Key Features.
The Information System for Research in Primary Care (SIDIAP) is a database of population-wide primary care electronic health records that was created to provide a useful tool for healthcare research.
SIDIAP includes data from 328 primary care centres managed by the Catalan Health Institute in Catalonia, Spain. The database contains pseudo-anonymized records for >8 million people since 2006, with 5.8 million people active in June 2021 (75% of the Catalan population). SIDIAP is representative of the general population living in Catalonia in terms of age, sex and geographic distribution.
SIDIAP is updated on a 6-monthly basis and the median follow-up time of the population is currently 15.5 years.
SIDIAP includes high-quality data on demographics, all-cause mortality, disease diagnoses, prescription and dispensation of drugs, laboratory tests, socio-economic indicators, vaccinations, lifestyle information, parent–child linkage and clinical parameters, among others. SIDIAP can be linked on a project-by-project basis to other data sources such as hospital discharges, mental health centres or specific disease registries.
Researchers from public institutions can request data access if they comply with certain requirements. Further information is available online (www.sidiap.org).
Data resource basics
Primary care in Catalonia, Spain
Spain has a universal taxpayer-funded health system that is decentralized to its 17 autonomous communities (the country's first level of political and administrative division).1 Primary care is free of charge and is the main entry point for accessing public non-emergency health-related services that are delivered through primary care. Persons can be referred to secondary care if necessary. Primary care centres are composed of general practitioners (GPs), paediatricians, dentists, nurses, social workers, auxiliary nurses and administrative staff. Additionally, as part of primary care, there are a set of support services such as sexual and reproductive health or home care at the end of life.
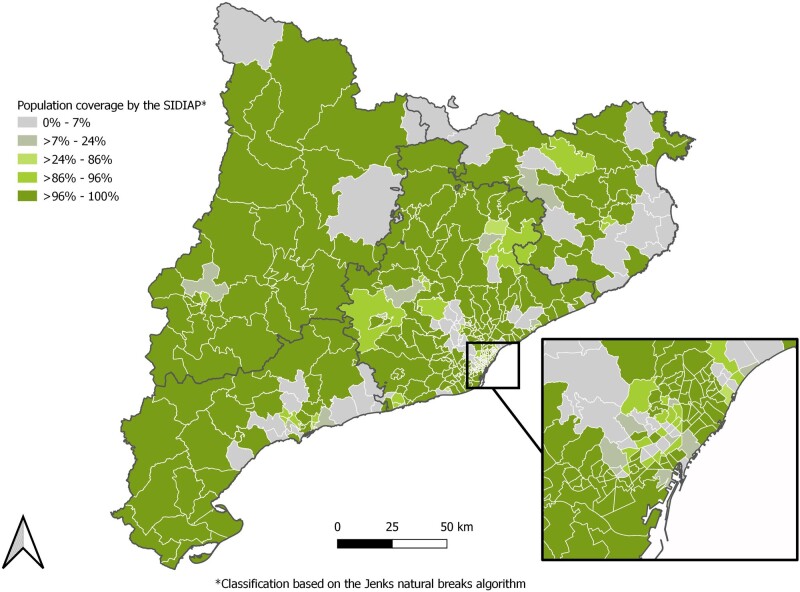
In Catalonia, an autonomous community in north-eastern Spain, the Institut Català de la Salut (ICS, Catalan Health Institute) is the largest healthcare provider.2 As seen in Figure 1, ICS covers most of the Basic Health Areas (territorial units by which primary healthcare services are organized in Catalonia) across Catalonia. It manages 328 primary care centres covering 5.8 million people, 75% of the population living in Catalonia (the remaining 25% is distributed among other providers whose services are hired by the Department of Health) (Figure 1 and Table 1).
Figure 1.
Population coverage by the Information System for Research in Primary Care database by Basic Health Area on 30 June 2021
Basic Health Areas are the territorial units by which primary healthcare services are organized in Catalonia. This delimitation is determined by the population's accessibility to health services, the efficiency of the organization of health resources and other factors (geographical, demographical, social and epidemiological).
Table 1.
Socio-demographic characteristics of the Information System for Research in Primary Care population, all-time and on 30 June 2021
| SIDIAP population |
||
|---|---|---|
| Total since 2006 | Current (as of 30 June 2021) | |
| Persons, n | 8 036 948 | 5 801 280 |
| Coverage of the population of Catalonia, % | – | 74.9 |
| Follow-up in years, median (IQR) | 15.2 (6.2–15.5) | 15.5 (12.7–15.5) |
| Sex, n (%) | ||
| Female | 4 029 112 (50.1) | 2 940 521 (50.7) |
| Male | 4 007 836 (49.9) | 2 860 759 (49.3) |
| Age in years, median (IQR) | 44 (27–63) | 44 (25–60) |
| Age in years, n (%) | ||
| <18 | 1 245 702 (15.5) | 1 009 303 (17.4) |
| 18–64 | 4 912 030 (61.1) | 3 676 712 (63.4) |
| >64 | 1 879 216 (23.4) | 1 115 265 (19.2) |
| Geographic region of nationality, n (%) | ||
| Spain | 6 476 556 (80.6) | 4 867 045 (83.9) |
| Europe (other than Spain) | 409 026 (5.1) | 241 816 (4.2) |
| America | 500 886 (6.2) | 278 905 (4.8) |
| Asia | 252 291 (3.1) | 156 298 (2.7) |
| Africa | 397 178 (4.9) | 256 687 (4.4) |
| Oceania | 1011 (0.0) | 529 (0.0) |
| Type of residential area, n (%) | ||
| Urban | 6 652 818 (82.8) | 5 125 779 (88.4) |
| Rural | 433 639 (5.4) | 332 827 (5.7) |
| Missing | 950 491 (11.8) | 342 674 (5.9) |
| Catalan regions, n (%) | ||
| Barcelona | 5 733 291 (71.3) | 4 365 572 (75.3) |
| Lleida | 518 810 (6.5) | 390 365 (6.7) |
| Girona | 667 816 (8.3) | 512 985 (8.8) |
| Tarragona | 706 192 (8.8) | 527 997 (9.1) |
| Missing | 410 839 (5.1) | 4361 (0.1) |
| Status on 30 June 2021 | ||
| Active | 5 801 280 (72.2) | 5 801 280 (100) |
| Transferred out | 1 545 850 (19.2) | – |
| Death | 689 818 (8.6) | – |
IQR, interquartile range.
The Information System for Research in Primary Care
The Information System for Research in Primary Care (SIDIAP; www.sidiap.org) database includes routinely collected data by >30 000 professionals from the ICS. During the 1990s, the ICS created a computerized programme [estació clínica d’atenció primària (e-CAP)] for the recording of information during primary care visits in a structured format that has been in use since 2005. In 2010, the ICS and the Institute for Primary Health Care Research Jordi Gol i Gurina (IDIAPJGol) created SIDIAP, which included the data collected through the e-CAP programme since 2006. SIDIAP was designed to provide a valid and reliable database of selected information from the patients’ electronic health records (EHRs) for research.3
Table 1 presents the main characteristics of the SIDIAP population. The database has information on 8 036 948 people, of whom 5 801 280 (72.2%) were still active as of 30 June 2021, 1 545 850 (19.2%) had been transferred out of the database (i.e. individuals who had moved out of the catchment area of SIDIAP) and 689 818 (8.6%) had died. Individuals are automatically incorporated into SIDIAP if they are registered in the public health system and have been assigned to a primary care centre of the ICS. The only requirement to do the self-registration in the public health system is to live in Catalonia (based on a census certificate). The registration process is free of charge and can be done online (without having to go to a primary care centre) or in person at a primary care centre. For births that take place in public healthcare facilities, the facility registers the newborn in the public health system. Individuals can subsequently leave the database when they move out of the catchment area (based on the census certificate) of SIDIAP or die. The median follow-up time of the population is 15.2 [interquartile range (IQR): 6.2–15.5] years (Table 1).
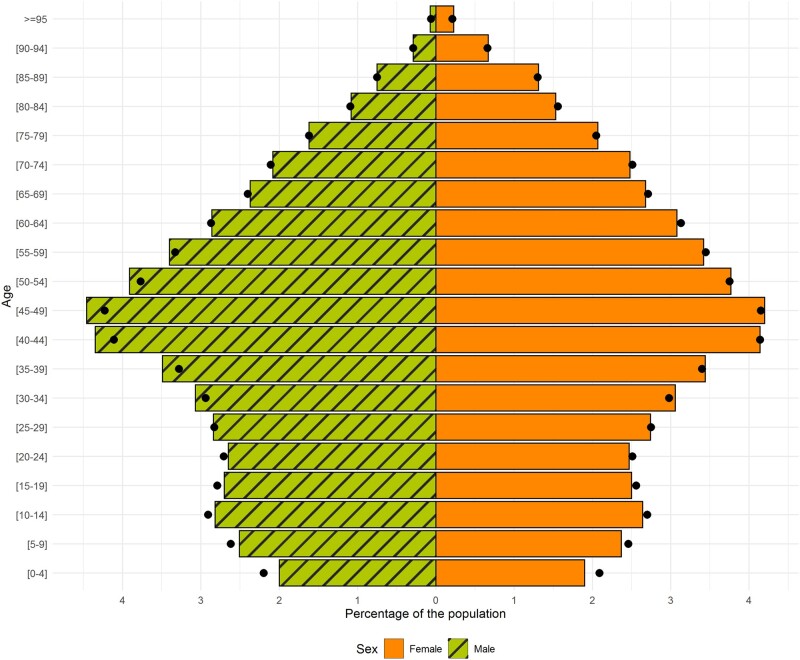
The current SIDIAP population (as of 30 June 2021) has a balanced sex distribution (50.7% are female) and a median age of 44 years (IQR: 25–60). The sex and age distribution of the SIDIAP population is similar to that of the general population in Catalonia (Figure 2). The large majority of the SIDIAP population is of Spanish nationality (83.9%), lives in urban areas (88.4%) and resides in the Barcelona region (75.3%) (Table 1). Interestingly, whereas the majority of the SIDIAP population resides in the Barcelona region, as seen in Figure 1, SIDIAP has a population coverage of ≤24% for several Basic Health Areas of Barcelona City.
Figure 2.
Age and sex distribution in the current Information System for Research in Primary Care population and in the general population of Catalonia
The Information System for Research in Primary Care data used for this graph was extracted on 30 June 2021. The data of the population of Catalonia were obtained from the Instituto Nacional de Estadística (National Institute of Statistics) website for the year 2020, ‘Población por comunidades, edad (grupos quinquenales), Españoles/Extranjeros, Sexo y Año’ tab, available from https://www.ine.es/jaxi/Tabla.htm?path=/t20/e245/p08/l0/&file=02002.px&L=0.
Data collected
SIDIAP is a dynamic database containing pseudo-anonymized data recorded in primary care centres (e.g. disease diagnoses, lifestyle information, clinical parameters, etc.) on a daily basis. It also contains external information related to the primary care visit such as pharmacy dispensations and results of laboratory tests, among others (Table 2). Although SIDIAP systematically collects data since 2006, information prior to this date is also available due to professionals recording data retrospectively and to the data transferred from paper to the EHRs in certain centres during the computerization process. The database is updated every 6 months and is structured in data domains, each containing the person’s pseudo-anonymized identifier, which allows linkage between them. Although the number of available data domains grows over time, a description of those most widely used is provided in Table 2.
Table 2.
Description of key data available in the Information System for Research in Primary Care database
| Type of data collected | Data domain in the SIDIAP database | Number of people with at least one entry for the data domain | Total number of entries for the data domain | Key information recorded in the data domain |
|---|---|---|---|---|
| Socio-demographics | Population | 8 036 948 | 8 036 948 | Date of birth, sex, date of entry to the database, date and cause of exit (death, transferral out of the database) if applicable, nationality (grouped into 11 categories) |
| Socio-economic variables | 8 036 948 | 8 036 948 | Individual income level (<18 000€, between 18 000€ and 100 000€, >100 000€ per year), deprivation indices measured at the census tract level (MEDEAa, IP2011b) and the basic health area level (ISC)c, requested pharmaceutical contribution (exempted, or contribution of 10%, 40%, 50%, 60%), maximum monthly pharmaceutical contribution (without limit, or up to 8.23€, 18.52€ or 61.75€) | |
| Regional variables | 8 036 948 | 18 036 948 | Type of residential area (rurald or urban), province (Barcelona, Tarragona, Girona, Lleida) and basic health area (n = 388) where the person resides; productive unit (333 categories, e.g. EAP Vallirana, EAP Salt, EAP La Garriga, etc.), sanitary scope (10 categories, e.g. Barcelona, Girona, Terres del Ebre, etc.). | |
| Complexity | 8 036 948 | 8 036 948 | Clinical risk group (indicator based on the person’s co-morbidities), state of fragility (categorizes persons into ‘with chronic complexity’, ‘with advanced chronic disease’ or none) and whether the person lives in a nursing home (yes, no) | |
| Health conditions | Primary care diagnoses | 7 531 524 | 171 215 580 | ICD-10 code, dates of start and end (if there is any), SIDIAP grouper (e.g. ‘Arthrosis’ includes two ICD-10 codes with its descendants) and type of productive unit (hospital, primary care team, mental health centre, etc.) that registers the health problem |
| Sick, maternity or paternity leaves | 3 191 357 | 16 651 062 | Coding of the health problem causing the sick leave (ICD-10) and dates of start and end of leave | |
| Medications and vaccines | Prescriptions | 7 083 845 | 320 380 795 | ATC code, treatment group (e.g. anxiety, hormonal replacement therapy, hypertension), dates of start and end of prescription, posology, frequency of intake and DDD of the medication, speciality of the health professional (e.g. general medicine, gynaecology, paediatrician) and setting (hospital, primary care team, mental health centre, etc.) of prescription |
| Dispensations | 6 928 471 | 1 082 492 571 | ATC code, dates of start and end of the dispensation, DDD, number of packages dispensed per month | |
| Adverse reactions | 240 047 | 316 214 | ATC code of the drug that produces the adverse reaction and date of occurrence | |
| Vaccinations | 6 443 204 | 116 615 695 | Code, date and dose of administration and grouper of the antigen of the vaccine | |
| Laboratory tests | Analytical variables | 5 703 343 | 1 129 564 883 | Biomarker/measurement (e.g. glucose, cholesterol, bilirubin, PCR), date of test/measurement, result value and unit of the test, and interpretation of the value (in four categories: positive, negative, indeterminate, inconclusive or through free text if applicable) |
| Serology | 2 051 806 | 11 170 439 | Serological test (e.g. hepatitis C antibodies, HIV) date and result (i.e. positive, negative, indeterminate) | |
| Clinical practice and lifestyle information | Clinical and lifestyle variables | 6 545 968 | 513 637 096 | Clinical measurement (e.g. systolic/diastolic blood pressure, weight, height) and lifestyle variables (e.g. alcohol consumption, smoking status) date and value of the registry |
| Request of complementary explorations/tests | 4 314 375 | 21 741 943 | Type (e.g. mammography, colonoscopy) and date of the exploration request | |
| Visits | Visits | 7 473 119 | 695 049 273 | Visited service, SIDIAP grouper (e.g. dermatology, nursing, general practice), type of productive unit (hospital, primary care team, mental health centre, etc.) that does the visit, place (online, in person at the health centre or at the residence of the person) where the visit takes place |
| Referrals | 4 738 748 | 22 042 118 | Health service to which the person is being referred to, date and cause (usually suspicion of an ICD-10 code) of referral | |
| Sexual and reproductive health | Variables of sexual and reproductive health assistance | 1 566 025 | 172 218 876 | Variable (e.g. year of menopause, breastfeeding, or result of the last mammography), date and value (if none, the result is reported in another variable containing text in free format) of the registry |
| Pregnancy | 427 193 | 649 630 | Date of last period and of estimated delivery, type of delivery (e.g. voluntary miscarriage, natural miscarriage, c-section), gestational age (in weeks), miscarriage risk (none, low, normal, moderate, high, very high), number of foetuses, trimestral obstetric ultrasounds [containing information about the mother (type of exploration, location of the placenta, type of amniotic fluid, etc.) and the foetus(es) (sex, cardiac activity, cephalic circumference, etc.)] | |
| Paediatric health (<15 years of age) | Paediatric health | 1 592 446 | 263 953 678 | Variables related to birth (e.g. gestational age at delivery, sex, weight, height), screening (e.g. neonatal deafness, cystic fibrosis, congenital hypothyroidism), development (e.g. reaction to external stimuli, object manipulation, sphincter control), nutrition (e.g. gluten intolerance, breastfeeding length, beikost consumption), school (e.g. course, integration, performance), hygiene (e.g. correct fingernail, oral and genital hygiene), leisure and sports (e.g. extracurricular and weekend activities, screen time) and sleeping patterns (e.g. dyssomnia, sleeping schedule, snoring) |
| Other data sources available through external linkage | Diagnoses and medical procedures at hospital dischargee | 3 223 390 | 59 748 073 | ICD-10-CM/PCS code, SIDIAP grouper, position of the diagnosis/procedure at the time of admission (used to prioritize the diagnoses/procedures that caused the admission), dates and circumstance (urgent, scheduled) of admission, and dates and circumstance (eight categories, including home, voluntary discharge or death) of discharge. From 2016 onwards, type of anaesthesia used, ICU admission and length of it, date of the first ICU admission |
| Hospital medication for outpatient dispensinge | 516 557 | 14 563 331 | ATC code, date of dispensation, content per box (tablets, syringes, suppositories, etc.), code of the pharmaceutical product, reason for the dispensation, price of the dispensation |
Deprivation index (based on five indicators related to work, education, housing conditions) calculated at the census tract level and available for urban areas.
Deprivation index available at the residential census tract level based on six indicators of employment and education for urban and rural areas.
Deprivation index calculated at the basic health area level for the assignment of the budgets of the primary healthcare teams in Catalonia valid for urban and rural areas.
Rural areas are defined as municipalities with a population density of <100 people per km2 and/or a population of <30 000 inhabitants.
Information recorded in all Catalan public hospitals registered in the minimum basic set of hospital discharge data (CMBD-AH) available through linkage to the Data Analysis Program for Health Research and Innovation (PADRIS) of the Department of Health.
ATC, Anatomical Therapeutic Chemical; DDD, defined daily dose; GP, general practitioner; ICD-10, International Classification of Diseases, 10th Revision (CM: Clinical Modification or PCS: Procedure Coding System); ICU, intensive care unit; IP2011, Deprivation Index 2011; ISC, Composed Socio-economic Index; HIV, human immunodeficiency virus; MEDEA, Mortalidad en áreas pequeñas Españolas y Desigualdades Socioeconómicas y Ambientales; PCR, polymerase chain reaction.
SIDIAP includes socio-demographic characteristics of the population such as the date of birth (only month and year can be provided to avoid re-identification), sex, nationality, type of residential area (rural or urban), dates of entry and exit (if applicable) and the status at the moment of the data extraction (active, transferred out of SIDIAP or dead). Socio-economic status is captured through individual and ecological indicators. The individual income level (<18 000€, between 18 000€ and 100 000€, >100 000€ per year) and type of occupation (active, retired) are obtained through the pharmaceutical co-payment information.4 Social class based on occupation is also available for those individuals who have taken sick leave at least once since 2014. The Mortalidad en áreas pequeñas Españolas y Desigualdades Socioeconómicas y Ambientales (MEDEA) deprivation index measures socio-economic status at the census tract level of both the residence and the primary care centre.5 In addition, the Índice de Privación of 2011 (IP2011) is available at the residential census tract level and the Índice de socioeconómico compuesto (ISC) is calculated at the primary care centre coverage area level.6,7
Health conditions are captured via diagnoses registered by healthcare professionals using the International Classification of Diseases (ICD) codification system (dates of beginning and end of diagnosis given by a GP can be obtained). Currently, the Tenth Revision, Clinical Modification version of the ICD-10 is being used.
The database also contains comprehensive information regarding prescriptions and dispensations of medications. This includes the drugs (dosage and drug units per day) prescribed by ICS healthcare professionals (mostly GPs although specialists can also initiate a prescription for chronic medications that are continued by GPs in the midterm and long term) that are financed by the Spanish National Health System and dispensed in community pharmacies (number of drug packages dispensed per month). For each drug, the corresponding code from the Anatomical Therapeutic Chemical (ATC) Classification System, defined daily dose recommended by the World Health Organization, the strength, the number of units per package and the administration route are available.
Data on therapeutic and requested procedures, physical examination results, routine measurements and laboratory tests are also captured. Therapeutic procedures include vaccinations (e.g. antigen and the number of administered doses) and health counselling information. Requested procedures comprise diagnostic imaging (e.g. echography, radiology, etc.), tests and scales (e.g. cognitive, pain, mental health, etc.) used in primary care, as well as other cardiovascular, digestive and respiratory diagnostic procedures (e.g. spirometry results, etc.). Physical examination results and routine measurements refer to blood pressure, weight, height, body mass index (BMI), measurements related to child growth and >500 other parameters (e.g. heart rate, cardiovascular risk calculator ‘REGICOR’, etc.). Laboratory tests include information such as cell count, serology and biochemistry, among others, that are collected in each laboratory and automatically integrated into the individual’s EHR.
SIDIAP also contains lifestyle information. The most widely used indicators include smoking status (categorized into never, former or current smoker) and alcohol intake risk (categorized into no risk, low risk or high risk). The latter is calculated based on the reported amount and the frequency of consumption of alcoholic drinks (e.g. on a daily basis), the type of alcoholic drink and/or whether the consumption is made in risky situations (e.g. pregnancy). This information is converted into standard units of alcohol ingested on a weekly basis and converted into levels of alcohol consumption.
Data regarding primary care visits are available, including the date of the visit and the type of professional consulted as well as the cause and date of referral to specialists.
The database includes detailed pregnancy information such as dates of last period and of estimated delivery, along with the type of delivery, the circumstance of the end of the pregnancy (e.g. type of delivery, abortion, etc.), gestational age and trimestral obstetric ultrasounds, among others. SIDIAP contains information about paediatric (<15 years of age) health (e.g. nutrition, development, screening tests, etc.), collected under the framework of the Programa de infancia amb salut (Childhood and Health Program).8 In addition, parent–child linkage is available for children and adolescents born or entering the database after 2006.
SIDIAP continues to incorporate new information into the database when needed (e.g. to answer new research questions or to monitor more closely a specific condition or disease, etc.) and possible. For instance, during the coronavirus disease 2019 (COVID-19) pandemic, SIDIAP incorporated additional information needed to investigate this disease (e.g. polymerase chain reaction test results, administered vaccines, etc.) in a timely fashion.
Free text that has been previously anonymized is available when sufficient detail cannot be obtained from the structured data. Further information to complement the structured data or to validate diagnoses needed for research can also be obtained through surveys sent to health professionals administered by the ICS.
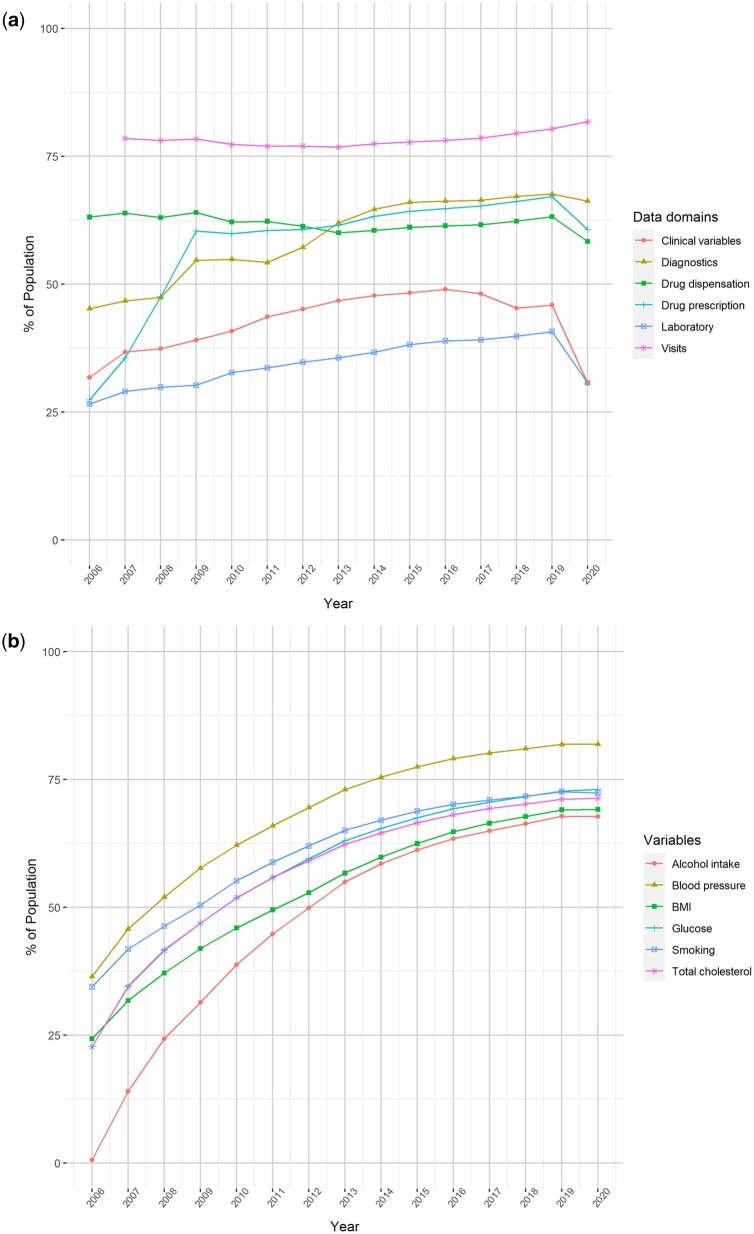
The growth in the recording of information in SIDIAP over time is shown in Figure 3a and b. For example, in 2019, ∼80% of the SIDIAP population had at least one visit to primary care and >60% had one clinical diagnosis and/or a prescription/dispensation for a medication (Figure 3a). A decrease in the amount of recorded information can be observed in 2020 (likely due to the COVID-19 pandemic). By 2019, 75% of the population had at least one record available of blood pressure and >60% had a record of alcohol intake, BMI, glucose, total cholesterol and/or smoking status (Figure 3b).
Figure 3.
Recording of key variables and data domains in the Information System for Research in Primary Care population by calendar year
(a) Proportion of the Information System for Research in Primary Care population with yearly registries of key data domains, by calendar year. (b) Proportion of the population with at least one recording of key variables by calendar year. The data domain ‘visits’ of Figure 3a include all kinds of visits (in person at the primary care centre or at home and telematic). The clinical variables domain shown in Figure 3b contains information about data collected in primary care visits (BMI, blood pressure, smoking status, alcohol intake, etc.). BMI, body mass index.
Linkage to other data sources
SIDIAP is a pseudo-anonymized database and does not contain individual personal data. Nevertheless, it can be linked to other data sources on a project-by-project basis through a Trusted Third Party (TTP) using the individuals’ unique personal identifier.
The information recorded in all Catalan public hospitals is registered in the minimum basic set of hospital discharge data (CMBD-AH) and is linked to SIDIAP through the Programa d'analítica de dades per a la recerca i la innovació en salut (PADRIS, Data Analysis Program for Health Research and Innovation) of the Catalan Department of Health.9,10 This linkage has been widely used for SIDIAP research and includes the date and cause of hospitalization and discharge, as well as the codes registered during the stay (in ICD-10-CM and ICD-10-PCS, respectively).11–19 Data from psychiatric hospitals, outpatient centres of mental health, dispensed medication in hospital settings and emergency rooms can also be obtained through the same linkage process.10 In addition, SIDIAP has been previously linked to disease registries of cancer, arthroplasties, dementia, kidney transplants and dialysis, among others.13–17,20,21 Finally, linkage to urban environment indicators (air pollution, noise, green spaces and built environment) at the census tract level22 and to external cohorts at the individual level have also been conducted. An example of the latter is the population-based prospective peripheral arterial disease study (ARTPER) cohort that includes 3786 individuals aged >49 years recruited in 28 primary care centres of Catalonia through random sampling. The participants were given an appointment for an interview, a blood sample extraction and a visit at which anthropometric indicators were measured (including the ankle arm index examination). The collected data were used to estimate the prevalence and associated risk factors of peripheral arterial disease in the general population.16
Data quality
Internal and external validation processes are carried out to determine the data quality of the SIDIAP information at each data update. These include stratifying the data by geographical regions and year in order to identify differences in data collection that need to be harmonized (e.g. recording of a specific information under different codes). The measurement units of variables measuring one characteristic are also homogenized (e.g. transformation of the data from every laboratory that measures haemoglobin to grams per decilitre). Visual inspection of all data included in the database by week is also conducted, allowing one to see temporal patterns in the registry of a certain variable. With this information, the SIDIAP team can issue recommendations to researchers about the most common variable(s) where certain information is recorded (e.g. there are several variables with information concerning the women’s menopausal status and with these visual inspection tools the SIDIAP team can inform the researchers about which related variables have the largest number of records and could be more helpful to capture menopause). Data availability (longitudinality and reliability), plausibility (range checks and unusual values) and consistency are inspected through visualization tools. In addition, before having access to the data for a requested project, research teams have access to a quality-control report. This document contains counts, years, percentiles, maximums and minimums, incidences and prevalences of the data requested for the project, allowing detection of inconsistencies in the data extraction prior to data delivery.
External validation processes of the SIDIAP database mainly include assessing the data recorded in SIDIAP through linkage to external gold standard data sources, by analysing free text or by sending questionnaires to health professionals. The quality of a wide number of data captured in SIDIAP (e.g. cancer, Alzheimer’s disease, dementia, cardiovascular risk factors and musculoskeletal disorders) has been demonstrated in validation studies.13–16,20,21,23,24
Data resource use
SIDIAP data have been extensively used by national and international institutions to generate real-world evidence. A non-exhaustive list of 223 peer-reviewed published articles and of 306 projects (of which 37 are still ongoing) using the SIDIAP database is available on the SIDIAP website (www.sidiap.org, ‘Projects’ and ‘Dissemination’ tabs). These publications cover a wide range of research topics such as cardiovascular diseases, diabetes, musculoskeletal disorders, respiratory problems, cancer, mental health, multimorbidity, COVID-19, vaccinations; and research areas including pharmacoepidemiology, evaluation of safety and comparative effectiveness research, characterization of a disease, drug utilization, temporal trends of disease, health economics and evaluation of healthcare services, among others.11,18,19,25–30
Strengths and weaknesses
Strengths
SIDIAP has several strengths. First, the database is representative of the population of Catalonia in terms of age, sex and geographic distribution (Figures 1 and 2). This favours the generalizability of the findings of the studies conducted using SIDIAP to the general population living in Catalonia but also to other comparable regions. Second, due to SIDIAP’s large size, this database can be used to answer research questions that would not be feasible in smaller-sized data sets. Third, the diverse type of data encompassed by this database is also an asset. Not only does SIDIAP include data typically recorded in EHRs (e.g. clinical diagnoses) but also contains socio-demographic information (e.g. socio-economic status or nationality) and lifestyle information (e.g. smoking status or alcohol intake). The parent–child linkage is also a major strength as it allows one to study the impact of parental health and early life exposures on health outcomes during childhood. Furthermore, SIDIAP contains data from external sources such as biomarkers’ information originating from laboratories or prescription and dispensation of drugs, which makes the assessment of drug exposure quite complete. Data from different settings (e.g. disease and hospitalization registries) can also be obtained through diverse linkages, enriching the data available for studies. Finally, SIDIAP is being mapped to different common data models used in European projects. At present, it has already been mapped to the international Observational Medical Outcomes Partnership–Common Data Model (OMOP-CDM), which facilitates and promotes multi-database studies, helps with data management and data analyses, and ensures confidentiality throughout the studies using a federated analysis approach.31
Weaknesses
The SIDIAP database also has weaknesses. Although the database is representative of the population living in Catalonia and regions with similar socio-demographics, it is not necessarily so of other regions of Spain or other countries. In addition, data missingness is a common issue of EHRs (e.g. BMI is not recorded for every participant in the database, as seen in Figure 3b) and a recent measurement of a variable of interest might not be available at the index date for a particular study (e.g. the last BMI measurement available might have been recorded years before the index date). However, methodological approaches such as multiple imputations can be implemented to reduce collider bias in research studies.32 Under-reporting of certain variables is also a limitation that can lead to the underestimation of the frequency of a certain exposure or condition (e.g. less severe behavioural or mental disorders might be more likely to go undiagnosed in clinical practice). Furthermore, individual validation of a complete list of events of interest, as conducted ad hoc in cohort or case–control studies, is not possible for large EHR databases and may lead to misclassification. However, algorithms to capture diseases or conditions can be tested in validation studies and allow the quantification of the data quality. Also, relevant information for research might be recorded in unstructured format (i.e. free text) by health professionals. Although advanced techniques to process these data are not yet available in SIDIAP, previously anonymized free text can be manually explored by researchers. Another limitation refers to clinical practice standards and coding that can change over time, giving rise to observed changes in the incidence of a certain condition that might be unrelated to its epidemiology. Finally, due to the primary care nature of this database, studies conducted with SIDIAP could lack the granularity to answer certain research questions. For instance, specialist prescribing, drugs administered in the hospital setting, drugs purchased over the counter and actual drug intake are not available in the database.
Data resource access
Any researcher is able to request SIDIAP data to conduct a study. A five-step procedure takes place before data access is granted: (i) the researcher(s) must send an application (standardized form available at www.sidiap.org and study protocol) to the SIDIAP team; (ii) the application is approved by SIDIAP’s Scientific Committee which evaluates the scientific quality and feasibility of the proposal; (iii) the study protocol is approved by the Clinical Research Ethics Committee of IDIAPJGol; (iv) the principal investigator of the study must sign a Good Practice form and, in some cases, an agreement between parties is needed; and (v) a meeting between the research team and the SIDIAP team is arranged to discuss the procedures and set the data extraction. Further information is available online (https://www.sidiap.org/index.php/menu-solicitudesen/application-proccedure) or by contacting Anna Moleras (sidiap@idiapjgol.org). Data access is limited to researchers from public organizations and collaboration with private institutions is possible when a study is required by a regulatory agency or for non-commercial studies within a European project financed by the European Commission.
In accordance with current European and national law, the data used in this study are only available for the researchers participating in this study. Thus, we are not allowed to distribute or make publicly available the data to other parties.
Ethics approval
The use of the data included in the Information System for Research in Primary Care (SIDIAP) is authorized by the Catalan Health Institute (ICS) and Data Analysis Program for Health Research and Innovation (PADRIS) who ensure the pseudo-anonymization of the information. When linkage with other public data sources is required, ICS or PADRIS act as a Trusted Third Party (TTP) to execute the linkage and provide the new data set already pseudo-anonymized; otherwise, informed consent of patients is needed to access their personal data, using the same TTP. SIDIAP does not provide information subject to re-identification and aggregations or deletions are applied in order to protect pseudo-anonymization. The data are managed in a secure server following all the present legal requirements of the General Data Protection Regulation (European Union) 2016/679 and of the Council of 27 April 2016 and Organic Law 3/2018 of 5 December on the protection of personal data and guarantee of Digital Rights.
This study was exempted from the approval of the Clinical Research Ethics Committee of the IDIAPJGol given that the data were directly analysed in the SIDIAP platform and only aggregated results were reported.
Acknowledgements
This study was carried out as part of the Doctoral Program in Biomedical Research Methodology and Public Health at the Autonomous University of Barcelona. We would like to thank all healthcare professionals of Catalonia who daily register information in the populations’ EHRs. We also want to thank all the research teams who have worked with SIDIAP and have helped to improve and maintain the database, and the Institut Català de la Salut (ICS) and the Programa d'analítica de dades per a la recerca i la innovació en salut (PADRIS) for providing access to the different data sources accessible through SIDIAP.
Conflict of interest
None declared.
Contributor Information
Martina Recalde, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain; Universitat Autònoma de Barcelona, Bellaterra, Spain.
Clara Rodríguez, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain.
Edward Burn, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain; Centre for Statistics in Medicine, NDORMS, University of Oxford, Oxford, UK.
Marc Far, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain.
Darío García, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain.
Jordi Carrere-Molina, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain.
Mencia Benítez, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain.
Anna Moleras, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain.
Andrea Pistillo, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain.
Bonaventura Bolíbar, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain; Universitat Autònoma de Barcelona, Bellaterra, Spain.
María Aragón, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain.
Talita Duarte-Salles, Fundació Institut Universitari per a la Recerca a l'Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain.
Data availability
See Data resource access above.
Author contributions
All authors were involved in the study design. M.R. wrote the first draft of the manuscript. C.R. performed the data analyses. M.R., C.R., M.A. and T.D.S. prepared the tables and figures. All authors interpreted the results, contributed to drafting the article and approved the final version of the manuscript.
Funding
The SIDIAP database creation, development and maintenance are currently funded by the IDIAPJGol that seeks to recoup part of its expenditures through research fees obtained from research projects. SIDIAP has obtained funding for studies from the National Institute of Health Carlos III, the Catalan Department of Health, the Innovative Medicines Initiative, the European Medicines Agency, the European Commission, as well as several universities, hospitals and national and international research organizations.
In the context of this study, M.R. was funded by Wereld Kanker Onderzoek Fonds, as part of the World Cancer Research Fund International grant programme (grant number: 2017/1630). T.D.S. acknowledges receiving financial support from the Instituto de Salud Carlos III (ISCIII; Miguel Servet 2021: CP21/00023). The funders had no role in study design, data collection, analysis, decision to publish or preparation of the manuscript.
References
- 1. Ministry of Health, Social Services, and Equality. National Health System of Spain. 2012. https://www.sanidad.gob.es/gl/organizacion/sns/docs/sns2012/SNS012__Ingles.pdf (28 October 2021, date last accessed).
- 2. Generalitat de C, Població de referència del Servei Català de la Salut per a l’any 2020. 2020. https://catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/registres_catalegs/documents/informe-poblacio-referencia-2021.pdf (28 October 2021, date last accessed).
- 3. Bolíbar B, Fina Avilés F, Morros R. et al. ; Grupo SIDIAP. Base de datos SIDIAP: La historia clínica informatizada de Atención Primaria como fuente de información para la investigación epidemiológica. Med Clin (Barc) 2012;138:617–21. [DOI] [PubMed] [Google Scholar]
- 4.CatSalut. Servei Català de la Salut. El model de copagament farmacèutic. 2020. https://catsalut.gencat.cat/ca/serveis-sanitaris/atencio-farmaceutica/financament-public-medicaments/model-copagament/ (28 October 2021, date last accessed).
- 5. Domínguez-Berjón MF, Borrell C, Cano-Serral G. et al. Construcción de un índice de privación a partir de datos censales en grandes ciudades españolas (Proyecto MEDEA). Gac Sanit 2008;22:179–87. [DOI] [PubMed] [Google Scholar]
- 6. Duque I, Domínguez-Berjón MF, Cebrecos A. et al. ; en nombre del Grupo de Determinantes Sociales de la Salud, iniciativa contexto de la Sociedad Española de Epidemiología. Índice de privación en España por sección censal en 2011. Gac Sanit 2021;35:113–22. [DOI] [PubMed] [Google Scholar]
- 7. Colls C, Mias M, García-Altés A.. Un índice de privación para reformar el modelo de financiación de la atención primaria en Cataluña. Gac Sanit 2020;34:44–50. [DOI] [PubMed] [Google Scholar]
- 8.Agència de Salut Pública de Catalunya (ASPCAT). Programa Infància amb Salut. 2018. https://salutpublica.gencat.cat/ca/ambits/promocio_salut/Infancia-i-adolescencia/Infancia/infancia-amb-salut/ (28 October 2021, date last accessed).
- 9.CatSalut. Servei Català de la Salut. Conjunt mínim bàsic de dades (CMBD). 2017. https://catsalut.gencat.cat/ca/proveidors-professionals/registres-catalegs/registres/cmbd/ (28 October 2021, date last accessed).
- 10. Agència de Qualitat i Avaluació Sanitàries de Catalunya (AQuAS). Programa d’analítica de dades per a la recerca i la innovació en salut. 2020. https://aquas.gencat.cat/ca/ambits/analitica-dades/padris/index.html (28 October 2021, date last accessed).
- 11. Recalde M, Davila-Batista V, Díaz Y. et al. Body mass index and waist circumference in relation to the risk of 26 types of cancer: a prospective cohort study of 3.5 million adults in Spain. BMC Med 2021;19:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia-Gil M, Comas-Cufí M, Blanch J. et al. Effectiveness of statins as primary prevention in people with different cardiovascular risk: a population-based cohort study. Clin Pharmacol Ther 2018;104:719–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Recalde M, Manzano-Salgado C, Díaz Y. et al. Validation of cancer diagnoses in electronic health records: results from the information system for research in primary care (SIDIAP) in northeast Spain. Clin Epidemiol 2019;11:1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ponjoan A, Garre-Olmo J, Blanch J. et al. Epidemiology of dementia: prevalence and incidence estimates using validated electronic health records from primary care. Clin Epidemiol 2019;11:217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramos R, Balló E, Marrugat J. et al. Validity for use in research on vascular diseases of the SIDIAP (Information System for the Development of Research in Primary Care): the EMMA study. Rev Esp Cardiol (Engl Ed) 2012;65:29–37. [DOI] [PubMed] [Google Scholar]
- 16. Pagès-Castellà A, Carbonell-Abella C, Avilés FF. et al. Burden of osteoporotic fractures in primary health care in Catalonia (Spain): a population-based study. BMC Musculoskelet Disord 2012;13:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robinson DE, Ali MS, Pallares N. et al. Safety of oral bisphosphonates in moderate-to-severe chronic kidney disease: a binational cohort analysis. J Bone Miner Res 2021;36:820–32. [DOI] [PubMed] [Google Scholar]
- 18. Ramos R, Comas-Cufí M, Martí-Lluch R. et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ 2018;362:k3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burn E, Tebé C, Fernandez-Bertolin S. et al. The natural history of symptomatic COVID-19 during the first wave in Catalonia. Nat Commun 2021;12:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prieto-Alhambra D, Judge A, Javaid M. et al. Predictors of poor outcome (high use of non-steroidal anti-inflammatory drugs) at year 1 following total knee/hip arthroplasty: the press-up cohort study. Osteoarthritis and Cartilage 2013;21:S155. [Google Scholar]
- 21. Ponjoan A, Garre-Olmo J, Blanch J. et al. How well can electronic health records from primary care identify Alzheimer’s disease cases? Clin Epidemiol 2019;11:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Bont J, Díaz Y, de Castro M. et al. Ambient air pollution and the development of overweight and obesity in children: a large longitudinal study. Int J Obes 2021;45:1124–32. [DOI] [PubMed] [Google Scholar]
- 23. Fina-Aviles F, Medina-Peralta M, Mendez-Boo L. et al. The descriptive epidemiology of rheumatoid arthritis in Catalonia: a retrospective study using routinely collected data. Clin Rheumatol 2016;35:751–57. [DOI] [PubMed] [Google Scholar]
- 24. Muñoz-Ortego J, Vestergaard P, Rubio JB. et al. Ankylosing spondylitis is associated with an increased risk of vertebral and nonvertebral clinical fractures: a population-based cohort study. J Bone Miner Res 2014;29:1770–76. [DOI] [PubMed] [Google Scholar]
- 25. Mata-Cases M, Franch-Nadal J, Real J. et al. Therapeutic inertia in patients treated with two or more antidiabetics in primary care: factors predicting intensification of treatment. Diabetes Obes Metab 2018;20:103–12. [DOI] [PubMed] [Google Scholar]
- 26. Lane JCE, Butler KL, Poveda-Marina JL. et al. Preschool obesity is associated with an increased risk of childhood fracture: a longitudinal cohort study of 466,997 children and up to 11 years of follow-up in Catalonia, Spain. J Bone Miner Res 2020;35:1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Monteagudo M, Nuñez A, Solntseva I. et al. Treatment pathways before and after triple therapy in COPD: a population-based study in primary care in Spain. Arch Bronconeumol (Engl Ed) 2021;57:205–13. [DOI] [PubMed] [Google Scholar]
- 28. Ortega Y, Aragonès E, Piñol JL, Basora J, Araujo A, Cabré JJ.. Impact of depression and/or anxiety on the presentation of cardiovascular events in a cohort with metabolic syndrome. StreX project: five years of follow-up. Prim Care Diabetes 2018;12:163–71. [DOI] [PubMed] [Google Scholar]
- 29. Troncoso-Mariño A, Roso-Llorach A, López-Jiménez T. et al. Medication-related problems in older people with multimorbidity in catalonia: a real-world data study with 5 years’ follow-up. J Clin Med 2021;10: 10.3390/jcm10040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braeye T, Emborg H-D, Llorente-García A. et al. Age-specific vaccination coverage estimates for influenza, human papillomavirus and measles containing vaccines from seven population-based healthcare databases from four EU countries—the ADVANCE project. Vaccine 2020;38:3243–54. [DOI] [PubMed] [Google Scholar]
- 31. Observational Health Data Sciences and Informatics. The Book of OHDSI. 2019. https://ohdsi.github.io/TheBookOfOhdsi/TheBookOfOhdsi.pdf (28 October 2021, date last accessed).
- 32. Pedersen AB, Mikkelsen EM, Cronin-Fenton D. et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol 2017;9:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
See Data resource access above.