Key Features.
South Asians comprise nearly 2 billion people worldwide and are at high risk of cardiometabolic disease, even at young ages and low body weights.
The CARRS cohort, a population-based representative sample of Chennai and Delhi in India and of Karachi in Pakistan, ages ≥20 years, was assembled in two waves [CARRS-1 (2010–12), n = 16 287, 13 720 with biospecimens; and CARRS-2 (2014–16), n = 14 587, 13 709 with biospecimens].
CARRS-1 has completed five follow-up assessments after the baseline visit. CARRS-2 has completed one follow-up assessment after the baseline visit. The cohort had high participation rates at recruitment (∼90% in both waves).
The CARRS Cohort has followed 30 874 individuals (27 429 with biospecimens) and accrued ∼115 000 person-years of follow-up, including a biorepository (n = 360 000 aliquots) in India.
CARRS provides scientific infrastructure and data for measuring incidence and secular trends of cardiometabolic diseases (CMD) and risk factors.
Researchers interested in the collaborative project can contact Dr KM Venkat Narayan [knaraya@emory.edu] or Dr Dorairaj Prabhakaran [dprabhakaran@ccdcinida.org].
Why was the cohort set up?
Emerging data, largely from the diaspora and largely cross-sectional, indicate that South Asians exhibit high rates of cardiovascular disease (CVD), diabetes, atypical dyslipidaemia profiles, and hepatic steatosis1–7 at lower body weight and younger ages relative to populations with European and other ancestries.8–10 It is hypothesized that these differences are rooted in some combination of distinctive pathophysiology, risk factor, phenotypic and socioeconomic characteristics.11–13 In addition, South Asia exhibits differences in health service use, health care costs and quality of care compared with high-income countries (HICs).14 Well-characterized prospective epidemiological cohorts help study the natural history of diseases and the evolution of care in the region. This can advance the knowledge and science of cardiometabolic diseases and provide the infrastructure for interdisciplinary and dynamic scientific explorations.15
The Center for cArdiometabolic Risk Reduction in South Asia (CARRS) is a population-based representative sociodemographically diverse cohort of 30 874 adults with prospective follow-up in three major cities in South Asia—Chennai and Delhi in India and Karachi in Pakistan.16
Who is in the cohort?
The cohort was representatively drawn from the adult populations aged 20 and older in Delhi (North India, population 20 million), Chennai (South India, population 8 million)17 and Karachi (Pakistan, population 16 million),18 three megacities with a cumulative population of 44 million individuals.
Study design and sampling
We used a population-based, multistage, cluster random sampling design based on local administrative boundaries to recruit adult men and women to be representative of each city. In 2010–12, CARRS-1 was recruited as a probability sample of n = 16 287 adults aged 20 years and older. In 2014–16, CARRS-2 was established as an independent probability sample of 14 587 newly recruited individuals, using methods identical to CARRS-1. Pregnant women and seriously ill individuals were excluded. Seriously ill individuals included bedridden individuals because of the difficulty in taking anthropometric measurements and future follow-up, as blood and other biological samples were collected in a camp.
Wards were the primary sampling units for Chennai and Delhi, and clusters were the primary sampling units for Karachi. In Chennai and Delhi, 20 wards were randomly selected from urban districts. Five Census enumeration blocks (CEBs) were randomly selected from each of the 20 randomly selected wards to get 100 CEBs from Chennai and Delhi. Finally, 20 households were selected per CEB in CARRS-1 (25 households in CARRS-2). In Karachi, 80 clusters were randomly selected and 25 households were randomly selected from each cluster. Two eligible participants, one man and one woman, aged 20 years or older, were selected from each household based on the ‘Kish method’, which has been used in the World Health Organization’s STEPS surveys.19 Census boundaries and population distribution were used to develop the sampling frame for wards and CEBs, and field staff enumerated households within CEBs to ensure up-to-date household maps and adequate coverage of the target population. Due to the random selection of CEBs there was no overlap in CEB or household selection between CARRS-1 and CARRS-2. We conducted household mapping for each of the newly selected CEBs to establish the sampling frame for random selection of households and participants.
How often have they been followed up?
Baseline assessments
Baseline assessments (demographics, risk factors, anthropometry and biospecimens) were conducted between September 2010 and December 2012 (Table 1).
Table 1.
Available study measures
| Domain | Variables | Measurement | CARRS-1 |
CARRS-2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1st FUP | 2nd FUP | 3rd FUP | 4th FUP | 5th FUPa | Baseline | 1st FUP | |||
| Year of measurement | 2010–12 | 2011–13 | 2013–14 | 2014 | 2016–17 | 2017–18 | 2014–16 | 2018–20 | ||
| Questionnaires | ||||||||||
| Sociodemographic | Contact information, age, sex, income, education, occupation, place of birth, marital status, household sizeb, number of childrenb | Questionnaire | A | A | A | |||||
| Health behaviours | Physical activity | IPAQ20; GPAQ21 | A | A | A | |||||
| Sedentary behaviour/standing | A | A | ||||||||
| Open place/garden | A | |||||||||
| Tobacco use, alcohol use | Questionnaires | A | A | A | A | A | A | A | A | |
| Sleep duration and quality | Questionnaire | A | A | |||||||
| Diet | Dietary habits; 24-h dietary recall (in subsample) | Modified Food frequency questionnaire (FFQ)and 24-h recall | A | A | A + S | |||||
| Household food insecurity | Household food insecurity Access Scale22 | A | ||||||||
| Family history | Family history of HTN; DM; dyslipidaemia; heart disease; stroke | Self-report | A | A | ||||||
| Physician diagnosis of previous disease | Previously diagnosed HTN; DM; dyslipidaemia; heart disease; stroke; COPD | Self-report/medical records | A | A | A | A | A | A | A | A |
| Medication status | Cholesterol, glucose, lipid, and BP-lowering medication | Self-report | A | A | A | A | A | A | A | A |
| Quality of life (QoL) | Health-related QoL | EQ-5D questionnaires23 | A | A | A | |||||
| Psychosocial | Stress, depression | PHQ-9 questionnaire24 | A | A | A | |||||
| Lifetime/past depression-UK Biobank25,26 | A | |||||||||
| Cancer | Cancer, cancer stigma | Self-report | A | A | A | A | ||||
| Complications | Foot ulcer, amputation, eyes: retinopathy and laser treatment | Self-report | A | A | A | A | A | A | A | A |
| Respiratory disease | COPD, Asthma and TB symptoms | Self-report | A | |||||||
| Treatment history and expenditure | Heart disease; stroke; diabetes; diabetic complications; high BP; chronic kidney disease | Self-report | A | A | ||||||
| Drug information | Names of drug taken in previous week; duration of drug intake | Self-report | A | A | A | A | ||||
| Female reproductive history | Self-report | A | A | A | A | A | ||||
| Fracture | Wrist/hip or spine | Self-report | A | A | ||||||
| Environmental | ||||||||||
| Ambient air pollution (available for Chennai and Delhi only) | Daily average PM2.5 concentrations at 1 km x 1 km spatial resolution (Delhi complete, Chennai ongoing) from 2010– | Β-attenuation monitors; gravimetric samplers27 | A | A | A | A | ||||
| Built environment (available for Chennai and Delhi only) | Vegetation index, Road Networks, Gridwise built-up area %, intersections, light intensity at night, locations of polluting sources | Satellite observations and land use maps28 | A | A | A | A | ||||
| Time Activity | Questionnaire | A | A | |||||||
| CVD risk factors | ||||||||||
| Anthropometry | Weight, height, waist and hip circumference | Tanita BC-418, Seca-213 Portable Stadiometer, Tape measure16 | A | A | A | A | A | |||
| Blood pressure | Systolic and diastolic BP | Automated Omron HEM-708016 | A | A | A | A | A | |||
| Glucose | Fasting blood glucose; HbA1c | Hexokinase16; HPLC16 | A | A | A | A | ||||
| Fasting insulin, 30-min insulin, 2-h insulin | ECL immunoassay29 | Ac | Ac | Ac | A | |||||
| Lipid markers | Total cholesterol; HDL, LDL cholesterol; triglycerides | Direct; Friedewald Equation16; Martin/Hopkins Equation if triglyceride >400 mg/dl30; enzymatic methods | A | A | A | A | ||||
| Renal function | Serum creatinine; urinary creatinine; microalbuminuria, albumin: creatinine ratio | Jaffe kinetic16 | A | A | A | A | ||||
| Spot urine31 | ||||||||||
| 24-ho urine | S | |||||||||
| Cystatin C | Immunoturbidimetric32 | S | S | |||||||
| Advanced lipids | Apolipoprotein A1; apolipoprotein B | Immunoturbidimetry | S | S | ||||||
| Advanced metabolic markers | 30-min glucose, 2-h blood glucose; fasting insulin, 30-min insulin, 2-ho insulin | Hexokinase,16 ECL immunoassay16 | Ac | Ac,d | ||||||
| Adipokines | Leptin, adiponectin | ELISA assay33 | S | |||||||
| Cotinine (saliva) | S | |||||||||
| Protein biomarkers (supported by Abbott Laboratories) | ||||||||||
| Inflammation thrombogenisis/immune | High-sensitivity C-reactive protein | Particle-enhanced immunoturbidimetry | A | |||||||
| Clinical vascular and myocardial disease and death | ||||||||||
| Cardiovascular events | Heart failure, MI, stroke, revascularization, angina, PAD | Symptoms,34,35 history, ECG, medical records | A | A | A | A | A | A | A | A |
| Mortality | Verbal autopsy | A | A | A | A | A | A | A | A | |
| Electrocardiogram (ECG) | ||||||||||
| ECG (only Delhi) | A | A | A | |||||||
FUP, follow-up; HTN, hypertension; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; BP, blood pressure; TB, tuberculosis; PM2.5, particulate matter with a diameter of 2.5 micrometres or less; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction; CVD, cardiovascular disease; PAD, peripheral artery disease; IPAQ, International Physical Activity Questionnaire; GPAQ, Global Physical Activity Questionnaire; EQ-5D, EuroQol five-dimension scale; PHQ-9, Patient Health Questionnaire; UK, United Kingdom; HPLC, high-performance liquid chromatography; ECL, electrochemiluminescence; A, available; S, sub-sample.
CARRS-1, fifth follow-up conducted in Chennai and Delhi site only;
Only available for CARRS-1 and -2 baseline.
Data available for Chennai only.
Available in a sub-sample for Delhi.
Longitudinal follow-up assessments
Approximately 12 months after the baseline examination, participants were contacted for follow-up via in-person or telephone interviews. All participants were continually followed longitudinally for the development of cardiometabolic disease risk factors, manifest disease and mortality. Five follow-up visits have been completed in CARRS-1 following baseline. The first, third and fifth follow-up visits involved only the administration of survey questionnaires and ascertainment of CVD events and deaths. At the second and fourth follow-up visits, we included biospecimen data collection along with annual questionnaires, anthropometry and ascertainment of CVD events and deaths. The sixth follow-up is ongoing (November 2020 on) and was started during the COVID-19 pandemic using a hybrid of telephone and in-person surveys, due to precautions concerning face-to-face contact.
Following a parallel process, the first follow-up visit of CARRS-2 participants was carried out during September 2018–July 2020.
What has been measured?
Data collection and procedures
Table 1 shows the measures, instruments and methods used in CARRS-1 and -2. Trained interviewers collected data from participants through detailed structured questionnaires on sociodemographic characteristics, lifestyle habits (e.g. physical activity, tobacco use, diet, alcohol use, health history, sleep and mental health), medical history (cardiometabolic diseases, cardiovascular events, cancer), quality of life, functioning and cost of disease and health care. Multilevel exposome assessments capture health exposures at the level of the individual [repeated measures of atherosclerotic cardiovascular disease (ASCVD) risk factors, health behaviours, sociodemographic factors), household (assets, air quality), and the environment (air pollution, neighbourhood food and the built environment]. Interviewers conducted physical assessments, including blood pressure, heart rate, height, weight, waist circumference, skin folds and body composition. Participant blood and urine samples were collected at neighbourhood camps during baseline visits following standardized procedures across sites. In follow-up visits, samples were collected from the home of participants. Samples were transported from the field to the laboratories for processing in cold conditions (4°C to 8°C) using cold icepacks. Further details are available in our earlier methods paper16 and on the study website.36
Biorepository
India (Chennai and Delhi)
A biorepository of over 360 000 aliquots of sera, plasma, buffy coat and urine samples of participants recruited in Chennai and Delhi are stored in accredited, well-maintained institutional laboratories. The CARRS biorepository uses best practices for standardizing sample collection, maintaining a cold chain for sample transfer and handling, maintaining sample records, using consumables, power and cooling back-up systems, and de-identifying stored samples. In the laboratory, samples are processed and transferred to deep freezers (–80°C) within 3 hour. The biospecimens are labelled with unique ID, with no personal details. Multiple aliquots are prepared and stored for each participant to prevent repeated freezing and thawing of samples. All the storage details are recorded on Excel sheets for easy retrieval. Additional laboratory details are provided in the Manual of Procedure (MOPs) and may be downloaded at CARRS website.36
Methods for in-country analysis were standardized across laboratories prior to sample collection. As a measure of internal quality control, two levels of commercially available internal controls for every parameter were analysed before analysing the samples. Control rules from Westgard37 for the rejection of runs were followed. In cases of rejection, the instrument was recalibrated, and internal controls were re-evaluated. Assay results were retrieved from the instrument in Excel format. The laboratories participated in an external quality assurance programme from RANDOX (UK)38 for all serum chemistry (monthly), lipids (fortnightly), HbA1c (monthly) and urinary parameters (fortnightly). For insulin, we participate in the UK NEQAS programme.39 The performance of the laboratories was well within specified limits.
Pakistan (Karachi)
The Karachi site has followed procedures similar to Chennai and Delhi. The samples were collected and transferred to the laboratory for storage according to the procedures set in the study protocol. In the laboratory, samples were processed and transferred to deep freezers (–80°C) in <3 . The facilities have a 24-h power supply to prevent the risk of thawing, and the freezer temperature is monitored twice per day and recorded for quality assurance.
Event and death ascertainment
The CARRS study event ascertainment protocols were standardized across sites and used multiple confirmatory approaches to classify new CVD events and deaths. In annual interviews with participants, for those reporting myocardial infarction (MI), stroke or a hospital visit since the latest follow-up, medical records [e.g. including biochemical and imaging tests such as creatine kinase-MB (CK-MB), troponins, electrocardiography (ECG), brain computed tomography or magnetic resonance imaging] were obtained. Medical records were then scanned by fieldworkers at participants’ homes. Next-of-kin interviews (verbal autopsies) were conducted among participants who had died, to identify causes of death.40,41 Based on the data collected by a questionnaire and any other available information, the cause of death or the sequence of causes that led to death are evaluated and classified by two physicians. If two different causes were reported, a third physician reviews the verbal autopsy and provides a tie-breaking ascertainment of the cause of death. Verbal autopsy data are available for 89% of deaths recorded among CARRS participants.
Quality assurance and quality control strategies
The CARRS Study quality assurance plan uses a framework that comprehensively considers each phase of the study, with detailed quality control and quality assurance strategies (Table 2). Standardized protocols are used for data collection and transfers, and technical laboratory staff were trained before any fieldwork and are re-trained intermittently. All laboratory methods, such as test kits and procedures for biospecimen collection, processing and storage, as well as methods of analysis across the three sites, are standardized. The details of quality control have been described in detail elsewhere.16
Table 2.
Quality assurance and control
| Phases |
|||
|---|---|---|---|
| Level | Design and planning | Data collection | Data analysis |
| Institutions |
|
|
|
| Investigators |
|
|
|
| Data collecting personnel |
|
|
|
| Survey instruments and equipment |
|
|
|
| Specimen handling |
|
|
|
| Laboratory |
|
|
|
| Documentation |
|
|
|
| Data storage |
|
|
|
| Data handling |
|
|
|
IRB, institutional review board; CITI, Collaborative Institutional Training Initiative; SOP, standard operating procedures; NABL, National Accreditation Board for Testing and Calibration Laboratories; CAP, College of American Pathologists.
Strategies for cohort retention
The CARRS study has so far achieved impressive participant retention rates. Our well-trained, enthusiastic field staff employ multiple retention strategies: (i) openly communicating with participants and responding quickly to questions or problems that may arise; ii) providing participants with their data, i.e. blood pressure, anthropometry and biospecimen (blood and urine) findings, at a promised time and explaining the results of the reports; (iii) providing a lifestyle modification pamphlet to participants with abnormal biochemical parameters and providing diet charts to participants who request this information; (iv) facilitating getting appointments with local physicians or at AIIMS if the reports suggest the need for medical attention; (v) providing means to contact study staff (e.g. issuing pre-stamped postcards) in the event of changes in residential, contact or health status; (vi) offering non-monetary incentives (e.g. a wall clock with the CARRS logo) to individuals who assisted in facilitating the camps (collecting blood samples and anthropometric data), as a token of appreciation; and (vii) conducting interviews and other measurements in the privacy of the participants’ homes as per their convenient time. Furthermore, three attempts are made to trace relocated participants through neighbours, relatives and employers.
Database development and data management
From 2011 to 2018, data were collected on paper-based questionnaires and transferred into an electronic database. Since 2018, data collection was transitioned entirely to tablet-based surveys. Tablet-based questionnaires facilitate the easier acquisition of data (eliminates the transfer of data from paper forms to electronic databases), execution of skip patterns and application of instant data-checking rules. The co-ordinating team at Delhi is responsible for centralized data management across all sites. Standard operating procedures for data management, data dictionary and annotated forms for baseline and respective follow-up questionnaires have been developed at the co-ordinating centre
What has it found?
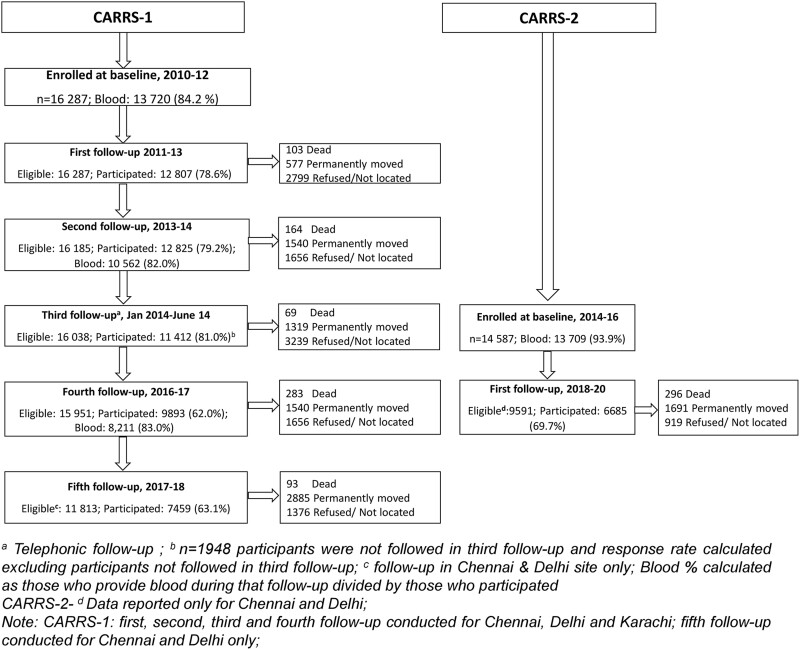
The overall number of participants recruited at baseline and at each follow-up is shown in Figure 1. In CARRS-1, 17 274 individuals from 10 002 households were approached in three study sites (7596 participants in Chennai, 5420 in Delhi and 4258 in Karachi). The overall response rate was 94.3% at the participant level. Overall, 13 720 of the participants (84.2%) recruited into the study contributed biospecimens (Supplementary Table S1, available as Supplementary data at IJE online).
Figure 1.
Flow chart of participants
In CARRS-2, 17 139 individuals in 13 842 households were approached: 5626 participants in Chennai, 6416 in Delhi and 5097 in Karachi. The overall response rate was 85.1% at the participant level. Table 3 shows the baseline characteristics of CARRS-1 and -2 participants. Thus far, the CARRS study has followed 30 874 individuals aged ≥20 years (27 429 with biospecimens) and accrued ∼115 000 person-years of follow-up (Table 4). In CARRS-1, 95% of the participants had at least one follow-up. Participants with at least one follow-up and those with no follow-up data were largely similar, with the exception of employment status (see Supplementary Table S2, available as Supplementary data at IJE online). In CARRS-2, the response rate for the first follow-up was 69.7% (Figure 1). The median (interquartile range) duration of follow-up (years) for CARRS-1 and -2 were 5.7 (5.1, 6.1) and 3.9 (3.6, 4.2) years, respectively (Table 4).
Table 3.
Baseline characteristics for CARRS-1 and -2
| CARRS-1 (N = 16 287) |
CARRS-2 (N = 14 587) |
|||
|---|---|---|---|---|
| Variable(s) | N | N | ||
| 16 287 | 14 587 | |||
| Age (years), mean (SD) | 42.4 (13.3) | 43.9 (13.4) | ||
| Male, n (%) | 7758 (47.7) | 6755 (46.3) | ||
| Education, n (%) | ||||
| Up to primary schooling | 3604 (22.1) | 3628 (24.8) | ||
| High school to secondary | 9924 (60.9) | 8386 (57.5) | ||
| Graduation and above | 2759 (16.9) | 2573 (17.7) | ||
| Income (INR), n (%) | ||||
| <10 000 | 11 537 (71.3) | 8212 (57.2) | ||
| 10 000–20 000 | 2667 (16.5) | 3694 (25.7) | ||
| ≥20 000 | 1975 (12.2) | 2450 (17.1) | ||
| Employed, n (%) | 7636 (46.9) | 6976 (46.5) | ||
| Current tobacco use, n (%) | 3757 (23.1) | 3463 (23.7) | ||
| Current alcohol use, n (%) | 2376 (14.6) | 1940 (13.3) | ||
| Anthropometric and biochemical measures | ||||
| Body mass index (kg/m2), mean (SD) | 12 472 | 25.5 (5.2) | 14 397 | 25.9 (5.4) |
| Height (cm), mean (SD) | 13 795 | 158.3 (9.4) | 14 478 | 159 (9.3) |
| Weight (kg), mean (SD) | 12 531 | 63.7(13.8) | 14 407 | 65.4 (14.5) |
| Systolic blood pressure, mean (SD) | 15 486 | 122.8(19.9) | 14 505 | 125.4 (20.1) |
| Diastolic blood pressure, mean (SD) | 15 486 | 81.4(11.9) | 14 505 | 81.1 (11.7) |
| Laboratory parameters | ||||
| Total cholesterol (mg/dL), mean (SD) | 13 717 | 179.8 (39.4) | 13 708 | 174.8 (39.2) |
| Triglycerides (mg/dL), median (P25, P75) | 13 716 | 121 (87, 172) | 13 708 | 119 (85.4, 170.4) |
| High-density lipoprotein cholesterol (mg/dL), mean (SD) | 13 713 | 43.3(11.4) | 13 706 | 42.3(10.9) |
| Low-density lipoprotein cholesterol (mg/dL), mean (SD) | 13 432 | 109.7(32.1) | 13 415 | 104.5 (32.4) |
| HbA1c %, mean (SD) | 13 633 | 6.2(1.5) | 13 693 | 6.1(1.5) |
| Fasting plasma glucose (mg/dL), mean (SD) | 13 720 | 109.2(43.7) | 13 709 | 109(46.8) |
| Plasma glucose at 30 min (mg/dL), mean (SD) | 4005c | 158.5(49) | 5982a | 161.5(48.6) |
| Plasma glucose at 120 min (mg/dL), mean (SD) | 4059c | 116.3(60.3) | 5903a | 121.3(61.7) |
| Insulin fasting (µU/ml), median (P25, P75) | 7858a | 8.6 (5.8, 12.7) | 4455b | 10 (6.4, 15.3) |
| Insulin at 30 min (µU/ml), median (P25, P75) | 3613c | 46.2 (32, 71.2) | 2714b | 74.2 (46.9, 115.4) |
| Insulin at 120 min (µU/ml), median (P25, P75) | 3613c | 35.7 (24.1, 59.7) | 2648b | 46 (25.9, 84.7) |
| Serum urea (mg/dL), mean (SD) | 13 708 | 22.5(9.1) | 13 705 | 23(8.5) |
| Serum creatinine (mg/dL), mean (SD) | 13 703 | 0.8(0.4) | 13 701 | 0.7(0.3) |
| Urine microalbumin (mg/dL) median (P25, P75) | 13 327 | 2.6 (1.1, 6.6) | 13 219 | 3 (0.7,6.9) |
| Urine creatinine (mg/dL), mean (SD) | 9843a | 74.6 (45.5, 122) | 13 220 | 83.0 (49.2, 137) |
| Apolipoprotein A (mg/dL), median (P25, P75) | 4244b | 123.9 (110.9, 138.7) | na | |
| Apolipoprotein B (mg/dL), median (P25, P75) | 4244b | 94.9 (78.8, 112.4) | na | |
| HsCRP (mg/L), median (P25, P75) | 9085a | 2.75 (1.21, 5.66) | na | |
Low-density lipoprotein cholesterol calculated using Friedwald formula.
HbA1c, glycated haemoglobin; HsCRP, high-sensitivity C-reactive protein; P25, 25th percentile; P75, 75th percentile; INR, Indian rupee; na, not available.
Data available only for Chennai and Delhi.
Data available for Delhi only.
Data available for Chennai only.
Table 4.
CARRS-1 & -2 duration of follow-up and deaths reported
| CARRS-1 | CARRS-2 | |
|---|---|---|
| Number surveyed at baseline | 16 287 | 14 587 |
| Number with blood taken at baseline | 13 720 | 13 709 |
| Duration of follow-up, years, median (P25, P75) | 5.7 (5.1, 6.1) | 3.9 (3.6, 4.2)a |
| Accumulated person-years since baseline | 89 488 | 24 117a |
| All cause deaths, n | 712 | 296a |
CARRS-1: first, second, third and fourth follow-up conducted for Chennai, Delhi and Karachi; fifth follow-up conducted for Chennai and Delhi only.
P25, 25th percentile; P75, 75th percentile.
CARRS-2 data reported only for Chennai and Delhi.
The CARRS study has enriched the literature on cardiometabolic diseases in urban South Asia. Cross-sectional findings indicate a scientifically informative distribution of exposures at individual and environmental levels, weight status and patterns of phenotypical presentation. The cohort has revealed that urban South Asians, on average, have a high lifetime risk of type 2 diabetes, even in normal weight individuals,8 and a high prevalence of type 2 diabetes (28%),42 dyslipidaemia,43,44 hypertension (29%)45 and hepatic steatosis at younger ages and relatively low body mass index (BMI) [underweight/normal weight prevalence (43%)].46 We have also documented a high prevalence of multimorbidity, with steep mortality risks as the number of morbidities grows and the link between chronic disease and quality of life.47 Significant findings from the CARRS study also indicate the relationship between socioeconomic disparities in health-related quality of life score, demonstrating the significantly lower health-related quality of life in key demographic groups and those with chronic conditions48 as well as the association between sleep and hypertension, drawing attention to lifestyle-related risk factors.49 Comparison of temporal changes in diabetes prevalence and achievement of diabetes care goals were conducted by comparing baseline data for CARRS-1 and -2; we found that between 2010 and 2016, the prevalence of self-reported diabetes increased as did glycaemic and lipid control among those with diabetes.50
Furthermore, the CARRS study has facilitated comparisons of resident South Asians with other populations through data pooling with global studies and cohorts. These include cross-national and multi-ethnic studies examining the role of vegetarian diet in cardiometabolic disease,51,52 risk factors for diabetes and prediabetes,53 the incidence of diabetes9,10 and the prevalence of chronic kidney disease.31,54,55 These studies have demonstrated the continued disproportionately high risk of cardiometabolic conditions and kidney disease in urban South Asians relative to other ethnic groups and relative to residents of other regions.
What are the main strengths and weaknesses?
Limitations
First, CARRS relies on self-reported measures of some behaviours (e.g. diet, physical activity, sleep). There is an opportunity in the future to add wearable technologies and more precise measurements, at least in subsamples, to validate the self-reported measures. Second, the cohort is representative of urban South Asia, limiting generalizablity to rural areas in the overall populations of these settings. Concurrently, we have adapted the CARRS protocol to measure non-communicable disease (NCD) burdens in rural populations in separate studies, such as the Solan Surveillance Study in Himachal Pradesh, India,56 and UDAY study in Sonipat and Vishakhapatnam.57 Furthermore, rapid socioeconomic development in South Asia is bringing expanded urbanization in previously rural areas and also rural-to-urban migration. Therefore, the lessons learned from the CARRS cohort will be valuable as the rest of the region also urbanizes. Over 60% of South Asia is projected to be urban by 2030. Third, whereas we have created a biorepository, we have not yet done genomic, epigenomic and metabolomic analyses, but plans are under way to make this possible. It has taken persistence and major effort to maintain and retain the cohort. One ongoing challenge is the need for infrastructural funding to sustain the cohort and to energize investigators to focus on grants related to scientific questions to answer, rather than always be on the lookout for programme funding.
Strengths
Over the past 10 years, CARRS has built a strong and extensive network of collaborators and interdisciplinary investigators, a robust field- and data-co-ordinating unit and robust and flexible international partnerships of high scientific quality.31,43,51,54,55,58,59 The study has been an invaluable resource in the region, fostering collaborations among 20 unique institutions across five continents and 70 investigators across multiple disciplines. These collaborations have already led to three large funded chronic disease intervention studies,60–63 four training grants64 and many smaller grants.
The cohort infrastructure has many scientific strengths. Those enrolled are representative of two major cities in India and one megacity in Pakistan, and its findings can be of value to major population growth centres in South Asia and beyond. Furthermore, CARRS was established using representative sampling methods to recruit adult samples at two time points, (CARRS-1 and -2), which allows for secular comparison and longitudinal follow-up with the accumulation of person-years which enables the study of incidence. The study also has recruited adults as young as 20 years old, much younger than the enrolment for most studies of CVD in adults. This is critical in a population where NCD and its risk factors emerge at younger ages than the global average. In addition, CARRS has high response rates at recruitment and high retention rates at follow-up. The study also has a biorepository of over 360 000 aliquots of sera, plasma, buffy coat and urine samples of the Indian participants stored in a well-maintained laboratory in India, which can be used in the future. The high-quality data collected from this study have enabled the application of conventional and customized statistical, machine learning and bioinformatics methods, as well as the pursuit of methodological innovations. Last, a very important part of this cohort is the collection of multiple sources of data on causes of death; the verbal autopsy data collected in CARRS overcome the issue of poor records on causes of deaths in India and Pakistan, where the majority of deaths occur at home.
Future directions
Together with conventional risk factors, stored biorepository data can be used to better understand the granular natural history and pathophysiological pathways (by incorporating molecular, protein-based and cellular biomarkers) of diverse CVD and diabetes phenotypes. In the near term, the cohort will be leveraged to investigate pathophysiological, environmental, genomic and sociobehavioural exposures on subclinical and clinical cardiometabolic disease phenotypes, with implications for the prevention and treatment of conditions such as heart failure and diabetes. The future establishment of an offspring cohort through recruiting the children of participants may facilitate investigations into the development of NCDs over the life course and familial origins of the disease.
Can I get hold of the data? Where can I find out more?
Data from CARRS-1 baseline and first three follow-up visits are available through the NHLBI BioLINCC.65 For additional information and collaboration, please contact the investigators: Dr KM Venkat Narayan, e-mail: [knaraya@emory.edu] and Dr Dorairaj Prabhakaran, email: [dprabhakaran@ccdcinida.org].
CARRS Investigators Group
Steering Committee: Dorairaj Prabhakaran, KM Venkat Narayan, Nikhil Tandon, V Mohan, Muhammed M Kadir, Mohammed K Ali. Names that follow of institutions, investigators and team members are listed in alphabetical order: Aga Khan University (AKU), Karachi: Zafar Fatami, Muhammed M Kadir; All India Institute of Medical Science (AIIMS), New Delhi: Deksha Kapoor, Lakshmy Ramakrishnan, Nikhil Tandon; Centre for Chronic Disease Control (CCDC)/Public Health Foundation of India (PHFI), New Delhi: Vamadevan S Ajay, Ruby Gupta, Naveen Kaushik, Dimple Kondal, Sailesh Mohan, Dorairaj Prabhakaran, Garima Rautela, Roopa Shivashankar; Emory University, Atlanta, USA: Mohammed K Ali, Mark Hutcheson, Unjali P Gujral, Ram Jaganathan, Shivani A Patel, Lisa Staimez, KM Venkat Narayan. Madras Diabetes Research Foundation (MDRF), Chennai: RM Anjana, M Deepa, V Mohan, R Pradeepa; data collection team: Chennai: M Kumar, JV Anthony, A Arul Dass, L Dhanasekar, L Gomathi, D Kalaivani, M Lenin, M Nandhakumar, K Parthiban, G Sampath, P Saravana Kumar, R Saravanan, G Shobana, T Suresh, S Tamilselvi, D Velmurugan, A Vivek, A Xavier Suresh, A Chiranjeevi A; laboratory staff: Akila N, Bharathy B, Irin Jayakumari A, Revathy M, SatishRaj S, Vijay Baskar S; data entry operator and scanner: Nirmala N. Delhi: field supervisor: Tripurari Prasad Singh; field interviewers: Anita Yadav, Anshu Arya, Kafal Kashyap, Makhan Kumar, Arun Dhillon, Arshit Kondal, Manju Sharma, Minakshi lal, Rukhsar Kazmi, Virendra Kumar, Sonia Mehta, Ravi Kumar, Lalit Kumar and Baldev Dhiman; laboratory staff: Savita Dhatwalia; Priyanka Nautiyal, Darshan Bisht; Geeta Singh, Narendra Dutt, Tarun Kumar, Ram Narayan Singh; data entry operators: Naresh Kumar, Sanjeev Sakral, Pankaj Sharma, Rajesh Singh; information technology and database development: Ramanathan K, Naveen Kaushik. Karachi: field supervisor: Mehboob John Samuel; field interviewers and laboratory assistants: Yousuf Sadiq, Shahirah Ziarat Khan, Nadia Khan, Zubida Parveen, Parkash, Muhammad Iqbal, Chambali, Mohan Lal, Naseem Doulat, Muhammad Ejaz, Shahaida, Ghulam Nabi, Shahina, Jehan Bibi, AKU laboratory; data entry operators and scanner: Shahirah, Mohan.
Ethics approval
Ethical approval for this study was provided by the Institutional Review Boards (IRBs) of PHFI (IRB00006658), AIIMS (IEC/NP-17/07.09.09), MDRF (MDRF/EC/EPI/2009/10) Chennai, India, AKU (1468-CHS-ERC-2010) and Emory University (IRB00044159). In addition, the study has received regulatory approval from the Health Ministry Screening Committee (HMSC) of India.
Supplementary data
Supplementary data are available at IJE online.
Author Contributions
D.P., K.M.V.N., M.K.A., N.T., V.M. designed the study. D.P., K.M.V.N., M.K.A., M.M.K., N.T., V.M., M.D., R.S., V.S.A., S.M. directed the study’s implementation. G.R., De.K., R.G. helped in study’s implementation. D.K., S.A.P., K.M.V.N. drafted the manuscript. D.K., S.A.P., K.M.V.N., M.K.A., D.P., N.T. designed the analytical strategy and helped to interpret the findings. D.P., N.T., M.K.A., V.M., U.P.G. and all listed authors provided critical comments for manuscript.
Funding
The CARRS Study was funded in part by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under Contract No. HHSN268200900026C, and the United Health Group, Minneapolis, MN, USA. Several members of the research team at PHFI, Emory University and CCDC were/are supported by the Fogarty International Clinical Research Scholars—Fellows programme (FICRS-F) through Grant Number 5R24TW007988 from NIH, Fogarty International Center (FIC) through Vanderbilt University, Emory’s Global Health Institute and D43 NCDs in India Training Program through Award Number 1D43HD05249 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) and FIC. However, the contents of this paper are solely the responsibility of the writing group and do not necessarily represent the official views of FIC, Vanderbilt University, Emory University, PHFI, NICHD or the NIH. K.M.V.N., M.K.A., S.A.P. were funded in part by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number P30DK111024. K.M.V.N. was funded in part for the ‘Worksite Lifestyle Program for Reducing Diabetes and Cardiovascular Risk in India’ project funded by NHLBI, NIH, Department of Health and Human Services under Award Number R01HL125442. S.A.P., K.M.V.N., M.K.A., N.T., D.P. were supported in part by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH), Award Number 5U01HL138635 under the Hypertension Outcomes for T4 Research within Lower Middle-Income Countries (Hy-TREC) program. R.S. was supported by a Wellcome Trust Capacity Strengthening Strategic Award Extension phase to the Public Health Foundation of India and a consortium of UK universities (WT084754/Z/08/A) and was supported by Grant Number 1 D43 HD065249 from the Fogarty International Center and the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health. D.K. has been supported by Fogarty International Center for PH leader Course, National Institutes of Health under Grant Number D43TW009135.
Supplementary Material
Acknowledgements
This study is co-ordinated by CoE-CARRS (Center of Excellence—Center for Cardiometabolic Risk Reduction in South Asia) based at the Public Health Foundation of India (PHFI), New Delhi, India in collaboration with the Centre for Chronic Disease Control (CCDC), New Delhi, Emory University, Atlanta, USA, All India Institute of Medical Sciences (AIIMS), New Delhi, Madras Diabetes Research Foundation (MDRF), Chennai, India and Aga Khan University, Karachi, Pakistan. We hereby, acknowledge the contributions of the field and research staff of the ‘CARRS Surveillance Investigators Group’ (a list of all members is included above). We would also like to acknowledge all the members of CARRS Team and CARRS participants for their contribution.
Conflict of interest
None declared.
Contributor Information
Dimple Kondal, Public Health Foundation of India, New Delhi, India; Centre for Chronic Disease Control, New Delhi, India.
Shivani A Patel, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Mohammed K Ali, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Deepa Mohan, Madras Diabetes Research Foundation, Chennai, India.
Garima Rautela, Centre for Chronic Disease Control, New Delhi, India.
Unjali P Gujral, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Roopa Shivashankar, Centre for Chronic Disease Control, New Delhi, India.
Ranjit Mohan Anjana, Madras Diabetes Research Foundation, Chennai, India.
Ruby Gupta, Public Health Foundation of India, New Delhi, India.
Deksha Kapoor, All India Institute of Medical Sciences, New Delhi, India.
Ajay S Vamadevan, Centre for Chronic Disease Control, New Delhi, India; Healthcare management, Goa Institute of Management, Sanquelim, Goa, India.
Sailesh Mohan, Public Health Foundation of India, New Delhi, India.
Muhammad M Kadir, Aga Khan University, Karachi, Pakistan.
Viswanathan Mohan, Madras Diabetes Research Foundation, Chennai, India.
Nikhil Tandon, All India Institute of Medical Sciences, New Delhi, India.
Dorairaj Prabhakaran, Public Health Foundation of India, New Delhi, India; Centre for Chronic Disease Control, New Delhi, India.
K M Venkat Narayan, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
References
- 1. Gujral UP, Vittinghoff E, Mongraw-Chaffin M. et al. Cardiometabolic abnormalities among normal-weight persons from five racial/ethnic groups in the United States: a cross-sectional analysis of two cohort studies. Ann Intern Med 2017;166:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Narayan KMV, Aviles-Santa L, Oza-Frank R. et al. ; Cardiovascular Disease in Asian and Pacific Islander Populations NHLBI Working Group. Report of a National Heart, Lung, And Blood Institute Workshop: heterogeneity in cardiometabolic risk in Asian Americans in the U.S. opportunities for research. J Am Coll Cardiol 2010;55:966–73. [DOI] [PubMed] [Google Scholar]
- 3. Shah AD, Kandula NR, Lin F. et al. Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: results from the MASALA and MESA studies. Int J Obes 2016;40:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta LS, Wu CC, Young S, Perlman SE.. Prevalence of diabetes in New York City, 2002-2008. Comparing foreign-born South Asians and other Asians with U.S.-born Whites, Blacks, and Hispanics . Diabetes Care 2011;34:1791–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Volgman AS, Palaniappan LS, Aggarwal NT. et al. ; American Heart Association Council on Epidemiology and Prevention; Cardiovascular Disease and Stroke in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation 2018;138:e1–e34. [DOI] [PubMed] [Google Scholar]
- 6. Joshi SR, Anjana RM, Deepa M. et al. ; ICMR-INDIAB Collaborative Study Group. Prevalence of dyslipidemia in urban and rural India: the ICMR-INDIAB study. PLoS One 2014;9:e96808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anjana RM, Deepa M, Pradeepa R, Mahanta J. et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol 2017;5:585–96. [DOI] [PubMed] [Google Scholar]
- 8. Luhar S, Kondal D, Jones R. et al. Lifetime risk of diabetes in metropolitan cities in India. Diabetologia 2021;64:521–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Narayan KMV, Kondal D, Kobes S. et al. Incidence of diabetes in South Asian young adults compared with Pima Indians. BMJ Open Diabetes Res Care 2021;9:e001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Narayan KMV, Kondal D, Daya N. et al. Incidence and pathophysiology of diabetes in South Asian adults living in India and Pakistan compared with US blacks and whites. BMJ Open Diabetes Res Care 2021;9:e001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Enas EA, Mohan V, Deepa M, Farooq S, Pazhoor S, Chennikkara H.. The metabolic syndrome and dyslipidemia among Asian Indians: a population with high rates of diabetes and premature coronary artery disease. J Cardiometab Syndr 2007;2:267–75. [DOI] [PubMed] [Google Scholar]
- 12. Unnikrishnan R, Anjana RM, Mohan V.. Diabetes in South Asians: is the phenotype different? Diabetes 2014;63:53–55. [DOI] [PubMed] [Google Scholar]
- 13. Prasad DS, Kabir Z, Dash AK, Das BC.. Abdominal obesity, an independent cardiovascular risk factor in Indian subcontinent: a clinico epidemiological evidence summary. J Cardiovasc Dis Res 2011;2:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kruk ME, Gage AD, Arsenault C. et al. High-quality health systems in the sustainable development goals era: time for a revolution. Lancet Glob Health 2018;6:e1196–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahmood SS, Levy D, Vasan RS, Wang TJ.. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 2014;383:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nair M, Ali MK, Ajay VS. et al. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health 2012;12:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Census of India. https://censusindia.gov.in/census_and_you/area_and_population.aspx (March 2021, date last accessed).
- 18. World Population Review. https://worldpopulationreview.com/world-cities/karachi-population (February 2021, date last accessed).
- 19. WHO: STEPwise Approach to Surveillance (STEPS). https://www.who.int/ncds/surveillance/steps/en/ (March 2021, date last accessed).
- 20. Hagströmer M, Oja P, Sjöström M.. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 2006;9:755–62. [DOI] [PubMed] [Google Scholar]
- 21. WHO. Global Physical Activity Questionnaire (GPAQ) Analysis Guide. Geneva: World Health Organization, 2012, 1–22.
- 22. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v. 3). Washington, D.C.: FHI 360/FANTA, 2007.
- 23.Preedy VR, Ronald R. Handbook of Disease Burdens and Quality of Life Measures. New York: Springer, 2010. [Google Scholar]
- 24. Maroufizadeh S, Omani-Samani R, Almasi-Hashiani A, Amini P, Sepidarkish M.. The reliability and validity of the Patient Health Questionnaire-9 (PHQ-9) and PHQ-2 in patients with infertility. Reprod Health 2019;16:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kishore J, Kapoor V, Reddaiah VP.. The composite international diagnostic interview (CIDI): its reliability and applicability in a rural community of northern India. Indian J Psychiatry 1999;41:350–57. [PMC free article] [PubMed] [Google Scholar]
- 26. UK Biobank-Mental Health Web-Based Questionnaire. https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/mental_health_online.pdf (January 2021, date last accessed).
- 27. Mandal S, Madhipatla KK, Guttikunda S, Kloog I, Prabhakaran D, Schwartz JD.. Ensemble averaging based assessment of spatiotemporal variations in ambient PM(2.5) concentrations over Delhi, India, during 2010-2016. Atmos Environ 2020;224:117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel O, Shahulhameed S, Shivashankar R. et al. Association between full service and fast food restaurant density, dietary intake and overweight/obesity among adults in Delhi, India. BMC Public Health 2017;18:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hulman A, Gujral UP, Narayan KMV. et al. Glucose patterns during the OGTT and risk of future diabetes in an urban Indian population: The CARRS study. Diabetes Res Clin Pract 2017;126:192–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin SS, Giugliano RP, Murphy SA. et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins Estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the FOURIER trial. JAMA Cardiol 2018;3:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anand S, Kondal D, Montez-Rath M. et al. Prevalence of chronic kidney disease and risk factors for its progression: a cross-sectional comparison of Indians living in Indian versus U.S. cities. PLoS One 2017;12:e0173554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis AV, James TJ, McGuire JB, Taylor RP.. Improved immunoturbidimetric assay for cystatin C. Ann Clin Biochem 2001;38:111–14. [DOI] [PubMed] [Google Scholar]
- 33. Sultana R, Kataki AC, Borthakur BB, Basumatary TK, Bose S.. Imbalance in leptin-adiponectin levels and leptin receptor expression as chief contributors to triple negative breast cancer progression in Northeast India. Gene 2017;621:51–58. [DOI] [PubMed] [Google Scholar]
- 34. Ammar KA, Jacobsen SJ, Mahoney DW. et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007;115:1563–70. [DOI] [PubMed] [Google Scholar]
- 35. Goldman L, Hashimoto B, Cook EF, Loscalzo A.. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation 1981;64:1227–34. [DOI] [PubMed] [Google Scholar]
- 36. Cardiometabolic Risk Reduction in South Asia: The CARRS Cohort. 2021. https://www.carrsprogram.org/study-documents (March 2021, date last accessed).
- 37. Westgard JO, Barry PL, Hunt MR, Groth T.. A multi-rule Shewhart chart for quality control in clinical chemistry. Clin Chem 1981;27:493–501. [PubMed] [Google Scholar]
- 38. RANDOX. 2021. https://www.randox.com/external-quality-assessment/ (March 2021, date last accessed).
- 39. UK NEQAS International Quality Expertise. https://ukneqas.org.uk/ (March 2021, date last accessed).
- 40. Jha P, Gajalakshmi V, Gupta PC. et al. ; RGI-CGHR Prospective Study Collaborators. Prospective study of one million deaths in India: rationale, design, and validation results. PLoS Med 2006;3:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gajalakshmi V, Peto R, Kanaka S, Balasubramanian S.. Verbal autopsy of 48 000 adult deaths attributable to medical causes in Chennai (formerly Madras), India. BMC Public Health 2002;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deepa M, Grace M, Binukumar B. et al. High burden of prediabetes and diabetes in three large cities in South Asia: The Center for cArdio-metabolic Risk Reduction in South Asia (CARRS) study. Diabetes Res Clin Pract 2015;110:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel SA, Shivashankar R, Ali MK. et al. ; CARRS Investigators. Is the “South Asian Phenotype” unique to South Asians?: comparing cardiometabolic risk factors in the CARRS and NHANES Studies. Glob Heart 2016;11:89–96.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fatmi Z, Kondal D, Shivashankar R. et al. Prevalence of dyslipidaemia and factors associated with dyslipidaemia among South Asian adults: The Center for Cardiometabolic Risk Reduction in South Asia Cohort Study. Natl Med J India 2020;33:137–45. [DOI] [PubMed] [Google Scholar]
- 45. Prabhakaran D, Jeemon P, Ghosh S. et al. Prevalence and incidence of hypertension: Results from a representative cohort of over 16,000 adults in three cities of South Asia. Indian Heart J 2017;69:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ali MK, Bhaskarapillai B, Shivashankar R. et al. ; CARRS investigators. Socioeconomic status and cardiovascular risk in urban South Asia: The CARRS Study. Eur J Prev Cardiol 2016;23:408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Singh K, Patel SA, Biswas S. et al. Multimorbidity in South Asian adults: prevalence, risk factors and mortality. J Public Health (Oxf) 2019;41:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh K, Kondal D, Shivashankar R. et al. Health-related quality of life variations by sociodemographic factors and chronic conditions in three metropolitan cities of South Asia: the CARRS study. BMJ Open 2017;7:e018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shivashankar R, Kondal D, Ali MK. et al. Associations of sleep duration and disturbances with hypertension in metropolitan cities of Delhi, Chennai, and Karachi in South Asia: cross-sectional analysis of the CARRS study. Sleep 2017;40:vsx119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anjana RM, Deepa M, Subashini R. et al. Temporal changes in diabetes prevalence and achievement of care goals in urban South Asia from 2010 to 2016. The Center for Cardio-metabolic Risk Reduction in South Asia Study. Diabet Med 2021;38:e14424. [DOI] [PubMed] [Google Scholar]
- 51. Gujral UP, Mohan V, Pradeepa R, Deepa M, Anjana RM, Narayan KM.. Ethnic differences in the prevalence of diabetes in underweight and normal weight individuals: The CARRS and NHANES studies. Diabetes Res Clin Pract 2018;146:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jaacks LM, Kapoor D, Singh K, Narayan KMV. et al. Vegetarianism and cardiometabolic disease risk factors: differences between South Asian and US adults. Nutrition 2016;32:975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gujral UP, Narayan KMV, Pradeepa RG. et al. Comparing type 2 diabetes, prediabetes, and their associated risk factors in Asian Indians in India and in the U.S.: The CARRS and MASALA Studies. Diabetes Care 2015;38:1312–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anand S, Shivashankar R, Ali MK. et al. ; CARRS Investigators. Prevalence of chronic kidney disease in two major Indian cities and projections for associated cardiovascular disease. Kidney Int 2015;88:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. O’Callaghan-Gordo C, Shivashankar R, Anand S. et al. Prevalence of and risk factors for chronic kidney disease of unknown aetiology in India: secondary data analysis of three population-based cross-sectional studies. BMJ Open 2019;9:e023353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Agarwal A, Jindal D, Ajay VS. et al. Association between socioeconomic position and cardiovascular disease risk factors in rural north India: The Solan Surveillance Study. PLoS One 2019;14:e0217834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mohan S, Jarhyan P, Ghosh S. et al. UDAY: a comprehensive diabetes and hypertension prevention and management program in India. BMJ Open 2018;8:e015919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Singh K, Patel SA, Biswas S. et al. Multimorbidity in South Asian Adults: prevalence, Risk Factors and Mortality. J Public Health (Oxf). 2019;41:8–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Berg CJ, Ajay VS, Ali MK. et al. A cross-sectional study of the prevalence and correlates of tobacco Use in Chennai, Delhi, and Karachi: Data from the CARRS study. BMC Public Health 2015;15:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shah S, Singh K, Ali MK. et al. ; CARRS Trial Writing Group. Improving diabetes care: multi-component cardiovascular disease risk reduction strategies for people with diabetes in South Asia: the CARRS multi-center translation trial. Diabetes Res Clin Pract 2012;98:285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patel SA, Sharma H, Mohan S. et al. The Integrated Tracking, Referral, and Electronic Decision Support, and Care Coordination (I-TREC) program: scalable strategies for the management of hypertension and diabetes within the government healthcare system of India. BMC Health Serv Res 2020;20:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dhawan J, Bray CL.. Relationship between angiographically assessed coronary artery disease, plasma insulin levels and lipids in Asians and Caucasians. Atherosclerosis 1994;105:35–41. [DOI] [PubMed] [Google Scholar]
- 63. Ali MK, Chwastiak L, Poongothai S. et al. ; for the INDEPENDENT Study Group. Effect of a collaborative care model on depressive symptoms and glycated hemoglobin, blood pressure, and serum cholesterol among patients with depression and diabetes in India: the INDEPENDENT randomized clinical trial. JAMA 2020;324:651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Walia GK, Mandal S, Jaganathan S. et al. Leveraging existing cohorts to study health effects of air pollution on cardiometabolic disorders: India Global Environmental and Occupational Health Hub. Environ Health Insights 2020;14:1178630220915688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. National Heart, Lung, and Blood Institute. https://biolincc.nhlbi.nih.gov/studies/ghcoe_new_delhi/ (March 2021, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.