Abstract
This study aimed to investigate the impact of intrapartum and post‐partum complications and newborn care practices on early initiation of breastfeeding (EIBF). Data for the study came from a prospective cohort study in Ethiopia that recruited and followed pregnant and post‐partum women from 2019 to 2021. Resident enumerators conducted interviews at enrolment in 2019 and follow‐ups at 6 weeks, 6 months and 1 year post‐partum. The present analysis is based on data from the baseline survey and 6 weeks follow‐up. Multivariable logistic regression was used to estimate the effects of newborn care practices and intrapartum and post‐ partum complications on EIBF (the proportion of newborns who initiated breastfeeding within the first hour of birth). Overall, 2660 mother–infant pairs were included in this analysis. After adjustment, EIBF was less likely among women who experienced intrapartum haemorrhage (adjusted odds ratio [AOR]: 0.76, 95% confidence interval [CI]: 0.59–0.97), malpresentation (AOR: 0.46, 95% CI: 0.30–0.72) and convulsions (AOR: 0.48, 95% CI: 0.34–0.66) during childbirth. Mother–newborn skin‐to‐skin contact increased the likelihood of EIBF (AOR: 1.47, 95% CI: 1.11–1.94). Women who experienced post‐partum haemorrhage (AOR: 0.63, 95% CI: 0.47–0.84), retained placenta for more than 30 min (AOR: 0.36, 95% CI: 0.24–0.52) and convulsions after delivery (AOR: 0.57, 95% CI: 0.41–0.79) were less likely to initiate breastfeeding early. Also, women who had a caesarean birth (AOR: 0.28, 95% CI: 0.18–0.41), delivered outside of a healthcare facility (AOR: 0.70, 95% CI: 0.50–0.99) or had twin birth (AOR: 0.43, 95% CI: 0.22–0.85) were less likely to initiate breastfeeding early. Skin‐to‐skin contact should be encouraged whenever possible, and women with obstetric complications should be encouraged and supported to initiate breastfeeding early.
Keywords: breastfeeding initiation, breastfeeding promotion, infant feeding, mother support groups, pregnancy, prospective study
Malpresentation,intrapartum haemorrhage, convulsions during childbirth, retained placenta for more than 30 mins, post‐ partum haemorrhage, and convulsions after childbirth were associated with a lower likelihood of breastfeeding initiation within the first hour of birth. Out‐of‐facility delivery, multiple births and caesarean delivery were associated with a lower likelihood of breastfeeding initiation within the first hour of birth. Women who practised skin‐to‐skin contact after birth were more likely to initiate breastfeeding within the first hour of birth than those who did not practise skin‐to‐skin contact.
Key messages
Malpresentation, intrapartum haemorrhage, and convulsions during childbirth were associated with a lower likelihood of breastfeeding initiation within the first hour of birth.
Retained placenta for more than 30 min, post‐partum haemorrhage, and convulsions after childbirth were associated with a lower likelihood of breastfeeding initiation within the first hour of birth.
Out‐of‐facility delivery, multiple births and caesarean delivery were associated with a lower likelihood of breastfeeding initiation within the first hour of birth.
Women who practised skin‐to‐skin contact after birth were more likely to initiate breastfeeding within the first hour of birth than those who did not practise skin‐to‐skin contact.
1. BACKGROUND
Immediate newborn care that includes early initiation of breastfeeding (EIBF) is essential for the health and survival of newborns. Globally, it has been shown that breastfeeding has prodigious benefits for both mothers and newborns. It supports the child's growth, protects the child against infections and the mother from post‐partum haemorrhage and breast and ovarian cancers (Ekholuenetale et al., 2021; Mallick et al., 2021; Rollins et al., 2016; Victora et al., 2016). It is estimated that breastfeeding can save up to a million deaths in children under age five each year (Victora et al., 2016). For example, a meta‐analysis in 2013 found a 44% lower risk of all‐cause neonatal mortality and a 45% lower risk of infection‐related neonatal mortality among children who initiated breastfeeding early (Debes et al., 2013). In a national prospective cohort study in the United States, early breastfeeding initiation was associated with a 26% lower risk of infant mortality (Li et al., 2022). Because of its immense benefits to infants and young children, the World Health Organization (WHO) recommends the initiation of breastfeeding within the first hour of birth (Unicef, 2018). However, despite the evidence and guidelines highlighting the importance of EIBF, the prevalence is suboptimal around the world (UNICEF & WHO, 2019). Data from the global breastfeeding scorecard for 2019 revealed that only 43% of newborns were breastfed within 1 h of birth, falling short of the 70% global target set for 2030 (UNICEF & WHO, 2019). In Africa, EIBF varies substantially from 24% in Chad to 86% in Burundi (Teshale & Tesema, 2021). In Ethiopia, the prevalence of EIBF is 51.9% (Getnet et al., 2020).
Several factors can influence the timing of breastfeeding initiation. However, many previous studies that investigated the factors influencing breastfeeding initiation in Ethiopia and elsewhere in Africa focused on distal factors such as maternal socioeconomic, demographic, behavioural and culture‐ related factors (Appiah et al., 2021; Habtewold et al., 2019; Nkoka et al., 2019; Teshale & Tesema, 2021; Woldeamanuel, 2020) while neglecting proximal factors such as childbirth and post‐partum complications and perinatal factors that have a direct impact on breastfeeding initiation. Intrapartum and post‐partum complications, for example, increase a woman's need for medical attention during and after childbirth, respectively, and can prevent her from bonding with and breastfeeding her newborn in the first hour. It has been shown that women with no obstetric complications are 57% more likely to initiate breastfeeding early than those with obstetric complications (Gurung et al., 2021). Also, studies have shown that prolonged labour, the use of opioid pain medication in labour, anaesthesia, assisted vaginal birth and caesarean section are associated with delayed breastfeeding initiation (Chapman & Pérez‐Escamilla, 1999; Dewey et al., 2003; Fan et al., 2020; Smith, 2007; Wiklund et al., 2009). In a Swedish study of 585 women, prolonged labour was linked to a nearly threefold increased risk of delayed breastfeeding initiation than nonprolonged labour (Wiklund et al., 2009).
Furthermore, health care practices during labour, birth and the immediate post‐partum can adversely affect breastfeeding initiation (Smith, 2007). In some hospitals, it is standard practise for babies to be separated from their mothers after birth for routine procedures (Er Moore Bergman et al., 2016; Kruse et al., 2005). However, studies have shown that mother–newborn skin‐to‐skin contact after birth (a practice where the baby is placed naked on the mother's bare chest at birth or soon afterwards covered in a warm blanket) can accelerate spontaneous breastfeeding and promote timely breastfeeding initiation (Mallick et al., 2021; Safari et al., 2018; Singh et al., 2017). During skin‐to‐skin contact, the movement of the infant's hands over the mother's breasts increases oxytocin secretion and breast milk production (Jonas et al., 2007). The prevalence of skin‐to‐skin contact within the first hour after birth in Ethiopia was 28.1% in a study done in 2019 (Bedaso et al., 2019).
Only a few studies in Africa have examined the influence of intrapartum and post‐partum complications on breastfeeding initiation. However, given the diversity of healthcare systems worldwide, it is critical to determine country‐specific factors associated with breastfeeding initiation for developing national policies and practice guidelines on breastfeeding. The current study investigated the impact of intrapartum and post‐partum complications and newborn care practices on EIBF (the proportion of newborns who initiated breastfeeding within the first hour of birth) in Ethiopia.
2. METHODS
2.1. Data source
Data for the study were drawn from the Performance Monitoring for Action Ethiopia (PMAET) Panel Cohort 1 survey (Addis Ababa University School of Public Health and The Bill & Melinda Gates Institute for Population and Reproductive Health at The Johns Hopkins Bloomberg School of Public Health, 2019, 2020). PMAET Panel Cohort 1 was a prospective cohort study that followed three groups of women from 2019 to 2021: (1) pregnant women, (2) women 0–4 weeks post‐partum and (3) women 5–9 weeks post‐partum. It was conducted in six regions of Ethiopia to measure critical reproductive, maternal and newborn health indicators (Addis Ababa University School of Public Health and The Bill & Melinda Gates Institute for Population and Reproductive Health at The Johns Hopkins Bloomberg School of Public Health, 2019). A multistage cluster sampling design was used to select 206 enumeration areas from the six regions. A census was conducted to identify all households with pregnant women or recently post‐partum women (0–9 weeks) in the enumeration areas. The census screened 32,791 women in 32,614 households and 2857 eligible women (99.3% response rate) aged 15–49 were enrolled in the panel survey. A total of 2669 women (93.4%) completed the 6‐week follow‐up survey. The selected sample consisted of 2240 pregnant women and 617 post‐partum women. Resident enumerators conducted interviews at enrolment in 2019 and follow‐ups at 6 weeks, 6 months and 1 year post‐partum (Addis Ababa University School of Public Health and The Bill & Melinda Gates Institute for Population and Reproductive Health at The Johns Hopkins Bloomberg School of Public Health, 2019). Women who were 5–9 weeks post‐partum at enrolment were eligible for the baseline survey and 6 weeks follow‐up at the same time. The present analysis pooled data from the baseline survey and 6 weeks follow‐up on women with information on breastfeeding initiation. The School of Public Health of Addis Ababa University and the Johns Hopkins Bloomberg School of Public Health conducted the baseline survey and follow‐ up studies. A detailed description of the survey's methods, including information about survey design, sampling and data collection, is published elsewhere (Addis Ababa University School of Public Health and The Bill & Melinda Gates Institute for Population and Reproductive Health at The Johns Hopkins Bloomberg School of Public Health, 2019, 2020).
2.2. Outcome and exposure variables
The outcome of this study was EIBF, defined as the initiation of breastfeeding within the first hour after birth. Women were asked ‘How long after birth did you first put {child_name} to the breast?’ to determine when breastfeeding was initiated. Responses to the question were recorded in ‘minutes’, ‘hours’, ‘days’, ‘not yet’, ‘do not know’ and ‘no response’. In this analysis, the responses were grouped into 'initiation within one hour’ and ‘initiation after one hour’. We excluded infants whose mothers responded, ‘not yet’, ‘do not know’ and ‘no response’. The main exposures of the study were the experience of intrapartum and immediate post‐partum complications and newborn care practices. Intrapartum complications included problems during labour and delivery such as intrapartum haemorrhage, leaking or ruptured membrane, malpresentation (a foetal part other than the head of the foetus engaging the maternal pelvis; Pilliod & Caughey, 2017), prolonged labour and convulsions. Immediate post‐partum complications were defined as complications that occurred within the first 24 h after delivery. Retained placenta, fever with foul discharge or abdominal pain, post‐partum haemorrhage and convulsion after birth were the post‐partum complications considered in the analysis. In addition, we explored mother–newborn skin‐to‐skin contact after birth, the timing of the baby's first bath, wrapping of the baby after birth, caesarean delivery, place of birth and multiple births for any association with breastfeeding initiation. For skin‐to‐skin contact after birth, mothers were asked, ‘Did someone place the baby naked on your chest against your skin immediately after delivery of the baby?’ and responses were coded ‘Yes’ or ‘No’.
2.3. Data analysis
Data from the baseline survey and 6‐week follow‐up were standardized and pooled into a single data set for analysis. We calculated the prevalence of breastfeeding initiation within the first hour of birth and summarised the characteristics of the study's sample by breastfeeding initiation. Multivariable binary logistic regression was used to estimate the effect of the exposures on breastfeeding initiation with the corresponding 95% confidence intervals (CIs) and p values. Statistical significance was set at p < 0.05. Several potential confounders were controlled in the multivariable model, including maternal age, marital status, place of residence, wealth status, maternal education, parity, multiple births, place of birth, baby's gender and mother's desire for the pregnancy (wantedness of pregnancy). All analyses accounted for the unequal sampling in certain areas, the stratification of rural and urban areas and the effect of clustering in the design. Stata 17 was used for statistical analysis.
3. RESULTS
Overall, 2660 mother–infant pairs were included in this study. Table 1 summarises the characteristics of the study sample. Over half of the women were between the ages of 20 and 30, and almost all (94.8%) were married. The sample was predominantly rural, with 40.8% of respondents having never attended school. Approximately 45.8% of the women lived in high‐income households, and 41.1% had one or no living children. At baseline, most women indicated that they wanted the pregnancy then, and about 54.3% delivered in a health care facility. Nearly all (98.4%) of the babies delivered were singletons, with slightly more than half (51.8%) being boys. There was a high prevalence of breastfeeding initiation within the first hour after birth among women with a higher level of education, those who were not married, urban dwellers, women who did not want pregnancy at the time and mothers who delivered single babies (Table 1 and Supporting Information: Table S1).
Table 1.
Characteristics of the study sample
| Overall sample | Breastfeeding initiation | |||
|---|---|---|---|---|
| Numbera | Percentb | Number breastfed within 1 ha | % breastfed within 1 hb | |
| Age of mother | ||||
| <20 | 265 | 10.6 | 158 | 66.8 |
| 20–30 | 1691 | 62.9 | 1020 | 67.7 |
| 31–48 | 704 | 26.6 | 427 | 65.0 |
| Educational level of the mother | ||||
| Never attended | 1063 | 40.8 | 618 | 67.5 |
| Primary | 970 | 38.7 | 600 | 66.1 |
| Secondary | 472 | 16.2 | 286 | 66.1 |
| Higher | 155 | 4.3 | 101 | 70.3 |
| Marital status | ||||
| Married | 2500 | 94.8 | 1494 | 66.5 |
| Not married | 158 | 5.2 | 111 | 73.3 |
| Place of residence | ||||
| Urban | 1001 | 22.2 | 662 | 70.2 |
| Rural | 1659 | 77.8 | 943 | 65.8 |
| Household wealth | ||||
| Low income | 928 | 35.9 | 553 | 66.4 |
| Middle income | 444 | 18.3 | 244 | 65.0 |
| High income | 1288 | 45.8 | 808 | 67.9 |
| Parity | ||||
| <2 | 1102 | 41.1 | 668 | 65.5 |
| 2–3 | 777 | 28.0 | 477 | 69.0 |
| 4+ | 778 | 31.0 | 458 | 66.7 |
| Pregnancy desired | ||||
| Then | 628 | 68.9 | 376 | 67.2 |
| Later or not at all | 228 | 31.1 | 148 | 71.6 |
| Place of childbirth | ||||
| Not in a health care facility | 981 | 45.7 | 560 | 62.8 |
| Health care facility | 1571 | 54.3 | 1,045 | 70.3 |
| Multiple births | ||||
| Single | 2604 | 98.4 | 1588 | 67.2 |
| Twin | 47 | 1.6 | 17 | 46.9 |
| Child's sex | ||||
| Boy | 1279 | 51.8 | 809 | 66.7 |
| Girl | 1236 | 48.2 | 796 | 67.0 |
Unweighted number.
Weighted percentage.
3.1. Intrapartum complications and breastfeeding initiation
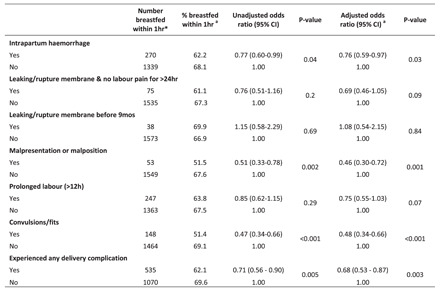
The impact of intrapartum complications on breastfeeding initiation is presented in Table 2. After adjusting for relevant confounders, there was evidence that women who experienced convulsions during delivery were less likely to initiate breastfeeding within the first hour after birth than those who did not experience convulsions (adjusted odds ratio [AOR]: 0.48, 95% CI: 0.34–0.66). Compared with women who did not experience malpresentation, those who experienced malpresentation at delivery had a 54% reduction in the odds of initiating breastfeeding within the first hour of birth (AOR: 0.46, 95% CI: 0.30–0.72). Women who suffered intrapartum haemorrhage had a 24% reduction in the odds of initiating breastfeeding within the first hour of birth than those who did not suffer intrapartum haemorrhage (AOR: 0.76, 95% CI: 0.59–0.97). In general, the odds of breastfeeding initiation within the first hour after birth was lower in women who experienced any delivery complication than in those without complications (AOR: 0.68, 95% CI: 0.53–0.87).
Table 2.
Association between childbirth complications and breastfeeding initiation
| Number breastfed within 1 ha | % breastfed within 1 hb | Unadjusted odds ratio (95% CI) | p Value | Adjusted odds ratio (95% CI)c | p Value | |
|---|---|---|---|---|---|---|
| Intrapartum haemorrhage | ||||||
| Yes | 270 | 62.2 | 0.77 (0.60–0.99) | 0.04 | 0.76 (0.59–0.97) | 0.03 |
| No | 1339 | 68.1 | 1.00 | 1.00 | ||
| Leaking/rupture membrane and no labour pain for >24 h | ||||||
| Yes | 75 | 61.1 | 0.76 (0.51–1.16) | 0.2 | 0.69 (0.46–1.05) | 0.09 |
| No | 1535 | 67.3 | 1.00 | 1.00 | ||
| Leaking/rupture membrane before 9mos | ||||||
| Yes | 38 | 69.9 | 1.15 (0.58–2.29) | 0.69 | 1.08 (0.54–2.15) | 0.84 |
| No | 1573 | 66.9 | 1.00 | 1.00 | ||
| Malpresentation or malposition | ||||||
| Yes | 53 | 51.5 | 0.51 (0.33–0.78) | 0.002 | 0.46 (0.30–0.72) | 0.001 |
| No | 1549 | 67.6 | 1.00 | 1.00 | ||
| Prolonged labour (>12 h) | ||||||
| Yes | 247 | 63.8 | 0.85 (0.62–1.15) | 0.29 | 0.75 (0.55–1.03) | 0.07 |
| No | 1363 | 67.5 | 1.00 | 1.00 | ||
| Convulsions/fits | ||||||
| Yes | 148 | 51.4 | 0.47 (0.34–0.66) | <0.001 | 0.48 (0.34–0.66) | <0.001 |
| No | 1464 | 69.1 | 1.00 | 1.00 | ||
| Experienced any delivery complication | ||||||
| Yes | 535 | 62.1 | 0.71 (0.56–0.90) | 0.005 | 0.68 (0.53–0.87) | 0.003 |
| No | 1070 | 69.6 | 1.00 | 1.00 | ||
Unweighted number.
Weighted percentage.
Adjusted for maternal age, marital status, place of residence, wealth status, maternal education, parity, multiple births, place of birth, baby's gender and mother's desire for the pregnancy.
3.2. Experience of immediate post‐partum complications and breastfeeding initiation
The association of immediate post‐partum complications with breastfeeding initiation is shown in Table 3. Women with a retained placenta for more than 30 min after delivery were 64% less likely than those with no retained placenta to initiate breastfeeding in the first hour after birth (AOR: 0.36, 95% CI: 0.24–0.52). Women with severe post‐partum haemorrhage were 37% less likely to initiate breastfeeding in the first hour after birth than those with no post‐partum haemorrhage (AOR: 0.63, 95% CI: 0.47–0.84). In the immediate post‐partum period, the odds of breastfeeding in the first hour of birth were lower (AOR: 0.57, 95% CI: 0.41–0.79) in women with convulsions than in those with no convulsions. We found evidence that women who experienced any immediate post‐partum complication were 39% less likely to initiate breastfeeding in the first hour after birth than those with no immediate post‐partum complication (AOR: 0.61, 95% CI: 0.47–0.79). There was no evidence of an association between high fever with foul discharge in the immediate post‐partum period and breastfeeding initiation.
Table 3.
Association between immediate post‐partum complications and breastfeeding initiation
| Number breastfed within 1 ha | % breastfed within 1 hb | Unadjusted odds ratio (95% CI) | p Value | Adjusted odds ratio (95% CI)c | p Value | |
|---|---|---|---|---|---|---|
| Retained placenta (>30 min) | ||||||
| Yes | 71 | 42.6 | 0.33 (0.23–0.48) | <0.001 | 0.36 (0.24–0.52) | <0.001 |
| No | 1535 | 69.1 | 1.00 | 1.00 | ||
| High fever with foul discharge/abdominal pain | ||||||
| Yes | 196 | 63.6 | 0.85 (0.61–1.18) | 0.33 | 0.87 (0.62–1.22) | 0.41 |
| No | 1409 | 67.4 | 1.00 | 1.00 | ||
| Post‐partum haemorrhage | ||||||
| Yes | 200 | 57.8 | 0.62 (0.47–0.83) | 0.001 | 0.63 (0.47–0.84) | 0.002 |
| No | 1411 | 68.7 | 1.00 | 1.00 | ||
| Convulsions/fits | ||||||
| Yes | 135 | 54.5 | 0.55 (0.40–0.77) | 0.001 | 0.57 (0.41–0.79) | 0.001 |
| No | 1475 | 68.4 | 1.00 | 1.00 | ||
| Any immediate post‐partum complication | ||||||
| Yes | 360 | 58.4 | 0.60 (0.47– 0.77) | <0.001 | 0.61 (0.47–0.79) | <0.001 |
| No | 1245 | 70.1 | 1.00 | 1.00 | ||
Unweighted number.
Weighted percentage.
Adjusted for maternal age, marital status, place of residence, wealth status, maternal education, parity, multiple births, place of birth, baby's gender and mother's desire for the pregnancy.
3.3. Obstetric and health care factors and breastfeeding initiation
Obstetric and health care factors associated with breastfeeding initiation are summarised in Table 4. Women who had skin‐to‐skin contact with their newborns after delivery were 47% more likely to initiate breastfeeding within the first hour (AOR: 1.47, 95% CI: 1.11–1.94) than those without skin‐to‐skin contact. Women who had a caesarean birth were 72% less likely than those who did not have a caesarean birth to initiate breastfeeding within the first hour after birth (AOR: 0.28, 95% CI: 0.18–0.41). Similarly, women who delivered outside of a healthcare facility (AOR: 0.70, 95% CI: 0.50–0.99) and those who delivered twins (AOR: 0.43, 95% CI: 0.22–0.85) were less likely to initiate breastfeeding within the first hour than those who delivered in a health care facility and those who had single birth, respectively. In the unadjusted and adjusted analyses, there was no evidence that the time to a baby's first bath and wrapping of the baby minutes after birth were associated with breastfeeding initiation.
Table 4.
Obstetric and healthcare factors associated with breastfeeding initiation
| Number breastfed within 1 ha | % breastfed within 1 hb | Unadjusted odds ratio (95% CI) | p Value | Adjusted odds ratio (95% CI)c | p Value | |
|---|---|---|---|---|---|---|
| Mother–newborn skin‐to‐skin | ||||||
| Yes | 908 | 72.8 | 1.60 (1.20–2.14) | 0.001 | 1.47 (1.11–1.94) | 0.007 |
| No | 688 | 62.5 | 1.00 | 1.00 | ||
| Minutes after the delivery baby was wrapped | ||||||
| 0–5 min | 1081 | 69.6 | 1.00 | 0.12 | 1.00 | 0.12 |
| 6–60 min | 441 | 64.6 | 0.80 (0.59–1.06) | 0.79 (0.59–1.06) | ||
| When a baby is given the first bath | ||||||
| Within 24 h | 608 | 67.5 | 1.00 | 0.71 | 1.00 | 0.21 |
| After 24 h | 984 | 66.4 | 0.95 (0.72–1.25) | 0.83 (0.61–1.11) | ||
| Caesarean section | ||||||
| Yes | 79 | 43.3 | 0.28 (0.19–0.42) | <0.001 | 0.28 (0.18–0.41) | <0.001 |
| No | 966 | 73.1 | 1.00 | 1.00 | ||
| Place of childbirth | ||||||
| Not in a health care facility | 560 | 62.8 | 0.72 (0.52–0.98) | 0.04 | 0.70 (0.50–0.99) | 0.04 |
| Health care facility | 1045 | 70.3 | 1.00 | 1.00 | ||
| Multiple births | ||||||
| Single | 1588 | 67.2 | 1.00 | 0.01 | 1.00 | 0.02 |
| Twin | 17 | 46.9 | 0.43 (0.22–0.85) | 0.43 (0.22–0.85) | ||
Unweighted number.
Weighted percentage.
Adjusted for maternal age, marital status, place of residence, wealth status, maternal education, parity, multiple births, place of birth, baby's gender and mother's desire for the pregnancy.
4. DISCUSSION
We investigated the impact of intrapartum and post‐partum complications and newborn care practices on breastfeeding initiation and found that women who experienced intrapartum haemorrhage, malpresentation and convulsions during childbirth were less likely to initiate breastfeeding early. In the immediate post‐partum period, we found that women with retained placenta for more than 30 min after delivery, those who suffered a post‐partum haemorrhage, and women who experienced convulsions after childbirth were less likely to initiate breastfeeding early. When we pooled the complications, it revealed that the experience of any intrapartum or post‐partum complication decreased the likelihood of early breastfeeding initiation. Furthermore, childbirth outside a healthcare facility, multiple births and caesarean delivery reduced the likelihood of early breastfeeding initiation. However, mother–newborn skin‐to‐skin contact after birth increased the likelihood of early breastfeeding initiation. The time to a baby's first bath was not associated with breastfeeding initiation.
Interventions for intrapartum complications often include the administration of drugs. For example, women with slow‐progressing labour are often subjected to induction and augmentation with synthetic oxytocin to accelerate the delivery process (Bell et al., 2014; Wiklund et al., 2009). Most of the drugs administered during labour cross the placenta, and babies exposed to these interventions due to prolonged labour, intrapartum haemorrhage or convulsions often have difficulty latching and sucking in the first few hours after birth (Smith, 2007). Several studies have reported delayed breastfeeding initiation among women who experienced obstetric complications and prolonged labour (Dewey et al., 2003; Gurung et al., 2021; Lau et al., 2018). Further, malpresentation can predispose foetuses to foetal distress and women to exhaustion and anxiety, resulting in delayed breastfeeding initiation. Women may choose to rest, and neonates will be too tired to latch on to the breast, suckle or swallow immediately after birth (Lau et al., 2018; Tzeng et al., 2017). In addition, intrapartum haemorrhage puts women at risk of hypovolaemic shock, stressing them out and delaying breastfeeding initiation.
Stressful labour and delivery, whether due to malpresentation or convulsions, can delay lactogenesis and the flow of breast milk (Hurst, 2007), resulting in delayed breastfeeding initiation. Furthermore, post‐partum haemorrhage, retained placenta and convulsions after delivery interrupt efforts towards successful breastfeeding initiation. It has been noted that women who suffer post‐partum complications are often separated from their babies for care activities, causing breastfeeding delays. In an earlier study, post‐partum haemorrhage delayed breastfeeding initiation for 22.5 h (Henry & Britz, 2013). In addition, the retention of placental tissue fragments can inhibit breastfeeding hormones from having their full effect, causing a delay in breast milk production and initiation of breastfeeding (Anderson, 2001; Hurst, 2007). Nevertheless, a study in Singapore reported no association between intrapartum complications and breastfeeding initiation (Lau et al., 2018). Differences in the healthcare systems and support offered to mothers with intrapartum complications may explain the discrepancy.
Similar to our findings, a meta‐analysis of 47 studies on the mode of delivery and 17 studies on skin‐to‐skin contact at birth found that women with spontaneous vaginal delivery were more likely to initiate breastfeeding early than those delivered through caesarean section (Cohen et al., 2018). In the meta‐analysis, skin‐to‐skin contact was associated with two times higher odds of early breastfeeding initiation than no skin‐to‐skin contact (Cohen et al., 2018). Skin‐to‐skin contact increases the mother's oxytocin level in the first hour (which decreases maternal anxiety, increases calmness and antagonizes the flight–fight effect), decreases stress in the baby, promotes optimal temperature, accelerates the maintenance of blood glucose levels and metabolic adaptation of the newborn (Jonas et al., 2007; Widström et al., 2019). Insufficient skin‐to‐skin contact probably contributed to the delayed breastfeeding initiation among women who had caesarean deliveries in this study. Because postcaesarean delivery care activities often disrupt bonding and contact between a mother and her newborn baby (Prior et al., 2012). Also, following a caesarean operation, women often experience difficulties with mobility and positioning, and babies delivered through caesarean section are often in poor conditions, hindering breastfeeding initiation (Tully & Ball, 2014). Consistent with our findings, an earlier study in Ethiopia and a pooled analysis of data from 30 sub‐Saharan African countries reported early breastfeeding initiation among women delivered in a healthcare facility (Bergamaschi et al., 2019; Woldeamanuel, 2020). In principle, facility birth provides a platform for women to receive supervised professional care and support to facilitate breastfeeding initiation. Outside‐facility deliveries are usually without professional support to educate and assist the mother to initiate breastfeeding early and may be associated with birth complications that further undermine early breastfeeding initiation.
Because pre‐eclampsia and pregnancy‐induced hypertension can result in convulsions during labour and post‐partum, early blood pressure monitoring and urine protein tests may be beneficial in detecting these disorders throughout pregnancy. Women with epilepsy should be identified early and the appropriate interventions instituted, with plans for alternate delivery. Lactation counselling is recommended following delivery to help increase breast milk production and facilitate early breastfeeding initiation. It is possible to increase the likelihood of early breastfeeding initiation if malpresentation is detected and managed early in pregnancy. Pregnant women with atypical presentations, such as oblique and transverse, should be prepared for safe alternative delivery or transfer to higher‐level facilities where expert services are available. Caesarean sections should only be performed when it is medically necessary. To minimize the impact of intrapartum and post‐partum complications on breastfeeding initiation, health care professionals who assist in childbirth should be adequately trained in basic and comprehensive emergency obstetric care practises, including blood transfusion, administration of parenteral anticonvulsants, manual placenta removal and assisted vaginal delivery.
Routine post‐partum procedures should be delayed until after the first hour or the first successful breastfeeding (Eidelman et al., 2012), and health care staff should encourage and assist women to practice skin‐to‐skin contact. The skin‐to‐skin contact should begin any time after delivery and remain uninterrupted for at least 60 min or until the baby has had the first breastfeeding. The use of drugs such as pethidine should be limited as this can sedate the infant (Righard & Alade, 1990). Because skin‐to‐skin contact can stimulate oxytocin secretion, it may help arrest post‐partum haemorrhage and facilitate the evacuation of retained placental tissues. It is best not to separate the newborn from the mother when dealing with post‐partum issues. Post‐natal mothers with delayed milk flow should be examined for retained placental fragments. Regular clinical meetings with obstetricians, nurses and midwives on infant feeding and care techniques will also be beneficial. In addition, the Baby‐Friendly Hospital Initiative should be adopted and practised. In the community, mother‐to‐mother support groups can assist first‐time mothers to prepare for skin‐to‐skin and early breastfeeding initiation.
The data for this study came from a population‐based cohort study with a large enough sample to be generalizable. However, there are some limitations worth mentioning. Because the data on breastfeeding initiation were obtained retrospectively, there is a possibility of recall bias, which can distort our estimates. However, because data regarding the study were collected near the time of birth, recall bias will be minimal. The data source lacked variables on the timing and duration of skin‐to‐skin contact and standard hospital procedures. Our findings on skin‐to‐skin contact would have been better understood if we had information on these variables. Also, data on birthweight and gestational age at birth would have been useful to the analysis as low‐birthweight and preterm babies need breastfeeding support. Future studies should consider the impact of timing and duration of skin‐to‐skin contact and routine postdelivery care procedures on breastfeeding initiation. Also, future studies should endeavour to collect data on breastfeeding initiation on the day of birth to minimize any recall bias.
5. CONCLUSION
Our analysis revealed that breastfeeding initiation was delayed among women who experienced intrapartum or post‐partum complications, caesarean delivery or gave birth outside of a healthcare facility. However, women who practised skin‐to‐skin contact after birth were more likely to initiate breastfeeding early.
AUTHOR CONTRIBUTIONS
Shamsudeen Mohammed conceived and designed the study, accessed the data and performed the statistical analysis in consultation with Alhassan S. Abukari and Agani Afaya. Alhassan S. Abukari discussed the results and reviewed all the drafts. Agani Afaya conducted the literature search and commented on the methodology. All authors reviewed and approved the final draft of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
We would like to thank Performance Monitoring for Action Ethiopia (PMA Ethiopia) for prompt approval and access to the data sets for the analysis.
Mohammed, S. , Abukari, A. S. , & Afaya, A. (2023). The impact of intrapartum and immediate post‐partum complications and newborn care practices on breastfeeding initiation in Ethiopia: A prospective cohort study. Maternal & Child Nutrition, 19, e13449. 10.1111/mcn.13449
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Performance Monitoring for Action Ethiopia (PMA Ethiopia), which is publicly available and can be accessed upon reasonable request.
REFERENCES
- Addis Ababa University School of Public Health and The Bill & Melinda Gates Institute for Population and Reproductive Health at The Johns Hopkins Bloomberg School of Public Health . (2019). Performance Monitoring for Action Ethiopia (PMA‐ET) household and female panel: Cohort 1—Baseline survey (version 2.0), PMAET‐Panel‐C1‐Baseline. 10.34976/h75w-8084 [DOI]
- Addis Ababa University School of Public Health and The Bill & Melinda Gates Institute for Population and Reproductive Health at The Johns Hopkins Bloomberg School of Public Health . (2020). Performance Monitoring for Action Ethiopia (PMA‐ET Panel: Cohort 1–6—Week follow‐up survey (version 1.0), PMAET‐Panel‐C1‐6wkFU. 10.34976/8r5s-dx31 [DOI]
- Anderson, A. M. (2001). Disruption of lactogenesis by retained placental fragments. Journal of Human Lactation, 17(2), 142–144. 10.1177/089033440101700210 [DOI] [PubMed] [Google Scholar]
- Appiah, F. , Ahinkorah, B. O. , Budu, E. , Oduro, J. K. , Sambah, F. , Baatiema, L. , Ameyaw, E. K. , & Seidu, A. A. (2021). Maternal and child factors associated with timely initiation of breastfeeding in sub‐Saharan Africa. International Breastfeeding Journal, 16(1), 55. 10.1186/s13006-021-00402-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedaso, A. , Kebede, E. , & Adamu, T. (2019). Assessment of skin‐to‐skin contact (SSC) during the postpartum stay and its determinant factors among mothers at public health institutions in Ethiopia. BMC Research Notes, 12(1), 136. 10.1186/s13104-019-4176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, A. F. , Erickson, E. N. , & Carter, C. S. (2014). Beyond labor: The role of natural and synthetic oxytocin in the transition to motherhood. Journal of Midwifery & Women's Health, 59(1), 35–42. 10.1111/jmwh.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi, N. , Oakley, L. , & Benova, L. (2019). Is childbirth location associated with higher rates of favourable early breastfeeding practices in Sub‐Saharan Africa? Journal of Global Health, 9(1), 1–9. 10.7189/jogh.09.010417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, D. J. , & Pérez‐Escamilla, R. (1999). Identification of risk factors for delayed onset of lactation. Journal of the American Dietetic Association, 9(4), 450–454. 10.1016/S0002-8223(99)00109-1 [DOI] [PubMed] [Google Scholar]
- Cohen, S. S. , Alexander, D. D. , Krebs, N. F. , Young, B. E. , Cabana, M. D. , Erdmann, P. , Hays, N. P. , Bezold, C. P. , Levin‐Sparenberg, E. , Turini, M. , & Saavedra, J. M. (2018). Factors associated with breastfeeding initiation and continuation: A meta‐analysis. The Journal of Pediatrics, 203, 190–196. 10.1016/j.jpeds.2018.08.008 [DOI] [PubMed] [Google Scholar]
- Debes, A. K. , Kohli, A. , Walker, N. , Edmond, K. , & Mullany, L. C. (2013). Time to initiation of breastfeeding and neonatal mortality and morbidity: A systematic review. BMC Public Health, 13(3), S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey, K. G. , Nommsen‐rivers, L. A. , Heinig, M. J. , & Cohen, R. J. (2003). Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics, 112(3), 607–619. [DOI] [PubMed] [Google Scholar]
- Eidelman, A. I. , Schanler, R. J. , Johnston, M. , Landers, S. , Noble, L. , Szucs, K. , & Viehmann L. (2012). Breastfeeding and the use of human milk. Pediatrics, 129(3), e827–e841. 10.1542/peds.2011-3552 [DOI] [PubMed] [Google Scholar]
- Ekholuenetale, M. , Mistry, S. K. , Chimoriya, R. , Nash, S. , Doyizode, A. M. , & Arora, A. (2021). Socioeconomic inequalities in early initiation and exclusive breastfeeding practices in Bangladesh: Findings from the 2018 demographic and health survey. International Breastfeeding Journal, 16, 73. 10.1186/s13006-021-00420-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Er Moore Bergman, N. , Anderson, G. , & Medley, N. (2016). Early skin‐to‐skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Reviews, 11(11), CD003519. 10.1002/14651858.CD003519.pub4.www.cochranelibrary.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, H. S. L. , Wong, J. Y. H. , Fong, D. Y. T. , Lok, K. Y. W. , & Tarrant, M. (2020). Association between intrapartum factors and the time to breastfeeding initiation. Breastfeeding Medicine, 15(6), 394–400. 10.1089/bfm.2019.0166 [DOI] [PubMed] [Google Scholar]
- Getnet, B. , Degu, A. , & Yenealem, F. (2020). Prevalence and associated factors of early initiation of breastfeeding among women delivered via cesarean section in South Gondar zone hospitals Ethiopia, 2020. Maternal Health, Neonatology and Perinatology, 6(1), 6. 10.1186/s40748-020-00121-3 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gurung, R. , Sunny, A. K. , Paudel, P. , Bhattarai, P. , Basnet, O. , Sharma, S. , Shrestha, D. , Sharma, S. , Malla, H. , Singh, D. , Mishra, S. , & Kc, A. (2021). Predictors for timely initiation of breastfeeding after birth in the hospitals of Nepal—A prospective observational study. International Breastfeeding Journal, 16(1), 85. 10.1186/s13006-021-00431-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtewold, T. D. , Mohammed, S. H. , Endalamaw, A. , Akibu, M. , Sharew, N. T. , Alemu, Y. M. , Beyene, M. G. , Sisay, T. A. , Birhanu, M. M. , Islam, M. A. , & Tegegne, B. S. (2019). Breast and complementary feeding in Ethiopia: New national evidence from systematic review and meta‐analyses of studies in the past 10 years. European Journal of Nutrition, 58(7), 2565–2595. 10.1007/s00394-018-1817-8 [DOI] [PubMed] [Google Scholar]
- Henry, L. , & Britz, S. P. (2013). Loss of blood = loss of breast milk? The effect of postpartum hemorrhage on breastfeeding success background. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 42, 92–110. 10.1111/1552-6909.12199 [DOI] [Google Scholar]
- Hurst, N. M. (2007). Recognizing and treating delayed or failed lactogenesis II. Journal of Midwifery & Women's Health, 52(6), 588–594. 10.1016/j.jmwh.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Jonas, W. , Wiklund, I. , Nissen, E. , Ransjö‐Arvidson, A. B. , & Uvnäs‐Moberg, K. (2007). Newborn skin temperature two days postpartum during breastfeeding related to different labour ward practices. Early Human Development, 83(1), 55–62. 10.1016/j.earlhumdev.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Kruse, L. , Denk, C. E. , Feldman‐Winter, L. , & Mojta Rotondo, F. (2005). Comparing sociodemographic and hospital influences on breastfeeding initiation. Birth, 32(2), 81–85. 10.1111/j.0730-7659.2005.00349.x [DOI] [PubMed] [Google Scholar]
- Lau, Y. , Tha, P. H. , Ho‐Lim, S. S. T. , Wong, L. Y. , Lim, P. I. , Citra Nurfarah, B. Z. M. , & Shorey, S. (2018). An analysis of the effects of intrapartum factors, neonatal characteristics, and skin‐to‐skin contact on early breastfeeding initiation. Maternal & Child Nutrition, 14(1), e12492. 10.1111/mcn.12492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Ware, J. , Chen, A. , Nelson, J. M. , Kmet, J. M. , Parks, S. E. , Morrow, A. L. , Chen, J. , & Perrine, C. G. (2022). Breastfeeding and post‐perinatal infant deaths in the United States, A national prospective cohort analysis. The Lancet Regional Health—Americas, 5, 100094. 10.1016/j.lana.2021.100094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick, L. , Wang, W. , Farid, S. , & Pullum T. (2021). Initiation of breastfeeding in low‐ and middle‐income countries: A time‐to‐event analysis. Global Health: Science and Practice, 9(2), 308–317. 10.9745/GHSP-D-20-00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkoka, O. , Ntenda, P. A. M. , Kanje, V. , Milanzi, E. B. , & Arora, A. (2019). Determinants of timely initiation of breast milk and exclusive breastfeeding in Malawi: A population‐based cross‐sectional study. International Breastfeeding Journal, 14, 37. 10.1186/s13006-019-0232-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilliod, R. A. , & Caughey, A. B. (2017). Fetal malpresentation and malposition. Obstetrics and Gynecology Clinics of North America, 44(4), 631–643. 10.1016/j.ogc.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Prior, E. , Santhakumaran, S. , Gale, C. , Philipps, L. H. , Modi, N. , & Hyde, M. J. (2012). Breastfeeding after cesarean delivery: A systematic review and meta‐analysis of world literature. The American Journal of Clinical Nutrition, 95(5), 1113–1135. 10.3945/ajcn.111.030254 [DOI] [PubMed] [Google Scholar]
- Righard, L. , & Alade, M. O. (1990). Effect of delivery room routines on success of first breast‐feed. The Lancet, 336(8723), 1105–1107. 10.1016/0140-6736(90)92579-7 [DOI] [PubMed] [Google Scholar]
- Rollins, N. C. , Bhandari, N. , Hajeebhoy, N. , Horton, S. , Lutter, C. K. , Martines, J. C. , Piwoz, E. G. , Richter, L. M. , & Victora, C. G. (2016). Why invest, and what it will take to improve breastfeeding practices? The Lancet, 387(10017), 491–504. 10.1016/S0140-6736(15)01044-2 [DOI] [PubMed] [Google Scholar]
- Safari, K. , Saeed, A. A. , Hasan, S. S. , & Moghaddam‐Banaem, L. (2018). The effect of mother and newborn early skin‐to‐skin contact on initiation of breastfeeding, newborn temperature and duration of third stage of labor. International Breastfeeding Journal, 13, 32. 10.1186/s13006-018-0174-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, K. , Khan, S. M. , Carvajal–Aguirre, L. , Brodish, P. , Amouzou, A. , & Moran, A. (2017). The importance of skin‐to‐skin contact for early initiation of breastfeeding in Nigeria and Bangladesh. Journal of Global Health, 7(2), 20505. 10.7189/jogh.07.020505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, L. J. (2007). Impact of birthing practices on the breastfeeding dyad. Journal of Midwifery & Women's Health, 52(6), 621–630. 10.1016/j.jmwh.2007.07.019 [DOI] [PubMed] [Google Scholar]
- Teshale, A. B. , & Tesema, G. A. (2021, March). Timely initiation of breastfeeding and associated factors among mothers having children less than two years of age in sub‐Saharan Africa: A multilevel analysis using recent Demographic and Health Surveys data. PLoS ONE, 16(3), 1–16. 10.1371/journal.pone.0248976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully, K. P. , & Ball, H. L. (2014). Maternal accounts of their breast‐feeding intent and early challenges after caesarean childbirth. Midwifery, 30(6), 712–719. 10.1016/j.midw.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng, Y. L. , Yang, Y. L. , Kuo, P. C. , Lin, Y. C. , & Chen, S. L. (2017). Pain, anxiety, and fatigue during labor: A prospective, repeated measures study. Journal of Nursing Research, 25(1), 59–67. 10.1097/jnr.0000000000000165 [DOI] [PubMed] [Google Scholar]
- UNICEF . (2018). Unicef for every child. Breastfeeding a mother’ s gift, for every child. https://data.unicef.org/wp-content/uploads/2018/05/180509_Breastfeeding.pdf
- UNICEF & WHO . (2019). Global breastfeeding collective (issue 3). Increasing commitment to breastfeeding through funding and improved policies and programmes. https://apps.who.int/iris/handle/10665/326049
- Victora, C. G. , Bahl, R. , Barros, A. J. D. , França, G. V. A. , Horton, S. , Krasevec, J. , Murch, S. , Sankar, M. J. , Walker, N. , & Rollins, N. C. (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. The Lancet, 387(10017), 475–490. 10.1016/S0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- Widström, A. M. , Brimdyr, K. , Svensson, K. , Cadwell, K. , & Nissen, E. (2019). Skin‐to‐skin contact the first hour after birth, underlying implications and clinical practice. Acta Paediatrica, 108(7), 1192–1204. 10.1111/apa.14754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund, I. , Norman, M. , Uvnäs‐Moberg, K. , Ransjö‐Arvidson, A. B. , & Andolf, E. (2009). Epidural analgesia: Breast‐feeding success and related factors. Midwifery, 25(2), e31–e38. 10.1016/j.midw.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Woldeamanuel, B. T. (2020). Trends and factors associated to early initiation of breastfeeding, exclusive breastfeeding and duration of breastfeeding in Ethiopia: Evidence from the Ethiopia Demographic and Health Survey 2016. International Breastfeeding Journal, 15(1), 3. 10.1186/s13006-019-0248-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from Performance Monitoring for Action Ethiopia (PMA Ethiopia), which is publicly available and can be accessed upon reasonable request.


