Implications.
The role of living microbes on swine health is undeniable. However, antimicrobial properties should not be solely based on selecting essential oils in swine production. Instead of higher doses required for their killing pathogens properties, using lower doses of essential oils could prevent inflammation and “leaky gut” induced by lipopolysaccharide.
It is critical to look at the total blend of raw materials available for feed formulation, their inclusion levels, costs, feed preference as well as the targeted feed acid-binding capacity value and then make the final decision based on science and field experience.
Minimizing mycotoxin contamination in feeds is an important component to control post-weaning diarrhea. Chemical approaches, such, as the use of sodium metabisulfite and biological approaches, such as the use of microorganisms for detoxification, have shown promise in reducing vomitoxin.
Introduction
Weaned piglets are highly susceptible to many stressors including bacterial pathogens, oxidative stress, and inflammation, which predisposes pigs to post-weaning diarrhea, eventually leading to reduced growth performance, high mortality and morbidity rates, and compromised animal welfare (Yang et al., 2015a, 2015b; Hassan et al., 2018). Since post-weaning diarrhea commonly results from the proliferation of pathogenic Escherichia coli, antibiotic growth promoters have been widely used in piglet diets, especially in nursery diets, to control incidences of diarrhea during the transition. Total consumption of antimicrobials in animal food production worldwide was estimated at 63,151 tons in 2010, with an increasing trend; the annual consumption of antimicrobials per kilogram body weight was 148 mg/kg for pigs (Van Boeckel et al., 2015). This practice may lead to the spread of antimicrobial-resistant bacterial pathogens in pigs and humans, challenging the sustainability of the pork industry (Yang et al., 2015a, 2015b). With environmental, health, and safety concerns, the public demands antibiotic-free pork (e.g., raised without antibiotics). However, the withdrawal of antibiotics from feeds can result in several challenges including compromised gut health and increased gut diseases. So far, we do not have a single “magic bullet” that can replace in-feed antibiotics. Although different types of alternatives to antibiotics (e.g., essential oils and probiotics) have been widely recognized as promising alternatives to antibiotics in feeds, an integrated approach to control post-weaning diarrhea should be taken, including supplementation of antibiotic alternatives, and measures related to nutrition, biosecurity, and management. In this review article, we reviewed the use of essential oils as antibiotic alternatives, the use of ingredients to lower dietary acid-binding capacity (ABC), and the use of innovative chemical and biological approaches to detoxify vomitoxin, which may be considered important parts of an integrated approach to control post-weaning diarrhea in piglets.
Using Essential Oils as Antibiotic Alternatives
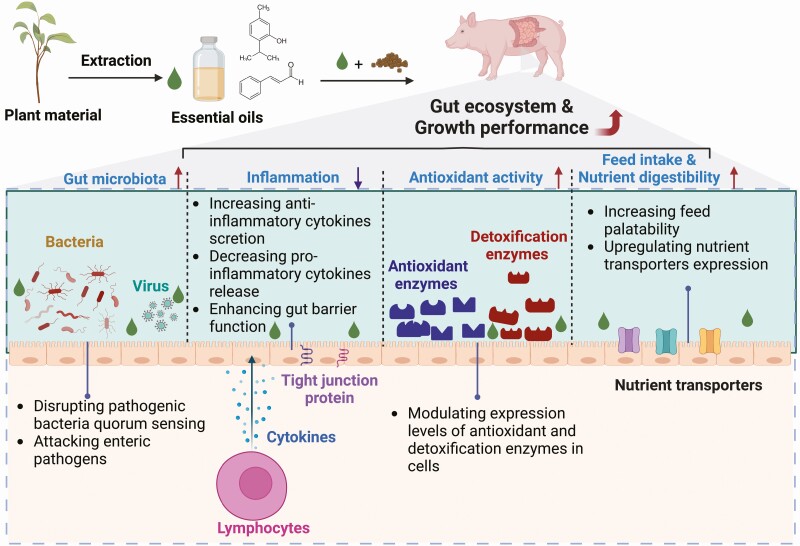
Essential oils have antioxidative, anti-inflammatory, and antimicrobial properties. Some essential oils (e.g., thymol, eugenol, and cinnamaldehyde) have been widely used to replace antibiotics in swine production mainly because of these essential oils’ antimicrobial properties (Yang et al., 2015a, 2015b; Hassan et al., 2018; Omonijo et al., 2018a). The adverse effects of pathogenic microbes on swine health are undeniable. However, antimicrobial properties should not be solely based on selecting alternatives to antibiotics in swine production (Gresse et al., 2017; Fouhse et al., 2016; Figure 1). Lipopolysaccharides, also called endotoxins, are cell wall components of Gram-negative bacteria (e.g., Salmonella and Escherichia) that are present everywhere in the environment including in the intestine, ground, air, and water, and have received much attention due to their ability to stimulate a low-grade inflammation in pigs (Huang et al., 2016; Nordgreen et al., 2018). One of the negative consequences of inflammation at the intestine level is increased intestinal permeability, or “leaky gut,” associated with impaired nutrient absorption and increases in diarrhea incidence (Gresse et al., 2017). Interestingly, in addition to antimicrobial properties, thymol (50 µM, 7.5 mg/kg), eugenol (100 µM, 16.4 mg/kg), and citral (10 to 20 µM, 1.52 to 3.04 mg/kg) can reduce inflammation associated with lipopolysaccharide or peptidoglycan in porcine intestinal epithelial cells (Omonijo et al., 2019; Hui et al., 2020; Li et al., 2022a, 2022b) and cinnamaldehyde (25 µM, 3.3 mg/kg) can improve intestinal mucosal barrier function (Sun et al., 2017). These levels are much lower than the minimum inhibitory concentration of thymol and eugenol against major pathogens (Yang et al., 2015a, 2015b; Omonijo et al., 2018a). Moreover, the minimum inhibitory concentration of most essential oils is much higher than the acceptable levels in the animal industry in terms of cost-effectiveness and feed palatability (Omonijo et al., 2018a). Since the industry’s acceptance of essential oils also depends on inclusion cost, those results are encouraging producers to use lower doses of essential oils to prevent inflammation and “leaky gut” induced by lipopolysaccharide instead of using higher doses required for their killing pathogens properties. Moreover, using higher doses has drawbacks on optimal feed intake and cost.
Figure 1.
Schematic diagram illustrating the four different potential mechanisms by which essential oils improve the gut ecosystem and growth performance of piglets.
The combined use of essential oils and other additives should lead to higher advantages. For two reasons, a combination of other alternatives with essential oils holds the most promise as a substitute for antibiotics in pig feeds. First, no single antibiotic alternative has been reported to be able to replace antibiotics completely. Second, a combination of products can have a synergistic effect that will reduce the effective dosages required to combat pathogens (e.g., organic acids and essential oils). For example, essential oil can change the structure and functions of bacterial cell membranes. This results in membrane swelling and thus increased membrane permeability, leading the bacteria toward an increased susceptibility to organic acids. Moreover, the hydrophobicity (being water-repellent) of essential oil is increased at low pH. Combining essential oils with organic acids will enable the essential oil to pass through the lipids of the bacterial cell membrane more easily.
Essential oils exhibit great potential to prevent post-weaning diarrhea. However, their direct inclusion in pig diets has compromised efficacy because of such factors as low stability, poor palatability, and low availability in the lower gut. Therefore, an effective and practical delivery method is very important for the use of essential oils in swine production. It is documented that the formulation of microparticles could effectively deliver thymol and lauric acid to the pig intestinal tract. Lauric acid not only acts as a carrier for thymol but also has synergistic antibacterial effects with thymol (Omonijo et al., 2018b). The stability of thymol in commercial lipid matrix microparticles (encapsulated essential oils and organic acids) was investigated during feed pelleting and feed storage and determined the intestinal release of thymol (Choi et al., 2020a). The thymol concentration was not significantly different in the mash and pelleted feeds, suggesting that the pelleting of feed did not affect total thymol in those lipid matrix microparticles. Encapsulated thymol was also stable in simulated pig gastric fluid (26.0% thymol released). The rest of the thymol was progressively released in the simulated intestinal fluids until completion, which was achieved within 24 h. In a pig experiment, 15.5% of thymol was released in the stomach, and 41.9% of thymol was delivered in the mid-jejunum section, demonstrating a slow release, and 2.2% of thymol was recovered in feces (Choi et al., 2020a). The lipid matrix microparticles maintained the stability of thymol during the feed pelleting process and storage and allowed a slow and progressive intestinal release of thymol in weaned pigs (Choi et al., 2020a). Subsequent studies were conducted to investigate further the effects of these commercial lipid matrix microparticles on growth performance, immune system, gut barrier function, nutrient digestion, and absorption in disease-challenged weaned piglets and demonstrated that the supplementation of those lipid matrix microparticles showed anti-diarrhea effects in disease-challenged weaned piglets (Choi et al., 2020b; Xu et al., 2020). Therefore, microencapsulated essential oil and organic acid combination can be a useful method to control post-weaning diarrhea in swine production.
Using Probiotics to Improve Gut Health
Direct-fed probiotics refer to live microorganisms supplied to the host with adequate amounts benefiting the host (Pluske, 2013). The benefits of providing direct-fed probiotics into swine diets are categorized into several aspects:1) benefiting gut health by modifying the composition of enteric microflora (Sartor, 2004); 2) promoting immunity (Yan and Polk, 2011), 3) increasing efficiency of nutrient digestion and utilization (Yadav and Jha, 2019) and 4) enhancing gut function and improving growth performance (Vohra et al., 2016). Direct-fed probiotics contain three main categories: Bacillus, lactic acid-producing bacteria, and yeast (Kerr et al., 2013). Bacillus is a potent producer of extracellular fiber-degrading enzymes, which can increase nutrient digestibility and utilization (Kiarie and Mills, 2019). In addition, Bacillus synthesizes enzymes that degrade feed and produces short-chain fatty acids through fermentation (Merchant et al., 2011). Those short-chain fatty acids are considered a useful energy source utilized by pigs to develop the large intestine (den Besten et al., 2013). Recent studies have identified the protective effect of Bacillus strains on intestinal cells challenged with enteric pathogenic bacteria, since they found the pre-treatment of Bacillus could upregulate tight junction protein expression and decrease quorum sensing (Chen et al., 2021; Li et al., 2022a, 2022b). For weaned piglets, including lactic acid-producing bacteria in their dies could relieve weaning stress, reduce diarrhea, and enhance growth performance (Yang et al., 2015a, 2015b). Lactic acid-producing bacteria are the primary bacteria in the nursing pig gut, and the lactic acid produced by lactic acid bacteria fermentation can inhibit the growth of intestinal pathogenetic bacteria and helps to aid immunity (Guevarra et al., 2019). That makes the supplementation of lactic acid-producing bacteria beneficial for the weaned piglets (Guevarra et al., 2019). The common forms of yeast supplied to pig diets include whole live yeast cells, heat-treated yeast cells, ground yeast cells, purified yeast cell cultures, and yeast extracts. Yeast supplementation has been reported to boost intestinal development by providing fermentation by-products such as short chain fatty acids to pigs and reducing post-weaning scour by supplying weaned piglets with beneficial nutrients such as specific sugars and nucleotides (Broadway et al., 2015). Accordingly, introducing probiotics in the creep feed is increasingly being explored (Barba-Vidal et al., 2018). However, the results of these studies are not consistent and further studies are still needed to interpret the mechanisms of action of probiotics and their interaction in various gut health situations.
Using Ingredients to Lower Dietary Acid-binding Capacity
Due to the decreased capacity of gastric acid secretion at weaning, weaned piglets have a higher pH value in the stomach than sow-reared piglets (Heo et al., 2013). Maintaining a lower gastric pH value is pivotal for the gut health of weaned piglets because this can positively affect the nutrition digestion and pathogenic bacteria inhibition. In contrast, the elevated gastric pH level makes weaned piglets more susceptible to enteric infections (Heo et al., 2013). Hence, not only the amino acid profile or the energy content of the diet but also other nutrients and key parameters should be considered (e.g., dietary ABC, Table 1). It refers to the ration’s resistance to a low pH in the pig’s stomach, is highly related to raw materials used in the feed and has a great impact on the pH of the stomach and feed digestibility. A high ABC can lead to lower digestibility of dry matter and crude protein and, therefore, adversely affect the growth performance of piglets. Moreover, a high ABC can increase the release of amine and ammonia that are toxic and could lead to diarrhea. The ABC value of feed ingredients and complete feeds can be calculated as the amount of acid in milliequivalents (meq) required to lower the pH of 1 kg of a sample to pH 4 and pH 3 based on the measurement value from a 0.5 g sample (Lawlor et al. 2005) and it can be used a nutrient constraint in feed formulation to select suitable ingredients. Therefore, lowering the ABC of the diets for new weanlings with the addition of feedstuffs with a low ABC in piglet feed formulations can be a good strategy for controlling diarrhea in weaned piglets (Huting et al., 2021).
Table 1.
Acid-binding capacity for different ingredients (Scholten et al., 2001; Lawlor et al., 2005; Karvelis, 2014; Hajati, 2018)
| Ingredient | ABC-3a | ABC-4b | Unit |
|---|---|---|---|
| Milk | |||
| Acid casein | 200 | 0 | meq/kg |
| Sows milk | 650 | 481 | meq/kg |
| Whey powder | 714 − 1,000 | 434 | meq/kg |
| cheese whey | 60.0 − 48.8 | meq/kg | |
| Milk replacer | 892 | 579 | meq/kg |
| Skim milk | 1,105 | 756 | meq/kg |
| Rennet casein | 1,929 | 1,423 | meq/kg |
| Cereals | |||
| Wheat Soft | 250 | meq/kg | |
| Wheat bran | 500 | meq/kg | |
| Wheat | 180 − 40 | 108 | meq/kg |
| liquid wheat starch | 77.0 − 74.5 | meq/kg | |
| Maize | 200 − 254 | 111 | meq/kg |
| Barley | 225 − 266 | 113 | meq/kg |
| Maize starch | 202 | 91 | meq/kg |
| Corn | 135 − 172 | meq/kg | |
| Corn distillers | 438 | 96 | meq/kg |
| Oat flakes | 180 | 72 | meq/kg |
| Root and pulp products | |||
| Sugar | 98 | 23 | meq/kg |
| Cassava | 393 | 167 | meq/kg |
| Beet pulp | 480 | 191 | meq/kg |
| Molasses | 790 | 399 | meq/kg |
| Citrus pulp | 873 | 373 | meq/kg |
| Mashed potato steam peel | 64.2 − 79.5 | meq/kg | |
| Vegetable protein | |||
| Sunflower meal | 852 | 482 | meq/kg |
| Rapeseed meal | 945 | 498 | meq/kg |
| Soybean meal | 1,068 | 642 | meq/kg |
| Soybean meal 42% | 980 − 1,240 | meq/kg | |
| Soybean meal 44% | 1,100 | meq/kg | |
| Soybean meal 48% | 1,025 − 1,035 | meq/kg | |
| Palm kernel | 485 | 250 | meq/kg |
| Peas | 515 | 278 | meq/kg |
| Maize gluten | 571 | 114 | meq/kg |
| Beans | 473 | 275 | meq/kg |
| Meat and fishmeal | |||
| Blood plasma | 1,150 − 1,350 | meq/kg | |
| Meat and bone meal | 920 | 595 | meq/kg |
| Fishmeal | 1,122.5 − 2,100 | 738 | meq/kg |
| Fishmeal 70/72% | 1,800 − 2,200 | meq/kg | |
| Fishmeal Peru origin | 1,800 − 2,000 | meq/kg | |
| Fat | |||
| Fat | 137 | 16 | meq/kg |
| Vegetable fat | 200 | meq/kg | |
| Choline chloride | 100 − 226 | 101 | meq/kg |
| Betaine | 600 | meq/kg | |
| Dextrose | 140 − 200 | meq/kg | |
| Microbial protein | |||
| Yeast | 130 | 150 | meq/kg |
| Amino acids | |||
| Lysine | 600 − 695 | 123 | meq/kg |
| Tryptophan | 1,024 | 179 | meq/kg |
| Methionine | 1,000 − 1,219 | 192 | meq/kg |
| Threonine | 1,100 − 1,386 | 218 | meq/kg |
| Minerals | |||
| Limestone | 18,500 − 22,000 | meq/kg | |
| Ferrous sulphate | 93 | −655 | meq/kg |
| Salt | 162 | 83 | meq/kg |
| Copper sulphate | 269 | 92 | meq/kg |
| Cobalt sulphate | 516 | 329 | meq/kg |
| Monoammonium phosphate | 815 | 46 | meq/kg |
| Ferrous oxide | 986 | 549 | meq/kg |
| Finisher minerals and vitamins | 5,123 | 3,357 | meq/kg |
| Weaner minerals and vitamins | 6,302 | 4,292 | meq/kg |
| Dicalcium phosphate | 3,813.6 − 10,150 | 3,098 | meq/kg |
| Sow minerals and vitamins | 7,503 | 5,413 | meq/kg |
| Potassium citrate | 7,851 | 5,703 | meq/kg |
| Mono dicalcium phosphate | 1,800 − 5,494 | meq/kg | |
| Sodium citrate | 8,745 | 6,334 | meq/kg |
| Defluorinated phosphate | 10,436 | 6,412 | meq/kg |
| Calcium formate | 9,000 − 12,069 | 3,983 | meq/kg |
| Calcium carbonate | 19,680 − 20,000 | meq/kg | |
| Manganese oxide | 10,887 | 6,678 | meq/kg |
| Sodium bicarbonate | 12,870 | 12,566 | meq/kg |
| Limestone flour | 15,044 | 12,932 | meq/kg |
| Zinc oxide | 13,000 − 17,908 | 16,321 | meq/kg |
| Acids | |||
| Orthophosphoric acid | −7,957 | −8,858 | meq/kg |
| Fumaric acid | −6,400 − −4,093 | −10,862 | meq/kg |
| Formic acid | −3,473 | −13,550 | meq/kg |
| Citric acid | −2,349 | −5,605 | meq/kg |
| Ascorbic acid | −4,000 − −2,249 | −217 | meq/kg |
| Malic acid | −2,550 | −7,214 | meq/kg |
| Lactic acid | −1,498 | −5,079 | meq/kg |
| Acetic acid | −141 | −2,283 | meq/kg |
| Propionic acid | −5 | −1,358 | meq/kg |
| Sorbic acid | 120 | −220 | meq/kg |
a Acid-binding capacity to pH 3.0; bAcid-binding capacity to pH 4.0.
Some mineral sources are key contributors to a high ABC value of feed, especially limestone (calcium carbonate), dicalcium phosphate (DCP), mono-dicalcium phosphate (MDCP) and zinc oxide (ZnO). So, it is very important to avoid limestone, DCP, and MDCP in formulating a weaner diet. Lowering calcium levels by decreasing the limestone content of the feed has a huge impact on the buffering capacity of the feed and on improving growth performance (Blavi et al., 2016). Using phytase super-dosing can provide multiple benefits including the reduction of limestone in diets. For example, the calcium and phosphorus levels (for the first 2 wk after weaning) should be reduced to 0.60% to 0.65% and 0.35% to 0.40%, respectively. At the same time, calcium formate (as a Ca source, 9,000 meq/kg) can partially replace limestone (18,000 to 20,000 meq/kg), which will inevitably lead to increased cost but will effectively reduce the feed ABC value.
Dietary supplementation of high levels of ZnO (2,000 to 3,000 mg/kg) has been widely used as an effective approach to reducing the incidences of post-weaning diarrhea (Laskoski et al., 2021). However, using a high dose of ZnO in piglet feeds has been associated with several negative effects including neutralizing the acid in the stomach because of a high ABC, being associated with post-weaning anemia, ZnO toxicity, zinc accumulation in the environment, interacting negatively with phytase, and antibiotic resistance (Maenz et al., 1999; Debski, 2016; Burrough et al., 2019). The maximal of 150 ppm zinc in the feed will be effective in June 2022 and the use of a high dose of zinc will be regulated in Canada. Although it becomes feasible to reduce the effective dosage of ZnO to combat post-weaning diarrhea and at the same time mitigate the negative impacts related to the high dosage of ZnO in feeds with the availability of new cost-effective technologies such as organic minerals, nanotechnology, and microencapsulation (Brown et al., 2019; Wang et al., 2022), there is still a need to develop strategies to replace higher doses of zinc in the feeds.
Organic acids have negative ABC (e.g., citric acid: −4,000 meq/kg and formic acid: −6,400 meq/kg). So, the addition of organic acids can decrease the ABC value of feed and then lower stomach pH (Desai et al., 2007). Sciopioni et al. (1978) reported a reduction in stomach pH from 4.6 to 3.5 with the addition of 1% citric acid and a pH reduction from 4.6 to 4.2 with 0.7% fumaric acid in the diet. However, we should consider the palatability of organic acids. Citric acid and tartaric acids improved feed preference; a high inclusion of some organic acids (e.g., formic acid), however, may reduce feed intake (Suarez et al., 2010). Higher early feed intake is very important for weaned piglets for promoting gut development and supporting better growth performance later on. On the other hand, inorganic acids (e.g., hydrochloric or phosphoric acid) can also reduce stomach pH but may also decrease feed preference (Suarez et al., 2010). Except for inhibition of pathogenic bacteria and reduction of ABC, organic acids can also act as an energy source in the gut of pigs as these are the intermediary products of tricarboxylic acid and improve mineral utilization (Pearlin et al., 2020). Therefore, several factors, including ABC value, palatability, bacteria inhibition, and other physiological functions, should be considered in the selection of organic acids in feeds.
Cereals and cereal by-products have a lower ABC when compared with the sources of minerals and proteins. So, it is important to keep feed crude protein levels as low as possible while maintaining a good, balanced supply of amino acids by using high protein raw materials and synthetic amino acids. When compared with fishmeal and soybean meal, potato protein, wheat gluten, and corn gluten have a lower ABC and therefore are highly recommended in weaner piglet feeds. However, potato protein, wheat gluten, and corn gluten may negatively affect feed intake as those protein sources are less preferred than fishmeal and soybean meal by piglets (Solà-Oriol et al., 2011). Moreover, it is not necessary to reject good high-value raw materials simply based on apparently high ABC as there are other ways to reduce the ABC of the feed.
Selecting ingredients to lower ABC is critical for optimizing the digestive function of the immature piglet. Although there is no clear-cut recommendation on the ABC values for nursery diet, it is critical to look at the total blend of raw materials available, their inclusion levels, costs, feed preference as well as the targeted feed ABC value and then make the final decision based on science and field experience.
Using Innovative Chemical and Biological Approaches to Detoxify Vomitoxin (DON)
The mycotoxin, deoxynivalenol (DON), commonly occurs on Fusarium-infected cereal grains (e.g., corn, wheat, barley), and the incidence of DON contamination of grains has been increasing in recent years (Biomin, 2021). It has been estimated that direct and secondary losses resulting from Fusarium Head Blight (a fungal disease of cereal crops, which also is an indicator of DON contamination) range from $50 to $300 million each year in Canada (Alberta Agriculture and Forestry, 2012). Moreover, the mycotoxin contamination in feeds and feed ingredients can reduce feed intake and compromise the immune system, which can make animals more susceptible to pathogens. Minimizing mycotoxin contamination in feeds is an important component to control post-weaning diarrhea. Typical negative effects of mycotoxin consumption include reduced feed intake, digestive dysfunction (e.g., gastroenteritis, gastrointestinal tract lesions, reduced nutrient absorption), immune suppression, and reduced growth performance (Sergent et al., 2006; Pinton et al., 2008; Johnston et al., 2010; NRC, 2012) with the primary physiological effect dependent on the mycotoxin present. DON levels as low as 0.6 to 2.0 ppm in complete feed cause a reduction in feed intake and growth rate (Pinton et al., 2008; Johnston et al., 2010). In addition to reduced feed intake and growth performance, consuming DON-contaminated feed results in damage to the intestinal tract epithelial cells resulting in alteration of intestinal growth and barrier function as well as increased susceptibility to enteric pathogen challenge (Pinton et al., 2012; Ghareeb et al., 2015). Damage to the intestine also results in a reduction in nutrient absorption (Ghareeb et al., 2015). Once absorbed, DON inhibits protein synthesis, causes kidney and liver damage, and can suppress immune function resulting in decreased ability to resist disease challenges (Chaytor et al., 2011). In general, the negative effects of mycotoxins are greater in younger animals (Chaytor et al., 2011). While strategies have been developed to reduce the effects of some mycotoxins (e.g., aflatoxin), such as toxin binders, these have limited effect on mitigating the negative effects of DON (Beaulieu et al., 2009). There is a need for effective and economical methods to reduce the impact of DON in feed and feed ingredients. Chemical approaches, such as the use of sodium metabisulfite (SMBS) (Rempe et al., 2013), and biological approaches, such as the use of microorganisms for detoxification (Yu et al., 2010; Li et al., 2011), have shown promise in reducing DON.
Sulphite reducing agents, including sodium sulphite (Na2SO3), sodium bisulphite (NaHSO3), and SMBS, have the capacity to cleave disulphide cross-linkages (Truong et al., 2016). Both in vitro and in vivo studies have demonstrated that SMBS is effective in DON detoxification (Table 2). Specifically, it has been shown that SMBS can destroy 70% to 100% of DON in processed grains or feeds in vitro with 0.45% to 0.9% levels at pH around 6.5 but not under acidic conditions (Dänicke et al., 2010a, 2010b; Schwartz et al., 2013; Frobose et al., 2015). Frobose et al. (2015) reported that adding a SMBS-based feed additive and pelleting can help overcome some of the negative effects of DON in the nursery pigs fed with naturally contaminated dried distillers grains with solubles (DDGS) (Frobose et al., 2015). Hydrothermally processing DON-contaminated diets with 1.0% SMBS restored ADFI and improved G:F in the nursery pigs (Frobose et al., 2017). Shawk et al. (2019) also reported that in diets with low DON concentrations (<1.5 mg/kg), SMB-based products increased ADG compared with control diets. Pigs fed high DON diets (4.17 mg/kg) had reduced performance compared with pigs fed low DON. Sodium metabisulfite (0.5%) in high DON diets (manufactured with corn containing an average of 4.17 mg/kg DON) provided a benefit in growth performance with ADG and G:F exceeding growth performance in the low DON diet (manufactured with corn containing an average of 2.46 mg/kg DON) (Becker et al., 2022). Mwaniki et al. (2021) reported that a feed additive containing SMB improved growth performance in the nursery piglets fed diets formulated with naturally contaminated corn (formulated with 5.5 mg/kg DON). The results from the above in vivo studies have demonstrated that feeding a supplement with relatively high levels of SMB to weanling pigs is safe and effective to detoxify DON. Although the response is still present even without pelleting in many situations, heat and moisture during the pelleting process seem to enhance the capacity of SMBS to detoxify DON as pelleted feeds were used in the above studies, suggesting SMBS detoxifying DON during the pelleting process not necessary in the gut. Because SMBS may be degraded quickly under aqueous acid conditions such as pig stomach to form sulfur dioxide and subsequently decompose into sodium oxide and sulfur dioxide (Dänicke et al., 2012), then damaging the metabolism of the liver and the functionality of the immune system, eventually leading to a decrease in health or growth performance (Davis et al., 2022). This may explain why more than 0.35% of unprotected SMBS in the diet can show toxic effects on pigs. Moreover, little SMBS will remain intact in the small intestine where an optimal pH environment exists for SMBS to detoxify DON (Yu et al., 2022). Thus, there is a need to deliver intact SMBS to the lower gut such as the small intestine to detoxify DON effectively through innovative delivery methods (Yu et al., 2021, 2022). Encapsulated SMBS with hydrogenated palm oil was stable in the simulated gastric fluid and allowed a progressive release of SMBS in the simulated intestinal fluid. The released SMBS in the simulated intestinal fluid effectively detoxified DON (Yu et al., 2021). However, the efficacy of DON detoxification by microparticles needs to be further investigated with pig experiments. Further, feeding high SMBS in the diet can decrease the bioavailability of thiamin (Til et al., 1972); therefore, thiamin is usually supplemented at greater concentrations or with a protected form in diets that are supplemented with SMBS.
Table 2.
Summary table of relevant literature on effects of sodium metabisulfite (SMBS) on deoxynivalenol (DON) levels and growth performance of pigs
| In vitro or in vivo | Diets/ingredients | Heat and humidity | Mash or pelleted | DON concentrations (ppm or mg/kg) | SMBS concentrations (g/kg or %) | Effects | References |
|---|---|---|---|---|---|---|---|
| In vivo | Corn-soybean meal | N/A | Phase.1: Pelleted, Phase.2: Meal |
Low: 1.12 ppm High: 2.34 ppm High + SMBS: 1.44 ppm |
0.5% | Improve feed efficiency, reduce total removals and mortality. | Becker et al., 2022 |
| In vivo | Corn-soybean meal with corn been contaminated | mash | Exp.1: Pelleted, Exp.2: Mash |
PC: 0.29 ppm NC: 2.86 ppm NC+SMBS: 1.21 ppm |
3 g/kg | Greater G:F and ATTD of dry matter, gross energy, and crude protein. |
Mwaniki et al., 2021 |
| In vivo | Barley-corn-soybean | N/A | Mash | Control: 0.29mg/kg Don: 4.49 mg/kg Control+ SMBS: 0.43 mg/kg Don + SMBS: 3.96 mg/kg |
3% | Reduce AID of some nutrients and intestinal absorption of DON. |
Bouchard et al., 2019 |
| In vivo | Corn-soybean meal | N/A | N/A | <0.5 mg/kg | 0.15%, 0.25%, 0.5% | Higher concentration of SMB for a longer duration; Have improved growth performance. |
Shawk et al., 2019 |
| In vivo | Wheat-soybean meal | Pelleted at 82 °C with a minimum conditioner retention time of 45 s | Pelleted | PC: <0.5 mg/kg NC: 4 mg/kg NC+SMBS: 0.35 mg/kg |
1% | DON was decreased by 92% when pelleted with SMB; Can alleviate DON effects on growth, restore feed intake and improve feed efficiency. | Frobose et al., 2017 |
| In vivo | Corn-soybean meal-DDGS, DDGS is the source of DON | N/A | Mash and Pellet | PC: <0.5 mg/kg NC: 3 mg/kg NC+0.25% SMBS: 3 mg/kg NC+0.5% SMBS: 3 mg/kg |
0.25% and 0.5% | No significant pellet × SMB interactions on growth performance and BW, but pelleting improved G:F and ADG, SMBS increased ADG. | Frobose et al., 2015 |
| In vivo | DDGS | N/A | Mash and Pellet | PC: <0.5 mg/kg NC: 4.8 mg/kg NC+ crumbled DDGS: 4.8 mg/kg NC+crumbled DDGS + 0.5% SMBS: 3 mg/kg |
2.5% (final concentration in diet: 0.77%) | Including SMB prior to pelleting DON-contaminated DDGS increased (P < 0.01) ADG and ADFI. |
Frobose et al., 2015 |
| In vivo & vitro | Triticale | 18 °C, 15% moisture for 4 wk | N/A | 0.156, 2.312, 0.084 and 0.275 mg/kg | 5% | Wet preservation in the presence of SBS could reduce DON level; restored growth performance to the level of their counterparts. | Dänicke et al., 2010a |
| In vitro | DDGS | Autoclave 1h at 121 °C | N/A | 20.6 mg/kg | 0.5%, 1%, 2.5%, 5%, 5% with 100 mL/kg distilled water | 9.8% reduction in DON, 82% were achieved when 5% SMBS were added before autoclaving. | Frobose et al., 2015 |
| In vitro | DDGS | Pelleting at 66 °C and 82 °C with retention times of 30 s and 60 s within temperature | N/A | 20.5 mg/kg | 1%, 2.5%, and 5% | Pelleting with SMBS inclusion can reduce DON level. | Frobose et al., 2015 |
| In vitro | Wheat kernels | 18 °C, 15%, 17.5% and 20% humidity | 2.09 mg/kg dry matter | 5% | Reduce 1.2–4.3% initial DON concentration, higher SBS-to-DON ratio and higher moisture content favour the derivatization of DON to DONS. |
Dänicke et al., 2010b | |
| In vitro | Triticale | 13% and 15% moisture and stored for up to 63 d | 6.63 mg/kg | 1%, 2%, 3%, 4%, 5% | DON concentration decreased with increasing amounts of supplemented SBS, moisture and longer duration. |
Dänicke et al., 2009 | |
| In vitro | Wheat | 100 °C, 22% moisture content for 15 min | 7.6 mg/kg | 1% | Reduce the DON-concentration to 0.28 mg/kg. | Dänicke et al., 2005 |
Biological approaches, such as using microorganisms to convert the toxins to non- or less toxic compounds, have become an attractive choice recently due to their high specificity, efficacy, and environmental soundness (Awad et al., 2010; He et al., 2016; Pierron et al., 2016; Vanhoutte et al., 2016; Zhu et al., 2016; Tian et al., 2022). It has been shown that the higher tolerance for DON observed in ruminants is due, in part, to the conversion of DON to nontoxic metabolites by rumen microorganisms (Chaytor et al., 2011). For instance, a bacterial strain BBSH797 from the bovine rumen (Fuchs et al., 2002) could transfer DON into its metabolite DOM-1. Additionally, some bacteria from the poultry industry also have the potential to detoxify DON into DOM-1, including a Clostridium sp. WJ06 from goose intestine in China (Li et al., 2017), a Bacillus sp. LS100 from the chicken intestine (Yu et al., 2010), and an Eggerthella sp. DII-9 from the chicken intestine (Gao et al., 2018). In general, the high pre-gastric bacterial count in both ruminants and poultry may be a major factor with respect to DON tolerance in these species (Maresca, 2013). Their detoxification principle is that the C12-C13 epoxy group is the main toxicity site of DON (Karlovsky, 2011), and DON can be deepoxidized to the metabolite DOM-1, which is considered to be a detoxification product. Indeed, many species of bacteria have been shown to possess the capability to enzymatically degrade mycotoxins (Shetty and Jespersen, 2006). The described animal gut microbes deeply oxidize DON under anaerobic conditions, which limits its practical application and only a few of them were identified that could detoxify DON-contaminated diets in vivo. A bacterial strain Coriobacteriaceum DSM 11798 (the active ingredient in Biomin BBSH 797) can be used as a feed additive in diets to remove the toxic effects of DON-contaminated diets in pigs by detoxifying DON to DOM-1 (Sayyari et al., 2018). Previously isolated microorganisms, including Bacillus sp. LS100, has been shown to have DON detoxifying properties in vitro (Yu et al., 2010). The concept of using the isolate for in vivo detoxifying DON has also been proven (Li et al., 2011) and found that microbial detoxification of contaminated feed could eliminate DON’s toxic effects on pigs. A U.S. patent has been granted for utilizing the bacterial isolates (Zhou et al., 2014). Since isolate LS100 possesses high efficiency and stability in detoxifying DON, direct feeding of the isolate through feed provides a unique opportunity for developing an effective microbial agent for field application to detoxify DON, which requires further pig studies to confirm the efficacy of detoxification in the pig gut.
Conclusion
Weaned piglets face many stressors including bacterial pathogens, oxidative stress, and inflammation. The withdrawal of antibiotics from feeds can result in challenges to control post-weaning diarrhea and an integrated approach should be taken to control post-weaning diarrhea. Regarding antibiotic alternatives, using lower doses of essential oils (e.g., combination with other bioactive compounds and microencapsulation) could prevent inflammation and “leaky gut” induced by lipopolysaccharide instead of higher doses required for their killing pathogens properties. With elevated pH levels in the stomach of weaned piglets, it is critical to look at the total blend of raw materials available, their inclusion levels, costs, feed preference as well as the targeted feed ABC value. Minimizing mycotoxin contamination in feeds is an important component to control post-weaning diarrhea. Chemical approaches, such, as the use of sodium metabisulfite and biological approaches, such as the use of microorganisms for detoxification, have shown promise in reducing vomitoxin. These strategies reviewed in this manuscript may be considered important parts of an integrated approach to control post-weaning diarrhea in piglets.
Acknowledgments
The authors thank the financial support from the Faculty of Agricultural and Food Sciences in the University of Manitoba, the Agriculture and Agri-Food Canada, Manitoba Pork, Innovation Canada, Natural Sciences and Engineering Research Council of Canada (NSERC), and Swine Innovation Porc. The authors also thank Tao Wang and Changning Yu for their help during the manuscript preparation.
Conflict of interest statement. None declared.
About the Authors

Liuqin He, Professor of College of Life Sciences, Hunan Normal University. She received her Ph.D. degree in ecology from the University of Chinese Academy of Sciences in 2018. She won Young Elite Scientists Sponsorship Program by China Association for Science and Technology, “Huxiang Young Talents Plan” of Hunan Province, and the Natural Science Foundation for Outstanding Youth Scholars of Hunan Province. She mainly engages in amino acid nutrition regulation and animal health. E-mail:285687180@qq.com

Xiaoya Zhao obtained her Ph.D. degree at the Department of Animal Science at the University of Manitoba in 2022 and she is currently working at South China Agricultural University as a postdoctoral fellow. During her Ph.D. study, her primary research interests are located in the roles of gut chemosensing in mammals’ growth and health, and she is also interested in identifying antibiotic alternatives. For her postdoctoral research, her interest turned to oncolytic virus therapy in cancer in pets. Currently, her postdoctoral research is about the impact of the tumor microenvironment on oncolytic viral therapy.

Li Jianzhong obtained his Ph.D. degree in aquatic biology from Sun Yat-Sen University in June 2003 and worked as a postdoctoral researcher at the National Institute of Basic Biology in Japan from October 2006 to October 2008. He is working at the School of Life Sciences, Hunan Normal University as a professor and a doctoral supervisor. His research interests are animal nutrition and gut health. He has published about 50 peer-reviewed journal articles as the first author or corresponding author and participated in the application of 6 national patents in China.

Dr. Chengbo Yang is n Associate Professor in Livestock Nutrition and Nutritional Biochemistry in the Department of Animal Science, University of Manitoba. Dr. Yang obtained his Ph.D. in monogastric animal nutrition at the University of Guelph in 2011. He joined the University of Manitoba in 2016 after several years of working in the industry. He is conducting research in the area of gut health and nutrient utilization relevant to nonruminants. He has acquired about 10.0 million dollars in research funding as PI and co-PI from NSERC, Canada Foundation for Innovation, USDA, Swine Cluster III, and other agencies. He currently serves as an editorial board member for two journals including the Canadian Journal of Animal Science. He has provided training to over 50 HQPs including high school students, undergraduate, and graduate students, postdoctoral fellows, technicians, and research associates, and published 89 peer-reviewed scientific publications. Dr. Yang received with 2018 Canadian Society of Animal Science Young Scientist Award, the 2018 Terry G. Falconer Memorial Rh Institute Foundation Emerging Researcher Awards for Natural Sciences, and the 2019 Merit Award in Research, Scholarly Work, and Creative Activities at the University of Manitoba.
Contributor Information
Liuqin He, Hunan Provincial Key Laboratory of Animal Intestinal Function and Regulation, Hunan International Joint Laboratory of Animal Intestinal Ecology and Health, College of Life Sciences, Hunan Normal University, Changsha 410081, China.
Xiaoya Zhao, College of Animal Science, South China Agricultural University, Tianhe District, Guangzhou 510642, China.
Jianzhong Li, Hunan Provincial Key Laboratory of Animal Intestinal Function and Regulation, Hunan International Joint Laboratory of Animal Intestinal Ecology and Health, College of Life Sciences, Hunan Normal University, Changsha 410081, China.
Chengbo Yang, Department of Animal Science, University of Manitoba, Winnipeg, MB R3T 2N2, Canada.
Literature Cited
- Alberta Agriculture and Forestry. 2012. Alberta Fusarium graminearum Management Plan. https://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/agdex5210/$file/110_632-3.pdf?OpenElement.
- Awad, W.A., Ghareeb K., Bohm J., and Zentek J... 2010. Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food. Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk. Assess. 27(4):510–520. doi: 10.1080/19440040903571747 [DOI] [PubMed] [Google Scholar]
- Barba-Vidal, E., Martin-Orue S.M., and Castillejos L... 2018. Review: Are we using probiotics correctly in post-weaning piglets? Animal 12(12):2489–2498. doi: 10.1017/S1751731118000873 [DOI] [PubMed] [Google Scholar]
- Beaulieu A.D., Patience J.F., and Gillis D... 2009. The efficacy of eight different feed additives on mitigating the effects of deoxynivalenol (DON). 28th Annual Centralia Swine Research Update. Kirkton, ON. [Google Scholar]
- Becker, L.L., DeRouchey J.M., Woodworth J.C., Tokach M.D., Goodband R.D., Vidal A., Gougoulias C., and Gebhardt J.T... 2022. Evaluation of dietary mycotoxin control strategies on nursery pig growth performance and blood measures. Transl. Anim. Sci. 6(3):txac081. doi: 10.1093/tas/txac081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIOMIN. 2021. World Mycotoxin Survey: Impact 2021. https://www.biomin.net/science-hub/world-mycotoxin-survey-impact-2021/. [Google Scholar]
- Bouchard, M.J., Chorfi Y., Letourneau-Montminy M.P., and Guay F... 2019. Effects of deoxynivalenol and sodium meta-bisulphite on nutrient digestibility in growing pigs. Arch. Anim. Nutr. 73(5):360–373. doi: 10.1080/1745039X.2019.1641369 [DOI] [PubMed] [Google Scholar]
- Blavi, L., Solà-Oriol D., and Pèrez J.F... 2016. Low calcium levels improve growth in piglets after weaning. J. Anim. Sci. 94(2):141–141. doi: 10.2527/msasas2016-300 [DOI] [Google Scholar]
- Broadway, P., Carroll J., and Sanchez N... 2015. Live yeast and yeast cell wall supplements enhance immune function and performance in food-producing livestock: a review. Microorganisms 3(3):417–427. doi: 10.3390/microorganisms3030417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, N., Wang Y., and Yang C... 2019. Different zinc sources may affect the acid buffering capacity of weanling pig diets. Can Hog J. Special summer edition, For the love of science 39–41. [Google Scholar]
- Burrough, E.R., De Mille C., and Gabler N.K... 2019. Zinc overload in weaned pigs: tissue accumulation, pathology, and growth impacts. J. Vet. Diagn. Invest. 31(4):537–545. doi: 10.1177/1040638719852144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor, A.C., Hansen J.A., van Heugten E., See M.T., and Kim S.W... 2011. Occurrence and decontamination of mycotoxins in swine feed. Asian-Australas. J. Anim. Sci. 24(5):723–738. doi: 10.5713/ajas.2011.10358 [DOI] [Google Scholar]
- Chen, Y., Liu S., Yu C., Azevedo P., Liu S., O K., Gong J., Hou Y., and Yang C... 2021. Evaluating the effectiveness of Lactobacillus zeae against enterotoxigenic Escherichia coli F4 infection in an in vitro porcine intestinal epithelial cell model. Food Sci. Technol. 1(2):215–228. doi: 10.1021/acsfoodscitech.0c00069 [DOI] [Google Scholar]
- Choi, J., Wang L., Ammeter E., Lahaye L., Liu S., Nyachoti M., and Yang C... 2020a. Evaluation of lipid matrix microencapsulation for intestinal delivery of thymol in weaned pigs. Transl. Anim. Sci. 4(1):411–422. doi: 10.1093/tas/txz176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J., Wang L., Liu S., Lu P., Zhao X., Liu H., Lahaye L., Santin E., Liu S., Nyachoti M.,. et al. 2020b. Effects of a microencapsulated formula of organic acids and essential oils on nutrient absorption, immunity, gut barrier function, and abundance of enterotoxigenic Escherichia coli F4 in weaned piglets challenged with E. coli F4. J. Anim. Sci. 98(9):skaa259. doi: 10.1093/jas/skaa259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dänicke, S., Hegewald A.K., Kahlert S., Kluess J., Rothkotter H.J., Breves G., and Doll S... 2010a. Studies on the toxicity of deoxynivalenol (DON), sodium metabisulfite, DON-sulfonate (DONS) and de epoxy-DON for porcine peripheral blood mononuclear cells and the intestinal porcine epithelial cell lines IPEC-1 and IPEC-J2, and on effects of DON and DONS on piglets. Food Chem. Toxicol. 48((8-9):2154–2162. doi: 10.1016/j.fct.2010.05.022 [DOI] [PubMed] [Google Scholar]
- Dänicke, S., Pahlow G., Beyer M., Goyarts T., Breves G., Valenta H., and Humpf H.U... 2010b. Investigations on the kinetics of the concentration of deoxynivalenol (DON) and on spoilage by moulds and yeasts of wheat grain preserved with sodium metabisulfite (Na2S2O5, SBS) and propionic acid at various moisture contents. Arch. Anim. Nutr. 64(3):190–203. doi: 10.1080/17450391003693159 [DOI] [PubMed] [Google Scholar]
- Dänicke, S., Pahlow G., Goyarts T., Rohweder D., Wilkerling K., Breves G., Valenta H., and Doll S... 2009. Effects of increasing concentrations of sodium metabisulphite (Na2S2O5, SBS) on deoxynivalenol (DON) concentration and microbial spoilage of triticale kernels preserved without and with propionic acid at various moisture contents. Mycotoxin Res. 25(4):215–223. doi: 10.1007/s12550-009-0030-2 [DOI] [PubMed] [Google Scholar]
- Dänicke, S., Valenta H., Gareis M., Lucht H.W., and Reichenbach H... 2005. On the effects of a hydrothermal treatment of deoxynivalenol (DON)-contaminated wheat in the presence of sodium metabisulphite (Na2S2O5) on DON reduction and on piglet performance. Anim. Feed Sci. Technol. 118(1–2):93–108. doi: 10.1016/j.anifeedsci.2004.09.011 [DOI] [Google Scholar]
- Dänicke, S., Kersten S., Valenta H., and Breves G... 2012. Inactivation of deoxynivalenol-contaminated cereal grains with sodium metabisulfite: a review of procedures and toxicological aspects. Mycotoxin Res. 28(4):199–218. doi: 10.1007/s12550-012-0139-6 [DOI] [PubMed] [Google Scholar]
- Davis, E.M., Liang Y., Wallace K.P., Zimmerman A.J., Siebecker M.G., Broadway P.R., Carroll J.A., and Ballou M.A... 2022. A porous ceramic particle with or without a preservative blend did not impair apparent digestibility of macro- and micro-nutrients of postweaned pigs. Transl. Anim. Sci. 6(3):txac078. doi: 10.1093/tas/txac078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten, G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., and Bakker B.M... 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54(9):2325–2340. doi: 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debski, B. 2016. Supplementation of pigs diet with zinc and copper as alternative to conventional antimicrobials. Pol. J. Vet. Sci. 19(4):917–924. doi: 10.1515/pjvs-2016-0113 [DOI] [PubMed] [Google Scholar]
- Desai D., Patwardhan D., and Ranade A... 2007. Acidifiers in animal nutrition – a guide for feed preservation and acidification to promote animal performance. In: Acidifiers in poultry diets and poultry production. Nottingham, England: Nottingham University Press; p. 63–69. [Google Scholar]
- Fouhse, J.M., Zijlstra R.T., and Willing B.P... 2016. The role of gut microbiota in the health and disease of pigs. Anim. Front. 6(3):30–36. doi: 10.2527/af.2016-0031 [DOI] [Google Scholar]
- Frobose, H.L., Fruge E.D., Tokach M.D., Hansen E.L., DeRouchey J.M., Dritz S.S., Goodband R.D., and Nelssen J.L... 2015. The influence of pelleting and supplementing sodium metabisulfite (Na2S2O5) on nursery pigs fed diets contaminated with deoxynivalenol. Anim. Feed Sci. Technol. 210:152–164. doi: 10.1016/j.anifeedsci.2015.09.020 [DOI] [Google Scholar]
- Frobose, H.L., Stephenson E.W., Tokach M.D., DeRouchey J.M., Woodworth J.C., Dritz S.S., and Goodband R.D... 2017. Effects of potential detoxifying agents on growth performance and deoxynivalenol (DON) urinary balance characteristics of nursery pigs fed DON-contaminated wheat. J. Anim. Sci. 95(1):327–337. doi: 10.2527/jas.2016.0664 [DOI] [PubMed] [Google Scholar]
- Fuchs, E., Binder E.M., Heidler D., and Krska R... 2002. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit. Contam. 19(4):379–386. doi: 10.1080/02652030110091154 [DOI] [PubMed] [Google Scholar]
- Gao, X., Mu P., Wen J., Sun Y., Chen Q., and Deng Y... 2018. Detoxification of trichothecene mycotoxins by a novel bacterium, Eggerthella sp. DII-9. Food Chem. Toxicol. 112:310–319. doi: 10.1016/j.fct.2017.12.066 [DOI] [PubMed] [Google Scholar]
- Ghareeb, K., Awad W.A., Bohm J., and Zebeli Q... 2015. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine. J. Appl. Toxicol. 35(4):327–337. doi: 10.1002/jat.3083 [DOI] [PubMed] [Google Scholar]
- Gresse, R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., and Blanquet-Diot S... 2017. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 25(10):851–873. doi: 10.1016/j.tim.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Guevarra, R.B., Lee J.H., Lee S.H., Seok M.J., Kim D.W., Kang B.N., Johnson T.J., Isaacson R.E., and Kim H.B... 2019. Piglet gut microbial shifts early in life: causes and effects. J. Anim. Sci. Biotechnol. 10:1. doi: 10.1186/s40104-018-0308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajati, H. 2018. Application of organic acids in poultry nutrition. Int. J. Avian Wildl. Biol. 3(4):324–329. doi: 10.15406/ijawb.2018.03.00114 [DOI] [Google Scholar]
- Hassan, Y.I., Lahaye L., Gong M.M., Peng J., Gong J., Liu S., Gay C.G., and Yang C... 2018. Innovative drugs, chemicals, and enzymes within the animal production chain. Vet. Res. 49(1):71. doi: 10.1186/s13567-018-0559-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, J.W., Hassan Y.I., Perilla N., Li X.Z., Boland G.J., and Zhou T... 2016. Bacterial epimerization as a route for deoxynivalenol detoxification: the influence of growth and environmental conditions. Front. Microbiol. 7:572. doi: 10.3389/fmicb.2016.00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo, J.M., Opapeju F.O., Pluske J.R., Kim J.C., Hampson D.J., and Nyachoti C.M... 2013. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 97(2):207–237. doi: 10.1111/j.1439-0396.2012.01284.x [DOI] [PubMed] [Google Scholar]
- Huang, B., Xiao D., Tan B., Xiao H., Wang J., Yin J., Duan J., Huang R., Yang C., and Yin Y... 2016. Chitosan oligosaccharide reduces intestinal inflammation that involves calcium-sensing receptor (CaSR) activation in lipopolysaccharide (LPS)-challenged piglets. J. Agric. Food Chem. 64(1):245–252. doi: 10.1021/acs.jafc.5b05195 [DOI] [PubMed] [Google Scholar]
- Hui, Q., Ammeter E., Liu S., Yang R., Lu P., Lahaye L., and Yang C... 2020. Eugenol attenuates inflammatory response and enhances barrier function during lipopolysaccharide-induced inflammation in the porcine intestinal epithelial cells. J. Anim. Sci. 98(8):skaa245. doi: 10.1093/jas/skaa245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huting, A.M.S., Middelkoop A., Guan X., and Molist F... 2021. Using nutritional strategies to shape the gastro-intestinal tracts of suckling and weaned piglets. Animals (Basel) 5(11):402. doi: 10.3390/ani11020402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, S.L., Patience J.F., Gillis D., De La Llata M., Hasen S.A., and Beaulieu A.D... 2010. The effects of deoxynivalenol on growth performance in nursery pigs. Amer. Soc. Anim. Sci. 88(3):71. Midwest Meeting(Abstr.). [Google Scholar]
- Karlovsky, P. 2011. Biological detoxification of the mycotoxin deoxynivalenol and its use in genetically engineered crops and feed additives. Appl. Microbiol. Biotechnol. 91(3):491–504. doi: 10.1007/s00253-011-3401-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvelis, G. 2014. Controlling dietary buffering capacity in piglet feeds. https://www.wattagnet.com/articles/20464-controlling-dietary-buffering-capacity-in-piglet-feeds. [Google Scholar]
- Kerr, B.J., Weber T.E., and Shurson G.C.. 2013. Evaluation of commercially available enzymes, probiotics, or yeast on apparent total-tract nutrient digestion and growth in nursery and finishing pigs fed diets containing corn dried distillers grains with solubles. Prof. Anim. Sci. 29(5):508–517. doi: 10.15232/S1080-7446(15)30272-2 [DOI] [Google Scholar]
- Kiarie, E.G., and Mills A... 2019. Role of feed processing on gut health and function in pigs and poultry: conundrum of optimal particle size and hydrothermal regimens. Front. Vet. Sci. 6:19. doi: 10.3389/fvets.2019.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskoski, F., Tokach M.D., Woodworth J.C., DeRouchey J.M., Dritz S.S., Gebhardt J.T., Goodband R.D., Faccin J.E.G., and Bortolozzo F.P... 2021. Effects of different diet alternatives to replace the use of pharmacological levels of zinc on growth performance and fecal dry matter of weanling pigs. Transl. Anim. Sci. 5(2):txab074. doi: 10.1093/tas/txab074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor, P.G., Lynch P.B., Caffrey P.J., O’Reilly J.J., and O’Connell M.K... 2005. Measurements of the acid-binding capacity of ingredients used in pig diets. Ir. Vet. J. 58(8):447–452. doi: 10.1186/2046-0481-58-8-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., Wang J., Huang L., Chen H., and Wang C... 2017. Effects of Adding Clostridium sp. WJ06 on intestinal morphology and microbial diversity of growing pigs fed with natural deoxynivalenol contaminated wheat. Toxins (Basel) 9(12):383. doi: 10.3390/toxins9120383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q., Yu C., Chen Y., Liu S., Azevedo P., Gong J., K O., and Yang C... 2022a. Citral alleviates peptidoglycan-induced inflammation and disruption of barrier functions in porcine intestinal epithelial cells. J. Cell. Physiol. 237(3):1768–1779. doi: 10.1002/jcp.30640 [DOI] [PubMed] [Google Scholar]
- Li, Q., Li L., Chen Y., Yu C., Azevedo P., Gong J., and Yang C... 2022b. Bacillus licheniformis PF9 improves barrier function and alleviates inflammatory responses against enterotoxigenic Escherichia coli F4 infection in the porcine intestinal epithelial cells. J. Anim. Sci. Biotechnol. 13(1):86. doi: 10.1186/s40104-022-00746-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X.Z., Zhu C., de Lange C.F., Zhou T., He J., Yu H., Gong J., and Young J.C... 2011. Efficacy of detoxification of deoxynivalenol-contaminated corn by Bacillus sp. LS100 in reducing the adverse effects of the mycotoxin on swine growth performance. Food. Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk. Assess. 28(7):894–901. doi: 10.1080/19440049.2011.576402 [DOI] [PubMed] [Google Scholar]
- Maenz, D.D., Engele-Schaan C.M., Newkirk R.W., and Classen H.L... 1999. The effect of minerals and mineral chelators on the formation of phytase-resistant and phytase-susceptible forms of phytic acid in solution and in a slurry of canola meal. Anim. Feed Sci. Technol. 81((3-4):177–192. doi: 10.1016/s0377-8401(99)00085-1 [DOI] [Google Scholar]
- Maresca, M. 2013. From the gut to the brain: journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins (Basel) 5(4):784–820. doi: 10.3390/toxins5040784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant, R.M., Yang L., Becker L.B., Berg R.A., Nadkarni V., Nichol G., Carr B.G., Mitra N., Bradley S.M., Abella B.S.,. et al. 2011. American heart association get with the guidelines-resuscitation. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit. Care Med. 39(11):2401–2406. doi: 10.1097/CCM.0b013e3182257459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwaniki, A.W., Buis Q.R., Trott D., Huber L.A., Yang C., and Kiarie E.G... 2021. Comparative efficacy of commercially available deoxynivalenol detoxifying feed additives on growth performance, total tract digestibility of components, and physiological responses in nursery pigs fed diets formulated with naturally contaminated corn. Transl. Anim. Sci. 5(2):txab050. doi: 10.1093/tas/txab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.). 2012. Nutrient requirements of swine. Washington (DC): Natl. Acad. Press. [Google Scholar]
- Nordgreen, J., Munsterhjelm C., Aae F., Popova A., Boysen P., Ranheim B., Heinonen M., Raszplewicz J., Piepponen P., Lervik A.,. et al. 2018. The effect of lipopolysaccharide (LPS) on inflammatory markers in blood and brain and on behavior in individually-housed pigs. Physiol. Behav. 195:98–111. doi: 10.1016/j.physbeh.2018.07.013 [DOI] [PubMed] [Google Scholar]
- Omonijo, F.A., Kim S., Guo T., Wang Q., Gong J., Lahaye L., Bodin J.C., Nyachoti M., Liu S., and Yang C... 2018a. Development of novel microparticles for effective delivery of thymol and lauric acid to pig intestinal tract. J. Agric. Food Chem. 66(37):9608–9615. doi: 10.1021/acs.jafc.8b02808 [DOI] [PubMed] [Google Scholar]
- Omonijo, F.A., Liu S., Hui Q., Zhang H., Lahaye L., Bodin J.C., Gong J., Nyachoti M., and Yang C... 2019. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during lipopolysaccharide (LPS)-induced inflammation. J. Agric. Food Chem. 67(2):615–624. doi: 10.1021/acs.jafc.8b05480. [DOI] [PubMed] [Google Scholar]
- Omonijo, F.A., Ni L., Gong J., Wang Q., Lahaye L., and Yang C... 2018b. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 4(2):126–136. doi: 10.1016/j.aninu.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin, B.V., Muthuvel S., Govidasamy P., Villavan M., Alagawany M., Ragab Farag M., Dhama K., and Gopi M... 2020. Role of acidifiers in livestock nutrition and health: A review. J. Anim. Physiol. Anim. Nutr. (Berl) 104(2):558–569. doi: 10.1111/jpn.13282 [DOI] [PubMed] [Google Scholar]
- Pierron, A., Mimoun S., Murate L.S., Loiseau N., Lippi Y., Bracarense A.P., Schatzmayr G., He J.W., Zhou T., Moll W.D.,. et al. 2016. Microbial biotransformation of DON: molecular basis for reduced toxicity. Sci. Rep. 6:29105. doi: 10.1038/srep29105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton, P., Accensi F., Beauchamp E., Cossalter A.M., Callu P., Grosjean F., and Oswald I.P... 2008. Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicol. Lett. 177(3):215–222. doi: 10.1016/j.toxlet.2008.01.015 [DOI] [PubMed] [Google Scholar]
- Pinton, P., Tsybulskyy D., Lucioli J., Laffitte J., Callu P., Lyazhri F., Grosjean F., Bracarense A.P., Kolf-Clauw M., and Oswald I.P... 2012. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 130(1):180–190. doi: 10.1093/toxsci/kfs239 [DOI] [PubMed] [Google Scholar]
- Pluske, J.R. 2013. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 4(1):1. doi: 10.1186/2049-1891-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempe, I., Brezina U., Kersten S., and Danicke S... 2013. Effects of a Fusarium toxin-contaminated maize treated with sodium metabisulphite, methylamine and calcium hydroxide in diets for female piglets. Arch. Anim. Nutr. 67(4):314–329. doi: 10.1080/1745039X.2013.818762 [DOI] [PubMed] [Google Scholar]
- Sartor, R.B. 2004. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126(6):1620–1633. doi: 10.1053/j.gastro.2004.03.024 [DOI] [PubMed] [Google Scholar]
- Sayyari, A., Faeste C.K., Hansen U., Uhlig S., Framstad T., Schatzmayr D., and Sivertsen T... 2018. Effects and biotransformation of the mycotoxin deoxynivalenol in growing pigs fed with naturally contaminated pelleted grains with and without the addition of Coriobacteriaceum DSM 11798. Food. Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk. Assess. 35(7):1394–1409. doi: 10.1080/19440049.2018.1461254 [DOI] [PubMed] [Google Scholar]
- Scholten, R.H., Rijnen M.M., Schrama J.W., Boer H., van der Peet-Schwering C.M., Den Hartog L.A., Vesseur P.C., and Verstegen M.W... 2001. Fermentation of liquid coproducts and liquid compound diets: Part 2. Effects on pH, acid-binding capacity, organic acids and ethanol during a 6-day storage period. J. Anim. Physiol. Anim. Nutr. (Berl) 85((5-6):124–134. doi: 10.1046/j.1439-0396.2001.00310.x [DOI] [PubMed] [Google Scholar]
- Schwartz, H.E., Hametner C., Slavik V., Greitbauer O., Bichl G., Kunz-Vekiru E., Schatzmayr D., and Berthiller F... 2013. Characterization of three deoxynivalenol sulfonates formed by reaction of deoxynivalenol with sulfur reagents. J. Agric. Food Chem. 61(37):8941–8948. doi: 10.1021/jf403438b [DOI] [PubMed] [Google Scholar]
- Sciopioni R., Zaghini G., and Biavati B.R... 1978. Researches on the use of acidified diets for early weaning of piglets. Nutr. Anim. 4:201–218. [Google Scholar]
- Sergent, T., Parys M., Garsou S., Pussemier L., Schneider Y.J., and Larondelle Y... 2006. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol. Lett. 164(2):167–176. doi: 10.1016/j.toxlet.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Shawk, D.J., Dritz S.S., Goodband R.D., Tokach M.D., Woodworth J.C., and DeRouchey J.M... 2019. Effects of sodium metabisulfite additives on nursery pig growth. Transl. Anim. Sci. 3(1):103–112. doi: 10.1093/tas/txy098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty, P.H., and Jespersen L... 2006. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 17(2):48–55. doi: 10.1016/j.jpgs.2005.10.004 [DOI] [Google Scholar]
- Solà-Oriol, D., Roura E., and Torrallardona D... 2011. Feed preference in pigs: effect of selected protein, fat, and fiber sources at different inclusion rates. J. Anim. Sci. 89(10):3219–3227. doi: 10.2527/jas.2011-3885 [DOI] [PubMed] [Google Scholar]
- Suarez, J., Roura E., and Torrallardona D... 2010. Dietary preferences of acids and salts in piglets. J. Anim. Sci. 88(2):651. (Abstr.). [Google Scholar]
- Sun, K., Lei Y., Wang R., Wu Z., and Wu G... 2017. Cinnamicaldehyde regulates the expression of tight junction proteins and amino acid transporters in intestinal porcine epithelial cells. J. Anim. Sci. Biotechnol. 8:66. doi: 10.1186/s40104-017-0186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Y., Zhang D., Cai P., Lin H., Ying H., Hu Q.-N., and Wu A... 2022. Elimination of Fusarium mycotoxin deoxynivalenol (DON) via microbial and enzymatic strategies: Current status and future perspectives. Trends Food Sci. Technol. 124:96–107. doi: 10.1016/j.jpgs.2022.04.002 [DOI] [Google Scholar]
- Til, H.P., Feron V.J., and de Groot A.P... 1972. The toxicity of sulphite. I. Long-term feeding and multigeneration studies in rats. Food Cosmet. Toxicol. 10(3):291–310. doi: 10.1016/s0015-6264(72)80250-5 [DOI] [PubMed] [Google Scholar]
- Truong, H.H., Cadogan D.J., Liu S.Y., and Selle P.H... 2016. Addition of sodium metabisulfite and microbial phytase, individually and in combination, to a sorghum-based diet for broiler chickens from 7 to 28 days post-hatch. Anim. Prod. Sci. 56(9):1484–1491. doi: 10.1071/an14841 [DOI] [Google Scholar]
- Van Boeckel, T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., and Laxminarayan R... 2015. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. 112(18):5649–5654. doi: 10.1073/pnas.1503141112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte, I., Audenaert K., and De Gelder L... 2016. Biodegradation of mycotoxins: tales from known and unexplored worlds. Front. Microbiol. 7:561. doi: 10.3389/fmicb.2016.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra, A., Syal P., and Madan A... 2016. Probiotic yeasts in livestock sector. Anim. Feed Sci. Technol. 219:31–47. doi: 10.1016/j.anifeedsci.2016.05.019 [DOI] [Google Scholar]
- Wang, L.L., Yang C., and Liu S... 2022. Development and antibacterial activity of zinc oxide nanoparticles encapsulated in core–shell microparticles for managing enterotoxigenic Escherichia coli-related post-weaning diarrhea. Appl. Nanosci. 12(5):1449–1458. doi: 10.1007/s13204-021-02303-7 [DOI] [Google Scholar]
- Xu, Y., Lahaye L., He Z., Zhang J., Yang C., and Piao X... 2020. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+). Anim. Nutr. 6(3):269–277. doi: 10.1016/j.aninu.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, S., and Jha R... 2019. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 10:2. doi: 10.1186/s40104-018-0310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, F., and Polk D.B... 2011. Probiotics and immune health. Curr. Gastroenterol. Rep. 27(6):496–501. doi: 10.1097/MOG.0b013e32834baa4d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F., Hou C., Zeng X., and Qiao S... 2015a. The use of lactic acid bacteria as a probiotic in swine diets. Pathogens 4(1):34–45. doi: 10.3390/pathogens4010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C., Chowdhury M.A., Huo Y., and Gong J... 2015b. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens 4(1):137–156. doi: 10.3390/pathogens4010137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C., Litke Q., Li Q., Lu P., Liu S., Diony F., Gong J., Yang C., and Liu S... 2022. Targeted delivery of sodium metabisulfite (SMBS) by pH-sensitive Eudragit L100-55 nanofibrous mats fabricated through advanced coaxial electrospinning. J. Mater. Sci. 57(5):3375–3395. doi: 10.1007/s10853-021-06785-2 [DOI] [Google Scholar]
- Yu, C., Lu P., Liu S., Li Q., Xu E., Gong J., Liu S., and Yang C... 2021. Efficiency of deoxynivalenol detoxification by microencapsulated sodium metabisulfite assessed via an In vitro bioassay based on intestinal porcine epithelial cells. ACS Omega 6(12):8382–8393. doi: 10.1021/acsomega.1c00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H., Zhou T., Gong J., Young C., Su X., Li X.Z., Zhu H., Tsao R., and Yang R... 2010. Isolation of deoxynivalenol-transforming bacteria from the chicken intestines using the approach of PCR-DGGE guided microbial selection. BMC Microbiol. 10:182. doi: 10.1186/1471-2180-10-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, T., Gong J., Yu H., and Li X.Z... 2014. Bacterial isolate and methods for detoxification of trichothecene mycotoxins. US patent publication No. US8642317B2. [Google Scholar]
- Zhu, Y., Hassan Y.I., Watts C., and Zhou T... 2016. Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients—a review of recent patents. Anim. Feed Sci. Technol. 216:19–29. doi: 10.1016/j.anifeedsci.2016.03.030 [DOI] [Google Scholar]