeneity
We did not perform a subgroup analysis. In subsequent updates of this review, when sufficient data are available, we will carry out the following subgroup analyses:
children and adults;
country of study; and
different responses in relation to different viral agents.
We will explore sources of heterogeneity in the assessment of the primary outcomes by subgroup analyses. Additionally, due to the limited number of included studies, we do not plan on performing a meta‐regression in the future.
Sensitivity analysis
We did not perform a sensitivity analysis. In future updates, we plan to conduct sensitivity analyses comparing the results using all trials as follows.
Trials with high methodological quality (studies classified as having a 'low risk of bias' versus those identified as having a 'high risk of bias') (Higgins 2022).
Trials that performed intention‐to‐treat versus per‐protocol analyses.
Parallel randomised clinical trials versus cluster‐randomised clinical trials.
We will also evaluate the risk of attrition bias, as estimated by the percentage of participants lost. We will exclude trials with a total attrition of more than 30% or where differences between the groups exceeded 10%, or both, from meta‐analysis, but will include these studies in the review.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table using the following outcomes: incidence of the common cold, vaccine safety, and mortality (all cause and vaccine related) (Table 1). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the evidence as it relates to the study that contributed data (Atkins 2004). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022), employing GRADEpro GDT software (GRADEpro GDT). We justified all decisions to down‐ or upgrade the certainty of the evidence using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
In the current update we identified a total of 4974 search results (Figure 1). We used Cochrane’s Screen4Me workflow to help identify potential reports of randomised trials. The results of the Screen4Me assessment process are shown in Figure 2. We assessed 6132 records and excluded 3657 records based on title and abstract screening. We assessed the full texts of 165 studies, and did not include any new studies in this update. We included only one trial in the review (Griffin 1970).
2.
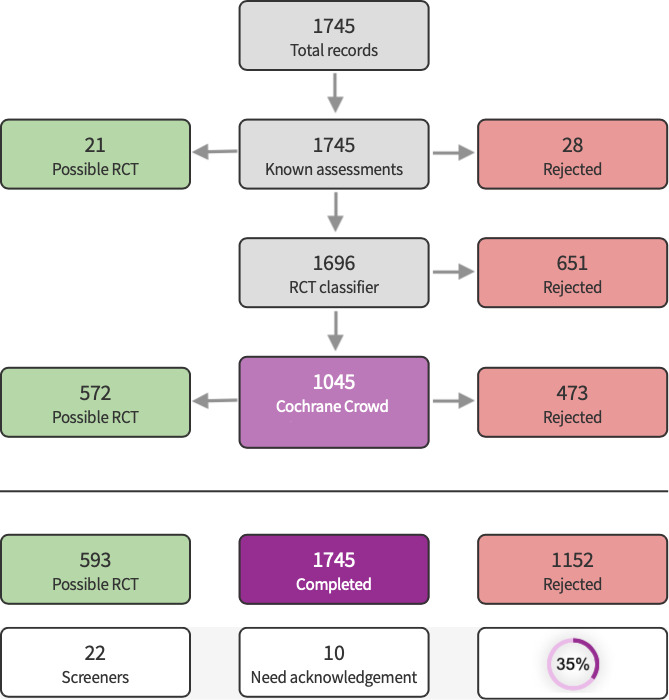
Screen4Me summary diagram.
Included studies
We included one RCT involving 2307 healthy young men (Griffin 1970). Griffin 1970 compared the effect of three adenovirus vaccines (live, inactivated type 4, and inactivated type 4 and 7) to placebo (an injection of physiological saline for the parenterally administered vaccines, and an identical‐appearing inert gelatin capsule for the orally administered vaccines). See Characteristics of included studies.
Excluded studies
We excluded 43 studies in the previous publication of this review (Belshe 1982; Belshe 1992; Belshe 2004a; Belshe 2004b; Clements 1991; DeVincenzo 2010; Doggett 1963; Dudding 1972; Falsey 1996; Falsey 2008; Fulginiti 1969; Glenn 2016; Gomez 2009; Gonzalez 2000; Greenberg 2005; Hamory 1975; Karron 1995a; Karron 1995b; Karron 1997; Karron 2003; Karron 2005; Karron 2015; Kumpu 2015; Langley 2009; Lee 2001; Lee 2004; Lyons 2008; Madhi 2006; Munoz 2003; Murphy 1994; Paradiso 1994; Piedra 1995; Pierce 1968; Power 2001; Ritchie 1958; Simoes 2001; Tang 2008; Top 1971; Tristram 1993; Watt 1990; Welliver 1994; Wilson 1960; Wright 1976).
We excluded 109 new studies in this update (Abarca 2020; Ahmad 2022; Aliprantis 2018; Aliprantis 2020; Ascough 2019; August 2017; Beran 2018; Bourne 1946; Cicconi 2020; Cunningham 2019; DeVincenzo 2019; Domachowske 2017; Domachowske 2018; Esposito 2019; EUCTR2008‐001714‐24‐GB; EUCTR2012‐001107‐20‐GB; EUCTR2013‐004036‐30‐GB; EUCTR2014‐005041‐41‐GB; EUCTR2015‐004296‐77‐GB; EUCTR2016‐000117‐76‐ES; EUCTR2016‐000117‐76‐PL; EUCTR2016‐001135‐12‐FR; EUCTR2016‐002733‐30‐ES; EUCTR2018‐001340‐62‐FI; Falloon 2017a; Falloon 2017b; Fries 2019; Israel 2016; Karppinen 2019; Karron 2020a; Karron 2020b; Langley 2016; Langley 2017; Langley 2018; Leroux‐Roels 2019; Madhi 2020; McFarland 2018; McFarland 2020a; McFarland 2020b; Munoz 2019; NCT00139347; NCT00308412; NCT00345670; NCT00345956; NCT00363545; NCT00366782; NCT00383903; NCT00420316; NCT00496821; NCT00641017; NCT00686075; NCT00767416; NCT01021397; NCT01139437; NCT01254175; NCT01290419; NCT01475305; NCT01709019; NCT01852266; NCT01905215; NCT02115815; NCT02266628; NCT02296463; NCT02419391; NCT02440035; NCT02472548; NCT02479750; NCT02491463; NCT02561871; NCT02593071; NCT02601612; NCT02624947; NCT02794870; NCT02830932; NCT02864628; NCT02873286; NCT02890381; NCT02926430; NCT02952339; NCT03026348; NCT03049488; NCT03191383; NCT03303625; NCT03334695; NCT03392389; NCT03403348; NCT03473002; NCT03572062; NCT03674177; NCT03814590; NCT04071158; NCT04086472; NCT04752644; NTR7173; Philpott 2016; Philpott 2017; Ruckwardt 2021; Sadoff 2021a; Sadoff 2021b; Samy 2020; Scaggs Huang 2021; Schwarz 2019; Shakib 2019; Shaw 2019; Swamy 2019; Van Der Plas 2020; Verdijk 2020; Williams 2020; Yu 2020). We identified 13 ongoing studies (NCT01893554; NCT03387137; NCT03422237; NCT03596801; NCT03916185; NCT04032093; NCT04126213; NCT04138056; NCT04681833; NCT04732871; NCT04980391; NCT05127434; NCT05238025). We contacted the authors of the ongoing trials; however, no preliminary results have been shared with us.
Risk of bias in included studies
Griffin 1970 had overall low methodological quality. See Figure 3 and Figure 4.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for the included study.
Allocation
We assessed Griffin 1970 as at unclear risk of bias for random sequence generation and allocation concealment as inadequate information prevented a judgement for this domain.
Blinding
We assessed Griffin 1970 as at low risk of bias for blinding of participants and personnel and blinding of outcome assessment.
Incomplete outcome data
We assessed Griffin 1970 as at low risk of attrition bias as there were no losses.
Selective reporting
We assessed Griffin 1970 as at unclear risk of reporting bias. The study protocol was not available, but it was clear that the published reports include all expected outcomes. However, some of the outcomes were described in a narrative fashion and did not specify incidence for each group.
Other potential sources of bias
We assessed Griffin 1970 as at unclear risk of other bias. The baseline characteristics of participants were not described, and there was no detailed information relating to assessment of selection bias, preventing an evaluation of whether both groups were comparable.
Effects of interventions
See: Table 1
Our results are based on one RCT with 2307 healthy people, which we assessed as providing very low‐certainty evidence (Griffin 1970). See Table 1.
Primary outcomes
1. Incidence of the common cold
We do not know if there is a difference between the adenovirus vaccine and placebo in reducing the incidence of the common cold (risk ratio 0.95, 95% confidence interval 0.45 to 2.02; 1 RCT, 2307 participants, 27 events; Analysis 1.1) (Griffin 1970). We downgraded the certainty of the evidence to very low due to unclear risk of bias; indirectness because the population of this study was only young and healthy men; and imprecision because confidence intervals were wide, and the number of events was low.
1.1. Analysis.

Comparison 1: Adenovirus vaccines versus placebo, Outcome 1: Incidence of the common cold
2. Vaccine safety
Griffin 1970 reported that there were no differences in adverse events between live vaccine preparation and placebo. We downgraded the certainty of the evidence to very low due to unclear risk of bias; indirectness because the population of this study was only young men; and imprecision because confidence intervals were wide, and the number of events was low.
3. Mortality
Griffin 1970 did not assess either vaccine‐related or all‐cause mortality.
Discussion
Summary of main results
One RCT met our inclusion criteria (Griffin 1970). The incidence of the common cold in Griffin 1970 was very low, probably due to the fact that only cases resulting in admission to the medical dispensary or hospital were included. Furthermore, the trial authors stated that more common cold cases were diagnosed incidentally when people were hospitalised for other causes, therefore mild cases of illness were not included.
Critical appraisal of Griffin 1970 did not support the use of any vaccine for preventing the common cold in healthy people. We did not find significant differences in the incidence of the common cold in people treated with adenovirus vaccines compared with placebo. Griffin 1970 did not evaluate main clinical outcomes such as mortality related to the vaccine. No differences in adverse events were reported. The relative effect of any of the vaccines for viruses that cause the common cold remains unclear.
Overall completeness and applicability of evidence
The included trial did not detect statistically significant differences between treatment groups (Griffin 1970).
When considering such neutral results, it is important to keep in mind that 'absence of evidence' is not 'evidence of absence' (Alderson 2004; Altman 1995; Westerterp 2020). The fact that this review did not detect any differences between intervention groups does not imply that placebo and adenovirus vaccine have the same effect on preventing the common cold.
The first possible explanation for not detecting any differences between intervention groups could be the lack of an appropriate sample size (Green 2002; Schulz 1995), which resulted in small differences in the incidence of the common cold and few events in the comparison groups. A remarkable paper from Freiman 1978 suggested that "many of the therapies labelled as 'no different from control' in trials using inadequate samples, have not received a fair test" and that "concern for the probability of missing an important therapeutic improvement because of small sample sizes deserves more attention in the planning of clinical trials". Moreover, most trials with negative results usually have insufficiently large sample sizes to detect at least 50% relative difference (Moher 1998; Sully 2014). It has also been suggested that the most important therapies adopted in clinical practice have only shown modest benefits (Kirby 2002).
Certainty of the evidence
The results for the primary outcomes of incidence of the common cold and vaccine safety were based on very low‐certainty evidence due to imprecision because confidence intervals were wide and the number of events was low; indirectness (the RCT considered in our review included only young, healthy men); and methodological limitations. Random sequence generation, allocation, sample size, and baseline characteristics of participants were not reported. Adverse events were not reported individually for each group. Overall, due to a lack of evidence, the balance between the benefits and harms of cold vaccines is uncertain.
Potential biases in the review process
Whilst performing a systematic review, several biases can emerge, such as 'significance‐chasing' biases (Ioannidis 2010). This group of biases include publication bias, selective outcome reporting bias, selective analysis reporting bias, and fabrication bias (Ioannidis 2010). Publication bias represents a major threat to the validity of systematic reviews, particularly those reviews that include small trials. However, in this systematic review we performed an exhaustive search and attempted to locate all studies to include new RCTs. The current evidence does not evaluate common cold outcomes.
Agreements and disagreements with other studies or reviews
Most studies on vaccines for the common cold evaluate respiratory syncytial virus (RSV) vaccines, followed by studies on adenovirus vaccines; only a few published studies evaluate human rhinovirus (HRV) and parainfluenza vaccines. In addition, these studies mainly aim to reduce lower respiratory tract infections or focus on immunological outcomes. For instance, Buchholz 2018 and McFarland 2020b are two RCTs of live‐attenuated RSV vaccines that focus on upper respiratory tract infections; however, these studies assessed immunological outcomes such as vaccine shedding, serum RSV antibodies, anti‐RSV F immunoglobulin G, surveillance period, adverse events, and reactogenicity. There is no general consensus on the outcomes to be considered in evaluating the efficacy of a vaccine. In this review we assessed the following outcomes: incidence of common cold after vaccination, vaccine safety, and mortality (all cause and vaccine related), which were not considered in most RCTs assessed for inclusion by full text. We focused on these outcomes due to the impact that the common cold has at the population level, with a high estimated economic loss due to missed working days (Kardos 2017; Tsuzuki 2020). Other systematic reviews on vaccines for preventing upper respiratory tract infection have assessed outcomes similar to those included in this review (Hao 2015; Thomas 2013).
We excluded 11 non‐RCTs that evaluated vaccines for upper respiratory tract infections (Belshe 1982; Clements 1991; Doggett 1963; Dudding 1972; Fulginiti 1969; Hamory 1975; Karron 1997; Ritchie 1958; Watt 1990; Wilson 1960; Wright 1976). Only one study evaluated the incidence of the common cold (Ritchie 1958), whilst the others focused on immunologic outcomes. The study conducted by Ritchie 1958 prepared an "autologous vaccine" developed from the nasal secretions of 125 healthy volunteers, who were then inoculated with this product, whilst 75 healthy volunteers served as a control. The results showed a lower incidence of common cold in the vaccine group than in the control group. This study was not an RCT and was thus excluded from our review.
Authors' conclusions
Implications for practice.
This Cochrane Review update found very limited evidence on the effects of vaccines for the common cold in healthy people. We included only one randomised controlled trial, which did not report differences between comparison groups. Our findings were based on only one trial with very low‐certainty evidence with an unclear risk of bias, indirectness, and imprecision. Griffin 1970 involved 2307 participants and assessed adenovirus vaccines compared with placebo, and showed that there may be no difference between the adenovirus vaccine and placebo in reducing the incidence of the common cold.
Implications for research.
This 2022 update highlights the need for well‐designed, high‐quality randomised clinical trials to assess the effectiveness and safety of vaccines to prevent the common cold in healthy people. Future trials should include outcomes such as common cold incidence, vaccine safety, mortality, and adverse events related to vaccine administration. Inert placebo use would also be beneficial to avoid dampening adverse events following immunisation. Future trials should be conducted by independent researchers and reported according to CONSORT guidelines (Moher 2012; PCORI 2012), and should adhere to the Foundation of Patient‐Centered Outcomes Research recommendations (Gabriel 2012).
What's new
| Date | Event | Description |
|---|---|---|
| 26 April 2022 | New search has been performed | We did not identify any new trials for inclusion in this update. We excluded 109 new studies and identified 13 ongoing studies. We recruited two new authors to update this review, and one of the previous review authors did not take part in this update. |
| 26 April 2022 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 2 September 2016 | New citation required but conclusions have not changed | We recruited three new authors to update this review. |
| 2 September 2016 | New search has been performed | We updated our searches and excluded three new trials (Glenn 2016; Karron 2015; Kumpu 2015). |
| 22 January 2015 | New search has been performed | Searches conducted. |
| 16 March 2011 | New citation required and major changes | Protocol taken over by a new team of review authors. |
| 26 February 2009 | Amended | Protocol withdrawn (Issue 3, 2009). |
Acknowledgements
The authors wish to thank Tom Jefferson and David Tyrell, who co‐authored the first published version of this review; Anne Lyddiatt, Gulam Khandaker, Lisa Jackson, Mark Griffin, and Meenu Singh for commenting on the draft protocol; and Theresa Wrangham, John Jordan, Viviana Rodriguez, and Meenu Singh for commenting on the first draft review. The authors wish to express their thanks to Liz Dooley, Managing Editor of the Cochrane Acute Respiratory Infections Group, for her comments, which improved the quality of this review.
For this 2022 update, we would like to acknowledge and thank the following people for their help in assessing the search results for this review via Cochrane’s Screen4Me workflow: Nikolaos Sideris, Anna Noel‐Storr, Shammas Mohammed, Sarah Moore, Yuan Chi, Mohammad Aloulou, Ana‐Marija Ljubenković, Vighnesh Devulapalli, Jens Bredbjerg Brock Thorsen, and Mykhailo Havrylets.
The following people conducted the editorial process for this 2022 update.
Sign‐off Editors (final editorial decision): Mark Jones (Bond University, Australia); Mieke van Driel (The University of Queensland, Australia).
Managing Editors (provided editorial guidance to authors, edited the review, selected peer reviewers, and collated peer‐reviewer comments): Liz Dooley (Bond University, Australia); Fiona Russell (Bond University, Australia).
Contact Editor (provided comments and recommended an editorial decision): Meenu Singh (Post Graduate Institute of Medical Education and Research, Chandigarh, India).
Statistical Editor (provided comments): Menelaos Konstantinidis (University of Toronto, Canada)
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Copy Edit Support
Peer reviewers (provided comments and recommended an editorial decision):
Clinical/content review: Roger E Thomas (University of Calgary, Canada).
Consumer review: Theresa Wrangham (USA).
Methods review: Rachel Richardson (Associate Editor, Cochrane, UK).
Search review: Justin Clark (Institute for Evidence‐Based Healthcare, Bond University, Australia).
One peer reviewer preferred to remain anonymous.
Appendices
Appendix 1. Glossary
| Term | Definition | Reference |
| Common cold | The common cold is a self‐limiting acute upper respiratory tract infection characterised by rhinorrhoea, nasal congestion, sneezing, cough, sore throat, fever, and malaise. | Heikkinen 2003 |
| Vaccination | Inoculation with a vaccine, i.e. a preparation of microbial antigen often combined with adjuvants administered to an individual in order to induce protective immunity against microbial infections. The antigen may be in the form of live, avirulent micro‐organisms or purified macromolecular components of micro‐organisms. | Abbas 2001 |
| Immune system | The collection of cells, tissues, and molecules that mediate resistance to infections | Abbas 2001 |
| Cell‐mediated immunity | The arm of the adaptative immune response whose role is to combat infections by intracellular microbes. This type of immunity is mediated by T lymphocytes. | Abbas 2001 |
| Antigenical variability | Microbes have evolved mechanisms to evade immunity. Many bacteria and viruses mutate their antigenic surface molecules and can no longer be recognised by antibodies produced in response to previous infection. | Abbas 2001 |
| Serotypes | An antigenically distinct subset of a species of an infectious organism that is distinguished from other subsets by serologic (i.e. serum antibody) tests. Humoral immune response to one serotype of microbes, e.g. influenza virus, may not be protective against another serotypes. | Abbas 2001 |
| Immune responses | Once a foreign organism has been recognised, the immune system enlists the participation of a variety of cells and molecules to mount an appropriate response in order to eliminate or neutralise the organism. | Goldsby 2000 |
| Antigenic molecules | Any molecule capable of being recognised by an antibody or T‐cell receptor. Any substance that elicits an immune response | Goldsby 2000; Roitt 2004 |
| Allergens | An antigen that elicits an immediate hypersensitivity (allergic) reaction. Allergens are proteins, or chemicals bound to proteins, that induce immunoglobulin E antibody production in atopic individuals. | Abbas 2001 |
| Immunopotentiation | Non‐specific immunostimulation given by various agents that can stimulate the immune response. It is believed that the mechanism of action is through some modification of local cytokines or growth of innate immune mechanisms. An increase in the functional capacity of the immune response |
Gorczynski 2007 |
| Opsonisation | The process by which particulate antigens are rendered more susceptible to phagocytosis The process of attaching opsonins, such as immunoglobulin G or complement fragments, to microbial surfaces to target microbes for phagocytosis |
Abbas 2001; Goldsby 2000 |
| Phagocytosis | Macrophages are capable of ingesting and digesting exogenous antigens, such as whole micro‐organisms and insoluble particles, and endogenous matter, such as injured or dead host cells, cellular debris, and activated clotting factors. The process by which certain cells of the innate immune system, including macrophages and neutrophils, engulf large particles (> 0.5‐micrometre diameter), such as intact microbes. The cell surrounds the particle by a cytoskeleton‐dependent process, leading to formation of an intracellular vesicle called a phagosome, which contains the ingested particle. |
Abbas 2001; Goldsby 2000 |
Appendix 2. Differences between clinical characteristics of the common cold and influenza
| Feature | Common cold | Influenza | References |
| Aetiological agent | > 100 viral strains; rhinovirus most common | 3 strains of influenza virus: influenza A, B, C |
Czubak 2021; DDCP 2010; Gwaltney 1967; Gwaltney 2000; Heikkinen 2003; Roxas 2007; Thompson 2003 |
| Site of infection | Upper respiratory tract | Entire respiratory system | |
| Symptom onset | Gradual: 1 to 3 days | Sudden: within a few hours | |
| Fever, chills | Occasional, low grade (< 100 ºF) | Fever is usually present with the flu (up to 80% of all flu cases). A temperature of 100 ºF or higher for 3 to 4 days is typically associated with the flu. | |
| Headache | Frequent, usually mild | Characteristic, more severe | |
| General aches, pains | Mild, if any | Characteristic, often severe and affecting the entire body | |
| Cough, chest congestion | Mild to moderate, with hacking cough | Common, may become severe | |
| Sore throat | Common, usually mild | Sometimes present | |
| Runny, stuffy nose | Very common, accompanied by bouts of sneezing | Sometimes present | |
| Fatigue, weakness | Mild, if any | Usual, may be severe and last 2 to 3 weeks | |
| Extreme exhaustion | Never | Frequent, usually in early stages of illness | |
| Season | Year around, peaks in winter months | Most cases between November and February | |
| Antibiotics helpful | No, unless secondary bacterial infection develops | No, unless secondary bacterial infection develops |
Appendix 3. Viral causes of the common cold
| Virus | Estimated annual proportion of cases | References |
| Rhinoviruses | 30% to 50%; during autumn 80%. Once considered to be limited to the upper airway, now recognised as an important cause of lower respiratory infections | Arruda 1997; Gwaltney 1985; Heikkinen 2003; Lemanske 2005; Mäkelä 1998; Monto 1993; Regamey 2008 |
| Coronaviruses | 7% to 18% in adults with upper respiratory infections. Responsible for 2.1% of hospital admissions for acute respiratory tract infections in all age groups | Larson 1980; Lau 2006; Mäkelä 1998; Nicholson 1997 |
| Influenza viruses | 5% to 15% | Heikkinen 2003 |
| Respiratory syncytial virus (RSV) | In low‐income countries, 15% to 20% In hospital the proportion of children aged between birth and 5 months with RSV acute lower respiratory tract infections ranged between 9% and 87%. Amongst children up to at least 5 years of age reported with RSV, on average 39% (range 20% to 62%) were < 6 months old; on average 24% of cases (range 14% to 38%) were children aged 6 to 11 months, thus an average of 63% of children were under 1 year of age. On average 20% (range 13% to 29%) of children were between 1 and 2 years of age. RSV accounts for approximately 10,000 deaths annually in people over the age of 65 years in the USA. RSV in adults, 5% infection annually |
Berman 1991; Falsey 2005; Thompson 2003 |
| Parainfluenza viruses | Acute respiratory infections cause 3% to 18% of all admissions to paediatric hospitals; however, this might vary at different times of the year. Parainfluenza viruses account for 17% of hospitalised illness‐associated virus isolation. In low‐income countries, 7% to 10% Parainfluenza viruses cause 50% to 74.2% of croup cases. |
Berman 1991; Denny 1983; Henrickson 2003 |
| Adenoviruses | In low‐income countries, 2% to 4% | Berman 1991 |
| Metapneumovirus | 10% short epidemic | Esper 2003; Kahn 2003; Nissen 2002; Risnes 2005 |
| Unknown | 20% to 30% | Mäkelä 1998; Monto 1993 |
Appendix 4. CENTRAL search strategy
#1 mh "Common Cold"
#2 "common cold*":ti,ab
#3 "coryza":ti,ab
#4 (acute near/5 ("upper respiratory infection*" or "upper respiratory tract infection*" or urti or uri)):ti,ab
#5 mh "Picornaviridae Infections"
#6 mh Rhinovirus
#7 rhinovir*
#8 "hrv":ti,ab
#9 mh "Paramyxoviridae Infections"
#10 mh "parainfluenza virus 1, human" or mh "parainfluenza virus 3, human"
#11 mh "parainfluenza virus 2, human" or mh "parainfluenza virus 4, human"
#12 "parainfluenza*":ti,ab
#13 mh coronavirus or mh "coronavirus 229e, human" or mh "coronavirus oc43, human"
#14 mh "Coronavirus Infections"
#15 coronavir*
#16 mh adenoviridae or mh "adenoviruses, human"
#17 mh adenoviridae or mh "adenoviruses, human"
#18 adenovir*
#19 mh "respiratory syncytial viruses" or mh "respiratory syncytial virus, human"
#20 mh "Respiratory Syncytial Virus Infections"
#21 ("respiratory syncytial virus*" or rsv):ti,ab
#22 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21
#23 mh Vaccines
#24 mh Vaccination
#25 (vaccin* or inocul* or immuni*):ti,ab
#26 #23 or #24 or #25
#27 #22 and #26 with Cochrane Library publication date Between Sep 2016 and April 2022
Appendix 5. MEDLINE (Ovid) search strategy
1 Common Cold/
2 common cold*.tw.
3 coryza.tw.
4 (acute adj5 (upper respiratory infection* or upper respiratory tract infection* or urti or uri)).tw.
5 Picornaviridae Infections/
6 Rhinovirus/
7 rhinovir*.tw.
8 hrv.tw.
9 Paramyxoviridae Infections/
10 parainfluenza virus 1, human/ or parainfluenza virus 3, human/
11 parainfluenza virus 2, human/ or parainfluenza virus 4, human/
12 parainfluenza*.tw.
13 coronavirus/ or coronavirus 229e, human/ or coronavirus oc43, human/
14 Coronavirus Infections/
15 coronavir*.tw.
16 exp adenoviridae/ or adenoviruses, human/
17 Adenovirus Infections, Human/
18 adenovir*.tw.
19 respiratory syncytial viruses/ or respiratory syncytial virus, human/
20 Respiratory Syncytial Virus Infections/
21 (respiratory syncytial virus* or rsv).tw.
22 or/1‐21
23 exp Vaccines/
24 exp Vaccination/
25 (vaccin* or inocul* or immuni*).tw.
26 or/23‐25 (724637)
27 randomized controlled trial.pt.
28 controlled clinical trial.pt.
29 randomi?ed.ab.
30 placebo.ab.
31 drug therapy.fs.
32 randomly.ab.
33 trial.ab.
34 groups.ab.
35 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34
36 exp animals/ not humans.sh.
37 35 not 36
38 22 and 26 and 37
39 limit 38 to dt=20160901‐20220426
Appendix 6. Embase (Elsevier) search strategy
#28 #27 AND (2016:py OR 2017:py OR 2018:py OR 2019:py OR 2020:py OR 2021:py OR 2022:py)
#27 #23 AND #26
#26 #24 OR #25
#25 random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR (((singl* OR doubl*) NEAR/1 blind*):ab,ti)
#24 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp
#23 #18 AND #22
#22 #19 OR #20 OR #21
#21 'vaccination'/de
#20 vaccin*:ab,ti OR immuni*:ab,ti OR inocul*:ab,ti
#19 'vaccine'/exp
#18 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17
#17 'respiratory syncytial virus':ab,ti OR 'respiratory syncytial viruses':ab,ti OR rsv:ab,ti
#16 'respiratory syncytial pneumovirus'/de OR 'respiratory syncytial virus infection'/de
#15 adenovir*:ab,ti
#14 'adenovirus'/exp OR 'human adenovirus infection'/de
#13 coronavir*:ab,ti
#12 'coronavirus'/de OR 'coronavirus infection'/de
#11 parainfluenza*:ab,ti
#10 'parainfluenza virus 1'/de OR 'parainfluenza virus 2'/de OR 'parainfluenza virus 3'/de OR 'parainfluenza virus4'/exp
#9 'parainfluenza virus'/exp
#8 'paramyxovirus infection'/de
#7 rhinovir*:ab,ti OR hrv:ab,ti
#6 'rhinovirus infection'/de OR 'human rhinovirus'/de
#5 coryza:ab,ti
#4 'acute upper respiratory infection':ab,ti OR 'acute upper respiratory infections':ab,ti OR 'acute upper respiratory tract infection':ab,ti OR 'acute upper respiratory tract infections':ab,ti OR ((acute NEAR/5 (urti OR uri)):ab,ti)
#3 'viral upper respiratory tract infection'/de OR 'upper respiratory tract infection'/de
#2 'common cold':ab,ti OR 'common colds':ab,ti
#1 'common cold'/de OR 'common cold symptom'/de
Appendix 7. CINAHL (EBSCO) search strategy
S34 S23 AND S33 Limiters ‐ Published Date: 20160101‐20220426
S33 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32
S32 (MH "Quantitative Studies")
S31 TI placebo* or AB placebo*
S30 (MH "Placebos")
S29 TI random* or AB random*
S28 TI (singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) or AB (singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*)
S27 TI (singl* blind* or doubl* blind* or trebl* blind* or tripl* blind*) or AB (singl* blind* or doubl* blind* or trebl* blind* or tripl* blind*)
S26 TI clinic* w1 trial* or AB clinic* w1 trial*
S25 PT clinical trial
S24 (MH "Clinical Trials+")
S23 S18 AND S22
S22 S19 OR S20 OR S21
S21 TI (vaccin* or immuni* or inocula*) or AB (vaccin* or immuni* or inocula*)
S20 (MH "Immunization")
S19 (MH "Vaccines+")
S18 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17
S17 TI (respiratory syncytial virus* or rsv ) or AB (respiratory syncytial virus* or rsv)
S16 (MH "Respiratory Syncytial Virus Infections")
S15 (MH "Respiratory Syncytial Viruses")
S14 TI adenovir* or AB adenovir*
S13 TI coronavir* or AB coronavir*
S12 (MH "Coronavirus+")
S11 (MH "Coronavirus Infections")
S10 TI parainfluenza* or AB parainfluenza*
S9 (MH "Paramyxovirus Infections")
S8 (MH "Paramyxoviruses")
S7 TI hrv or AB hrv
S6 TI rhinovir* or AB rhinovir*
S5 (MH "Picornavirus Infections")
S4 TI (upper respiratory tract infection* or upper respiratory infection*) or AB (upper respiratory tract infection* or upper respiratory infection*)
S3 TI coryza or AB coryza
S2 TI common cold* or AB common cold*
S1 (MH "Common Cold")
Appendix 8. LILACS (BIREME) search strategy
((mh:"Common Cold" OR "common cold" OR "common colds" OR coryza OR "Resfriado Común" OR "Resfriado Comum" OR "Coriza Aguda" OR "Upper Respiratory Tract Infections" OR "upper respiratory tract infection" OR "Infecciones del Tracto Respiratorio Superior" OR "Infecciones de las Vías Respiratorias Superiores" OR "Infecções do Trato Respiratório Superior" OR "Infecções das Vias Respiratórias Superiores" OR "Infecções das Vias Aéreas Superiores" OR "Infecções do Sistema Respiratório Superior" OR mh:"Picornaviridae Infections" OR "Infecciones por Picornaviridae" OR "Infecções por Picornaviridae" OR "Picornavirus Infections" OR mh:rhinovirus OR rhinovir* OR "Virus de la Coriza" OR "Virus del Resfriado Común" OR "Vírus da Coriza" OR "Vírus do Resfriado Comum" OR hrv OR mh:"Paramyxoviridae Infections" OR parainfluenza* OR mh:"Parainfluenza Virus 1, Human" OR mh:"Parainfluenza Virus 2, Human" OR mh:"Parainfluenza Virus 3, Human" OR mh:"Parainfluenza Virus 4, Human" OR mh:"Coronavirus Infections" OR coronavir* OR mh:coronavirus OR mh:"Coronavirus 229E, Human" OR mh:"Coronavirus OC43, Human" OR mh:"Coronavirus NL63, Human" OR mh:adenoviridae OR mh:"Adenoviruses, Human" OR mh:"Adenovirus Infections, Human" OR adenovir* OR mh:"Respiratory Syncytial Viruses" OR "Virus Sincitiales Respiratorios" OR "Vírus Sinciciais Respiratórios" OR "Virus Sincitial Respiratorio" OR "Vírus Sincicial Respiratório" OR mh:"Respiratory Syncytial Virus, Human" OR "respiratory syncytial virus" OR "Virus Humano Respiratorio Sincitial" OR mh:"Respiratory Syncytial Virus Infections" OR "Infecciones por Virus Sincitial Respiratorio" OR "Infecções por Vírus Respiratório Sincicial" OR rsv) AND (mh:vaccines OR vaccin* OR vacunas OR vacinas OR mh:d20.215.894* OR mh:vaccination OR vacunación OR vacinação OR mh:"Mass Vaccination" OR mh:immunization OR inmunización OR imunização OR mh:e02.095.465.425.400* OR mh:e05.478.550* OR mh:n02.421.726.758.310* OR mh:n06.850.780.200.425* OR mh:n06.850.780.680.310* OR mh:sp2.026.182.113* OR mh:sp8.946.819.838* OR immuni* OR inmuni* OR imuni*) ) AND ( db:("LILACS") AND type_of_study:("clinical_trials")) AND (year_cluster:[2016 TO 2022])
Data and analyses
Comparison 1. Adenovirus vaccines versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of the common cold | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Griffin 1970.
| Study characteristics | ||
| Methods |
Design: double‐blind RCT (2 arms) Country: USA (1 site) Clinical setting: Great Lakes Naval Training Center Follow‐up: 9 weeks' basic‐training period Intention‐to‐treat: yes Randomisation unit: participant Analysis unit: participant |
|
| Participants | Great Lakes Naval Training Center, new recruits Randomised: 2307 participants Vaccines group: 1139 (49.3%) Placebo group: 1168 (50.7%) Participants receiving intervention: 1139 Vaccines group: 1139 (49.3%) Placebo group: 1168 (50.7%) Lost postrandomisation: 0% Analysed participants: Vaccines group: 1139 (49.3%) Placebo group: 1168 (50.7%) Age median (mean (SD)): did not report Gender (number of men): 2307 Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions | Experimental group: the vaccines used were composed of orally administered live adenovirus 4, parenterally administered inactivated adenovirus 4, and parenterally administered inactivated adenovirus 4 and 7 preparations Control group: placebo Co‐interventions:
|
|
| Outcomes | This RCT did not specify primary or secondary outcomes. Incidence of admissions of participants with respiratory illness (not only hospitalised participants):
Toxic effects |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Epidemiologic design of this study consisted of the random assignment of one half of the recruits ..." (p 982) Insufficient information to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind procedure was followed with paramedical personnel administering the appropriate vaccine or placebo to recruits on their third day after arrival at Great Lakes, just prior to initiation of basic training" (p 982) Quote: "Placebo for the parenterally administered vaccines consisted of an injection of physiological saline, and that for the orally administered vaccine consisted of an identical appearing inert gelatin capsule" (p 982) Comment: blinding of participants and key study personnel ensured, and it is unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding of outcome assessment was performed with the use of placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes. However, some outcomes are described in a narrative fashion and not per group. Quote: "... there was no observable toxic reaction to this new live vaccine preparation within the study design." (p 985) |
| Other bias | Unclear risk | The sample size was not reported. There is no table with baseline characteristics of the participants. |
RCT: randomised controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abarca 2020 | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| Ahmad 2022 | Phase 2a ‐ did not assess incidence of common cold |
| Aliprantis 2018 | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| Aliprantis 2020 | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| Ascough 2019 | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| August 2017 | Phase II study ‐ did not assess incidence of common cold, did not define common cold |
| Belshe 1982 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Belshe 1992 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Belshe 2004a | Assessed lower respiratory tract infection |
| Belshe 2004b | Phase II study ‐ did not assess incidence of common cold, did not define common cold |
| Beran 2018 | Phase II study ‐ did not assess incidence of common cold, did not define common cold |
| Bourne 1946 | Assessed common cold symptoms caused by bacterial infections |
| Cicconi 2020 | Phase I ‐ did not assess incidence of common cold |
| Clements 1991 | Wrong design, not RCT |
| Cunningham 2019 | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| DeVincenzo 2010 | Wrong design, experimental infection |
| DeVincenzo 2019 | Duplicate record ‐ conference abstract of Sadoff 2021a |
| Doggett 1963 | Wrong study design, not RCT |
| Domachowske 2017 | Assessed lower respiratory tract infection |
| Domachowske 2018 | Assessed lower respiratory tract infection |
| Dudding 1972 | Wrong design, not RCT |
| Esposito 2019 | Assessed common cold symptoms caused by bacterial infections |
| EUCTR2008‐001714‐24‐GB | Phase I study ‐ did not assess incidence of common cold |
| EUCTR2012‐001107‐20‐GB | Phase II study ‐ did not assess incidence of common cold, did not define common cold |
| EUCTR2013‐004036‐30‐GB | Phase II study ‐ did not assess incidence of common cold, did not define common cold |
| EUCTR2014‐005041‐41‐GB | Phase II study ‐ did not assess incidence of common cold, did not define common cold |
| EUCTR2015‐004296‐77‐GB | Phase II study ‐ did not assess incidence of common cold, did not define common cold |
| EUCTR2016‐000117‐76‐ES | Phase I/II study ‐ did not assess incidence of common cold, did not define common cold |
| EUCTR2016‐000117‐76‐PL | Assessed lower respiratory tract infection |
| EUCTR2016‐001135‐12‐FR | Phase II study ‐ did not assess incidence of common cold, did not define common cold |
| EUCTR2016‐002733‐30‐ES | Phase I/II study ‐ did not assess incidence of common cold, did not define common cold |
| EUCTR2018‐001340‐62‐FI | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Falloon 2017a | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| Falloon 2017b | Phase II study ‐ did not assess incidence of common cold, did not define common cold |
| Falsey 1996 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Falsey 2008 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Fries 2019 | Assessed lower respiratory tract infection |
| Fulginiti 1969 | Wrong study design, not RCT |
| Glenn 2016 | Assessed lower respiratory tract infection |
| Gomez 2009 | Assessed lower respiratory tract infection |
| Gonzalez 2000 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Greenberg 2005 | Phase II study ‐ did not assess incidence of common cold, did not define common cold |
| Hamory 1975 | Wrong study design, not RCT |
| Israel 2016 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Karppinen 2019 | Assessed respiratory symptoms caused by bacterial infections |
| Karron 1995a | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| Karron 1995b | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| Karron 1997 | Wrong study design, not RCT |
| Karron 2003 | Wrong study design, not RCT |
| Karron 2005 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Karron 2015 | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| Karron 2020a | Wrong study design, not RCT |
| Karron 2020b | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| Kumpu 2015 | Assessed respiratory symptoms caused by bacterial infections |
| Langley 2009 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Langley 2016 | Duplicate record ‐ clinical trial register of Langley 2018 |
| Langley 2017 | Assessed lower respiratory tract infection |
| Langley 2018 | Assessed lower respiratory tract infection |
| Lee 2001 | Did not assess incidence of common cold or vaccine safety |
| Lee 2004 | Wrong study design, not RCT |
| Leroux‐Roels 2019 | Assessed lower respiratory tract infection |
| Lyons 2008 | Phase I study ‐ did not assess incidence of common cold, did not define common cold |
| Madhi 2006 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Madhi 2020 | Phase III ‐ did not assess incidence of common cold, did not define common cold |
| McFarland 2018 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| McFarland 2020a | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| McFarland 2020b | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Munoz 2003 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Munoz 2019 | Phase II ‐ did not assess incidence of common cold, did not define common cold |
| Murphy 1994 | Wrong study design, not RCT |
| NCT00139347 | Wrong intervention, this study assessed human rotavirus associated with gastroenteritis |
| NCT00308412 | Assessed lower respiratory tract infection |
| NCT00345670 | Assessed lower respiratory tract infection |
| NCT00345956 | Wrong intervention, this study assessed human rotavirus associated with gastroenteritis |
| NCT00363545 | Wrong intervention, this study assessed human rotavirus associated with gastroenteritis |
| NCT00366782 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT00383903 | Wrong intervention, this study assessed human rotavirus associated with gastroenteritis |
| NCT00420316 | Wrong intervention, this study assessed human rotavirus causing gastroenteritis |
| NCT00496821 | Duplicate record ‐ clinical trial register of DeVincenzo 2010 |
| NCT00641017 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT00686075 | Did not assess incidence of common cold or vaccine safety |
| NCT00767416 | Phase I and II ‐ did not assess incidence of common cold, did not define common cold |
| NCT01021397 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT01139437 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT01254175 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT01290419 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT01475305 | Terminated, and no results available |
| NCT01709019 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT01852266 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT01905215 | Duplicate record ‐ clinical trial register of Langley 2017 |
| NCT02115815 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT02266628 | Phase II ‐ did not assess incidence of common cold, did not define common cold |
| NCT02296463 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT02419391 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT02440035 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT02472548 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT02479750 | Wrong intervention, not a vaccine |
| NCT02491463 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT02561871 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT02593071 | Assessed lower respiratory tract infection |
| NCT02601612 | Assessed lower respiratory tract infection |
| NCT02624947 | Duplicate record ‐ clinical trial register of Madhi 2020 |
| NCT02794870 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT02830932 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT02864628 | Withdrawn, no reasons specified |
| NCT02873286 | Phase II ‐ did not assess incidence of common cold, did not define common cold |
| NCT02890381 | Duplicate record ‐ clinical trial register of Cunningham 2019 |
| NCT02926430 | Phase II ‐ did not assess incidence of common cold, did not define common cold |
| NCT02952339 | Assessed lower respiratory tract infection |
| NCT03026348 | Phase II ‐ did not assess incidence of common cold, did not define common cold |
| NCT03049488 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT03191383 | Withdrawn due to instability of the PreF antigen during manufacturing |
| NCT03303625 | Phase I and II ‐ did not assess incidence of common cold, did not define common cold |
| NCT03334695 | Duplicate record ‐ clinical trial register of Sadoff 2021a |
| NCT03392389 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT03403348 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT03473002 | Duplicate record ‐ clinical trial register of Scaggs 2020 |
| NCT03572062 | Terminated |
| NCT03674177 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| NCT03814590 | Phase I and II ‐ did not assess incidence of common cold, did not define common cold |
| NCT04071158 | Phase II ‐ did not assess incidence of common cold, did not define common cold |
| NCT04086472 | Wrong study design, not RCT |
| NCT04752644 | Phase IIa ‐ did not assess incidence of common cold |
| NTR7173 | Duplicate record ‐ clinical trial register of Verdijk 2020 |
| Paradiso 1994 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Philpott 2016 | Duplicate record ‐ conference abstract of Philpott 2017 |
| Philpott 2017 | Wrong intervention, study assessed influenza |
| Piedra 1995 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Pierce 1968 | Did not assess incidence of common cold or vaccine safety |
| Power 2001 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Ritchie 1958 | Wrong design, not RCT |
| Ruckwardt 2021 | Phase I ‐ did not assess incidence of common cold |
| Sadoff 2021a | Phase II ‐ did not assess incidence of common cold, did not define common cold |
| Sadoff 2021b | Phase II ‐ did not assess incidence of common cold, did not define common cold |
| Samy 2020 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| Scaggs Huang 2021 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| Schwarz 2019 | Phase II ‐ did not assess incidence of common cold, did not define common cold |
| Shakib 2019 | Did not assess incidence of common cold or vaccine safety |
| Shaw 2019 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| Simoes 2001 | Wrong study design, not RCT |
| Swamy 2019 | Duplicate record ‐ conference abstract of Madhi 2020 |
| Tang 2008 | Unknown phase ‐ did not assess incidence of common cold, did not define common cold |
| Top 1971 | Did not assess incidence of common cold or vaccine safety |
| Tristram 1993 | Assessed lower respiratory tract infection |
| Van Der Plas 2020 | Duplicate record ‐ conference abstract of Verdijk 2020 |
| Verdijk 2020 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| Watt 1990 | Wrong study design, not RCT |
| Welliver 1994 | Did not assess incidence of common cold or vaccine safety |
| Williams 2020 | Phase I ‐ did not assess incidence of common cold, did not define common cold |
| Wilson 1960 | Wrong study design, not RCT |
| Wright 1976 | Wrong study design, not RCT |
| Yu 2020 | Did not assess incidence of common cold or vaccine safety |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01893554.
| Study name | Evaluating the safety and immune response to a single dose of a respiratory syncytial virus (RSV) vaccine in infants and children |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 105 |
| Interventions | Biological: RSV ΔNS2 Δ1313 I1314L vaccine; other: placebo |
| Outcomes | Frequency and severity of vaccine‐related solicited AEs; proportion of participants that develop 4‐fold or greater rises in RSV neutralising antibody titre following vaccination |
| Starting date | June 2013 |
| Contact information | Jocelyn San Mateo; 410‐614‐4306; jsanmate@jhsph.edu |
| Notes |
NCT03387137.
| Study name | Evaluating the infectivity, safety, and immunogenicity of a respiratory syncytial virus vaccine (RSV 6120/∆NS2/1030s) in RSV‐seropositive children and RSV‐seronegative infants and children |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 45 |
| Interventions | Biological: RSV 6120/∆NS2/1030s; other: placebo |
| Outcomes | Grades of: study product‐related solicited AEs (RSV‐seropositive participants); study product‐related solicited AEs (RSV‐seronegative participants); study product‐related unsolicited AEs (RSV‐seropositive participants); study product‐related unsolicited AEs (RSV‐seronegative participants); study product‐related SAEs (RSV‐seropositive participants). Frequency of infection with: RSV (RSV‐seropositive participants); with RSV (RSV‐seronegative participants). Peak titre of vaccine virus shed (RSV‐seropositive participants). Duration of virus shedding in nasal washes (RSV‐seropositive participants). RSV‐neutralising serum antibody titre (RSV‐seropositive participants). IgG serum antibody titres to RSV F glycoprotein ELISA (RSV‐seropositive participants) |
| Starting date | 13 October 2017 |
| Contact information | Ruth A Karron |
| Notes |
NCT03422237.
| Study name | Evaluating the infectivity, safety, and immunogenicity of the recombinant live‐attenuated RSV vaccines RSV ΔNS2/Δ1313/I1314L or RSV 276 in RSV‐seronegative infants and children 6 to 24 months of age |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 80 |
| Interventions | Biological: RSV ΔNS2/Δ1313/I1314L; biological: RSV 276; other: placebo |
| Outcomes | Grades of: study product‐related solicited AEs; product‐related unsolicited AEs; product‐related SAEs; number of participants infected with RSV; peak titre of vaccine virus shed; duration of virus shedding in nasal washes. Frequency of: ≥ 4‐fold rise in RSV serum neutralising antibody titre; RSV neutralising antibody responses; ≥ 4‐fold rise in serum antibody titres to RSV F glycoprotein; antibody responses to RSV F glycoprotein |
| Starting date | 4 October 2017 |
| Contact information | Ruth A Karron |
| Notes |
NCT03596801.
| Study name | Evaluating the infectivity, safety and immunogenicity of respiratory syncytial virus vaccines, RSV 6120/∆NS1 and RSV 6120/F1/G2/∆NS1, in RSV‐seropositive children and RSV‐seronegative infants and children |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 75 |
| Interventions | Biological: RSV 6120/∆NS1; biological: RSV 6120/F1/G2/∆NS1; other: placebo |
| Outcomes | Grades of study product‐related: solicited AEs; unsolicited AEs; SAEs. Frequency of infection with RSV. Peak titre of vaccine virus shed. Duration of virus shedding in nasal washes. Frequency of ≥ 4‐fold rise in RSV‐neutralising antibody titre. Frequency of ≥ 4‐fold rise in IgG antibody responses to RSV F glycoprotein. Frequency of symptomatic, medically attended respiratory and febrile illness in the RSV‐seronegative (group 2) vaccine and placebo recipients who experience natural infection with wt RSV during the RSV season. Severity of symptomatic, medically attended respiratory and febrile illness in the RSV‐seronegative (group 2) vaccine and placebo recipients who experience natural infection with wt RSV during the RSV season. Frequency of antibody responses in the RSV‐seronegative vaccine and placebo recipients who experience natural infection with wt RSV during the RSV season. Measurement of mucosal antibody titres to vaccine |
| Starting date | 25 June 2018 |
| Contact information | Kristi Herbert; 410‐502‐3333; kherber1@jhu.edu |
| Notes |
NCT03916185.
| Study name | Safety and immunogenicity of a single dose of the recombinant live‐attenuated RSV vaccines RSV ΔNS2/Δ1313/I1314L, RSV 6120/ΔNS2/1030s, RSV 276 or placebo, delivered as nose drops to RSV‐seronegative children 6 to 24 months of age |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 160 |
| Interventions | Biological: RSV ΔNS2/Δ1313/I1314L vaccine; RSV 6120/ΔNS2/1030s vaccine; RSV 276 vaccine; other: placebo |
| Outcomes | Primary: frequency of Grade 1 or higher solicited AEs [Time Frame: measured through Day 28]; frequency of Grade 2 or higher lower respiratory illnesses [Time Frame: measured through Day 28]; frequency of serious AEs [Time Frame: measured through Day 56]; frequency of ≥ 4‐fold rise in serum RSV‐neutralising antibody titre [Time Frame: measured through Day 56] Secondary: frequency of ≥ 4‐fold rise in serum RSV F IgG [Time Frame: measured through Day 56]; titre of serum RSV F IgG [Time Frame: measured at the Day 56 visit]; titre of serum RSV‐neutralising antibodies [Time Frame: measured at the Day 56 visit]; frequency of RSV‐MAARI [Time Frame: measured through the last day of the RSV season, which will occur between 5 and 12 months after study entry, depending on when the participant enrolls in the study]; maximum grade (if more than 1 illness within a participant) of RSV‐MAARI [Time Frame: measured through the last day of the RSV season, which will occur between 5 and 12 months after study entry, depending on when the participant enrolls in the study]; frequency of RSV‐MAALRI [Time Frame: measured through the last day of the RSV season, which will occur between 5 and 12 months after study entry, depending on when the participant enrolls in the study]; maximum grade (if more than 1 illness within a participant) of RSV‐MAALRI [Time Frame: measured through the last day of the RSV season, which will occur between 5 and 12 months after study entry, depending on when the participant enrolls in the study] |
| Starting date | 16 May 2019 |
| Contact information | Coleen Cunningham and Ruth Karron |
| Notes |
NCT04032093.
| Study name | A phase 2B placebo‐controlled, randomised study of a RSV vaccine in pregnant women |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 650 |
| Interventions | Biological: RSV vaccine; other: placebo |
| Outcomes | Percentage of participants reporting: local reactions and systemic events from day of vaccination (Day 1) until Day 7; AEs within 1 month after vaccination; obstetric complications, MAEs and SAEs throughout the study. Percentage of infant participants: with specific birth outcomes; with AE from birth to 1 month of age; with SAE, AE of special interest (congenital anomalies, developmental delay), and MAE through 12 months of age. Immune responses measured by RSV neutralising antibody titres in maternal participants. Geometric mean ratio for RSV neutralising antibody titres in maternal participants |
| Starting date | 7 August 2019 |
| Contact information | Pfizer |
| Notes |
NCT04126213.
| Study name | Study of safety, reactogenicity and immunogenicity of GlaxoSmithKline's (GSK) respiratory syncytial virus (RSV) maternal unadjuvanted vaccine in healthy pregnant women (aged 18 to 40 years) and their infants |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 420 |
| Interventions | Biological: RSVPreF3 formulation 2; biological: RSVPreF3 formulation 3; other: placebo |
| Outcomes | Percentage of maternal participants reporting: solicited administration site events; solicited systemic events; with haematological and biochemical laboratory abnormality at baseline; with haematological and biochemical laboratory abnormality at Day 8; unsolicited AEs; at least 1 SAE; with AEs leading to study withdrawal; with at least 1 MAE; pregnancy outcomes; pregnancy‐related AESIs; neonatal AESIs; at least 1 SAE; AEs leading to study withdrawal; at least 1 MAE. RSVPreF3 IgG‐specific antibody concentration in terms of GMCs at Day 1, before vaccination for each group, and by age category. RSVPreF3 IgG antibody GMCs at Day 31. RSVPreF3 IgG antibody GMCs at delivery. RSV‐A neutralising antibody GMTs at Day 1, before vaccination. RSV‐A neutralising antibody GMTs at Day 31. RSV‐A neutralising antibody GMTs at delivery. RSVPreF3 IgG antibody GMCs in infants born to maternal participants. RSV‐A neutralising antibody GMTs in infants born to maternal participants. Geometric mean ratio between cord blood and maternal RSVPreF3 IgG‐specific antibody concentrations |
| Starting date | 5 November 2019 |
| Contact information | GlaxoSmithKline |
| Notes |
NCT04138056.
| Study name | A study of a vaccine against respiratory syncytial virus (RSV) when given alone and together with a vaccine against diphtheria, pertussis and tetanus (Tdap) viruses followed by a 2nd dose of the RSV vaccine to healthy non‐pregnant women |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 509 |
| Interventions | Biological: RSVPreF3 formulation 3; biological: RSVPreF3 formulation 2; biological: Boostrix‐ex‐US; other: placebo |
| Outcomes | Percentage of participants with: at least 1 solicited local AE for each study group, after the 1st vaccination; at least 1 solicited general AE for each study group, after the 1st vaccination. Percentage of participants with: any unsolicited AEs for each study group, after the 1st vaccination; at least 1 SAE for each study group, after the 1st vaccination; at least 1 solicited local AE for each study group, after the 2nd vaccination; at least 1 solicited general AE for each study group, after the 2nd vaccination; any unsolicited AEs for each study group, after the 2nd vaccination; at least 1 SAE for each study group, after the 2nd vaccination. Humoral immune response in terms of RSV A neutralising antibody GMTs for each group, at screening. RSV A neutralising antibody GMTs for each group at Day 8, after the 1st vaccination. RSV A neutralising antibody GMTs for each group at Day 31, after the 1st vaccination. Humoral immune response in terms of RSV PreF3 IgG antibody GMCs for each group, at screening. RSV PreF3 IgG GMCs for each group, at Day 8, after the 1st vaccination. RSV PreF3 IgG GMCs for each group, at Day 31, after the 1st vaccination |
| Starting date | 5 November 2019 |
| Contact information | GlaxoSmithKline |
| Notes |
NCT04681833.
| Study name | Safety and efficacy of BARS13 in the elderly |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 120 |
| Interventions | Drug: recombinant respiratory syncytial virus vaccine (BARS13)/placebo; drug: recombinant respiratory syncytial virus vaccine (BARS13); drug: placebo |
| Outcomes | Incidence and severity of vaccine‐related AEs including the following solicited AEs; incidence and severity of vaccine‐related AEs including the following solicited AEs; incidence and severity of vaccine‐related AEs including the following solicited AEs; occurrence of any SAE; occurrence of any clinically significant clinical laboratory abnormalities |
| Starting date | 24 May 2021 |
| Contact information | Xuefen Huai: +8618351991682; xuefenhuai@advaccine.com Alex Cheng: +86 17600221846; alexcheng@advaccine.com |
| Notes |
NCT04732871.
| Study name | A phase 3, randomised, open‐label, multi‐country study to evaluate the immunogenicity, safety, reactogenicity and persistence of a single dose of the RSVPreF3 OA investigational vaccine and different revaccination schedules in adults aged 60 years and above |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 1720 |
| Interventions | Biological: RSVPreF3 OA investigational vaccine |
| Outcomes | Humoral immune response in terms of RSV‐A neutralising antibody GMTs; RSV‐A neutralising antibody GMTs; humoral immune response in terms of RSV‐B neutralising antibody titres; humoral immune response in terms of RSV‐B neutralising antibody titres; humoral immune response in terms of RSVPreF3 IgG antibody GMCs; humoral immune response in terms of RSV‐A neutralising antibody GMTs; cell‐mediated immune response in terms of frequency of RSVPreF3‐specific cluster of differentiation (CD)4+ and/or CD8+ T cells expressing at least 2 activation markers; number of participants with at least 1 solicited administration‐site event and solicited systemic event; number of participants with SAEs; number of participants with a fatal SAE, related SAE, and related pIMDs |
| Starting date | 15 February 2021 |
| Contact information | GlaxoSmithKline |
| Notes |
NCT04980391.
| Study name | A phase III, double‐blind, randomised, placebo‐controlled study to evaluate the safety, reactogenicity and immune response of a single intramuscular dose of unadjuvanted RSV maternal vaccine, in high‐risk pregnant women aged 15 to 49 years and infants born to the vaccinated mother |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 353 |
| Interventions | Biological: RSV MAT; drug: placebo |
| Outcomes | Percentage of maternal participants reporting solicited administration site events; percentage of maternal participants reporting solicited systemic events; percentage of maternal participants reporting unsolicited AEs; percentage of maternal participants reporting SAEs, (S)AEs leading to study withdrawal, and medically attended adverse events; percentage of maternal participants reporting pregnancy outcomes; percentage of maternal participants reporting pregnancy‐related AESIs; humoral immune response in terms of RSV MAT IgG‐specific antibody concentrations at pre‐dosing (Day 1) for maternal participants; geometric mean ratio between cord blood and maternal RSV MAT IgG‐specific antibody concentrations; humoral immune response in terms of RSV‐A neutralising antibody titres at delivery for infant participants |
| Starting date | 3 August 2021 |
| Contact information | GlaxoSmithKline |
| Notes |
NCT05127434.
| Study name | A phase 2/3, randomised, observer‐blind, placebo‐controlled study to evaluate the safety and efficacy of mRNA‐1345, an mRNA vaccine targeting respiratory syncytial virus (RSV), in adults ≥ 60 years of age |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 34,000 |
| Interventions | mRNA‐1345; placebo |
| Outcomes | Number of participants with solicited local and systemic adverse reactions up to 7 days postinjection; number of participants with unsolicited AEs up to 28 days postinjection; number of participants with medically attended AEs, AESIs, SAEs, and AEs leading to withdrawal up to 24 months postinjection; VE of mRNA‐1345 to prevent a first episode of RSV‐LRTD within the period of 14 days postinjection up to 12 months postinjection; VE of mRNA‐1345 to prevent RT‐PCR confirmed protocol‐defined RSV‐LRTD, defined as 100*(1 − RR), where RR is the ratio of attack rates in the mRNA‐1345 group and the placebo group; VE of mRNA‐1345 to prevent a first episode of RSV‐ARD within the period of 14 days postinjection up to 12 months postinjection; VE of mRNA‐1345 to prevent RT‐PCR confirmed protocol‐defined RSV‐ARD, defined as 100*(1 − RR), where RR is the ratio of attack rates in the mRNA‐1345 group and the placebo group; VE of mRNA‐1345 to prevent hospitalisations associated with RSV‐ARD or RSV‐LRTD within the period of 14 days postinjection up to 12 months postinjection; VE of mRNA‐1345 to prevent RT‐PCR confirmed protocol‐defined RSV‐ARD or RSV‐LRTD, defined as 100*(1 − RR), where RR is the ratio of attack rates in the mRNA‐1345 group and the placebo group; GMT of serum RSV neutralising and binding antibodies (Abs); geometric mean fold‐rise of postbaseline/baseline Ab titres; proportion of participants with ≥ 4‐fold increases in Ab titres from baseline |
| Starting date | 17 November 2021 |
| Contact information | Moderna Clinical Trials Support Center: 1‐877‐777‐7187; clinicaltrials@modernatx.com |
| Notes |
NCT05238025.
| Study name | A randomised, double‐blind, phase 3 trial to assess clinical efficacy, safety and reactogenicity of the recombinant MVA‐BN® ‐RSV vaccine in adults ≥ 60 years of age |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 20,000 |
| Interventions | Biological: MVA‐BN‐RSV vaccine; biological: Tris buffered saline |
| Outcomes | Occurrence of LRTD; occurrence of ARD; occurrence of any SAEs; occurrence of complications and hospitalisations; occurrence of any grade 3 or higher adverse events; RSV‐specific T‐cell responses; RSV‐specific serum neutralising antibody titres; RSV‐specific serum IgG antibody titres; occurrence of solicited systemic adverse events |
| Starting date | April 2022 |
| Contact information | Heinz Weidenthaler: 004989255446 ext 300; hwe@bavarian‐nordic.com |
| Notes |
AEs: adverse events AESIs: adverse events of special interest ARD: acute respiratory disease ARs: adverse reactions CS: clinically significant ELISA: enzyme‐linked immunosorbent assay GMCs: geometric mean concentrations GMTs: geometric mean titres IgG: immunoglobulin G LRTD: lower respiratory tract disease MAE: medically attended adverse event pIMDs: potential immune‐mediated disorders RSV: respiratory syncytial virus RSV‐ARD: respiratory syncytial virus‐associated acute respiratory disease RSV‐LRTD: respiratory syncytial virus‐associated lower respiratory tract disease RSV‐MAARI: respiratory syncytial virus‐associated medically attended acute respiratory illness RSV‐MAALRI: respiratory syncytial virus‐associated medically attended acute lower respiratory illness RT‐PCR: reverse transcription polymerase chain reaction SAEs: serious adverse events Tdap: diphtheria, pertussis, and tetanus VE: vaccine efficacy wt RSV: wild‐type respiratory syncytial virus
Differences between protocol and review
2022 update:
Two new authors contributed to the 2022 update: Diana Buitrago‐Garcia and Camila Montesinos‐Guevara.
Changes to outcomes: We split the outcome of mortality into: 1) due to all causes, and 2) vaccine related.
Studies' search and selection: We used Cochrane’s Screen4Me workflow to help assess the initial search results. We only included randomised controlled trials that reported data on the incidence of common cold, thus we excluded early‐phase studies which did not assess the incidence of common cold.
Unit of analysis issues: In future updates in which multi‐arm studies are included, differences between the study arms should be explored, compared to placebo. If no differences are found, data of all active arms should be pooled and compared with a placebo. If differences are found in one arm, results should be compared separately with placebo. In addition, for future updates with cluster‐randomised controlled trials, if the sample size has been adequately calculated, data should be pooled using the generic inverse‐variance approach. If sample size is incorrect, ‘effective sample size’ should be calculated following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Dealing with missing data: In future updates, we will undertake a complete‐case analysis and an intention‐to‐treat analysis in the case of available data, or perform multiple imputation methods if needed. If numerical outcome data are missing, such as standard deviations or correlation coefficients, and these could not be obtained from the authors, we will calculate them from other available statistics such as P values, following the methods in the Cochrane Handbook for Systematic Reviews of Interventions.
Assessment of heterogeneity: If in future updates we identify substantial heterogeneity as per the Cochrane Handbook (50% to 90%), we will explore this by performing a prespecified subgroup analysis (Higgins 2022).
Subgroup analysis and investigation of heterogeneity: Due to the limited number of included studies, we do not plan to perform a meta‐regression in the future.
Sensitivity analysis: In future updates, we will perform a sensitivity analysis comparing parallel randomised clinical trials versus cluster‐randomised clinical trials.
2016 update:
Three new authors contributed to the 2016 update: Juan VA Franco, Maria L Felix, and Maria José Martinez‐Zapata.
We considered risk of bias for blinding as unclear in the 2013 publication of this review. In the 2016 update, we reassessed this as low because the study used a placebo.
We added two additional primary outcomes, vaccine safety and vaccine‐related mortality, to the summary of findings table.
2014 update:
We did not search Scirus for this update as the service became unavailable in 2014.
Contributions of authors
Conceiving the review: DSR
Designing the review: DSR, CVG, MLF, RH
Co‐ordinating the review: DSR, CMG
Data collection for the review: DBG, CMG
Screening search results: MLF, MJMZ, CMG, DBG
Appraising quality of papers: DSR, CVG, RH, MJMZ
Extracting data from papers: DSR
Writing to authors of papers for additional information: CMG
Obtaining and screening data on unpublished studies: MLF, MJMZ
Data management for the review: DSR
Entering data into Review Manager 5: DSR, MJMZ
Interpretation of data: all authors
Providing a methodological perspective: MJMZ, DBG, CMG, DSR
Providing a clinical perspective: CVG, MLF, RH
Writing the review: CMG, DBG, MJMZ, DSR
All authors contributed to the improvement of this updated review and approved the final version of the review.
Sources of support
Internal sources
-
Cochrane Ecuador, Ecuador
Methodological
-
Centro de Investigación en Salud Pública y Epidemiología Clínica (CISPEC). Facultad de Ciencias de la Salud Eugenio Espejo, Universidad UTE., Ecuador
Methodological
External sources
-
Instituto de Salud Carlos III, Spain
Mª José Martinez Zapata is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CPII20/00023).
Declarations of interest
Camila Montesinos‐Guevara: declared that they have no conflict of interest. Diana Buitrago‐Garcia: declared that they have no conflict of interest. Maria L Felix: declared that they have no conflict of interest. Claudia V Guerra: declared that they have no conflict of interest. Ricardo Hidalgo: declared that they have no conflict of interest. Maria José Martinez‐Zapata: declared that they have no conflict of interest. Daniel Simancas‐Racines: declared that they have no conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Griffin 1970 {published data only}
- Griffin JP, Greenberg BH. Live and inactivated adenovirus vaccines. Clinical evaluation of efficacy in prevention of acute respiratory disease. Archives of Internal Medicine 1970;125(6):981-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abarca 2020 {published data only}
- Abarca K, Rey-Jurado E, Muñoz-Durango N, Vázquez Y, Soto JA, Gálvez N, et al. Safety and immunogenicity evaluation of recombinant BCG vaccine against respiratory syncytial virus in a randomized, double-blind, placebo-controlled phase I clinical trial. EClinicalMedicine 2020;27:100517. [DOI: 10.1016/j.eclinm.2020.100517] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmad 2022 {published data only}
- Ahmad A, Eze K, Noulin N, Horvathova V, Murray B, Baillet M, et al. EDP-938, a respiratory syncytial virus inhibitor, in a human virus challenge. New England Journal of Medicine 2022;386(7):655-66. [DOI: 10.1056/NEJMoa2108903] [PMID: ] [DOI] [PubMed] [Google Scholar]
Aliprantis 2018 {published data only}
- Aliprantis A, Wolford D, Caro L, Maas B, Ma H, Vora K, et al. A randomized, double-blind, placebo-controlled trial to assess the safety and tolerability of a respiratory syncytial virus (RSV) neutralizing monoclonal antibody (MK-1654) in healthy subjects. Open Forum Infectious Diseases 2018;5(Suppl 1):572. [DOI: 10.1093/ofid/ofy210.1627] [DOI] [Google Scholar]
Aliprantis 2020 {published data only}
- Aliprantis AO, Shaw CA, Griffin P, Farinola N, Railkar RA, Cao X, et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Human Vaccines and Immunotherapeutics 2020;17(5):1248-61. [DOI: 10.1080/21645515.2020.1829899] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ascough 2019 {published data only}
- Ascough S, Vlachantoni I, Kalyan M, Haijema BJ, Wallin-Weber S, Dijkstra-Tiekstra M, et al. Local and systemic immunity against respiratory syncytial virus induced by a novel intranasal vaccine a randomized, double-blind, placebo-controlled clinical trial. American Journal of Respiratory and Critical Care Medicine 2019;200(4):481-92. [DOI: 10.1164/rccm.201810-1921OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
August 2017 {published data only}
- August A, Glenn GM, Kpamegan E, Hickman SP, Jani D, Lu H, et al. A phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine 2017;35(30):3749-59. [DOI: 10.1016/j.vaccine.2017.05.045] [DOI] [PubMed] [Google Scholar]
Belshe 1982 {published data only}
- Belshe RB, Van Voris LP, Mufson MA. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. Journal of Infectious Diseases 1982;145(3):311-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Belshe 1992 {published data only}
- Belshe RB, Karron RA, Newman FK, Anderson EL, Nugent SL, Steinhoff M, et al. Evaluation of a live attenuated, cold-adapted parainfluenza virus type 3 vaccine in children. Journal of Clinical Microbiology 1992;30(8):2064-70. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Belshe 2004a {published data only}
- Belshe RB, Newman FK, Anderson EL, Wright PF, Karron RA, Tollefson S, et al. Evaluation of combined live, attenuated respiratory syncytial virus and parainfluenza 3 virus vaccines in infants and young children. Journal of Infectious Diseases 2004;190(12):2096-103. [PMID: ] [DOI] [PubMed] [Google Scholar]
Belshe 2004b {published data only}
- Belshe RB, Newman FK, Tsai TF, Karron RA, Reisinger K, Roberton D, et al. Phase 2 evaluation of parainfluenza type 3 cold passage mutant 45 live attenuated vaccine in healthy children 6-18 months old. Journal of Infectious Diseases 2004;189(3):462-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beran 2018 {published data only}
- Beran J, Lickliter JD, Schwarz TF, Johnson C, Chu L, Domachowske JB, et al. Safety and immunogenicity of 3 formulations of an investigational respiratory syncytial virus vaccine in nonpregnant women: results from 2 phase 2 trials. Journal of Infectious Diseases 2018;217(10):1616-25. [DOI: 10.1093/infdis/jiy065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bourne 1946 {published data only}
- Bourne LB. Trial of an oral vaccine against bacterial infection accompanying the common cold. Nature 1946;157:591. [DOI: 10.1038/157591b0] [DOI] [PubMed] [Google Scholar]
Cicconi 2020 {published data only}
- Cicconi P, Jones C, Sarkar E, Silva-Reyes L, Klenerman P, De Lara C, et al. First-in-human randomized study to assess the safety and immunogenicity of an investigational respiratory syncytial virus (RSV) vaccine based on chimpanzee-adenovirus-155 viral vector–expressing RSV fusion, nucleocapsid, and antitermination viral proteins in healthy adults. Clinical Infectious Diseases 2020;70(10):2073–81. [DOI: 10.1093/cid/ciz653] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clements 1991 {published data only}
- Clements ML, Belshe RB, King J, Newman F, Westblom TU, Tierney EL, et al. Evaluation of bovine, cold-adapted human, and wild-type human parainfluenza type 3 viruses in adult volunteers and in chimpanzees. Journal of Clinical Microbiology 1991;29(6):1175-82. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunningham 2019 {published data only}
- Cunningham CK, Karron R, Muresan P, McFarland EJ, Perlowski C, Libous J, et al. Live-attenuated respiratory syncytial virus vaccine with deletion of RNA synthesis regulatory protein M2-2 and cold passage mutations is overattenuated. Open Forum Infectious Diseases 2019;6(6):ofz212. [DOI: 10.1093/ofid/ofz212] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeVincenzo 2010 {published data only}
- DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proceedings of the National Academy of Sciences of the United States of America 2010;107(19):8800-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeVincenzo 2019 {published data only}
- DeVincenzo J, Gymnopoulou E, De Paepe E, Murray B, Bastian AR, Haazen W. A randomized, double-blind, placebo-controlled study to evaluate the efficacy of a single immunization of AD26.RSV.Pref against RSV infection in a viral challenge model in healthy adults. Open Forum Infectious Diseases 2019;6(Suppl):27-8. [DOI: 10.1093/ofid/ofz359.061] [DOI] [Google Scholar]
Doggett 1963 {published data only}
- Doggett JE, Bynoe ML, Tyrrell DA. Some attempts to produce an experimental vaccine with rhinoviruses. British Medical Journal 1963;1(5322):34-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Domachowske 2017 {published data only}
- Domachowske JB, Khan A, Esser MT, Jensen KM, Takas T, Villafana T, et al. A single dose monoclonal antibody (mAb) immunoprophylaxis strategy to prevent RSV disease in all infants: results of the first in infant study with MEDI8897. Open Forum Infectious Diseases 2017;4(Suppl 1):S37. [DOI: 10.1093/ofid/ofx162.089] [PMID: ] [DOI] [Google Scholar]
Domachowske 2018 {published data only}
- Domachowske JB, Khan AA, Jensen K, Takas T, Villafana T, Dubovsky F, et al. A single dose monoclonal antibody immunoprophylaxis strategy to prevent respiratory syncytial virus disease in all infants: results of the first in infant study with MEDI8897. Pediatrics 2018;141(1):256. [DOI: 10.1542/peds.141.1_MeetingAbstract.256] [DOI]
Dudding 1972 {published data only}
- Dudding BA, Bartelloni PJ, Scott RM, Top FH Jr, Russell PK, Buescher EL. Enteric immunization with live adenovirus type 21 vaccine I. Tests for safety, infectivity, immunogenicity, and potency in volunteers. Infection and Immunity 1972;5(3):295-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 2019 {published data only}
- Esposito S, Bianchini S, Bosis S, Tagliabue C, Coro I, Argentiero A, et al. A randomized, placebo-controlled, double-blinded, single-centre, phase IV trial to assess the efficacy and safety of OM-85 in children suffering from recurrent respiratory tract infections. Journal of Translational Medicine 2019;17(1):284. [DOI: 10.1186/s12967-019-2040-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR2008‐001714‐24‐GB {published data only}
- EUCTR2008-001714-24-GB. A phase II, double-blind placebo-controlled study to determine the prophylactic efficacy of oral BTA798 in an experimental rhinovirus challenge model. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2008%E2%80%90001714%E2%80%9024%E2%80%90GB (first received 18 June 2008).
EUCTR2012‐001107‐20‐GB {published data only}
- EUCTR2012-001107-20-GB. A phase 2a randomised, double-blind, placebo-controlled repeat dose trial of the activity of MDT-637 in healthy subjects challenged with RSV-A (Memphis 37b) - phase 2a trial of MDT-637 in healthy subjects challenged with RSV. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2012%E2%80%90001107%E2%80%9020%E2%80%90GB (first received 2 August 2013).
EUCTR2013‐004036‐30‐GB {published data only}
- EUCTR2013-004036-30-GB. A randomised, phase 2a, double-blind, placebo-controlled study to evaluate the safety, pharmacokinetics and antiviral activity of multiple doses of orally administered ALS-008176 against respiratory syncytial virus infection in the virus challenge model - phase 2a, double blind, placebo-controlled, viral challenge study. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2013%E2%80%90004036%E2%80%9030%E2%80%90GB (first received 29 November 2013).
EUCTR2014‐005041‐41‐GB {published data only}
- EUCTR2014-005041-41-GB. A phase 2a, randomized, double-blinded, placebo-controlled study to evaluate the antiviral activity, safety, and pharmacokinetics of repeated doses of orally administered JNJ-53718678 against respiratory syncytial virus infection in the virus challenge model in healthy adult subjects. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2014%E2%80%90005041%E2%80%9041%E2%80%90GB (first received 24 April 2015).
EUCTR2015‐004296‐77‐GB {published data only}
- EUCTR2015-004296-77-GB. A randomised, phase 2a, double‐blind, placebo‐controlled study to evaluate the safety and antiviral activity against respiratory syncytial virus infection, and the pharmacokinetics of multiple oral doses of BTA‐C585 in the virus challenge model. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2015%E2%80%90004296%E2%80%9077%E2%80%90GB (first received 8 February 2016).
EUCTR2016‐000117‐76‐ES {published data only}
- EUCTR2016-000117-76-ES. A study to evaluate safety, reactogenicity and immunogenicity of GSK biologicals’ RSV investigational vaccine based on viral proteins encoded by chimpanzee-derived adenovector (ChAd155-RSV) (GSK3389245A) in RSV-seropositive infants. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2016%E2%80%90000117%E2%80%9076%E2%80%90ES (first received 5 August 2016).
EUCTR2016‐000117‐76‐PL {published data only}
- EUCTR2016-000117-76-PL. A study to evaluate safety and immunogenicity of GSK biologicals’ RSV investigational vaccine in RSV-seropositive infants aged 12 to 23 months. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2016%E2%80%90000117%E2%80%9076%E2%80%90PL (first received 6 June 2018).
EUCTR2016‐001135‐12‐FR {published data only}
- EUCTR2016-001135-12-FR. A study to rank different dosages of antigen of GlaxoSmithKline (GSK) biologicals’ investigational respiratory syncytial virus (RSV) vaccine (GSK3003891A), based on their immune response and safety, when administered to healthy adult women. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2016%E2%80%90001135%E2%80%9012%E2%80%90FR (first received 14 December 2016).
EUCTR2016‐002733‐30‐ES {published data only}
- EUCTR2016-002733-30-ES. A study to evaluate the safety, reactogenicity and immunogenicity of the GSK investigational vaccine GSK3003891A in healthy pregnant women and infants born to vaccinated mothers. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2016%E2%80%90002733%E2%80%9030%E2%80%90ES (first received 8 February 2017).
EUCTR2018‐001340‐62‐FI {published data only}
- EUCTR2018-001340-62-FI. A study to evaluate different dose levels of GlaxoSmithKline (GSK) biologicals' investigational respiratory syncytial virus (RSV) vaccine (GSK3888550A), based on the safety of the vaccine and the antibodies (body defences) that the body produces following the vaccine administration, when given to healthy non-pregnant women. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2018%E2%80%90001340%E2%80%9062%E2%80%90FI (first received 17 October 2018).
Falloon 2017a {published data only}
- Falloon J, Talbot HK, Curtis C, Ervin J, Krieger D, Dubovsky F, et al. Dose selection for an adjuvanted respiratory syncytial virus F protein vaccine for older adults based on humoral and cellular immune responses. Clinical and Vaccine Immunology 2017;24(9):e00157-17. [DOI: 10.1128/CVI.00157-17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falloon 2017b {published data only}
- Falloon J, Yu J, Esser MT, Villafana T, Yu L, Dubovsky F, et al. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. Journal of Infectious Diseases 2017;216(11):1362-70. [DOI: 10.1093/infdis/jix503] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falsey 1996 {published data only}
- Falsey AR, Walsh EE. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in ambulatory adults over age 60. Vaccine 1996;14(13):1214-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Falsey 2008 {published data only}
- Falsey AR, Walsh EE, Capellan J, Gravenstein S, Zambon M, Yau E, et al. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (RSV) vaccines - nonadjuvanted vaccine or vaccine adjuvanted with alum-given concomitantly with influenza vaccine to high-risk elderly individuals. Journal of Infectious Diseases 2008;198(9):1317-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fries 2019 {published data only}
- Fries LF, Cho I, Thomas DN, Wen JL, Spindler MS, Fix AB, et al. Third trimester immunization with an respiratory syncytial virus F protein vaccine for the prevention of RSV lower respiratory tract infection in infants. Open Forum Infectious Diseases 2019;6(Suppl):921-2. [DOI: 10.1093/ofid/ofz360.2315] [DOI] [Google Scholar]
Fulginiti 1969 {published data only}
- Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. American Journal of Epidemiology 1969;89(4):435-48. [PMID: ] [DOI] [PubMed] [Google Scholar]
Glenn 2016 {published data only}
- Glenn GM, Fries LF, Thomas DN, Smith G, Kpamegan E, Lu H, et al. A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. Journal of Infectious Diseases 2016;213(3):411-22. [DOI: 10.1093/infdis/jiv406] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gomez 2009 {published data only}
- Gomez M, Mufson MA, Dubovsky F, Knightly C, Zeng W, Losonsky G. Phase-I study MEDI-534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatric Infectious Disease Journal 2009;28(7):655-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gonzalez 2000 {published data only}
- Gonzalez IM, Karron RA, Eichelberger M, Walsh EE, Delagarza VW, Bennett R, et al. Evaluation of the live attenuated cpts 248/404 RSV vaccine in combination with a subunit RSV vaccine (PFP-2) in healthy young and older adults. Vaccine 2000;18(17):1763-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Greenberg 2005 {published data only}
- Greenberg DP, Walker RE, Lee MS, Reisinger KS, Ward JI, Yogev R, et al. A bovine parainfluenza virus type 3 vaccine is safe and immunogenic in early infancy. Journal of Infectious Diseases 2005;191(7):1116-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hamory 1975 {published data only}
- Hamory BH, Hamparian VV, Conant RM, Gwaltney JM. Human responses to two decavalent rhinovirus vaccines. Journal of Infectious Diseases 1975;132(6):623-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Israel 2016 {published data only}
- Israel S, Rusch S, DeVincenzo J, Boyers A, Fok-Seang J, Huntjens D, et al. Effect of oral JNJ-53718678 (JNJ-678) on disease severity in healthy adult volunteers experimentally inoculated with live respiratory syncytial virus (RSV): a placebo-controlled challenge study. Open Forum Infectious Diseases 2016;3(Suppl 1):650. [DOI: 10.1093/ofid/ofw172.513] [DOI] [Google Scholar]
Karppinen 2019 {published data only}
- Karppinen S, Toivonen L, Schuez-Havupalo L, Teros-Jaakkola T, Waris M, Auranen K, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against all respiratory tract infections in children under two years of age. Vaccine 2019;37(22):2935-41. [DOI: 10.1016/j.vaccine.2019.04.026] [DOI] [PubMed] [Google Scholar]
Karron 1995a {published data only}
- Karron RA, Wright PF, Hall SL, Makhene M, Thompson J, Burns BA, et al. A live attenuated bovine parainfluenza virus type 3 vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. Journal of Infectious Diseases 1995;171(5):1107-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karron 1995b {published data only}
- Karron RA, Wright PF, Newman FK, Makhene M, Thompson J, Samorodin R, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in healthy infants and children. Journal of Infectious Diseases 1995;172(6):1445-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karron 1997 {published data only}
- Karron RA, Wright PF, Crowe JE, Clements-Mann ML, Thompson J, Makhene M, et al. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. Journal of Infectious Diseases 1997;176(6):1428-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karron 2003 {published data only}
- Karron RA, Belshe RB, Wright PF, Thumar B, Burns B, Newman F, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatric Infectious Disease Journal 2003;22(5):394-405. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karron 2005 {published data only}
- Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. Journal of Infectious Diseases 2005;191(7):1093-104. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karron 2015 {published data only}
- Karron RA, San Mateo J, Thumar B, Schaap-Nutt A, Buchholz UJ, Schmidt AC, et al. Evaluation of a live-attenuated human parainfluenza type 1 vaccine in adults and children. Journal of Pediatric Infectious Diseases Society 2015;4(4):e143-6. [DOI: 10.1093/jpids/piu104] [PMID: ] [DOI] [PMC free article] [PubMed]
Karron 2020a {published data only}
- Karron RA, Atwell JE, McFarland EJ, Cunningham CK, Muresan P, Perlowski C. Live-attenuated vaccines prevent respiratory syncytial virus-associated illness in young children. American Journal of Respiratory and Critical Care Medicine 2021;203(5):594-603. [DOI: 10.1164/rccm.202005-1660OC] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karron 2020b {published data only}
- Karron RA, Luongo C, Mateo JS, Wanionek K, Collins PL, Buchholz UJ. Safety and immunogenicity of the respiratory syncytial virus vaccine RSV/DELTANS2/DELTA1313/I1314L in RSV-seronegative children. Journal of Infectious Diseases 2020;222(1):82-91. [DOI: 10.1093/infdis/jiz408] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumpu 2015 {published data only}
- Kumpu M, Kekkonen RA, Korpela R, Tynkkynen S, Järvenpää S, Kautiainen H, et al. Effect of live and inactivated Lactobacillus rhamnosus GG on experimentally induced rhinovirus colds: randomised, double blind, placebo-controlled pilot trial. Beneficial Microbes 2015;6(5):631-9. [DOI: 10.3920/BM2014.0164] [PMID: ] [DOI] [PubMed] [Google Scholar]
Langley 2009 {published data only}
- Langley JM, Sales V, McGeer A, Guasparini R, Predy G, Meekison W, et al. A dose-ranging study of a subunit respiratory syncytial virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults > or = 65 years of age. Vaccine 2009;27(42):5913-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Langley 2016 {published data only}
- Langley J, Macdonald L, Weir G, Mackinnon-Cameron D, Ye L, McNeil S, et al. A phase I randomized, observer-blind, controlled, dose escalation trial of the safety and tolerability of two intramuscular doses of DPXRSV (A), a respiratory syncytial virus (RSV) vaccine containing RSV SH antigen and a novel adjuvant depovax, or SH antigen and a novel adjuvant DepoVax, or SH antigen co-administered with aluminum hydroxide, or placebo to healthy adults ≥ 50–64 years of age. Open Forum Infectious Diseases 2016;3:ofw172.97. [DOI: 10.1093/ofid/ofw172.973] [DOI] [Google Scholar]
Langley 2017 {published data only}
- Langley JM, Aggarwal N, Toma A, Halperin SA, McNeil SA, Fissette L, et al. A randomized, controlled, observer-blinded phase 1 study of the safety and immunogenicity of a respiratory syncytial virus vaccine with or without alum adjuvant. Journal of Infectious Diseases 2017;215(1):24-33. [DOI: 10.1093/infdis/jiw453] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langley 2018 {published data only}
- Langley J, Macdonald L, Weir G, Mackinnon-Cameron D, Ye L, Mcneil S, et al. A respiratory syncytial virus vaccine based on the small hydrophobic protein ectodomain presented with a novel lipid-based formulation is highly immunogenic and safe in adults: a first-in-humans study. Journal of Infectious Diseases 2018;218(3):378-87. [DOI: 10.1093/infdis/jiy177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2001 {published data only}
- Lee MS, Greenberg DP, Yeh SH, Yogev R, Reisinger KS, Ward JI, et al. Antibody responses to bovine parainfluenza virus type 3 (PIV3) vaccination and human PIV3 infection in young infants. Journal of Infectious Diseases 2001;184(7):909-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2004 {published data only}
- Lee FE, Walsh EE, Falsey AR, Betts RF, Treanor JJ. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Research 2004;63(3):191-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leroux‐Roels 2019 {published data only}
- Leroux-Roels G, De Boever F, Maes C, Nguyen TL-A, Baker S, Gonzalez Lopez A. Safety and immunogenicity of a respiratory syncytial virus fusion glycoprotein F subunit vaccine in healthy adults: results of a phase 1, randomized, observer-blind, controlled, dosage-escalation study. Vaccine 2019;37(20):2694-703. [DOI: 10.1016/j.vaccine.2019.04.011] [DOI] [PubMed] [Google Scholar]
Lyons 2008 {published data only}
- Lyons A, Longfield J, Kuschner R, Straight T, Binn L, Seriwatana J, et al. A double-blind, placebo-controlled study of the safety and immunogenicity of live, oral type 4 and type 7 adenovirus vaccines in adults. Vaccine 2008;26(23):2890-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Madhi 2006 {published data only}
- Madhi SA, Cutland C, Zhu Y, Hackell JG, Newman F, Blackburn N, et al. Transmissibility, infectivity and immunogenicity of a live human parainfluenza type 3 virus vaccine (HPIV3cp45) among susceptible infants and toddlers. Vaccine 2006;24(13):2432-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Madhi 2020 {published data only}
- Madhi SA, Polack FP, Piedra PA, Munoz FM, Trenholme AA, Simões E, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. New England Journal of Medicine 2020;383(5):426-39. [DOI: 10.1056/NEJMoa1908380] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McFarland 2018 {published data only}
- McFarland EJ, Karron RA, Muresan P, Cunningham CK, Valentine ME, Perlowski C, et al. Live-attenuated respiratory syncytial virus vaccine candidate with deletion of RNA synthesis regulatory protein M2-2 is highly immunogenic in children. Journal of Infectious Diseases 2018;217(9):1347-55. [DOI: 10.1093/infdis/jiy040] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McFarland 2020a {published data only}
- McFarland EJ, Karron RA, Muresan P, Cunningham CK, Perlowski C, Libous J, et al. Live respiratory syncytial virus attenuated by M2-2 deletion and stabilized temperature sensitivity mutation 1030s is a promising vaccine candidate in children. Journal of Infectious Diseases 2020;221(4):534-43. [DOI: 10.1093/infdis/jiz603] [DOI] [PMC free article] [PubMed] [Google Scholar]
McFarland 2020b {published data only}
- McFarland EJ, Karron RA, Muresan P, Cunningham CK, Perlowski C, Libous J, et al. Live-attenuated respiratory syncytial virus vaccine with M2-2 deletion and with small hydrophobic noncoding region is highly immunogenic in children. Journal of Infectious Diseases 2020;221(12):2050-9. [DOI: 10.1093/infdis/jiaa049] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munoz 2003 {published data only}
- Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine 2003;21(24):3465-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Munoz 2019 {published data only}
- Muňoz FM, Swamy GK, Hickman SP, Agrawal S, Piedra PA, Glenn GM, et al. Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. Journal of Infectious Diseases 2019;220(11):1802-15. [DOI: 10.1093/infdis/jiz390] [DOI] [PubMed] [Google Scholar]
Murphy 1994 {published data only}
- Murphy BR, Hall SL, Kulkarni AB, Crowe JE, Collins PL, Connors M, et al. An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Research 1994;32(1):13-36. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT00139347 {published data only}
- NCT00139347. A multi-country & multi-center study to assess the efficacy, immunogenicity & safety of two doses of GSK biologicals' oral live attenuated HRV vaccine given concomitantly with routine EPI vaccinations including OPV in healthy infants. clinicaltrials.gov/show/NCT00139347 (first received 31 August 2005).
NCT00308412 {published data only}
- NCT00308412. Safety of and immune response to a human parainfluenza virus vaccine (rHPIV3cp45) in healthy infants [Phase 1 study to determine the safety, infectivity, and tolerability of two doses of live attenuated recombinant cold passaged (cp) 45 parainfluenza type 3 virus vaccine, rHPIV3cp45, lot PIV3 102A, delivered as nose drops to infants 6 to 12 months of age, and to HPIV3 seronegative infants and children 6 to 36 months of age]. clinicaltrials.gov/show/NCT00308412 (first received 29 March 2006).
NCT00345670 {published data only}
- NCT00345670. Study to evaluate the safety of MEDI-534 vaccine against respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) in healthy children [A phase I, randomized, double-blind, placebo-controlled, dose-escalation study to evaluate the safety, tolerability, immunogenicity, and viral shedding of MEDI-534, a live, attenuated intranasal vaccine against respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3), in healthy RSV and PIV3 seropositive 1-9 year-old children]. clinicaltrials.gov/show/NCT00345670 (first received 28 June 2006).
NCT00345956 {published data only}
- NCT00345956. To evaluate immunogenicity, reactogenicity & safety of 2 doses of GSK Bio HRV liquid vaccine given to infants (Vietnam) [A placebo-controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GSK biologicals' oral live attenuated human rotavirus (HRV) liquid vaccine, when given to healthy infants, in Vietnam]. clinicaltrials.gov/show/NCT00345956 (first received 29 June 2006).
NCT00363545 {published data only}
- NCT00363545. To assess immunogenicity, reactogenicity & safety of 2 formulations of GSK's HRV vaccine as 2-dose vaccination (Infants) [A study to assess the immunogenicity, reactogenicity and safety of 2 different formulations of GSK biologicals' live attenuated HRV vaccine, given as a two-dose primary vaccination, in healthy infants previously uninfected with HRV]. clinicaltrials.gov/ct2/show/NCT00363545 (first received 15 August 2006).
NCT00366782 {published data only}
- NCT00366782. Safety of and immune response to a cow/human parainfluenza virus vaccine (rB/HPIV3) in healthy infants, children, and adults [A phase 1 study of the safety and immunogenicity of the recombinant live-attenuated chimeric bovine/human parainfluenza type 3 virus vaccine, rB/HPIV3, lot PIV3 #101A, delivered as nose drops to adults 18 to 49 years of age, HPIV3-seropositive children 15 to 59 months of age, and HPIV3-seronegative infants and children 6 to 36 months of age]. clinicaltrials.gov/ct2/show/NCT00366782 (first received 21 August 2006).
NCT00383903 {published data only}
- NCT00383903. Evaluate safety & immunogenicity of 2 or 3 doses of GSK HRV vaccine in healthy infants in South Africa [A phase II, randomized, double-blind, placebo-controlled study of safety, reactogenicity and immunogenicity of 2 or 3 doses of GSK biologicals' oral live attenuated human rotavirus vaccine at 10E6.5 CCID50 viral concentration in healthy infants (approximately 5-10 weeks old) in the Republic of South Africa]. clinicaltrials.gov/ct2/show/NCT00383903 (first received 4 October 2006).
NCT00420316 {unpublished data only}
- NCT00420316. Long-term efficacy and safety of subjects approximately 3 years after priming with 2 doses of GSK Bio's HRV vaccine [To assess long-term efficacy & safety of subjects approximately 3 years after priming with 2 doses of GlaxoSmithKline (GSK) biologicals' oral live attenuated human rotavirus (HRV) vaccine (Rotarix) in the primary vaccination study (102247)]. clinicaltrials.gov/ct2/show/NCT00420316 (first received 11 January 2007).
NCT00496821 {published data only}
- NCT00496821. Intranasal ALN-RSV01 administered to adult volunteers experimentally inoculated with respiratory syncytial virus [A study to investigate the safety and efficacy of intranasal ALN-RSV01 administered to adult volunteers experimentally inoculated with respiratory syncytial virus]. clinicaltrials.gov/ct2/show/NCT00496821 (first received 4 July 2007).
NCT00641017 {published data only}
- NCT00641017. Safety of and immune response to recombinant live-attenuated parainfluenza type 1 virus vaccine [A phase I study of the safety and immunogenicity of the recombinant live-attenuated human parainfluenza type 1 virus vaccine, rHPIV1 84/del170/942A, lot PIV1 #104A, delivered as nose drops to adults 18 to 49 years of age, HPIV1-seropositive children 15 to 59 months of age, and hpiv1-seronegative infants and children 6 to 59 months of age]. clinicaltrials.gov/ct2/show/NCT00641017 (first received 21 March 2008).
NCT00686075 {published data only}
- NCT00686075. A study to evaluate the safety, tolerability, immunogenicity and vaccine-like viral shedding of MEDI-534, against respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3), in healthy 6 to < 24 month-old children and in 2 month-old infants [A phase 1/2a, randomized, double-blind, placebo-controlled, dose-escalation study to evaluate the safety, tolerability, immunogenicity and vaccine-like viral shedding of MEDI-534, a live, attenuated intranasal vaccine against respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3), in healthy 6 to < 24 month-old children and in 2 month-old infants]. clinicaltrials.gov/ct2/show/NCT00686075 (first received 29 May 2008).
NCT00767416 {published data only}
- NCT00767416. A randomized, double-blind, placebo-controlled study to evaluate safety of MEDI-559 in healthy 1 to < 24 month-old children [A phase 1/2a, randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, immunogenicity, and viral shedding of MEDI-559, a live attenuated intranasal vaccine against respiratory syncytial virus in healthy 1 to < 24 month-old children]. clinicaltrials.gov/ct2/show/NCT00767416 (first received 7 October 2008).
NCT01021397 {published data only}
- NCT01021397. Safety of and immune response to recombinant live attenuated parainfluenza type 3 virus vaccine in healthy infants and children [Phase I study to determine the safety, infectivity, and tolerability of 2 doses of live attenuated recombinant cold-passaged (cp) 45 human parainfluenza type 3 virus vaccine, rHPIV3cp45, lot PIV3#102A, delivered as nose drops to HPIV3-seronegative infants and children 6 to 36 months of age, at a 6 month interval]. clinicaltrials.gov/ct2/show/NCT01021397 (first received 30 November 2009).
NCT01139437 {published data only}
- NCT01139437. Safety of a live attenuated human parainfluenza virus type 2 (HPIV2) vaccine for adults, children, and infants [A phase I study of the safety and immunogenicity of the recombinant live-attenuated human parainfluenza type 2 virus vaccine, rHPIV2 15C/948L/Δ1724 lot PIV2#109C, delivered as nose drops to adults 18 to 49 years of age, HPIV2-seropositive children 15 to 59 months of age, and HPIV2-seronegative infants and children 6 to 59 months of age]. clinicaltrials.gov/ct2/show/NCT01139437 (first received 8 June 2010).
NCT01254175 {published data only}
- NCT01254175. Evaluating the safety and immunogenicity of a human parainfluenza type 3 (HPIV3) virus vaccine in infants and children [Phase 1 study to determine the safety, infectivity, immunogenicity and tolerability of 2 doses of live attenuated recombinant cold-passaged (cp) 45 human parainfluenza type 3 virus vaccine, rHPIV3cp45, lot PIV3#102A, delivered as nose drops to HPIV3-seronegative infants and children 6 to 36 months of age, at a 6 month interval]. clinicaltrials.gov/ct2/show/NCT01254175 (first received 6 December 2010).
NCT01290419 {published data only}
- NCT01290419. Safety study of respiratory syncytial virus (RSV)-fusion (F) protein particle vaccine [A phase 1 randomized, observer-blinded,placebo-controlled trial to evaluate the safety and immunogenicity of a recombinant respiratory syncytial virus f protein particle vaccine in healthy adults]. clinicaltrials.gov/ct2/show/NCT01290419 (first received 7 February 2011).
NCT01475305 {published data only}
- NCT01475305. Intranasal challenge of healthy adults with respiratory syncytial virus (RSV) [A phase 1 randomized, placebo-controlled, double-blind study to evaluate the safety and efficacy of MEDI-557 in healthy adults intranasally challenged with respiratory syncytial virus (RSV)]. clinicaltrials.gov/ct2/show/NCT01475305 (first received 21 November 2011).
NCT01709019 {published data only}
- NCT01709019. RSV-F vaccine and influenza vaccine co-administration study in the elderly [A phase I randomized, observer-blinded, dose-ranging study to evaluate the immunogenicity and safety of an RSV-F protein nanoparticle vaccine, with or without aluminum adjuvant, and co-administered with a licensed inactivated influenza vaccine, in healthy subjects ≥ 60 years of age]. clinicaltrials.gov/ct2/show/NCT01709019 (first received 17 October 2012).
NCT01852266 {published data only}
- NCT01852266. Evaluating the safety and immune response to a single dose of a respiratory syncytial virus (RSV) vaccine in RSV-seronegative infants and children [A phase I study of the safety and immunogenicity of a single dose of the recombinant live-attenuated respiratory syncytial virus vaccine RSV cps2, lot RSV#005A, delivered as nose drops to RSV-seronegative infants and children 6 to 24 months of age]. clinicaltrials.gov/ct2/show/NCT01852266 (first received 13 May 2013).
NCT01905215 {published data only}
- NCT01905215. Study to evaluate the safety, reactogenicity and immunogenicity of GlaxoSmithKline (GSK) biologicals' investigational respiratory syncytial virus (RSV) vaccines [An observer-blind study to evaluate the safety, reactogenicity and immunogenicity of GSK biologicals' respiratory syncytial virus (RSV) investigational vaccine (GSK3003891A) in healthy men]. clinicaltrials.gov/ct2/show/NCT01905215 (first received 23 July 2013).
NCT02115815 {published data only}
- NCT02115815. A study to evaluate the safety of the respiratory syncytial virus vaccine MEDI7510 in older adults [A phase 1a study to evaluate the safety of the respiratory syncytial virus vaccine MEDI7510 in older adults]. clinicaltrials.gov/ct2/show/NCT02115815 (first received 16 April 2014).
NCT02266628 {published data only}
- NCT02266628. Placebo-controlled study to evaluate the safety and immunogenicity of the RSV-F vaccine in elderly adults [A phase II randomized, observer-blind, placebo-controlled study to evaluate the immunogenicity and safety of respiratory syncytial virus (RSV) F vaccine in healthy elderly subjects and to estimate the incidence rate of medically-attended RSV disease in vaccine and placebo recipients]. clinicaltrials.gov/ct2/show/NCT02266628 (first received 17 October 2014).
NCT02296463 {published data only}
- NCT02296463. A phase I randomized, observer-blinded, dose-ranging study in healthy subjects 24 to < 72 months of age [A phase I randomized, observer-blinded, dose-ranging study to evaluate the immunogenicity and safety of a respiratory syncytial virus (RSV) recombinant fusion (F) nanoparticle vaccine, with or without aluminum adjuvant, in healthy subjects 24 to < 72 months of age]. clinicaltrials.gov/ct2/show/NCT02296463 (first received 20 November 2014).
NCT02419391 {published data only}
- NCT02419391. Trial to evaluate the safety, tolerability and immunogenicity of the recombinant MVA BN® RSV vaccine [A randomized, single-blind, placebo-controlled phase i trial to evaluate the safety, tolerability and immunogenicity of the recombinant MVA BN® RSV vaccine in healthy adult subjects]. clinicaltrials.gov/ct2/show/NCT02419391 (first received 17 April 2015).
NCT02440035 {published data only}
- NCT02440035. A study to evaluate the safety, tolerability and immunogenicity of Ad35.RSV.FA2 regimens boosted with Ad26.RSV.FA2 in healthy adult participants [Phase 1, first in human study to evaluate the safety, tolerability and immunogenicity of Ad35.RSV.FA2 regimens boosted with Ad26.RSV.FA2 in healthy adult volunteers]. clinicaltrials.gov/ct2/show/NCT02440035 (first received 17 April 2015).
NCT02472548 {published data only}
- NCT02472548. A study to evaluate the safety and reactogenicity of DPX-RSV(A), a respiratory syncytial virus [A phase I randomized, observer-blind, controlled, dose escalation trial of the safety and tolerability of two intramuscular doses of DPX-RSV(A), a respiratory syncytial virus vaccine containing respiratory syncytial virus (RSV) SHe antigen and a novel adjuvant DepoVaxTM, or SHe a antigen co-administered with aluminum hydroxide, or placebo to healthy adults ≥50-64 years of age]. clinicaltrials.gov/ct2/show/NCT02472548 (first received 16 June 2015).
NCT02479750 {published data only}
- NCT02479750. Evaluation of ColdZyme® on experimentally induced common cold [Evaluation of ColdZyme® on experimentally induced common cold - a double-blind, randomized, placebo-controlled study in healthy volunteers]. clinicaltrials.gov/ct2/show/NCT02479750 (first received 24 June 2015).
NCT02491463 {published data only}
- NCT02491463. A study to assess the safety, reactogenicity and immunogenicity of GlaxoSmithKline (GSK) biologicals' RSV investigational vaccine (ChAd155-RSV) (GSK3389245A) in healthy adults [A study to evaluate safety, reactogenicity and immunogenicity of GSK biologicals' RSV investigational vaccine based on viral proteins encoded by chimpanzee-derived adenovector (ChAd155-RSV) (GSK3389245A) in healthy adults]. clinicaltrials.gov/ct2/show/NCT02491463 (first received 8 July 2015).
NCT02561871 {published data only}
- NCT02561871. A study to evaluate the safety, tolerability and immunogenicity of Ad26.RSV.FA2 followed by Ad35.RSV.FA2 in healthy adult volunteers [Phase 1, first in human study to evaluate the safety, tolerability and immunogenicity of Ad26.RSV.FA2 followed by Ad35.RSV.FA2 in healthy adult volunteers]. clinicaltrials.gov/ct2/show/NCT02561871 (first received 28 September 2015).
NCT02593071 {published data only}
- NCT02593071. Safety and immunogenicity of the RSV-F vaccine in older adults previously treated with the same vaccine or placebo in the prior year [A phase II randomized, observer-blind, placebo-controlled study to evaluate the immunogenicity and safety of a respiratory syncytial virus (RSV) recombinant F nanoparticle vaccine in healthy older adult subjects previously treated with the same vaccine, or placebo, in the prior year; and to estimate the incidence rate of RSV disease and vaccine efficacy in subjects based on their RSV F vaccine experience over two consecutive years]. clinicaltrials.gov/ct2/show/NCT02593071 (first received 30 October 2015).
NCT02601612 {published data only}
- NCT02601612. Safety and immunogenicity of the RSV D46cpΔM2-2 Vaccine in RSV-seropositive children and RSV-seronegative infants and children [A phase I study of the safety and immunogenicity of a single dose of the live recombinant RSV D46cpΔM2-2 vero grown virus vaccine (lot RSV #008A), delivered as nose drops to RSV-seropositive children 12 to 59 months of age and RSV-seronegative infants and children 6 to 24 months of age]. clinicaltrials.gov/ct2/show/NCT02601612 (first received 10 November 2015).
NCT02624947 {published data only}
- NCT02624947. A study to determine the safety and efficacy of the RSV F vaccine to protect infants via maternal immunization [A phase 3, randomized, observer-blind, placebo-controlled study to determine the immunogenicity and safety of a respiratory syncytial virus (RSV) F nanoparticle vaccine with aluminum in healthy third-trimester pregnant women; and safety and efficacy of maternally transferred antibodies in preventing rsv disease in their infants]. clinicaltrials.gov/ct2/show/NCT02624947 (first received 9 December 2015).
NCT02794870 {published data only}
- NCT02794870. Evaluating the infectivity, safety and immunogenicity of a recombinant live-attenuated respiratory syncytial virus vaccine in RSV-seronegative infants 6 to 24 months of age [Phase I placebo-controlled study of the infectivity, safety and immunogenicity of a single dose of a recombinant live-attenuated respiratory syncytial virus vaccine, LID ΔM2-2 1030s, lot RSV#010A, delivered as nose drops to RSV-seronegative infants 6 to 24 months of age]. clinicaltrials.gov/ct2/show/NCT02794870 (first received 9 June 2016).
NCT02830932 {published data only}
- NCT02830932. Dose-ranging trial of safety & immunogenicity of an oral adenoviral-vector based RSV vaccine (VXA-RSV-f) [A phase 1, randomized, double-blind, placebo-controlled, dose-ranging trial to determine the safety and immunogenicity of an adenoviral-vector based respiratory syncytial virus (RSV) F protein vaccine (VXA-RSV-f) expressing protein F and dsRNA adjuvant administered orally to healthy volunteers]. clinicaltrials.gov/ct2/show/NCT02830932 (first received 13 July 2016).
NCT02864628 {published data only}
- NCT02864628. RSV-MVA-BN vaccine phase I trial, intranasal application in adults [A partially randomized, partly placebo controlled phase i trial to evaluate the safety, tolerability and immunogenicity of the recombinant MVA-BN® RSV vaccine after intranasal and intramuscular administration]. clinicaltrials.gov/ct2/show/NCT02864628 (first received 12 August 2016).
NCT02873286 {published data only}
- NCT02873286. RSV-MVA-BN vaccine phase II trial in ≥ 55 year old adults [A randomized, single-blind, placebo controlled, dose-ranging phase II trial in ≥ 55 year old adults to evaluate the safety and immunogenicity of the recombinant MVA-BN-RSV vaccine]. clinicaltrials.gov/ct2/show/NCT02873286 (first received 19 August 2016).
NCT02890381 {published data only}
- NCT02890381. Evaluating the infectivity, safety and immunogenicity of a recombinant live-attenuated respiratory syncytial virus vaccine (RSV LID cp ΔM2-2) in RSV-seronegative infants 6 to 24 months of age [Phase I placebo-controlled study of the infectivity, safety and immunogenicity of a single dose of a recombinant live-attenuated respiratory syncytial virus vaccine, LID cp ΔM2-2, lot RSV#009B, delivered as nose drops to RSV-seronegative infants 6 to 24 months of age]. clinicaltrials.gov/ct2/show/NCT02890381 (first received 7 September 2016).
NCT02926430 {published data only}
- NCT02926430. A study to evaluate the safety, tolerability and immunogenicity of two vaccinations of Ad26.RSV.preF one year apart in adults aged 60 years and older in stable health [A randomized, double-blind, first-in-human phase 1 study to evaluate the safety, tolerability and immunogenicity of two vaccinations of Ad26.RSV.preF one year apart in adults aged 60 years and older in stable health]. clinicaltrials.gov/ct2/show/NCT02926430 (first received 6 October 2016).
NCT02952339 {published data only}
- NCT02952339. Evaluating the infectivity, safety and immunogenicity of a recombinant live-attenuated respiratory syncytial virus vaccine (RSV LID cp ΔM2-2) in RSV-seronegative infants 6 to 24 months of age [Phase I placebo-controlled study of the infectivity, safety and immunogenicity of a single dose of a recombinant live-attenuated respiratory syncytial virus vaccine, LID ΔM2-2 1030s, lot RSV#010A, delivered as nose drops to RSV-seronegative infants and children 6 to 24 months of age]. clinicaltrials.gov/ct2/show/NCT02952339 (first received 2 November 2016).
NCT03026348 {published data only}
- NCT03026348. Safety and immunogenicity study to evaluate single- or two-dose regimens of RSV F vaccine with and without aluminum phosphate or Matrix-M1™ adjuvants in clinically-stable older adults. clinicaltrials.gov/ct2/show/NCT03026348 (first received 20 January 2017).
NCT03049488 {published data only}
- NCT03049488. Dose, safety, tolerability and immunogenicity of a stabilized prefusion RSV F subunit protein vaccine, VRC-RSVRGP084-00-VP (DS-Cav1), alone or with alum adjuvant, in healthy adults [VRC 317: a phase I randomized, open-label clinical trial to evaluate dose, safety, tolerability and immunogenicity of a stabilized prefusion RSV F subunit protein vaccine, VRC-RSVRGP084-00-VP (DS-Cav1), alone or with alum adjuvant, in healthy adults]. clinicaltrials.gov/ct2/show/NCT03049488 (first received 10 February 2017).
NCT03191383 {published data only}
- NCT03191383. A study to evaluate the safety, reactogenicity and immunogenicity of the GlaxoSmithKline (GSK) biologicals' respiratory syncytial virus (RSV) investigational vaccine (GSK3003891A) in healthy pregnant women and infants born to vaccinated mothers [An observer-blind study to assess the safety, reactogenicity and immunogenicity of GSK biologicals' investigational RSV vaccine (GSK3003891A), in healthy pregnant women and infants born to vaccinated mothers]. clinicaltrials.gov/ct2/show/NCT03191383 (first received 19 June 2017).
NCT03303625 {published data only}
- NCT03303625. A study to evaluate the safety, tolerability and immunogenicity of an investigational RSV vaccine candidate (Ad26.RSV.preF) in adults 18 to 50 years of age, and RSV-seropositive toddlers 12 to 24 months of age [A randomized, double-blind, phase 1/2a study to evaluate the safety, tolerability and immunogenicity of Ad26.RSV.preF in adults 18 to 50 years of age, RSV-seropositive toddlers 12 to 24 months of age]. clinicaltrials.gov/ct2/show/NCT03303625 (first received 6 October 2017).
NCT03334695 {published data only}
- NCT03334695. An exploratory study to evaluate the prophylactic efficacy of a single immunization of Ad26.RSV.preF against respiratory syncytial virus infection in a virus challenge model in healthy 18 to 50 year-old adults [An exploratory, phase 2a, randomized, double-blind, placebo-controlled study to evaluate the prophylactic efficacy of a single immunization of Ad26.RSV.preF against respiratory syncytial virus infection in a virus challenge model in healthy 18 to 50 year-old adults]. clinicaltrials.gov/ct2/show/NCT03334695 (first received 17 November 2017).
NCT03392389 {published data only}
- NCT03392389. Safety, reactogenicity, and immunogenicity of mRNA-1653 in healthy adults [A phase 1, randomized, observer-blind, placebo-controlled, dose-ranging study to evaluate the safety, reactogenicity, and immunogenicity of mRNA-1653, a combined human metapneumovirus and human parainfluenza virus type 3 vaccine, when administered to healthy adult]. clinicaltrials.gov/ct2/show/NCT03392389 (first received 8 January 2018).
NCT03403348 {published data only}
- NCT03403348. A first-in-human study of orally administered JNJ-64417184 to evaluate the safety, tolerability, and pharmacokinetics of single and multiple ascending doses, and the antiviral activity of multiple doses in a respiratory syncytial virus (RSV) challenge study in healthy participants [A randomized, double-blind, placebo-controlled, first-in-human, 6-part study of orally administered JNJ-64417184 to evaluate the safety, tolerability, and pharmacokinetics of single and multiple ascending doses, and the antiviral activity of multiple doses in a respiratory syncytial virus (RSV) challenge study in healthy subjects]. clinicaltrials.gov/ct2/show/NCT03403348 (first received 18 January 2018).
NCT03473002 {published data only}
- NCT03473002. A safety and immunogenicity study of intranasal sendai virus vectored respiratory syncytial virus (SeVRSV) vaccine in healthy adults [A phase I double-blind placebo controlled trial to evaluate the safety and immunogenicity of intranasal sendai virus vectored respiratory syncytial virus (SeVRSV) vaccine in healthy adults]. clinicaltrials.gov/ct2/show/NCT03473002 (first received 21 March 2018).
NCT03572062 {published data only}
- NCT03572062. A study to evaluate the safety and immunogenicity of an adjuvanted RSV vaccine in healthy older adults [A phase 1/2, placebo-controlled, randomized, observer-blind, dose-finding, first-in-human study to describe the safety, tolerability, and immunogenicity of an adjuvanted respiratory syncytial virus (RSV) vaccine in healthy older adults]. clinicaltrials.gov/ct2/show/NCT03572062 (first received 28 June 2018).
NCT03674177 {published data only}
- NCT03674177. A study to evaluate different dose levels of GlaxoSmithKline (GSK) biologicals' investigational respiratory syncytial virus (RSV) vaccine (GSK3888550A), based on the vaccine safety and the antibodies (body defences) produced following vaccine administration [A study to evaluate the safety, reactogenicity and immunogenicity of GSK biologicals' investigational unadjuvanted RSV maternal vaccine compared to placebo when administered to healthy non-pregnant women]. clinicaltrials.gov/ct2/show/NCT03674177 (first received 17 September 2018).
NCT03814590 {published data only}
- NCT03814590. A study to assess the safety, reactogenicity and immune response of GlaxoSmithKline (GSK) biologicals' investigational respiratory syncytial virus (RSV) vaccine (GSK3844766A) in older adults [Phase I/II, observer-blind, safety, reactogenicity and immunogenicity study of GSK biologicals' respiratory syncytial virus (RSV) vaccine GSK3844766A in subjects aged 18-40 or 60-80 years]. clinicaltrials.gov/ct2/show/NCT03814590 (first received 24 January 2019).
NCT04071158 {published data only}
- NCT04071158. A study of a RSV vaccines when given together with TDAP in healthy non-pregnant women aged between 18 to 49 years [A phase 2b, placebo-controlled, randomized, observer-blind study to evaluate the safety, tolerability, and immunogenicity of a respiratory syncytial virus (RSV) vaccine when administered concomitantly with tetanus, diphtheria, and acellular pertussis vaccine (TDAP) in healthy nonpregnant women 18 through 49 years of age]. clinicaltrials.gov/ct2/show/NCT04071158 (first received 28 August 2019).
NCT04086472 {published data only}
- NCT04086472. Phase 2a respiratory syncytial virus (RSV) human challenge study of MK-1654 in healthy participants (MK-1654-005) [A phase 2a double-blind, randomized, placebo-controlled study to evaluate the efficacy and safety of MK-1654 in healthy participants inoculated with experimental respiratory syncytial virus]. clinicaltrials.gov/ct2/show/NCT04086472 (first received 11 September 2019).
NCT04752644 {published data only}
- NCT04752644. Phase 2a study of MVA-BN-RSV vaccination and RSV challenge in healthy adults [A phase 2a, randomised, double-blinded, placebo-controlled study to assess the safety, immunogenicity and efficacy of the recombinant MVA-BN®-RSV vaccine against respiratory syncytial virus infection in the virus challenge model in healthy adult participants]. clinicaltrials.gov/ct2/show/NCT04752644 (first received 12 February 2021).
NTR7173 {published data only}
- NTR7173. Clinical study to evaluate safety, tolerability and effects on the immune system of a common cold vaccine in healthy adults [Randomised, double-blind, placebo-controlled study to evaluate the safety, tolerability, immunogenicity and shedding of live-attenuated RSV vaccine in healthy adults]. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR7173 (first received 18 May 2018).
Paradiso 1994 {published data only}
- Paradiso PR, Hildreth SW, Hogerman DA, Speelman DJ, Lewin EB, Oren J, et al. Safety and immunogenicity of a subunit respiratory syncytial virus vaccine in children 24 to 48 months old. Pediatric Infectious Disease Journal 1994;13(9):792-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Philpott 2016 {published data only}
- Philpott E, Englund J, Tielsch J, Katz J, Khatry S, Leclerq SC, et al. Effect of febrile rhinovirus illness during pregnancy on adverse birth outcomes in Nepal. Open Forum Infectious Diseases 2016;3(Suppl 1):1751. [DOI: 10.1093/ofid/ofw194.131] [DOI] [Google Scholar]
Philpott 2017 {published data only}
- Philpott EK, Englund JA, Katz J, Tielsch J, Khatry S, LeClerq SC, et al. Febrile rhinovirus illness during pregnancy is associated with low birth weight in Nepal. Open Forum Infectious Diseases 2017;4(2):ofx073. [DOI: 10.1093/ofid/ofx073] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piedra 1995 {published data only}
- Piedra PA, Glezen WP, Kasel JA, Welliver RC, Jewel AM, Rayford Y, et al. Safety and immunogenicity of the PFP vaccine against respiratory syncytial virus (RSV): the western blot assay aids in distinguishing immune responses of the PFP vaccine from RSV infection. Vaccine 1995;13(12):1095-101. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pierce 1968 {published data only}
- Pierce WE, Rosenbaum MJ, Edwards EA, Peckinpaugh RO, Jackson GG. Live and inactivated adenovirus vaccines for the prevention of acute respiratory illness in naval recruits. American Journal of Epidemiology 1968;87(1):237-46. [PMID: ] [DOI] [PubMed] [Google Scholar]
Power 2001 {published data only}
- Power UF, Nguyen TN, Rietveld E, Swart RL, Groen J, Osterhaus AD, et al. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. Journal of Infectious Diseases 2001;184(11):1456-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ritchie 1958 {published data only}
- Ritchie JM. Autogenous vaccine in prophylaxis of the common cold. Lancet 1958;1(7021):615-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ruckwardt 2021 {published data only}
- Ruckwardt T, Morabito K, Phung E, Crank M, Costner P, Holman L, et al. Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respiratory Medicine 2021;9(10):1111-20. [DOI: 10.1016/S2213-2600(21)00098-9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadoff 2021a {published data only}
- Sadoff J, De Paepe E, DeVincenzo J, Gymnopoulou E, Menten J, Murray B, et al. Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26.RSV.preF in a human challenge study. Journal of Infectious Diseases 2022;226(3):396-406. [DOI: 10.1093/infdis/jiab003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadoff 2021b {published data only}
- Sadoff J, De Paepe E, Haazen W, Omoruyi E, Bastian AR, Comeaux C. Safety and immunogenicity of the Ad26.RSV.preF investigational vaccine coadministered with an influenza vaccine in older adults. Journal of Infectious Diseases 2021;223(4):699-708. [DOI: 10.1093/infdis/jiaa409] [PMID: ] [DOI] [PubMed] [Google Scholar]
Samy 2020 {published data only}
- Samy N, Reichhardt D, Schmidt D, Chen LM, Silbernagl G, Vidojkovic S, et al. Safety and immunogenicity of novel modified vaccinia Ankara-vectored RSV vaccine: a randomized phase I clinical trial. Vaccine 2020;38(11):2608-19. [DOI: 10.1016/j.vaccine.2020.01.055] [DOI] [PubMed] [Google Scholar]
Scaggs Huang 2021 {published data only}
- Scaggs Huang F, Bernstein DI, Slobod KS, Portner A, Takimoto T, Russell CJ, et al. Safety and immunogenicity of an intranasal sendai virus-based vaccine for human parainfluenza virus type I and respiratory syncytial virus (SeVRSV) in adults. Human Vaccines & Immunotherapeutics 2021;17(2):554-9. [DOI: 10.1080/21645515.2020.1779517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarz 2019 {published data only}
- Schwarz TF, McPhee RA, Launay O, Leroux-Roels G, Talli J, Picciolato M, et al. Immunogenicity and safety of 3 formulations of a respiratory syncytial virus candidate vaccine in non-pregnant women: a phase II, randomized trial. Journal of Infectious Diseases 2019;220(11):1816-25. [DOI: 10.1093/infdis/jiz395] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shakib 2019 {published data only}
- Shakib JH, Varner MW, Fiuza M, Trenholme AA, Baqui A, Frech S, et al. Immunoglobulin A, immunoglobulin G, and neutralizing antibodies to respiratory syncytial virus increase in human milk following immunization with an RSV F protein vaccine. International Journal of Stroke 2019;221(6):669-70. [DOI: 10.1016/j.ajog.2019.10.080] [DOI] [Google Scholar]
Shaw 2019 {published data only}
- Shaw C, Lee H, Knightly C, Kalidindi S, Zaks T, Smolenov I, et al. Phase 1 trial of an mRNA-based combination vaccine against HMPV and PIV3. Open Forum Infectious Diseases 2019;6(Suppl 2):S970. [DOI: 10.1093/ofid/ofz360.2431] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simoes 2001 {published data only}
- Simoes EA, Tan DH, Ohlsson A, Sales V, Wang EE. Respiratory syncytial virus vaccine: a systematic overview with emphasis on respiratory syncytial virus subunit vaccines. Vaccine 2001;20(5-6):954-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Swamy 2019 {published data only}
- Swamy GK, Munoz FM, Polack F, Madhi SA, Trenholme AA, Simoes EA, et al. Safety of third trimester immunization with a respiratory syncytial virus F protein vaccine and protection of infants over the first 180 days of life against all-cause lower respiratory tract infection. American Journal of Obstetrics & Gynecology 2019;221(6):670. [DOI: 10.1016/j.ajog.2019.10.081] [DOI] [Google Scholar]
Tang 2008 {published data only}
- Tang RS, Spaete RR, Thompson MW, MacPhail M, Guzzetta JM, Ryan PC, et al. Development of a PIV-vectored RSV vaccine: preclinical evaluation of safety, toxicity, and enhanced disease and initial clinical testing in healthy adults. Vaccine 2008;26(50):6373-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Top 1971 {published data only}
- Top FH Jr, Buescher EL, Bancroft WH, Russell PK. Immunization with live types 7 and 4 adenovirus vaccines. II. Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. Journal of Infectious Diseases 1971;124(2):155-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tristram 1993 {published data only}
- Tristram DA, Welliver RC, Mohar CK, Hogerman DA, Hildreth SW, Paradiso P. Immunogenicity and safety of respiratory syncytial virus subunit vaccine in seropositive children 18-36 months old. Journal of Infectious Diseases 1993;167(1):191-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Der Plas 2020 {published data only}
- Van Der Plas J, Verdijk P, Van Brummelen E, Roestenberg M, Burggraaf J, Kamerling I. First-in-human, randomized study to assess the safety, tolerability, immunogenicity, and shedding of a live attenuated respiratory syncytial virus vaccine. British Journal of Clinical Pharmacology 2020;86(6):1189-90. [DOI: 10.1111/bcp.14266] [DOI] [Google Scholar]
Verdijk 2020 {published data only}
- Verdijk P, Plas JL, Brummelen E, Jeeninga RE, Haan C, Roestenberg M, et al. First-in-human administration of a live-attenuated RSV vaccine lacking the G-protein assessing safety, tolerability, shedding and immunogenicity: a randomized controlled trial. Vaccine 2020;38(39):6088-95. [DOI: 10.1016/j.vaccine.2020.07.029] [PMID: ] [DOI] [PubMed] [Google Scholar]
Watt 1990 {published data only}
- Watt PJ, Robinson BS, Pringle CR, Tyrrell DA. Determinants of susceptibility to challenge and the antibody response of adult volunteers given experimental respiratory syncytial virus vaccines. Vaccine 1990;8(3):231-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Welliver 1994 {published data only}
- Welliver RC, Tristram DA, Batt K, Sun M, Hogerman D, Hildreth S. Respiratory syncytial virus-specific cell-mediated immune responses after vaccination with a purified fusion protein subunit vaccine. Journal of Infectious Diseases 1994;170(2):425-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Williams 2020 {published data only}
- Williams K, Bastian AR, Feldman RA, Omoruyi E, Paepe E, Hendriks J, et al. Phase 1 safety and immunogenicity study of a respiratory syncytial virus vaccine with an adenovirus 26 vector encoding prefusion F (Ad26.RSV.preF) in adults aged ≥ 60 years. Journal of Infectious Diseases 2020;222(6):979-88. [DOI: 10.1093/infdis/jiaa193] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilson 1960 {published data only}
- Wilson JS, Grant PJ, Miller DL, Taylor CE, McDonald JC. Trial of adenovirus vaccine in Royal Air Force recruits. British Medical Journal 1960;1(5179):1081-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1976 {published data only}
- Wright PF, Shinozaki T, Fleet W, Sell SH, Thompson J, Karzon DT. Evaluation of a live, attenuated respiratory syncytial virus vaccine in infants. Journal of Pediatrics 1976;88(6):931-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yu 2020 {published data only}
- Yu J, Powers JH, Vallo D, Falloon J. Evaluation of efficacy endpoints for a phase IIb study of a respiratory syncytial virus vaccine in older adults using patient-reported outcomes with laboratory confirmation. Value Health 2020;23(2):227-35. [DOI: 10.1016/j.jval.2019.09.2747] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01893554 {published data only}
- NCT01893554. Evaluating the safety and immune response to a single dose of a respiratory syncytial virus (RSV) vaccine in infants and children [A phase I study of the safety and immunogenicity of a single dose of the recombinant live-attenuated respiratory syncytial virus vaccine RSV ΔNS2 Δ1313 I1314L, lot RSV#006A, delivered as nose drops to RSV-seropositive children 12 to 59 months of age, RSV-seronegative infants and children 6 to 24 months of age, and infants 4 to 6 months of age]. clinicaltrials.gov/ct2/show/NCT01893554 (first received 9 July 2013).
NCT03387137 {published data only}
- NCT03387137. Evaluating the infectivity, safety, and immunogenicity of a respiratory syncytial virus vaccine (RSV 6120/∆NS2/1030s) in RSV-seropositive children and RSV-seronegative infants and children [Phase I placebo-controlled study of the infectivity, safety and immunogenicity of a single dose of a recombinant live-attenuated respiratory syncytial virus vaccine, 6120/∆NS2/1030s, lot RSV#012A, delivered as nose drops to RSV-seropositive children 12 to 59 months of age and RSV-seronegative infants and children 6 to 24 months of age]. clinicaltrials.gov/ct2/show/NCT03387137 (first received 29 December 2017).
NCT03422237 {published data only}
- NCT03422237. Evaluating the infectivity, safety, and immunogenicity of the recombinant live-attenuated respiratory syncytial virus (RSV) vaccines RSV ΔNS2/Δ1313/I1314L or RSV 276 in RSV-seronegative infants and children 6 to 24 months of age [Randomized phase I study of the infectivity, safety, and immunogenicity of a single dose of the recombinant live-attenuated respiratory syncytial virus (RSV) vaccines RSV ΔNS2/Δ1313/I1314L or RSV 276 or placebo, delivered as nose drops to RSV-seronegative infants and children 6 to 24 months of age]. clinicaltrials.gov/ct2/show/NCT03422237 (first received 5 February 2018).
NCT03596801 {published data only}
- NCT03596801. Evaluating the infectivity, safety and immunogenicity of respiratory syncytial virus vaccines, RSV 6120/∆NS1 and RSV 6120/F1/G2/∆NS1, in RSV-seropositive children and RSV-seronegative infants and children [Phase I placebo-controlled study of the infectivity, safety and immunogenicity of a single dose of a recombinant live-attenuated respiratory syncytial virus vaccine, RSV 6120/∆NS1, lot RSV#018A, or RSV 6120/F1/G2/∆NS1, lot RSV#016A, delivered as nose drops to RSV-seropositive children 12 to 59 months of age and RSV-seronegative infants and children 6 to 24 months of age]. clinicaltrials.gov/ct2/show/NCT03596801 (first received 24 July 2018).
NCT03916185 {published data only}
- NCT03916185. Safety and immunogenicity of a single dose of the recombinant live-attenuated respiratory syncytial virus (RSV) vaccines RSV ΔNS2/Δ1313/I1314L, RSV 6120/ΔNS2/1030s, RSV 276 or placebo, delivered as nose drops to RSV-seronegative children 6 to 24 months of age [Randomized phase I/II study of the safety and immunogenicity of a single dose of the recombinant live-attenuated respiratory syncytial virus (RSV) vaccines RSV ΔNS2/Δ1313/I1314L, RSV 6120/ΔNS2/1030s, RSV 276 or placebo, delivered as nose drops to RSV-seronegative children 6 to 24 months of age]. clinicaltrials.gov/ct2/show/NCT03916185 (first received 16 April 2019).
NCT04032093 {published data only}
- NCT04032093. A phase 2B placebo-controlled, randomized study of a respiratory syncytial virus (RSV) vaccine in pregnant women [A phase 2B, randomized, placebo-controlled, observer-blinded trial to evaluate the safety, tolerability, and immunogenicity of a respiratory syncytial virus (RSV) vaccine in pregnant women 18 through 49 years of age and their infants]. clinicaltrials.gov/ct2/show/NCT04032093 (first received 25 July 2019).
NCT04126213 {published data only}
- NCT04126213. Study of safety, reactogenicity and immunogenicity of GlaxoSmithKline's (GSK) respiratory syncytial virus (RSV) maternal unadjuvanted vaccine in healthy pregnant women (aged 18 to 40 years) and their infants [A phase II, randomised, observer-blind, placebo controlled multi-country study to assess the safety, reactogenicity and immunogenicity of a single intramuscular dose of GSK biologicals' investigational RSV maternal unadjuvanted vaccine (GSK3888550A), in healthy pregnant women aged 18 to 40 years and infants born to vaccinated mothers]. clinicaltrials.gov/ct2/show/NCT04126213 (first received 15 October 2019).
NCT04138056 {published data only}
- NCT04138056. A study of a vaccine against respiratory syncytial virus (RSV) when given alone and together with a vaccine against diphtheria, pertussis and tetanus (Tdap) viruses followed by a 2nd dose of the RSV vaccine to healthy non-pregnant women [A phase II study of a primary dose of investigational RSV maternal vaccine, given alone or with Boostrix, with a 2nd dose investigational RSV maternal vaccine]. clinicaltrials.gov/ct2/show/NCT04138056 (first received 24 October 2019).
NCT04681833 {published data only}
- NCT04681833. Safety and efficacy of BARS13 in the elderly [A randomised, double-blind, placebo-controlled, dose-ranging phase II study in 60 to 80-year-old adults to assess the safety and immunogenicity of BARS13]. clinicaltrials.gov/ct2/show/NCT04681833 (first received 23 December 2020).
NCT04732871 {published data only}
- NCT04732871. Immunogenicity, safety, reactogenicity and persistence of an investigational respiratory syncytial virus (RSV) vaccine in adults aged 60 years and above [A phase 3, randomized, open-label, multi-country study to evaluate the immunogenicity, safety, reactogenicity and persistence of a single dose of the RSVPreF3 OA investigational vaccine and different revaccination schedules in adults aged 60 years and above]. clinicaltrials.gov/ct2/show/NCT04732871 (first received 1 February 2021).
NCT04980391 {published data only}
- NCT04980391. A study on the safety and immune response to an unadjuvanted RSV maternal vaccine, in high-risk pregnant women aged 15 to 49 years and infants born to the vaccinated mothers [A phase III, double-blind, randomized, placebo-controlled study to evaluate the safety, reactogenicity and immune response of a single intramuscular dose of unadjuvanted RSV maternal vaccine, in high-risk pregnant women aged 15 to 49 years and infants born to the vaccinated mother]. clinicaltrials.gov/ct2/show/NCT04980391 (first received July 28 2021).
NCT05127434 {published data only}
- NCT05127434. A study to evaluate the safety and efficacy of mRNA-1345 vaccine targeting respiratory syncytial virus (RSV) in adults ≥60 years of age [A phase 2/3, randomized, observer-blind, placebo-controlled study to evaluate the safety and efficacy of mRNA-1345, an mRNA vaccine targeting respiratory syncytial virus (RSV), in adults ≥60 years of age]. clinicaltrials.gov/ct2/show/NCT05127434 (first received 19 November 2021).
NCT05238025 {published data only}
- NCT05238025. Phase 3 MVA-BN-RSV vaccine efficacy trial [A randomized, double-blind, phase 3 trial to assess clinical efficacy, safety and reactogenicity of the recombinant MVA-BN® -RSV vaccine in adults ≥60 years of age]. clinicaltrials.gov/ct2/show/NCT05238025 (first received 14 February 2022).
Additional references
Abbas 2001
- Abbas A, Lichtman A. Glossary. In: Basic Immunology. Vol. 1. Philadelphia: WB Saunders Company, 2001. [Google Scholar]
Adler 2018
- Adler FR, Stockmann C, Ampofo K, Pavia AT, Byington CL. Transmission of rhinovirus in the Utah BIG-LoVE families: consequences of age and household structure. PLOS ONE 2018;13(7):e0199388. [DOI: 10.1371/journal.pone.0199388] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alderson 2004
- Alderson P. Absence of evidence is not evidence of absence. BMJ 2004;328(7438):476-7. [DOI: 10.1136/bmj.328.7438.476] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexandrino 2016
- Alexandrino AS, Santos R, Melo C, Bastos JM. Risk factors for respiratory infections among children attending day care centres. Family Practice 2016;33(2):161-6. [DOI: 10.1093/fampra/cmw002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Allan 2014
- Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. Canadian Medical Association Journal 2014;186(3):190-9. [DOI: 10.1503/cmaj.121442] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1995
- Altman DG, Bland JM. Absence of evidence is not evidence of absence. BMJ 1995;311(7003):485. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Armah 2016
- Armah G, Lewis KD, Cortese MM, Parashar UD, Ansah A, Gazley L, et al. A randomized, controlled trial of the impact of alternative dosing schedules on the immune response to human rotavirus vaccine in rural Ghanaian infants. Journal of Infectious Diseases 2016;213(11):1678-85. [DOI: 10.1093/infdis/jiw023] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arruda 1997
- Arruda E, Pitkäranta A, Witek TJ Jr, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. Journal of Clinical Microbiology 1997;35(11):2864-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berman 1991
- Berman S. Epidemiology of acute respiratory infections in children of developing countries. Reviews of Infectious Diseases 1991;13(Suppl 6):454-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Biserni 2020
- Biserni GB, Dondi A, Masetti R, Bandini J, Dormi A, Conti F, et al. Immune response against adenovirus in acute upper respiratory tract infections in immunocompetent children. Vaccines (Basel) 2020;8(4):602. [DOI: 10.3390/vaccines8040602] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchholz 2018
- Buchholz UJ, Cunningham CK, Muresan P, Gnanashanmugam D, Sato P, Siberry GK, et al. Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. Journal of Infectious Diseases 2018;217(9):1338-46. [DOI: 10.1093/infdis/jiy066] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2014
- Chu HY, Steinhoff MC, Magaret A, Zaman K, Roy E, Langdon G, et al. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. Journal of Infectious Diseases 2014;210(10):1582-9. [DOI: 10.1093/infdis/jiu316] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2020
- Collins ND, Adhikari A, Yang Y, Kuschner RA, Karasavvas N, Binn LN, et al. Live oral adenovirus type 4 and type 7 vaccine induces durable antibody response. Vaccines (Basel) 2020;8(3):411. [DOI: 10.3390/vaccines8030411] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Czubak 2021
- Czubak J, Stolarczyk K, Orzeł A, Frączek M, Zatoński T. Comparison of the clinical differences between COVID-19, SARS, influenza, and the common cold: a systematic literature review. Advances in Clinical and Experimental Medicine 2021;30(1):109-14. [DOI: 10.17219/acem/129573] [PMID: ] [DOI] [PubMed] [Google Scholar]
DDCP 2010
- Division of Disease Control and Prevention, Utah Department of Health. Difference between cold and flu symptoms. health.utah.gov/epi/diseases/flu/ColdvsFlu.pdf (accessed 10 October 2021).
DeGeorge 2019
- DeGeorge KC, Ring DJ, Dalrymple SN. Treatment of the common cold. American Family Physician 2019;100(5):281-9. [PMID: ] [PubMed] [Google Scholar]
Denny 1983
- Denny FW, Murphy TF, Clyde WA, Collier AM, Henderson FW. Croup: an 11 year study in a pediatric practice. Pediatrics 1983;71(6):871-6. [PMID: ] [PubMed] [Google Scholar]
Dicpinigaitis 2015
- Dicpinigaitis PV, Eccles R, Blaiss MS, Wingertzahn MA. Impact of cough and common cold on productivity, absenteeism, and daily life in the United States: ACHOO Survey. Current Medical Research and Opinion 2015;31(8):1519-25. [DOI: 10.1185/03007995.2015.1062355] [PMID: ] [DOI] [PubMed] [Google Scholar]
Eccles 2009
- Eccles R. Mechanisms of symptoms of common cold and flu. Common Cold 2009;10:23–45. [DOI: 10.1007/978-3-7643-9912-2_2] [PMID: ] [DOI] [Google Scholar]
Edlmayr 2011
- Edlmayr J, Niespodziana K, Popow-Kraupp T, Krzyzanek V, Focke-Tejkl M, Blaas D, et al. Antibodies induced with recombinant VP1 from human rhinovirus exhibit cross-neutralisation. European Respiratory Journal 2011;37(1):44-52. [DOI: 10.1183/09031936.00149109] [PMID: ] [DOI] [PubMed] [Google Scholar]
Esper 2003
- Esper F, Boucher D, Weibel C, Martinello RA, Kahn JS. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics 2003;111(6 Pt 1):1407-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Falsey 2005
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. New England Journal of Medicine 2005;352(17):1749-59. [PMID: ] [DOI] [PubMed] [Google Scholar]
Freiman 1978
- Freiman JA, Chalmers TC, Smith H Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials. New England Journal of Medicine 1978;299(13):690-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gabriel 2012
- Gabriel SE, Normand SL. Getting the methods right - the foundation of patient-centered outcomes research. New England Journal of Medicine 2012;367(9):787-90. [DOI: 10.1056/NEJMp1207437] [DOI] [PubMed] [Google Scholar]
Giraud‐Gatineau 2020
- Giraud-Gatineau A, Colson P, Jimeno MT, Zandotti C, Ninove L, Boschi C, et al. Comparison of mortality associated with respiratory viral infections between December 2019 and March 2020 with that of the previous year in Southeastern France. International Society for Infectious Diseases 2020;96:154-6. [DOI: 10.1016/j.ijid.2020.05.001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2013
- Glanville N, McLean GR, Guy B, Lecouturier V, Berry C, Girerd Y, et al. Cross-serotype immunity induced by immunization with a conserved rhinovirus capsid protein. PLOS Pathogens 2013;9(9):e1003669. [DOI: 10.1371/journal.ppat.1003669] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldsby 2000
- Goldsby R, Kingt T, Osborne B. Overview of the immune system. In: Kuby Immunology. 4th edition. Vol. 3. New York, NY: WH Freeman Co, 2000. [Google Scholar]
Gomes 2017
- Gomes AC, Mohsen M, Bachmann MF. Harnessing nanoparticles for immunomodulation and vaccines. Vaccines (Basel) 2017;5(1):6. [DOI: 10.3390/vaccines5010006] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorczynski 2007
- Gorczynski RM, Stanley J. Inmunología Basada en la Resolución de Problemas. Vol. 138. Madrid: Elsevier Saunders, 2007. [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 15 October 2016. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Green 2002
- Green SB. Design of randomized trials. Epidemiologic Reviews 2002;24(1):4-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gwaltney 1967
- Gwaltney JM, Hendley J, Simon G, Jordan WSJ. Rhinovirus infections in an industrial population II. Characteristics of illness and antibody response. JAMA 1967;202(6):494-500. [PMID: ] [PubMed] [Google Scholar]
Gwaltney 1985
- Gwaltney JM. Virology and immunology of the common cold. Rhinology 1985;23(4):265-71. [PMID: ] [PubMed] [Google Scholar]
Gwaltney 2000
- Gwaltney JM. The common cold. In: Mandell GL, Bennett JE, Dolin R, editors(s). Principles and Practices of Infectious Diseases. 5th edition. New York, NY: Churchill Livingstone, 2000:651-6. [Google Scholar]
Hao 2015
- Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD006895. [DOI: 10.1002/14651858.CD006895.pub3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Heikkinen 2003
- Heikkinen T, Jarvinen A. The common cold. Lancet 2003;361(9351):51-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henrickson 2003
- Henrickson KJ. Parainfluenza viruses. Clinical Microbiology Reviews 2003;16(2):242-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Ioannidis 2010
- Ioannidis JP. Meta-research: the art of getting it wrong. Research Synthesis Methods 2010;1(3-4):169-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jaume 2020
- Jaume F, Valls-Mateus M, Mullol J. Common cold and acute rhinosinusitis: up-to-date management in 2020. Current Allergy and Asthma Reports 2020;20(7):28. [DOI: 10.1007/s11882-020-00917-5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaume 2021
- Jaume F, Quintó LL, Alobid I, Mullol J. Direct costs of acute rhinosinusitis in Spain - a prospective and observational study (PROSINUS). Journal of Investigational Allergology and Clinical Immunology 2021;31(6):481-8. [DOI: 10.18176/jiaci.0525] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jefferson 2012
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD004879. [DOI: 10.1002/14651858.CD004879.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferson 2020
- Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD006207. [DOI: 10.1002/14651858.CD006207.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2017
- Johnston NW, Olsson M, Edsbäcker S, Gerhardsson de Verdier M, Gustafson P, McCrae C, et al. Colds as predictors of the onset and severity of COPD exacerbations. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:839-48. [DOI: 10.2147/COPD.S127146] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahn 2003
- Kahn JS. Human metapneumovirus: a newly emerging respiratory pathogen. Current Opinion in Infection Diseases 2003;16(3):255-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kang 2009
- Kang SM, Compans RW. Host responses from innate to adaptive immunity after vaccination: molecular and cellular events. Molecular Cell 2009;27(1):5-14. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kardos 2017
- Kardos P, Malek FA. Common cold - an umbrella term for acute infections of nose, throat, larynx and bronchi. Pneumologie 2017;71(4):221-6. [DOI: 10.1055/s-0042-116112] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karron 2011
- Karron RA, Casey R, Thumar B, Surman S, Murphy BR, Collins PL, et al. The cDNA-derived investigational human parainfluenza virus type 3 vaccine rcp45 is well tolerated, infectious, and immunogenic in infants and young children. Pediatric Infectious Disease Journal 2011;30(10):e186-91. [DOI: 10.1097/INF.0b013e31822ea24f] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirby 2002
- Kirby A, Gebski V, Keech AC. Determining the sample size in a clinical trial. Medical Journal of Australia 2002;177(5):256-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
L'Huillier 2015
- L'Huillier AG, Tapparel C, Turin L, Boquete-Suter P, Thomas Y, Kaiser L. Survival of rhinoviruses on human fingers. Clinical Microbiology and Infection 2015;21(4):381-5. [DOI: 10.1016/j.cmi.2014.12.002] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambert 2007
- Lambert SB, Allen KM, Druce JD, Birch CJ, Mackay IM, Carlin JB, et al. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens. Pediatrics 2007;120(4):e929-37. [DOI: 10.1542/peds.2006-3703] [PMID: ] [DOI] [PubMed] [Google Scholar]
Larson 1980
- Larson HE, Reed SE, Tyrrell DA. Isolation of rhinoviruses and coronaviruses from 38 colds in adults. Journal of Medical Virology 1980;5(3):221-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2006
- Lau SK, Woo PC, Yip CC, Tse H, Tsoi HW, Cheng VC, et al. Coronavirus HKU1 and other coronavirus infection in Hong Kong. Journal of Clinical Microbiology 2006;44(6):2063-71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Lemanske 2005
- Lemanske RF Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. Journal of Allergy and Clinical Immunology 2005;116(3):571-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lu 2020
- Lu C, Norbäck D, Zhang Y, Li B, Zhao Z, Huang C, et al. Common cold among young adults in China without a history of asthma or allergic rhinitis - associations with warmer climate zone, dampness and mould at home, and outdoor PM10 and PM2.5. Science of the Total Environment 2020;749:141580. [DOI: 10.1016/j.scitotenv.2020.141580] [PMID: ] [DOI] [PubMed] [Google Scholar]
Luka 2020
- Luka MM, Kamau E, Adema I, Munywoki PK, Otieno GP, Gicheru E, et al. Molecular epidemiology of human rhinovirus from 1-year surveillance within a school setting in rural coastal Kenya. Open Forum Infectious Diseases 2020;7(10):ofaa385. [DOI: 10.1093/ofid/ofaa385] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mäkelä 1998
- Mäkelä MJ, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, Kimpimäki M, et al. Viruses and bacteria in the etiology of the common cold. Journal of Clinical Microbiology 1998;36(2):539-42. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazur 2018
- Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infectious Diseases 2018;18(10):e295-311. [DOI: 10.1016/S1473-3099(18)30292-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
McLean 2012
- McLean GR, Walton RP, Shetty S, Peel TJ, Paktiawal N, Kebadze T, et al. Rhinovirus infections and immunisation induce cross-serotype reactive antibodies to VP1. Antiviral Research 2012;95(3):193-201. [DOI: 10.1016/j.antiviral.2012.06.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Moher 2012
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2012;10(1):28-55. [DOI: 10.1016/j.ijsu.2011.10.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Monto 1993
- Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiology and Infection 1993;110(1):145-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muňoz 2019
- Muňoz FM, Swamy GK, Hickman SP, Agrawal S, Piedra PA, Glenn GM, et al. Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. Journal of Infectious Diseases 2019;220(11):1802-15. [DOI: 10.1093/infdis/jiz390] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nebeker 2004
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Annals of Internal Medicine 2004;140(10):795-801. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nicholson 1997
- Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective population based study of disease burden. BMJ 1997;315(7115):1060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nissen 2002
- Nissen MD, Siebert DJ, Mackay IM, Sloots TP, Withers SJ. Evidence of human metapneumovirus in Australian children. Medical Journal of Australia 2002;176(4):188. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane centralised search service showed high sensitivity identifying randomized controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021
- Noel-Storr A, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomized trials. Journal of Clinical Epidemiology 2021;133:130-9. [DOI: 10.1016/j.jclinepi.2021.01.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papadopoulos 2017
- Papadopoulos NG, Megremis S, Kitsioulis NA, Vangelatou O, West P, Xepapadaki P. Promising approaches for the treatment and prevention of viral respiratory illnesses. Journal of Allergy and Clinical Immunology 2017;140(4):921-32. [DOI: 10.1016/j.jaci.2017.07.001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
PCORI 2012
- Methodology Committee of the Patient-Centered Outcomes Research Institute (PCORI). Methodological standards and patient-centeredness in comparative effectiveness research: the PCORI perspective. JAMA 2012;307(15):1636-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pichichero 2009
- Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis? Pediatrics 2009;124(6):1633-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Plotkin 2010
- Plotkin SA. Correlates of protection induced by vaccination. Clinical and Vaccine Immunology 2010;17(7):1055-65. [DOI: 10.1128/CVI.00131-10] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plotkin 2020
- Plotkin SA. Updates on immunologic correlates of vaccine-induced protection. Vaccine 2020;38(9):2250-7. [DOI: 10.1016/j.vaccine.2019.10.046] [PMID: ] [DOI] [PubMed] [Google Scholar]
Regamey 2008
- Regamey N, Kaiser L. Rhinovirus infections in infants: is respiratory syncytial virus ready for the challenge? European Respiratory Journal 2008;32(2):249-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ren 2017
- Ren L, Yang D, Ren X, Li M, Mu X, Wang Q, et al. Genotyping of human rhinovirus in adult patients with acute respiratory infections identified predominant infections of genotype A21. Scientific Reports 2017;7:41601. [DOI: 10.1038/srep41601] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rey‐Jurado 2017
- Rey-Jurado E, Kalergis AM. Immunological features of respiratory syncytial virus-caused pneumonia - implications for vaccine design. International Journal of Molecular Sciences 2017;18(3):556. [DOI: 10.3390/ijms18030556] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reynolds 2016
- Reynolds KA, Beamer PI, Plotkin KR, Sifuentes LY, Koenig DW, Gerba CP. The healthy workplace project: reduced viral exposure in an office setting. Archives of Environmental & Occupational Health 2016;71(3):157-62. [DOI: 10.1080/19338244.2015.1058234] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Risnes 2005
- Risnes KR, Radtke A, Nordbø SA, Grammeltvedt AT, Døllner H. Human metapneumovirus - occurrence and clinical significance. Tidsskrift for den Norske Lægeforening 2005;20:2769-72. [PMID: ] [PubMed] [Google Scholar]
Roitt 2004
- Roitt I, Delves P. Glossary. In: Essential Immunology. 10th edition. Vol. 466. Blackwell, 2004. [Google Scholar]
Roxas 2007
- Roxas M, Jurenka J. Colds and influenza: a review of diagnosis and conventional, botanical and nutritional considerations. Alternative Medicine Review 2007;12(1):25-48. [PMID: ] [PubMed] [Google Scholar]
Rubner 2017
- Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. Journal of Allergy and Clinical Immunology 2017;139(2):501-7. [DOI: 10.1016/j.jaci.2016.03.049] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Russell 2006
- Russell KL, Hawksworth AW, Ryan MA, Strickler J, Irvine M, Hansen CJ, et al. Vaccine-preventable adenoviral respiratory illness in US military recruits, 1999-2004. Vaccine 2006;24(15):2835-42. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shibata 2018
- Shibata M, Iwane T, Higuchi R, Suwa K, Nakajima K. Potential common factors associated with predisposition to common cold in middle-aged and elderly Japanese: a community-based cross-sectional study. Medicine (Baltimore) 2018;97(20):e10729. [DOI: 10.1097/MD.0000000000010729] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stepanova 2019
- Stepanova E, Isakova-Sivak I, Rudenko L. Overview of human rhinovirus immunogenic epitopes for rational vaccine design. Expert Review of Vaccines 2019;18(9):877-80. [DOI: 10.1080/14760584.2019.1657014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stjärne 2012
- Stjärne P, Odebäck P, Ställberg B, Lundberg J, Olsson P. High costs and burden of illness in acute rhinosinusitis: real-life treatment patterns and outcomes in Swedish primary care. Primary Care Respiratory Journal 2012;21(2):174-9. [DOI: 10.4104/pcrj.2012.00011] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stobart 2017
- Stobart CC, Nosek JM, Moore ML. Rhinovirus biology, antigenic diversity, and advancements in the design of a human rhinovirus vaccine. Frontiers in Microbiology 2017;5(8):2412. [DOI: 10.3389/fmicb.2017.02412] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sully 2014
- Sully BG, Julious SA, Nicholl J. An investigation of the impact of futility analysis in publicly funded trials. Trials 2014;17(15):61. [DOI: 10.1186/1745-6215-15-61] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Szelazek 2017
- Szelazek B, Kabala W, Kus K, Zdzalik M, Twarda-Clapa A, Golik P, et al. Structural characterization of human coronavirus NL63 N protein. Journal of Virology 2017;91(11):e02503-16. [DOI: 10.1128/JVI.02503-16] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2019
- Tang J, Chen J, He T, Jiang Z, Zhou J, Hu B, et al. Diversity of upper respiratory tract infections and prevalence of Streptococcus pneumoniae colonization among patients with fever and flu-like symptoms. BMC Infectious Diseases 2019;19(1):24. [DOI: 10.1186/s12879-018-3662-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2013
- Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long-term care institutions. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD005187. [DOI: 10.1002/14651858.CD005187.pub4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomas 2021
- Thomas J, McDonald S, Noel-Storr A, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduced workload with minimal risk of missing studies: development and evaluation of a randomized controlled trial classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2021;133:140-51. [DOI: 10.1016/j.jclinepi.2020.11.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2003
- Thompson WW. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289(2):179-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
To 2017
- To KK, Yip CC, Yuen KY. Rhinovirus - from bench to bedside. Journal of the Formosan Medical Association 2017;116(7):496-504. [DOI: 10.1016/j.jfma.2017.04.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tomita 2012
- Tomita K, Sano H, Iwanaga T, Ishihara K, Ichinose M, Kawase I, et al. Association between episodes of upper respiratory infection and exacerbations in adult patients with asthma. Journal of Asthma 2012;49(3):253-9. [DOI: 10.3109/02770903.2012.661009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsuzuki 2020
- Tsuzuki S, Kimura Y, Ishikane M, Kusama Y, Ohmagari N. Cost of inappropriate antimicrobial use for upper respiratory infection in Japan. BMC Health Services Research 2020;20(1):153. [DOI: 10.1186/s12913-020-5021-1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Veiga 2021
- Veiga A, Martins LG, Riediger I, Mazetto A, Debur M, Gregianini TS. More than just a common cold: endemic coronaviruses OC43, HKU1, NL63, and 229E associated with severe acute respiratory infection and fatality cases among healthy adults. Journal of Medical Virology 2021;93(2):1002-7. [DOI: 10.1002/jmv.26362] [PMID: ] [DOI] [PubMed] [Google Scholar]
Visseaux 2017
- Visseaux B, Burdet C, Voiriot G, Lescure FX, Chougar T, Brugière O, et al. Prevalence of respiratory viruses among adults, by season, age, respiratory tract region and type of medical unit in Paris, France, from 2011 to 2016. PLOS ONE 2017;12(7):e0180888. [DOI: 10.1371/journal.pone.0180888] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warner 2019
- Warner SM, Wiehler S, Michi AN, Proud D. Rhinovirus replication and innate immunity in highly differentiated human airway epithelial cells. Respiratory Research 2019;20(1):150. [DOI: 10.1186/s12931-019-1120-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Westerterp 2020
- Westerterp KR. Absence of evidence is no evidence for absence of the phenomenon. American Journal of Clinical Nutrition 2020;112(3):501-2. [DOI: 10.1093/ajcn/nqaa165] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wooden 2018
- Wooden SL, Koff WC. The human vaccines project: towards a comprehensive understanding of the human immune response to immunization. Human Vaccines & Immunotherapeutics 2018;14(9):2214-6. [DOI: 10.1080/21645515.2018.1476813] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmermann 2020
- Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatric Infectious Disease Journal 2020;39(5):355-68. [DOI: 10.1097/INF.0000000000002660] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zumla 2016
- Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses - drug discovery and therapeutic options. Nature Reviews Drug Discovery 2016;15(5):327-47. [DOI: 10.1038/nrd.2015.37] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Felix 2011
- Felix ML, Guerra CV, Hinojosa MA, Cabezas CI, Hidalgo R, Samaniego DH, et al. Vaccines for the common cold. Cochrane Database of Systematic Reviews 2011, Issue 4. Art. No: CD002190. [DOI: 10.1002/14651858.CD002190.pub3] [DOI] [Google Scholar]
Simancas‐Racines 2013
- Simancas-Racines D, Guerra CV, Hidalgo R. Vaccines for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD002190. [DOI: 10.1002/14651858.CD002190.pub4] [DOI] [PubMed] [Google Scholar]
Simancas‐Racines 2017
- Simancas-Racines D, Franco JV, Guerra CV, Felix ML, Hidalgo R, Martinez-Zapata MJ. Vaccines for the common cold. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD002190. [DOI: 10.1002/14651858.CD002190.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


