Abstract
The ability of Cryptococcus neoformans to synthesize polymerized melanin in vitro has been associated with virulence, but it is unclear whether this fungus synthesizes polymerized melanin during infection. To study this question, we used two approaches: one involved the generation of monoclonal antibodies (MAbs) to melanin for use in immunohistochemical studies of C. neoformans-infected rodents, and the other sought to isolate fungal melanin from infected tissues. Digestion of in vitro-melanized C. neoformans cells with proteases, denaturant, and hot concentrated acid yields melanin particles that retain the shape of fungal cells and are therefore called melanin ghosts. BALB/c mice were immunized with melanin ghosts, and two immunoglobulin M MAbs to melanin were generated from the spleen of one mouse. Immunofluorescence analyses of lung and brain tissues of rodents infected with wild-type melanin-producing (Mel+) C. neoformans strains demonstrated binding of the MAbs to the fungal cell wall. No binding was observed when infections were performed with mutant albino (Mel−) C. neoformans strains. Particles with striking similarity to melanin ghosts were recovered after digestion of lung and brain tissues from Mel+ C. neoformans-infected rodents and were reactive with the MAbs to melanin. No particles were recovered from tissues infected with Mel− C. neoformans. A Mel+ C. neoformans strain grown on lung or brain homogenate agar became lightly pigmented and also yielded particles similar to melanin ghosts upon digestion, providing additional evidence that lung and brain tissues contain substrate for C. neoformans melanization. These results demonstrate that C. neoformans synthesizes polymerized melanin during infection, which has important implications for pathogenesis and antifungal drug development.
Cryptococcus neoformans is an encapsulated fungus that causes life-threatening meningoencephalitis, predominantly in immunocompromised individuals (reviewed in reference 29). C. neoformans has a laccase (CNLAC1) (47) that catalyzes the synthesis of polymerized melanin in vitro when the cells are grown in the presence of phenolic substrates, such as 3,4-dihydroxyphenylalanine (l-dopa) (reviewed in reference 46). The ability of C. neoformans to synthesize polymerized melanin in vitro has been used over the last three decades as an identifying characteristic of this fungus (12).
Synthesis of polymerized melanin in vitro has been associated with virulence by both classical genetic and gene disruption studies. In the early 1980s, several studies of murine models of infection demonstrated increased virulence of wild-type melanin-producing (Mel+) C. neoformans strains compared to their respective albino (Mel−) mutants, as measured by survival of infected mice (19, 20, 34). In mice that died after infection with the Mel− strains, 50% of the cryptococcal cells present in their brains had reverted to the Mel+ phenotype. These revertants regained the ability to synthesize melanin in vitro and had virulence comparable to that of the wild-type strains (34). More recently, disruption of the CNLAC1 gene was associated with a significant reduction in virulence (36). Although these studies clearly implicated the ability to synthesize polymerized melanin in vitro as being associated with virulence, it is unknown whether C. neoformans can synthesize polymerized melanin during infection. Numerous in vitro studies have analyzed mechanisms by which melanin could function in the pathogenesis of C. neoformans infections. Transmission electron microscopic analysis has demonstrated that melanin is deposited in the fungal cell wall (44), where it could provide cell wall support and integrity. Melanins are free-radical scavengers (reviewed in references 27 and 33). Melanized C. neoformans cells are less susceptible to nitrogen- and oxygen-derived oxidants (5, 14, 16, 17, 32, 41, 44), microbicidal peptides (4), and macrophage-mediated phagocytosis (44) than nonmelanized cells. In vivo studies have demonstrated that a strain of C. neoformans that produces a high melanin content in vitro can down-regulate components of the afferent phase of T-cell-mediated immunity, namely, production of tumor necrosis factor alpha and lymphoproliferation (13). These studies suggest that melanin may function in vivo to protect C. neoformans from host defense mechanisms. Furthermore, melanized C. neoformans cells are less susceptible to killing by the antifungal drug amphotericin B than nonmelanized cells (42), which suggests that melanization could contribute to the persistence of infection despite antifungal therapy. Melanins, however, have high affinity for certain drugs and chemicals, such as the antipsychotic drug trifluoperazine (22), and melanized C. neoformans cells are more susceptible to the toxic effects of trifluoperazine than nonmelanized cells (43). This suggests that melanins could be a target for antifungal drug development.
Several studies have attempted to detect melanization in vivo. Studies using the Masson-Fontana silver stain to detect melanization of C. neoformans cells in human and murine tissues gave positive results, but these were not totally specific results due to reactions with other cell wall components (21). Dark-walled cells have been observed in several cases of human cryptococcal meningitis, but the identity of the pigment was not established (23). A previous study from our laboratory used melanin-binding peptides to demonstrate the formation of melanin-like compounds by a Mel+ C. neoformans strain during pulmonary infections in mice (31). That study also demonstrated that cryptococcal infections induce strong antibody responses to melanin in mice, providing additional evidence of the presence of fungal melanin in vivo. Another group reported that Mel+ C. neoformans strains form melanin precursors during infections in mice, consistent with laccase activity in vivo, but they did not find polymerized melanin in the cryptococcal cells recovered from brain tissue (26). Furthermore, this group suggested that laccase contributes to virulence by mechanisms other than melanin production, such as by converting the host's normal catecholamines into potentially toxic quinones (26) or by protecting C. neoformans from antifungal activity of alveolar macrophages (25).
In view of the apparent discrepancy of results, we investigated whether C. neoformans synthesizes polymerized melanin during infection. We undertook two new approaches: one involved the generation of monoclonal antibodies (MAbs) to polymerized melanin for use in immunohistochemical studies of C. neoformans-infected rodents, and the other sought to isolate fungal melanin from infected tissues. Our results demonstrated that the MAbs to melanin bind to Mel+, but not to Mel−, C. neoformans strains in infected tissues. Furthermore, particles with striking similarity to melanin ghosts were recovered from Mel+ C. neoformans-infected tissues. These results demonstrate that C. neoformans synthesizes polymerized melanin during infection. Hence, the protective mechanisms ascribed to melanin in vitro may also apply in vivo.
(The data in this paper are from a thesis to be submitted by Ángel L. Rosas in partial fulfillment of the requirements for the degree of doctor of philosophy in the Sue Golding Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University, Bronx, N.Y.)
MATERIALS AND METHODS
C. neoformans.
Wild-type melanin-producing (Mel+) C. neoformans strains H99, JEC21, and CN110 (serotype A); 24065 (serotype B); NIH 34 (serotype C); and 24067 (serotype D) were obtained from the American Type Culture Collection (Rockville, Md.).
C. neoformans strain HMC4 is a mutant albino (Mel−) of H99 generated by random insertional gene disruption (3).
C. neoformans strain HMC6 is a mutant albino (Mel−) of JEC21 generated using targeted disruption of CNLAC1. Briefly, a 6.1-kb genomic fragment from C. neoformans 3501 containing the entire CNLAC1 gene was subcloned into the EcoRV site of pBluescript (a generous gift from Peter R. Williamson, University of Chicago, Chicago, Ill.). A 2-kb URA5 genomic fragment was amplified by PCR to introduce NcoI restriction sites onto both ends, and this fragment was ligated into the single NcoI site located within the coding region of CNLAC1. This disruption construct was used to transform C. neoformans C21 (a spontaneous ura5 auxotroph derived by passage of JEC21 on 5-fluoro-orotic acid agar), using biolistic DNA delivery, as described previously (40). All transformants were selected on uridine dropout media, and stable transformants were screened on Niger seed agar (Becton Dickinson, Cockeysville, Md.). Disruption of CNLAC1 was confirmed by Southern blot and PCR analyses.
Growth of C. neoformans with or without l-dopa.
To confirm the Mel+ or Mel− phenotype of the C. neoformans strains, 102 cells were grown on 1.5 Bacto Agar (Difco Laboratories, Detroit, Mich.) containing a defined minimal medium (15.0 mM glucose, 10.0 mM MgSO4, 29.4 mM KH2PO4, 13.0 mM glycine, and 3.0 μM vitamin B1 [pH 5.5]) with or without 1.0 mM l-dopa (Sigma Chemical Co., St. Louis, Mo.) for 7 days at 30°C.
Isolation of l-dopa melanin ghosts.
Mel+ C. neoformans 24067 cells were grown in minimal medium with or without 1.0 mM l-dopa for 15 days at 30°C in a rotary shaker at 150 rpm. Only cells grown with l-dopa became melanized. Fungal cells were collected by centrifugation at 2,500 rpm for 30 min, washed three times with phosphate-buffered saline (PBS) (pH 7.4), and suspended in 1.0 M sorbitol–0.1 M sodium citrate (pH 5.5). Novozym 234 (Calbiochem, La Jolla, Calif.) was added at 10 mg/ml, and the suspension was incubated overnight at 30°C to generate protoplasts. The protoplasts were collected by centrifugation, washed three times with PBS, and incubated in 4.0 M guanidine thiocyanate (denaturant) (Sigma Chemical Co.) for 12 h at room temperature with frequent vortexing. Cell debris were collected by centrifugation, washed three times with PBS, and treated with 1.0 mg of proteinase K (Boehringer Mannheim Co., Indianapolis, Ind.) per ml (reaction buffer was 10.0 mM Tris, 1.0 mM CaCl2, and 0.5% sodium dodecyl sulfate [pH 7.8]) overnight at 37°C. The debris were then washed three times with PBS and boiled in 6.0 M HCl for 1 h. When applied to nonmelanized cells, the combination of protease, denaturant, and hot acid digestion results in the complete solubilization of fungal cells. However, for melanized cells, this protocol yields hollow melanin particles that retain the shape of fungal cells and are therefore called melanin ghosts (45). Melanin ghosts were collected by centrifugation, washed extensively with PBS, dialyzed against distilled water for 10 days, and lyophilized using a Flexi-Dry microprocessor (FTS Systems Inc., Stone Ridge, N.Y.).
Immunization.
Five 6- to 8-week-old female BALB/c mice (National Cancer Institute, Rockville, Md.) were immunized with an intraperitoneal injection of 300 μg of l-dopa melanin ghosts suspended in a 1:1 (vol/vol) emulsion of complete Freund's adjuvant (Sigma Chemical Co.) and PBS, followed by additional immunization doses of 300 μg of l-dopa melanin ghosts in 1:1 (vol/vol) emulsions of incomplete Freund's adjuvant (Sigma Chemical Co.) and PBS at weeks 2, 4, and 6 after the initial immunization. At week 7, the mice were bled from the retro-orbital plexus and their sera were analyzed for antibodies to melanin by enzyme-linked immunosorbent assay (ELISA) as described below. The mouse with the highest antibody titer to melanin was boosted again at week 8 and used to generate hybridomas.
Production of hybridomas.
One day after the last immunization, the mouse was killed by cervical dislocation and the spleen was removed. Spleen cells were fused to myeloma cells at a ratio of 4:1 in the presence of 50% polyethyleneglycol to generate hybridomas. The cell mixture was suspended in a defined complete hypoxanthine-aminopterin-thymidine (HAT) medium (Dulbecco's modified Eagle's medium with l-glutamine [Mediatech, Washington, D.C.] containing 20% heat-inactivated fetal bovine serum [Harlan Bioproducts for Science, Indianapolis, Ind.], 10% NCTC-109 without l-glutamine [Life Technologies GIBCO BRL, Grand Island, N.Y.], HAT [Sigma Chemical Co.], 1% nonessential amino acids [Life Technologies GIBCO BRL], and 1% penicillin-streptomycin [Life Technologies GIBCO BRL]) for selection of hybridomas, plated in 96-well tissue culture-treated plates (Becton Dickinson), and incubated in a 10% CO2 incubator at 37°C. Hybridomas were fed with complete HAT medium as necessary. Supernatants were screened for the presence of MAbs to melanin by ELISA.
Melanin ELISAs.
A suspension of 5 × 106 l-dopa melanin ghosts in water was plated in each well of a polystyrene 96-well ELISA plate (Corning Glass Works, Corning, N.Y.) and incubated overnight at room temperature to allow the l-dopa melanin ghosts to dry. The l-dopa melanin ghosts were then heat fixed to the polystyrene solid-phase support by incubating the plates at 60°C for 30 min. Wells were blocked to prevent nonspecific binding by adding a suspension of 2% bovine serum albumin (BSA) (ICN Biomedicals, Aurora, Ohio) in water, followed by addition of 5% powdered milk in water. Each blocking step involved incubation for 2 h at room temperature. Plates blocked with BSA and milk without melanin were used as controls. The plates were washed three times with 0.1% Tween 20 in Tris-buffered saline after each incubation. Hybridoma supernatants were diluted 1:100 in PBS, added to a well of the melanin-coated and control plates, and incubated for 1.5 h at 37°C. After washing, a 1:1,000 dilution of alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) and IgM (Southern Biotechnologies Associates Inc., Birmingham, Ala.) was added to the wells and incubated for 1.5 h at 37°C. Antibody binding was detected by addition of p-nitrophenyl phosphate (Sigma Chemical Co.) (reaction buffer was 1.0 mM MgCl2 and 50.0 mM Na2CO3 [pH 9.8]). After 30 min of incubation, the solutions were transferred to clear plates and the optical densities were measured at 405 nm with a Ceres 900HDi EIA Workstation (Bio-Tek Instruments Inc., Winooski, Vt.). The contents of those wells containing hybridomas producing antibodies that bound to l-dopa melanin but not to the blocking solutions were subcloned twice by soft agar cloning to recover homogenous hybridoma cell lines. The hybridomas were again tested for production of MAbs to melanin. The heavy- and light-chain isotype of each MAb was determined using alkaline phosphatase-conjugated antibodies specific for the heavy-chain constant region and for κ and λ light chains. The MAbs to melanin (6D2 and 11B11) (10 μg/ml) were also tested for binding to other melanins by coating each well of the ELISA plates with suspensions of 50 μg of synthetic melanin (Sigma Chemical Co.) and 100 μg of melanin from Sepia officinalis (Sigma Chemical Co.) in water and following the protocol described above. MAb 5C11, which binds mycobacterial lipoarabinomannan (9), was used as an IgM negative control.
Immunofluorescence analyses of melanins and C. neoformans.
Suspensions of 106 l-dopa melanin ghosts, 50 μg of synthetic melanin, and 100 μg of melanin from S. officinalis in water were fixed to poly-l-lysine-coated slides (Sigma Chemical Co.), blocked against nonspecific binding with SuperBlock Blocking Buffer in PBS (Pierce, Rockford, Ill.) for 4 h at room temperature, and incubated with 20 μg of MAb 6D2 or 11B11 per ml overnight at 4°C. MAb 5C11 was used as a negative control. Prior to incubation, the MAbs were conjugated to the Alexa 488 dye (Molecular Probes, Inc., Eugene, Oreg.). Melanin particles were washed three times with PBS to eliminate unbound antibody, and then a mounting solution (50% glycerol, 50% PBS, and 0.1 M N-propyl gallate) and coverslip were applied to the slide and the samples were examined using an AX70 microscope (Olympus America Inc., Melville, N.Y.) with a fluorescein isothiocyanate filter at a magnification of ×1,000. Immunofluorescence analyses were also performed on Mel+ and Mel− C. neoformans cells grown with or without l-dopa for 7 days, either as whole cells or as frozen sections. Whole cells were fixed to poly-l-lysine-coated slides and treated as described above. For frozen sections, cells were washed twice with PBS, collected by centrifugation, and embedded in TBS Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, N.C.). Sections 4 μm thick were fixed to poly-l-lysine-coated slides, washed for 5 min with PBS, and treated as described above.
Reduction of pentameric MAbs to l-dopa melanin.
One-milliliter solutions of MAb 6D2 or 11B11 (20 μg/ml) were diluted 1:1 in PBS with or without 0.15 M β-mercaptoethanol for 1 h at 37°C. β-Mercaptoethanol depolymerizes the pentameric form of IgM (38). Pentameric or reduced MAbs were then incubated with l-dopa melanin ghosts and analyzed by immunofluorescence as described above.
C. neoformans infections.
Mel+ and Mel− C. neoformans cells were grown in Sabouraud dextrose broth (Difco Laboratories) for 2 days at 30°C in a rotatory shaker at 150 rpm. Female BALB/c mice (6 to 8 weeks old) (National Cancer Institute) were infected intravenously with 5 × 105 Mel+ or Mel− C. neoformans cells. Groups of three to five mice were then killed by cervical dislocation at days 1 to 7, 14, and 21 after infection with Mel+ C. neoformans 24067 and at day 7 after infection with the other strains. SCID mice (6 to 8 weeks old) (National Cancer Institute) were infected with Mel+ C. neoformans 24067 and killed at day 7 after infection. Five adult female Fischer rats (National Cancer Institute) were infected intraperitonealy with 107 Mel+ C. neoformans 24067 cells and killed with lethal injections of pentobarbital sodium (Nembutal) solution (Abbott Laboratories, North Chicago, Ill.) at days 1, 3, 7, 14, and 21 after infection. Mice and rats were housed in the animal facility of our institution, and all experimental procedures adhered to protocols approved by the Animal Care and Use Committee at our institution.
Immunofluorescence analyses of infected tissues.
Samples of the lungs and brains of Mel+ or Mel− C. neoformans-infected rodents were collected for immunofluorescence analyses. Tissue sections 4 μm thick were deparaffinized in xylenes and rehydrated by serial incubations in solutions of decreasing ethanol concentration. The samples were treated with 20 μg of proteinase K per ml for 1 h at room temperature and then heated in 10 mM citric acid in a microwave oven for 5 min. The samples were incubated with SuperBlock Blocking Buffer in PBS for 4 h at room temperature to block nonspecific binding and then with 20 μg of MAb 6D2 or 11B11 per ml for 1 h at 37°C. MAb 5C11 was used as a negative control. As before, MAbs 6D2, 11B11, and 5C11 were conjugated to the Alexa 488 dye prior to incubation. Tissue samples were washed three times with PBS, the mounting solution and coverslip were applied, and the samples were examined using an Olympus AX70 microscope with a fluorescein isothiocyanate filter at a magnification of ×1,000.
Immunogold transmission electron microscopy of infected tissues.
To localize the site of MAb binding on C. neoformans cells in vivo, immunogold labeling was performed on infected lung tissues. Mel+ C. neoformans 24067 cells were grown in Sabouraud dextrose broth for 2 days at 30°C in a rotary shaker at 150 rpm. Five female C57BL/6 mice (6 to 8 weeks old) (National Cancer Institute) were infected intratracheally with 104 fungal cells, as described previously (7). Mice were killed at day 7 after infection. Ultrathin tissue sections were placed on nickel grids, incubated with 10% H2O2 for 10 min, and then washed with PBS. Grids were etched in a saturated solution of sodium periodate for 10 min, washed, and blocked in a suspension of 2% goat serum, 1% BSA, and 4% milk in PBS for 1 h at room temperature. Grids were then incubated in 5 μg of MAb 11B11 per ml overnight at 4°C. Polyclonal murine IgM (ICN Biomedicals) was used as a negative control. After being washed with PBS, the grids were incubated in a 1:1,000 dilution of goat anti-mouse IgM conjugated to 5-nm gold (Goldmark Biologicals, Phillipsburg, N.J.) for 2 h at room temperature. Grids were then washed with PBS, fixed in 2% gluteraldehyde, and examined using a 100 CX transmission electron microscope (JEOL, Tokyo, Japan). Further tissue samples were stained as described above, except that the H2O2 and sodium periodate incubations were omitted. MAb 11B11 and polyclonal IgM were used at a concentration of 10 μg/ml, and goat anti-mouse IgM conjugated to gold was used at a 1:100 dilution.
Digestion of infected tissues.
To isolate C. neoformans melanin from infected tissues, the lungs and brains of infected rodents were homogenized by mechanical grinding. Aliquots of homogenized organs were plated on Sabouraud dextrose agar (Difco Laboratories) and incubated for 2 days at 30°C to test for the presence of C. neoformans cells. Infected tissues were treated with 1.0 mg of proteinase K per ml at 65°C for 4 h, incubated in 4.0 M guanidine thiocyanate for at least 2 h at room temperature with frequent vortexing, and then boiled in 6.0 M HCl for 1 h. The resulting material was washed three times with PBS, incubated in 2.5% gluteraldehyde for 1 h at room temperature, and applied to poly-l-lysine-coated slides. The samples were dehydrated by serial incubations in solutions of increasing ethanol concentration, dried in a Tousimis Samdri-790 Critical Point Drier (Tousimis Research Co., Rockville, Md.), coated with gold-palladium in a Desk-1 Sputter Coater (Denton Vacuum Inc., Cherry Hill, N.J.), and examined using a JSM-6400 scanning electron microscope (JEOL). Particle sizes were calculated using the scale bar from the scanning electron micrograph as reference. As controls, lungs and brains from uninfected BALB/c mice were mixed with in vitro-melanized or nonmelanized Mel+ C. neoformans 24067 cells, digested, and examined as described above.
Immunofluorescence analyses were performed on the material isolated from the digested organs as described above.
Growth of C. neoformans in organ homogenates.
To establish whether mouse organs contain substrate for C. neoformans melanization, the brains and lungs from five uninfected BALB/c mice (6 to 8 weeks old) (National Cancer Institute) were placed in 5 ml of minimal medium containing 0.2% biphenyl (Eastman Kodak Co., Rochester, N.Y.), 0.050% vancomycin (Fugisawa USA Inc., Deerfield, Ill.), and 0.05% imipenem-cilastin (Merck and Co., West Point, Pa.); homogenized by mechanical grinding; mixed with equal volume of 3% Bacto Agar, and poured onto petri dishes (Becton Dickinson). Plates containing medium alone with or without l-dopa served as controls. Mel+ C. neoformans 24067 cells were grown on the plates for 25 days; collected from the agar surface; treated with proteases, denaturant, and hot concentrated acid; and analyzed by scanning electron microscopy (SEM) as described above.
Statistical analyses.
Values for the melanin ELISAs are presented as the means ± standard deviations of 12 measurements from each experiment. P values were calculated by Student's t test using Primer of Statistics: The Program Version 3.0 (McGraw-Hill Inc., New York, N.Y.) for comparison of melanin binding of MAbs 6D2 and 11B11 to that of MAb 5C11. P values of less than 0.05 were considered significant.
RESULTS
Melanization of C. neoformans in vitro.
To confirm the Mel+ or Mel− phenotype of each of the C. neoformans strains used in this study, cells were grown in minimal medium agar plates with or without l-dopa for 7 days. All Mel+ strains (i.e., H99, JEC21, CN110, 24065, NIH 34, and 24067) grown in medium with l-dopa began to produce pigment after day 5 of incubation, and by day 7 the cells were heavily pigmented (by eye inspection), which is indicative of melanin production (data not shown). No melanization was observed with Mel+ strains grown without l-dopa or with Mel− strains (i.e., HMC4 and HMC6) grown with or without l-dopa.
Generation of MAbs to melanin.
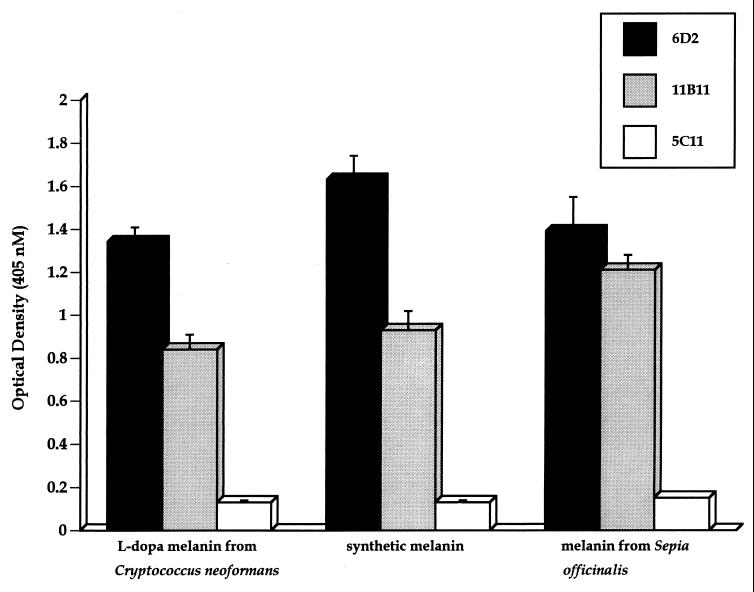
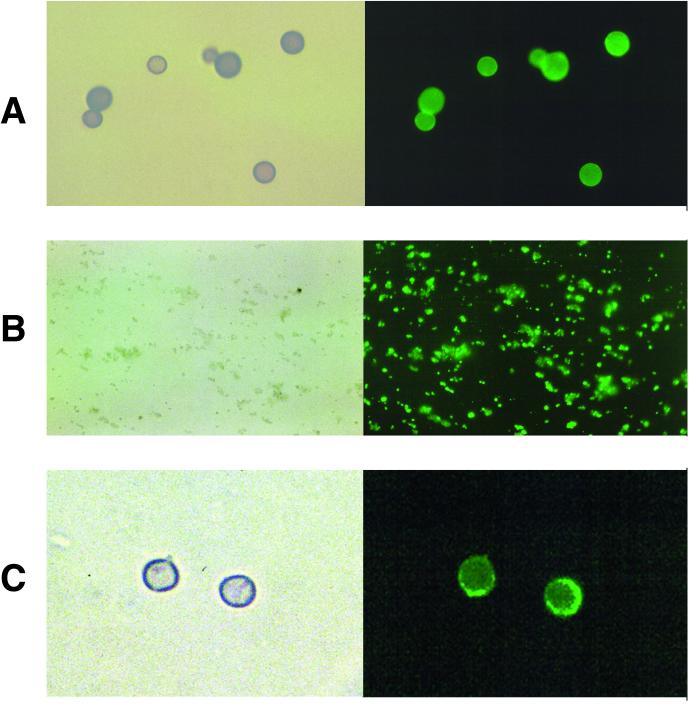
Immunization of BALB/c mice with melanin ghosts isolated from in vitro-melanized Mel+ C. neoformans 24067 cells induced high antibody responses to melanin, as described previously (30). Two hybridomas producing IgM MAbs (6D2 and 11B11) that bound to C. neoformans l-dopa melanin were isolated by screening hybridoma supernatants on melanin plates. These MAbs bound to l-dopa melanin ghosts, synthetic melanin, and melanin from S. officinalis by ELISA (Fig. 1). MAb 6D2 had the highest affinity for all three types of melanins tested. Minimal binding was observed with the negative control MAb 5C11 by ELISA. Immunofluorescence analyses confirmed binding of the MAbs to these melanins (data not shown for melanin from S. officinalis) and to in vitro-melanized C. neoformans cells (i.e., Mel+ cells grown with l-dopa) (Fig. 2). No binding was observed to nonmelanized C. neoformans cells (i.e., Mel+ cells grown without l-dopa or Mel− cells grown with or without l-dopa) or to cells incubated with the negative control MAb 5C11 (data not shown). Reduction of MAbs 6D2 and 11B11 to their monomeric forms by treatment with β-mercaptoethanol abrogated binding to l-dopa melanin ghosts (data not shown).
FIG. 1.
Reactivity of MAbs to natural and synthetic melanins by ELISA. The concentration of the MAbs was 10 μg/ml. Each value is the mean ± standard deviation of 12 measurements from one experiment. P values were calculated by Student's t test for comparison of melanin binding of MAbs 6D2 and 11B11 to that of negative control MAb 5C11. P values were less than 0.05 for all comparisons.
FIG. 2.
Light and fluorescent microscopy of l-dopa melanin ghosts from Mel+ C. neoformans 24067 cells (A), synthetic melanin (B), and 7-day-old in vitro-melanized Mel+ C. neoformans 24067 cells (C) incubated with 20 μg of MAb 11B11 per ml. Magnification, ×1,000.
Immunofluorescence and immunogold analyses of infected tissues.
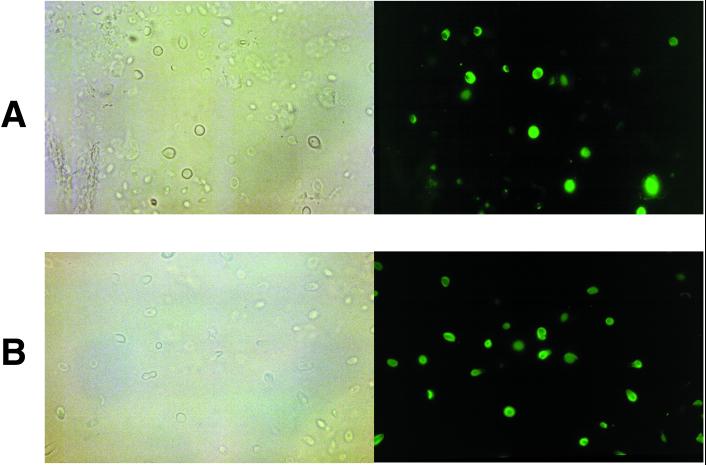
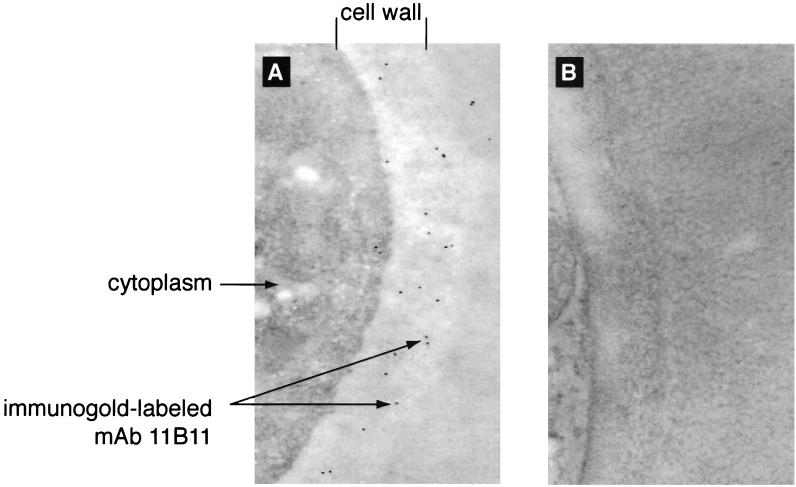
MAbs 6D2 and 11B11 were used to investigate whether C. neoformans produces melanin in the lungs and brains of infected rodents. Significant amounts of all Mel+ or Mel− C. neoformans cells were found in the lungs and brains of rodents at every time period of infection, as determined by CFU on Sabouraud dextrose agar. MAbs 6D2 and 11B11 bound to the cell wall of Mel+ C. neoformans cells in lung and brain tissues (Fig. 3) as early as 2 to 3 days after infection. No binding was observed to the Mel− C. neoformans cells or with the negative control MAb 5C11 (data not shown) at any time period of infection. Immunogold labeling of Mel+ C. neoformans 24067 in infected lung tissue also revealed binding of MAb 11B11 limited to the fungal cell wall (Fig. 4). No binding was observed with polyclonal IgM used as a negative control.
FIG. 3.
Light and fluorescent microscopy of Mel+ C. neoformans 24067-infected BALB/c mouse lung (A) and brain (B) tissues incubated with 20 μg of MAb 11B11 per ml at day 21 after infection. Not all fungal cells are stained by MAb 11B11. This may reflect heterogeneity in melanization in vivo and/or differences in the plane of magnification at which the photographs were taken. Magnification, ×1,000.
FIG. 4.
Immunogold labeling of Mel+ C. neoformans 24067 in infected C57BL/6 mouse lung tissue incubated with 5 μg of MAb 11B11 (A) or negative control MAb 5C11 (B) per ml at day 7 of infection. Magnification, ×60,000.
Recovery of melanin ghost-like particles from infected tissues.
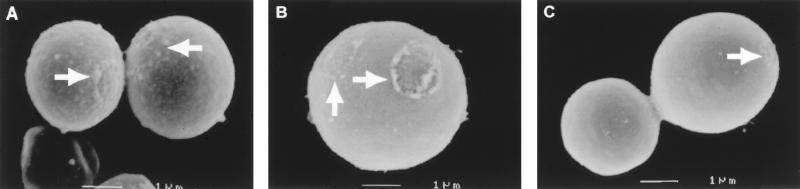
Protease, denaturant, and hot acid treatment of in vitro-melanized C. neoformans cells produces hollow particles that are structurally similar to cells and are therefore called melanin ghosts (45). SEM analyses of protease-, denaturant-, and acid-treated organs from Mel+ C. neoformans-infected rodents revealed the presence of structures that closely resembled melanin ghosts both in size and shape, in the lungs and brains (Fig. 5B and C). These melanin ghost-like particles were observed as early as 3 days after infection. The melanin ghost-like particles had average sizes of 4.3 ± 1.0 μm (n = 20) in the lungs and 2.6 ± 0.8 μm (n = 20) in the brains, which is consistent with the ratio in size difference of C. neoformans cells in these organs during infections in mice (35). SEM revealed surface structures reminiscent of the budding scars noted in melanin ghosts, consistent with their putative origin from fungal cells. No melanin ghost-like particles were recovered from organs infected with Mel− C. neoformans cells or from uninfected organs. As additional controls, in vitro-melanized and nonmelanized Mel+ C. neoformans 24067 cells were mixed with lungs and brains of uninfected BALB/c mice and then digested. Melanin ghosts were isolated only from mixtures of melanized cells and organs (Fig. 5A).
FIG. 5.
(A) SEM of l-dopa melanin ghosts isolated from in vitro-melanized Mel+ C. neoformans 24067 cells mixed with brain tissue from uninfected BALB/c mice. (B and C) Melanin ghost-like structures isolated from the lung (B) and brain (C) tissues of BALB/c mice at day 21 after infection with Mel+ C. neoformans 24067. Arrows depict budding scars. Magnifications, ×15,000 (A and B) and ×10,000 (C).
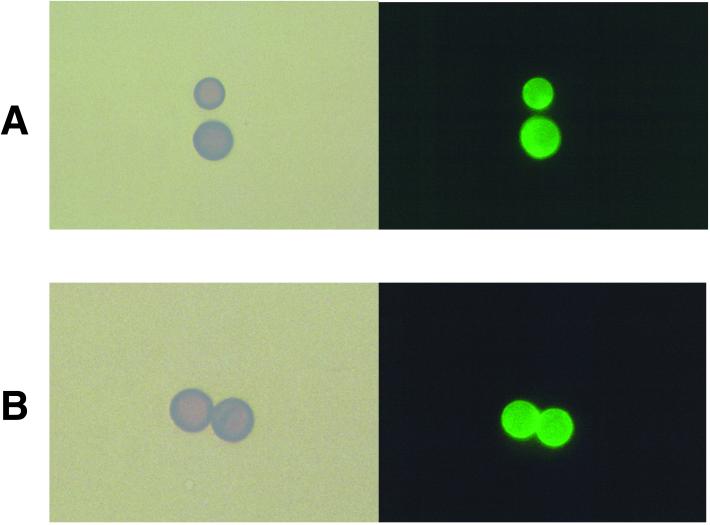
Immunofluorescence analyses revealed that the MAbs to melanin bound to the melanin ghost-like particles formed in vivo (Fig. 6). No binding was observed with the negative control MAb 5C11 (data not shown).
FIG. 6.
Light and fluorescent microscopy of melanin ghost-like particles isolated from Mel+ C. neoformans 24067-infected BALB/c mouse lung (A) and brain (B) tissues incubated with 20 μg of MAb 11B11 per ml at day 21 after infection. Magnification, ×1,000.
To investigate whether melanin synthesis by C. neoformans occurred only in response to an attack by the host's immune system, SCID (severely combined immunodeficient) mice and their parental BALB/c mice were infected with Mel+ C. neoformans 24067 for 7 days. Melanin ghost-like particles were recovered from the lungs and brains of infected BALB/c and SCID mice, indicating that these particles also formed in immunodeficient mice (data not shown).
Growth of C. neoformans in organ homogenates.
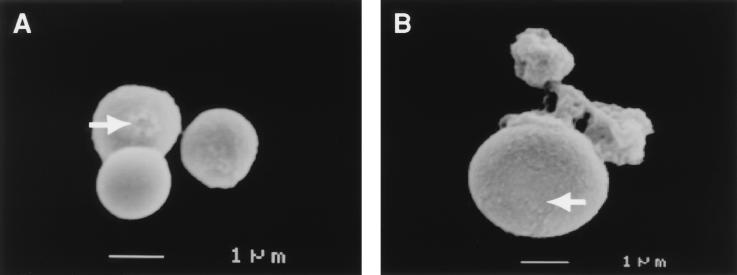
Mel+ C. neoformans 24067 cells grown for 25 days on lung and brain homogenate agar plates produced a light brown pigment, consistent with melanization. To establish whether the pigment was melanin, cells grown on organ homogenate agar were collected and digested with protease, denaturant, and hot concentrated acid. A dark residue was observed (by eye inspection), which consisted of particles similar to melanin ghosts (Fig. 7). No dark residue or melanin ghost-like particles were observed from cells grown on agar without organ homogenate.
FIG. 7.
SEM of melanin ghost-like particles isolated from Mel+ C. neoformans 24067 cells grown on lung (A) and brain (B) homogenate agar plates for 25 days. Arrows depict budding scars. Magnifications, ×10,000 (A) and ×11,000 (B).
Reproducibility of results.
ELISA, immunofluorescence, and immunogold studies were done at least twice. Melanin ghost-like particles were isolated from Mel+ C. neoformans-infected tissues as demonstrated by SEM in 20 experiments consisting of seven mouse studies and one rat study. The results were consistent and reproducible.
DISCUSSION
The production of polymerized melanin has been associated with virulence for several pathogenic fungi and bacteria. Both classical and genetic disruption studies have demonstrated that laccase activity is important for the virulence of C. neoformans. However, evidence of the synthesis of polymerized melanin in vivo has been more elusive. Recently, two studies have provided evidence of laccase activity during experimental C. neoformans infections. Nosanchuk et al. used melanin-binding peptides and serological analyses to demonstrate production of melanin-like compounds during Mel+ C. neoformans infections in mice (31). Liu et al. demonstrated laccase-derived products in Mel+ C. neoformans cells recovered from infected mice but did not observe polymerized melanin (26). In this study we investigated whether C. neoformans synthesizes polymerized melanin during infection, and we used protease-, denaturant-, and acid-resistant melanin ghost particles as our reference for polymerized melanin.
To study melanization in vivo, we generated MAbs to melanin suitable for immunohistochemical studies. A previous study had demonstrated that fungal melanin is immunogenic in mice (30). Two hybridomas producing IgM MAbs (6D2 and 11B11) to melanin were generated by fusing splenocytes harvested from mice immunized with l-dopa melanin ghosts to myeloma cells. Two other groups have reported MAbs to phaeomelanin (24) and melanin precursors (18), but to our knowledge these are the first MAbs generated in response to polymerized fungal melanin. Our MAbs bind to C. neoformans l-dopa melanin ghosts, synthetic melanin, and melanin from S. officinalis, indicating that these melanin types share antigenic structures. Immunofluorescence analyses demonstrated that the MAbs bound to Mel+, but not to Mel−, C. neoformans cells in rodent tissues. Binding was observed as early as 2 to 3 days after infection. Immunogold electron microscopy demonstrated that MAb 11B11 bound almost exclusively to the fungal cell wall in infected tissue, where melanin is deposited in in vitro-melanized cells (44). Only the pentameric (unreduced) form of the MAbs bound to l-dopa melanin ghosts, indicating that avidity is important for binding to melanin. Since monomeric IgM does not bind to melanin, we conclude that MAbs 6D2 and 11B11 interact with melanin through more than one binding site. This implies that melanin epitopes are closely spaced in C. neoformans cells in tissue and is consistent with the presence of polymerized melanin.
To provide more direct evidence that polymerized melanin was made during infection, we attempted to isolate fungal melanin particles from infected rodent tissues. This experimental approach took advantage of the fact that melanin is resistant to the protease, denaturant, and hot concentrated acid treatment used to destroy rodent tissue while preserving the melanin particle. We successfully isolated dark particles with a strong resemblance to melanin ghosts from the lungs and brains of Mel+ C. neoformans-infected rodents. We conclude that these particles are the in vivo equivalent of C. neoformans melanin ghosts made in vitro on the basis of (i) recovery from Mel+, but not from Mel−, C. neoformans-infected tissues; (ii) resistance to protease, denaturant, and hot concentrated acid treatment; (iii) dark color; (iv) shape, dimensions, and surface scars similar to those of melanin ghosts isolated from in vitro-melanized fungal cells; and (v) reactivity with the MAbs to melanin.
Since C. neoformans melanin synthesis requires exogenous laccase substrate (reviewed in reference 46), the observation that fungal cells synthesize polymerized melanin in vivo implies that substrate is present in lung and brain tissue. To investigate whether these organs contained sufficient substrate to support melanization, we cultured a Mel+ C. neoformans strain on agar containing organ homogenate. Growth in this medium resulted in pigmentation, albeit not to the extent observed in medium with l-dopa. C. neoformans laccase has low selectivity for substrate and may generate pigment from structurally different compounds in tissue, such as catecholamines and other neurotransmitter precursors (reviewed in reference 46). Mouse and rat brains, for instance, have various precursors that could serve as substrate for melanization, including norepinephrine, dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, serotonin, and 5-hydroxyindolacetic acid, whose concentrations are 750, 1,530, 42, 308, 820, and 117 ng/g of brain, respectively (2, 8, 10, 11, 28, 37, 39). Furthermore, pigmented cells obtained from the organ homogenates yielded melanin ghost-like particles upon digestion. These results indicate that organ homogenates contain sufficient precursors to support melanization of C. neoformans cells.
We conclude that C. neoformans synthesizes polymerized melanin during infection on the basis of the following lines of evidence: (i) melanin-like compounds were detected on the cell wall of Mel+ C. neoformans cells by using both melanin-binding peptides (31) and MAbs; (ii) melanin ghost-like particles can be isolated from rodent tissues infected with Mel+ C. neoformans strains but not from tissues infected with Mel− C. neoformans strains or from uninfected tissues; (iii) rodent lung and brain homogenates contain compounds that support melanization in vitro; (iv) Mel+ C. neoformans infection elicits strong antibody responses to fungal melanin in mice (31); and (v) treatment with an inhibitor of melanin polymerization prolongs survival of lethally infected mice (J. D. Nosanchuk and A. Casadevall, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. F-49, p. 305, 1999). In addition, elegant biochemical studies by Liu et al. showed the presence of laccase-derived products in Mel+ C. neoformans cells recovered from infected mice, indicating that laccase is active in vivo (26). Those investigators, however, did not observe synthesis of polymerized melanin on gross examination of the recovered fungal cells or by absorbance after solubilization with Soluene 350. Although the experimental causes for the discrepancy between their results and ours are not fully understood, we note that the two groups used different techniques to study melanization. The discrepancy in the results appears to be quantitative, and both studies agree that laccase is active in vivo and almost certainly functions in virulence and pathogenesis. Part of the problem in this field is semantic and involves the definition of melanin, a compound that is insoluble and notoriously difficult to study (6; reviewed in reference 46). It remains to be established whether the melanin made in vivo is similar to l-dopa melanin made in vitro. Since tissue may contain multiple substrates for melanization, it is possible (and likely) that in vivo polymers are different from l-dopa melanin. Hence, the potential chemical and structural differences between in vitro and in vivo melanin introduce yet another variable that must be considered in future studies. The demonstration that C. neoformans synthesizes polymerized melanin in vivo is important for our understanding of pathogenesis because it suggests that the observations made with melanized cells in vitro regarding protection against host defense mechanisms and iron metabolism (15) may be applicable to cells in tissue. In addition to synthesizing melanin, laccase may function in virulence by catalyzing the formation of melanin precursors that could be toxic to the host (26) or by protecting C. neoformans from antifungal activity of alveolar macrophages (25).
Melanin synthesis in C. neoformans is regulated by the G-protein α subunit GPA1 and cyclic AMP (1). The finding that C. neoformans can synthesize polymerized melanin in vivo suggests a need for studies to define how melanin synthesis is regulated during infection and how this enigmatic pigment affects the host response to infection. Furthermore, the results imply that melanin could serve as a target for antifungal drug research.
ACKNOWLEDGMENTS
Arturo Casadevall is supported by NIH grants AI33774, AI13342, and HL59842 and is the recipient of a Burroughs Wellcome Fund Scholar Award in Experimental Therapeutics. Ángel L. Rosas is supported by NIH grant 5T32GM07491. Joshua D. Nosanchuk is supported by NIH grant AI01489 and a grant from the Infectious Diseases Society of America. Marta Feldmesser is supported by NIH grant AI01341. Gary M. Cox and Henry C. McDade are supported by Public Health Services grants AI044975-01 and AI01334 from the National Institute of Allergy and Infectious Diseases.
We are very grateful to David Goldman for his assistance with the rat experiments, and to Jorge Bermúdez for his outstanding preparations of tissue sections.
Ángel L. Rosas and Joshua D. Nosanchuk contributed equally to this work.
REFERENCES
- 1.Alspaugh J A, Perfect J R, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang J-Y, Martin P, Bernasconi R, Braun D G. High-sensitivity amino acid analysis: measurement of amino acid neurotransmitter in mouse brain. FEBS Lett. 1981;132:117–120. doi: 10.1016/0014-5793(81)80441-3. [DOI] [PubMed] [Google Scholar]
- 3.Cox G M, Toffaletti D L, Perfect J R. Dominant selection system for use in Cryptococcus neoformans. J Med Vet Mycol. 1996;34:385–391. [PubMed] [Google Scholar]
- 4.Doering T L, Nosanchuk J D, Roberts W K, Casadevall A. Melanin as a potential cryptococcal defense against microbicidal proteins. Med Mycol. 1999;37:175–181. [PubMed] [Google Scholar]
- 5.Emery H S, Shelburne C P, Bowman J P, Fallon P G, Schulz C A, Jacobson E S. Genetic study of oxygen resistance and melanization in Cryptococcus neoformans. Infect Immun. 1994;62:5694–5697. doi: 10.1128/iai.62.12.5694-5697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enochs W S, Ningles M J, Swartz H M. A standardized test for the identification and characterization of melanins using electron paramagnetic (EPR) spectroscopy. Pigment Cell Res. 1993;6:91–99. doi: 10.1111/j.1600-0749.1993.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 7.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucoronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 8.Freeman G B, Gibson G E. Selective alteration of mouse brain neurotransmitter release with age. Neurobiol Aging. 1987;8:147–152. doi: 10.1016/0197-4580(87)90024-8. [DOI] [PubMed] [Google Scholar]
- 9.Glatman-Freedman A, Martin J M, Rifka P F, Bloom B R, Casadevall A. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J Clin Microbiol. 1996;34:2795–2802. doi: 10.1128/jcm.34.11.2795-2802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glowinski J, Iversen L L. Regional studies of catecholamines in the rat brain. Neurochemistry. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 11.Haubrich D R, Denzer J S. Simultaneous extraction and fluorometric measurement of brain serotonin, catecholamines, 5-hydroxyindoleacetic acid and homovanillic acid. Anal Biochem. 1973;55:306–312. doi: 10.1016/0003-2697(73)90320-5. [DOI] [PubMed] [Google Scholar]
- 12.Hopfer R L, Groschel D. Six-hour pigmentation test for the identification of Cryptococcus neoformans. J Clin Microbiol. 1975;2:96–98. [PMC free article] [PubMed] [Google Scholar]
- 13.Huffnagle G B, Chen G H, Curtis J L, McDonald R A, Strieter R M, Toews G B. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–3616. [PubMed] [Google Scholar]
- 14.Jacobson E S, Emery H S. Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans. J Bacteriol. 1991;173:401–403. doi: 10.1128/jb.173.1.401-403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson E S, Hong J D. Redox buffering by melanin and Fe(II) in Cryptococcus neoformans. J Bacteriol. 1997;179:5340–5346. doi: 10.1128/jb.179.17.5340-5346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson E S, Jenkins N D, Todd J M. Relationship between superoxide dismutase and melanin in a pathogenic fungus. Infect Immun. 1994;62:4085–4086. doi: 10.1128/iai.62.9.4085-4086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson E S, Tinnell S B. Antioxidant function of fungal melanin. J Bacteriol. 1993;175:7102–7104. doi: 10.1128/jb.175.21.7102-7104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kammeyer A, Oomen L A, Pavel S. Preparation of monoclonal mouse antibodies against two specific eu-melanin related compounds. J Immunol Methods. 1992;156:61–67. doi: 10.1016/0022-1759(92)90011-h. [DOI] [PubMed] [Google Scholar]
- 19.Kwon-Chung K J, Polacheck I, Popkin T J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence in mice. J Bacteriol. 1982;150:1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon-Chung K J, Rhodes J C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986;51:218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon-Chung K J, Hill W B, Bennett J E. New, special stain for histopathological diagnosis of cryptococcosis. J Clin Microbiol. 1981;13:383–387. doi: 10.1128/jcm.13.2.383-387.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson B S. Interaction between chemicals and melanin. Pigment Cell Res. 1993;6:127–133. doi: 10.1111/j.1600-0749.1993.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee S C, Dickson D W, Casadevall A. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenic implications. Hum Pathol. 1996;27:839–847. doi: 10.1016/s0046-8177(96)90459-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Jimbow K. Development and characterization of a murine monoclonal antibody against phaeomelanin and its precursor 5-s-cysteinyldopa. Melanoma Res. 1993;3:463–469. doi: 10.1097/00008390-199311000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Tewari R P, Williamson P R. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect Immun. 1999;67:6034–6039. doi: 10.1128/iai.67.11.6034-6039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Wakamatsu K, Ito S, Williamson P. Catecholamine oxidative products, but not melanin, are produced by Cryptococcus neoformans during neuropathogenesis in mice. Infect Immun. 1999;67:108–112. doi: 10.1128/iai.67.1.108-112.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longuet-Higgins H C. On the origin of the free radical properties of melanins. Arch Biochem Biophys. 1960;86:231–232. doi: 10.1016/0003-9861(60)90410-0. [DOI] [PubMed] [Google Scholar]
- 28.Merritt J H, Medina M A, Frazer J W. Neurotransmitter content of mouse brain after inactivation by microwave heating. Res Commun Chem Pathol Pharmacol. 1975;10:751–754. [PubMed] [Google Scholar]
- 29.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosanchuk J D, Rosas A L, Casadevall A. The antibody response to fungal melanin in mice. J Immunol. 1998;160:6026–6031. [PubMed] [Google Scholar]
- 31.Nosanchuk J D, Valadon P, Feldmesser M, Casadevall A. Melanization of Cryptococcus neoformans in murine infection. Mol Cell Biol. 1999;19:745–750. doi: 10.1128/mcb.19.1.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polacheck I, Platt Y, Aronovitch J. Catecholamines and virulence of Cryptococcus neoformans. Infect Immun. 1990;58:2919–2922. doi: 10.1128/iai.58.9.2919-2922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porebska-Budny M, Sakina N L, Stepien K B, Dontsov A E, Wilczok T. Antioxidative activity of synthetic melanins. Cardiolipin liposome model. Biochim Biophys Acta. 1992;1116:11–16. doi: 10.1016/0304-4165(92)90121-a. [DOI] [PubMed] [Google Scholar]
- 34.Rhodes J C, Polacheck I, Kwon-Chung K J. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect Immun. 1982;36:1175–1184. doi: 10.1128/iai.36.3.1175-1184.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera J, Feldmesser M, Cammer M, Casadevall A. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect Immun. 1998;66:5027–5030. doi: 10.1128/iai.66.10.5027-5030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salas S D, Bennett J E, Kwon-Chung K J, Perfect J R, Williamson P R. Effects of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid G, Bahner U, Peschkes J, Heidland A. Neurotransmitter and monoaminergic amino acid precursor levels in rat brain: effects of chronic renal failure and of malnutrition. Miner Electrolyte Metab. 1996;22:115–118. [PubMed] [Google Scholar]
- 38.Scott D W, Gershon R K. Determination of the total and mercaptoethanol-resistant antibody in the same serum sample. Clin Exp Immunol. 1970;6:313–316. [PMC free article] [PubMed] [Google Scholar]
- 39.Siddiqui A, Clark J S, Gilmore D P. Post-mortem changes of neurotransmitter concentrations in the rat brain regions. Acta Physiol Hung. 1990;75:179–185. [PubMed] [Google Scholar]
- 40.Toffaletti D L, Rude T H, Johnston S A, Durack D T, Perfect J R. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol. 1993;175:1405–1411. doi: 10.1128/jb.175.5.1405-1411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun. 1994;62:3004–3007. doi: 10.1128/iai.62.7.3004-3007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Casadevall A. Growth of Cryptococcus neoformans in presence of l-dopa decreases its susceptibility to amphotericin B. Antimicrob Agents Chemother. 1994;38:2648–2650. doi: 10.1128/aac.38.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to the melanin-binding compounds trifluoperazine and chloroquine. Antimicrob Agents Chemother. 1996;40:541–545. doi: 10.1128/aac.40.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun. 1995;63:3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Aisen P, Casadevall A. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect Immun. 1996;64:2420–2424. doi: 10.1128/iai.64.7.2420-2424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wheeler M, Bell A A. Melanins and their importance in pathogenic fungi. Curr Top Med Mycol. 1988;2:338–387. doi: 10.1007/978-1-4612-3730-3_10. [DOI] [PubMed] [Google Scholar]
- 47.Williamson P R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol. 1994;176:656–664. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]