Abstract
Prospective Anti-viral compound 3, 5 Dimethyl Pyrazolium 3, 5 Dichloro Salicylate (DPDS) was synthesized and characterized using FT-IR, FT-Raman, UV and NMR spectra. To escort the experimental results, computational methods were performed using B3LYP with 6-311G (d, p) basis set expending Gaussian09w package to attain geometry of the molecule. Vibrational assignments for all the vibrational modes have been made of PED results obtained from SQM method. On contrary, FMO analysis, global chemical reactivity descriptors, Aromaticity and Natural charge analysis were studied. Molecular stability and bond strength have been inquired by executing NBO analysis. Topological features of DPDS were intended by MEP, ELF and LOL maps. UV–vis spectrum was predicted by TD-DFT method in gaseous phase and compared with the experimental spectrum for displaying the involved electronic transitions in the compound. The interactions within the DPDS molecule were investigated via RDG analysis. Molecular docking was performed with SARS-CoV-2 proteins and docking parameters were obtained. Drug likeness was carried out based on Lipinski's rule of five and the ADMET factors were also predicted.
Keywords: ELF, RDG, DFT, IR, Docking, ADMET
Graphical abstract
1. Introduction
In recent times, viral infections are reflected as one of the prime extortions to social existence and health global. Controlling of such viral infections epitomizes a rich field of scientific research because of viruses’ mutability that springs new drug-resistant strains. Pyrazole derivatives have attracted more attention due to their expediency in the pharmaceutical research. Naturally acquired pyrazoles exhibits organic activities and used as efficient chemotherapeutics. Among azoles, pyrazoles are rarely found in nature probably due to their difficulty in formation of N-N bond by living organisms [1]. Pyrazole derivatives are also important in treating muscle pain, fever and arthritis but the recent literature shows these derivatives are not only analgesic and anti-inflammatory but also parades anti-viral, anti-bacterial, anti-tumor and anti-microbial activities.The competency to kill infectious agents and preventing their proliferation inside the living organisms and environment is the anti-microbial properties. Thus, multitude of compounds is investigated for their potential applications as therapeutic agents in treating infectious diseases especially those caused by multi-resistant virus and bacteria [2]. Salicylic acid is an organic acid used in pharmaceutical laboratories and is the formative material for making drugs like aspirin [3] and is the natural and safest chemical used for postharvest quality maintenance in the field of horticulture [4]. 3, 5-dichloro salicylic acid has extensive applications and has been recognized as a potential enzyme inhibitor and burgeoning usage in cosmetic industries [5]. Effect of pyrazole is studied and reveals that the pyrazolium anion is less reactive towards nucleophiles and is more to electrophiles whereas it has broad spectrum of biological activity comprising anti-viral, anti-bacterial and anti-tumor to engender novel primes possessing pyrazole nucleus with high efficacy. Since pyrazole a heterocyclic moiety, it epitomizes the fundamental configuration for the number of drugs and establishes a pertinent synthetic path in curative diligence [6].
Literature screening divulges that the crystal structure of 3, 5-Dimethyl Pyrazolium 3, 5-Dichloro Salicylate (DPDS) has not been reported so far and this meagerness observed in the literature stimulated us to make vibrational spectroscopic research based on the molecule, to give a precise consignment of the fundamental bands in FT-IR and FT-Raman spectra. Geometry of the organic structures requires diverse techniques and approaches en route for their strength in gaseous state. Redistribution of electron density was designed using NBO (Natural Bond Orbital) analysis. MO (Molecular Orbital) analysis was accomplished to intend the biological activity of the molecule and the impact of transition of electrons from π →π* were studied using UV-vis (Ultraviolet Visible) spectrum. Further Aromaticity and Natural Charge Analysis were also counted in order to determine the aromatic character and charge distribution of the molecule correspondingly. Distribution of electrons and reactive sites on the surface were analyzed using ESP (Electrostatic potential), ELF (Electron localization function) and LOL (Localized orbital locator). Repulsive, attractive, and van der Waals strong and weak interactions in DPDS were investigated by RDG (Reduced Density Gradient) analysis. Drug likeness and molecular docking methodology were empowered to form drug potential and bioactivity of DPDS. Insilico ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicology) analysis was also anticipated to crisscross whether the compound is a vigorous drug to treat SARS-CoV-2.
2. Experimental details
2.1. Synthesis
Slow evaporation- solution growth technique was used to grow single crystal. 3, 5 –Dichloro Salicylic acid (DS) and 3, 5-Dimethyl Pyrazole (DP) were taken in 1:1 stoichiometric proportions and dissolved homogeneously using methanol as a solvent. Their reaction mixture was thoroughly mixed together using mechanical stirrer up to 3 h at room temperature to get a clear solution. The synthetic scheme of DPDS was depicted in Scheme 1 . This solution was filtered through a quantitative filter paper (Whatman no.40) and filtrate was kept aside without any disturbance for the growth of crystal in a dust free environment at ambient temperature. Well defined, transparent crystals were collected at the end of 6th day and the collected crystals were recrystallized using dry methanol to get good quality crystals.
Scheme 1.
Synthetic scheme for DPDS.
2.2. Characterization techniques
FT-IR spectrum of DPDS was recorded on a Perkin Elmer FT-IR 8000 spectrophotometer in the range of 4000–400 cm−1using KBr pellet technique at room temperature. FT-Raman spectrum has been recorded on a Bruker RFS 27: Standalone FT-Raman Spectrometer with Nd: YAG laser source at 1064 nm and resolution 2 cm−1 in the region 4000–50 cm−1. Electronic absorption spectrum was measured in methanol using JASCO UV-Vis spectrophotometer in the range of 200–800 nm.13Cand 1H NMR spectra were recorded employing a Bruker AV III 400 MHz spectrometer in deuterated dimethyl sulphoxide (DMSO) as solvent using TMS as an internal standard.
3. Computational details
Theoretical calculations for DPDS were carried out with the aid of Gaussian09w package [7] using B3LYP method in conjunction with 6–311G (d, p) basis set. Natural bond orbital (NBO) analysis [8] was also achieved and the molecular visualization was ended by the Gauss view6 program [9]. MOLVIB programming was preferred for the vibrational spectral assignments by utilizing potential energy distribution (PED) analysis [10]. MO analysis and Molecular electrostatic potential (MEP) mapping were calculated using the same B3LYP level of theory. Auto Dock Suite 4.2.1 was used to find the minimum binding energy, inhibition constant and various parameters of the ligand-protein docking interactions [11].The ligand-protein binding sites have been visualized using PYMOL graphic software [12]. ELF, LOL and the RDG and sign (λ2)ρ functions were computed using Multiwfn [13] and the RDG scatter graph was drawn with the VMD (Visual Molecular Dynamics) program [14].
4. Results and discussions
4.1. Geometry optimization
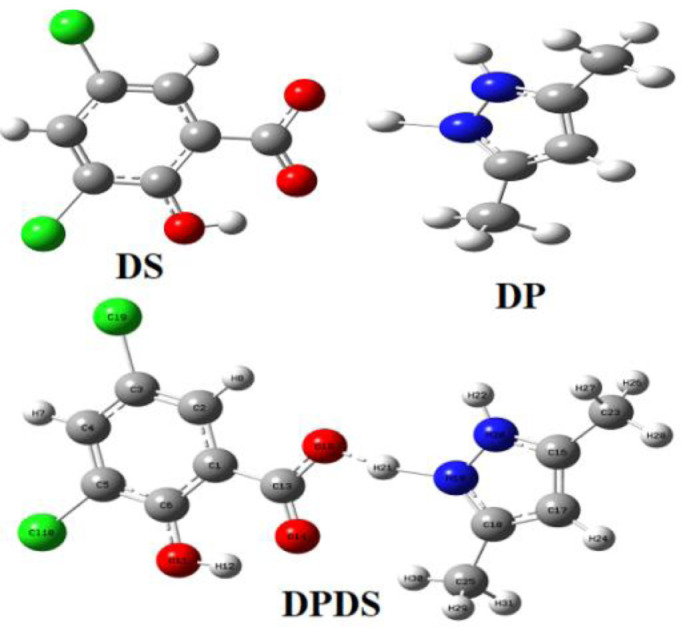
Optimized molecular structures of DS, DP & DPDS with atomic symbols and labels are shown in Fig. 1, respectively.
Fig. 1.
Optimized Molecular Structures of DS, DP and DPDS.
The self-consistent field energy of DPDS -1720.34 a.u is combined with the energy of DS and DP refers to -1415.40 a.u and -304.92 a.u, respectively. BSSE and counterpoise corrected energy for DPDS have been calculated as 0.004 a.u and -1720.34 a.u, respectively. Wavenumbers in the solid phase must be higher than the gaseous phase due to the isolation of molecule in the solid state. Optimized Bond length with bond angles of DPDS are tabulated in Table 1 and dihedral angles of DPDS are tabulated in Table S1 which are compared with the XRD data of 3,5 Dichlorosalicylic acid [16] and Dichlorobis(3,5-dimethylpyrazole)copper(II) [18] besides their RMS values are calculated.
Table 1.
Comparison of optimized bond length and bond angle of DPDS with the XRD data of 3,5 Dichlorosalicylic acid and Dichlorobis(3,5-dimethylpyrazole)copper(II).
| Bond length | Theoretical value (Å) |
Expt (Å) | RMS (Å) | Bond Angle | Theoretical value (°) |
Expt (°) | RMS (°) | ||
|---|---|---|---|---|---|---|---|---|---|
| DPDS | DS/DP | DPDS | DS/DP | ||||||
| C1-C2 | 1.401 | 1.409 | 1.393 | 0.00006 | C2-C1-C6 | 120.58 | 120.58 | 120.22 | 0.1296 |
| C1-C6 | 1.415 | 1.415 | 1.399 | 0.00025 | C2-C1- C13 | 120.70 | 120.40 | 119.83 | 0.1296 |
| C1-C13 | 1.482 | 1.485 | 1.474 | 0.00006 | C6-C1- C13 | 118.70 | 118.66 | 119.93 | 1.5129 |
| C2-C3 | 1.380 | 1.381 | 1.376 | 0.00001 | C1-C2-C3 | 119.77 | 119.69 | 119.82 | 1.5129 |
| C2-H8 | 1.080 | 1.055 | 0.930 | 0.02250 | C1-C2- H8 | 119.18 | 119.13 | 120.11 | 0.8649 |
| C3-C4 | 1.395 | 1.394 | 1.376 | 0.00036 | C3-C2- H8 | 121.04 | 121.17 | 120.07 | 0.8649 |
| C3-Cl9 | 1.760 | 1.745 | 1.733 | 0.00072 | C2-C3-C4 | 120.86 | 120.80 | 121.12 | 0.0676 |
| C4-C5 | 1.384 | 1.386 | 1.380 | 0.00001 | C2-C3-Cl9 | 120.03 | 120.00 | 119.01 | 0.0676 |
| C4-H7 | 1.081 | 1.088 | 0.931 | 0.02250 | C4-C3-Cl9 | 119.10 | 119.18 | 119.87 | 0.5929 |
| C5-C6 | 1.408 | 1.398 | 1.390 | 0.00032 | C3-C4-C5 | 119.52 | 119.51 | 118.65 | 0.5929 |
| C5-Cl10 | 1.748 | 1.745 | 1.737 | 0.00012 | C3-C4- H7 | 120.61 | 120.63 | 120.65 | 0.0016 |
| C6-O11 | 1.332 | 1.331 | 1.342 | 0.00010 | C5-C4-H7 | 119.85 | 119.81 | 120.7 | 0.0016 |
| O11-H12 | 0.989 | 0.982 | 0.921 | 0.00462 | C4-C5-C6 | 121.47 | 121.82 | 122.2 | 0.5329 |
| H12-O14 | 1.689 | 1.689 | 1.912 | 0.04972 | C4-C5-Cl10 | 119.55 | 119.54 | 119.43 | 0.5329 |
| C13-O14 | 1.232 | 1.239 | 1.232 | 0.00000 | C6-C5-Cl10 | 118.97 | 118.13 | 118.37 | 0.3600 |
| C13-O15 | 1.316 | 1.315 | 1.305 | 0.00012 | C1-C6-C5 | 117.77 | 117.62 | 117.98 | 0.3600 |
| O15…H21 | 1.013 | - | 0.820 | 0.03724 | C1-C6- O11 | 123.08 | 122.46 | 123.85 | 0.5929 |
| C16-C17 | 1.381 | 1.380 | 1.390 | 0.00008 | C5-C6- O11 | 119.14 | 118.10 | 118.16 | 0.5929 |
| C16-N20 | 1.35 | 1.350 | 1.368 | 0.00048 | C6- O11-H12 | 106.51 | 106.91 | 106.42 | 0.0081 |
| C16-C23 | 1.494 | 1.494 | 1.496 | 0.00000 | C1- C13-O14 | 122.03 | 122.42 | 122.3 | 0.0081 |
| C17-C18 | 1.415 | 1.419 | 1.352 | 0.00396 | C1- C13-O15 | 114.82 | 114.82 | 114.7 | 0.0144 |
| C17-H24 | 1.078 | 1.078 | 0.931 | 0.02160 | O14- C13-O15 | 123.13 | 123.30 | 123 | 0.0144 |
| C18-N19 | 1.331 | 1.329 | 1.347 | 0.00003 | C13-O15…H21 | 111.75 | - | 109.54 | 4.8841 |
| C18-C25 | 1.496 | 1.497 | 1.501 | 0.00002 | C17- C16-N20 | 105.56 | 105.59 | 106.28 | 0.5184 |
| N19-N20 | 1.355 | 1.354 | 1.342 | 0.00016 | C17- C16-N23 | 131.75 | 131.85 | 130.75 | 1.0000 |
| N19-H21 | 1.690 | - | - | - | N20- C16-N23 | 122.68 | 122.65 | 121.94 | 1.0000 |
| N20-H22 | 1.007 | 1.007 | 2.010 | 1.00600 | C16- C17-C18 | 106.17 | 106.17 | 107.01 | 0.7056 |
| C23-H26 | 1.094 | 1.094 | 0.960 | 0.01795 | C16- C17-H24 | 126.56 | 126.87 | 126.45 | 0.7056 |
| C23-H27 | 1.094 | 1.090 | 0.959 | 0.01822 | C18- C17-H24 | 127.26 | 127.68 | 126.53 | 0.5329 |
| C23-H28 | 1.089 | 1.084 | 0.960 | 0.01664 | C17-C18-N19 | 110.00 | 110.58 | 110.91 | 0.5329 |
| C25-H29 | 1.093 | 1.093 | 0.959 | 0.01795 | C17-C18-C25 | 128.70 | 128.73 | 128.31 | 0.1521 |
| C25-H30 | 1.089 | 1.084 | 0.960 | 0.01664 | N19- C18-C25 | 121.28 | 121.96 | 121.78 | 0.1521 |
| C25-H31 | 1.093 | 1.093 | 0.959 | 0.01795 | C18-N19-N20 | 105.61 | 105.60 | 105.29 | 0.1024 |
| C18-N19-H21 | 134.62 | 134.53 | 134.85 | 0.1024 | |||||
| N20-N19-H21 | 119.75 | 119.65 | - | - | |||||
| C16-N20-N19 | 112.63 | 112.68 | 111.5 | 1.2769 | |||||
| C16-N20-H22 | 127.94 | 127.96 | 127.81 | 0.0169 | |||||
| N19-N20-H22 | 119.41 | 119.60 | 120.57 | 0.0169 | |||||
| O15…H21-N19 | 168.23 | - | - | - | |||||
| C16-C23-H26 | 111.57 | 111.61 | 110.5 | 1.1449 | |||||
| C16-C23-H27 | 111.57 | 111.71 | 109.45 | 0.0144 | |||||
| C16-C23-H28 | 109.97 | 109.71 | 109.55 | 0.0144 | |||||
| H26-C23-H27 | 107.85 | 107.02 | 109.51 | 0.1156 | |||||
| H26-C23-H28 | 107.85 | 107.70 | 109.4 | 0.1156 | |||||
| H27-C23-H28 | 107.85 | 107.75 | 109.4 | 0.2025 | |||||
| C18-C25-H29 | 110.72 | 110.75 | 109.36 | 0.2025 | |||||
| C18-C25-H30 | 110.49 | 111.40 | 109.38 | 1.2321 | |||||
| C18-C25-H31 | 110.73 | 110.66 | 109.4 | 1.2321 | |||||
| H29-C25-H30 | 108.69 | 108.40 | 109.58 | 0.7921 | |||||
| H29-C25-H31 | 107.39 | 107.41 | 109.63 | 0.7921 | |||||
| H30-C25-H31 | 108.69 | 108.41 | 109.47 | 0.0484 | |||||
Deliberated bond length O15…H21 is feeble than other wave numbers, indicates the formation of intermolecular hydrogen bonding O15…H21-N21 with improved stabilization. Hydrogen bond length O15…H21 is obtained as 1.013 Å which is emphatically deficient than that of van der Waals radii (2.75 Å) [15] and is extraordinary compared to the experimental value 0.820 Å [16] with a RMS value surpassed around 0.03724 Å at very high resolution. Also geometrical bond angles O15...H21-N19 spectacles extreme of 168.23 ° which is terribly amplified from the trigonal angle 120 ° owed to intermolecular interactions between pyrazole and benzene ring moieties. These results boot the contingency of intermolecular hydrogen bond formation which gets a closer comprehension and enterprise the molecules with enhanced biological contour.
Bond angles C1-C6-O11 (123.08 °) and C5-C6-O11 (119.14 °) with experimental values 123.85 ° and 118.16 °, respectively are diverged from the intended trigonal angle owing to the influence of hydroxyl group in the phenyl ring. This divergence scanted the bond length O11-H12 (0.9898 Å) and bond angle C6- O11-H12 (106.51 °) which are deflated to radical values due to the deprotonation of salicylic acid to form hydrogen bond between oxygen in the carboxylate anion and hydrogen in the alcohol group, thereby forming intramolecular interaction H12…O14 with bond length 1.689 Å with a RMS value 0.04972 Å at high resolution stimulates the bioactive nature of DPDS molecule [17]. RMS value for the bond angle C13-O15…H21 gets increased to 4.8841 ° due to the formation of salicylate anion at very low resolution leads to the inter-molecular interaction.
Adjoining these interactions C3-Cl9 (1.760 Å) & C5-Cl10 (1.748 Å) holds the extreme bond length ensuring their experimental values 1.733 Å & 1.737 Å, respectively which are in line for the virtue of electron withdrawing nature of chlorine which increases the double bond character by redistributing π electrons in maximum shared orbitals, thus deteriorate the bond length of chlorine as compared to other bond lengths. Bond length assigned to C18-C25 (1.4962 Å) & C16-C23 (1.4941 Å) remains shrinking owing to the resonance of dimethyl group annexed to the pyrazole ring. Bond length for C18-N19 (1.3319 Å) is subordinate to the bond length C16-N20 (1.3573 Å) which designates the effect of double bond in the pyrazole ring. RMS value increases to 1.0 Å amidst N20-H22 with bond distance 1.007 Å and C16-N20-N19 with bond angle 112.63 ° obligating experimental value 2.010 Å and 111.5 °, respectively [18] which is hefty and random since the diffraction terms ought to slight or no impact on the geometry at low resolution [19].
Bond lengths C18-N19 (1.331 Å) and C16-N20 (1.357 Å) were stretched while equated to their experimental values 1.347 Å and 1.368 Å, respectively designates substantial electron delocalization in the pyrazole ring structure. Bond angles H21-N19-C18 (134.62 °) and H21-N19-N20 (119.75 °) are exaggerated distortedly in line for the incidence of nitrogen in the pyrazole ring whereas scale down in bond angles C17- C16-N20 (105.56 °) and C18-N19-N20 (105.56 °) stand owed to the steric interaction in virtue of intermolecular hydrogen bonding interactions.
Dihedral angle is an imperative constraint to unfold the conformation of voluminous organic and bioorganic molecules. Furthermost of the dihedral angles of phenyl ring are about virtually -179.99 ° and 0 ° point towards structure of salicylate ion is planar. Carboxylate anion devoted to the phenyl ring has antiperiplanar conformations which are flagged by the torsional angles C2-C1-C13- O 14 (-179.98 °) & C6-C1- C13-O 15 (-179.98 °). The dimethyl groups attached to the pyrazole ring are found to be out of plane are indicated by the torsional angles C17-C16-C23-H26 (-119.97 °), C17-C16-C23-H27 (-119.31 °), N20-C16-C23-H26 (-60.06 °), N20-C16-C23-H27 (60.65 °), C17-C18-C25-H29 (59.32 °), C17-C18-C25-H31 (-59.68 °), N19-C18-C25-H29 (-120.68 °) and N19-C18-C25-H31 (120.30 °) [20].
4.2. NBO profile
NBO analysis contributes the proficient schemes to examine the chemical characteristics of the bonding and different properties like intra and intermolecular charge transfer, basicity, stability, reactivity and correlation between donor and acceptor. The interactions between filled and vacant orbitals are tabulated in Table 2 .
Table 2.
Second order perturbation theory analysis of Fock matrix.
| Donor (i) | ED(i) (e) | Acceptor (j) | ED(j) (e) | E(2)a (kcal/mol) | E(j)-E(i)b (a.u) | F(ij)c (a.u) |
|---|---|---|---|---|---|---|
| σ(C1 – C2) | 1.96303 | σ*(C1 – C13) | 0.06263 | 2.25 | 1.12 | 0.045 |
| σ*(C3 – Cl9) | 0.03318 | 5.60 | 0.84 | 0.061 | ||
| LP(1)O14 | 1.95987 | σ *(O11 - H12) | 0.05796 | 4.30 | 1.10 | 0.062 |
| π (C1 – C2) | 1.65916 | π*(C5 – C6) | 0.44564 | 24.93 | 0.26 | 0.074 |
| σ(C1 – C6) | 1.96778 | σ*(C1 – C2) | 0.02188 | 4.29 | 1.26 | 0.066 |
| π(C5 – C6) | 1.60297 | π*(C3 – C4) | 0.42826 | 27.78 | 0.28 | 0.080 |
| σ(O11 – H12) | 1.98573 | σ*(C5 – C6) | 0.04282 | 5.82 | 1.25 | 0.077 |
| LP (1)Cl9 | 1.99215 | σ*(C3 – C4) | 0.03087 | 1.51 | 1.47 | 0.042 |
| LP (3)Cl9 | 1.93777 | π*(C3 – C4) | 0.42826 | 11.21 | 0.33 | 0.060 |
| LP (2)Cl10 | 1.96664 | σ*(C4 – C5) | 0.02608 | 4.02 | 0.88 | 0.053 |
| LP (3)Cl10 | 1.92703 | π*(C5 – C6) | 0.44564 | 11.60 | 0.32 | 0.060 |
| LP (1)O11 | 1.97308 | σ*(C1 – C6) | 0.03779 | 7.79 | 1.10 | 0.083 |
| LP (2) O11 | 1.81257 | π*(C5 – C6) | 0.44564 | 40.21 | 0.31 | 0.107 |
| LP (2) O14 | 1.85119 | σ*(C13 – O15) | 0.06444 | 24.60 | 0.72 | 0.121 |
| LP (1) O15 | 1.96086 | σ*(C13 – O14) | 0.02806 | 8.82 | 1.11 | 0.089 |
| LP (2) O15 | 1.74384 | π*(C13 – O14) | 0.33800 | 64.50 | 0.30 | 0.126 |
| σ*(C13 –O15) | 0.02806 | LP (1) H21 | 0.56515 | 13.93 | 1.16 | 0.133 |
| LP (3) O15 | 1.65021 | LP*(1) H21 | 0.56515 | 437.42 | 0.67 | 0.511 |
| π* (C18 - N19) | 1.90252 | π* (C16 - C17) | 0.05796 | 89.77 | 0.03 | 0.071 |
| LP (1) N19 | 1.73150 | LP*(1) H21 | 0.56515 | 286.67 | 0.61 | 0.404 |
E(2) means energy of hyper conjugative interaction (stabilization energy).
E(j) - E(i) is the energy difference between donor i and acceptor j.
F(i,j) is the Fock matrix element between i and j NBO orbital's.
Scope of hyper conjugation is governed by the contributing capability of Lewis type orbitals and the acquiescent capability of non-Lewis type orbitals which is measured in terms of interaction energy E(2). Inordinate hyper conjugative interaction energy advance the level of delocalization which consequently grander the stability of molecular system [21]. Overlapping of orbitals σ(C1 – C2) to σ*(C1 – C13) and σ*(C3 – Cl9) leads to the interaction energy of 2.25 kcal/mol and 5.60 kcal/mol, respectively. Utmost pivotal interactions in DPDS is stuck between the lone pairs LP(3)O15 and LP*(1) H21 ensuing very high stabilization energy 437.42 kcal/mol which forms intermolecular hydrogen bonding O15…H21-N19 resulting in intermolecular charge transfer. This loftier energy shows that the hyper conjugative interaction transpires amongst the electron donating and the acceptor group which enriches the bioactivity of the DPDS molecule [22].
Anti-bonding orbital σ*(C13 –O15) overlaps with the lone pair orbital LP (1) H21 outcomes with a negative hyper conjugation with a stabilization energy 13.93 kcal/mol and is illuminated using inductive effect of oxygen atom. Interaction between two lone pair electrons LP (1) N19 and unfilled p-orbital LP*(1) H21 grades to stronger stabilization with an interaction energy 286.67 kcal/mol and leads to reduction in hybridization and the non-periodic power of these bonds ensure inferences for the exposure of molecules [23]. Orbital overlapping between lone pair LP (1) O14 to anti-bonding orbital σ *(O11 - H12) results in the stabilization energy of 4.30 kcal/mol triggering intra-molecular charge transfer and leads to hyper conjugative intra-molecular hydrogen bonding interaction [24]. Rehybridization plays a dominant role in weakening and elongation of hydrogen bond observed in Table 3 . NBO analysis of DPDS in comparison with DS and DP clearly shows the sign for the formation of strong H-bonded interactions.
Table 3.
Composition of H-bonded NBOs in terms of natural atomic hybrids.
| Bond (A-B) | DS/DP |
DPDS |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EDA % | EDB % | spn | A |
B |
EDA % | EDB % | spn | A |
B |
|||||
| s% | p% | s% | p% | s% | p% | s% | p% | |||||||
| C13-O15 | 66.79 | 33.21 | sp2.41 | 25.08 | 74.72 | 35.03 | 64.90 | 66.74 | 33.26 | sp2.16 | 29.31 | 70.49 | 34.35 | 65.57 |
| C18-N19 | 59.35 | 40.65 | sp1.97 | 29.61 | 70.28 | 38.85 | 61.06 | 61.19 | 38.81 | sp1.94 | 28.75 | 71.15 | 41.25 | 58.69 |
| N19- N20 | 45.30 | 54.70 | sp2.86 | 30.59 | 69.33 | 22.39 | 77.46 | 47.24 | 52.76 | sp2.85 | 22.96 | 76.92 | 29.83 | 70.09 |
The influence of rehybridization results in destructive effects, leads to contraction and strengthening of C13-O15 bond by the impact of s-character of the hybrid orbital decreases from sp2.41 to sp2.16. Though the contraction and elongation of bonds is due to the effect of rehybridization and hyper conjugation, rehybridization dominates and overshadows the latter. This is well reflected in the geometry as bond C13-O15 which contracts by 0.25 Å with respect to DS and manifests the delocalization of electron density. The s-character of C18-N19 hybrid orbital falls from sp1.97 to sp1.96 hints to the contraction of C18-N19 bond due to the reduction in s-character of carbon and polarization of the C-N bond in the progression of N-H…O inter-molecular hydrogen bond formation. Polarization promulgates over bonds leads to rehybridization with hybrid orbital declines from sp2.86 to sp2.85 and p-character increases to 69.33% with s-character 30.59% in the N-N hybrid bond. Because the total s- and p-characters at every nitrogen atom are sealed, this shrinkage in the s-character leads to a reflex upsurge in the other hybrid orbitals at N including the N-H bond. This escalation leads to inter-molecular hydrogen bonding by shortening and strengthening N-H bonds.
4.3. Electronic properties
4.3.1. Frontier molecular orbitals
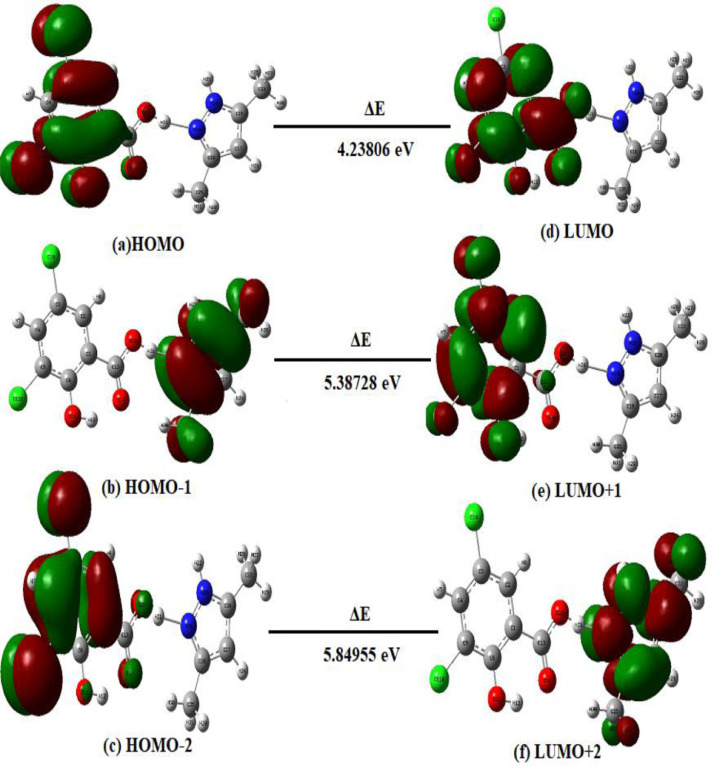
Highest occupied molecular orbitals (HOMO) and Lowest unoccupied molecular orbitals (LUMO) are the Frontier molecular orbitals which are vibrant to depict kinetic stability and chemical reactivity by their energy gap. The positive and negative has shown in red and green congealed segments, respectively. HOMO is related to the more reactive molecule with electrophiles whereas the lower energy profile related to less reactive molecule with nucleophiles [25]. A molecule with small frontier orbital gap is more polarisable and is generally associated with high chemical reactivity [26]. Ordinarily the conjugated molecules are characterised by less H→L separation, which is significant to the gradation of intra-molecular charge relocation from the end-capping electron-donor groups to the proficient electron-acceptor group [27].
Fig. 2 illustrates the Frontier Molecular Orbital transitions within the molecule. It is inferred from the figure that HOMO localised over the phenyl ring and hydroxyl group and LUMO localised over the phenyl ring and carboxylic acid group besides DS. HOMO→LUMO transition implies that electron density has spread over and charge transfer occurs from hydroxyl group to the carboxylic acid group, thereby no charge interactions in DP moiety. Calculated HOMO and LUMO energies are -9.12983 eV and -4.89177 Ev, respectively while the energy gap serves as a stability index. In fact, a small HOMO-LUMO gap implies high molecular stability in the sense of virtuous reactivity in chemical reactions [28]. The figured band gap energy is 4.23806 eV which embodies the low energy electronic transitions inherently belongs to π→π* transitions and is chemically more reactive [29] affords biological delineation with a trifling band gap. The overlying orbital loops has occurred in the benzene ring of DS moiety and the HOMO LUMO transition endorses the revelation of resonance expedited with hydrogen bonding in course of electron density transfer. HOMO-1→ LUMO+1 and HOMO-2→LUMO+2 transitions were also implemented with their band gap energies 5.38738 eV and 5.84955 eV, respectively.
Fig. 2.
Plot of Frontier Molecular Orbitals of DPDS.
4.3.2. Global reactivity descriptors
Molecular descriptors of global reactivity act as mediators between the kinetic stability and chemical reactivity of the molecule and were calculated from Frontal Orbital energy using Koopman's theorem [30].The global descriptors are Ionisation Potential (IP), Electron Affinity (EA), Electronegativity (χ), Global hardness (ɳ), Softness (σ), Chemical Potential (µ) and Global Electrophilicity index (ω) [31] can be calculated using B3LYP/6-311G (d, p) and listed in the Table S2.
Following Parr and Pearson [32] Chemical potential describes the emerging inclination of electron from a firm system and is -7.01080 eV which notifies the stability does not disintegrate impulsively into its fundamental at its least possible assessment. The hardness insinuates the resistance towards the dislocation of electron cloud in the chemical systems under slight perturbations that stumble during chemical course of action. DPDS molecule is more reactive and highly polarizable due to lanky hardness of 7.01080 eV and stumpy softness value 0.14263 eV [33]. Global electrophilicity index is the better descriptor of global chemical reactivity which is 3.50539 eV describes the biological activity of DPDS compound [34] for DPDS and measures the equilibrium in energy when the system acquires additional electronic charge from the entourage. It also encompasses the dexterity of an electrophile to acquire additional electronic charge and the resistance of the system to swap electronic charge with the entourage.
4.4. UV-visible spectral analysis
UV absorption spectra for the optimized molecule DPDS were calculated with the aid of computational method to determine the low-lying excited states of DPDS theoretically [35] and recorded in gas phase. The electronic transitions confirm the absorption peaks experimentally and theoretically are shown in Fig 3 . NBO analysis indicates that molecular orbitals are generally composed of π and σ atomic orbitals but in UV-vis region molecules allows strong π →π* transitions with the high extinction coefficients and higher the coefficients, more wavelength is absorbed [36]. The absorption spectra exhibits an intense peaks at 270 nm which affirms the protonation of nitrogen in the pyrazole ring due to π→π* transitions.
Fig. 3.
UV spectra of DPDS.
Excitation energies, absorbance and oscillator strength (f) for DPDS molecule were tabularized in Table 4 using Gauss Sum [37]. Absorption peak (λmax) in the UV-vis spectrum predicts electronic transition supreme at 265 nm with an oscillator strength f = 0.0576 with a major contribution of 86% from HOMO to LUMO shows good agreement with the experimental data at 270 nm. Other wavelengths obtained via computational method are 235.88, 232.97, 229.33, 225.12 and 219.05 nm (in gas). In view of calculated absorption spectra the maximal oscillator strength is 0.0722 at the wavelength 225 nm with a major contribution of about 52% from HOMO to LUMO+1 transitions. Minimal oscillator strength is zero at wavelength 235 nm with a major contribution 60% from HOMO to LUMO+4 electronic transition.
Table 4.
UV-vis excitation energy and oscillator strength for DPDS.
| Experimental |
Energy | Theoretical |
Oscillator strength | Symmetry | Major Contributions | ||
|---|---|---|---|---|---|---|---|
| λmax (nm) | Band gap (eV) | λmax (nm) | Band gap (eV) | ||||
| 270 | 4.23806 | 37720 | 265 | 4.67924 | 0.0576 | Singlet-A | H-1→L+1 (12%), HOMO→LUMO (86%) |
| 42394 | 235 | 5.27659 | 0.0000 | Singlet-A | HOMO→L+2 (25%), HOMO→L+3 (12%), HOMO→L+4 (60%) | ||
| 42922 | 232 | 5.34482 | 0.0008 | Singlet-A | HOMO→L+3 (83%), HOMO→L+4 (12%) | ||
| 43603 | 229 | 5.41484 | 0.0060 | Singlet-A | HOMO→L+1 (16%), HOMO→L+2 (56%), HOMO→L+4 (26%) | ||
| 44420 | 225 | 5.51111 | 0.0722 | Singlet-A | H-1→LUMO (30%), HOMO→L+1 (52%), HOMO→L+2 (15%) | ||
| 45650 | 219 | 5.66210 | 0.0014 | Singlet-A | H-4→L+3 (31%), H-1→L+3 (63%) | ||
4.5. Vibrational spectral analysis
Calculated frequencies of molecular structures are paralleled with the observed frequencies by NCA based on SQMFF (Scaled Quantum Mechanical force field) calculations recommended by Rauhat and Pulay [38]. Root mean square (RMS) error of scaled wavenumber is 8 cm−1 and this scaling factor is used to correct the anharmonicity and neglected part of electron correlation [39]. The detailed assignments stipulated by the potential Energy Distribution (PED) are depicted in Table S3 and the experimental and theoretical FT-IR & FT-Raman spectra are shown in the Figs. 4 and 5 .
Fig. 4.
FT-IR spectra of DPDS.
Fig. 5.
FT-Raman spectra of DPDS.
4.5.1. C-H vibration
Aromatic hetero structure offers C-H stretching vibrations in the region 3100–3000 cm−1 [40]. In DPDS, the C-H stretching vibration emerges at the medium range 3067 cm−1 in the Raman spectrum where it shows the stretching mode flashes with strong Raman intensity and 99% PED contribution. Vibrations assigned to aromatic C-H stretching in the region 3076 cm−1 predicted theoretically are in good alliance with the experimental assignment 3067 cm−1. DPDS molecule possesses a dimethyl group in the pyrazole ring and the CH stretching expose at lower frequencies for about 3000–2800 cm−1 than those of the aromatic ring are typically downshifted owing to electronic possessions [41]. CH3 in-plane and out-of-plane stretching displays around medium region 2960 cm−1 in the FT-IR and at the weak 2840 cm−1 in the Raman region. Scaled frequencies for the in-plane and the out-of-plane stretching occur at 2960 & 2955 cm−1, respectively and are correlated with the experimental values.
4.5.2. C – C and C=C vibrations
C-C and C=C stretching modes are expected in the range from 1650–1200 cm−1 [42]. The strong bands at 1586 and 1448 cm−1 belongs to tetra-substituted benzene ring which reveals the hyper conjugative interaction between the rings with the C – C stretching. The weak band 1220 cm−1 in the FT-Raman region shows C-C stretching which contributes 91% PED. The bands 1548, 1382 & 1256 cm−1 belong to the C=C stretching in the pyrazole group effects to hyperconjugation. C=C in-plane deformation prevails in the range 760–600 cm−1 and out-of-plane deformation turns below 600 cm−1 endorses by Shimanouchi et.al [43] which is higher frequencies than the out-of-plane vibrations. C-C-C in-plane bending vibration is observed at 752 cm−1 and out-of-plane bending vibrations of the ring is observed as a strong peak at 395 cm−1 with 86% PED contribution. In addition, these frequencies show the substitutions in the ring to some extend which affect the ring modes of vibration with good agreement by the literature.
4.5.3. C-Cl vibration
C-Cl stretching vibrations are expected in the range 765–505 cm−1 [44] and authorizes that the CH bond has the sign of polarity opposite to that of the carbon‐halogen bonds. In DPDS, strong IR spectral band is observed at 731 cm−1 and the medium Raman bands observed at 730 and 593 cm−1which is well correlated with scaled wavenumbers at 757, 725 and 592 cm−1 with 69% PED contribution. C-Cl in-plane bending vibrations are assigned as strong Raman bands at 284 and 144 cm−1 and the C-Cl out-of-plane bending vibration is assigned at medium band 361 cm−1 which are good parallelism with the scaled wavenumbers.
4.5.4. C-N & N-N vibrations
For the aromatic compound which bears a C-N group, a band of good intensity has been absorbed in the region 1400–1200 cm−1 [45] where the identification of C-N stretching is very much entangled since the mixing of bands is possible in this region [46]. For the DPDS, the bands observed at 1383 and 1256 cm−1 in IR region and at 1382 and 1257 cm−1 in Raman region while scaled values at 1378 and 1247 cm−1 coincides well with the experimental values. This result affirms the values are hard up in the direction of the higher end of the range which is due to the interaction of C-C modes. Similarly for the C=N stretching, Demir et.al observed the C=N stretching in pyrazole ring is predictable at 1610 cm−1 [47]. In DPDS, C=N stretching mode for the pyrazole ring is observed in the region 1548 cm−1. This is rather least in their wavenumber and may be due to the intermolecular interaction C=N-H…O between the rings. N-N stretching vibrations transpires at the weak bands 1112 cm−1 in IR and Raman spectra and its scaled frequency has supposed at 1125 cm−1 with 87% PED contribution and are in good line with experimental.
4.5.5. COO− vibrations
Carbonyl stretching frequency is very sensitive to the factors that disturb the nature of carbonyl group and its precise frequency is characteristic to the type of the carbonyl compound being studied. C=O group of saturated aromatic carboxylic acid absorbs strongly in the region 1730–1680 cm−1 [48]. In DPDS, theoretically predicted wave number 1724 cm−1 is identified as C=O stretching vibration and equated with the experimental band as a very strong at 1700 cm−1 in FT-IR spectrum and 1710 cm−1 in FT-Raman spectrum gets correlated. Broadening and red-shifted nature of this band depicts the level of intermolecular hydrogen bonding N-H…O with the formation carboxylate anion by donating hydrogen atom to the pyrazole moiety [49]. For the DPDS, the aromatic C-O band observed in the FT-IR region at 1275 cm−1 and the scaled at 1276 cm−1 and related to the expected in the range 1320–1210 cm−1. C=O in-plane deformation is observed as medium at 360 cm−1in FT-IR and strong in FT-Raman at 361 cm−1 and is correlated by the scaled frequency at 362 cm−1 with 72% PED contribution. C=O out-of-plane deformation is weakly observed in FT-IR at 752 cm−1 and scaled frequency 757 cm−1 which is inactive in Raman spectrum due to the C=O in the vicinity of halogen substituted aromatic structure.
4.5.6. N-H vibration
For secondary amine formation in pyrazole ring, N-H stretching and N-H bending vibration occurs in the range 3500–3300 cm−1 and 1580–1490 cm−1, respectively [49]. For DPDS, symmetric stretching mode of NH group is observed at 3449 cm−1 as a medium in Raman and scaled value at 3450 cm−1 with 97% PED contribution. O…H-N bending occurs as a strong peak at 869 cm−1 in IR and 870 cm−1 in Raman spectrum with the scaled value at 872 cm−1. N23-H12 stretching and bending proceed in the range below 100 cm−1 with a contribution of 58% and 42%, respectively and this lower magnitude be existent due to the intermolecular N-H…O bonding between two ring moieties.
4.5.7. OH vibration
Hydroxyl bonds are very important in dipole interactions to stabilize the molecular structures and have higher stretching frequencies when its relative intensity increases due to the differences in force constants. [50]. For the DPDS, intramolecular hydrogen bonding occurs in the hydroxyl group and their stretching vibration expected at 3590–3400 cm−1 [51]. Therefore stretching occurs at band 3448 cm−1 in FT-IR spectrum which is strong and unaffected by concentration changes. In-plane deformation the band occurs in 1586 cm−1 which is strong in IR and medium in Raman spectra related to the scaled values 1588 cm−1 with 35% PED contribution. This deformation exceeds the expected range due to the intramolecular interaction O-H…O.
4.6. Electrostatic potential map analysis
ESP surfaces show the charge distributions of molecules three dimensionally and their interaction of molecules with one another can be determined. It can also be used to determine the nature of the chemical bond [52]. ESP is to probe the relative electron density in a molecule and to understand the concepts of Lewis acids and bases on top of hydrogen bonded interactions amidst the molecules [53]. ESP contour mapping is a substantial tool in molecular modeling studies to predict the interactions of distant geometries.
ESP view is mapped up with the optimized geometry using B3LYP computational method and shown in Fig. 6 with colour code mapped in the range between -0.06882e and 0.06882e. Electron rich zone is the negative region concentrated over the carboxylic oxygen atoms and indicate that oxygen atom is surrounded by greater surface of positive charge and is a site for electrophilic attack. Positive potential sites is the electron poor zone mainly focused over the hydrogen atoms of methyl group attached to the carbon atom of the pyrazolium moiety and be susceptible to nucleophile attack. The nucleophilic and electrophilic reactive sites illustrate the binding site [54] of the molecule besides act as a tool for giving information about intermolecular hydrogen interaction amongst salicylate and pyrazolium ions over and above leads to biological activity of the molecule.
Fig. 6.
Electrostatic potential for DPDS.
4.7. Aromaticity
Aromatic compounds have a cyclic and conjugated set of p-orbitals that embodies the π-system. Aromaticity quantification is positioned by the geometric indexes that are agitated in the drift of aromatic compounds making equal distances of the bonds in an aromatic ring [55]. There are many structural criteria to evaluate the aromaticity for the six-membered and five-membered heterocycles. For DPDS HOMA (Harmonic Oscillator Model of Aromaticity) index is preferred to estimate the delocalization of the molecules. HOMA divided into geometric and energetic terms that are not correlated with each other and the index of aromaticity is described [56]. Energetic and Geometric terms are in least number explains the decrease of resonance energy and slight increase of bond length alteration which is caused by the contingency of C-H through hydroxyl and carboxylic acid group, which derange the electron density distribution within the ring and inhibits the aromaticity. The HOMA value for supreme benzene molecule is unity but the phenyl ring shows lesser striction in this value. Experimental and theoretical HOMA indexes values of phenyl ring are 0.9873 and 0.9778, respectively which emphasize benzene belongs to aromatic compound.
By virtue of magnetic and geometric criteria, pyrazoles and triazoles are 80–85% aromatic relative to benzene in the midst of heterocyclic compounds [57] and for the pyrazole derivatives the disparity of supremacy is perceived. Analysis of the geometric data reveal that experimental HOMA value of the pyrazole derivatives is 0.4729 and the theoretical value is 0.8693. The theoretical HOMA exhibits pyrazole is an electron withdrawing substituent which attract π-electrons from the phenyl ring, prominent to the formation of structures not gratifies the Huckel's rule 4n + 2 [58]. The depreciate HOMA value on trial could not be premeditated as non-aromatic [59]. Owing to the fact they undergo other chemical reactions such as dimethyl substitution and intermolecular interaction N-H…O. It is witnessed that π-electron delocalization is very sensitive to the nature of the substituent and to intermolecular interactions. By this the observed HOMA value emphasizes pyrazole ring belongs to aromatic compound.
4.8. Natural charge analysis
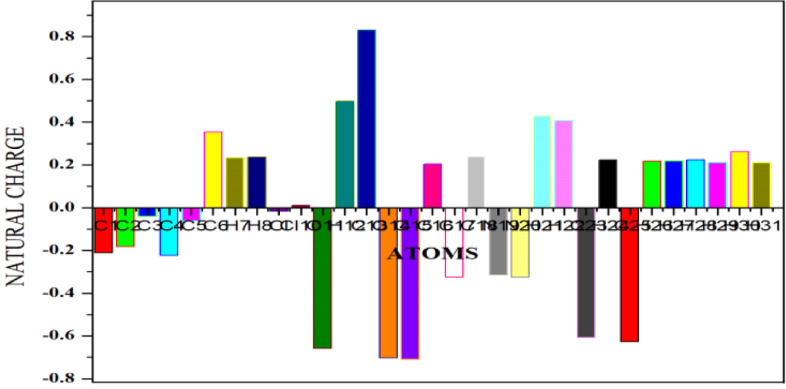
To study the charge distribution of DPDS, it is better to use Natural Charge Analysis since it do not exhibit dependence on basis set [60]. The atomic charges are calculated by natural population analysis by using B3LYP/6-311G (d, p) method are plotted in Fig. 7 and tabulated in Table S4.
Fig. 7.
Natural charge Distribution for DPDS.
C13 shows maximum positive charge (0.83017 e) than other atoms due to the charge formation over the electronegative oxygen atoms. In carboxylic acid group, carbon atom progress a partial positive charge and oxygen atom develops partial negative charge. Deprotonation takes place in the acid group and becomes carboxylate anion which makes the carbon atom more positive. Minimum positive charge over Cl10 is due to the substitution reaction in which hydrogen atom is replaced by chlorine atom in the benzene ring which grades the polarization of chlorine by the Lewis base. Maxima negative charge (-0.70089 e) is stated in the atom O15 of the carboxylate anion which interacts with nitrogen of pyrazole ring through intermolecular interactions. Since oxygen and nitrogen are electronegative atoms, they are electrostatically attracted to the hydrogen atom and bear a large negative charge. The minima negative charge (-0.00196 e) is observed in the atom Cl9 and is more electronegative than carbon which pulls the bonded electrons closer to itself. Substitution of hydrogen atom by chlorine atom makes the carbon more electropositive and befalls minima negative charge.
4.9. NMR chemical shift analysis
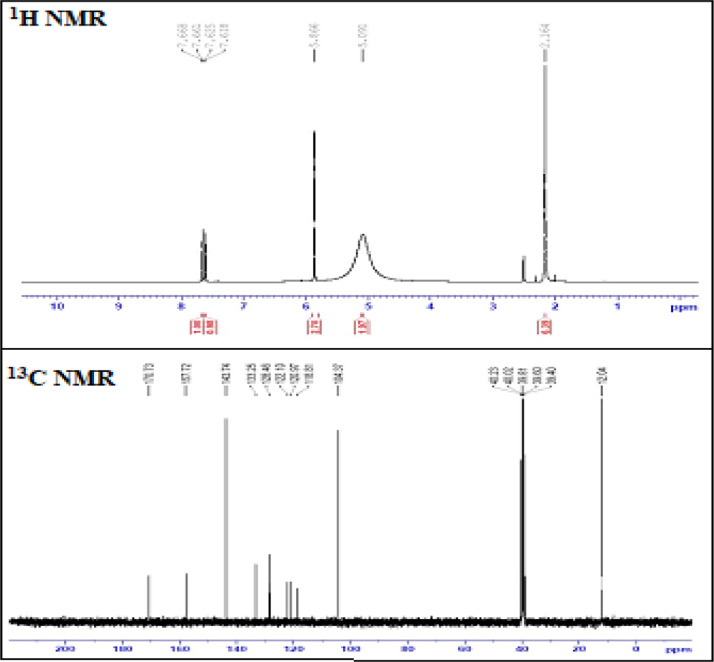
1H and 13C NMR spectra contribute a tectonic data with disparate hydrogen and carbon atoms in a molecule and their chemical shifts are shown in Fig. 8. For reliable calculation of magnetic properties it is acknowledged that literal prognosis of molecular geometries are important [61]. Chemical shifts are very advantageous to afford the facts of diverse protons and carbons extant in the molecule. Chemical shifts in the spectrum are due to the de-shielding and shielding by electrons.
Fig. 8.
Experimental NMR spectra of DPDS.
1H NMR spectrum of DPDS advertises distinct type of protons in which signal at δ 2.164 ppm is is due to proton impurity in the DMSO – d6 solvent. Hydrogen peaks in the 1H spectrum develops in the range 6.95–7.40 ppm which reciprocates to the aromatic region whereas greater shielding is owing to the anisotropy of aromatic ring π electrons [62]. In DPDS, 1H spectrum of pyrazole ring reveals a wide-ranging signal at δ 5.091 ppm which is the symptomatic of two identical hydrogen atoms in amino group (H22 and H21), whereas it appears in deshielded region because of adjacent amino group (NH+). The carboxylate group primarily arises in the range 10–12 ppm [63] however in DPDS H21 exhibits a peak at δ 5.866 ppm which is deshielded in supreme because of the electronegative oxygen atom in the carboxylate anion. 1H spectrum shows doublet signals at δ 7.68 ppm and δ 7.618ppm which are assigned to H8 and H7 , respectively. These signals are deshielded due to the adjacent chlorine atoms in the salicylate group moiety. The signals at δ 2.55 ppm, δ2.35 ppm and δ2.164 ppm are attributed to the methyl groups attached to the pyrozolium moiety where it acquires electron donating resonance effect and shows a substantial lowering in chemical shift.
13C NMR spectrum of DPDS advertises ten distinct carbon signals to indicate carbon points present in the compound. The signal for DMSO solvent is assigned at δ 39.40 ppm. The presence of C13 signal at δ 170.73 ppm in the highly deshielded region delivers the emergence of carboxylate anion in the compound. The C6 (δ 157.72 ppm) and C16 (δ 143.74 ppm) signals are intensely deshielded by virtue of O-H grouping and the transacted methyl group in the pyrazole ring moiety, respectively. The emergence of prime intensity signals in the region δ 120–130 ppm is indicative of the carbons in the aromatic ring. The signals in the low field region (– 20–50 ppm) manifest the being of methyl, methylene and methane carbons present in the compound [64]. Signals at C3 (δ 133.25 ppm) and C5 (δ 128.46 ppm) are granted to phenyl ring bordening on the electronegative chlorine atom which leads to deshielding of carbon by attracting all electron clouds towards chlorine atom and results in the increase of chemical shift value. Correspondingly signal at δ 118.81 ppm is due to the aromatic carbon C1 and the downfield signal at δ 104.37 ppm is due to the carbon atoms attached to the methyl groups in the pyrazolium moiety. The upfield signal at δ 12.04 ppm is attached with methyl group of base moiety of C23 and C25.
4.10. Thermal (TG/DTA) analysis
Thermal characteristics of DPDS crystal was studied by using TG and DTA under nitrogen atmosphere at a heating rate of 10 °C /min from 30 to 500 °C. TG-DTA thermo gram is shown in the Fig. 9 . The compound DPDS was stable up to 95 °C whereas the endothermic dip at 105 °C in DTA indicates the melting point of the compound. Single stage decomposition is observed between 90 °C and up to 230 °C, which corroborate the bulk degradation of the material and was further established by a broad endothermic dip at 200 °C in DTA. The weight loss in the bulk decomposition is around 80% when the temperature was increased from 160 to 250 °C. Volatile fragments such as CO, CO2, NO, CH4 are the decomposition products. Thus, the thermal analysis validates the applicability of DPDS for any biotic purpose.
Fig. 9.
TG/DTA spectrum of DPDS.
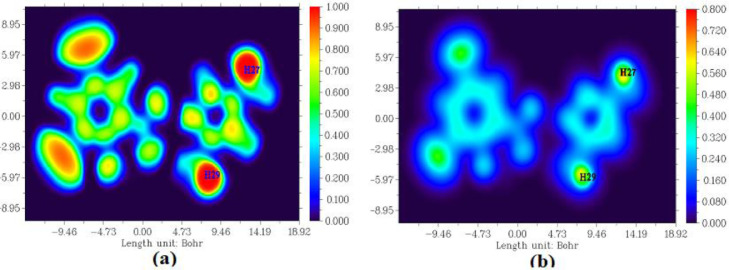
4.11. ELF and LOL analysis
ELF (electron localization function) and LOL (localized orbital locator) maps concede the zones of molecular space and their colour shade maps are presented in Fig. 10 . This topological surface analysis is based on the covalent bonds where the revelation of electron pair is high. The chemical content of LOL is analogous to that of ELF, as both counts upon the kinetic-energy density. ELF is dredged up owing to the fact of electron pair density and LOL decodes the gradients of localized orbitals inflamed when overlap [65].
Fig. 10.
(a) ELF and (b) LOL colour filled maps for DPDS.
The value of ELF, τ(r) ranges from 0.0 to 1.0, where relatively comprehensive values in the interval 0.5 and 1.0 indicate the contours containing bonding and nonbonding localized electrons. The diminutive values (< 0.5) describe the contours where electrons are intended to be delocalized [66]. LOL, ƞ(r), attains large values (> 0.5) in contours where the electron density is dominated by electron localization [67]. High localization of electrons is due to the endurance of covalent bond in the contours with comprehensive values [68]. From the Fig. 10a, the high ELF regions are seen around hydrogen atoms H27and H29 indicating the presence of highly localized bonding and nonbonding electrons and the blue regions around few chlorine atoms Cl9 and Cl10 show the delocalized electron cloud around it. From the Fig. 10b, it is seen that the central region of a hydrogen atom is white; indicating that electron density exceeds the upper limit of colour scale (0.80 au.) and the blue circles around hydrogen atoms (H27 and H29) shows the electron depletion region between inner shell and valance shell. The most part of the covalent region present between the carbon and chlorine atoms are indicated by green colour.
4.12. Reduced density gradient na
A visual approach known as RDG analysis is implemented to make the non-covalent interactions more specific and to explore intra and inter non-bonded interactions in a molecular system [69]. The gradient isosurfaces and scatter graphs of DPDS molecule are shown in Fig. 11 .
Fig. 11.
Gradient isosurfaces and scatter graphs of DPDS.
RDG scatter graph is generated between RDG versus sign (λ2)ρ, where sign(λ2)ρ is the second Eigen value of the electron density which provides useful information regarding the strength and nature of the interactions. The value and sign of sign (λ2)ρ are used to explain the nature of interactions,sign (λ2)ρ > 0 for repulsive interaction, sign (λ2)ρ < 0 for attractive interaction and sign (λ2)ρ nearly zero for vander Waals weak interaction [70]. The function of λ2(r) ranges between −0.035 and0.02 0 a.u in RDG scatter spectra which is divided in to three colours red, green and blue. In the RDG isosurfaces, the red spike shows the steric repulsion observed in the centres of aromatic and pyrazole rings. The RDG scatter graph manifests the red contour between 0.02 and 0.05 au clarifies the higher repulsive exchange contribution. This plot illustrates the steric repulsive force between the aromatic carbon atoms between the rings. The blue spike manifests the strong attraction between N-H…O constitutes inter-molecular hydrogen bonding. In the RDG scatter graph blue contour between -0.05 and -0.03 au confirms the presence of strong hydrogen bonding. The red-green mixed spikes are observed near the C-Cl interactions in the aromatic ring. The red-green mixed flaky area between 0.00 and 0.015 au confirms the absence of hydrogen bonding and the C-H formation in the aromatic ring are due to the interaction between hydrogen atoms and the π-electrons. The RDG graph results confirm the interacting regions in the molecular structure DPDS.
4.13. Molecular docking analysis
Molecular docking leads to further drug development with the bio molecular interactions for the rational drug design [71,72]. It is an enticing framework to be acquainted which is used to predict the binding energy, free energy and stability of the complexes. PDB structures of the target proteins are downloaded from RCSB (Research Collaboratory for Structural Bioinformatics) protein data bank. The ID's for downloaded SARS-CoV-2 hydrolase inhibitor proteins are 7JRN, 6XBI, 6XMK and 6XFN with various drug molecules. Fig. 12 represents the Ramachandran plots for the four centred proteins and the preparation of ligand with minimal energy for docking was done.
Fig. 12.
Ramachandran plots of SARS-CoV-2 hydrolase inhibitor proteins.
By observing the Ramachandran plots for all the four proteins, 85–95% of the amino acids lie in the allowed region (red) and only very few lie in the partially allowed vicinity (yellow) and therefore 7JRN, 6XBI, 6XMK and 6XFN have 88.3%, 90.6%, 90.7% and 91.7% of the amino acids in the allowed region, respectively. Majority of the amino acids in the allowed regions indicate the stability of the proteins chosen for the binding interaction.
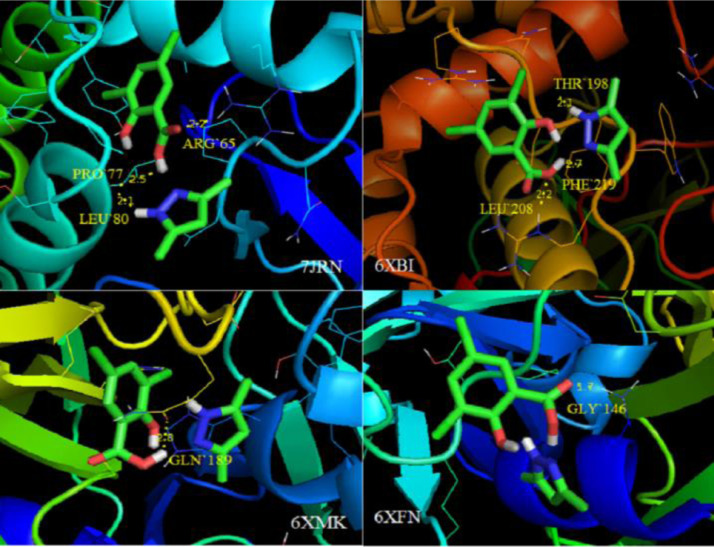
Auto Dock Tools is used to remove the ligand and water molecules present in the target proteins, which was also used to add the polar hydrogen bond and Geisteger charges. The docking parameters such as binding energy and refRMS of the molecule with respect to the targeted proteins are listed in Table 5 . The interactions of ligand DPDS with SARS-CoV-2 proteins are shown in Fig. 13 and the yellow dotted interactive line in the figure indicates the formation of intermolecular hydrogen bond between DPDS and SARS-CoV-2 hydrolase inhibitor proteins.
Table 5.
Docking parameters of DPDS docked into SARS-CoV-2 hydrolase inhibitor proteins.
| Protein (PDB ID) | Bonded residues | Bond distance (Å) | Estimated inhibition constant (milli Molar) | Binding energy (kcal/mol) | Inter molecular energy (kcal/mol) | Reference RMSD |
|---|---|---|---|---|---|---|
| 7JRN | LEU`80 | 2.1 | 48.22 | -5.89 | -7.38 | 31.92 |
| PRO`77 | 2.5 | |||||
| ARG`65 | 2.2 | |||||
| 6XBI | THR`198 | 2.1 | 242.51 | -4.93 | -6.42 | 33.88 |
| LEU`208 | 2.2 | |||||
| PHE`219 | 2.7 | |||||
| 6XMK | GLN`189 | 2.8 | 377.41 | -4.67 | -6.16 | 36.69 |
| 6XFN | GLY`146 | 1.7 | 465.11 | -4.55 | -6.04 | 18.93 |
Fig. 13.
Molecular Docking of SARS-CoV-2 hydrolase inhibitor proteins.
Amongst the targeted protein inhibitors, 7JRN protein exhibits least binding energy -5.89 kcal/mol with three amino acids LEU`80, PRO`77 and ARG`65 encompassing N-H…O and O…H interactions with an intermolecular energy -7.38 k cal/mol and prominent RMSD value 31.92. At this juncture, the energy released due to the bond formation or relatively interaction of ligand and the protein is termed as the binding energy whereas higher the binding energy, the stronger the interaction. The intermolecular energy is estimated for the combination of ligand and protein in their bound conformation which shows that higher the interaction energy lowers the bond stiffness. This shows the relation between binding energy and the interaction energy between ligand and the protein [73,74]. It can be seen that the hydrogen bond is formed between the hydrogen atom and oxygen atom from the ligand and the hydrolase inhibitor. Looking into the docking conformation of the ligand with the proteins reveals that the atoms N19, H21 and O15 are the only active sites undergoing hydrogen bond formation. In general hydrogen bonds are formed between the hydrogen which is bound to a more electronegative atom like nitrogen or oxygen and another atom bearing a lone pair of electron. This similarity has been observed between the DPDS and the SARS-CoV-2 hydrolase inhibitor proteins. Ligand DPDS shows good binding affinity towards all the four targeted proteins, indicating anti-viral features and the reactive site analysis with topological studies complements DPDS assured towards amino acids and awards to be a noble antiviral drug.
4.14. Drug likeness
To unmask an active drug, reckoning of drug likeness is efficacious for which Lipinski's rule of 5 was used. In consonance with this rule, either ligand is considered as a pharmaceutic drug if it is apt for marked preconditions counting molecular weight < 500 Dalton, number of H-bond acceptor < 10, number of H-bond donors < 5 and lipophilicity laid out as log P < 5 [75]. Utterly these limitations deputize the drug likeness trial and the values akin to the antiviral drug DPDS is tabulated in Table 6 . Resultantly, the number of hydrogen bond donors and hydrogen bond acceptors for DPDS found to be 3 and 4 for each. Values of log P was perceived to be 1.32, which is a shade of lipophilic feature and Molar refractivity raised as 72 and settled within the appropriate range. DPDS ligand accede this rule and firmly established as a vigorous anti-viral drug.
Table 6.
Drug likeness parameters of DADS.
| Descriptors | Calculated | Expected |
|---|---|---|
| Molecular mass(Dalton) | 301 | <500 |
| Hydrogen bond donor | 3 | <5 |
| Hydrogen bond acceptor | 4 | <10 |
| Log P | 1.32 | <5 |
| Molar refractivity | 72 | 40-130 |
4.15. ADMET contour
To uncover a neoteric drug and its buildup, researchers must assay the liveliness of a drug in the body to gage welfare and toxicity. Drug assimilation and pharmacokinetics studies, such as ADME and toxicology studies, are the perilous phase in this process. Records poised states if a drug is doable and affords explicit goals for the future study and progression [76] and ADMET factors are tabulated in Table 7 .
Table 7.
ADMET Factors.
| ADMET | Factors | DPDS |
|---|---|---|
| Absorption | Water solubility (log mol/L) | -2.987 |
| Caco-2 permeability (log Papp in 10−6cm/s) | 0.909 | |
| Human intestinal absorption (%) | 98.692 | |
| P-glycoprotein substrate | YES | |
| P-glycoprotein I/II inhibitors | NO | |
| Distribution | CNS permeability (log PS) | -2.426 |
| Human VDss (log L/kg) | -2.018 | |
| Fraction unbound (human)(Fu) | 0.307 | |
| Metabolism | CYP substrates/inhibitors | NO |
| Excretion | Total clearance (log ml/min/kg) | 0.2 |
| Toxicity | AMES toxicity | NO |
| Max. tolerated dose (human)(log mg/Kg/day) | 1.177 | |
| Oral rat Acute Toxicity (LD50)(mol/kg) | 2.651 | |
| hERG l/ll inhibitors | NO | |
| Hepatotoxicity | NO |
Amidst absorption all the constituents of the ligand trot out water solubility (log S) greater than -5, which reflect their solubility in water at 25 ˚C [77] and the predicted water solubility of a compound results as -2.987 (log mol/L). Alike absorbance in the small intestine is one of the cardinal evolution to incline bioavailability of drug in the rear oral profit [78]. Thus the prediction for the fraction of DPDS that absorbed by virtue of the human small intestine is 98.6%. Conjointly Caco-2 permeability value presumes the logarithm of permeability coefficient 0.909 in Caco -2 monolayer of the cells (log Papp in 10−6 cm/s). DPDS compound is not a p-glycoprotein I/II inhibitors and act as substrates which functions as a biological barrier by extruding toxins and xenobiotics out of cells. While distributing the drug throughout the body, most drugs in plasma abide in serenity by unbounding or bounding to serum proteins. Bounding to the serum proteins in less would transverse cellular membranes [74]. DPDS compound results less bound to the serum proteins and is impotent to infiltrate the CNS. The steady state volume of distribution (VDss) is the complete dose of a drug needed to be circulated homogeneously in blood and plasma. It is considered low if log VDss is less than -0.15 and high if it is more than 0.45. Higher VDss value grants better drug distribution in tissue rather than plasma and cause renal failure and dehydration [78].DPDS spectacles VDss IS -2.081 (log L/kg) which is less and scattered homogeneously in blood plasma and tissues. Total clearance enumerates the amputation of drug from blood or plasma where the drug riddance practice grades from kidney and liver [79]. ADMET result foresees the total clearance as 0.2 (log ml/min/kg). Toxicity of the compound can be tested by AMES toxicity which predicts if the compound has mutagenic potential or not [77]. ADMET result predicts that the compound has flimsy inhibitor and non-inhibitor of hERG inhibition and is non-mutagenic which does not act as a carcinogen. Also the compound is not hepatotoxic as it does not intrude the normal function of liver. Maximum tolerated dose is freaked out and is about 1.177 (log mg/Kg/day) which is an estimate of the toxic dose inception in humans. ADMET results interpreted that DPDS compound is an innocuous incitation to treat SARS-CoV-2.
5. Conclusion
DPDS was synthesized by slow evaporation method and their molecular structure has been optimized using the DFT technique and the calculated bond length, bond angle and dihedral angle sustains a good stability with good agreement with the experimental values. Rehybridization overshadows hyper conjugation and is well reflected in the geometry of DPDS as bond O11-H12 contracts with respect to DS due to on intra-molecular hydrogen bonding O11-H12…O14. Charge transfer interactions were dissected to scrutinize hydrogen bonding networks and the strong N-H…O inter-molecular hydrogen bonding get earlier perception into these interactions to project the molecules with biotic profile. Second order perturbation theory results the transfer of electron density from lone pair nitrogen atom to the anti-bonding orbital of O-H bond which produces a strong evidence for inter-molecular hydrogen bonding. HOMO-LUMO energy gap (ΔE) value is demoted and directs that DPDS molecule yields virtuous biological activity of the molecule. Electron distribution and reactive sites on the surface of the DPDS were analysed using ESP, ELF and LOL contour maps. Colour code for the ESP was mapped and confirmed the concepts of Lewis acids and bases with in the molecule. Electronic spectra was evaluated using DFT method displays that the absorption spectra exhibits an intense peaks at 360 transitions. Aromaticity evaluated and agreed the phenyl ring and the pyrazole ring belongs to the aromatic compounds while Natural Charge analysis confirmed that C13 and O15 are the most electropositive and electronegative atoms respectively. Thermal analysis showed that the DPDS crystal can retain its stability up to 95 °C which is recognized using TG/DTA curve. Structure elucidation of DPDS using IR, Raman and NMR spectroscopic techniques were used for the data analysis and spectral interpretation which contributes the outcome of the functional groups in DPDS. In this context, predictions of 1H and 13C NMR chemical shifts and scaled frequencies have been demonstrated to be a viable strategy for the relative configuration of new molecules with good linearity amongst scaled and experimental vibration frequencies was also observed. Broadening and red-shifted nature of the bands depicts the level of intermolecular hydrogen bonding O-H…N with the molecular structure in gaseous nature. RDG approach allowed analyzing the weak attractive interactions, strong attraction, and steric repulsion existed in DPDS. Molecular docking study with SARS-CoV-2 hydrolase inhibitor protein 6XA4 shows substantial anti-viral activity exhibiting least binding energy -5.89 kcal/mol in the active sites. From docking result, it is evident that nitrogen atom in the pyrazole moiety involved in hydrogen bond formation with the target enzyme and concludes pyrazolium moiety acts as a good anti-viral medication. Drug Likeness and ADMET analyses analyzes revealed that DPDS is superlative in absorption and total clearance rate which high spot the consequential aptitude for antiviral activity counter to SARS-CoV-2. These consummations propound supplemental powerful assays for the declaration of activity of antiviral constituents contrast to SARS-CoV-2 affords evidence concerning drug pharmacokinetics.
CRediT authorship contribution statement
X.D. Divya Dexlin: Conceptualization, Data curation, Formal analysis, Writing – original draft. J.D. Deephlin Tarika: Investigation, Data curation. S. Madhan Kumar: . A. Mariappan: Methodology, Supervision. T. Joselin Beaula: Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank Dr. I. Hubert Joe, Associate Professor, Department of Physics, University of Kerala for providing laboratory for the DFT based calculations using Gaussian’09 software package.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molstruc.2021.131165.
Appendix. Supplementary materials
References
- 1.Kumar V., Kaur K., Gupta G.K., Sharma A.K. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013;69:735–753. doi: 10.1016/j.ejmech.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 2.Mocanu A.M., Luca C. Potential Antimicrobial activity of some new 3,5- dimethyl pyrazole derivatives. Eur. J. Med. Chem. 2018;3(2):03–07. ISSN: 2537-4338. [Google Scholar]
- 3.Schror K. 2nd ed. John Wiley and sons; New York: 2016. Acetylsalicylic Acid. [Google Scholar]
- 4.Ding C.K., Wang C.Y. The dual effects of methyl salicylate on ripening and expression of ethylene biosynthetic genes in tomato fruit. Plant Sci. 2003;164:589–596. doi: 10.1016/S0168-9452(03)00010-4. [DOI] [Google Scholar]
- 5.Paul B.K., Ray D., Guchhait N. Spectral deciphering of the interaction between an intramolecular hydrogen bonded ESIPT drug, 3,5-dichlorosalicylic acid, and a model transport protein. J. Phys.Chem, Chem. Phys. 2012;14:8892–8902. doi: 10.1039/C2CP23496C. [DOI] [PubMed] [Google Scholar]
- 6.Faisal M., Saeed A., Hussain S., Dar P., Larik F.A. Recent developments in synthetic chemistry and biological activities of pyrazole derivatives. J. Chem. Sci. 2019;131(70):1–30. doi: 10.1007/s12039-019-1646-1. [DOI] [Google Scholar]
- 7.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Roobb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., Nakatsuji H., Caricato M., Li X., Hratchian H.P., Izmaylov A.F., Bloino J., Zheng G., Sonnenberg J.L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J.A, Peralta J.E., Ogliaro F., Bearpark M., Heyd J.J., Brothers E., Kudin K.N., Staroverov V.N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J.C., Iyengar S.S, Tomasi J., Cossi M., Rega N., Millam J.M., Klene M., Knox J.E., Crosss J.B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R.E., Yazyev O., Austin A.J., Cammi R., Pomelli C., Morokuma K., Zakrzewski V.G., Voth G.A., Salvador P., Dannenberg J.J., Dapprich S., Daniels A.D, Farkas O., Foresman J.B., Ortiz J.V., Cioslowski J., Fox D.J. Gaussian, Inc.; Wallingford CT: 2009. Gaussian 09, Revision A.02. [Google Scholar]
- 8.Sundius T.T. Scaling of ab initio force fields by MOLVIB. Vib. Spectrosc. 2002;29:89–95. doi: 10.1016/S0924-2031(01)00189-8. [DOI] [Google Scholar]
- 9.Dennington R., Keith T.A., Millam J.M. Semichem. Inc; Shawnee mission, KS, gaussview: 2019. [Google Scholar]
- 10.Glendening E.D., Reed A.E., Carpenter J.E., Weinhold F. NBO Version3.1. TCI, University of Wisconsin; Madison: 1998. [Google Scholar]
- 11.Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., Olson A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- 12.LLC; Schrodinger: 2009. The PYMOL Molecular Graphics System. [Google Scholar]
- 13.Lu Tian, Chen Feiwu. Multiwfn: a multifunctional wavefunction analyser. J. Comput. Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 15.Bondi A. Van der Waals volumes and radii. J. Phys. Chem. 1964;68(3):441–451. doi: 10.1021/j100785a001. [DOI] [Google Scholar]
- 16.https://pubchem.ncbi.nlm.nih.gov/compound/3_5-Dichlorosalicylicacid.
- 17.Stato H., Dybal J., Murakami R., Noda I., Ozaki Y. Infrared and Raman spectroscopy and quantum chemistry calculation studies of C-H…O hydrogen bondings and thermal behavior of biodegradable polyhydroxyalkanoate. J. Mol. Struct. 2005;35:744–747. doi: 10.1016/j.molstruc.2004.10.069. [DOI] [Google Scholar]
- 18.Zhao N., Eichhorn D.M. Dichlorobis(3,5-dimethylpyrazole)copper(II) Acta Cryst. 2005;E61:m822–m823. doi: 10.1107/S1600536805009621. [DOI] [Google Scholar]
- 19.Tickle I.J. Experimental determination of optimal root-mean-square deviations of macromolecular bond lengths and angles from their restrained ideal values. Acta Cryst. 2007;D63:1274–1281. doi: 10.1107/S0907444907050196. [DOI] [PubMed] [Google Scholar]
- 20.Novak P., Jednacak T. In: (Ed.) Z., editor. Vol. 85. IAPC Publishing; Zagreb: 2012. (Physico Chemical Methods in Drug Discovery and Development). [DOI] [Google Scholar]
- 21.Beaula T.J., Muthuraja P., Dhandapani M., Bena Jothy V. Effect of charge transfer with spectral analysis on the antibacterial compound 4-(Dimethyl amino) pyridine: 3,5-Dinitrobenzoic acid: experimental and theoretical perspective. J. Mol. Struct. 2018;1171:511–526. doi: 10.1016/j.molstruc.2018.06.026. [DOI] [Google Scholar]
- 22.Velraj G., Soundharam S., Sridevi C. Investigation of structure, vibrational,electronic, NBO and NMR analyzes of 2-chloro-4-nitropyridine (CNP), 2-chloro-4-methyl-5-nitropyridine (CMNP) and 3-amino-2-chloro-4-methylpyridine (ACMP) by experimental and theoretical approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;137:790–803. doi: 10.1016/j.saa.2014.08.075. [DOI] [PubMed] [Google Scholar]
- 23.Doerkson E.S., Fortenberry R.C., Reduction in Hybrdization: Lone Pairs Interacting with Empty p Orbitals, pre-print, January 2020, 10.26434/chemrxiv.11594841.
- 24.Govindarajan M., Karabacak M., Suvitha A, Periandy S., FT-IR FT-Raman, initio ab. HF and DFT studies, NBO, HOMO-LUMO and electronic structure calculations on 4-chloro-3-nitrotoluene. Spectrochim. Acta Part A. 2012;89:137–148. doi: 10.1016/j.saa.2011.12.067. [DOI] [PubMed] [Google Scholar]
- 25.Rauk A. 2nd ed. John Wiley & Sons; New York: 2001. Orbital Interaction Theory of Organic Chemistry. [Google Scholar]
- 26.Gece G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008;50(11):2981–2992. doi: 10.1016/j.corsci.2008.08.043. [DOI] [Google Scholar]
- 27.Choi C.H., Kertesz M.J. Conformational Information from vibrational spectra of Styrene, trans-Stilbene, and cis-Stilbene. J. Phys. Chem. 1997;A101(20) 3823–3821, doi:10.1021/jp970620v. [Google Scholar]
- 28.Arjunan V., Devi L., Subbalakshmi R., Rani T., Mohan S. Synthesis, Vibrational, NMR, quantum chemical and structure-activity relation studies of 2-hydroxy-4-methoxyacetophenone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;130:164–177. doi: 10.1016/j.saa.2014.03.121. [DOI] [PubMed] [Google Scholar]
- 29.El-Gammal O.A., Rakha T.H., Metwally H.M., Abu El-Reash G.M. Synthesis, characterization, DFT and biological studies of isatinpicolinohydrazone and its Zn(II), Cd(II) and Hg(II) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;127:144–156. doi: 10.1016/j.saa.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Bally T., Nitsche S., Roth K., Haselbach E. Excited states of polyene radical cations: limitations of Koopmans’ theorem. J. Am. Chem. Soc. 1984;106(14):3927–3933. doi: 10.1021/ja00326a007. [DOI] [Google Scholar]
- 31.Carneiro S.S., Marinho M.M., Marinho E.S. Electronic/structural characterization of antiparkinsonian drug istradefylline: a semi-empirical study. Int. J. Recent Res. Rev. X. 2017;(4):9–14. ISSN 2277 –8322. [Google Scholar]
- 32.Parr R.G., Pearson J. Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983;105:7512–7516. doi: 10.1021/ja00364a005. [DOI] [Google Scholar]
- 33.Barim E., Akman F. Synthesis, characterization and spectroscopic investigation of N-(2-acetylbenzofuran-3-yl)acrylamide monomer: molecular structure, HOMO- LUMO study, TD-DFT and MEP analysis. J. Mol. Struct. 2019;1195:506–513. doi: 10.1016/j.molstruc.2019.06.015. [DOI] [Google Scholar]
- 34.Sakthivel S., Alagesan T., Muthu S., Abraham Christina Susan, Geetha E. Quantum mechanical, spectroscopic study (FT-IR and FT - Raman), NBO analysis, HOMO-LUMO, first order hyperpolarizability and docking studies of a non-steroidal anti-inflammatory compound. J. Mol. Struct. 2018;1156:645–656. doi: 10.1016/j.molstruc.2017.12.024. [DOI] [Google Scholar]
- 35.Muthuraja P., Beaula T.J., Sethuraman M., Jothy V.B., Dhandapani M. Hydrogen bonding interactions on 1H-1, 2, 3-triazole based crystals: Featuring experimental and theoretical analysis. Curr. Appl. Phys. 2018;18:774–778. doi: 10.1016/j.cap.2018.03.005. [DOI] [Google Scholar]
- 36.Mohan J. Narosa Publishing House; New Delhi: 2009. Organic Spectroscopy Principles and Appliances. [Google Scholar]
- 37.O'Boyle N.M., Tenderholt A.L., Langner K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008;29(5):839–845. doi: 10.1002/jcc.20823. [DOI] [PubMed] [Google Scholar]
- 38.Rauhut G., Pulay P.J. Transferable scaling factors for density functional derived vibrational force fields. J. Phys. Chem. 1995;99(10):3093–3100. doi: 10.1021/j100010a019. [DOI] [Google Scholar]
- 39.Merrick J.P., Moran D., Radom L.J. An evaluation of harmonic vibrational frequency scale factors. J. Phys. Chem. 2007;A111(45):11683–11700. doi: 10.1021/jp073974n. [DOI] [PubMed] [Google Scholar]
- 40.Varsanyi Gyorgy, Lang L. Wiley &Sons; New York: 1974. Assignment for Vibrational Spectra of Seven Hundred Benzene Derivatives. [Google Scholar]
- 41.Krishnakumar V., Dheivamalar S., Xavier R.J., Balachandran V. Analysis of vibrational spectra of 4-amino-2, 6-dichloropyridine and 2-chloro-3, 5-dinitropyridine based on density functional theory calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006;65(1):147–154. doi: 10.1016/j.saa.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 42.Bellamy L.J. 3rd ed. Springer, Wiley & Sons; New York: 1975. The Infrared Spectra of Complex Molecules. [Google Scholar]
- 43.Shimanouchi T., Kakiuti Y., Gamo I.J. Out-of-plane CH vibrations of benzene derivatives. J. Chem. Phys. 1956;25(6):1245–1252. doi: 10.1063/1.1743187. [DOI] [Google Scholar]
- 44.Tonannavar J., Yenagi J., Sortur V., Jadhav V.B., Kulkarni M.V. Vibrational spectra, normal modes, ab initio and DFT calculations for 6-Chloro- and 7-Chloro-4-bromomethylcoumarins. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010;77(2):351–358. doi: 10.1016/j.saa.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Krishnakumar V., Balachandran V. Analysis of vibrational spectra of 5-fluoro, 5-chloro and 5-bromo-cytosines based on density functional theory calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005;61(5):1001–1006. doi: 10.1016/j.saa.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 46.Sathyanarayana D.N. New Age International Publishers; New Delhi: 2004. Vibrational Spectroscopy: Theory and Applications. [Google Scholar]
- 47.Demir D., Tinmaz F., Dege N., Ilhan I.O. Vibrational spectroscopic studies, NMR, HOMO–LUMO, NLO and NBO analysis of 1-(2-nitrobenzoyl)-3,5-diphenyl-4,5-dihydro-1H-pyrazole with use X-ray diffractions and DFT calculations. J. Mol. Struct. 2016;1108:637–648. doi: 10.1016/j.molstruc.2015.12.057. [DOI] [Google Scholar]
- 48.Smith B.C. CRC Press; 2018. Infrared Spectral Interpretation: A Systematic Approach. [Google Scholar]
- 49.Socrates G. John Wiley &Sons, Ltd.; UK: 1980. Infrared Characteristic Group Frequencies. [Google Scholar]
- 50.Beaula T.J., James C., IR FT. FT-Raman spectra and chemical computations of herbicide 2-phenoxy propionic acid – A DFT approach. Spectrochim. Acta Part A. 2014;122:661–669. doi: 10.1016/j.saa.2013.10.126. [DOI] [PubMed] [Google Scholar]
- 51.Socrates G. 3rd ed. John Wiley & sons; Ltd., UK: 2004. Infrared and Raman Characteristic Group Frequencies. [Google Scholar]
- 52.Kuruvilla Tintu K., Prasana Johanan Christian, Muthu S., George Jacob. Vibrational spectroscopic (FT-IR, FT-Raman) and quantum mechanical study of 4-(2-chlorophenyl)-2-ethyl-9-methyl-6H-thieno[3,2-f] [1,2,4]triazolo[4,3-a][1,4] diazepine. J. Mol. Struct. 2018;1157:519–529. doi: 10.1016/j.molstruc.2018.01.001. [DOI] [Google Scholar]
- 53.Luque F.J., Lopez J.M., Orozco M. Perspective on Electrostatic interactions of a solute with a continum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Theor. Chem. Acc. 2000;103:343–345. doi: 10.1007/s002149900013. [DOI] [Google Scholar]
- 54.Sevvanthi S., Muthu S., Raja M. Molecular docking, vibrational spectroscopy studies of (RS)-2-(tertbutylamino)-1-(3-chlorophenyl) propan-1-one: A potential adrenaline uptake inhibitor. J. Mol. Struct. 2018;1173:251–260. doi: 10.1016/j.molstruc.2018.07.001. [DOI] [Google Scholar]
- 55.Montero Campillo M.M., Otero J.Rodriguez, CabaleiroLago E.M. Ab initio and DFT study of the aromaticity of some fulvalenes derived from Methylidenecyclopropabenzene. J. Mol. Model. 2007;13:919–926. doi: 10.1007/s00894-007-0211-x. [DOI] [PubMed] [Google Scholar]
- 56.Krygowski T.M., Cyranski M. Separation of the energetic and geometric contributions to the aromaticity of π-electron carbocyclics. Tetrahedron. 1996;52(5):1713–1722. doi: 10.1016/0040-4020(95)01007-6. [DOI] [Google Scholar]
- 57.Maria K.Ayub. Aromaticities of five membered heterocycles through dimethyldihydropyrenes probe by magnetic and geometric criteria. J. Chem. 2015;11 doi: 10.1155/2015/456961. Article ID 456961. [DOI] [Google Scholar]
- 58.H. Szatylowicz, A. Jezuita, AT.M. Krygowski, On the relations between aromaticity and substituent effect, Struct. Chem. 30, 1529–1548, 10.1007/s11224-019-01360-7.
- 59.Frizzo C.P., Martins M.A.P. Aromaticity in heterocycles: new HOMA index parametrization. Struct. Chem. 2012;23:375–380. doi: 10.1007/s11224-011-9883-z. [DOI] [Google Scholar]
- 60.Irikura K.K., Frurip D.J. Vol. 677. American Chemical Society; Washington: 1998. (Computational Thermochemistry: Prediction and Estimation of Molecular Thermodynamics). [Google Scholar]
- 61.Muthuraja P., Beaula T.J., Sethuram M., Jothy V.B., Dhandapani M. Hydrogen bonding interactions on 1H-1,2,3-triazole based crystals: featuring experimental and theoretical analysis. Curr. Appl. Phys. 2018;18:774–784. doi: 10.1016/j.molstruc.2017.02.067. [DOI] [Google Scholar]
- 62.Ahmed A.B., Feki H., Abid Y., Boughzala H., Minot C., Mlayah A. Crystal structure, vibrational spectra and theoretical studies of l-histidinium dihydrogen phosphate-phosphoric acid. J. Mol. Struct. 2009;920:1–7. doi: 10.1016/j.molstruc.2008.09.029. [DOI] [Google Scholar]
- 63.Flouria N.K., Flouria S. Studium Press India Pvt. Ltd.; New Delhi: 2013. Spectroscopy Fundamentals and Data Interpretation. [Google Scholar]
- 64.Muthuraja P., Shanmugavadivu T., Beaula T.J., Jothy V.B., Dhandapani M. Influence of intramolecular hydrogen bonding interaction on the molecular properties of N-p-tolyl-5-oxo pyrrolidine-3-carboxylic acid: a theoretical and experimental study. Chem. Phys. Lett. 2018;691:114–121. doi: 10.1016/j.cplett.2017.11.003. [DOI] [Google Scholar]
- 65.Silvi B., Savin A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature. 1994;371:683–686. doi: 10.1038/371683a0. [DOI] [Google Scholar]
- 66.Fathima Rizwana B., Prasana J.C., Muthu S., Abrahama C.S. Molecular docking studies, charge transfer excitation and wave function analyzes (ESP, ELF, LOL) on valacyclovir: a potential antiviral drug. Comput. Biol. Chem. 2019;78:9–17. doi: 10.1016/j.compbiolchem.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Jacobsen Heiko. Localized-orbital locator (LOL) profiles of chemical bonding. Can. J. Chem. 2008;86:695–702. doi: 10.1139/v08-052. [DOI] [Google Scholar]
- 68.Fathima Rizwana B., Prasana J.C., Muthu S., Abrahama C.S. Wavefunction analysis, charge transfer and molecular docking studies on famciclovir and entecavir: Potential anti-viral drugs. Chem. Data Collect. 2020;26(1-13) doi: 10.1016/j.cdc.2020.100353. [DOI] [Google Scholar]
- 69.Contreras Garcia J., Boto R.A., Izquierdo Ruiz V., Reva I., Woller T., Alonso M. A benchmark for the non-covalent interaction (NCI) index or… is it really all in the geometry? Theor. Chem. Acc. 2016;135(10):1–14. doi: 10.1007/s00214-016-1977-7. [DOI] [Google Scholar]
- 70.Jia Z., Pang H., Li H., Wang X. A density functional theory study on complexation processes and intermolecular interactions of triptycene-derived oxacalixarenes. Theor. Chem. Acc. 2019;138 doi: 10.1007/s00214-019-2502-6. Article No. 113. [DOI] [Google Scholar]
- 71.Trott O., Olson A.J. AutoDockVina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput.Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., lson A.J. Autodock4 and AutoDockTools4: automated docking with selective receptor flexiblity. SDRP J. Comput. Chem. Mol. Model. 2009;16:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cournia Z., Allen B., Sherman W. Relative Binding free energy calculations in drug discovery: recent advances and practical considerations. J. Chem. Inf. Model. 2017;57(12):2911–2937. doi: 10.1021/acs.jcim.7b00564. [DOI] [PubMed] [Google Scholar]
- 74.Maiolo F.D., Painelli A. Intermolecular energy transfer in real time. J. Chem. Theory Comput. 2018;14(10):5339–5349. doi: 10.1021/acs.jctc.8b00540. [DOI] [PubMed] [Google Scholar]
- 75.Lipinski C.A. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov. Today: Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 76.https://www.technologynetworks.com/drug-discovery/articles/what-is-adme-336683.
- 77.Pires Douglas E.V, Blundell T.L., Ascher D.B. pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph based signatures. J. Med. Chem. 2015;58:4066–4072. doi: 10.1021/acs.jmedchem.5b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radchenko E., Dyabina A., Palyulin V., Zefirov N. Prediction of human intestinal absorption of drug compounds. Russ. Chem. Bull. 2016;65:576–580. doi: 10.1007/s11172-016-1340-0. [DOI] [Google Scholar]
- 79.Smith D.A., Beaumont K., Maurer T.S., Di L. Clearance in drug design: mini perspective. J. Med. Chem. 2018;62:2245–2255. doi: 10.1021/acs.jmedchem.8b01263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.