Abstract
Objective:
Nutrition and physical activity are key components of daily diabetes care in young children with type 1 diabetes (T1D). Normative developmental behavioral challenges related to nutrition and physical activity complicate management of T1D. The current pilot study evaluated the feasibility, acceptability, and indications of behavior change of an intervention aimed at improving nutrition and physical activity in young children with T1D.
Methods:
Thirty-six parents of young children (ages 2–5 years, M=4.2) with T1D from two clinics in the Washington, DC area were randomized to receive the Type One Training (TOTs) program or Usual Care (UC). Assessments included recruitment and completion rates, participant acceptability, and outcomes including glycemic variability via continuous glucose monitoring, nutritional intake via remote food photography, physical activity via accelerometers, and parental report on behavior and psychosocial functioning.
Results:
Despite recruitment challenges, the TOTs program was feasible to administer, with high program and assessment completion rates. Acceptability ratings were very high but differed by recruitment site. Participants randomized to TOTs had an increase in percent of time in target glycemic range and reduction in behavioral feeding problems between baseline and follow-up while those randomized to UC did not. Participants in UC demonstrated a decrease in in moderate to vigorous physical activity at follow-up.
Conclusions:
The TOTs program demonstrated preliminary feasibility and acceptability. Future research will examine components of treatment for evidence of efficacy and target the intervention to those most likely to benefit.
Type 1 diabetes (T1D) occurs in 1 in 500–600 children and incidence is increasing annually, with higher growth in young children ages 0–9 years (Dabelea et al., 2014; Mayer-Davis et al., 2017). T1D management requires strict adherence to daily complex and time-consuming regimens to delay or prevent the onset of acute and chronic T1D-related complications (Chiang et al., 2014). For young children with T1D, parents assume full responsibility for daily blood glucose (BG) monitoring and insulin administration. Only 23% of children ages 2–5 meet current recommendations for glycemic targets (A1c < 7.5%; Miller et al., 2015) with children this age demonstrating a mean A1c of 8.2% (Foster et al., 2019). Children who have A1c outside of glycemic targets, particularly those recently diagnosed, are at higher risk for development of serious diabetes-related complications (Svensson et al., 2004). Therefore, families of young children with diabetes may benefit from additional supports for daily T1D management during these early years.
Behavioral interventions supporting T1D management among parents of young children could significantly impact both staying within glycemic targets and child development to reduce the incidence of immediate and long-term consequences of T1D. There are indications of impact of suboptimal glycemic management on the developing brain (Jaser & Jordan, 2021). To date, only a few small-scale behavioral interventions have targeted parents of young children with T1D. Although these interventions have demonstrated potentially promising psychosocial outcomes (Monaghan et al., 2011; Sullivan-Bolyai et al., 2004), concomitant improvements in children’s glycemic management remain elusive and understudied with few notable exceptions (e.g., Patton et al., 2014). Two critical areas in diabetes management that have not yet been addressed directly in existing interventions for young children with T1D include the promotion of healthy eating and engagement in consistent physical activity. Healthy eating and physical activity serve as both the building blocks for optimal health and cornerstones of diabetes management.
One of the most important predictors of long-term health complications is high BG levels following meals (Dzygalo & Szypowska, 2014). Nutrition management is a key component of diabetes management, and predicting a young child’s food intake before mealtime can be very challenging for parents. Breakfast may be particularly challenging as it most commonly includes highly palatablebut less healthy options that quickly increase BG levels with negative effects on BG trajectories through the afternoon and into the next day (Nilsson et al., 2012; Sweenie et al., 2014). Research also indicates the importance of protein consumption at breakfast for reducing post-prandial glycemic variability, indicating an opportunity for improving glycemic variability by increasing protein intake at breakfast (Monzon, Smith, Powers, Dolan, & Patton, 2020). Moreover, parents of young children with T1D report greater negative child mealtime behaviors including food refusal, difficulties following parental requests, and throwing tantrums, as well as lower confidence managing these behaviors compared to parents of children without diabetes (Patton et al., 2004; Powers et al., 2002; Sundberg et al., 2014).
Regular engagement in physical activity is an important component of optimal diabetes management, and may result in health benefits such as improved cardiovascular health and insulin sensitivity for children with T1D (Colberg et al., 2016). However, participating in consistent physical activity can be challenging, especially as physical activity tends to decline beginning as young as ages 3–4 (Taylor et al., 2013). Young children have unpredictable physical activity patterns (Bailey et al., 1995; Baquet et al., 2007) and parents may avoid activity due to fears of hypoglycemia, or may not appropriately alter their diabetes management in response to physical activity (Yardley et al., 2013). There are gaps in knowledge regarding the association of physical activity and glycemic management in young children with T1D and evaluations of the impact of interventions that examine linkages among eating, physical activity, and glycemic management in these young children will be critical for informing future clinical guidelines (Tully et al., 2016).
The current study reports on the evaluation of feasibility, acceptability, and preliminary efficacy of the Type One Training (TOTs) program, a multicomponent healthy eating and physical activity intervention for parents of young children with T1D. This was the second phase of the overall project that had a goal to develop a feasible and acceptable intervention ready for evaluation in a larger efficacy trial at the end of the funding period. This second phase included conducting a pilot randomized controlled trial (RCT); the first involved development and pre-pilot testing with 10 participants (Tully et al., 2018). It was hypothesized that the TOTs program would be feasible, acceptable, and the measures used to assess clinical outcomes would show potential for utility as indicators of change, particularly to those randomized to the TOTs program in comparison with those randomized to Usual Care (UC).
Methods
Participants
Primary caregivers (in the current study, all were parents) of young children with T1D were recruited from a diabetes clinic located within a pediatric academic hospital. An additional local affiliate clinic was added as a recruitment site due to slow recruitment over the first 8 months of the study due to the small population of patients in the target age group (see Figure 1). In total, 36 parent-child dyads participated (M parent age = 36.3 (6.2), M child age = 4.2 (0.9) (see Table 1)). Fifty-three percent of the families recruited were from the primary recruitment site and 47% from the affiliate outpatient clinic. There were differences by site in terms of income and method of measuring glycemic variability (see Table 1). Families were eligible to participate if their child was 2–5 years of age and had been diagnosed with T1D for at least 1 year. Caregivers had to be at least 21 years of age and fluent in English. Exclusions from participation included if the child had been diagnosed with a developmental delay or had a co-occurring serious medical illness that would impact their daily management of diabetes (i.e. cancer, cystic fibrosis). There was no hemoglobin A1c (HbA1c) requirement nor exclusion of any insulin regimens or technologies.
Figure 1.
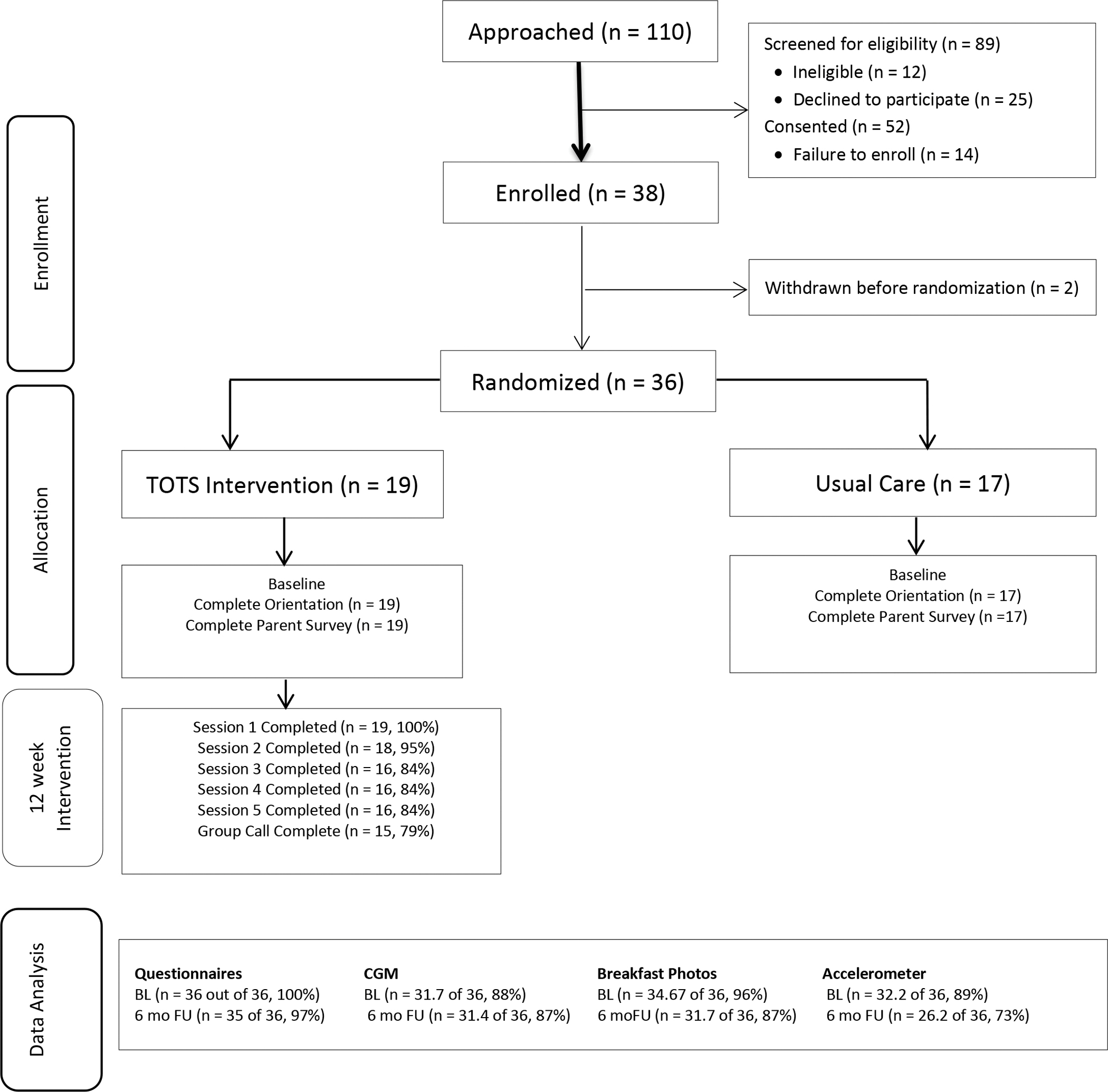
CONSORT Diagram.
Table 1.
Participant Demographics
| Variable | N (%) | Primary Site (N=19) | Affiliate Site (N=17) | p-value |
|---|---|---|---|---|
|
| ||||
| Annual Household Income * | ||||
| >$100,000 (%) | 15 (45.5) | 5 (26.3) | 10 (71.4) | 0.02 |
| ≤100,000 (%) | 18 (54.65) | 14 (73.7) | 4 (28.6) | |
|
| ||||
| Child Race | ||||
| White | 26 (74.3) | 11 (61.1) | 15 (88.2) | 0.12 |
| Black or African-American | 8 (22.2) | 7 (36.8) | 1 (5.9) | |
| Asian | 2 (5.9) | 1 (5.3) | 1 (5.9) | |
|
| ||||
| Child Ethnic Background | ||||
| Hispanic or Latino | 2 (5.6) | 1 (5.3) | 1 (5.9) | 1.00 |
| Not of Hispanic or Latino | 34 (94.4) | 18 (94.7) | 16 (94.1) | |
|
| ||||
| Mean Child Age (years) | 4.7 (0.9) | 4.3 (0.9) | 5.1 (0.8) | 0.05 |
|
| ||||
| Mean Parent Age (years) | 36.3 (6.2) | 34.3 (6.8) | 38.5 (4.7) | 0.04 |
|
| ||||
| Child Gender | ||||
| Male | 23 (63.9) | 12 (63.2) | 11 (64.7) | 0.92 |
| Female | 13 (36.1) | 7 (36.8) | 6 (35.3) | |
|
| ||||
| Mean # of caregivers in the home ** | ||||
| 1 | 4 (11.1) | 2 (10.5) | 2 (11.8) | 0.82 |
| 2 | 29 (80.6) | 15 (79.0) | 14 (82.4) | |
| 3+ | 5 (8.4) | 2 (10.6) | 1 (5.9) | |
|
| ||||
| Marital Status (%) | ||||
| Married | 25 (71.4) | 10 (55.6) | 15 (88.2) | 0.06 |
| Not Married | 10 (28.6) | 8 (44.4) | 2 (11.8) | |
|
| ||||
| Education | ||||
| 12th grade/partial college | 9 (25.0) | 7 (36.8) | 2 (11.8) | 0.13 |
| College/Graduate/Professional | 27 (75.0) | 12 (63.2) | 15 (88.2) | |
|
| ||||
| Work Schedule | ||||
| Full-time | 21 (58.3) | 11 (57.9) | 10 (58.8) | 0.74 |
| Part-time | 3 (8.3) | 1 (5.3) | 2 (11.8) | |
| Not employed/Student | 12 (33.3) | 7 (36.8) | 5 (29.4) | |
|
| ||||
| Regimen | ||||
| Insulin Pump | 9 (25.0) | 5 (26.3) | 4 (23.5) | 0.16 |
| Fixed dose insulin regimen | 9 (25.0) | 7 (36.8) | 2 (11.8) | |
| Multiple daily injections/Basal-Bolus | 18 (50.0) | 7 (36.8) | 11 (64.7) | |
|
| ||||
| CGM | ||||
| Yes, Currently | 25 (69.4) | 10 (52.6) | 15 (88.2) | 0.06 |
| No | 11 (30.86) | 9 (47.4) | 2 (11.8) | |
This cutoff was determined using the approximate median income for the region in which the study was conducted.
Caregiver refers to individuals who self-identified as providing care for the child with T1D.
Procedures
The hospital’s Institutional Review Board approved the study protocol and all participants provided written informed consent. Participants were recruited by study staff using clinic lists to identify eligible patients, sending recruitment letters, and conducting follow-up phone calls to describe the project, assess eligibility and interest, and obtain verbal consent to participate.
Caregivers completed baseline procedures, including online self-report questionnaires via REDCap (Harris et al., 2009), and an in-person study orientation session lead by a trained research assistant at the diabetes clinic. At orientation, caregivers were randomized on a 1:1 basis to receive the intervention (TOTs) or UC. Assessment was repeated at 3- (follow up 1) and 6-months (follow up 2) following randomization which occurred following the intervention. Caregivers completed additional assessments of their child’s breakfast nutrition, physical activity, and glycemic variability for the week following randomization.
TOTs Intervention Design
The TOTs parenting support intervention integrated tailored behavioral strategies that promote healthy eating, physical activity, and glycemic management. Specifically, the proximal goals were to 1) increase protein and decrease carbohydrate intake at breakfast and 2) increase moderate to vigorous physical activity, with the distal goal being to improve glycemic management and reduce time spent out of target ranges for blood glucose. The program included five phone sessions with a master’s level trained clinical social worker (referred to as “phone counselor”) and one in-person session with a Certified Diabetes Care and Education Specialist and the counselor. One of the sessions was a group call among two to three participants. The phone counselor received extensive training on T1D management, including shadowing providers, attending diabetes education sessions, and reviewing written materials. The phone counselor also received training on behavioral intervention tailoring and goal setting. The phone sessions lasted 40–60 minutes and the in-person session lasted about 1.5 hours. The interventionist completed a fidelity checklist after each session indicating the topics covered during the session. All sessions were scheduled at the participant’s convenience over 9–12 weeks to allow for flexibility for participant needs and schedules. Each session was designed to use a combination of behavioral and educational strategies including facts about T1D in young children, parent day-to-day management of T1D, parenting skills such as managing child behavior, self-monitoring, mealtime strategies, and developing consistent routines and habits (see Figure 2). In conjunction with the sessions, participants received three text messages each week with tips, encouragement, and links with access to the study website that included reiterating messages delivered during the intervention. For example, participants were encouraged to be persistent with trying new foods and provided links to the study website that reminded participants of helpful approaches in encouraging new foods.
Figure 2.
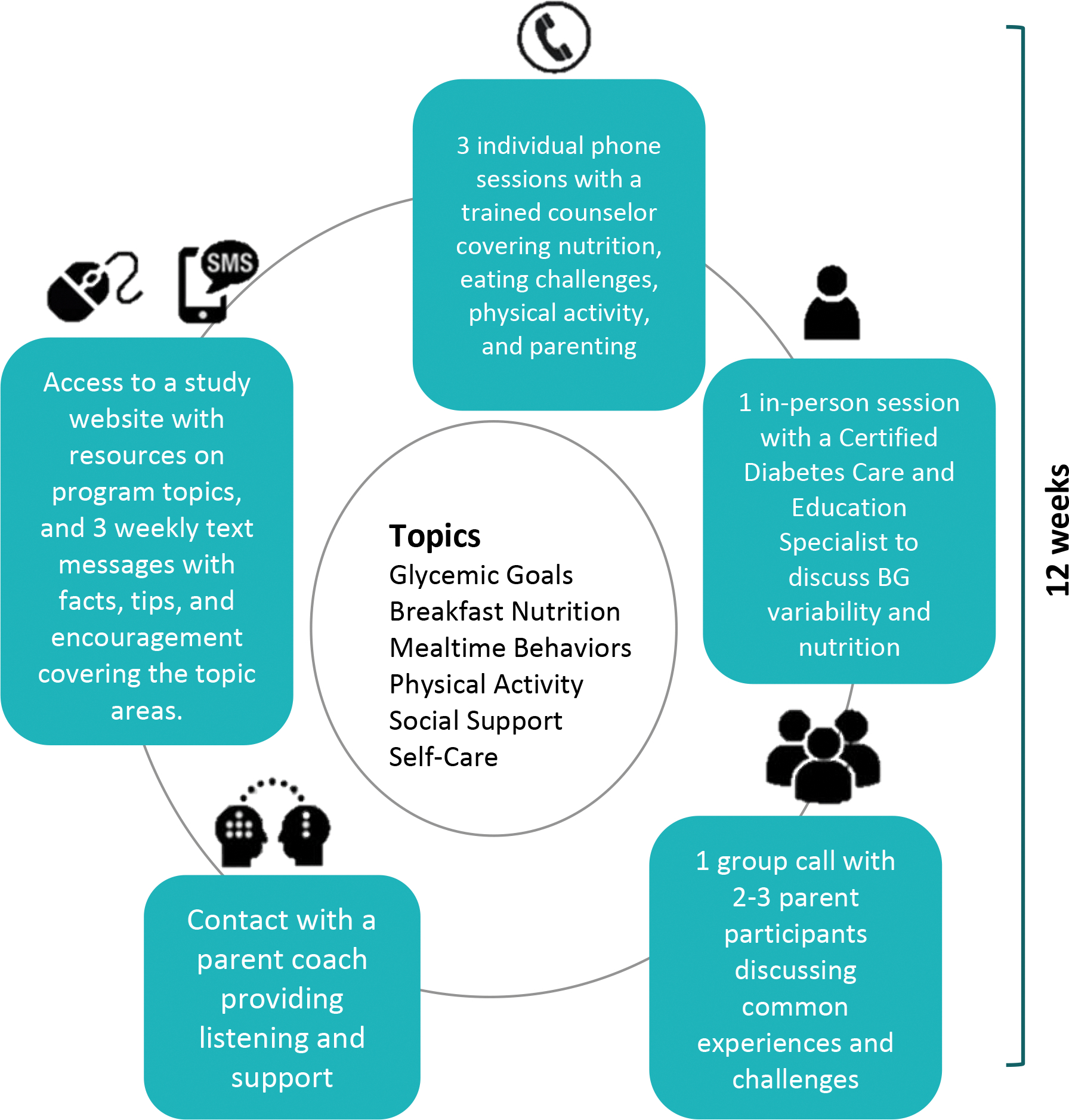
Overview of TOTs intervention
Participants also worked with a trained parent coach who provided support and T1D management strategies with a focus on healthy eating and physical activity. Parent coaches were parents of children who were diagnosed with T1D at a young age and were referred to the project by the medical team. Training involved a half-day meeting and independent work covering research conduct and ethics training (via the CITI program), training in active listening, and support skills. They were asked to contact their assigned participants by phone, text message, or email to provide ongoing social support throughout the program and followed a schedule for frequency of initiating contact across the intervention period. Parent coaches received supervision by a licensed clinical psychologist who was part of the study team.
Measures
Demographic and Medical Variables
Parents provided self-report of demographic information. Medical record reviews were conducted by research staff to gather data on HbA1c as an overall index of glycemic management and height and weight to calculate the child’s body mass index percentile (BMI%). Glycemic variability was assessed using glucose data that were collected via Continuous Glucose Monitors (CGMs), which are minimally invasive devices that measure blood glucose levels every 5–15 minutes (Rewers et al., 2014). CGM data were collected for a period of 5 days through sharing data from personal devices (67% at baseline and 69% at follow-up 2), a blinded trial provided by the study (25% at baseline and 17% at follow-up), or not obtained (8% at baseline and 14% at follow-up). Time in range was calculated as an average of the percent of time in range across the five-day wear period.
Feasibility and Acceptability
Feasibility.
Recruitment feasibility was evaluated by examining rates of recruitment and points of participant attrition prior to baseline assessment via the CONSORT table (Figure 1). Retention feasibility was assessed by attrition by group following enrollment, adherence to phone treatment sessions, and rates and patterns of missing data.
Acceptability.
For all participants randomized to receive the intervention, specific aspects of acceptability were assessed via self-report (Streisand & Mednick, 2006) including: (a) the perceived usefulness of specific components of the intervention, (b) frequency of use of strategies, and (c) logistical arrangements (e.g., timing/length of sessions, use of text messaging).
Preliminary Outcome Variables
Nutrition intake was assessed via Remote Food Photography Method ((RFPM), Martin et al., 2012; Martin et al., 2008), a technology used to measure food intake in real world settings. RFPM has been used across a diverse range of populations such as children and adults with obesity, Type 2 diabetes, and in preschool children (Martin et al., 2012; Rose et al., 2018). The RFPM relies on participants using smartphones to take before and after photographs of their meals. The images are transmitted to Pennington Biomedical Research Center (PBRC) to analyze energy and nutrition intake (Martin et al., 2012; Martin et al., 2008). Participants texted breakfast photographs directly to our study staff for this study, who then sent the photographs and descriptions to PBRC for analyses. Participants collected three days of breakfast intake at baseline and follow-up. For the current study, given the focus of the intervention on increasing protein consumption and decreasing carbohydrates at breakfast, percent of protein and carbohydrates at breakfast were used as the nutrition outcomes of interest.
Glycemic management was assessed by HbA1c levels in addition to the percentage of time in range over a 5-day period, as determined by ISPAD guidelines as 70–180 mg/dL (DiMeglio et al., 2018) as an indicator of glycemic variability.
Physical activity was assessed through use of accelerometers, which track and record the frequency and magnitude of movement to estimate the duration and intensity of activity (sedentary, light, moderate, and vigorous) (Pulakka et al., 2013). Child participants in this study were provided small belts to place the accelerometer device in for 5 days. Though accelerometers have been used to assess physical activity and sleep in young children in prior research (Trost et al., 2011), there is no one formula that is well validated for both moderate-to-vigorous physical activity (MVPA) and sedentary time. Therefore, two separate formulas for scoring were used for the current study. The Pate Preschool formula was used in the calculation of MVPA and the Evenson formula was utilized for sedentary minutes (Alhassan et al., 2012; Cliff et al., 2009; Evenson et al., 2008; Hnatiuk et al., 2014; Pate et al., 2016; Schmutz et al., 2017). Percent of time spent in sedentary and moderate-to-vigorous physical activity were used as outcome measures.
Psychosocial
Child Eating and Parent Feeding Behaviors.
The Behavioral Pediatrics Feeding Assessment Scale (BPFAS) is a 35-item parent self-report measure used to assess child behavior around feeding and mealtime and parents’ feelings about or strategies used for addressing eating-related behavior problems (Crist & Napier-Phillips, 2001). The scale has demonstrated acceptable reliability and validity within normative and pediatric samples (α > 0.80) (Davis et al., 2014; Dobbelsteyn et al., 2008; Patton et al., 2006). Within the current sample, internal reliability was acceptable (α = 0.88–0.91).
Parent Mood.
Parent depressive symptoms have been associated with outcomes in previous parent-focused behavioral interventions and may be a secondary outcome or covariate in a future efficacy study (Mackey et al., 2016). Parent depressive symptoms were assessed using the Center for Epidemiological Studies-Depression Scale (CES-D) (Radloff, 1977). This 20-item self-report measures depressive affect, somatic symptoms, positive affect, and interpersonal relations. This measure has been used in previous studies evaluating behavioral interventions with parents of youth with T1D (e.g., Grey, Jaser, Whittemore, Jeon, & Lindemann, 2011). Internal consistency coefficients range from .85–.90 (Bartlett et al., 2001; Friis & Nanjundappa, 1986; Radloff, 1977). Internal consistency within the current sample was acceptable (α = 0.88).
Data Analytic Plan
Feasibility, acceptability data, and primary outcomes (A1c, % time in BG range, % of breakfast composed of protein, fat, and carbohydrates, minutes of MVPA and sedentary time, and child feeding challenges as measured by the BPFAS) were evaluated looking at descriptive data by group and using paired t-tests.
Results
The primary outcome of feasibility was examined using the recruitment and enrollment data (see Figure 1). Of families eligible for the study, 68% (n=52) consented to participate, but only 73% (n=38) of those who provided initial consent were enrolled and 69% (n=36 of 52) were randomized. The most common reason families declined to participate was study time burden.
Feasibility once enrolled was excellent, with high completion rates of the program and minimal missing data. For the intervention group, 79% completed all sessions and 84% completed all but one session (100% completed session 1, 95% completed session 2, 84% completed sessions 3, 4, and 5, and 79% participated in the group call). All participants completed baseline questionnaires, and 97% finished questionnaires at the follow-up. Feasibility of the assessments using technology was also high with CGM (85% at baseline and 87% at follow up), RFPM (94% at baseline and 87% at follow up), and accelerometers (89% at baseline and 73% at follow up).
Acceptability with regards to participant perspective was also excellent, with some differences by recruitment site. Overall, 84% reported that they agreed or strongly agreed with the statement that they would suggest the use of the TOTs program to other parents of young children with T1D, felt that the program had a positive impact on their child’s T1D management, and were glad they participated in the program. Differences between sites were observed in some aspects of the perception of utility of the content of the program, with 100% of participants from the primary site reporting that they found the mealtime behavior and picky eating component useful while only 55% of parents from the affiliate site reported this component as useful. All participants from the primary site reported that the program met or exceeded their expectations, while only 64% of participants from the affiliate site reported that it met or exceeded expectations.
There were significant baseline differences by site in some demographics (the primary site had younger parents and more families with lower incomes; see Table 1), A1c (t(34) = 3.1, p<.01), and child eating problems as measured by the BPFAS (t(34) = 1.7, p<.10). To evaluate the utility of the outcome measures, we examined indicators of significant change from baseline to the second follow-up, by conducting paired t-tests comparing baseline and follow up for both TOTs and UC. In the TOTs group, there was a significant difference in % of time in range as measured by CGM (t(13) = 2.43, p<.05), with increases in time spent in range observed between baseline and follow up. There was no significant difference in changes to % time in range in the UC group. Likewise, participants in the TOTs group had significant improvements in BPFAS scores for both parent (t(17) = 3.90, p<.01) and child (t(17) = 3.06, p<.01) problems, as well as decreases in parental depressive symptoms (t(17) = 2.38, p<.05). In contrast, the only paired t-test that showed a significant change from baseline to follow up 2 in the UC group was MVPA in which participants showed a decrease in the percentage of time they spent engaged in MVPA (t(16) = 2.25, p<.05).
Discussion
Young children with T1D face a number of challenges adhering to their regimens and optimizing their glycemic management (Silverstein et al., 2005). Nutrition and physical activity are key factors critical to current health, as well as setting the foundation for future health (Dzygalo & Szypowska, 2014; Yardley et al., 2013). Parents of young children T1D often encounter normative developmental challenges that can make high quality nutrition and adequate physical activity difficult (Streisand & Monaghan, 2014). The results of the current pilot study demonstrate that a primarily phone-based intervention targeted to parents of young children with T1D aimed at improving nutrition and physical activity can be feasible to administer. The majority of parents reporting enthusiasm of the program and perceived benefit from participating.
A few notable findings can inform future research. Namely, there was unexpected difficulty with recruitment, with a number of families reporting perceived time burden of the intervention or the assessments as a key barrier to participation. Retention rates were similar to those from prior studies, but recruitment was lower than might be expected based on previous studies and the pre-pilot for the current study (Herbert et al., 2016; Tully et al., 2018). Adjustments to the assessments or the length of the intervention by shortening to accommodate busy schedules may help achieve planned enrollment or implementation in a clinic setting. Additionally, because the intervention was developed using previous research and qualitative feedback from families at the primary site, it may not have been as generalizable to other populations as anticipated. Specifically, one of the key findings of the current pilot study was that there were site differences, particularly in sociodemographic variables, at baseline. These differences may have affected how the intervention was perceived and the benefit provided. For example, the families at the primary site, on whose input the intervention was primarily based, had higher acceptability ratings than families at the affiliate site. Potential mechanisms of this finding will need to be evaluated in future research. Future research needs to ensure that the development stages of intervention development be broader with regards to demographics of the participants involved in the development phase if the resulting intervention is to be generalizable to a larger population than that for which it was originally developed (Stuart et al., 2015).
As this was a pilot study, it was not powered to detect statistical differences, but rather is meant to test the feasibility and acceptability of the intervention and examine any indication of hypothesized effect of the intervention on outcomes of interest (Mudd, 2017). In terms of signals for outcomes of interest to include in future efficacy trials, glycemic variability appears to have potential as a marker of impact of the intervention. Specifically, there was an increase in time spent in range for those in the TOTs group but not within the UC group. The nutrition variables demonstrated less variability between baseline and follow-up between either group, which could either indicate less of an impact of the intervention, a need to have a larger sample size to control for insulin regimen, as this has been found to be related to nutritional intake (Katz et al., 2014), or a different method of evaluating nutritional intake. Notably, child and parent behavior around mealtimes and eating as measured by the BPFAS also evidenced sufficient variability and potential response to the intervention in the expected direction. An important potential covariate that also indicated need for further study is caregiver depressive symptoms as measured by the CES-D. These are all variables that should be considered for use in a future efficacy trial.
These preliminary findings, particularly the acceptability ratings, indicate who might benefit from the intervention to target in a future trial. Specifically, families from the primary site, families with a child who had higher baseline A1c, more reported child eating problems, and higher scores on the parental depression measure may find this intervention more appealing and more useful. These findings were consistent with the preliminary findings noted in the pre-pilot of 10 participants who all received the TOTs program and were all recruited from the primary site (Tully et al., 2018). There were also differences in insulin regimen that may alter the impact of changes to nutrition on glycemic targets, such as fat intake having differing effects in those on pumps as compared to those using injections for insulin (Katz et al., 2014) and should be considered in future research. These findings indicate that the intervention has potential high value for families who may require additional support for suboptimal glycemic management, problematic child eating behaviors, and/or parental depression at baseline.
Given that recruitment was difficult, future research that is more targeted towards populations who might benefit from the intervention need to be carefully considered for designs that maximize power in small sample sizes. Additionally, minimizing assessment burden by utilizing the information generated by the current study to focus assessment protocols may help with recruitment. Possible future directions could include considering alternative study designs, such as a Multiphase Optimization Strategy (MOST) design, which utilizes a factorial design to test specific components of an intervention. This would allow for optimizing the number of components needed in the intervention, reducing the time burden by selecting only the components that contribute to the efficacy of the intervention. The MOST design is also well suited for evaluating the effect of an intervention on nested, or clustered, groups (Dziak et al., 2012). The MOST design could permit inclusion of those from a particular clinic population and incorporate pre-intervention screening methods (for example, screening for presence of problematic child eating behaviors) to improve targeting and power.
There are a number of clinical implications raised by the current study. For example, clinics could incorporate routine screening for behavioral eating problems in young children with T1D, akin to depression screening in older children. This type of screening could identify those families in need of targeted interventions to address picky eating or behavioral eating concerns that could be delivered in person or via telemedicine.
Limitations and Future Research.
The primary limitation to the current study is the small sample size. The current study was not powered to detect significant differences overall or, importantly, examine multiple potential moderators to treatment efficacy, such as site, regimen, and presence of baseline difficulties. Given site differences, the sample also does not allow for determination of how much of the intervention is generalizable beyond the population at the primary site. Future research will need to evaluate potential moderators, maximize benefit from the smallest number of intervention components to reduce participant burden, and assess for generalizability.
Table 2.
Baseline (BL) and 6-month Follow-Up (FU) values by group in M (SD).
| Variable | Overall | |||
|---|---|---|---|---|
| TOTS N=19 |
Usual Care N=17 |
|||
| BL | FU | BL | FU | |
| A1c | 8.2 (1.0) | 8.4 (1.4) | 8.3 (1.1) | 8.6 (1.4) |
| Avg. % time in range (CGM) | 39.8 (15.5) | 46.2 (15.8) | 41.0 (13.9) | 43.6 (18.5) |
| Avg. % Protein at Breakfast | 14.4 (4.0) | 15.9 (4.9) | 16.2 (4.4) | 15.4 (6.4) |
| Avg. % Carbohydrate at Breakfast | 50.4 (11.8) | 44.4 (15.1) | 48.6 (11.8) | 50.0 (16.6) |
| Avg. % Fat at Breakfast | 35.2 (9.5) | 39.7 (14.2) | 35.1 (12.8) | 34.6 (13.0) |
| Avg. % of time Sedentary | 43.7 (7.9) | 45.1 (9.0) | 44.7 (5.8) | 49.1 (10.3) |
| Avg. % of time in MVPA | 14.8 (4.9) | 14.4 (6.3) | 15.4 (4.7) | 12.8 (4.5) |
| BPFAS Child Problems | 5.1 (5.8) | 1.9 (2.6) | 3.9 (3.5) | 2.4 (2.8) |
| BPFAS Parent Problems | 2.1 (2.1) | 0.4 (0.7) | 1.9 (2.4) | 0.9 (1.5) |
| CES-D | 20.7 (8.0) | 17.3 (4.1) | 21.4 (12.4) | 19.8 (8.7) |
Note – Bolded pairs of means indicate significant difference (p<.05) between baseline and follow-up using paired t-tests.
Acknowledgements:
We are grateful for the expertise and contributions from Drs. Kathy Knafl, Leann Birch, and Janet Silverstein, as well as Ms. Jane Turek, and Ms. Megan Watts in the intervention development. We also thank the families of young children with type 1 diabetes who gave their time, opinions, trust, and research participation to help in the development of the TOTS program.
Funding:
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (grant number DP3 DK103998) to the last author. ClinicalTrials.gov Identifier: NCT02343146
References
- ADA. (2019). 12. Children and adolescents. Diabetes Care, 40(Supplement 1), S105–S113. [DOI] [PubMed] [Google Scholar]
- Alhassan S, Nwaokelemeh O, Ghazarian M, Roberts J, Mendoza A, & Shitole S (2012). Effects of locomotor skill program on minority preschoolers’ physical activity levels. Pediatric Exercise Science, 24(3), 435–229. [DOI] [PubMed] [Google Scholar]
- Bailey RC, Olson JODI, Pepper SL, Porszasz JANOS, Barstow TJ, & Cooper DM (1995). The level and tempo of children’s physical activities: an observational study. Medicine and Science in Sports and Exercise, 27, 1033–1033. [DOI] [PubMed] [Google Scholar]
- Baquet G, Stratton G, Van Praagh E, & Berthoin S (2007). Improving physical activity assessment in prepubertal children with high-frequency accelerometry monitoring: a methodological issue. Preventive Medicine, 44(2), 143–147. [DOI] [PubMed] [Google Scholar]
- Bartlett SJ, Kolodner K, Butz AM, Eggleston P, Malveaux FJ, & Rand CS (2001). Maternal depressive symptoms and emergency department use among inner-city children with asthma. Archives of Pediatrics & Adolescent Medicine, 155(3), 347–353. [DOI] [PubMed] [Google Scholar]
- Chiang J, Kirkman M, Laffel L, & Peters A (2014). Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care, 37, 2034–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff DP, Reilly JJ, & Okely AD (2009). Methodological considerations in using accelerometers to assess habitual physical activity in children aged 0–5 years. Journal of Science and Medicine in Sport, 12(5), 557–567. [DOI] [PubMed] [Google Scholar]
- Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, & Tate DF (2016). Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care, 39(11), 2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist W & Napier-Phillips A (2001). Mealtime behaviors of young children: A comparison of normative and clinical data. Journal of Developmental & Behavioral Pediatrics, 22, 279–286. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, … Crume T (2014). Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA, 311(17), 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A, Canter K, Stough C, Gillette MD, & Patton S (2014). Measurement of mealtime behaviors in rural overweight children: An exploratory factor analysis of the behavioral pediatrics feeding assessment scale. Journal of Pediatric Psychology, 39, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMeglio LA, Acerini CL, Codner E, Craig ME, Hofer SE, Pillay K, & Maahs DM (2018). ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatric Diabetes, Suppl.27, 105–114. [DOI] [PubMed] [Google Scholar]
- Dobbelsteyn C, Peacocke SD, Blake K, Crist W, & Rashid M (2008). Feeding difficulties in children with CHARGE syndrome: Prevalence, risk factors, and prognosis. Dysphagia, 23(2), 127–135. [DOI] [PubMed] [Google Scholar]
- Dziak JJ, Nahum-Shani I, & Collins LM (2012). Multilevel factorial experiments for developing behavioral interventions: Power, sample size, and resource considerations. Psychological Methods, 17, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzygalo K, & Szypowska A (2014). Impact of insulins glulisine and aspart on postprandial glycemia after a high-glycemic index meal in children with type 1 diabetes. European Journal of Endocrinology, 170, 539–545. [DOI] [PubMed] [Google Scholar]
- Evenson K, Catellier D, Gill K, Ondrak K, & McMurray R (2008). Calibration of two objective measures of physical activity for children. Journal of Sports Sciences, 26, 1557–1565. [DOI] [PubMed] [Google Scholar]
- Foster NC, Beck RW, Miller KM, et al. (2019). State of type 1 diabetes management and outcomes from the T1D exchange. Diabetes Technology & Therapeutics, 21(2), 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R, & Nanjundappa G (1986). Diabetes, depression and employment status. Social Science & Medicine, 23(5), 471–475. [DOI] [PubMed] [Google Scholar]
- Goonetilleke R, Pollitzer M, & Mann N (2004). Insulin for toddlers with difficult diabetes. Diabetes Care, 27(6), 1505. [DOI] [PubMed] [Google Scholar]
- Grey M, Jaser SS, Whittemore R, Jeon S, & Lindemann E (2011). Coping skills training for parents of children with type 1 diabetes: 12-month outcomes. Nursing Research, 60(3), 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert LJ, Gillespie C, Monaghan M, Holmes C, & Streisand R (2016). Factors associated with recruitment and retention in randomized controlled trials of behavioral interventions for patients with pediatric type 1 diabetes. Journal of Clinical Psychology in Medical Settings, 23(2), 112–125. [DOI] [PubMed] [Google Scholar]
- Hnatiuk JA, Salmon J, Hinkley T, Okely AD, & Trost S (2014). A review of preschool children’s physical activity and sedentary time using objective measures. American Journal of Preventive Medicine, 47(4), 487–497. [DOI] [PubMed] [Google Scholar]
- Jaser SS & Jordan LC (2021). Brain health in children with type 1 diabetes: Risk and protective factors. Current Diabetes Reports, 21, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, Mehta S, Nansel T, Quinn H, Lipsky LM, & Laffel LM (2014). Associations of nutrient intake with glycemic control in youth with type 1 diabetes: differences by insulin regimen. Diabetes Technology & Therapeutics, 16(8), 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey ER, Herbert L, Monaghan M, Cogen F, Wang J, & Streisand R (2016). The feasibility of a pilot intervention for parents of young children newly diagnosed with type 1 diabetes. Clinical Practice in Pediatric Psychology, 4(1), 35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Correa JB, Han H, Allen HR, Rood JC, Champagne CM, … Bray GA (2012). Validity of the Remote Food Photography Method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity, 20(4), 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Han H, Coulon SM, Allen HR, Champagne CM, &, & Anton SD (2008). A novel method to remotely measure food intake of free-living individuals in real time: the remote food photography method. British Journal of Nutrition, 101(3), 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, & Pihoker C (2017). Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. New England Journal of Medicine, 376(15), 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, … Tamborlane WV (2015). Current state of type 1 diabetes treatment in the US: updated data from the T1D Exchange clinic registry. Diabetes Care, 38(6), 971–978. [DOI] [PubMed] [Google Scholar]
- Monaghan M, Hilliard ME, Cogen FR, & Streisand R (2011). Supporting parents of very young children with type 1 diabetes: Results from a pilot study. Patient Education and Counseling, 82(2), 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzon AD, Smith LB, Powers SW, Dolan LW, & Patton SR (2020). The association between glycemic variability and macronutrients in young children with T1D. Journal of Pediatric Psychology, 45(7), 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd L (2017). Uses and Misuses of Pilot Studies. https://nccih.nih.gov/research/blog/uses-pilot-studies. Accessed February 25, 2020.
- Nilsson A, Radeborg K, & Björck I (2012). Effects on cognitive performance of modulating the postprandial blood glucose profile at breakfast. European Journal of Clinical Nutrition, 66, 1039–1043. [DOI] [PubMed] [Google Scholar]
- Pate RR, Brown WH, Pfeiffer KA, Howie EK, Saunders RP, Addy CL, & Dowda M (2016). An intervention to increase physical activity in children: a randomized controlled trial with 4-year-olds in preschools. American Journal of Preventive Medicine, 51, 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton S, Dolan L, Mitchell M, Byars K, Standiford D, & Powers S (2004). Mealtime interactions in families of preschoolers with T1 diabetes. Pediatric Diabetes, 5, 190–198. [DOI] [PubMed] [Google Scholar]
- Patton S, Dolan L, & Powers S (2006). Parent report of mealtime behaviors in young children with type 1 diabetes mellitus: Implications for better assessment of dietary adherence problems in the clinic. Journal of Developmental & Behavioral Pediatrics, 27, 202–208. [DOI] [PubMed] [Google Scholar]
- Patton S, Odar C, Midyett LK, & Clements MA (2014). Pilot study results for a novel behavior plus nutrition intervention for caregivers of young children with type 1 diabetes. Journal of Nutrition Education and Behavior, 46(5), 429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SW, Byars KC, Mitchell MJ, Patton SR, Standiford DA, & Dolan LM (2002). Parent report of mealtime behavior and parenting stress in young children with type 1 diabetes and in healthy control subjects. Diabetes Care, 25(2), 313–318. [DOI] [PubMed] [Google Scholar]
- Pulakka A, Cheung YB, Ashorn U, Penpraze V, Maleta K, Phuka JC, & Ashorn P (2013). Feasibility and validity of the ActiGraph GT 3X accelerometer in measuring physical activity of Malawian toddlers. Acta Paediatrica, 102(12), 1192–1198. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Rewers MJ, Pillay K, De Beaufort C, Craig ME, Hanas R, Acerini CL, & Maahs DM (2014). Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatric Diabetes, 15(S20), 102–114. [DOI] [PubMed] [Google Scholar]
- Rose MH, Streisand R, Aronow L, Tully C, Martin CK, & Mackey E (2018). Preliminary feasibility and acceptability of the remote food photography method for assessing nutrition in young children with type 1 diabetes. Clinical Practice in Pediatric Psychology, 6(3), 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz EA, Leeger-Aschmann CS, Radtke T, Muff S, Kakebeeke TH, Zysset AE, & Munsch S (2017). Correlates of preschool children’s objectively measured physical activity and sedentary behavior: a cross-sectional analysis of the SPLASHY study. International Journal of Behavioral Nutrition and Physical Activity, 14(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, & Clark N (2005). Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care, 28(1), 186–212. [DOI] [PubMed] [Google Scholar]
- Streisand R, & Mednick L (2006). Development of the diabetes education, counseling, information delivery and evaluation (DECIDE) program: a health promotion intervention for preadolescents with type 1 diabetes. Journal of Clinical Psychology in Medical Settings, 13(2), 180–190. [Google Scholar]
- Streisand R, & Monaghan M (2014). Young children with type 1 diabetes: challenges, research, and future directions. Current Diabetes Reports, 14, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart EA, Bradshaw CP, & Leaf PJ (2015). Assessing the generalizability of randomized trial results to target populations. Prevention Science, 16(3), 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Grey M, Deatrick J, Gruppuso P, Giraitis P, & Tamborlane W (2004). Helping other mothers effectively work at raising young children with type 1 diabetes. The Diabetes Educator, 30(3), 476–484. [DOI] [PubMed] [Google Scholar]
- Sundberg F, Augustsson M, Forsander G, Cederholm U, & Axelsen M (2014). Children under the age of seven with diabetes are increasing their cardiovascular risk by their food choices. Acta Paediatrica, 103(4), 404–410. [DOI] [PubMed] [Google Scholar]
- Svensson M, Eriksson JW, & Dahlquist G (2004). Early glycemic control, age at onset, and development of microvascular complications in childhood-onset type 1 diabetes: a population-based study in northern Sweden. Diabetes Care, 27(4), 955–962. [DOI] [PubMed] [Google Scholar]
- Sweenie R, Mackey E, & Streisand R (2014). Parent–child relationships in type 1 diabetes: Associations among child behavior, parenting behavior, and pediatric parenting stress. Families, Systems, & Health, 32(1), 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R, Williams S, Farmer VL, & Taylor BJ (2013). Changes in physical activity over time in young children: a longitudinal study using accelerometers. PloS one, 8, e81567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost SG, Loprinzi PD, Moore R, & Pfeiffer KA (2011). Comparison of accelerometer cut points for predicting activity intensity in youth. Medicine & Science in Sports & Exercise, 43(7), 1360–1368. [DOI] [PubMed] [Google Scholar]
- Tully C, Aronow L, Mackey E, & Streisand R (2016). Physical activity in youth with type 1 diabetes: A review. Current Diabetes Reports, 16 (9), 1–8. [DOI] [PubMed] [Google Scholar]
- Tully C, Mackey ER, Aronow L, Monaghan M, Henderson C, Cogen F, Wang J, & Streisand R (2018). Parenting intervention to improve nutrition and physical activity for preschoolers with type 1 diabetes: A feasibility study. Journal of Pediatric Health Care, 32, 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tine ML, McNicholas F, Safer DL, & Agras WS (2017). Follow-up of selective eaters from childhood to adulthood. Eating Behaviors, 26, 61–65. [DOI] [PubMed] [Google Scholar]
- Yardley J, Mollard R, MacIntosh A, MacMillan F, Wicklow B, Berard L, & McGavock J (2013). Vigorous intensity exercise for glycemic control in patients with type 1 diabetes. Canadian Journal of Diabetes, 37(6), 427–432. [DOI] [PubMed] [Google Scholar]


