CONSPECTUS:
Intracellular cargo delivery is an essential step in many biomedical applications including gene editing and biologics therapy. Examples of cargo include nucleic acids (RNA and DNA), proteins, small biomolecules, and drugs, which can vary substantially in terms of their sizes, charges, solubility, and stability. Viruses have been used traditionally to deliver nucleic acids into cells, but the method suffers from limitations such as small cargo size, safety concerns, and viral genome integration into host cells, all of which complicate therapeutic applications. Commercially available techniques using biochemicals and bulk electroporation are, in general, poorly compatible with primary cells such as human induced pluripotent stem cells and immune cells, which are increasingly important candidates for adoptive cell therapy.
Nanostructures, with dimensions ranging from tens of nanometers to a few micrometers, may play a critical role in overcoming cellular manipulation and delivery challenges and provide a powerful alternative to conventional techniques. A critical feature that differentiates nanostructures from viral, biochemical, and bulk electroporation techniques is that they interface with cells at a scale measuring ten to hundreds of nanometers in size. This highly local interaction enables application of stronger and more direct stimuli such as mechanical force, heat, or electric fields than would be possible in a bulk treatment. Compared to popular viral, biochemical, and bulk electroporation methods, nanostructures were found to minimally perturb cells with cells remaining in good health during postdelivery culture. These advantages have enabled nanostructures such as nanowires and nanotubes to successfully interface with a wide variety of cells, including primary immune cells and cardiomyocytes, for in vitro and in vivo applications.
This Account is focused on using nanostructures for cargo delivery into biological cells. In this Account, we will first outline the historical developments using nanostructures for interfacing with cells. We will highlight how mechanistic understanding of nano–bio interactions has evolved over the last decade and how this improved knowledge has motivated coupling of electric and magnetic fields to nanostructures to improve delivery outcomes. There will also be an in-depth discussion on the merits of nanostructures in comparison to conventional methods using viruses, biochemicals, and bulk electroporation.
Finally, motivated by our observations on the lack of consistency in reporting key metrics such as efficiency in literature, we suggest a set of metrics for documenting experimental results with the aim to promote standardization in reporting and ease in comparing. We suggest the use of more sophisticated tools such as RNA transcriptomics for thorough assessment of cell perturbation attributed to intracellular delivery. We hope that this Account can effectively capture the progress of nanostructure-mediated cargo delivery and encourage new innovations.
Graphical Abstract
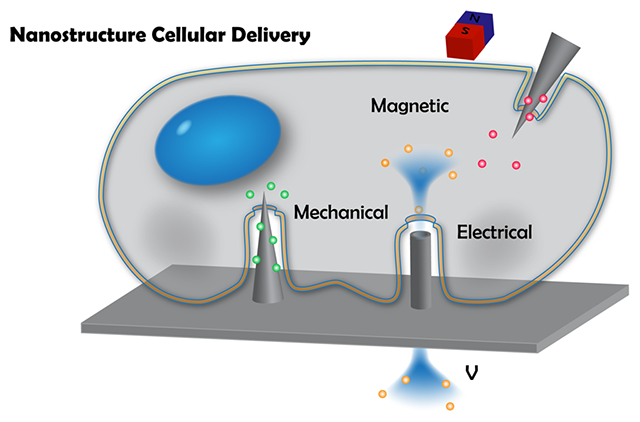
1. HISTORICAL DEVELOPMENTS OF NANOSTRUCTURES FOR CELLULAR DELIVERY
Intracellular cargo delivery with nucleic acids, proteins, and small molecule sensors is a critical step to influence and monitor cellular functions and fates. The rapid advances in biological therapies and gene editing methods, such as clustered regularly interspaced short palindromic repeats (CRISPR) and chimeric antigen receptor T cell (CAR-T) therapy, require delivery of biomolecular cargo across the cell membrane and potentially to different subcellular locations, such as the nuclei and mitochondria. Manipulation of complex biological phenomena in a rigorous and reproducible manner requires techniques that easily and efficiently deliver cargo into targeted intracellular spaces with significantly less cell perturbation than conventional viral, biochemical, and bulk electroporation techniques. Recent delivery methods have facilitated development of innovative biomedical technologies including treatments like immunotherapy1 and personalized disease modeling.2
The first case of intracellular delivery was arguably reported in 1911 when cargo was injected into an individual cell using fire-polished glass micropipettes.3 Motivated by the low throughput and labor-intensive nature of microinjection, researchers subsequently developed a plethora of other transfection techniques, which can be classified into three major classes according to their mechanisms: viral, biochemical, and physical methods. Viral methods capitalize on evolutionarily directed protein machinery to encapsulate and deliver nucleic acids into cells.4 Biochemical methods utilize polymers, proteins, or lipid vesicles to deliver biochemical complexes, while physical methods exploit high intensity energies or forces created with electrical, magnetic, or optical fields to generate transient pores in the cell membrane for cargo transport.5
Although a variety of delivery methods exists, current techniques have limitations that prevent them from achieving high delivery efficiency without compromising cell health. For instance, viral multiplicity of infection (i.e., the number of viral particles to cells), biochemical concentrations, or voltages during bulk electroporation have to be high to attain high efficiency, yet these have a trade-off of greater cytotoxicity. Unique limitations also exist for each specific technique.5 For instance, viruses have a maximum cargo packing capacity of ~15 kilobase-pairs (kpb) and their protein machinery may only allow them to efficiently deliver to a specific class of cells. Biochemicals are generally unable to deliver to primary cells, and conjugating a high density of cargo to chemical polymers can also result in aggregation and protein misfolding.6 The most common physical technique is electroporation, yet it is common to have >80% total cell death and difficulty delivering into more sensitive cell types. Electroporation can also lead to Joule heating and bubble formation, which adversely affect the homogeneity of electric fields and delivery outcomes.
Over the past ten years, a rapidly expanding effort has been made to develop nanostructures for cellular delivery. A wide variety of nanomaterials have been developed for this purpose, including nanowires, nanostraws, nanospears, and nanopores. These new devices have become possible by progress in nanofabrication techniques like vapor–liquid–solid nanowire synthesis, chemical vapor deposition, and atomic layer deposition. An important feature that distinguishes nanostructures from existing techniques is that they interface with cells highly locally, with size scales measuring tens of nanometers. By limiting the nano–bio interface to such a small scale, cell health may be improved by limiting the extent of the perturbation. At the same time, the magnitude of the physical effect, such as electric field strength or mechanical pressure, may be extremely high in that local region, allowing for more effective cargo delivery. In Figure 1, we outline the chronological developments of nanostructures and how mechanistic understanding has evolved in the past decade.
Figure 1.
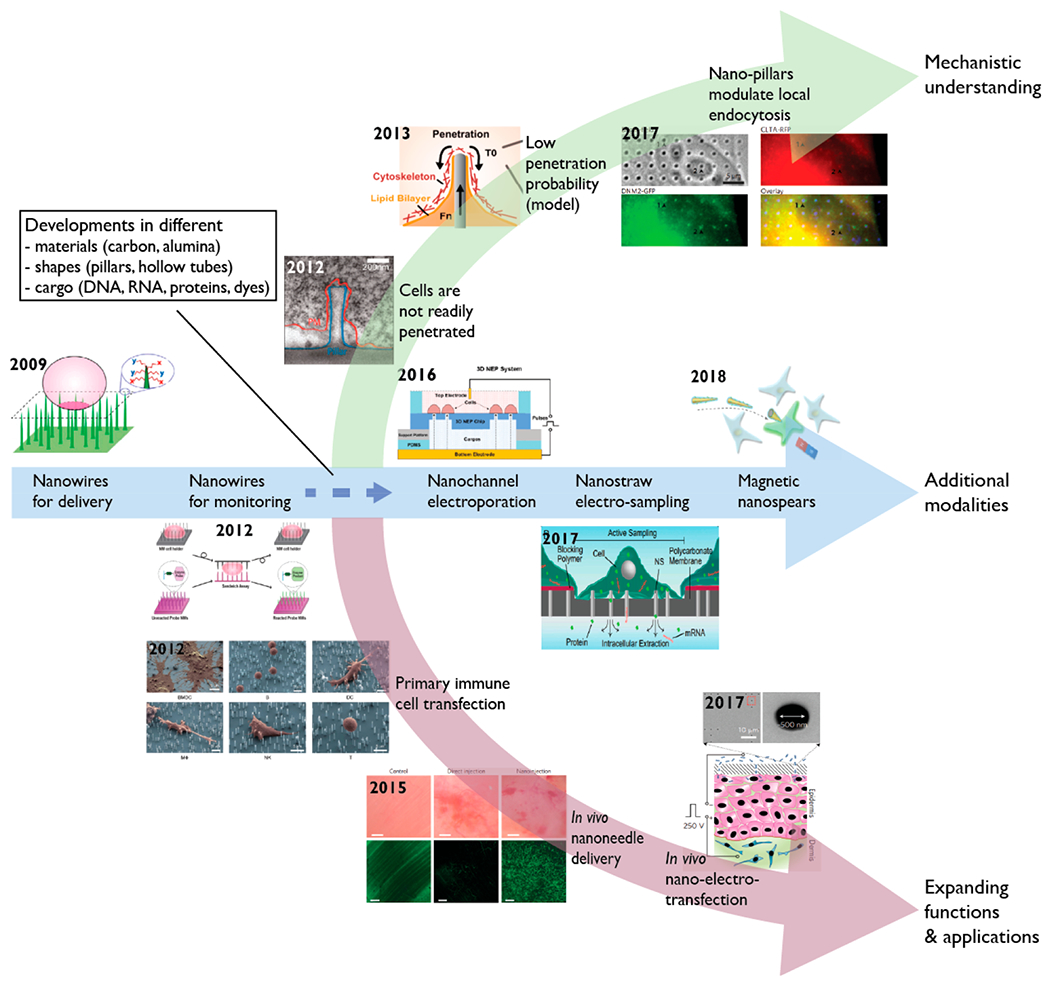
Historical development of nanostructures for intracellular delivery and sampling. Nanowires were described for cargo delivery7 and enzymatic monitoring11 by direct physical penetration through cell membrane. Throughout the past decade, nanostructures of different shapes and materials have been fabricated to deliver diverse cargo including nucleic acids, proteins, and small molecules to cell lines and primary cells. The use of electron microscopy12,13 and computational modeling14 demonstrated that although nanostructures could penetrate cell membrane, the frequency is low. This motivated coupling of electrical fields15,16 and magnetic forces17 to nanostructures to enhance delivery outcomes. In 2015, the first two in vivo applications using biodegradable nanoneedles for delivering DNA18 and quantum dots19 were reported, paving the route for future technologies such as nanostructures coupled with electrical control for in vivo transfection. Images adapted with permission from the following: ref 7, Copyright 2010 Proceedings of the National Academy of Sciences; ref 11, Copyright 2013 American Chemical Society; ref 12, Copyright 2012 American Chemical Society; ref 14, Copyright 2013 American Chemical Society; ref 15, Copyright 2016 Royal Society of Chemistry; ref 16, Copyright 2017 Proceedings of the National Academy of Sciences; ref 17, Copyright 2018 American Chemical Society; ref 22, Copyright 2012 American Chemical Society; ref 18, Copyright 2015 Nature Publishing Group; ref 31, Copyright 2015 Nature Publishing Group; ref 38, Copyright 2017 Nature Publishing Group.
The most common nanomaterial methods have relied on either high mechanical stresses or electric fields at the nanomaterial–cell interface to cause local membrane rupture. These small pores allow exogenous cargo materials to enter the cell, through diffusion7 or electrophoresis.8,9 Since these mechanisms are largely agnostic to the molecular cargo, nanostructures enable delivery or co-delivery of a wide variety of materials that have not been traditionally possible or require laborious redesign. This is a key advantage, as there is an increasing interest to deliver mixed cargo such as mRNA and proteins of the cas9 enzyme together with guide RNA for CRISPR applications.10 More details on the merits of nanostructures with direct comparisons to viral, biochemical, and bulk electroporation methods will be discussed in section 2.
Figure 1 outlines the historical development of nanostructures for cellular delivery. In 2005, Cai et al. first showed that magnetic carbon nanotubes could be magnetically guided to puncture cell membrane to transfect primary neurons and ex vivo B cells with DNA plasmids.20 Shortly afterward, Kim et al. found that cells could be cultured on top of nanowire arrays, although little cargo delivery was detected.21 Following that, Shalek et al. demonstrated the use of aligned vertical silicon nanowires for delivering biomolecular cargo into cells.7 The team coated nucleic acids and peptides onto nanowires followed by growing cells onto those surface-modified nanowires. Nanowires were believed to readily penetrate cells, enabling intracellular delivery through passive diffusion of biomolecules from nanowires to intracellular spaces. Using the same nanowire technology, the same team later transfected diverse primary immune cells including T cells, B cells, natural killer cells, and macrophages.22 At the same time, Na and colleagues adapted silicon nanowires to sandwich cells for monitoring intracellular enzymatic activity of protease, phosphatase, and protein kinase.11 Following this, there was rapid development in creating nanostructures with different dimensions, materials (such as carbon23 and alumina24), and shapes (like needles19 and straws24). Most of these technologies purported that enhanced delivery efficiency was attributed to the mechanism of physical penetration by nanostructures followed by intracellular cargo diffusion.
However, in 2012, Hanson et al. showed with electron microscopy that neurons were not easily penetrated by nanopillars (50–500 nm in diameter) and that cells either rest on pillars or wrap their cell membranes around them (Figure 2a),12 while other groups reported that neurons could be penetrated by nanowires (Figure 2b).25 Many of these early observations appeared to be at odds with each other, yet if the probability of penetration was low, microscopy methods could miss some penetration events. However, Lin et al. found that electroporation was needed for intracellular access for measuring electrical action potentials from cardiomyocytes, corroborating the TEM measurements.26 Most recently, Dipalo et al. fabricated a variety of nanostructures with diameters ranging from 80 to 800 nm and found through electron microscopic images that cell membranes were highly deformable and cardiomyocytes were able to conform tightly to the shapes of all nanostructures.13 Theoretical14 and computational models27 both suggest that penetration is possible yet requires active application of forces on the order of 100 pN. Recent studies suggest that nanostructures could also modulate local endocytosis to influence intracellular delivery.28
Figure 2.
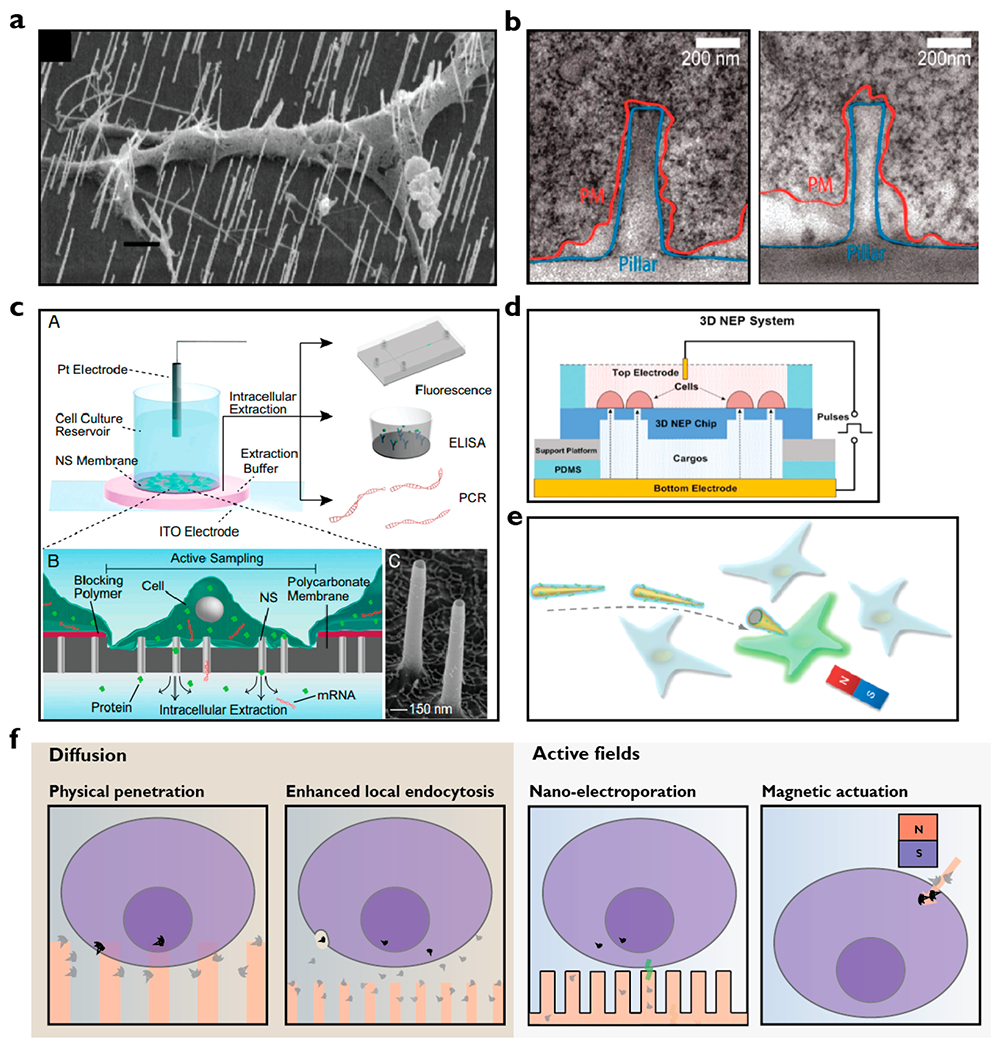
Modes of intracellular cargo delivery using nanostructures. (a) Electron microscopic image showing penetration of neuron by nanowires25 (scale bar, 1 μm) in contrast to the images in panel b where neurons were found wrapping their membrane around nanopillars.12 (c) The nanostraw electroporation platform where cultured cells on the platform are exposed to local electric fields for controlled nanoelectroporation for delivery and extraction of materials such as mRNA and proteins into and out of the cells.16 Electron microscopic image shows nanostraws of about 1.5 μm in height and 150 nm in diameter. (d) Nanochannel electroporator capable of high throughput (40[IPS] 000 cells/cm2) delivery of cargo into cells.15 (e) Magnetic nanospears functionalized with plasmid encoding for green fluorescent protein (GFP). A benchtop magnet can be used to guide the nanospears for targeted single cell transfection.17 (f) Proposed mechanisms of intracellular cargo delivery using nanostructures. Cargo delivery is likely to occur through both physical penetrations followed by passive diffusion and cargo diffusion followed by endocytosis. By coupling of active fields, intracellular cargo delivery can also occur through nanoelectroporation and magnetic actuation. Note that the illustration is for nonadherent or suspension cells. Adherent cells typically conform to the shapes of the nanostructures after an hour of culture. Images adapted with permission from the following: ref 12, Copyright 2012 American Chemical Society; ref 15, Copyright 2016 Royal Society of Chemistry; ref 16, Copyright 2017 Proceedings of the National Academy of Sciences; ref 17, Copyright 2018 American Chemical Society; ref 25, Copyright 2007 American Chemical Society.
To resolve these seemingly conflicting results, our group developed a method to directly test whether the membrane was penetrated at the location of the nanostructures over a large number of cells simultaneously, eliminating selection bias and providing quantifiable statistics.29 This assay delivered Co2+ ions, which are cell-impermeant, through a 100 nm hollow nanostraw to GFP-expressing cells. If the nanostraw penetrated the membrane, a dark spot formed as the Co2+ quenched the GFP fluorescence, and each “spot” could be counted and compared to the number of nanostraws under the cells to get a quantitative penetration efficiency. These tests found that spontaneous penetration can occur but is a very low frequency event, with occurrence around 7%, in agreement with mechanical calculations.14,29 This effect was dependent on nanostraw size and cell adhesiveness, as slightly larger nanostraws (200 nm) had 0% efficiency. These measurements resolved many of the observations, highlighting that it was quite difficult, yet possible, for nanostructures on planar substrates to spontaneously penetrate the cell wall.
Lately, there is emerging evidence suggesting that physical deformations of the nuclear envelope could increase the porosity of nuclear pore complexes. For example, transient disruption of plasma and nuclear membrane might have enhanced transfection efficiency during microfluidic dielectrophoresis.30 It has also been found that nanopillars can impose dramatic nuclear deformations and that nuclear morphology can be influenced by nanopillar geometry.31 In the future, advanced microscopic techniques can be combined with various tools for mechanobiology32 to visualize possible changes in plasma and nuclear membranes in the presence of magnetomechanical forces and whether mechanical forces can preferentially enhance the entry of cargo into the nuclei.
Capitalizing on emerging evidence that nanostructure penetration through a cell membrane is a low probability event, the community soon began to couple additional modalities such as electrical fields and magnetic forces. Our group developed the nanostraw electroporation system, using applied voltages to create very high local electric fields through nanostraws, generating transient membrane pores as well as electrophoretically injecting charged cargo species like DNA directly into cells.8 Because of the physical nature of the delivery, this platform can be used for precise dosage control by varying reagent concentrations, electrical pulse time duration, and voltages.9 Interestingly, by reversing the sign of the applied voltage, the same platform could be used for nondestructive sampling of intracellular DNA or RNA (Figure 2c).16 Other members in the field have integrated electrical control with nanostructures for better intracellular access,33,34 including nanochannel electroporation35 and dielectrophoretic nanoelectroporation36 for transfecting immune cells. This has been extended to a high throughput array version of the nanochannel electroporator to transfect primary cardiomyocytes (Figure 2d).15,37 Besides electrical fields, magnetic forces have also been coupled to nanostructures to aid cargo delivery. For example, Xu et al. fabricated magnetic nickel nanospears (Figure 2e), which can be guided by a benchtop magnet for single cell targeting and co-delivery of biomolecules with high efficiency and cell viability.17
Another major area of development is use of nanostructures for in vivo applications (Figure 3). Chiappini et al. first described the design of biodegradable nanoneedles to deliver quantum dots19 and DNA plasmids18 in vivo. They demonstrated the ability of nanoneedles to deliver the VEGF-165 plasmid DNA to induce sustained neovascularization, leading to a localized six-fold increase in blood perfusion to a targeted muscle region.18 Gallego-Perez et al. also integrated nanostructures with electrical control to deliver cargo to promote vascularization of necrotic tissues.38 Most recently, Tang et al. demonstrated the use of nanoneedles for therapeutic heart regeneration after acute myocardial infarction in vivo.39
Figure 3.
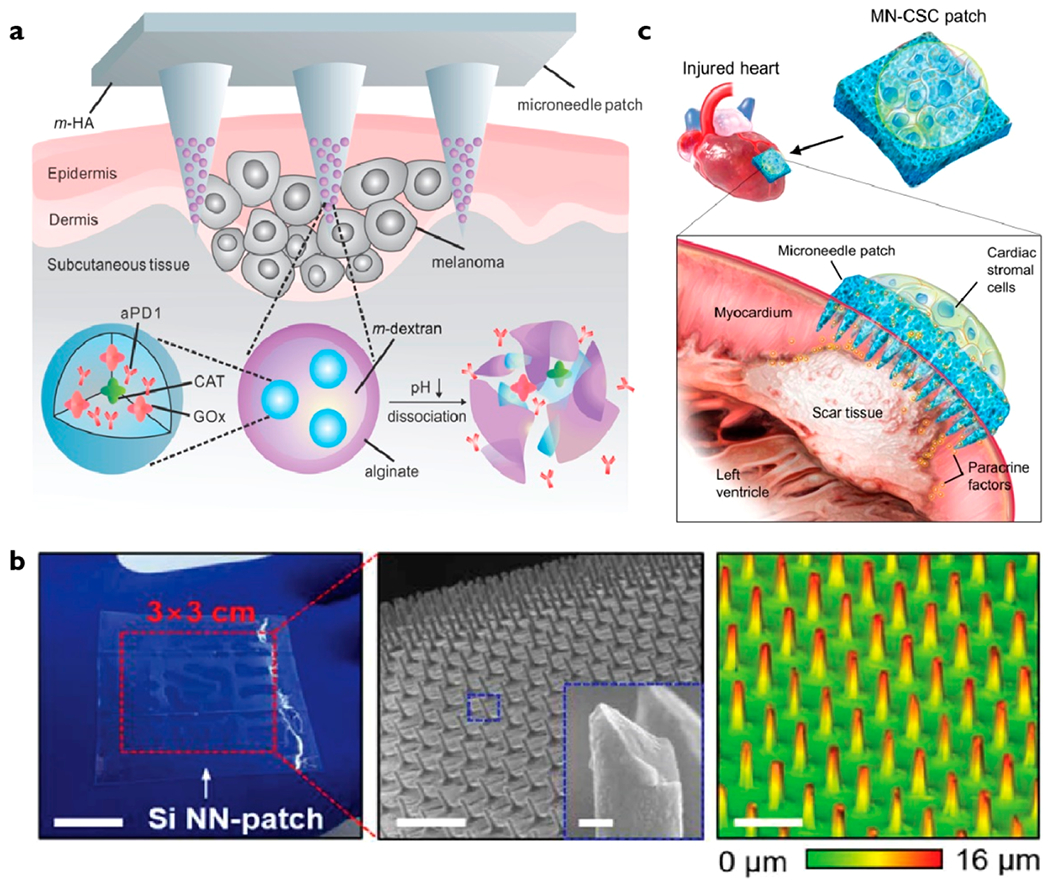
Examples of micro- and nanostructure for in vivo cargo delivery. (a) Biocompatible hyaluronic acid microneedle patch integrated with pH-sensitive dextran nanoparticles encapsulating anti-PD1 antibody and glucose oxidase (GOx). GOx generates an acidic environment by converting blood glucose to gluconic acid, which promotes dissociation of nanoparticles and release of anti-PD1 for immunotherapy. The microneedle patch can be applied under the skin to target skin cancer cells.40 (b) Silicon nanoneedles were coated with poly(dimethyl siloxane) (PDMS), enabling them to become flexible and conform to irregular surfaces of tissues.41 (c) Microneedle patch to deliver paracrine secretions from cardiac stromal cells to injured cardiomyocytes to promote angiomyogenesis.39 Images adapted with permission from the following: ref 40, Copyright 2016 American Chemical Society; ref 41, Copyright 2018 American Association for the Advancement of Science; ref 39, Copyright 2018 American Association for the Advancement of Science.
Microneedles have also been integrated with nanoparticles to deliver anti-PD-1 antibody to boost the efficacy of immunotherapy against melanoma (Figure 3a).40 Recently, Kim and colleagues developed a vertically ordered silicon-based nanoneedle patch to deliver biomolecules to tissues (Figure 3b).41 A key innovation is the use of elastomer poly(dimethyl siloxane) (PDMS) for coating nanoneedles to provide mechanical flexibility and optical transparency. Tang et al. also creatively designed a microneedle patch with cultured cardiac stromal cells for therapeutic heart regeneration after acute myocardial infarction in vivo (Figure 3c).39 The microneedles act as channels for paracrine secretions from therapeutic cardiac stromal cells to diffuse to myocardium to promote angiomyogenesis, reduce scar formation, and augment cardiac functions.
2. BENEFITS OF NANOSTRUCTURES FOR INTRACELLULAR CARGO DELIVERY
Cell transfection techniques aim to achieve high cargo delivery efficiency with minimal cell perturbation. In this section, we examine the benefits of nanoscale mechanism for transfection, focusing on cell perturbation and applicability to diverse cell types and environment.
Cells can experience perturbation during delivery owing to cytotoxicity of cargo and the mechanism of transfection. Viral vectors such as adeno-associated viruses (AAVs) and lentiviruses are known to induce host immune stresses as they integrate viral genetic materials into the host genome and hijack the host’s protein machinery for intensive viral protein production.42 The problem of cytotoxicity is not limited to viral vectors. For instance, the ratio of biochemical agents such as lipid vesicles to biomolecules must be carefully optimized to minimize cytotoxicity after intracellular unpacking, which can lead to a sudden spike in the local concentrations of biochemical complexes.43 Conventional physical methods, such as bulk electroporation, employ high intensity electric fields up to a few thousand volts, creating a broad distribution of hole sizes in the cell membrane. Cells with larger holes die, while those with very small holes may not have any delivery, giving very inhomogeneous results. When membrane pores remain open, it can lead to significant cell stress as cells can no longer maintain homeostasis, especially ionic balance.44
On the other hand, when cells interface with nanostructures, they either experience highly localized mechanical penetration, such as through nanowires or magnetic nanospears17 or local electric fields such as through nanostraws.16 Particularly, nanoelectroporation techniques restrict voltages within small nanochannels, thus minimizing cell perturbation compared to bulk electric fields.35 Due to these factors, the cell viability postdelivery is uniformly very high, often >90%.9
Existing viral, biochemical, and bulk electroporation techniques are not easily adaptable to diverse cell types, requiring development of new vectors or protocols for each respective cell type. For example, viral vectors exhibit tropism, which enables viruses to efficiently transfect specific cell types but not others depending on the expressed cell receptors and cell cycle progression.45 Biochemical agents such as Lipofectamine are useful for delivering cargo to cell lines but have limited utility for primary cells.5 Physical methods such as optotransfection and bulk electroporation are amenable to different cell types; however, cells can exhibit dramatically different sensitivities to high intensity fields or forces, and there is often a need for careful optimization of transfection conditions.44 For instance, although bulk electroporation is known to work relatively well for cell lines, it typically provides <20% net transfection efficiency in primary T-cells. This limitation has motivated recent integration of bulk electroporation with double-stranded DNA to minimize aggregation of ribonucleoproteins for CRISPR-mediated genetic engineering of primary T-cells. Even with this, only 22% net efficiency was achieved, highlighting the challenges of transfecting primary immune cells.46
Nanostructures can be an effective tool to interface with diverse cell types as they do not rely on biological machineries, such as membrane receptors or lipid composition and charges that can be highly cell specific. For instance, it is known that cells at different stages of cell cycle have different membrane envelope permeability and they express varying density of membrane receptors.47 These biological variations can greatly influence reproducibility with viruses and biochemical agents. On the other hand, delivery mechanisms using nanostructures can take advantage of physical perturbation, such as electrical fields and mechanical forces, which are less affected by these types of variations. For example, nanostraw delivery have been successfully applied diverse primary cells including human induced pluripotent stem cells (hiPSC), human embryonic stem cells, hiPSC-cardiomyocytes, and mouse primary neurons and astrocytes with efficiency as high as 85% (Figure 4a).9 Using nanowires, Shalek et al. also demonstrated delivery of nucleic acids to a variety of primary immune cells including T cells, natural killer cells, and dendritic cells, which are currently being used or tested under clinical trials for cancer immunotherapy (Figure 4b,c).22 These examples show that by varying the physical dimensions of nanostructures and strengths of external modalities such as intensity and duration of nanoelectrical stimulations, nanostructures can effectively interface with a variety of cell types with significantly different lipid compositions, surface adherence, and shapes, which would not be possible with other transfection methods such as those using viruses.
Figure 4.
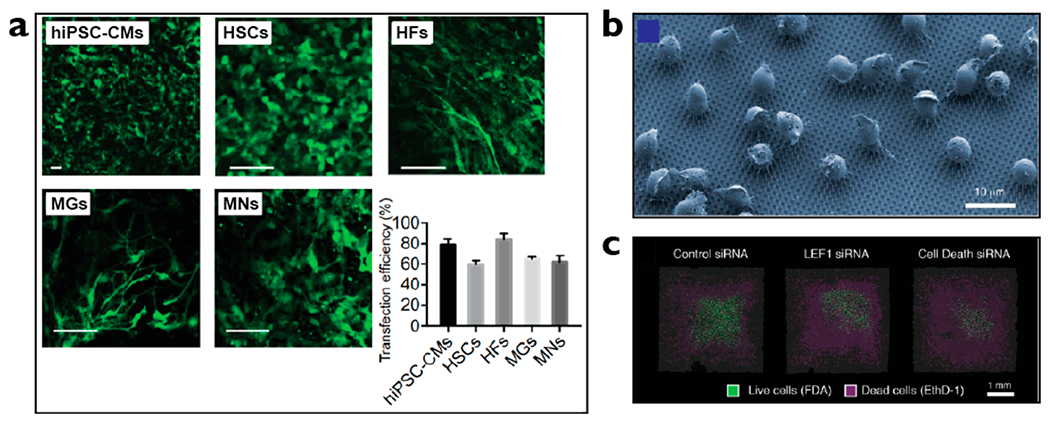
Nanostructures deliver cargo with high efficiency to a variety of clinically useful, primary cells including neurons and immune cells. (a) Nanostraw electroporation platform delivered mRNA encoding for green fluorescent protein (GFP) into human induced pluripotent stem cells differentiated into cardiomyocytes (hiPSC-CMs), human embryonic stem cells (HSCs), human fibroblasts (HFs), mouse primary glial cells (MGs), and mouse primary neurons (MNs) with efficiency ranging between 60% and 85%.9 (b) Electron microscopy image showing B cells seeded onto nanowires.22 (c) Confocal images show delivery of apoptosis-inducing siRNA (far right) led to higher cell death compared to control (far left).22 Images reproduced with permission from the following: ref 9, Copyright 2018 American Association for the Advancement of Science; ref 22, Copyright 2012 American Chemical Society.
3. ENHANCED DELIVERY ASSESSMENTS
The field of transfection science started in the 1900s, and despite being a mature field, many key metrics are not consistently reported, often making it difficult to assess comparative performance between techniques. Publications sometimes describe transfection efficiency only considering the fraction of live cells that express the particular gene, ignoring the large fraction of initial cells that die, while others report a small subset of cells from microscopy or only a select fraction report flow cytometry data. In addition, very few studies examine the functional health of the cells post-transfection or analyze the cell expression perturbation. In Table 1, we suggest several key metrics to help standardize reporting and assess overall cell health. While tools such as live/dead assays and propidium iodide are routinely used to evaluate newly developed transfection technologies, adoption of more sophisticated tools such as RNA transcriptomics would provide a dramatically improved view of the downstream effects of delivery method.
Table 1.
Checklist for Reporting Transfection Literature
| parameters | aims and rationale | definitions | tools |
|---|---|---|---|
| Delivery Characteristics | |||
| efficiency | maximize efficiency | expressed as total number of transformed cells over total cell number (both dead and alive)a and days post-transfection | fluorescence signals; indel rates for genetic engineering applications |
| viability | maximize viability | expressed as total number of viable cells over total cell number (both dead and alive) and days post-transfectionb | propidium iodide |
| net efficiency | highlights trade-off between efficiency and viability | expressed as a product of efficiency and viability | see above |
| metabolism | minimize perturbation to metabolic states of cells | expressed as a function to control cells without transfection treatment | MTTc colorimetric assays; ATP determination kits |
| cell perturbation | minimize cell perturbation | expressed as a function to control cells without transfection treatment; we also recommend measuring cell stress at different time points, that is, from 1 h up to 48 h post-transfection. | calcium indicators; reactive oxygen species assays; RNA transcriptomics |
| proliferation | transient and minimal impact on cell proliferation | expressed as a function of control cells without transfection treatment | total cell count with FACS; estimated cell count with microscopy cell counting |
| Cargo Characterizations | |||
| type | affects the choice of transfection | nucleic acids (i.e. RNA, DNA), proteins, ribonucleoproteins (RNPs), small molecules | not applicable |
| size and dispersity | affects delivery efficiency | nucleic acids, proteins and RNPs, and quantum dots should be reported in kilobase-pair (kbp), kilodalton (kDa), and nanometers (nm), respectively | polymerase chain reaction (PCR) and gel electrophoresis; dynamic light scattering |
| concentration | affects cargo aggregation, delivery efficiency, and cytotoxicity | total mass (μg) and density (g/cm3) | absorbance using spectrophotometer |
| labeling | affects delivery efficiency, cytotoxicity, and cell-type specificity | types of ligands, such as nuclear-localization signals and antibodies, and density of ligands | transmittance and standard curves using titer kit |
| Biological Characterizations | |||
| cell density | affects cell–cargo interactions and cell–nanostructure interface | expressed as a function of seeding density and % of cell culture flask surface area occupied | microscopic counting |
| cell source and origin | affects reproducibility | ATCC (American Type Culture Collection) designation | validation by 3rd party |
| passage number and age | affects reproducibility as older cells are known to have different biological properties such as metabolic rate | we recommend using cell lines with passage number <30; in general, when cells are dividing slower, we recommend changing to a new batch of cells | record passage number and any observations regarding cell health |
| Mycoplasma testing | affects reproducibility across laboratories | Mycoplasma are bacteria resistant to antibiotics commonly used in cell culture medium; they can dramatically alter cell physiology | routine testing with PCR (every 2 months) |
| media composition | affects cell growth and recovery after transfection | composition such as serum, supplements (like antioxidants), and growth factors (like interleukin) with concentrations, product code, and batch number | manufacturer’s data sheet |
It is important to note that in some literature, such as T-cell transfection, the efficiency is often calculated ignoring any dead cells, which can dramatically increase efficiency estimates and produce much smaller absolute numbers of cells.4
The days post-transfection should be reported because cells undergo cell divisions and the % transfected and viable cells can be very different 1 day or 4 days post-transfection.
MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
3.1. Intracellular Calcium Level Indicators
Calcium is an important second messenger implicated in numerous signaling events related to cell stress and apoptosis.48 Calcium levels in the cytosol are typically maintained at around 100 nM, which is significantly lower than that in organelles (endoplasmic reticulum, mitochondria) and in the extracellular environment, which have calcium levels in the micromolar and millimolar range, respectively. During transfection, calcium levels can increase due to viral entry into cells, endocytosis of biochemical complexes, and pore opening due to physical penetration or electroporation.49 The larger the membrane pores and the longer they remain open, the greater the influx of calcium. Intracellular calcium level is thus a good indication of the magnitude of cell perturbation soon after delivery, when the cell has not yet re-equilibrated.49
There are a few ways to measure intracellular calcium concentrations. The most convenient way is using synthetic fluorescent indicators of calcium, with Fura-2 and fluo-4 being popular options.50 These are synthetic dye molecules that bind to calcium and, upon binding, become fluorescent. They are suitable for measuring acute calcium levels as they diffuse rapidly into cells and can be used in virtually any cell type. The other option is to use genetically encoded calcium indicators, such as GCaMP. GCaMP is created from the fusion of GFP and calmodulin, a protein that binds to calcium. Through directed evolution, several versions of GCaMP with different kinetics and photobleaching exist for different purposes.51 GCaMP is particularly suitable for studying intracellular calcium levels on the same cell populations longitudinally as they are continually being replenished by cells. A drawback of the GCaMP technique is, however, that the protein complex must first be expressed in cells and cell sorting is necessary to select for successfully transfected cells for good signal-to-noise ratio to assess calcium stress signals.
3.2. RNA Transcriptomics
Assessment of mRNA expressions after transfection are just beginning to be performed using micro-RNA chips, which highlight a powerful way to assess both short-term and long-term perturbation effects.42 While an exciting first step, the limits of fixed species detected on the chip could introduce biases and omit critical mRNAs. With the advent of next-generation sequencing, RNA transcriptomics can now be performed at a lower price and faster rate and with more accurate output. More importantly, the library of mRNA is much more comprehensive, potentially revealing unknown complex biological relations between transfection technique and cell state perturbation. For instance, Cromer et al. showed that viral and bulk electroporation transfection greatly affected the metabolic genes of cells.42 In particular, they found that ribonucleoproteins used in CRISPR genetic engineering applications can elicit a strong DNA damage response transcriptionally. Most recently, DiTommaso et al. showed that human primary T-cells experienced significant transcriptional dysregulation after transfection with bulk electroporation, though the effects of gene misexpressions started to subside after 24 h.52 This is particularly insightful as it is known that immunotherapy using T-cells can elicit deadly side effects due to impaired gene and cytokine regulation.53 RNA transcriptomics is therefore an important tool to monitor perturbation to cell states after cargo delivery and help optimize the post-transfection media cocktail to improve cell viability.
4. CONCLUSIONS AND OUTLOOK
Nonperturbative and efficient biomolecular delivery into cells has evolved from a niche technique to a critical step in modern molecular biology and cellular therapies, including CRISPR/Cas-9 and CAR-T therapy. Nanostructures are increasingly being developed to overcome limitations in efficiency, cell types, and cargo types associated with using conventional viral, biochemical, and bulk electroporation transfection techniques. While the field is rapidly evolving, challenges for the field still remain. Foremost is to transition from simply reporting successful delivery of materials to focusing on cell functionality after transformation. For therapeutic purposes, the end goal is healthy and effective cells, which may mean using smaller quantities of genetic material and milder delivery conditions. This is becoming apparent in areas like T-cells for CAR-T, where the delivery method may be at least partially responsible for the high degree of cellular exhaustion observed. Transport of DNA from the cytoplasm to the nucleus is another significant unresolved hurdle. By improving our understanding in this area, we can dramatically reduce the quantities of delivery material needed while enhancing transfection efficiency.
Another challenge is the number of cells that can be transformed at once using nanostructures. Planar nanostructure arrays only transform the monolayer of cells in direct contact with them, thus the number of cells transformed is proportional to the device area. While easily capable of transforming millions of cells at once, reaching billions of cells becomes logistically complex. However, these extremely large numbers needed may be reduced as the fraction of fully healthy cells increases. New innovations for nanostructures that do not rely on monolayer contact could also greatly increase the number of transformed cells.
The field of nanostructure-meditated delivery into cells is witnessing a dramatic increase in effort and importance for both research and clinical applications. As the impact grows, we hope to see new innovations emerge to address some of the current issues. Effortless delivery and sampling of materials through the cell wall could lead to a golden age of cellular transformations, increasing their functionality, safety, and effectiveness. We hope that this Account captures the progress of nanostructure-mediated intracellular delivery and sampling to encourage greater innovations within the field.
ACKNOWLEDGMENTS
The work was supported by NIH Grant R21 EB02533201 and NSF STTR Grant 1549696 and Bio-X Interdisciplinary Initiatives Program, and A.T. was supported by the National University of Singapore Overseas Postdoctoral Fellowship.
Biographies
Biographies
Andy Tay received his Ph.D. from the University of California, Los Angeles, in 2017 with support from the National University of Singapore (NUS) Overseas Graduate Scholarship. He subsequently went to on Stanford University for his postdoctoral training where he developed nanoscale magnetic materials for immunoengineering.
Nicholas Melosh received his B.S. degree in Chemistry from Harvey Mudd College in 1996, then went on to earn a Ph.D. in Materials Science at UC, Santa Barbara. He was a postdoctoral scholar at UCLA and Caltech from 2001 to 2003 and joined the Materials Science and Engineering Department at Stanford University in 2003. Professor Melosh’s interests include interfacing inorganic structures with biology, self-assembly, and diamondoids. He is a Terman Fellow, Reid and Polly Anderson Faculty Scholar, and Chambers Faculty Scholar at Stanford.
Footnotes
The authors declare the following competing financial interest(s): N.M. is a co-founder of Navan Inc, which develops nanostraw delivery solutions.
REFERENCES
- (1).Wang X; Rivière I Clinical Manufacturing of CAR T Cells: Foundation of a Promising Therapy. Molecular Therapy - Oncolytics 2016, 3, 16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Rafii S; Lyden D Therapeutic Stem and Progenitor Cell Transplantation for Organ Vascularization and Regeneration. Nat. Med 2003, 9, 702–712. [DOI] [PubMed] [Google Scholar]
- (3).Barber MA A Technic for the Inoculation of Bacteria and Other Substances into Living Cells. J. Infect. Dis 1911, 8, 348–360. [Google Scholar]
- (4).Vaughan EE; Geiger CR; Miller AM; Loh-Marley PL; Suzuki T; Miyata N; Dean DA Microtubule Acetylation through HDAC6 Inhibition Results in Increased Transfection Efficiency. Mol. Ther 2008, 16, 1841–1847. [DOI] [PubMed] [Google Scholar]
- (5).Stewart MP; Sharei A; Ding X; Sahay G; Langer R; Jensen KF In Vitro and Ex Vivo Strategies for Intracellular Delivery. Nature 2016, 538, 183–192. [DOI] [PubMed] [Google Scholar]
- (6).Gao C; Yan D Hyperbranched Polymers: From Synthesis to Applications. Prog. Polym. Sci 2004, 29, 183–275. [Google Scholar]
- (7).Shalek AK; Robinson JT; Karp ES; Lee JS; Ahn D-R; Yoon M-H; Sutton A; Jorgolli M; Gertner RS; Gujral TS; MacBeath G; Yang EG; Park H Vertical Silicon Nanowires as a Universal Platform for Delivering Biomolecules into Living Cells. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 1870–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Xie X; Xu AM; Leal-Ortiz S; Cao Y; Garner CC; Melosh NA Nanostraw-Electroporation System for Highly Efficient Intracellular Delivery and Transfection. ACS Nano 2013, 7, 4351–4358. [DOI] [PubMed] [Google Scholar]
- (9).Cao Y; Chen H; Qiu R; Hanna M; Ma E; Hjort M; Zhang A; Lewis RS; Wu JC; Melosh NA Universal Intracellular Biomolecule Delivery with Precise Dosage Control. Sci. Adv 2018, 4, eaat8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Yin H; Song CQ; Dorkin JR; Zhu LJ; Li Y; Wu Q; Park A; Yang J; Suresh S; Bizhanova A; Gupta A; Bolukbasi M; Walsh S; Bogorad RL; Gao G; Weng Z; Dong Y; Koteliansky V; Wolfe SA; Langer R; Xue W; Anderson DG Therapeutic Genome Editing by Combined Viral and Non-Viral Delivery of CRISPR System Components in Vivo. Nat. Biotechnol 2016, 34, 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Na YR; Kim SY; Gaublomme JT; Shalek AK; Jorgolli M; Park H; Yang EG Probing Enzymatic Activity inside Living Cells Using a Nanowire-Cell “Sandwich” Assay. Nano Lett. 2013, 13, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hanson L; Lin ZC; Xie C; Cui Y; Cui B Characterization of the Cell-Nanopillar Interface by Transmission Electron Microscopy. Nano Lett. 2012, 12, 5815–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Dipalo M; McGuire AF; Lou HY; Caprettini V; Melle G; Bruno G; Lubrano C; Matino L; Li X; De Angelis F; Cui B; Santoro F Cells Adhering to 3D Vertical Nanostructures: Cell Membrane Reshaping without Stable Internalization. Nano Lett. 2018, 18, 6100–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Xie X; Xu AM; Angle MR; Tayebi N; Verma P; Melosh NA Mechanical Model of Vertical Nanowire Cell Penetration. Nano Lett. 2013, 13, 6002–6008. [DOI] [PubMed] [Google Scholar]
- (15).Chang L; Bertani P; Gallego-Perez D; Yang Z; Chen F; Chiang C; Malkoc V; Kuang T; Gao K; Lee LJ; Lu W 3D Nanochannel Electroporation for High-Throughput Cell Transfection with High Uniformity and Dosage Control. Nanoscale 2016, 8, 243–252. [DOI] [PubMed] [Google Scholar]
- (16).Cao Y; Hjort M; Chen H; Birey F; Leal-Ortiz SA; Han CM; Santiago JG; Paşca SP; Wu JC; Melosh NA Nondestructive Nanostraw Intracellular Sampling for Longitudinal Cell Monitoring. Proc. Natl. Acad. Sci U. S. A 2017, 114, E1866–E1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Xu X; Hou S; Wattanatorn N; Wang F; Yang Q; Zhao C; Yu X; Tseng HR; Jonas SJ; Weiss PS Precision-Guided Nanospears for Targeted and High-Throughput Intracellular Gene Delivery. ACS Nano 2018, 12, 4503–4511. [DOI] [PubMed] [Google Scholar]
- (18).Chiappini C; De Rosa E; Martinez JO; Liu X; Steele J; Stevens MM; Tasciotti E Biodegradable Silicon Nanoneedles Delivering Nucleic Acids Intracellularly Induce Localized in Vivo Neovascularization. Nat. Mater 2015, 14, 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Chiappini C; Martinez JO; De Rosa E; Almeida CS; Tasciotti E; Stevens MM Biodegradable Nanoneedles for Localized Delivery of Nanoparticles in Vivo: Exploring the Biointerface. ACS Nano 2015, 9, 5500–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Cai D; Mataraza JM; Qin ZH; Huang Z; Huang J; Chiles TC; Carnahan D; Kempa K; Ren Z Highly Efficient Molecular Delivery into Mammalian Cells Using Carbon Nanotube Spearing. Nat. Methods 2005, 2, 449–454. [DOI] [PubMed] [Google Scholar]
- (21).Kim W; Ng JK; Kunitake ME; Conklin BR; Yang P Interfacing Silicon Nanowires with Mammalian Cells. J. Am. Chem. Soc 2007, 129, 7228–7229. [DOI] [PubMed] [Google Scholar]
- (22).Shalek AK; Gaublomme JT; Wang L; Yosef N; Chevrier N; Andersen MS; Robinson JT; Pochet N; Neuberg D; Gertner RS; Amit I; Brown JR; Hacohen N; Regev A; Wu CJ; Park H Nanowire-Mediated Delivery Enables Functional Interrogation of Primary Immune Cells: Application to the Analysis of Chronic Lymphocytic Leukemia. Nano Lett. 2012, 12, 6498–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Golshadi M; Wright LK; Dickerson IM; Schrlau MG High-Efficiency Gene Transfection of Cells through Carbon Nanotube Arrays. Small 2016, 12, 3014–3020. [DOI] [PubMed] [Google Scholar]
- (24).Vandersarl JJ; Xu AM; Melosh NA Nanostraws for Direct Fluidic Intracellular Access. Nano Lett. 2012, 12, 3881–3886. [DOI] [PubMed] [Google Scholar]
- (25).Hällström W; Mårtensson T; Prinz C; Gustavsson P; Montelius L; Samuelson L; Kanje M Gallium Phosphide Nanowires as a Substrate for Cultured Neurons. Nano Lett. 2007, 7, 2960–2965. [DOI] [PubMed] [Google Scholar]
- (26).Lin ZC; Xie C; Osakada Y; Cui Y; Cui B Iridium Oxide Nanotube Electrodes for Sensitive and Prolonged Intracellular Measurement of Action Potentials. Nat. Commun 2014, 5, 3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Pogodin S; Baulin VA Can a Carbon Nanotube Pierce through a Phospholipid Bilayer? ACS Nano 2010, 4, 5293–5300. [DOI] [PubMed] [Google Scholar]
- (28).Gopal S; Chiappini C; Penders J; Leonardo V; Seong H; Rothery S; Korchev Y; Shevchuk A; Stevens MM Porous Silicon Nanoneedles Modulate Endocytosis to Deliver Biological Payloads. Adv. Mater 2019, 31, 1970086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Xu AM; Aalipour A; Leal-Ortiz S; Mekhdjian AH; Xie X; Dunn AR; Garner CC; Melosh NA Quantification of Nanowire Penetration into Living Cells. Nat. Commun 2014, 5, 3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ding X; Stewart MP; Sharei A; Weaver JC; Langer RS; Jensen KF High-Throughput Nuclear Delivery and Rapid Expression of DNA via Mechanical and Electrical Cell-Membrane Disruption. Nat. Biomed. Eng 2017, 1, 0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hanson L; Zhao W; Lou HY; Lin ZC; Lee SW; Chowdary P; Cui Y; Cui B Vertical Nanopillars for in Situ Probing of Nuclear Mechanics in Adherent Cells. Nat. Nanotechnol 2015, 10, 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Tay A; Schweizer FE; Di Carlo D Micro- and Nano-Technologies to Probe the Mechano-Biology of the Brain. Lab Chip 2016, 16, 1962–1977. [DOI] [PubMed] [Google Scholar]
- (33).Caprettini V; Cerea A; Melle G; Lovato L; Capozza R; Huang JA; Tantussi F; Dipalo M; De Angelis F Soft Electroporation for Delivering Molecules into Tightly Adherent Mammalian Cells through 3D Hollow Nanoelectrodes. Sci. Rep 2017, 7, 8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Boukany PE; Morss A; Liao WC; Henslee B; Jung H; Zhang X; Yu B; Wang X; Wu Y; Li L; Gao K; Hu X; Zhao X; Hemminger O; Lu W; Lafyatis GP; Lee LJ Nanochannel Electroporation Delivers Precise Amounts of Biomolecules into Living Cells. Nat. Nanotechnol 2011, 6, 747–754. [DOI] [PubMed] [Google Scholar]
- (35).Zhao X; Huang X; Wang X; Wu Y; Eisfeld AK; Schwind S; Gallego-Perez D; Boukany PE; Marcucci GI; Lee LJ Nanochannel Electroporation as a Platform for Living Cell Interrogation in Acute Myeloid Leukemia. Adv. Sci 2015, 2, 1500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Chang L; Gallego-Perez D; Zhao X; Bertani P; Yang Z; Chiang CL; Malkoc V; Shi J; Sen CK; Odonnell L; Yu J; Lu W; Lee LJ Dielectrophoresis-Assisted 3D Nanoelectroporation for Non-Viral Cell Transfection in Adoptive Immunotherapy. Lab Chip 2015, 15, 3147–3153. [DOI] [PubMed] [Google Scholar]
- (37).Chang L; Gallego-Perez D; Chiang CL; Bertani P; Kuang T; Sheng Y; Chen F; Chen Z; Shi J; Yang H; Huang X; Malkoc V; Lu W; Lee LJ Controllable Large-Scale Transfection of Primary Mammalian Cardiomyocytes on a Nanochannel Array Platform. Small 2016, 12, 5971–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Gallego-Perez D; Pal D; Ghatak S; Malkoc V; Higuita-Castro N; Gnyawali S; Chang L; Liao WC; Shi J; Sinha M; Singh K; Steen E; Sunyecz A; Stewart R; Moore J; Ziebro T; Northcutt RG; Homsy M; Bertani P; Lu W; Roy S; Khanna S; Rink C; Sundaresan VB; Otero JJ; Lee LJ; Sen CK Topical Tissue Nano-Transfection Mediates Non-Viral Stroma Reprogramming and Rescue. Nat. Nanotechnol 2017, 12, 974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Tang J; Wang J; Huang K; Ye Y; Su T; Qiao L; Hensley MT; Caranasos TG; Zhang J; Gu Z; Cheng K Cardiac Cell–Integrated Microneedle Patch for Treating Myocardial Infarction. Sci. Adv 2018, 4, eaat9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Wang C; Ye Y; Hochu GM; Sadeghifar H; Gu Z Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [DOI] [PubMed] [Google Scholar]
- (41).Kim H; Jang H; Kim B; Kim MK; Wie DS; Lee HS; Kim DR; Lee CH Flexible Elastomer Patch with Vertical Silicon Nanoneedles for Intracellular and Intratissue Nanoinjection of Biomolecules. Sci. Adv 2018, 4, eaau6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Cromer MK; Vaidyanathan S; Ryan DE; Curry B; Lucas AB; Camarena J; Kaushik M; Hay SR; Martin RM; Steinfeld I; Bak RO; Dever DP; Hendel A; Bruhn L; Porteus MH Global Transcriptional Response to CRISPR/Cas9-AAV6-Based Genome Editing in CD34+ Hematopoietic Stem and Progenitor Cells. Mol. Ther 2018, 26, 2431–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Yamano S; Dai J; Moursi AM Comparison of Transfection Efficiency of Nonviral Gene Transfer Reagents. Mol. Biotechnol 2010, 46, 287–300. [DOI] [PubMed] [Google Scholar]
- (44).Stewart MP; Langer R; Jensen KF Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem. Rev 2018, 118, 7409–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Robbins PD; Ghivizzani SC Viral Vectors for Gene Therapy. Pharmacol. Ther 1998, 80, 35–47. [PubMed] [Google Scholar]
- (46).Roth TL; Puig-Saus C; Yu R; Shifrut E; Carnevale J; Li PJ; Hiatt J; Saco J; Krystofinski P; Li H; Tobin V; Nguyen DN; Lee MR; Putnam AL; Ferris AL; Chen JW; Schickel JN; Pellerin L; Carmody D; Alkorta-Aranburu G; Del Gaudio D; Matsumoto H; Morell M; Mao Y; Cho M; Quadros RM; Gurumurthy CB; Smith B; Haugwitz M; Hughes SH; Weissman JS; Schumann K; Esensten JH; May AP; Ashworth A; Kupfer GM; Greeley SAW; Bacchetta R; Meffre E; Roncarolo MG; Romberg N; Herold KC; Ribas A; Leonetti MD; Marson A Reprogramming Human T Cell Function and Specificity with Non-Viral Genome Targeting. Nature 2018, 559, 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Bai H; Schiralli Lester GM; Petishnok LC; Dean DA Cytoplasmic Transport and Nuclear Import of Plasmid DNA. Biosci. Rep 2017, 37, BSR20160616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Ermak G; Davies KJA Calcium and Oxidative Stress: From Cell Signaling to Cell Death. Mol. Immunol 2002, 38, 713–721. [DOI] [PubMed] [Google Scholar]
- (49).Tang SKY; Marshall WF Self-Repairing Cells: How Single Cells Heal Membrane Ruptures and Restore Lost Structures. Science 2017, 356, 1022–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Tay A; Di Carlo D Remote Neural Stimulation Using Magnetic Nanoparticles. Curr. Med. Chem 2017, 24, 537. [DOI] [PubMed] [Google Scholar]
- (51).Chen TW; Wardill TJ; Sun Y; Pulver SR; Renninger SL; Baohan A; Schreiter ER; Kerr RA; Orger MB; Jayaraman V; Looger LL; Svoboda K; Kim DS Ultrasensitive Fluorescent Proteins for Imaging Neuronal Activity. Nature 2013, 499, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).DiTommaso T; Cole JM; Cassereau L; Buggé JA; Hanson JLS; Bridgen DT; Stokes BD; Loughhead SM; Beutel BA; Gilbert JB; Nussbaum K; Sorrentino A; Toggweiler J; Schmidt T; Gyuelveszi G; Bernstein H; Sharei A Cell Engineering with Microfluidic Squeezing Preserves Functionality of Primary Immune Cells in Vivo. Proc. Natl. Acad. Sci. U. S. A 2018, 115, E10907–E10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Suntharalingam G; Perry MR; Ward S; Brett SJ; Castello-Cortes A; Brunner MD; Panoskaltsis N Cytokine Storm in a Phase 1 Trial of the Anti-CD28 Monoclonal Antibody TGN1412. N. Engl. J. Med 2006, 355, 1018–1028. [DOI] [PubMed] [Google Scholar]


