Abstract
Enteric gram-negative bacilli cause a severe, often life-threatening pneumonia. An improved understanding of the pathogenesis of this infection may lead to improved treatment. Nearly all of the responsible gram-negative bacilli possess capsular polysaccharides and/or an O-specific antigen as part of their lipopolysaccharide (LPS). We hypothesized that these surface polysaccharides may modulate the pulmonary host response. To investigate this, a rat pneumonitis model was used, and pulmonary neutrophil influx, a critical aspect of host defense, was measured. To assess for the effect of the capsule and O-specific antigen on this host response, three proven, isogenic derivatives that are deficient in capsular polysaccharide alone (CP9.137), the O-specific antigen moiety of the LPS alone (CP921), and both the capsular polysaccharide and O-specific antigen (CP923), as well as their wild-type parent (CP9), were used as challenge strains at various intratracheal challenge inocula (CI). Total lung myeloperoxidase (MPO), a surrogate marker for neutrophils, was measured for 15 h post-bacterial challenge. To determine the effect of capsule and the O-specific antigen on the measured MPO levels, a mathematical model was developed and used to describe the MPO levels as a function of time for each CI of each of the four strains. The results from this analysis demonstrated that in the absence of the K54 capsule, 80.7 times the CI is necessary to achieve the same maximum MPO level relative to K54 positive strains (P < 0.0001). In contrast, a diametric effect was observed in the absence of the O-specific antigen, where 0.13 times the CI was necessary to achieve the same maximum MPO level relative to O4-positive strains (P = 0.0032). No interactive effect was observed between the capsule and the O-specific antigen. These findings demonstrate that these surface polysaccharides modulate pulmonary neutrophil influx and suggest that the K54 capsular polysaccharide is a proinflammatory mediator and that the O4-specific antigen attenuates the proinflammatory response. If these speculations are substantiated, an understanding of how the capsule and the O-specific antigen modulate host response could have significant therapeutic implications. The potential use of biologic modulators directed against the host response, as well as approaches based on inactivating bacterial components (e.g., surface polysaccharides) in attempts to modify sepsis syndromes, could be developed.
Enteric gram-negative bacilli are a group of pathogens that are capable of causing severe, life-threatening pneumonia (8, 24, 26). Despite the availability of active antimicrobial agents, there has been little improvement in outcome from this infection over the last 10 to 15 years. As a result, this syndrome continues to cause significant morbidity and mortality and strongly contributes to the economic burden of our national health care system. An improved understanding of the pathogenesis of this infection may result in improved treatment or prevention of infection.
The importance of neutrophils in protecting against infection in the lung is as great as any other site in the body (23). The increased susceptibility and severity of infection that occurs in the setting of chemotherapy-induced neutropenia or genetically inherited disorders of neutrophil function (e.g., chronic granulomatous disease) are unequivocal proof. We are now capable of modulating the host response to bacterial infection, including both neutrophil numbers and their state of activation. However, as is true for many biologic response systems, an appropriately balanced response, which in this case is maximal bacterial clearance while minimizing pulmonary damage, is needed for optimal outcome (4, 5). Prior to considering manipulating the host biologic response as a treatment modality in gram-negative pneumonitis, it is critical to understand the mechanism by which the host responds to bacterial challenge and how certain bacterial components modulate this response. Although our understanding of the pulmonary inflammatory response, which leads to the influx of neutrophils, into the lung has significantly progressed (13, 23, 25), other than studies on lipid A there is little information available on other gram-negative factors that may affect this crucial host response. This information is needed for the logical development of rapid diagnostic tests, which in turn will enable the clinician to effectively utilize a variety of novel therapeutics, including immune modulators.
We hypothesized that bacterial surface polysaccharides may modulate the host inflammatory response in gram-negative pneumonitis. To test this hypothesis, we utilized a rat model of acute pulmonary infection and measured pulmonary neutrophil influx over the course of infection. An extraintestinal human isolate of Escherichia coli (CP9, O4/K54/H5) was used as a model pathogen, in part because E. coli is one of the commonly isolated agents in nosocomial gram-negative pneumonia (2) but more importantly because three proven isogenic derivatives have been generated that are deficient in capsular polysaccharide alone (CP9.137), the O-specific antigen moiety of the lipopolysaccharide alone (CP921), and both the capsular polysaccharide and the O-specific antigen (CP923). Challenge with these strains and subsequent measurement of myeloperoxidase (MPO), a surrogate marker for neutrophils, and bacterial growth enabled us to evaluate the ability of these defined bacterial traits to modulate neutrophil influx.
MATERIALS AND METHODS
Bacterial strains.
A human bacteremic isolate of E. coli (CP9, O4/K54/H5) and isogenic derivatives have been used as model pathogens for these studies (19, 21). CP9 is well characterized and is virulent in a variety of in vivo infection models (18, 20). It possesses a group 3 capsule (K54), O4-specific antigen, alpha-hemolysin, cytotoxic necrotizing factor, P pilus (class I PapG adhesin), Prs pilus (class III PapG adhesin), and type 1 pilus and is complement resistant. Transposon mutagenesis and transduction was used to generate proven isogenic derivatives of CP9 deficient in the K54 antigen alone (CP9.137), the O4 antigen alone (CP921), or both the K54 and O4 antigens (CP923) (19, 21). Previous studies established that the LPS in O4 antigen deficient mutants consists of intact lipid A and core polysaccharide moieties plus either a single or a portion of a single O4 antigen pentasaccharide (21).
Pulmonary infection model.
An established rat (Long-Evans) model for studying pulmonary damage was used and modified for these studies (9–11, 14). Long-Evans rats (250 to 300 g) were anesthetized with 5% halothane in 100% oxygen until unconscious and then maintained at 2% halothane. The animals were suspended from the front teeth via a suture on a 60°C incline board. The trachea was exposed surgically, and a 4-in. piece of 1-0 silk was slipped under the trachea using a curved hemostat. The challenge inoculum (1.2 ml/kg) was introduced intratracheally via a 1-ml syringe and 26-gauge needle, and the incision was closed with surgical staples. Experimental animals were challenged with various challenge inocula (CI; 1.3 × 106 to 3.0 × 108 CFU) of CP9 (wild type [wt]), CP9.137 (capsule deficient), CP921 (O-specific antigen deficient), and CP923 (capsule and O-specific antigen deficient) to establish the consequences of infection for each of these strains. Control animals were anesthetized identically to experimental animals but did not receive a bacterial challenge. Animals that died within 15 min after bacterial challenge were excluded from the analysis since these deaths were attributed to the challenge procedure and not to the infecting bacteria themselves. Each experimental animal was prospectively assigned a time for subsequent sampling. At harvest the animal was anesthetized as described above, and betadine was used to sterilize its abdomen and chest. The descending aorta and vena cava were exposed, and appropriate samples were removed (e.g., blood gas, blood culture, blood for serum, etc.). For removal of the lungs, the thoracic cavity was exposed, and 10 ml of 1× phosphate-buffered saline (PBS; pH 7.4) was injected into the right ventricle to “flush” the lung vasculature. The trachea, lungs, and heart were removed en bloc, and the lungs were subsequently dissected free and weighed. If appropriate, sterile saline was added to yield a total weight of 10 g. The lung tissue was then homogenized (Polytron PT200; Brinkmann Instruments, Westbury, N.Y.) for 3 s three times on ice (setting 6). Pilot experiments have demonstrated that homogenization resulted a 100% yield of the bacterial challenge inoculum.
(i) Assessment of neutrophil influx.
Total lung MPO activity was measured at 1, 3, 6, 9, 12, and 15 h postchallenge for a given strain and CI. Groups of three animals were preassigned to each of the six harvest times; therefore, in each experiment the MPO activity was determined in 18 animals. CP9 (wt), CP9.137 (capsule deficient), CP921 (O-specific-antigen deficient), and CP923 (capsule and O-specific-antigen deficient) were each evaluated at several different CI as follows: for CP9 (four experiments), 1.3 × 106, 5.6 × 106, 1.2 × 107, and 5.3 × 107; for CP9.137 (three experiments), 1.2 × 107, 7.7 × 107, and 2.3 × 108; for CP921 (five experiments), 3.0 × 106, 9.6 × 106, 3.4 × 107, 1.3 × 108, and 2.1 × 108; and for CP923 (three experiments), 1.3 × 107, 6.7 × 107, and 3.0 × 108. The lung MPO levels provide a precise means of measuring organ content of recruited neutrophils. Total lung MPO activity was quantified by the method of Goldblum et al. (7). Lung homogenates were assayed for MPO activity using a spectrophotometer reading with 0-dianisidine hydrochloride (Sigma) at 460 nm. Units were calculated based on the rate of increase over time.
(ii) Determination of bacterial titers.
Dissected total lung tissue was weighed, and sterile normal saline was added to yield a total weight of 10 g. The lung tissue was homogenized as described above, and serial 10-fold dilutions were performed in 1× PBS for determination of the CFU per milliliter. These titers were multiplied by 10 to determine total lung titers.
(iii) Analysis of the effect of capsular polysaccharide and O-specific antigen on MPO.
To quantify the effects of capsular polysaccharide and O-specific antigen on MPO activity, the effects were modeled using functions presented in the Appendix (see below). As a first approximation, the effects of CP9.137, CP921, and CP923 were modeled as if each were a dilution or concentration of CP9. For each strain, the model for maximum MPO activity was a linear function of the common logarithm of the challenge inoculum. The function used to describe the increase in MPO activity before the maximum is reached was modeled using a logistic function of the logarithm of time. The model for MPO activity was fitted using nonlinear regression by using SPSS for Windows. The effects of deleting the capsule and deleting the O-specific antigen and the synergy between these two effects were estimated by reparameterizing the model. These effects were tested for statistical significance by dividing each estimate by its standard error and comparing the resulting Z score to a standard normal distribution.
RESULTS
Establishing relevance of the rat pneumonia model.
The pertinent features of gram-negative pneumonia include bacterial growth, pulmonary damage, and an ensuing inflammatory response. To establish whether these characteristics pertained to our rat pneumonitis model, experimental animals were challenged with various CI (1.3 × 106 to 5.3 × 107 CFU) of CP9 (wt). Groups of three animals were preassigned to each of the six harvest times (1, 3, 6, 9, 12, and 15 h), and the consequences of infection were measured.
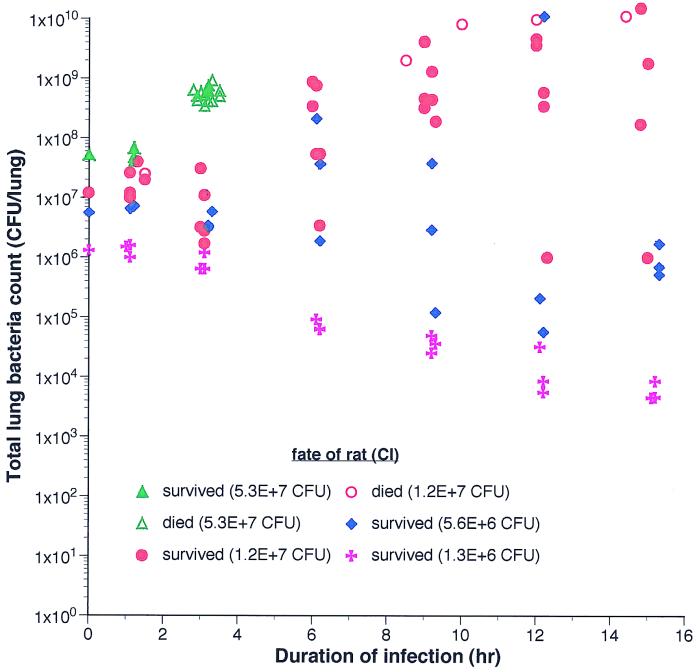
At a CI of 5.3 × 107 CFU of CP9, all 18 animals were either premoribund or dead by 3 h (Fig. 1). A CI of 1.2 × 107 CFU (two experiments, 35 animals) resulted in proliferation at ≥6 h in 88% (21 of 24) of the animals. Although in some animals there was a transient decrease in bacterial titer at 3 h, the subsequent growth trend was upward (Fig. 1). Blood cultures were positive in only one animal (1 of 35, 2.8%). In two additional experiments (CI of approximately 1.0 × 107 CFU) with death as the endpoint (18 animals), the mean bacterial titer of CP9 at death was 1.1 × 1010 CFU, and deaths occurred over the timeframe of ≥8.5 to 24 h in >90% of the animals that died. At a CI of 5.6 × 106 CFU (one experiment, 17 animals) clearance was observed in 13 of 17 animals, and at a CI of 1.3 × 106 CFU (one experiment, 18 animals) clearance occurred in all 18 animals (Fig. 1). Therefore, a CI of approximately 1.0 × 107 CFU was most appropriate for a relevant bacterial pneumonia model. A higher CI resulted in fulminant death, and a lower CI generally resulted in bacterial clearance.
FIG. 1.
Growth of CP9 in vivo at various CI in the rat pneumonitis model. Intratracheal challenge was performed as described in Materials and Methods. Each color represents animals given the same indicated CI. Each point represents the bacterial titer from a single animal that was harvested at a prospectively assigned time (closed symbols) or was harvested immediately at the time of death (open symbols).
After challenge with 1.2 × 107 CFU of CP9, a significant increase in pulmonary damage occurred over time as manifested by increases in lung weight, bronchoalveolar lavage protein content, the endothelial permeability index, and a decrease in oxygenation compared to the controls (Table 1). A significant inflammatory response, as measured by neutrophil influx, also occurred (Table 1). These parameters demonstrated that this model represented a progressive bacterial proliferation and pulmonary damage with subsequent death due to respiratory failure, a result similar to untreated gram-negative pneumonitis in humans.
TABLE 1.
Measures of pulmonary damage after challenge with CP9 (1.0 × 107 CFU)
| Group | Mean lung wt (g)a | Permeability indexb | PO2/FIO2 (mm Hg)c | BAL protein (mg/ml) | Neutrophils in BALd (mean no./ml) |
|---|---|---|---|---|---|
| Control | 1.3 ± 0.03 | 0.31 ± 0.05 | 400 ± 48 | 5.2 ± 1.1 | 0 |
| CP9 (wt) | 5.3 ± 0.10 | 10.1 ± 2.0 | 84 ± 6.9 | 74.7 ± 18.4 | (3.2 ± 0.9) × 107 |
Mean weight in grams ± the standard error in animals that died.
Measured at 6 h post-CI administration.
FIO2 = 0.8.
Mean number measured at 6 h post-CI administration. BAL, bronchoalveolar lavage fluid.
Initial neutrophil influx is dependent on the bacterial CI.
The measurement of pulmonary MPO was used as a surrogate marker for the influx of neutrophils into the lung. Baseline MPO levels (0 h) were consistently low (34.0 ± 4.6). After bacterial challenge, MPO levels increased, reaching a plateau level 6 to 8 h postchallenge. Maximal MPO activity increased approximately 10- to 25-fold, depending on the challenge strain and the CI utilized.
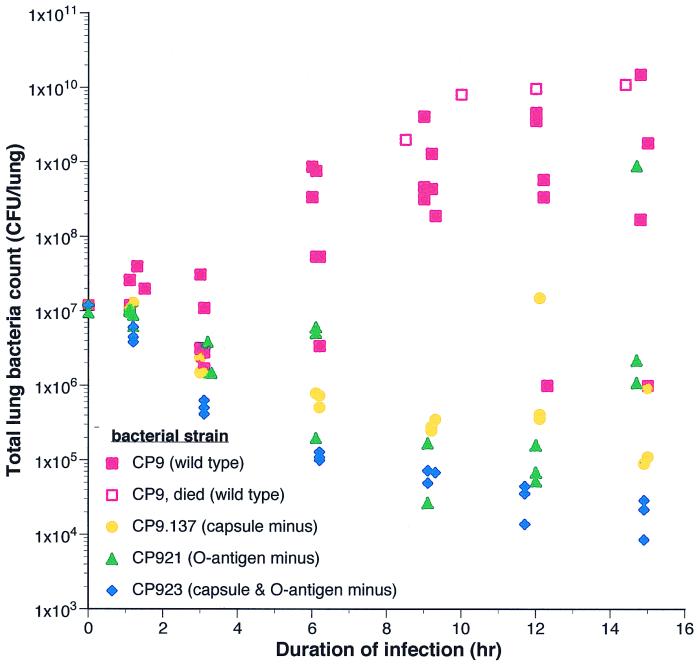
Bacterial growth or clearance varied depending on the challenge strain (Fig. 2) and the CI. Therefore, it was critical to establish whether MPO activity (over the 15-h timeframe of these experiments) was dependent on the initial CI or the bacterial load over time (the total number of bacteria over the course of the experiment which is dependent on the degree of subsequent growth or clearance of the bacteria). The latter measurement is conceptually equivalent to the area under the curve. Analysis of MPO activity from CI in which both clearance and growth occurred for a given strain lent significant insight into this matter. This occurred for CP9 at CI of 5.6 × 106 and 1.2 × 107 (Fig. 1) and for CP9.137 at a CI of 7.7 × 107. Presumably, these CI were on the threshold at which the resident host defenses were able to contain the infecting bacterium. Comparison of samples in which bacterial clearance was achieved versus those in which the challenge strain was able to proliferate demonstrated no difference in MPO activity (Table 2). Therefore, this analysis demonstrated that MPO activity, at least for the 15-h time course of these experiments, depended primarily on the initial CI.
FIG. 2.
Growth of CP9, CP9.137 (capsule deficient), CP921 (O-specific antigen deficient), and CP923 (capsule and O-specific antigen deficient) in vivo in the rat pneumonitis model. Each color represents animals challenged with approximately 1.0 × 107 CFU a given bacterial strain. Each point represents the bacterial titer from a single animal that was harvested at a prospectively assigned time (closed symbols) or was harvested immediately at the time of death (open symbols).
TABLE 2.
MPO activity is dependent on initial CI and not on bacterial load over time
| Strain | CI (CFU/lung) | Avg titer with clearance (CFU/lung) | Avg titer with growth (CFU/lung) | MPO activity with bacterial clearance (U/lung) | MPO activity with bacterial growth (U/lung) |
|---|---|---|---|---|---|
| CP9 (wt) | 5.6 × 106 | 2.8 × 105 | 2.8 × 109 | 559 ± 76 | 582 ± 60 |
| CP9 | 1.2 × 107 | 1.8 × 106 | 5.9 × 108 | 622 ± 69 | 644 ± 38 |
| CP9.137 | 7.7 × 107 | 2.9 × 107 | 3.1 × 108 | 454 ± 24 | 493 ± 20 |
Assessing the effect of the K54 capsular polysaccharide and the O4-specific antigen on neutrophil influx.
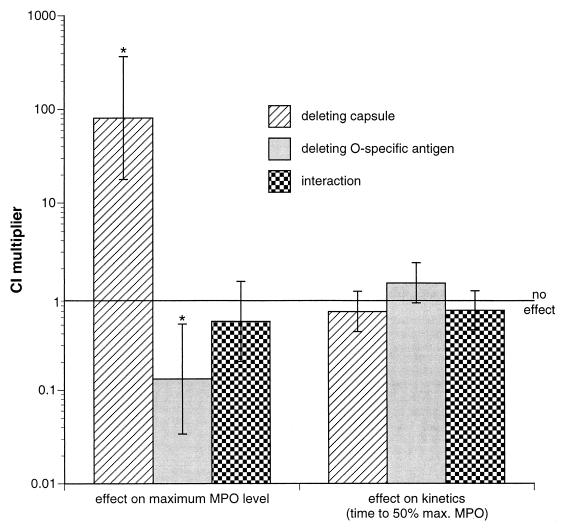
MPO levels were measured and analyzed after challenge with CP9 in four experiments, CP9.137 in three experiments, CP921 in five experiments, and CP923 in three experiments. A modeling function was developed to describe the MPO data, expressing the lung MPO level as a function of basal lung MPO level (Y0), duration of E. coli challenge (t), and the log of the challenge inoculum (X). Of the parameters utilized to define this function (b, c, Rn, Qn; see Appendix) only Rn, which describes the strain-specific effect on maximal lung MPO, was found to be different between strains CP9, CP9.137, CP921, and CP923. Since these strains differ solely in whether they produce capsule, O-specific antigen, or both it was decided to further analyze these data in terms of the effect of capsule and/or the O-specific antigen on the maximal lung MPO. The usefulness of this approach was that (i) analysis was more powerful since data obtained for all of the strains were utilized and (ii) interactions (synergism, antagonism) between capsule and O-specific antigen could be identified. These advantages would be lost by utilizing a strain-to-strain comparison. To perform the analysis in this manner, Rn and Qn were reparameterized to rn and qn and then reanalyzed (see Appendix). Statistical tests were performed to formally test (i) the effect of capsule and the O-specific antigen on the kinetics of increase of MPO production, (ii) the effect of capsule and the O-specific antigen on the maximal MPO levels, and (iii) whether an interaction between capsule and O-specific antigen occurs. A summary of these data is presented in Table 3. These tests compared (i) a grouping of strains that were deficient in the capsule to the grouping of strains that contained the capsule (i.e., a comparison of CP9.137 and CP923 to CP9 and CP921), (ii) a grouping of strains that were deficient in the O-specific antigen to the grouping of strains that contained the O-specific antigen (i.e., a comparison of CP921 and CP923 to CP9 and CP9.137), and (iii) a grouping of strains that are deficient in either the capsule or the O-specific antigen to the grouping of strains that were deficient in neither or both (i.e., a comparison of CP9.137 and CP921 to CP9 and CP923). The results in Table 3 indicate that there was no statistically significant effect on the kinetics of increase in MPO activity in the presence or absence of capsule. However, the K54 capsule did exert a statistically significant effect on the maximal MPO level. In the absence of the K54 capsule, 80.7 times (101.9071 = 80.7) the challenge inoculum is necessary to achieve the same maximum MPO level, relative to a K54-positive strains (P < 0.0001). The results in Table 3 also indicate that there was no statistically significant effect on the kinetics of increase in MPO activity in the presence or absence of O-specific antigen. However, the O4-specific antigen did exert a statistically significant effect on the maximal MPO level. In the absence of the O-specific antigen, 0.13 times (10−0.8787 = 0.13) the challenge inoculum was necessary to achieve the same maximum MPO level, relative to a O4-positive strains (P = 0.0032). The absence of the O-specific antigen had an opposite effect on neutrophil influx as the loss of the capsule. No synergy was observed between effects of the capsule and the O-specific antigen. These findings are graphically depicted in Fig. 3.
TABLE 3.
Capsule and O-specific antigen significantly alter maximum MPO levels but do not significantly change time to 50% of maximum MPO increases
| Effecta | Estimate | SE | Z score | Pb |
|---|---|---|---|---|
| Effect on maximum MPO level | ||||
| Deleting capsule | 1.9071 | 0.3333 | 5.722 | <0.0001 |
| Deleting O-specific antigen | −0.8787 | 0.2978 | −2.951 | 0.0032 |
| Synergy | −0.2655 | 0.2172 | −1.222 | 0.9028 |
| Effect on time to 50% of increase | ||||
| Deleting capsule | −0.1593 | 0.1095 | −1.455 | 0.1445 |
| Deleting O-specific antigen | 0.1456 | 0.1095 | 1.330 | 0.1834 |
| Synergy | −0.1459 | 0.1057 | −1.381 | 0.1672 |
Estimates are expressed as the common logarithm of amount by which the challenge inoculum must be multiplied to produce the same result when the capsule or O-specific antigen is deleted.
The effects of deleting the capsule, deleting the side chain, and the synergy were tested for statistical significance by dividing each estimate by its standard error and comparing the resulting Z score to a standard normal distribution. P < 0.05 was considered to be statistically significant.
FIG. 3.
The effect of deleting the K54 capsular polysaccharide or the O4-specific antigen on pulmonary neutrophil influx (determined by both maximum MPO level and the time to 50% maximum MPO level) in the rat pneumonitis model. The CI multiplier is the factor by which the challenge inoculum must be multiplied to achieve the same response that occurred in the presence of the K54 capsule or O4-specific antigen (e.g., in the absence of the K54 capsule, 80.7 times the challenge inoculum is necessary to achieve the same maximum MPO level relative to a K54 positive strains). A potential interaction between the K54 capsule and O4 specific antigen was also assessed. ∗, P < 0.005.
These results can also be expressed in terms of the CI of each strain which is required to produce a given maximum MPO level. For example, when comparing strains differing in only the presence or absence of capsule, the CI required to produce a maximum of 500 U of total lung MPO would be 1.34 × 106 CFU for CP9 and 1.15 × 108 CFU for CP9.137 (86 times as high relative to CP9), and the CI required would be 1.88 × 105 CFU for CP921 and 1.43 × 107 CFU for CP923 (76 times as high relative to CP921). Similarly, when comparing strains differing in only the presence or absence of O-specific antigen, the CI required to produce a maximum of 500 U of total lung MPO would be 1.34 × 106 CFU for CP9 and 1.88 × 105 CFU for CP921 (0.14 times as high relative to CP9), and the CI required would be 1.15 × 108 CFU for CP9.137 and 1.43 × 107 CFU for CP923 (0.12 times as high relative to CP9.137).
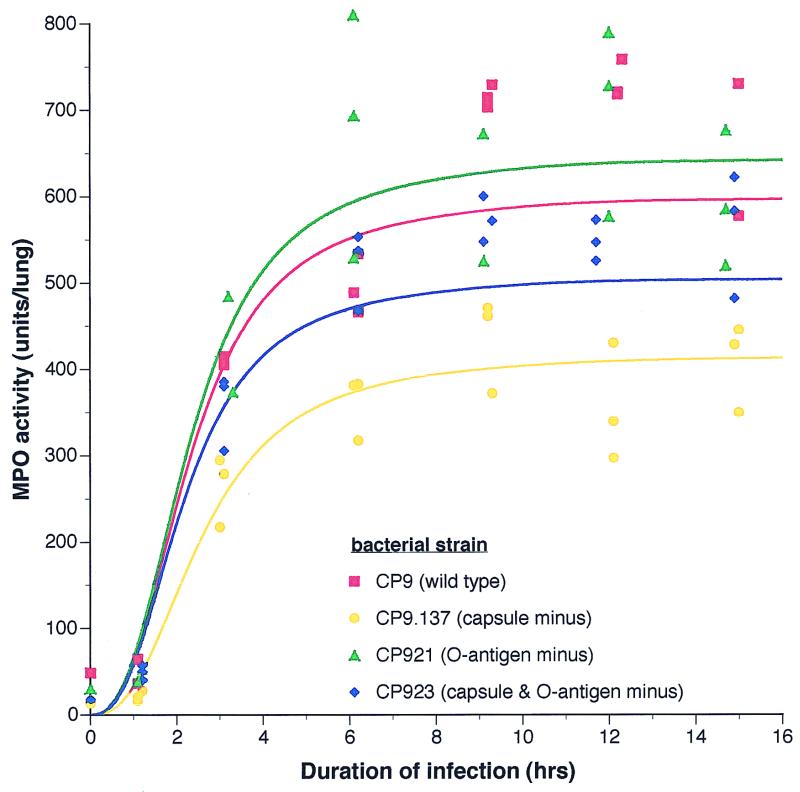
An illustration of this fitted model for the MPO activity of each strain at approximately equivalent CI (1.0 × 107 CFU) is shown in Fig. 4. Since there were no effects of capsule or the O-specific antigen on the kinetics of increase in MPO activity, this illustration uses a reduced model with those effects removed. A qualitative assessment of the effect of capsule can be appreciated from this graph. Diminished MPO levels were observed in the absence of the K54 capsule, and increased MPO levels were observed in the absence of the O-specific antigen.
FIG. 4.
An example of the fitted model for the MPO activity of CP9 (wt), CP9.137 (capsule deficient), CP921 (O-specific-antigen deficient), and CP923 (capsule and O-specific-antigen deficient) at an approximate CI of 1.0 × 107 CFU. The derivation of this model is described in detail in Materials and Methods and The Appendix.
DISCUSSION
The findings from this study demonstrate that the effect of the K54 capsule is an increase and the effect of the O4-specific antigen is a decrease in pulmonary neutrophil influx. These data strongly support the hypotheses that the K54 capsular polysaccharide is a proinflammatory mediator that stimulates the host defense response and that the O4-specific antigen attenuates the proinflammatory response and downregulates the host defense response. Confirmation of these hypotheses will require additional studies on host defense factors and studies of the mechanism by which capsule and O-specific antigen modulate pulmonary neutrophil influx. In addition, further experiments are needed to establish whether these results are relevant to other gram-negative surface polysaccharides. However, if this is the case, our findings will be of considerable importance since capsular polysaccharide and the O-specific antigen are nearly ubiquitous surface components of the gram-negative bacilli capable of causing pneumonia and a variety of other extraintestinal infections.
We chose to use whole live organisms in this study for several reasons. Most importantly, a live-organism challenge will present the bacterial component in question in the most physiologic manner and mimics the clinical reality of gram-negative pneumonitis. The host will be exposed to both shed and attached surface polysaccharides. Further, the host will be exposed to the native form of these components. It has been previously demonstrated that the form of bacterial components affects the host response (6). The process of killing whole organisms or the purification of individual components will likely result in alterations to the native form. Lastly, the use of purified components always raises the problem of potential contamination by other bacterial products. The use of a live set of defined, isogenic mutants that are deficient in various components of interest avoids these potential problems which otherwise is very difficult to control for.
Previously published data from our laboratory indirectly supports the hypothesis that the K54 capsule is proinflammatory. Mice were injected intraperitoneally with killed CP9 (wt) or two independent isogenic capsule-deficient derivatives (CP9.108 and CP9.C56). Since killed organisms were utilized, subsequent death was likely due to a bacterial-product-mediated host response. At high CI (7.0 × 109 and 7.7 × 109 CFU, respectively) the mean survival of the capsule-deficient strains (2.44 and 2.0 days, respectively) was significantly longer than that of CP9 (1.39 days) (P < 0.001) (20).
Although it is generally conceded that multiple microbial determinants activate the host pulmonary response, most of the available data involves the role of lipid A or peptidoglycan. Bacterial endotoxin has been shown to be a potent proinflammatory mediator with resultant activation of alveolar macrophages and the complement cascade, and subsequent recruitment and activation of neutrophils and monocytes (1). The signal transduction pathway of the proinflammatory effects of lipid A has been relatively well worked out and is mediated in part through CD14 which is present on macrophages and other lipid A-responsive cells (15). A number of studies on both gram-positive and gram-negative pathogens have demonstrated that peptidoglycan, alone or in a polysaccharide-peptidoglycan complex (e.g., lipoteichoic acid but not the capsular polysaccharides), is able to stimulate a proinflammatory response. Studies have focused on chronic noninfectious inflammatory processes (e.g., arthritis) (22) and on mediators of gram-positive related septic shock (3), although some infectious processes have also been evaluated (12, 17). Various studies have demonstrated activation of macrophages, release of various proinflammatory mediators including cytokines (e.g., tumor necrosis factor alpha and interleukin-1β), nitric oxide, prostaglandins (e.g., prostaglandin E2), and leukotrienes (e.g., leukotriene B4) and a subsequent influx of neutrophils (3, 12, 17, 22). The CD14 signal transduction pathway has also been implicated as effecting these responses (16). However, we are unaware of data that demonstrate that capsule increases pulmonary neutrophil influx or stimulates the host proinflammatory response. Data have been published on Cryptococcus neoformans capsule which, in contrast, reduces tumor necrosis factor alpha and interleukin-1β production from human monocytes in vitro (27).
Previously published results from our laboratory also support the hypothesis that the O-specific antigen attenuates the host proinflammatory response. Mice were challenged with CP9 (wt), CP920 (O4 deficient), and CP921(O4 deficient) via intraperitoneal injection, and the 50% lethal doses (LD50s) were determined. Contrary to predictions, the LD50s of CP920 (3.2 × 106 CFU) and CP921 (5.2 × 106 CFU) were significantly lower than that of CP9 (1.7 × 107 CFU) (P < 0.05) (21).
To date, the O-specific antigen is usually thought of as a bacterial component that protects against various host defense factors. Findings from this model system have substantiated that fact, as demonstrated by the increased clearance of CP921 from the lung compared to CP9 (Fig. 2). However, in addition to this important role, an effect of the O-specific antigen is to decrease pulmonary neutrophil influx. This finding, in combination with the LD50 data described above, suggests that the O-specific antigen attenuates the host inflammatory response. The mechanism by which this effect is exerted will be interesting and is presently being evaluated. It may or may not be independent of lipid A. It is possible that the O-specific-antigen effect is mediated by altering the biologic activity of lipid A. Previous studies have demonstrated that the form of lipid A is important in its localization within the host (6). Alternatively, the O-specific antigen may act independently of lipid A but through the same signal transduction pathway (e.g., a lipid A binding protein/CD14 pathway) or through a completely independent pathway. It is interesting to note that most gram-negative systemic pathogens possess an O-specific antigen, whereas mucosal gram-negative pathogens generally do not. It is tempting to speculate as to whether it is advantageous for a systemic pathogen to possess a component that attenuates the host response. Potential benefits include a reduction in bactericidal activity generated by the host and/or a lower probability that the host response becomes unregulated (e.g., septic shock). Such a response may lead to premature death of the host, thereby decreasing the likelihood of bacterial transmission to a new individual. If these speculations are substantiated, an understanding of how the O-specific antigen attenuates host response could have significant implications for both the potential use of biologic modulators directed against the host response and approaches based on inactivating bacterial components (e.g., lipid A) in attempts to modify sepsis syndromes.
ACKNOWLEDGMENTS
This work was supported by grants AI 42059 (T.A.R.) and HL 48889 (P.R.K.) from the National Institutes of Health, a Merit Review Grant (T.A.R.) from the Department of Veterans Affairs, and from the Lucille P. Markey Charitable Trust.
We appreciate the continued support of Tim Murphy and Bruce Holm.
Appendix
Description of the methods used to develop and fit the model for MPO activity. To describe the effects of the four strains on MPO levels, a empirical model was constructed. As a first approximation, the effects of CP9.137, CP921, and CP923 were modeled as if each were a dilution or concentration of CP9. For each strain, the MPO levels observed appeared to increase until about 6 h after administration of the CI and then remain at similar levels for several hours (Fig. 3). The maximum MPO level for each strain was modeled as a linear function of the common logarithm of CI. The function used was: Ymax = Y0 + b(X − Rn) (for n = 1, 2, 3, and 4), where Ymax is the maximum MPO level, Y0 is the MPO level at time zero, X is the common logarithm of the CI, n = 1 for strain 1 (CP9), n = 2 for strain 2 (CP9.137), n = 3 for strain 3 (CP921), n = 4 for strain 4 (CP923), b is a slope parameter used to estimate the relationship of maximum MPO level to the common logarithm of the CI, and Rn is a parameter used to estimate a strain-specific effect on the maximum MPO level for each strain.
In this model, the maximum MPO level is thus Ymax = Y0 + b(X − R1) for CP9, Ymax = Y0 + b(X − R2) for CP9.137, Ymax = Y0 + b(X − R3) for CP921, and Ymax = Y0 + b(X − R4) for CP923.
To explore whether it was reasonable to describe the maximum MPO level as a linear function of the common logarithm of the CI for each strain, the relationship between the CI and the MPO level was first studied for all observations using the MPO levels for all animals sacrificed at 6 h or later after administration of the CI. For each strain, these MPO levels and their means were graphed against the common logarithm of the CI to see if the function appeared to be linear, and the fitting linear regression was fitted. For each strain, this relationship appeared to be reasonably described by a linear function (Fig. A1). To test the goodness of fit of the linear relationship between maximum MPO and logarithm of the CI, additional parameters were added to allow for a separate maximum MPO at each CI of each strain (with the relationship between maximum MPO and the common logarithm of the CI not necessarily being linear). When the additional parameters were added and the relationship was thus not assumed to be linear, the fit was not significantly improved (F = 0.11513, df = 4,98, P = 0.97).
FIG. A1.
Demonstration that maximum MPO level can be described as a linear function of the common logarithm of the CI for each strain. This illustration uses CP921 as the challenge strain. The × symbols represent MPO activity from a single animal at the given CI. The closed circles are the mean of MPO activity at the indicated CI.
The next step in building the model was to describe the pattern by which the MPO levels were increasing from the time of the CI until the plateau was reached. For each strain, the MPO levels observed appear to increase over time in a way which resembles a logistic function of the logarithm of time. The relationship of MPO level and time was thus modeled as:
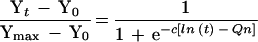 |
where Yt is the MPO level at time t, Ymax is the maximum MPO level, Y0 is the MPO level at time zero, t is the time (in hours) after CI instillation, n = 1 for strain 1 (CP9), n = 2 for strain 2 (CP9.137), n = 3 for strain 3 (CP921), n = 4 for strain 4 (CP923), c is a parameter used to estimate the slope constant of the logistic function, and Qn is a parameter used for each strain to estimate the log time at which the MPO level reaches 50% of its maximum increase.
Combining the two parts of the model above and solving for Yt, the full model used was:
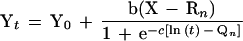 |
where the constants and variables are as described above.
The parameters R1, R2, R3, and R4 represent the strain-specific effect on the maximum MPO level for strains CP9, CP9.137, CP921, and CP923, respectively. The parameters Q1, Q2, Q3, and Q4 represent the log time at which the MPO level reaches 50% of its maximum increase for strains CP9, CP9.137, CP921, and CP923, respectively.
To test the effects on the maximum MPO level of deleting the capsule, of deleting the O-specific antigen, and of the synergy between these two effects, the model was reparameterized. The parameters R1, R2, R3, and R4 were replaced by the parameters r1, r2, r12, and r0, where r1 = [(R2 − R1] + (R4 − R3)]/2 is the average effect of deleting the capsule, r2 = [(R3 − R1) + (R4 − R2)]/2 is the average effect of deleting the side chain, r12 = [(R1 − R2) + (R4 − R3)]/2 = [(R1 − R3) + (R4 − R2)]/2 is the synergy between the effects of deleting the capsule and deleting the side chain, and r0 is the value of R averaged over all observations.
To test the effects of deleting the capsule, of deleting the side chain, and of the synergy between these two effects on the time to 50% of the maximum MPO increase, the model was reparameterized. The parameters Q1, Q2, Q3, and Q4 were replaced by the parameters q1, q2, q12, and q0, where q1 = [(Q2 − Q1) + (Q4 − Q3)]/2 is the average effect of deleting the capsule, q2 = [(Q3 − Q1) + (Q4 − Q2)]/2 is the average effect of deleting the side chain, q12 = [(Q1 − Q2) + (Q4 − Q3)]/2 = [(Q1 − Q3) + (Q4 − Q2)]/2 is the synergy between the effects of deleting the capsule and deleting the side chain, and q0 is the value of Q averaged over all observations.
The model was fitted using nonlinear regression using SPSS for Windows. The significance of the effects of deleting the capsule, of deleting the side chain, and of the synergy between these two events were tested by dividing each estimate by its standard error and then comparing the resulting Z score to a standard normal distribution.
REFERENCES
- 1.Brigham K L, Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986;133:913–927. [PubMed] [Google Scholar]
- 2.Cook D, Guyatt G, Marshall J, Leasa D, Fuller H, Hall R, Peters S, Rutledge F, Griffith L, McLellan A, Wood G, Kirby A. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. New Engl J Med. 1998;338:791–797. doi: 10.1056/NEJM199803193381203. [DOI] [PubMed] [Google Scholar]
- 3.De Kimpe S J, Kengatharan M, Thiemermann C, Vane J R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deitch E A. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Freeman B D, Correa R, Karzai W, Natanson C, Patterson M, Banks S, Fitz Y, Danner R, Wilson L, Eichacker P Q. Controlled trials of rG-CSF and CD11b-directed MAb during hyperoxia and E. coli pneumonia in rats. J Appl Physiol. 1996;80:2066–2076. doi: 10.1152/jappl.1996.80.6.2066. [DOI] [PubMed] [Google Scholar]
- 6.Ge Y, Ezzell R M, Tompkins R G, Warren H S. Cellular distribution of endotoxin after injection of chemically purified lipopolysaccharide differs from that after injection of live bacteria. J Infect Dis. 1994;169:95–104. doi: 10.1093/infdis/169.1.95. [DOI] [PubMed] [Google Scholar]
- 7.Goldblum S E, Wu K M, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol. 1985;59:1978–1985. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- 8.Graybill J R, Marshall L W, Charache P, et al. Nosocomial pneumonia. Am Rev Resp Dis. 1973;108:1130–1140. doi: 10.1164/arrd.1973.108.5.1130. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy T P, Johnson K J, Kunkel R, et al. Acute acid aspiration lung injury in the rat: biphasic pathogenesis. Anesth Analg. 1989;69:87–92. [PubMed] [Google Scholar]
- 10.Knight P R, Druskovich G, Tait A R, et al. The role of neutrophils, oxidents and proteases in the pathogenesis of acid pulmonary injury. Anesthesiology. 1992;77:772–778. doi: 10.1097/00000542-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Knight P R, Rutter T, Tait A R, et al. Pathogenesis of gastric particulate lung injury: A comparison and interaction with acid pneumonitis. Anesth Analg. 1993;77:754–760. doi: 10.1213/00000539-199310000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Leake E R, Holmes K, Lim D J, DeMaria T F. Peptidoglycan isolated from nontypeable Haemophilus influenzae induces experimental otitis media in the chinchilla. J Infect Dis. 1994;170:1532–1538. doi: 10.1093/infdis/170.6.1532. [DOI] [PubMed] [Google Scholar]
- 13.Lukas N W, Ward P A. Inflammatory mediators, cytokines, and adhesion molecules in pulmonary inflammation and injury. Adv Immunol. 1996;62:257–304. doi: 10.1016/s0065-2776(08)60432-0. [DOI] [PubMed] [Google Scholar]
- 14.Nader-Djalal N, Knight P R, Davidson B A, Johnson K J. Hyeroxia exacerbates microvascular lung injury following acid aspiration. Chest. 1997;112:1607–1614. doi: 10.1378/chest.112.6.1607. [DOI] [PubMed] [Google Scholar]
- 15.Raetz C R H. Bacterial endotoxins: extraordinary lipids that activate eucaryotic signal transduction. J Bacteriol. 1993;175:5745–5753. doi: 10.1128/jb.175.18.5745-5753.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rietschel E T, Schletter J, Weidemann B, El-Samalouti V, Mattern T, Zahringer U, Seydel U, Brade H, Flad H D, Kusumoto S, Gupta D, Dziarski R, Ulmer A J. Lipopolysaccharide and peptidoglycan: CD14-dependent bacterial inducers of inflammation. Microb Drug Resist. 1998;4:37–44. doi: 10.1089/mdr.1998.4.37. [DOI] [PubMed] [Google Scholar]
- 17.Roord J J, Apicella M, Scheld W M. The induction of meningeal inflammation and blood-brain barrier permeability by Haemophilus influenzae type b peptidoglycan. J Infect Dis. 1994;170:254–256. doi: 10.1093/infdis/170.1.254-a. [DOI] [PubMed] [Google Scholar]
- 18.Russo T A, Brown J J, Jodush S T, Johnson J R. The O4 specific antigen moiety of lipopolysaccharide but not the K54 group 2 capsule is important for urovirulence in an extraintestinal isolate of Escherichia coli. Infect Immun. 1996;64:2343–2348. doi: 10.1128/iai.64.6.2343-2348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo T A, Guenther J E, Wenderoth S, Frank M M. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Mol Microbiol. 1993;9:357–364. doi: 10.1111/j.1365-2958.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 20.Russo T A, Liang Y, Cross A S. The presence of K54 capsular polysaccharide increases the pathogenicity of Escherichia coli in vivo. J Infect Dis. 1994;169:112–118. doi: 10.1093/infdis/169.1.112. [DOI] [PubMed] [Google Scholar]
- 21.Russo T A, Sharma G, Brown C R, Campagnari A A. The loss of the O4 antigen moiety from the lipopolysaccharide of an extraintestinal isolate of Escherichia coli has only minor effects on serum sensitivity and virulence in vivo. Infect Immun. 1995;63:1263–1269. doi: 10.1128/iai.63.4.1263-1269.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwab J H. Phlogistic properties of peptidoglycan-polysaccharide polymers from cells walls of pathogenic and normal-flora bacteria which colonize humans. Infect Immun. 1993;61:4535–4539. doi: 10.1128/iai.61.11.4535-4539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shelhamer J H, Toews G B, Masur H, Suffredini A F, Pizzo P A, Walsh T J, Henderson D K. Respiratory disease in the immunosuppressed host. Ann Intern Med. 1992;117:415–431. doi: 10.7326/0003-4819-117-5-415. [DOI] [PubMed] [Google Scholar]
- 24.Stamm W E, Martin S M, Bennett J V. Epidemiology of nosocomial infections due to gram-negative bacilli: aspects relevant to development and use of vaccines. J Infect Dis. 1977;136:S151–S160. doi: 10.1093/infdis/136.supplement.s151. [DOI] [PubMed] [Google Scholar]
- 25.Standiford T J, Kunkel S L, Greenberger M J, Laichalk L L, Streiter R M. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996;59:24–28. doi: 10.1002/jlb.59.1.24. [DOI] [PubMed] [Google Scholar]
- 26.Stevens R M, Terex D, Skillman J J, et al. Pneumonia in an intensive care unit. Arch Intern Med. 1974;134:106–111. [PubMed] [Google Scholar]
- 27.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Downregulation by cryptococcal polysaccharide of tumor necrosis factor-alpha and interleukin-1 beta secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]