Abstract
Interleukin-10 (IL-10) is associated with inhibition of cell-mediated immunity and downregulation of the expression of costimulatory molecules required for T-cell activation. When IL-10-deficient (IL-10KO) mice are infected with Toxoplasma gondii, they succumb to a T-cell-mediated shock-like reaction characterized by the overproduction of IL-12 and gamma interferon (IFN-γ) associated with widespread necrosis of the liver. Since costimulation is critical for T-cell activation, we investigated the role of the CD28-B7 and CD40-CD40 ligand (CD40L) interactions in this infection-induced immunopathology. Our studies show that infection of mice with T. gondii resulted in increased expression of B7 and CD40 that was similar in wild-type and IL-10KO mice. In vivo blockade of the CD28-B7 or CD40-CD40L interactions following infection of IL-10KO mice with T. gondii did not affect serum levels of IFN-γ or IL-12, nor did it prevent death in these mice. However, when both pathways were blocked, the IL-10KO mice survived the acute phase of infection and had reduced serum levels of IFN-γ and alanine transaminase as well as decreased expression of inducible nitric oxide synthase in the liver and spleen. Analysis of parasite-specific recall responses from infected IL-10KO mice revealed that blockade of the CD40-CD40L interaction had minimal effects on cytokine production, whereas blockade of the CD28-B7 interaction resulted in decreased production of IFN-γ but not IL-12. Further reduction of IFN-γ production was observed when both costimulatory pathways were blocked. Together, these results demonstrate that the CD28-B7 and CD40-CD40L interactions are involved in the development of infection-induced immunopathology in the absence of IL-10.
Interleukin-10 (IL-10) was first identified as the product of Th2 CD4+ T-cell clones which could inhibit the production of gamma interferon (IFN-γ) by Th1 CD4+ T cells (41). As a consequence of this early characterization of the biological function of IL-10, it has been generally regarded as a Th2-type cytokine. Further studies demonstrated that other cells, including macrophages, dendritic cells, B cells, human TH0, and TH1 clones, as well as a newly defined T-regulatory subpopulation of CD4+ T cells, also produce IL-10 (19, 23, 26, 39, 40). The ability of IL-10 to downmodulate the production of IFN-γ during an immune response is not due to a direct inhibitory effect on T cells but rather is a consequence of its ability to inhibit accessory cell functions, including the production of cytokines (i.e., tumor necrosis factor alpha [TNF-α], IL-1, and IL-12) and expression of costimulatory molecules that are necessary for optimal stimulation of T cells to produce IFN-γ (11, 15, 16, 26, 45).
The critical role of IL-10 in the regulation of cell-mediated immunity was revealed by studies in which IL-10 knockout (IL-10KO) mice were generated (12, 35). In those studies, the absence of IL-10 resulted in the development of a severe inflammatory bowel disease, as a consequence of a pathogenic Th1-type response. IL-10KO mice are also extremely susceptible to the development of septic shock and deleterious skin reactions when exposed to contact sensitizing agents (2). Furthermore, a role for IL-10 in the prevention of immune hyperactivity was illustrated by studies in which IL-10KO mice infected with Toxoplasma gondii or Trypanosoma cruzi developed a lethal shock-like reaction characterized by high levels of IL-12, the production of pathogenic levels of IFN-γ by CD4+ T cells, and the development of large necrotic foci and cellular infiltrates in the liver and lungs (18, 28, 42). These findings demonstrated that T-cell hyperactivity contributed to the death of IL-10KO mice following infection with T. gondii (18, 42) or T. cruzi (28).
The demonstration that IL-12 and IFN-γ were involved in this infection-induced shock correlates with the ability of IL-10 to downregulate the production of these cytokines. However, IL-10 can also inhibit accessory cell expression of major histocompatibility complex class I and class II molecules and B7 molecules, and it can act as an antagonist of the CD40-CD40 ligand (CD40L) interaction, pathways which are required for activation of T-cell responses (14, 32, 34, 43). Therefore, we hypothesized that in the absence of IL-10, these costimulatory pathways could be dysregulated and may contribute to the development of the CD4+ T-cell-mediated, infection-induced immunopathology seen in IL-10KO mice. To test this hypothesis, we analyzed how the absence of IL-10 affected the expression of B7-1 (CD80), B7-2 (CD86), and CD40 following infection and if blockade of the CD28-B7 and CD40-CD40L interactions would alter the development of infection-induced shock in IL-10KO mice. The results reveal that following infection of IL-10KO mice, expression of B7 and CD40 molecules on accessory cells was not dysregulated and that blockade of costimulation through CD40 or CD28 alone failed to rescue IL-10KO mice from the infection-induced mortality. However, the blockade of both costimulatory pathways protected these mice; this effect was characterized by a significant reduction of serum levels of IFN-γ, inducible nitric oxide synthase (iNOS) expression in the liver and spleen, and reduced hepatic damage as measured by serum levels of alanine transferase (ALT). Thus, the CD40-CD40L and the CD28-B7 costimulatory pathways are constitutive elements required for the development of infection-induced immunopathology in IL-10KO mice.
MATERIALS AND METHODS
Mice.
Female Swiss Webster, CBA/CaJ, and C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, Maine). C57BL/6 mice deficient in IL-10 due to a disruption of the IL-10 gene were provided by DNAX (1). These mice were generated by backcrossing C57BL/6-129/Ola IL-10KO mice onto the C57BL/6 background for seven generations (35). Mice were bred and maintained within Thoren caging units at the University Laboratory Animal Resource facilities, University of Pennsylvania. Each experimental group contained three to eight male mice between 6 and 8 weeks of age.
Parasites.
The ME49 strain of T. gondii was maintained in infected Swiss Webster and CBA/CaJ mice. ME49 cysts were prepared from brains of donor mice as previously described (3, 4). Mice were infected with 20 cysts by intraperitoneal injection in a volume of 0.2 ml. Soluble toxoplasma antigen (STAg) was prepared from RH strain tachyzoites as previously described (50). The activity of STAg was titrated to determine the optimal concentration for splenocyte proliferation and cytokine production (20 to 25 μg/ml).
Histology and measurement of ALT levels.
At the time of sacrifice, samples of livers, lungs, and spleens were removed from each mouse and prepared for hematoxylin and eosin or immunohistochemical staining as previously described (27). Briefly, tissues were fixed overnight in Accustain 10% formalin neutral buffered solution (Sigma Diagnostics, St. Louis, Mo.) and then embedded in paraffin. Paraffin sections (5 μm) were stained with hematoxylin and eosin for visualization of pathological changes. Immunohistochemical staining of paraffin sections was done with polyclonal rabbit anti-iNOS (Transduction Laboratories, Lexington, Ky.) as a primary antibody to detect iNOS expression in various tissues. Biotinylated goat anti-rabbit (Vector Laboratories Inc., Burlingame, Calif.) was used as a detecting antibody with diaminobenzamide (Vector Laboratories) as the chromogen.
Sera from IL-10KO mice were collected at various time points during the course of infection, and serum ALT levels were measured to assess the extent of hepatic damage. Serum samples were processed by the Veterinary Clinical Pathology Department, University of Pennsylvania School of Veterinary Medicine, to quantitate ALT levels in the serum.
Reagents.
Complete RPMI 1640 (Life Technologies, Gaithersburg, Md.) medium contained 10% heat-inactivated fetal calf serum (HyClone Laboratories, Logan, Utah), sodium pyruvate, nonessential amino acids, penicillin (10 U/ml), streptomycin (100 μg/ml), and amphotericin B (25 ng/ml) (BioWhittaker, Walkersville, Md.). Anti-CD40L (MR1) (TSD Biosciences, Newark, Del.) was used at a concentration of 20 μg/ml during in vitro T-cell recall responses. HuCTLA-4Ig, a fusion protein comprised of the human CTLA-4 extracellular domain and Fc portion of human immunoglobulin G (IgG), was supplied by Bristol Myers Squibb Research Institute (Princeton, N.J.) and used at a concentration of 20 μg/ml in vitro. Human chimeric L6 (ChiL6; Bristol Myers Squibb), rat IgG (Sigma), and hamster IgG (Jackson Laboratory) were used as control antibodies. IL-10KO mice that had been infected with ME49 were given 200 μg of anti-CD40L, 300 μg of huCTLA-4Ig, the combination of these treatments, or the appropriate isotype control intraperitoneally on days 6 and 8 postinfection. The dose of CTLA-4Ig and anti-CD40L used in vivo was based on previous studies in which a dose of 300 μg of CTLA-4Ig or 200 μg of anti-CD40L antibody was shown to inhibit the CD28-B7 and CD40-CD40L pathways (8, 22, 47).
Analysis of T-cell responses.
Spleens from infected animals were harvested, dissociated into a single-cell suspension, and depleted of erythrocytes using 0.83% (wt/vol) ammonium chloride (Sigma). Cells were washed three times and resuspended in complete RPMI 1640 before being plated at a cell density of 2 × 105 cells per well in a final volume of 200 μl in 96-well plates (Costar, Costar, N.Y.). Cells were stimulated with soluble anti-CD3 (145-2C11) at a final concentration of 1 μg/ml or STAg for 24 to 48 h at 37°C in 5% CO2 using different experimental conditions. IFN-γ levels were measured using a two-site enzyme-linked immunosorbent assay as previously described (49). IL-12 (p40) levels were measured using monoclonal antibody C17.8 as a capture antibody and biotinylated C15.6 as a detecting antibody (hybridomas provided by Giorgio Trinchieri, Wistar Institute, Philadelphia, Pa.).
FACS analysis.
Splenocytes depleted of erythrocytes or peritoneal exudate cells from uninfected and infected animals were resuspended in fluorescence-activated cell sorting (FACS) buffer (1× phosphate-buffered saline, 0.2% bovine serum albumin fraction V, 4 mM sodium azide) to a final concentration of 107 cells/ml. Then, 106 cells were preincubated with saturating concentrations of Fc Block for 20 min on ice and stained with various conjugated antibodies against F4/80, CD40, B220, CD86 (Caltag, South San Francisco, Calif.), or CD80 (Pharmingen, San Diego, Calif.) for 20 min on ice. Cells were washed with FACS buffer and analyzed using a FACScalibur flow cytometer (Becton Dickinson, San Jose, Calif.). Antibodies were used at dilutions empirically determined to give optimal staining for flow cytometric analyses. Results were analyzed using CELL Quest software (Becton Dickinson). Mean fluorescence intensity (MFI) was determined by dividing the fluorescence intensity of cells stained with antibody against CD80, CD86, or CD40 by the fluorescence intensity of cells stained with the isotype control.
Intracellular detection of cytokines.
Erythrocyte-depleted splenocytes from IL-10KO mice infected for 7 days were plated in a 96-well plate (Costar) at a density of 4 × 105 cells per well in a final volume of 200 μl. Cells were stimulated with STAg (25 μg/ml) for 72 h, and phorbol myristate acetate (50 ng/ml; Sigma), ionomycin (50 ng/ml; Sigma), and brefeldin A (10 μg/ml; Sigma) were added to cultures during the last 5 h of stimulation. Cells were harvested, washed, resuspended in FACS buffer, and then stained with fluorescein isothiocyanate-labeled anti-CD8, allophycocyanin-labeled anti-CD4, or fluorescein isothiocyanate-labeled anti-NK1.1 (Pharmingen) for 20 min on ice. Cells were then washed with FACS buffer, fixed with 1% (wt/vol) paraformaldehyde, washed again, and permeabilized with 0.1% (wt/vol) saponin in FACS buffer. After permeabilization, cells were stained with phycoerythrin-conjugated anti-IFN-γ (Pharmingen) for 30 min on ice. Cells were washed once with 0.1% saponin buffer and then with FACS buffer. Analysis of the cells was performed using a FACScalibur flow cytometer (Becton Dickinson).
Statistics.
INSTAT software (GraphPad, San Diego, Calif.) was used to calculate statistical significance. A paired Wilcoxon Mann-Whitney test was used to analyze the statistical significance of differences between serum cytokine levels. An unpaired Student t test was used to determine significant differences in serum ALT levels. A log rank test was used to determine statistical significance in survival curve experiments. A P value of less than 0.05 was considered significant.
RESULTS
Expression of CD80, CD86, and CD40 following infection with T. gondii.
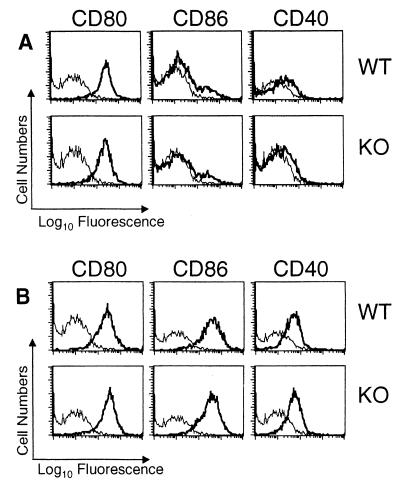
Previous in vitro studies have reported that IL-10 downregulates expression of B7 (13), as well as that of CD40 (34). Therefore, to assess the role of IL-10 in the regulation of these molecules during infection, we infected IL-10KO mice intraperitoneally with T. gondii and analyzed expression of B7 on antigen-presenting cells. Our results reveal that F4/80+ peritoneal exudate cells from uninfected mice express high levels of CD80 and low basal levels of CD86, and expression of these molecules was similar between wild-type (WT) and IL-10KO mice (Fig. 1A). Following infection of WT and IL-10KO mice with T. gondii, we detected no further upregulation of CD80 expression on F4/80+ peritoneal exudate cells from 7-day-infected mice, while CD86 expression was markedly upregulated (Fig. 1B). Interestingly, there were no differences in the level of CD80 or CD86 expression between infected WT and IL-10KO mice. Analysis of CD80 and CD86 expression on F4/80+ splenocytes revealed that there was also an upregulation of both molecules at day 7 postinfection, although no major differences in levels of expression between WT and IL-10KO mice were observed (MFIs for CD80, 1.4 [WT] and 1.7 [IL0-10KO]; MFIs for CD86, 1.0 [WT] and 1.1 [IL-10KO]).
FIG. 1.
Analysis of CD80, CD86, and CD40 expression on F4/80+ peritoneal macrophages following infection with T. gondii. Peritoneal exudate cells from uninfected (A) or 7-day-infected (B) WT and IL-10KO mice were stained for CD80, CD86, and CD40 expression on F4/80+ cells as described in Materials and Methods. Results shown are representative of three separate experiments containing three to five mice per group. Thick lines represent CD40, CD80, or CD86; thin lines represent isotype control stainings.
When expression levels of CD80 and CD86 were analyzed on B220+ spleen cells, our results revealed that uninfected peritoneal B220+ cells from WT and IL-10KO mice expressed similar low basal levels of CD80 and CD86 (MFIs for CD80, 0.49 [WT] and 0.51 [IL-10KO]; MFIs for CD86, 0.85 [WT] and 0.89 [IL-10KO]). At day 7 postinfection, an upregulation of these molecules was detected on B220+ spleen cells, although no differences in the levels of expression of these molecules between WT and IL-10KO mice were detected (MFIs for CD80, 0.66 [WT] and 0.64 [IL-10KO]; MFIs for CD86, 2.09 [WT] and 1.17 [IL-10KO]).
Next, we examined the expression levels of CD40 in WT and IL-10KO mice. In uninfected mice, the CD40 molecule was detected at low basal levels on F4/80+ peritoneal exudate cells (Fig. 1A) and F4/80+ splenocytes of WT and IL-10KO mice (MFIs, 0.6 [WT] and 0.5 [IL-10KO]). At day 7 postinfection, expression of CD40 was upregulated on F4/80+ peritoneal exudate cells (Fig. 1B) and F4/80+ splenocytes, but the levels of expression were similar between infected WT and IL-10KO mice (MFIs, 1.1 [WT] and 0.9 [IL-10KO]). Further analysis of CD40 expression on B220+ spleen cells from uninfected mice revealed that there were low basal levels of expression on these cell populations and that CD40 levels were comparable in WT and IL-10KO mice (MFIs, 0.3 [WT] and 0.4 [IL-10KO]). Following infection with T. gondii, there was a minor increase in the levels of CD40 on B220+ splenocytes (MFIs, 0.4 [WT] and 1.2 [IL-10KO]); in repeat experiments, there was no significant difference between infected WT and IL-10KO mice. Together, these results demonstrate that levels of expression of CD80, CD86, and CD40 are similar in IL-10KO and WT mice following infection with T. gondii.
Effect of in vivo administration of CTLA-4Ig or anti-CD40L on the course of infection.
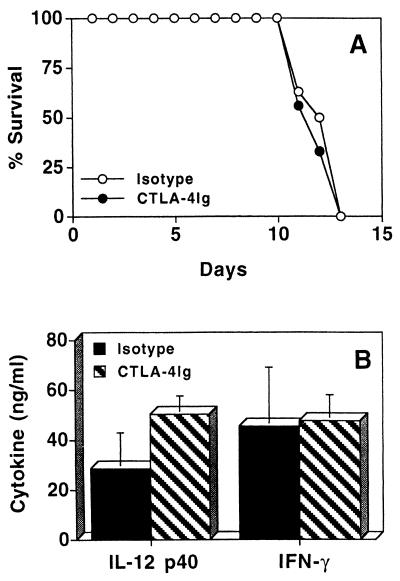
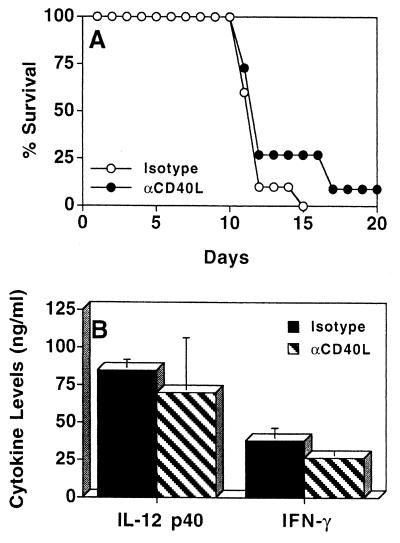
Infection of IL-10KO mice with T. gondii results in the generation of CD4+ T cells that mediate the infection-induced immunopathology seen in these mice (18). Since costimulation is involved in the activation of T-cell responses, the contribution of the CD28-B7 and CD40-CD40L pathways to the development of this T-cell-mediated pathological response was assessed by treating infected IL-10KO mice with CTLA-4Ig or anti-CD40L. Since previous studies have demonstrated that the immune response to T. gondii in IL-10KO mice starts to diverge from that of WT mice by days 5 to 7 postinfection (18, 42), we administered the treatments at this time. Maximum serum levels of IFN-γ and IL-12 occurred at day 8 postinfection (data not shown). Therefore, serum cytokine levels in IL-10KO mice following T. gondii infection were routinely assessed at day 8 postinfection. When infected IL-10KO mice were treated with CTLA-4Ig at days 6 and 8 postinfection, time to death of the IL-10KO mice was not changed (Fig. 2A) and the differences in serum levels of IFN-γ and IL-12 were not significant compared to littermate controls (Fig. 2B). Histological analyses of livers and lungs revealed severe pathology characterized by large foci of necrosis and cellular infiltration at days 7 and 11 postinfection but no difference in the severity of pathology between experimental groups (data not shown). Similarly, when infected IL-10KO mice were treated with anti-CD40L at 6 and 8 days postinfection, no significant difference in mortality was detected (Fig. 3A). Serum levels of IFN-γ and IL-12 in mice treated with anti-CD40L or the isotype control during the course of infection were assayed, and no significant differences were observed (Fig. 3B). In addition, livers and lungs from IL-10KO mice treated with anti-CD40L or with the isotype control had large numbers of cellular infiltrates and inflammation which were comparable in severity (data not shown).
FIG. 2.
Effect of CTLA-4Ig treatment on survival of IL-10KO mice infected with T. gondii. (A) IL-10KO mice infected with T. gondii were treated with 300 μg of CTLA-4Ig (n = 8) or ChiL6 (n = 9) at days 6 and 8 postinfection, and survival was monitored (P ≥ 0.05). (B) Serum levels of IFN-γ and IL-12 p40 at day 8 postinfection from T. gondii-infected IL-10KO mice treated with CTLA-4Ig (n = 8) or ChiL6 (n = 9) at 6 and 8 days postinfection. Results shown are pooled data from two separate experiments ± standard deviation.
FIG. 3.
Effect of anti-CD40L (αCD40L) treatment on IL-10KO mice infected with T. gondii. (A) IL-10KO mice infected with T. gondii were treated with 200 μg of anti-CD40L (n = 11) or isotype control hamster IgG (n = 11) at days 6 and 8 postinfection, and survival was monitored (P ≥ 0.05). (B) Serum levels of IFN-γ and IL-12 p40 at day 8 postinfection from infected IL-10KO mice treated with anti-CD40L (n = 11) or hamster IgG (n = 10) at days 6 and 8 postinfection. Results shown are pooled data from three separate experiments ± standard deviation.
Effects of CTLA-4Ig plus anti-CD40L during T. gondii infection.
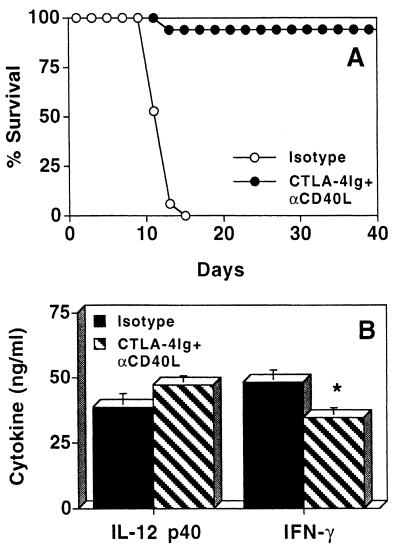
Although treatment with CTLA-4Ig or anti-CD40L did not significantly alter survival of IL-10KO mice infected with T. gondii, coadministration of these antagonists on days 6 and 8 resulted in the survival of >95% of infected IL-10KO mice (Fig. 4A). However, this treatment regimen when given on days 0 and 5 postinfection resulted in a less consistent survival rate (50% from two experiments). The protective effect of treatment on days 6 and 8 was not associated with a decrease in serum levels of IL-12 but was associated with a small reduction in serum levels of IFN-γ at day 8 postinfection (Fig. 4B). A paired Wilcoxon Mann-Whitney test of five independent experiments revealed that this difference was statistically significant (P < 0.05). Interestingly, histological analyses of livers and lungs from mice that received CTLA-4Ig plus anti-CD40L exhibit extensive inflammation and cellular infiltrates comparable to findings for control infected IL-10KO mice (data not shown). However, assessment of serum ALT levels, as a quantitative assay for hepatic damage, revealed that serum ALT levels in IL-10KO mice were 113 ± 4.29 U/ml before infection with T. gondii but increased to 391 ± 46.93 U/ml by day 11 postinfection. When infected IL-10KO mice were treated with CTLA-4Ig plus anti-CD40L, serum ALT levels were significantly reduced to 284.6 ± 17.88 U/ml (P < 0.03). ALT levels are pooled data ± standard error from three independent experiments; and an unpaired Student t test was performed to determine statistical significance. In addition, immunohistochemical analysis of iNOS expression in the liver and spleen (Fig. 5) and lungs (data not shown) revealed that IL-10KO mice treated with CTLA-4Ig plus anti-CD40L had reduced levels of iNOS-positive cells compared with IL-10KO mice that had received the sham treatment. No differences in levels of iNOS expression were seen in infected IL-10KO mice treated with CTLA-4Ig or anti-CD40L alone compared with control treated animals (data not shown). It should be noted that infected IL-10KO mice treated with CTLA-4Ig plus anti-CD40L still developed cachexia which appeared as severe as that in mice that received the control treatment and more severe than the disease seen in WT mice. Nonetheless, the treated animals survived this acute phase of infection, and histological analysis of livers at day 30 postinfection showed that normal hepatic architecture was restored (data not shown).
FIG. 4.
Effects of CTLA-4Ig plus anti-CD40L (αCD40L) in IL-10KO mice infected with T. gondii. (A) IL-10KO mice infected with T. gondii were treated with 300 μg of CTLA-4Ig plus 200 μg of anti-CD40L (n = 18) or appropriate isotype control (n = 17) at days 6 and 8 postinfection, and survival was monitored. Mice treated with CTLA-4Ig plus anti-CD40L survived >30 days (P ≤ 0.0001). (B) Serum levels of IFN-γ and IL-12p40 at day 8 postinfection from infected IL-10KO mice treated with CTLA-4Ig plus anti-CD40L (n = 18) or isotype control (n = 17) at days 6 and 8 postinfection. Results shown are pooled data from three separate experiments ± standard error. The asterisk indicates P < 0.05 using a paired Wilcoxon Mann-Whitney test.
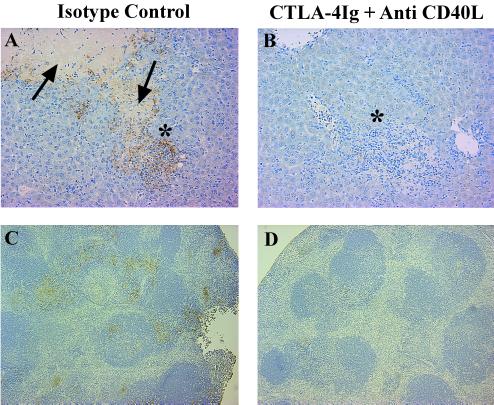
FIG. 5.
Effects of CTLA-4Ig plus anti-CD40L on iNOS expression in livers and spleens of IL-10KO mice infected with T. gondii. IL-10KO mice were infected with T. gondii and treated with 300 μg of CTLA-4Ig plus 200 μg of anti-CD40L or appropriate isotype control on day 6 postinfection. On day 7 postinfection, livers (A and B) and spleens (C and D) of infected IL-10KO mice treated with an isotype control (A and C) or CTLA-4Ig plus anti-CD40L treated mice (B and D) were harvested and stained for iNOS expression as described in Materials and Methods. Magnification, ×200. Arrows indicate areas of coagulative necrosis. Asterisks show areas of cellular infiltrations. Brown precipitate indicates iNOS-positive cells.
Effects of CD40-CD40L and CD28-B7 blockade on production of cytokines in vitro.
The observed reduction of serum levels of IFN-γ in infected IL-10KO mice treated with CTLA-4Ig plus anti-CD40L is likely due to inhibition of T-cell function during infection. However, blockade of the CD28-B7 interactions also inhibits NK cell function during toxoplasmosis (29), and so it is possible that following administration of CTLA-4Ig plus anti-CD40L, production of IFN-γ by NK cells is also affected. Therefore, to determine the source of IFN-γ produced in response to STAg, we stimulated splenocytes from 7-day-infected IL-10KO mice with STAg for 72 h and performed intracellular staining to identify the source(s) of IFN-γ in these cultures. In a typical experiment of two performed, FACS analysis revealed that 44% of the IFN-γ-positive cells were CD4+ T cells, 44% were CD8+ T cells, and 13% were NK1.1+ cells; the addition of CTLA-4Ig and anti-CD40L to these cultures did not alter these percentages. These data have to be interpreted with care since the percentage of IFN-γ-positive cells does not always correlate with the protein levels detected in these cultures (Table 1). Nonetheless, these results suggest that the major sources of IFN-γ produced in response to antigen are CD4+ and CD8+ T cells.
TABLE 1.
Effects of CTLA-4Ig and anti-CD40L on the production of IFN-γ and IL-12 by IL-10KO mice in response to STAga
| In vivo treatment | In vitro treatmentb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ (ng/ml)
|
IL-12p40 (ng/ml)
|
|||||||
| Isotype | CTLA-4Ig | αCD40L | CTLA-4Ig + αCD40L | Isotype | CTLA-4Ig | αCD40L | CTLA-4Ig + αCD40L | |
| Isotype control | 111.73 ± 29.65 | 89.93 ± 23.64c | 115.10 ± 90.93 | 78.67 ± 21.01d | 36.52 ± 5.92 | 38.26 ± 6.19 | 36.59 ± 5.55 | 32.81 ± 5.90e |
| CTLA-4Ig + αCD40L | 101.64 ± 21.96 | 81.66 ± 19.54f | 105.19 ± 22.65 | 69.85 ± 15.67c | 39.49 ± 7.13 | 40.15 ± 7.61 | 38.59 ± 7.46 | 35.28 ± 7.16 |
Splenocytes from 7-day-infected IL-10KO mice treated with CTLA-4Ig plus anti-CD40L (αCD40L) or the isotype control at day 6 postinfection were stimulated with STAg (25 μg/ml) and cocultured with CTLA-4Ig and anti-CD40L for 24 h.
Pooled data from four or five independent experiment ± standard error.
P ≤ 0.005.
P ≤ 0.001.
P ≤ 0.008.
P ≤ 0.01.
Since costimulation is involved in the regulation and activation of T-cell responses, and T cells are the major source of IFN-γ, we assessed the contribution of B7 and CD40L to the activation of T cells seen in IL-10KO mice following infection with T. gondii. In these experiments, we treated IL-10KO mice infected with T. gondii in vivo with an isotype control, CTLA-4Ig, anti-CD40L, or both CTLA-4Ig and anti-CD40L on day 6 postinfection and assessed their production of IL-12 and IFN-γ in antigen-specific recall responses. These experiments revealed that in vivo administration of CTLA-4Ig or anti-CD40L alone did not alter the ex vivo responses of splenocytes to STAg (data not shown). In addition, cytokine production by spleen cells from infected mice that were treated in vivo with CTLA-4Ig plus anti-CD40L in response to STAg produced high levels of IFN-γ and IL-12 which were comparable to those in splenocytes from the infected control group (Table 1). These results demonstrate that in vivo treatment with CTLA-4Ig or anti-CD40L alone or in combination does not alter ex vivo splenocyte responses to STAg.
To further dissect the role of these costimulatory pathways in the regulation of IFN-γ and IL-12 production, the effects of anti-CD40L or CTLA-4Ig was assessed in vitro using splenocytes from IL-10KO mice treated in vivo either with the isotype control or with CTLA-4Ig plus anti-CD40L. Stimulation with STAg resulted in the production of high levels of IL-12 and IFN-γ, and the inclusion of anti-CD40L had no significant effect on the production of these cytokines (Table 1). However, the addition of CTLA-4Ig to these cultures resulted in a significant reduction in the production of IFN-γ but not IL-12 (Table 1). When anti-CD40L and CTLA-4Ig were used in combination, the reduction in IFN-γ levels was greater than that observed with CTLA-4Ig alone; this effect was most pronounced with splenocytes from IL-10KO mice treated in vivo with CTLA-4Ig plus anti-CD40L (Table 1). It is interesting that although CTLA-4Ig or anti-CD40L alone did not significantly alter production of IL-12, there was a small inhibitory effect when the two were coadministered in culture (Table 1). Nonetheless, these in vitro studies demonstrate that simultaneous blockade of the CD28-B7 and CD40-CD40L pathways is required for maximal reduction of IFN-γ produced in response to STAg by splenocytes from IL-10KO mice and this correlates with the ability of these treatments to reduce liver damage, reduce iNOS expression, and prevent the infection-induced death of these mice.
DISCUSSION
The role of costimulation in the regulation of the immune response to T. gondii remains unclear. However, recent studies by others and our laboratory have begun to address these issues. Subauste and colleagues reported that human monocytes infected with T. gondii upregulate their expression of B7 molecules (52). In addition, studies by the same group using peripheral blood mononuclear cells from hyper-IgM patients demonstrated that CD40 is also upregulated following infection with T. gondii (53). Similarly, results presented here reveal that infection with T. gondii resulted in a broad upregulation of CD80, CD86, and CD40 expression in both WT and IL-10KO mice (Fig. 1). However, upregulation of B7 and CD40 expression is not restricted to infected cells but rather appears to be a global effect, since at day 7 postinfection, less than 2% of the macrophages in the peritoneal cavity are infected (U. Wille, E. N. Villegas, B. Streipen, D. S. Roos, and C. A. Hunter, submitted for publication). There are several possible mechanisms whereby the upregulation of these molecules may occur. Soluble antigens of T. gondii have been reported to affect macrophage function and may have a role in the upregulation of B7 molecules during infection. Alternatively, IFN-γ has been shown to upregulate macrophage expression of B7, and since the production of systemic levels of IFN-γ occurs early after infection, this may also be responsible for the widespread upregulation of these molecules.
Given that previous reports showed that IL-10 suppresses expression of B7 (13), it was surprising that in the absence of IL-10, expression of B7 as well as CD40 was not increased. Although IL-10 has been associated with inhibition of the immune response during toxoplasmosis (7, 17, 18, 30, 33), these results suggest that IL-10 is not involved in the regulation of B7 or CD40 expression during acute toxoplasmosis. Similar to our findings, other workers reported that there was no evidence of dysregulated expression of B7 after infection of IL-10KO mice with Listeria monocytogenes (9). Together, these findings question the role of IL-10 in the regulation of B7 and CD40 expression during infection.
Since B7 and CD40 have been described to be involved in optimal activation of T-cell responses (13, 54, 56), we assessed the contribution of these molecules during the T-cell-mediated infection-induced shock-like reaction seen in IL-10KO mice. Studies presented here demonstrate that interference with both the CD28-B7 and the CD40-CD40L pathways resulted in survival of IL-10KO mice infected with T. gondii. Although the administration of CTLA-4Ig plus anti-CD40L affected mortality, these mice still developed severe clinical disease and pathology. Nevertheless, the combination of these treatments resulted in survival of IL-10KO mice and correlated with a significant reduction in the production of IFN-γ, decreased iNOS expression in the liver and spleen, and lower serum ALT levels. We propose that the reduction of these proinflammatory factors and reduced hepatic damage allow these mice to survive the acute phase of infection. Alternatively, interference with these costimulatory pathways may affect other inflammatory mechanisms that contribute to the death of IL-10KO mice infected with T. gondii. For example, IL-10 is implicated in the prevention of T-cell-mediated apoptosis (25, 43); thus, blockade of these costimulatory pathways may reduce the ability of T cells to induce apoptosis. Moreover, blockade of these pathways may also affect the ability of T cells to proliferate or to stimulate the production of other proinflammatory factors such as oxygen intermediates, IL-1, IL-6, or TNF-α (20, 37). Thus, further studies are required to determine whether blockade of the CD28-B7 and CD40-CD40L pathways alters other T-cell-mediated effector cell functions that contribute to the death of IL-10KO mice in this model.
Subauste and colleagues recently reported that the upregulation of B7 and CD40 by peripheral blood mononuclear cells following infection with T. gondii is required for the optimal production of IFN-γ and IL-12 in these cultures (52, 53). However, results from this laboratory have revealed that CD40LKO and CD28KO mice are resistant to the acute phase of infection and that WT mice have significant components of IL-12 and IFN-γ production that are independent of these costimulatory pathways (44, 55). Similarly, in IL-10KO mice, the early IL-12-driven, IFN-γ-dependent mechanism of resistance to toxoplasmosis appears to be largely independent of the CD28-B7 and CD40-CD40L costimulatory pathways.
The relationship between the CD28-B7 and the CD40-CD40L pathways in this model, as well as in models of allo- and autoimmunity, remains unclear. Previous studies by Ding and colleagues have shown that the interaction of CD28 with B7 can lead to the expression of CD40L on T cells (13). This then allows the interaction of CD40L on T cells with CD40 on accessory cells to further upregulate expression of CD80, CD86, and other costimulatory molecules (21). Thus, these two pathways are clearly linked (21, 46, 56). However, our studies show that blockade of either CD28-B7 or CD40-CD40L interaction did not alter the outcome of infection in the IL-10KO mice (Fig. 2 and 3); only blockade of both pathways resulted in survival of IL-10KO mice. In agreement with our findings, other studies which have analyzed the role of costimulatory pathways in autoimmune oophoritis and graft rejection demonstrated that disease can be prevented only when both the CD28-B7 and CD40-CD40L pathways are blocked (22, 36). Similarly, studies on murine lupus and graft-versus-host disease showed that blockade of CD28 and CD40L was required for optimal inhibition of disease (10, 47, 48). Evidence that these costimulatory pathways are not linked is provided by studies which demonstrate that although the CD40-CD40L interaction is important for the induction of IL-12 and resistance to infection with Leishmania spp. (6, 31, 51), mice deficient in CD28 still develop protective immunity to this parasite (5). Thus, the requirement for simultaneous blockade of these pathways for effective inhibition of allo- and autoimmunity, as well as during infection (these studies), indicates that although they are interrelated, the CD28 and CD40 pathways are independent regulators of T-cell-dependent immune responses. In conclusion, both the CD28-B7 and CD40-CD40L interactions contribute to the development of the lethal, T-cell-mediated shock-like reaction observed in the IL-10KO mice. The successful prevention of immunopathology through the blockade of the CD28-B7 and CD40-CD40L interactions indicates that these pathways represent suitable targets to manage infection-induced pathology.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI 42334-01, the Marie Lowe Center for Cancer Research, and Center for Molecular Studies in Digestive and Liver Diseases grant P30 DK50306. E.N.V. is supported by NIH predoctoral fellowship award AI09562, U.W. is supported by the Deutsche Forschungsgemeinschaft, and C.A.H. is a Burroughs Welcome New Investigator in Molecular Parasitology. DNAX is supported by Schering Plough Corporation.
We thank Thad Radzanowski for expert technical assistance and members of the Hunter, Farrell, and Scott labs for helpful discussions during these studies.
REFERENCES
- 1.Berg D J, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach M W, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ Th1-like responses. J Clin Investig. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg D J, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the responses to LPS in murine models of endotoxic shock and the Schwartzman reaction but not endotoxin tolerance. J Clin Investig. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blewett D A, Miller J K, Harding J. Simple technique for the direct isolation of toxoplasma tissue cysts from fetal ovine brains. Vet Rec. 1983;112:98–100. doi: 10.1136/vr.112.5.98. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, Sharma S D, Remington J S. Differential regulation of the L3T4-T cell subset by B-cells in different mouse strains bearing the H-2k haplotype. J Immunol. 1986;137:2991–2997. [PubMed] [Google Scholar]
- 5.Brown D R, Green J M, Moskowitz N H, Davis M, Thompson C B, Reiner S L. Limited role of CD28-mediated signals in T helper subset differentiation. J Exp Med. 1996;184:803–810. doi: 10.1084/jem.184.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell K A, Ovendale P J, Kennedy M K, Fanslow W C, Reed S G, Mallazewski C R. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 7.Candolfi E, Hunter C A, Remington J S. Roles of gamma interferon and other cytokines in suppression of the spleen cell proliferative responses to concanavalin A and toxoplasma antigen during acute toxoplasmosis. Infect Immun. 1995;63:751–756. doi: 10.1128/iai.63.3.751-756.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corry D B, Reiner S L, Linsley P S, Locksley R M. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- 9.Dai W, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10 deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 10.Daikh D I, Finck B K, Linsley P S, Hollenbaugh D, Wofsy D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J Immunol. 1997;159:3104–3108. [PubMed] [Google Scholar]
- 11.D'Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson N J, Leach M W, Fort M M, Thompson-Snipes L, Kuhn R, Muller W, Berg D J, Rennick D M. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Green J M, Thompson C B, Shevach E M. B7/CD28-dependent and -independent induction of CD40 ligand expression. J Immunol. 1995;155:5124–5132. [PubMed] [Google Scholar]
- 14.Ding L, Linsley P S, Huang L-Y, Germain R N, Shevach E M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 15.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 16.Fiorentino D F, Zlotnik A, Vieira P, Mossman T R, Howard M, Moore K W, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 17.Gazzinelli R T, Oswald I P, James S L, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ-activated macrophages. J Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- 18.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent of CD4+ T cells and accompanied by overproduction of IL-12, IFNγ, and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 19.Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli M T, Aste-Amezaga M, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-γ and interleukin-10. J Exp Med. 1996;183:2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grewal I S, Flavell R A. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 21.Grewal I S, Flavell R A. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 22.Griggs N D, Agersborg S S, Noelle R J, Ledbetter J A, Linsley P S, Tung K S K. The relative contribution of the CD28 and gp39 costimulatory pathways in the clonal expansion and pathogenic acquisition of self-reactive T cells. J Exp Med. 1996;183:801–810. doi: 10.1084/jem.183.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries J E, Roncarolo M G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 24.Grunvald D, Chiaramonte M, Hieny S, Wysocka M, Trinchieri G, Vogel S N, Gazzinelli R T, Sher A. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. Infect Immun. 1996;64:2010–2018. doi: 10.1128/iai.64.6.2010-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasko G, Virag L, Egnaczyk G, Salzman A L, Szabo C. The crucial role of IL-10 in the suppression of the immunological responses in mice exposed to staphylococcal enterotoxin B. Eur J Immunol. 1998;28:1417–1425. doi: 10.1002/(SICI)1521-4141(199804)28:04<1417::AID-IMMU1417>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Howard M, O'Garra A, Ishida H, Malefyt R W, de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992;12:239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- 27.Hunter C A, Abrams J S, Beaman M H, Remington J S. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii importance of T-cell-independent regulation of resistance of T. gondii. Infect Immun. 1993;61:4038–4044. doi: 10.1128/iai.61.10.4038-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter C A, Ellis-Nayes L A, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo F G. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–3316. [PubMed] [Google Scholar]
- 29.Hunter C A, Ellis-Neyer L, Gabriel K E, Kennedy M K, Grabstein K H, Linsley P S, Remington J S. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J Immunol. 1997;158:2285–2293. [PubMed] [Google Scholar]
- 30.Hunter C A, Subauste C S, Van Cleave V H, Remington J S. Production of gamma-interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy M K, Picha K S, Fanslow W C, Grabstein K H, Alderson M R, Clifford K N, Chin W A, Mohler M M. CD40/CD40 ligand interactions are required for T cell-dependent production of interleukin-12 by mouse macrophages. Eur J Immunol. 1996;26:370–378. doi: 10.1002/eji.1830260216. [DOI] [PubMed] [Google Scholar]
- 33.Khan I A, Matsuura T, Kasper L H. IL-10 mediates immunosuppression following primary infection with Toxoplasma gondii in mice. Parasitol Immunol. 1995;17:185–195. doi: 10.1111/j.1365-3024.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 34.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin 10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 36.Larsen C P, Elwood E T, Alexander D Z, Ritchie S C, Hendrix R, Tucker-Burden C, Cho H R, Aruffo A, Hollenbaugh D, Linsley P S, Winn K J, Pearson T C. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–437. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 37.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 38.Li Z-Y, Manthey C L, Perera P Y, Sher A, Vogel S N. Toxoplasma gondii soluble antigen induces a subset of lipopolysaccharide-inducible genes and tyrosine phosphoproteins in peritoneal macrophages. Infect Immun. 1994;62:3434–3440. doi: 10.1128/iai.62.8.3434-3440.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyaard L, Hovenkamp E, Otto S A, Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL12-induced immune responses. J Immunol. 1996;156:2776–2782. [PubMed] [Google Scholar]
- 40.Mingari M C, Maggi E, Cambiaggi A, Annunziato F, Schiavetti F, Manetti R, Moretta L, Romangnani S. Development of in vitro of human CD4+ thymocytes into functionally mature Th2 cells. Exogenous interleukin-12 is required for priming thymocytes to produce both Th1 cytokines and interleukin-10. Eur J Immunol. 1996;26:1083–1087. doi: 10.1002/eji.1830260519. [DOI] [PubMed] [Google Scholar]
- 41.Moore K W, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 42.Neyer L E, Grunig G, Fort M, Remington J S, Rennick D, Hunter C A. Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect Immun. 1997;65:1657–1682. doi: 10.1128/iai.65.5.1675-1682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poe J C, Wagner D H J, Miller R W, Stout R D, Suttles J. IL-4 and IL-10 modulation of CD40-mediated signaling of monocyte IL-1b synthesis and rescue from apoptosis. J Immunol. 1997;159:846–852. [PubMed] [Google Scholar]
- 44.Reichmann G, Walker W, Villegas E N, Craig L, Cai G, Alexander J, Hunter C A. The CD40/CD40L interaction is required for resistance to toxoplasmic encephalitis. Infect Immun. 2000;68:1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rennick D, Berg D, Holland G. Interleukin 10: an overview. Prog Growth Factor Res. 1992;4:207–227. doi: 10.1016/0955-2235(92)90020-i. [DOI] [PubMed] [Google Scholar]
- 46.Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–477. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 47.Saito K, Sakurai J, Ohata J, Kohsaka T, Hashimoto H, Okumura K, Abe R, Azuma M. Involvement of CD40 ligand-40 and CTLA-4-B7 pathways in murine acute graft-versus-host disease induced by allogeneic T cells lacking CD28. J Immunol. 1998;160:4225–4231. [PubMed] [Google Scholar]
- 48.Saito K, Yagita H, Hashimoto H, Okumura K, Azuma M. Effect of CD80 and CD86 blockade and anti-interleukin treatment on mouse acute graft-versus-host disease. Eur J Immunol. 1997;26:3098–3106. doi: 10.1002/eji.1830261241. [DOI] [PubMed] [Google Scholar]
- 49.Sander B, Hoiden I, Anderson U, Moller E, Abrams J S. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. J Immunol Methods. 1993;166:201–214. doi: 10.1016/0022-1759(93)90361-a. [DOI] [PubMed] [Google Scholar]
- 50.Sharma S D, Mullenax J, Auaujo F G, Erlich A A, Remington J S. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983;131:977–983. [PubMed] [Google Scholar]
- 51.Soong L, Xu J-C, Grewal I S, Kima P, Sun J, Ruddie N H, McMahon-Pratt D, Flavell R A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 52.Subauste C S, de Waal Malefyt R, Fuh F. Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. J Immunol. 1998;160:1831–1840. [PubMed] [Google Scholar]
- 53.Subauste C S, Wessendarp M, Sorensen R U, Leiva L E. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. J Immunol. 1999;162:6690–6700. [PubMed] [Google Scholar]
- 54.Van Gool S W, Vandernberghe P, De Boer M, Ceuppens J L. CD80, CD86, CD40 provide accessory signals in a multiple-step-T-cell activation model. Immunol Rev. 1996;153:47–83. doi: 10.1111/j.1600-065x.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 55.Villegas E N, Elloso M M, Reichmann G, Peach R, Hunter C A. Role of CD28 in the generation of effector and memory responses required for resistance to Toxoplasma gondii. J Immunol. 1999;163:3344–3353. [PubMed] [Google Scholar]
- 56.Yang Y, Wilson J M. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]