Abstract
Human C-reactive protein (CRP), an early clinical indicator of infectious or inflammatory conditions has been recently identified as a key biomarker associated with the development of COVID-19. The rapid and accurate determination of CRP level in blood serum is an urgent need to predict timely the risk of disease worsening. The emergence of nanotechnological tools has provided an attractive perspective in designing portable bioanalytical assays with fast response time, high sensitivity and specificity, and multiplexing capability for accurate, on-site disease diagnosis and monitoring. Due to their versatile optical properties, plasmonic nanoparticles (PNPs) are appealing candidates for biosensing applications. This review summarizes the advances in the application of PNPs for CRP detection and quantification. Particularly, we review the improvements attained in the detection of CRP using aggregation-based colorimetric, localized surface plasmon resonance (LSPR), plasmon-assisted fluorescence and chemiluminescence, and surface-enhanced Raman scattering (SERS) spectroscopic methods.
Keywords: C-reactive protein, plasmonic nanoparticles, colorimetric detection, LSPR, MEF, SERS
Graphical Abstract
1. Introduction
As morbidity and mortality associated with local organ infections have meaningfully increased in the past years, early diagnosis has become a major challenge in view of prevention, disease management, and prognosis of inflammatory infections. Inflammation is considered as a non-specific immune response to different harmful stimuli such as pathogens, irritants, or damaged cells as a part of the body's defence mechanism. Diagnosis, prognosis, and treatment of inflammation or inflammatory infections are related to the measurement of biomarkers in biological specimens. Human C-reactive protein (CRP) is an early clinical indicator of infectious or inflammatory conditions related to various diseases and pathological conditions (e.g. sepsis, cardiovascular diseases, viral infections, etc.) [1], which has been also recently identified as a key biomarker associated with the development of coronavirus disease 2019 (COVID-19) [2]. CRP is a plasma protein composed of five identical monomeric sub-units with cyclic pentameric symmetry [3], mainly synthesized in the liver upon an inflammatory stimulus [4]. After reaching the region of the inflammatory reaction through the blood flow, CRP dissociates into the monomers that play a direct role in the inflammatory response.
The CRP levels in blood plasma for humans with no inflammation are lower than 10 mg/l, in case of viral infection between 10–40 mg/l, active inflammation and bacterial infection produce levels of 40–200 mg/l, while in severe bacterial infection and burn its level raises above 200 mg/l. However, in response to trauma, necrosis of tissue and acute inflammatory events the CRP blood level can suddenly increase up to 1000-fold over the baseline by 48 h after an acute event [5]. On the other hand, chronic low-level of CRP below 5 mg/l has been also shown to play an essential role in the development of coronary heart disease, ischemic stroke, and acute myocardial infarction, therefore CRP can be used also as a predictive marker for the future development of these diseases [6]. Moreover, in the case of COVID-19 patients, the blood level of CRP may predict timely the risk of disease worsening and helps in triage of patients. Although cannot be the sole basis for accurate COVID-19 diagnosis, the CRP level can provide valuable quantitative information on the severity or critical trends of COVID-19 infection. In particular, the patients with high levels of CRP (≥26.9 mg/l) had significantly elevated risks of developing into severe cases when compared with patients with low levels [7], [8], [9]. Since different diseases have different corresponding CRP detection ranges and CRP is also present at trace levels in plasma, there is a need to develop clinical tests with a broad linear detection range and high detection sensitivity to ensure accurate CRP analysis which could be helpful in making accurate clinical decisions for appropriate medication administration.
The current methods for the determination of CRP level, based on immunochemical laboratory techniques such as ELISA, immunoturbidimetric and chemiluminescent assay, are complex, expensive, time-consuming and require experienced personnel, therefore not suitable for point-of-care (POC) clinical diagnosis. As an emerging approach, the development of smart biosensing protocols for fast, routine, accurate, low sample volume, on-site, real-time identification and quantification with high specificity and sensitivity of biological entities (e.g. molecules, viruses, bacteria, etc.) related to pathological conditions represents one of the “holy grails” of modern medical diagnostics.
Due to the continuous progress in the field of nanotechnology, several nanomaterials with enhanced optical, mechanical, electrical and electrochemical properties have been successfully developed and applied in biosensing applications [10]. Among them, plasmonic nanoparticles (PNPs) are very attractive candidates for such applications since they integrate into a single system several desirable properties [11]. For example, the surface chemistry of PNPs allows their functionalization with target molecules by both chemical conjugation methods or physical adsorption strategies [12]. To confer recognition function against CRP, PNPs can be functionalized with CRP-specific antibodies, aptamers or phosphocholine groups.
On the other hand, PNPs feature outstanding optical properties determined by their localized surface plasmon resonance (LSPR) [13,14]. LSPR is an optical phenomenon occurring when the electromagnetic field of the light interacts with metallic NPs smaller than the wavelength of light [15]. This interaction induces a collective coherent oscillation of the surface conduction electron in resonance with the frequency of light, manifesting as a well-defined plasmon band in the UV-Vis-NIR spectrum. Noble metal NPs which exhibit well defined optical properties on account of their LSPR are widely exploited in sensing applications [16]. For instance, the interaction of biomolecules with PNPs can induce aggregation or shape transformation (etching) of the particles, both phenomena being accompanied by a drastic color change of the colloidal solution, enabling their use as colorimetric sensors simply by a standard UV-Vis spectrometer or even by the naked eye. Another way to explore PNPs as nanosensors is related to their high sensibility to the refractive index changes occurring in their close vicinity as a consequence of binding with analyte molecules, inducing a spectral shift in their LSPR maximum [16]. In this case, the detection can be spectroscopically monitored via the LSPR peak shift as a function of the biomarker concentration. The high electromagnetic field created around NPs, especially at the hot-spots of the PNPs (in the gaps created by aggregation or at the corners and edges of PNPs with anisotropic shape) is recognized to have considerable effects on the organic molecules in their closed vicinity. This can give rise to a number of outstanding plasmon-enhanced optical phenomena, which can be exploited in biosensing applications in order to achieve high sensibility [17]. For instance, metal enhanced fluorescence (MEF) may occur when fluorophores are placed at an optimal distance from the surface of PNPs in the near-field, characterized by an increased fluorescence intensity and a decreased fluorescence lifetime [18]. Surface-enhanced Raman scattering (SERS) is another highly sensitive spectroscopic technique taking advantage of the highly amplified electromagnetic fields of PNPs [19]. Besides its high sensibility, allowing the detection of SERS signal even from a single molecule, this technique is also able to identify molecular species and provide structural information. Both surface-enhanced spectroscopic techniques have attracted considerable research interest to implement them into biosensing applications.
In this review, we present an overview of the recent developments of high-sensitivity biosensing assays for the detection and quantification of CRP based on PNPs. Specifically, we focused onto the colorimetric and LSPR-based approaches relying on the modification of the optical properties (colour, LSPR) of PNPs induced by the specific interaction with the CRP. Two other high sensitivity spectroscopic detection methods were also reviewed, based on the plasmon-enhanced optical phenomena occurring due to the amplified electromagnetic fields around PNPs, namely MEF and SERS. Summary of the discussed assays for CRP detection based on PNPs is presented in Table 1 .
Table 1.
Analytical characteristics for different CRP biosensors reported in the literature. The lowest LOD reported for each detection principle is highlighted.
| Optical Technique/ Principle | Plasmonic platform | CRP recognition element | Linear Dynamic Range | LOD | Ref. |
|---|---|---|---|---|---|
| Colorimetric |
AuNPs | O-phosphorylethanolamine | 50-450 ng/ml | 50 ng/ml | [28] |
| AuNPs | poly(2-methacryloyloxyethyl phosphorylcholine)-b-poly(N-methacryloyl-(L)-tyrosine methylester) | <10 nM-> 100 nM | 20 - 40 nM | [29] | |
| AuNPs | antibody | 10 ng/ml-5 µg/ml | - | [31] | |
| AuNPs | aptamer | 0.889-20.7 μg/ml | 1.2 µg/ml | [32] | |
| AuNPs | Detection and capture antibodies | - | 1 ng/ml | [33] | |
| AuNPs | Detection and capture antibodies | 0-100 μg/ml | 1.15 μg/ml | [34] | |
| AuNPs | Antibody, antigen and single-stranded DNA | - | 326 pg/ml | [35] | |
| AuNPs | antibodies | 0-0.5 ng/ml | 32.0 pg/ml | [36] | |
| AuNPs | antibodies | 0-2 µg/ml | 54 ng/ml | [37] | |
| AuNPs | biotinylated antibodies | - | 3•10−8 g/ml | [38] | |
| LSPR | triangular Ag nanoplates | Phosphocholine (PC) | 5 ng | [42] | |
| Au-edge-coated triangular Ag nanoplate | cytidine 5’-diphosphocholine | 3.310−3 mg/l | [43] | ||
| AuNR | single chain variable fragment (scFv) | 1 ng/ml | [44] | ||
| AuNS | MUA linkers | 41-124.2 ng/ml | 41 ng/ml | [45] | |
| Au nanobipyramids | CRP antibody | 100 pg/ml-100 ng/ml | 87 pg/ml | [46] | |
| Au deposited nanostructured anodicaluminum oxide substrates | CRP antibody | 100 ag/ml | [47] | ||
| AuNP deposited onto transparent substrates | Anti-CRP | 0.001μg/ml | [48] | ||
| Au truncated icosahedra NPs assembled in an array | Anti-CRP | 2-160 mg/l | [49] | ||
| plasmonic Au nanohole array | antibody | 36 pg/ml | [50] | ||
| Fluorescence | AuNPs | Antibody | - | - | [53] |
| Au chip | Antibody | 33.3 zM-800 pM | 33.3 zM | [54] | |
| AgNPs | Antibody | - | 0.24 µg/l | [55] | |
| AgNPs | Antibody | 0.1-10 ng/ml | 30 pg/ml | [56] | |
| Ti-Ag-Ti-SiO2 chip | Polymer | - | 10 pM | [57] | |
| AuNPs | Aptamer | 3 pM-6 nM | 1.77 pM | [58] | |
| AuNPs | Antibody | 3.5-455 nM | - | [59] | |
| Au-Fe3O4NPs | Antibody | 9.5-2375 pM | 2.5 pM | [60] | |
| AgNPs | Antibody | 7 ×10−7-0.07 mg/ml | 0.05 ng/ml | [61] | |
|
AuNPs | antiCRP | 0.2 ng/ml | [64] | |
| Ag nano aggregates | Label-free | 0.01 ng/ml | [69] | ||
| Au-coated magnetic nanostars | antibodies | 27 pM | [76] | ||
| Core-shell Au@AgAuNPs | Detection and capture antibodies | 7.7 pM | [70] | ||
| AgNPs | antibodies | 1.56-25 ng/ml | 1.09 ng/ml | [66] | |
| AuNPs | antibodies | 1-1000 ng/ml | [65] | ||
| core-shell Ag@Au | antibodies | 5 pg/ml-10 μg/ml | 478 fg/ml | [71] | |
| Au nanoplates | anti-CRP | 10−17 M | [78] | ||
| Fe3O4@Au core-shell | antibodies | 0.01 ng/ml | [74] | ||
| Au-Ag core-shell | antibodies | 0.01-1000 ng/ml | 53.4 fg/ml | [77] | |
| Au nano-bridged nanogaps particles + Ag coated magnetic NPs |
aptamers | 10 fM-10 nM | 10 fM (1.14 pg/ml) | [75] |
2. Aggregation-based colorimetric detection of CRP
Colorimetric detection based on the aggregation of PNPs is one of the most powerful, rapid, and sensitive methods for real-time and on-site detection of various biomarkers simply by observing the color change of the colloidal solution with the naked eye [20], [21], [22]. The principle of this technique relies on the target-mediated aggregation of colloidal PNPs modified with a (bio)recognition element upon specific receptor-analyte binding. As the color of colloidal PNPs is strongly dependent on their morphology and electromagnetic coupling, the aggregation of PNPs is accompanied by an obvious color change of the colloidal solution detectable with the naked eye. On the other hand, the aggregation of the NPs leads to the emergence of a new plasmonic band supported by interconnected NPs, which can be evidenced by UV-Vis-NIR spectroscopy. Therefore, the concentration of the targeted (bio)analyte can be quantified by monitoring the ratio between the LSPR absorption maximum of aggregated and individual NPs. Due to their ultrahigh extinction coefficients (e.g., 2.7×108 M−1cm−1 for gold NPs (AuNPs)), PNPs enable an ultrasensitive colorimetric detection of target biomarkers with a limit of detection (LOD) in the nanomolar-picomolar range [23], [24], [25], [26], [27]. Among PNPs, spherical NPs, especially those made from gold (Au) are the most well-known sensing units in colorimetric assays based on the aggregation of NPs.
In the following, we summarize some successful colorimetric assays proposed for the specific detection and quantification of CRP.
A good example is the approach reported by Raj and Sreenivasan [28]. In their method, citrate-capped spherical AuNPs with a diameter of 39±3 nm were first functionalized with 16-mercaptohexadecanoic acid. The NPs affinity towards CRP was assured by tethering O-phosphorylethanolamine (PEA) onto their surface via carbodiimide chemistry. The colorimetric detection of CRP was achieved through specific molecular interaction between CRP and PEA which results in a color change of the colloidal solution due to NPs aggregation, detectable with naked eyes. As the aggregation of AuNPs is consistent with the shift and broadening of the plasmonic resonant band, UV-Vis-NIR spectroscopy was also used to evaluate the performance of the sensing method. The results showed that the developed colorimetric assay provides combined colorimetric and spectroscopic detection and quantification of CRP with the LOD of 50 ng/ml and a linear range of detection from 50 to 450 ng/ml. Besides CRP determination in aqueous solution, the designed approach enables the measurement of CRP in blood samples in the nanogram range. At the same time, the study conducted by Reed and co-workers provided important findings on biomolecular interaction between CRP and lipid-coated AuNPs [29]. Specifically, their work demonstrates that CRP can recognize and bind to the lipid-coated AuNPs via a reversible calcium-bridging mechanism. Although this study does not focus on CRP detection, the information it provides is useful for the subsequent design of CRP sensors. For instance, the calcium dependent binding of CRP to some biomolecules was later exploited by Yusa et al. to construct a colorimetric sensor for label-free detection of CRP in aqueous solution [30]. In this work, biomimetic block copolymer-protected AuNPs were prepared using a thiol-terminated biomimetic block copolymer, poly(2-methacryloyloxyethyl phosphorylcholine)-b-poly(N-methacryloyl-(L)-tyrosine methylester) as both, reducing and stabilizing agent. The obtained NPs exhibit remarkable colloidal stability over a wide range of pH and at a high salt concentration. The results showed that the prepared NPs facilitate the sensitive detection of CRP with a LOD between 20 and 40 nM. Significantly, the dynamic range for CRP quantification corresponds to the clinically relevant upregulation of CRP from normal (<10 nM) to acute-phase levels (>100 nM).
Antibody-conjugated AuNPs were proposed by Kim et al. as sensing units to develop a homogeneous colorimetric immunoassay for qualitative analysis of CRP in aqueous solution and serum samples [31]. In their concept, the authors exploit the pentameric structure of CRP and the immunoreaction of CRP with AuNP probes to promote the aggregation of the NPs as a function of CRP concentration. Specifically, one of the monomeric units of CRP binds to antibody-modified AuNPs, while the rest of the tetrameric portion of CRP promotes the aggregation of the NPs, leading to a red-shift of the LSPR band and a visible color change of the colloidal solution. To overcome the limitations caused by the hook and improve the linear range of CRP assay, the authors intentionally induced the aggregation of AuNPs by pre-spiking the serum sample with CRP. The designed colorimetric assay achieved a dynamic range for CRP detection of 10 ng/ml to 5 µg/ml. Recently, Daniel-da-Silva and co-workers have demonstrated a facile, fast, sensitive, and selective colorimetric assay for CRP detection using as sensing elements citrate–capped AuNPs modified with a guanine (G)-rich single-stranded DNA (ssDNA) aptamer [32]. The specificity of the designed sensor toward CRP was achieved through the high affinity of the aptamer against CRP. The ability of the aptamer to switch its conformation upon specific binding to targeted CRP was exploited here to induce the CRP-mediated aggregation of the aptamer-modified PNPs in buffer salts. CRP detection was performed by naked eye assessment of the aggregation induced color change of the colloidal AuNPs, while the concentration of CRP was quantified via UV-Vis-NIR extinction spectroscopy by monitoring the absorbance ratio between A670 nm and A520 nm. The proposed strategy for CRP determination yielded a linear sensing range of 0.889–20.7 μg/ml and a LOD of 1.2 µg/ml. The suitability of the designed colorimetric assay for practical applications was also demonstrated by the determination of CRP in diluted human urine.
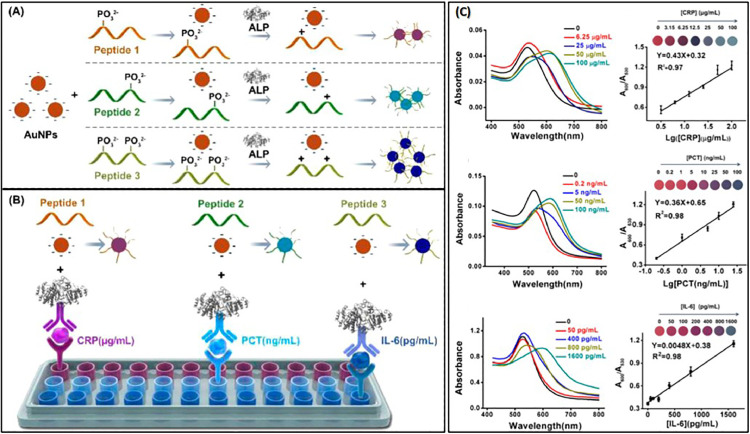
Initially used as colorimetric transducers for quantification of a specific biomarker, PNPs were later integrated into various colorimetric immunoassays that allow simultaneous detection of multiple biomarkers with high specificity and selectivity. In this matter, Long et al. proposed an enzyme-free alternative to enzyme-linked immunosorbent assay (ELISA) for selective detection of CRP, prostatic specific antigen (PSA) and α-fetoprotein (AFP) [33]. In their method, called metal-linked immunosorbent assay (MeLISA), alkyne-functionalized AuNPs were exploited as colorimetric sensing units, while silver NPs (AgNPs) of spherical shape were introduced to promote a signal amplification mechanism that replaces the enzyme element in ELISA. Remarkable, the developed MeLISA strategy enables the fastest AuNPs-based colorimetric assay ever reported until 2016, yielding a LOD of 1 ng/ml for CRP, 0.1 ng/ml for PSA and 0.1 ng/ml for AFP, respectively. Later, Jiang et al. fabricated a colorimetric immunoassay that enables the simultaneous detection of three inflammatory markers (CRP, procalcitonin (PCT) and interleukin-6 (IL-6)) by the naked eye with a broad detection range [34]. The proposed assay involves the use of negatively charged AuNPs, a series of phosphorylated short peptides and alkaline phosphatase (ALP), a widely used labeling enzyme in immunoassays. The principle of peptide-ALP-AuNPs immunoassay (PAAI) for simultaneous detection of CRP, PCT, and IL-6 is schematically illustrated in Fig. 1 (A).
Fig. 1.
Principle of peptide-ALP-AuNPs immunoassay (PAAI) for simultaneous detection of the inflammatory markers (IL-6, PCT, and CRP). (A) The scheme of AuNPs-based signal readout. Negatively charged phosphotyrosine or phosphoserine in the short peptides acts as a molecular switch for the AuNPs aggregation. When ALP removes the negatively charged phosphate group from the peptide, AuNPs aggregation is triggered after addition of the resulting dephosphorylated peptide. (B) The scheme of PAAI based on three kinds of peptides for simultaneous detection of IL-6, PCT, and CRP. (C) The color, spectra change, and corresponding relationship between the concentrations of CRP, IL-6, PCT and the A600nm/A530nm.
Reprinted with permission from ref. [34]. Copyright (2018) American Chemical Society
According to their concept, ALP removes the negatively charged phosphotyrosine or phosphoserine groups in the short peptides thus yielding a positively charged product. This, in turn, causes the aggregation of negatively charged AuNPs accompanied by a visible color change of the colloidal solution from red to blue and the emergence of a new plasmonic band supported by interconnected NPs. The quantitative analysis of CRP, PCT, and IL-6 in aqueous solutions and serum samples was performed by monitoring the aggregation ratio (A600 nm/A530 nm). By using three peptides with different sensitivities (the most sensitive, a moderately sensitive, and the most insensitive) quantitative analysis of multiple biomarkers in serum samples with a broad and controllable detection range from pg/ml to μg/ml was accomplished (Fig. 1(B) and (C)). The proposed colorimetric immunoassay provides reproducible, selective detection of CRP, PCT, and IL-6 with the LOD of 1.15 μg/ml for CRP, 0.24 ng/ml for PCT and 12.51 pg/ml for IL-6. Owing to its high specificity, sensibility, tunable detection range and multiplexing capacity, the designed colorimetric immunoassay could be promising for applications in clinical diagnostic.
Recently, Mao et al. fabricated a multiplexed colorimetric immunoassay for the simultaneous detection of CRP and IL-6 with high specificity and selectivity [35]. The design of this colorimetric immunoassay involves the use of bifunctional spherical AuNPs decorated with antibodies and single-stranded DNA (ssDNA) as detection probes, AuNPs conjugated with complementary ssDNA as signal amplification probes and antibody microarrays. The mechanism of detection relies on dual signal amplification to improve the sensitivity of the protein microarray. Specifically, AuNPs labeled with antibodies and ssDNA bind to the targeted molecules and then it hybridized with complementary ssDNA conjugated AuNPs, thus increasing the signal of the protein microarrays. On the basis of this, AuNPs initiated Au reduction and subsequent deposition, leading to the second amplification of the output signal of the immunoassay. Finally, the colorimetric visualization was performed with a microscope. and the data were analyzed using a Gray analysis 5.0 software. The principle of detection is illustrated in Fig. 2 .
Fig. 2.
Schematic representation of the detection process of CRP and IL-6.
Reprinted with permission from ref. [35]. Copyright (2020) Elsevier.
By exploiting the cooperation of the two mechanisms of signal amplification a highly sensitive analysis of CRP and IL-6 in aqueous solution could be accomplished with the LOD of pg/ml (326 pg/ml for CRP and 8 pg/ml for IL-6). The clinical applicability of the designed multiplexed immunoassay was evaluated in 28 clinical serum samples and the results showed a good agreement with those obtained by the ELISA kit. At the same time, Park et al. developed a colorimetric immunoassay using AuNPs conjugated with 6X-histidine (6X-his) peptide as colorimetric transducers, and nickel-horseradish peroxidase (Ni2+-HRP) as enhancer of the colorimetric signal in ELISA [36]. The performance of the developed assay was assessed by quantifying CRP in standard and serum samples and comparing the results with conventional ELISA assay. The results showed that the developed colorimetric immunoassay enables an increase of 12-fold sensitivity for detecting CRP compared to ELISA, yielding a LOD of 32 pg/ml in human serum samples and a wide linear range of detection (0-0.5 ng/ml).
Microfluidic immunoassays were also fabricated and tested for colorimetric determination of CRP with the aim to fulfill the requirements for POC diagnosis. For instance, Lin et al. demonstrated a proof-of-concept for a new, innovative paper-based microfluidic immunoassay employing AuNPs integrated into a lateral flow assay [37]. In their design, the color changes caused by NPs aggregation were detected using a commercial smartphone. The fabricated colorimetric immunoassay enables qualitative and quantitative analysis of CRP in aqueous solution, plasma, and whole blood with a LOD of 54 ng/ml and a linear range of detection up to 2 µg/ml. Later, Russell and de la Rica designed a novel type of colorimetric transducer consisting of a piece of paper printed in toner with a specially designed pattern [38]. The results showed that the fabricated paper transducer can detect the plasmon variations caused by the aggregation of AuNPs using a mobile device as the reader. The suitability of the proposed printable colorimetric transducer toward practical applications was demonstrated by quantitative determination of CRP with a similar LOD compared to a competitive ELISA (3×10−8 g/ml).
3. Localized Surface Plasmon Resonance (LSPR)-Based Detection of CRP
Besides their own size, shape, and dielectric properties, the LSPR of a metallic NPs also depends on the local refractive index (RI) change leading to some well-quantifiable spectral shifts of the LSPR extinction peak. The principle of LSPR sensors is based on the quantification of LSPR peak modifications upon binding of analyte molecules to the surfaces of NPs conjugated with specific receptors. The local RI sensitivity of metal NPs defines the sensing depth of the LSPR sensor, therefore the dependence of RI sensitivity on the properties of NPs has been extensively investigated. It was found that the RI sensitivity increases for nanocrystals with higher curvatures [39], larger polarizabilities [40], and longer plasmon wavelengths [41]. In general, NPs with high shape anisotropy (eq. nanoplates, bipyramids, etc) or increased aspect ratio (width/height) results in LSPR red-shifted to longer wavelengths. To date, various nanostructures with high RI sensitivity have been developed and applied in the LSPR-based detection of CRP. For instance, Fournet et al. developed highly sensitive solution phase LSPR bioassays based on triangular silver nanoplates (TSNPs) for the detection of CRP. Their results revealed that TSNPs with the highest aspect ratios exhibit the highest LSPR sensitivities, thus the lowest detection level of CRP was reached (5 ng) [42]. Another approach to increase the RI sensitivity of plasmonic NP was addressed by Zhang et al. [43]. Namely, TSNPs with various lengths with LSPR from 600 nm to 1197 nm were subjected to galvanic replacement reaction to obtain Au-edge-coated-TSNP. Detailed characterization regarding the stability and ensemble RI sensitivity over figures of merit was examined using discrete dipole approximation calculations and single nanostructures dark field microscopy measurements. The obtained results reveal that Au-edge-coated-TSNPs possess higher sensitivity and stability by hindering NPs etching in saline solutions. The highest ensemble RI sensitivity value of 1816 nmRIU−1 was measured for an Au-edge-coated TSNPs sol with a LSPR maximum at 1197 nm. This Au-edge-coated-TSNP based nowash detection method allowed for rapid and sensitive detection of high sensitive CRP (hs-CRP) at concentrations as low as 3.3×10−3 mg/l.
As the sensitivity of LSPR sensing drastically decreases with the distance between the surface of the NP and the target binding event, Byun et al. have chosen as recognition element for CRP detection a short single-chain variable fragment (scFv) instead of a full-length antibody [44]. The synthesised scFv contains cysteine tags that enable conjugation to Au nanorods (AuNR) surface, but also feature recognition sites for CRP target binding. The assay relies on the LSPR peak-shift of scFv-functionalized AuNR induced by the binding of CRP. Due to the shortness of the antibody fragment (3 nm instead of 15 nm in the case of full-length antibody), the obtained system was found to display a much higher sensitivity compared to one using a full-length antibody as a capture receptor. CRP in human serum was quantitatively detected at concentrations lower than 1 ng/ml.
The LSPR detection systems are also influenced by the employed surface chemistry method, therefore its optimization for maximum protein immobilization and retention is relevant as well. Garifullaina et al. conducted a detailed study and compared four representative Au surface functionalization methods in attaching biomolecules to nonspherical plasmonic Au nanostructures (AuNS): simple physical adsorption, microcontact printing, and two thiol linkers 11-mercaptoundecanoic acid (MUA method) and thiolated PEG acid linkers (HS−PEG−COOH method), respectively [45]. The efficiencies of the different surface chemistry methods were estimated based on the LSPR shifts before and after incubation of AuNS in anti-hCRP. The study concluded that binding via the MUA linkers resulted in the most efficient and reproducible antibody immobilization, retaining the specificity toward hCRP giving a LOD and LOQ of 41.0 and 124.2 ng/ml, respectively. Another study uses Au nanobipyramids (AuNBP) to create a sensitive sandwich LSPR immunosensor for CRP detection [46]. Here an enzyme catalysed precipitation reaction was applied to overcome the limitation of sensing distance in the case of large proteins. 4-chloro-1-naphthol (4-CN) was precipitated onto the surface of CRP antibody functionalized AuNBP substrate which induced additional large change of local RI, manifested in a strong LSPR-shift after the sandwich structure formed. By monitoring the LSPR-shift, this sensing method enabled the quantitative analysis of CRP from 100 pg/ml to 100 ng/ml with good linearity, reaching a LOD of 87 pg/ml. Yeom et al. also applied the sandwich assay approach to increase the sensitivity of their LSPR sensor system [47]. The reported sensor consists of Au deposited nanostructured anodicaluminum oxide (AAO) substrates functionalized with CRP antibodies. The binding of the CRP antigen induces changes in the RI of the sensing membrane, and the LSPR-shift in the reflection spectrum of the membrane can be measured in real-time. By applying the sandwich structure using AuNP-labeled CRP antibodies, the sensitivity of the sensor increased 1.84 times reaching a LOD of 100 ag/ml.
Portability and rapidity of such infections marker detection sensors are a key requirement, particularly in the management of life-threatening infections and sepsis. Therefore, the development of POC testing devices is needful for early diagnosis of diseases. Oh et al. recently developed a selective portable LSPR sensor chip for the sensitive detection of CRP using a cuvette cell system, as shown in Fig. 3 [48]. The sensor is based on AuNP deposited onto transparent substrates in form of stripes functionalized with anti-CRP. The detection sensitivity of the sensor chip was evaluated for various CRP concentrations, with the low detection limit of 0.001 μg/ml confirming the superiority of the detection platform developed in this study.
Fig. 3.
Schematic illustration of the anti-CRP-based LSPR sensor chip for CRP detection.
Reprinted with permission from ref. [48]. Copyright (2019) Frontiers Media SA.
Furthermore, James-Pemberton et al. recently developed a multiplexed biophotonic assay platform for a clinically useful triage of CRP detection from diluted whole blood sample, requiring no preparation and giving result in only 8 minutes [49]. The assay is based on the LSPR of Au truncated icosahedra NP with a diameter of approximately 60 nm assembled in an array and functionalised with a self-assembled monolayer to allow EDC–NHS coupling of anti-CRP to the surface. Control samples functionalised with BSA or FBR were also evaluated in order to correct for variations in temperature, non-specific binding and variations in the illumination field. The detection technique is based on the variation in the intensity of the scattered light from the array elements when illuminated in total-internal reflection mode, calibrated for refractive index sensitivity. The accuracy and precision of the CRP sensor assay were assessed with 54 blood samples containing spiked CRP in the range 2-160 mg/l. The mean accuracy was 0.42 mg/l with Confidence Interval (CI) at 95% from 14.7 to 13.8 mg/l and the precision had a Coefficient of Variation (CV) of 10.6% with 95% CI 0.9% - 20.2%. Another portable digital NP-enhanced plasmonic imager for rapid detection of the inflammatory sepsis-related biomarkers, PCT and CRP from blood serum has been recently developed by Belushkin et al. [50]. The detection principle is based on a plasmonic imaging mechanism based on antibody-conjugated AuNP binding in the presence of the biomarker to plasmonic Au nanohole array surface functionalized with complementary antibodies (Fig. 4 (A)). Individual AuNP bound inside or close to the nanoholes create strong local intensity contrast allowing digital detection of single analyte molecules. The bioassay is performed in a single step without signal amplification or washing procedures and enables quantification of individual molecule binding on the sensor surface in complex media. This compact and low-cost device, a prototype DENIS reader (Fig. 4(B)), can identify biomarker level in less than 15 min, achieving an outstanding limit of detection of 21.3 pg/ml for PCT and 36 pg/ml for CRP.
Fig. 4.
Portable digital NP-enhanced plasmonic imager for biomarkers detection. (A) PCT and CRP, which are blood-circulating protein biomarkers secreted by the host body in response to systemic inflammation, are detected using DENIS. A single-step bioassay directly in human serum enables rapid molecular results, critical for the early diagnosis of sepsis, by detecting individual Au-NPs binding to Au-NHA. (B) A prototype DENIS reader developed for highly sensitive and multiplexed detection of biomarkers. The device uses a CMOS camera and a narrow-band LED source to record the transmitted images from a nanoplasmonic chip. (C) SEM image of a Au-NHA area after a bioassay showing the bound NPs. Inset shows a single NP bound inside a nanohole. (D) Plasmonic image of a Au-NHA area with bound NPs. The binding of Au-NPs on Au-NHAs causes local transmission suppression through distortion of plasmonic excitations in the Au-NHA and can be digitally detected using far-field imaging. The inset shows a normalized intensity contrast induced by a single NP trapped in a nanohole.
Reprinted with permission from ref [50]. Copyright (2020) John Wiley & Son.
4. Plasmon-assisted fluorescence and chemiluminescence based detection of CRP
Fluorescence is the emission of electromagnetic radiation generated by the radiative relaxation of excited electrons inside a material. Particularly, once the material absorbs light, electrons transition to the first (S1) or second excited singlet level. After an extremely short period of time, they relax to the ground vibrational state of S1. Afterwards, the electrons return to their fundamental electronic state through a radiative process, causing energy release under the form of fluorescence emission.
Fluorescence-method is one of the most often applied analytical approaches for the detection of biomarkers [51]. The sensing mechanism is based on linking the analyte concentration with the emission intensity. The relationship can be direct through fluorescence amplification (higher analyte concentration, higher emission intensity) or indirect through fluorescence quenching (higher analyte concentration, lower emission intensity). However, fluorescence-based sensors present limitations when it comes to sensitivity in the CRP molecule's clinical region of interest due to low quantum efficiency, photobleaching and autofluorescence [52]. Therefore, it is absolutely necessary the development of new methods that could solve this deficiency of fluorescence-based immunoassays. Lately, it was found that the addition of PNPs in the proximity of fluorophores resulted in two possible processes: metal enhanced fluorescence (MEF) when an ideal distance between the plasmonic NP and the fluorophore is obtained (10-90 nm) or fluorescence quenching when the fluorophore-NP distance is too short (0-10 nm). Both phenomena are able to enhance the sensitivity of fluorescence-based immunoassays if they are used under proper experimental conditions.
In 2008, Hong et. al. developed the first MEF-based biosensor for the quantification of CRP molecules [53]. Briefly, they formed a sandwich protein complex captured by its specific antibody to metal NPs (Au and Ag) on one side and a fluorophore (Cyanine 5 or Alexa 647) on the other side. Next, they thoroughly studied how the NPs metal type (Au and Ag) and the solvent can enhance the sensitivity of fluorophore-mediated biosensors, superior enhancements being obtained when AuNPs dissolved in 1-butanol were used. In the end, they translated the sensor into an automated prototype for the simultaneous detection and quantification of four cardiac markers (CRP, myoglobin, cardiac Troponin I, and B-type natriuretic peptide). The preliminary results demonstrated that their system could quantify all the markers within 10 minutes with the same precision as single-detection sensors. Beside precision, the detection limit of an immunoassay should be as low as possible. The detection of a single CRP molecule was obtained using a 20 nm Au-nanopatterned biochip proposed by Heo et. al. [54]. The target molecule was identified via a total internal reflection fluorescence microscope based on evanescent field-enhanced fluorescence imaging. The same antibody-sandwich method was used to capture the CRP molecule between the Au chip and the Alexa 488 fluorophore. By reducing the CRP concentrations, the relative fluorescence intensities linearly decreased in the range of 33.3 zM - 800 pM, with a correlation coefficient of 0.9925. The determined LOD was 33.3 zM, the equivalent of a single CRP molecule. Noteworthy, they observed that for CRP concentration below 500 zM, the optimum incubation time between the CRP antigen and the antibody needs to be increased from 1 to 4 h. Moreover, their technique was successfully implemented on the detection of low CRP concentrations even in human serum samples.
The NPs geometry is another factor that has a huge impact on the MEF effect and, in consequence, on the detection's sensitivity. Spherical NPs were found by Zhang et. al. to exhibit improved fluorescence enhancement compared to triangular ones, due to increased scattering [55]. Therefore, spherical AgNPs were deposited on a microplate and the platform was used to perform, for the first time, solid-phase sandwich assay via MEF for the detection of human CRP. In this case, the protein sandwich-capture was performed with mouse-specific antibodies between the AgNPs and a DY-647 fluorophore. The solid-phase sandwich assays revealed a 0.24 µg/l LOD when enhanced by AgNPs and a 4.5 µg/l one in the absence of NPs. In order to obtain these results, a complex purification was performed after the deposition of each component, which could have led to system destabilization. An easier purification process was described by Zhao et. al., who managed to combine magnetic beads and AgNPs functionalized with specific antibodies (Fig. 5 ) to detect CRP molecules via a Ag+ turn “on” fluorescence method [56]. The use of physically and chemically stable magnetic beads provided the possibility of easy and efficient purification, separation and concentration processes, while AgNPs triggered and enhanced the fluorescence emission. Once the sandwich-type immune-complex was formed, it was magnetically separated and dissolved in a solution containing an inactivated Rhodamine B-based fluorophore dissolved in hydrogen peroxide (H2O2). The interaction between AgNPs and H2O2 generated numerous Ag+, turning “on” the fluorophore emission, whose intensity was correlated with the detected CRP concentration. Using this novel method, they obtained good linear response range between 0.1-10 ng/ml, while the calculated LOD was just 30 pg/ml. Moreover, the same device was able to detect specifically even α-fetoprotein in real human samples with extremely high accuracy, but cheaper than the commercial methods, demonstrating great promise even in clinical applications.
Fig. 5.
Schematic illustration of Ag+ triggered fluorescence detection for protein biomarkers.
Reprinted with permission from ref [56]. Copyright (2017) Ivyspring International Publisher.
All the aforementioned immunoassays use antibodies as an antigen-capture agent. Even though antibodies are extremely specific, they are unstable and expensive. A cheaper bio-chemical hybrid CRP sensing device was reported by Matsuura et. al. [57]. They used a synthetic polymer instead of antibodies on one side of the protein-sandwich as a CRP-specific ligand, making it a more affordable method. The artificial polymer Poly(2-methacryloyloxyethyl-phosphorylcholine) was grafted on a four layers chip (Ti, Ag, Ti, SiO2) using controlled radical polymerization and the CRP molecule was captured between the polymer and the specific antibody. Afterwards, by adding a biotin-streptavidin complex between the antibody and the Cy5 fluorophore to increase the NP-fluorophore distance, they managed to reduce the background fluorescence making the system more sensitive and obtaining a LOD of 10 pM. This work was the first one to use a ligand as a substitute of the specific antibody to capture and detect via MEF the CRP molecules, making a step forward towards the development of more affordable CRP fluorescence-based sensing devices. Another substitute for antibodies is aptamers. They exhibit lower development time, smaller sizes, and higher stability. The first DNA aptamer-based optical turn “off” nanosensor was released by Ghosh et. al in 2019 [58]. The device was composed of a deoxyribonucleic acid aptamer linked on the 3’ terminus to a AuNP and on the 5’ one to a quantum dot (QD). The principle used in this work was fluorescence resonance energy transfer (FRET) and, therefore, increasing the CRP concentration resulted in a decrease of the QD's photoluminescence. The linear quenching behaviour was obtained between 3 pM and 6 nM, while the LOD was calculated to be 1.77 pM. Remarkably, only 5 µl of sample was required to perform the measurement. Moreover, the nanosensor showed promising results even against CRP spiked human samples, exhibiting 10% fluorescence quenching at 10 pM of target molecule. Unfortunately, at high concentrations, a limitation called the Hook effect is encountered due to the total saturation of the capture agent. Bravin et. al. developed a multi-component sandwich immunoassay, which could detect a wide range of CRP concentrations without being affected by the Hook effect [59]. The sandwich system was based on an antiCRP-functionalized AuNPs and an antiCRP-conjugated FRET donor-acceptor complex. In consequence, the resulting FRET fluorescence is quenched by the AuNPs through the nanomaterial-surface energy transfer phenomenon. The linear quenching effect was obtained in the clinical range of interest between 3.5-455 nM. Furthermore, the assay showed high accuracy and reproducibility when tested on real human serum samples, obtaining an error percentage of just 3 ± 3%.
Another method that is widely used in the detection of biomarkers is Chemiluminescence (CL). CL is electromagnetic radiation, released under the form of light after a chemical reaction. This method presents fast times of detection combined with low costs. Therefore, Xing et. al developed a CL immunoassay for the detection of CRP based on antibody functionalized Au-Fe3O4 core-shell NPs as magneto-plasmonic nanocarriers and glycerophosphoryl (GPC) as blocking agent [60]. When ultrasensitive detection is wanted, non-specific protein adsorption on the sensing platform could be a big problem due to the increased noise which reduces the diagnose performance. By eliminating non-specific adsorption of CRP molecules, the LOD of the device decreased by 3.8 times from 9.5 (without GPC) to 2.5 pM (with GPC). The linear response was obtained between 9.5 to 2375 pM with a correlation coefficient as high as 0.9980. The sensing device exhibited great efficiency also against clinical samples, even surpassing the Immunoturbidimetric Assay which was approved by the US Food and Drug Administration for clinical use. However, CL-based immunoassays present sensitivity limitations due to low reaction yield and low quantum yield. Thus, a novel metal enhanced CL (MEC) signal tag was designed by Zong et. al. to be used as an ultrasensitive immunoassay for the detection of CRP [61]. The target molecule was captured in an antibody-sandwich between a CL substrate and a plasmonic complex with the role of CL enhancer. The plasmonic part was provided by the hybridization of two kinds of AgNPs probes: (A) DNA-hemin/DNA-A/biotin-DNA-AgNPs and (B) DNA-hemin/DNA-B-AgNPs. Evaluating the CL intensity against CRP concentrations, lead to a wide linear detection range from 7×10−7 to 7×10−2 mg/ml. The LOD was estimated to be around 0.05 ng/ml. In interaction with different markers such as CRP, human myoglobin, and myoglobin isoenzyme of creatine kinase, the proposed immunosensor chip presented high CRP selectivity. Furthermore, when tested against real human samples the MEC immunoassay obtained great results with a relative error of just 4.94%.
5. Surface Enhanced Raman Scattering (SERS) based detection of CRP
The enhanced electromagnetic field around PNPs generated as a consequence of collective oscillations of conduction electrons, known as surface plasmon resonances, is recognized to have considerable effects on the organic molecules in their closed vicinity, giving rise to a number of remarkable phenomena with particular interest in imaging and detection applications. Specifically, the Raman scattering from molecules located in the amplified electromagnetic field near PNPs can be drastically enhanced, termed as Surface Enhanced Raman Scattering (SERS) [62], allowing even single-molecule detection [63]. Compared to the aforementioned spectroscopic detection techniques, Raman spectroscopy excels by its high specificity, with the ability to identify and provide structural information about molecular species from their unique vibrational Raman fingerprint. Therefore, SERS-based biosensing approaches not only enables ultralow detection, but also can identify molecular species and provide additional information about conformation etc. The SERS technique was also widely explored in the detection of infection biomarkers such as CRP, using different plasmonic nanostructured optimized for high enhancement.
The first SERS bioassay for quantitative human CRP analysis was reported in 2008 by Campbell et al. [64]. In this study, SERS was used with the aim of improving CRP detection in an ELISA system by investigating the coloured label generated by the enzymatic reaction. A common substrate for alkaline phosphatase (AP) is converted in SERS active species upon the action of AP following the detection of CRP by AP-antiCRP antibodies. By replacing the colorimetric detection step in ELISA with SERS, the limit of detection for CRP is highly improved, from 7 ng/ml to 0.2 ng/ml. An enzymatic strategy to activate reduction caged reporters in SERS bioassays for CRP detection was also reported by Guo et al. [65]. Through enzymatic activation of a Raman inactive reporter using horseradish peroxidase (HRP), leucomalachite green (LMG), new MG Raman active agents that give a strong SERS signal are obtained. For CRP detection immunoassay, CRP biomarker is captured with antibodies attached on agarose beads which form a sandwich with HRP conjugated detection antibodies. HRP activates the SERS reporters in the presence of H2O2 which mixed with AuNPs generates a reliable signal with a strong peak at 1615 cm−1 and a linear dependence on the CRP concentration in the 1-1000 ng/ml range. Due to their versatility, metallic NPs can also be successfully used as artificial enzymes, called nanozymes. For CRP detection, the catalytic activity of AgNPs was exploited in a surface based Ag-linked immunosorbent assay (SLISA) [66]. Specifically, AgNPs were functionalized with antibodies specific for CRP target antigen to replace the usual enzymes used in a traditional ELISA. These bind to the CRP immobilised on the substrate by the capture antibody and when 3,3′,5,5′-tetramethylbenzidine (TMB) substrate is added, the AgNPs catalyse the oxidation of TMB by H2O2. The reaction product, analysed by SERS, is dependent on the AgNPs concentration which is related to the detected CRP. Therefore, by replacing the conventional enzymes with AgNPs, a low LOD of 1.09 ng/ml for CRP is achieved.
Aggregated PNPs present a high density of hot-spots at the junctions and gaps created between interconnected NP with enhanced electromagnetic fields which can drastically enhance the Raman signal of entrapped molecules, therefore are widely explored as SERS substrates in sensing applications. For instance, Benford et al. used SERS active aggregated AuNPs to detect CRP as a cardiac biomarker together with b-type natriuretic peptide (BNP) and cardiac troponin (cTn) for detection of acute coronary syndrome, employing a nanofluidic device [67]. Later, the same authors improved this platform to specifically capture CRP using agarose beads functionalized with an anti-CRP antibody [68]. The captured biomarker displaces a peptide fragment that contains the binding epitope of the antibody labeled with Rhodamine-6G (R6G). In the presence of the analyte, an increase in the SERS signal of R6G is noticed, proportional to the amount of the detected analyte. Ag nanoaggregates were also exploited by Kim and co-authors [69] to develop a label-free SERS detection chip for CRP detection in serum. Specifically, they functionalized a glass coverslip with phosphocholine coated Ag nano aggregates to selectively capture CRP in a capillary gap created to control the flow of the CRP solutions. Due to a less than 4 nm distance between the nanoaggregates and CRP, and a small difference between the radius of the aggregated AgNPs (20 nm) and the size of CRP (12 nm), this chip presents a high sensitivity with increased chances of protein attachment to the interparticle hotspots with maximized field enhancement. The minimum detection amount of CRP was 0.01 ng/ml in buffer and 0.1 ng/ml in 1% serum when aggregated AgNPs were layered in a 200-300 nm thickness. Interestingly, higher sensitivity and resolvability were recorded for the 2800-3000 cm−1 range, compared to fingerprint (1000-1600 cm−1) and low frequency (<900 cm−1) regions. The highest sensitivity was reported for asymmetric C–H stretching mode at 2930 cm−1. The cross-reactivity was also tested, and the chip proved an excellent selectivity and specificity for CRP.
A key issue in SERS-based detection bioassays represents the development of SERS-substrates which provide highly amplified, reproducible, stable and quantifiable SERS signals. To respond to these queries, researchers proposed to synthesize core-shell metallic nanostructures with well-defined narrow interior nanogaps. Raman reporters localized in these built-in hot spots in the core-shell nanostructure junction experience extremely enhanced local electromagnetic fields providing a strong SERS signal. Moreover, reporter molecules are well-retained in these gaps, protected by the shell from leakage, degradation and uncontrolled aggregation-induced enhancement, therefore they offer stable and quantitative SERS signal. This approach was also recently employed in the development of sensing SERS nanoplatforms for CRP detection, reaching very low LOD values.
For instance, Li et al. [70] reported a new SERS-active core-shell PNP-based on an etching-assisted approach for multiplex analyte detection. They labelled Au cores with Rhodamine B as Raman reporter and formed a Ag shell on top by depositing Ag atoms on the Au cores in the presence of Pluronic F127. Then, the Ag shell was etched with HAuCl4 to form Au@AgAuNPs with nanometric gaps inside which induces an increased Raman signal of trapped reporters, reaching a LOD of 7.7 pM. These SERS tags were also successfully employed for multiplex quantitative detection of CRP and PSA and as cancer cell imaging agents after conjugation with specific aptamers. Another core-shell SERS nanotag-based biosensor was also developed by Liu et al. [71] for CRP detection using photonic crystal beads (PCBs) as carriers. SERS nanotags were built on a Au core with a Ag shell and Nile blue A dye embedded at their interface to provide a strong Raman signal. The SERS tags were conjugated with detection antibodies to form an immunocomplex with capture antibodies loaded on PCBs when CRP is present. In this way, the signal from the nanotags can be recorded under excitation with a 785 nm laser line and the detected CRP quantified based on the Raman intensity of 595 cm−1 peak. A high sensitivity and a LOD much better than previously reported values of 478 fg/ml can be obtained by using this biosensor. Additionally, the biosensor shows high stability, reproducibility, reliability, and a high signal-to-noise ratio. Also, it performs well over a wide range of clinical CRP concentrations, ranging from 5 pg/ml to 10 μg/ml, and has a correlation coefficient with the ELISA clinical reference method of 99.82% when is tested on five real human serum samples with CRP concentration ranging from 70.2 pg/ml to 7.7 μg/ml. Raman reporter-embedded Au-core Ag-shell NPs were also employed by Cong et al. for the design of a rapid and accurate POC SERS-based lateral flow assay for selective quantitative detection of CRP [72]. The specificity of the fabricated SERS nanotags toward CRP was achieved by conjugating them with a CRP antibody. CRP quantification was performed by monitoring the SERS intensity of a characteristic Raman reporter band following the exposure of NPs to different concentrations of CRP. The designed SERS-based lateral flow assay provides reproducible, selective and high-sensitive detection of CRP with the LOD of 0.01 ng/ml. The suitability of the presented SERS-assay for practical applications was also demonstrated by the determination of CRP in plasma samples of irradiated nonhuman primates.
By endowing PNPs with magnetic properties has brought new advances in SERS-based detection of CRP, enabling rapid separation enrichment of targets from a complex solution.
For example, a smart, label-free SERS immunosensor for rapid and hypersensitive analysis of CRP was demonstrated by Yang et al. using porous magnetic Ni@C nanospheres and calcium carbonate (CaCO3) microcapsules as the SERS sensing platform and AgNP-coated silicon wafer as an enhancer of the SERS signal [73]. The fabricated immunosensor enables specific capture of CRP via antibody-antigen interactions, yielding a LOD of 0.01 pg/ml and a linear range for CRP quantification from 0.1 pg/ml to 1 μg/ml. Liu et al have recently proposed a novel Fe3O4@Au core-shell NP based SERS - lateral flow immunoassay biosensor (Fig. 6 ) for simultaneous and quantitative detection of two infection biomarkers: serum amyloid A (SAA) and C-reactive protein (CRP), respectively [74]. The Fe3O4@Au nanotag is composed of three main elements, specifically, a superparamagnetic Fe3O4@Au core as the active SERS substrate, a layer of Raman reporter molecules 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) adsorbed on the Au shell and surface-modified monoclonal antibodies which specifically recognize SAA/CRP (Fig. 6(A)). The superparamagnetic component served as a separation tool for magnetic enrichment of SAA/CRP in unprocessed blood samples. SERS-based quantified analysis of target infection biomarkers revealed a 100- and 1000-fold sensitivity enhancement by the proposed sensor compared to those of standard colloidal Au-based lateral flow assays, reaching a LOD of 0.1 ng/ml and 0.01 ng/ml for SAA and CRP, respectively.
Fig. 6.
Schematic illustration of (a) the preparation of antibody-conjugated Fe3O4@Au SERS nanotags, and (b) the detection principle of the Fe3O4@Au-based SERS-LFA strip for simultaneous quantification of SAA/CRP.
Reprinted with permission from ref [74]. Copyright (2020) American Chemical Society.
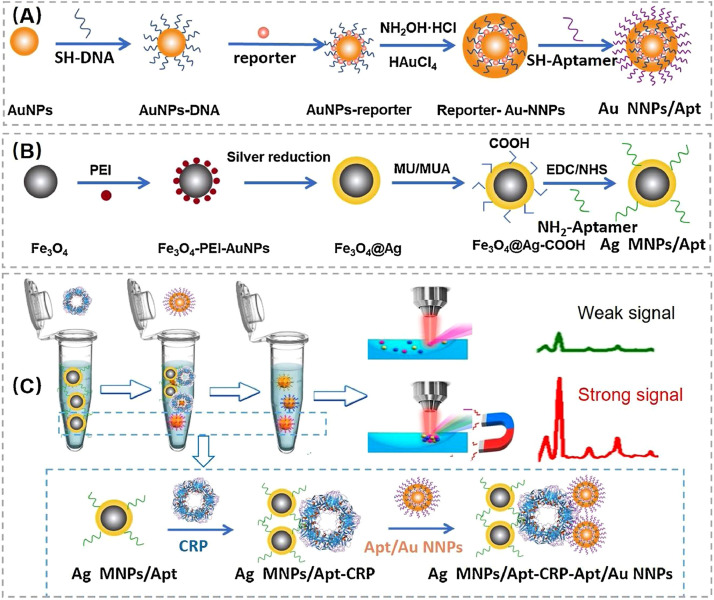
By combining Ag coated magnetic NPs (Ag MNPs) and Au nano-bridged nanogaps particles (Au NNPs) an innovative aptamer-based SERS biosensor has been recently developed by Hu et al showing high affinity to CRP [75]. As schematically presented in Fig. 7 (A) and (B), the sensor is composed of 4-ATP-labeled Au NNPs with narrow nanogap as SERS signal probe and Ag MNPs as a capture substrate, respectively. The approach of using Ag MNPs instead of conventional Fe3O4 NP, also served for magnetic separation, Au NNPs-CRP-Ag MNPs complex enhanced the intensity of the SERS signal 13 times compared to the Au NNPs-CRP-Fe3O4 complex. For specific recognition of CRP, both SERS tag and magnetic capture substrate were modified with aptamers against CRP. Selective and specific detection of CRP was accomplished by SERS measurement based on sandwich complex strategy (Fig. 7(C)), reaching an ultra-low LOD of 10 fM (1.14 pg/ml) and exhibits high accuracy in the detection of actual human serum samples.
Fig. 7.
Synthesis of (A) Au NNPs SERS tag and (B) Ag MNPs magnetic capture substrate. (C) Schematic illustration of protein detection via Ag MNPs-CRP-AuNNPs "sandwich" structure by SERS.
Reprinted with permission from ref [75]. Copyright (2021) Elsevier.
Another common approach for the preparation of SERS substrates relies on the use of mesoporous templates greatly increasing active sites and enlarging adsorption capacity. Owning to its outstanding biochemical properties, a biomaterial template, M13 bacteriophage, was used to fabricate a novel SERS substrate to develop a biosensor for sepsis related biomarker detection (CRP, procalcitonin (PCT) and soluble triggering receptor expressed on myeloid cells-1 (sTREM-1)) [76]. The SERS substrate developed on M13 phages was decorated with Au-coated magnetic nanostars and functionalized with specific antibodies to capture sepsis biomarkers. Three different SERS tags were prepared by bioconjugating antibodies and Raman reporter molecules on the AuNPs surface using EDC/NHS method. In the presence of specific biomarkers, a sandwich immunoassay is formed, and the SERS signal appears after excitation with a 785 nm laser line. The recorded SERS spectra show distinct peaks for all corresponding tags of biomarkers. In the case of CRP detection, a 4-ATP SERS signal is detected at 1132 cm−1 with good specificity and sensitivity and a limit of detection of 27 pM.
By combining the advantages offered by an ordered nanoporous template and core-shell SERS nanotags, Chen et al. have recently reported multiplexed SERS-based vertical flow immunoassay system for detection of CRP along with three other inflammation biomarkers, interleukin-6 (IL-6), serum amyloid A (SAA), and procalcitonin (PCT) [77]. As presented in Fig. 8 , the sensor substrate is composed of a nanoporous anodic aluminum oxide (AAO) with 350 nm pore size with double through nanochannels functionalized with multiplex capture antibodies, the resulting structure allowing the analytes to penetrate through the vertical pores. The second component, the Raman dyes encoded Au-Ag core-shell SERS nanotags are used as labels for biomarker detection. The detection assay was performed by dropping the sample and the functionalized SERS nanotags onto the AAO substrate, followed by washing and measuring the SERS signal of nanotags captured in the nanochannels. As also sustained by theoretical analysis, the electromagnetic field of the encoded core-shell SERS nanotags is enhanced in the presence of AAO template. Furthermore, the vertical channels improve the reaction binding kinetics and the washing efficiency. Consequently, the four biomarkers were detected with LODs of 53.4, 4.72, 48.3, and 7.53 fg/ml for the simultaneous detection of CRP, IL-6, SAA, and PCT, respectively covering in all cases a linear dynamic range of at least five orders of magnitude.
Fig. 8.
Schematic illustration of nanoporous AAO-based multiplex vertical flow assay (VFA) for the detection of four inflammatory biomarkers with Raman dyes encoded core-shell SERS nanotags. Characteristic Raman peaks of NBA at 593 cm−1, 4-MBA at 1075 cm−1, DNTB at 1341 cm−1, and MB at 1621 cm−1, are used to encode CRP, IL-6, SAA, and PCT, respectively.
Reprinted with permission from ref [77]. Copyright (2020) John Wiley & Son.
An innovative PNP architecture, ultraflat and ultraclean single-crystalline Au nanoplates with no grain boundaries, has been reported by Hwang et al. for completely specific and attomolar detection of CRP [78]. The high sensing performance of the nanoplatforms relies on the carefully optimized immobilization of the anti-CRP onto Au nanoplate in order to maximize the binding capacity for CRP and to eliminate the nonspecific binding of other proteins or Au NPs. Anti-CRP-attached Rhodamine B isothiocyanate (RBITC) tagged AuNP served as a probe in CRP detection assay providing SERS signal only when the NPs-on-nanoplate architecture is formed upon specific binding with CRP. The optimized anti-CRP-immobilized Au nanoplate achieved a LOD of 10−17 M in CRP detection.
6. Conclusions and perspectives
The literature reports a large number of plasmon-assisted biosensors able to quantify CRP with the aim to improve the performances compared to the existing analytical methods, regarding sensibility, accuracy, rapidity and portability. Aggregation-based colorimetric assays are simple, rapid and the color change of the colloidal solution can be also observed with the naked eye. This strategy was used both for the specific detection of a target biomarker, both for multiplexed detection and signal amplification in ELISA immunoassays. The best LOD for CRP as low as 32 pg/ml using colorimetric assays was reached by Park et al. using AuNPs conjugated with 6X-histidine peptide as colorimetric transducers, and nickel-horseradish peroxidase as enhancer of the colorimetric signal in ELISA [36]. For LSPR-based detection of CRP various nanostructures have been also developed with increased RI sensitivity in order to enhance the sensibility of the biosensor. The lowest LOD reported using LSPR sensor for CRP detection was of 100 ag/ml, achieved by Yeom et al., using Au deposited nanostructured anodicaluminum oxide substrates and applying the sandwich structure to increase the sensitivity of the sensor [47]. PNPs were also successfully used as enhancers of the fluorescence signal leading to the development of fluorescent-based assays with very low LOD for CRP detection. For instance, the detection of a single CRP molecule (33.3 zM) was obtained using a 20 nm Au-nanopatterned biochip proposed by Heo et. al. [54]. Different plasmonic nanostructured optimized for high SERS enhancement were also widely explored in the detection of CRP. Among them, the best LOD of 10−17 M was yielded by Hwang et al. using a completely specific detection nanoplatform based on ultraflat and ultraclean single-crystalline Au nanoplates with carefully optimized immobilization of the anti-CRP on their surface.
Portability and rapidity of CRP detection assays have particular importance in the management of life-threatening infections, therefore special attention has been accorded to the development of POC testing devices using PNPs. Such POC assays seem to be promising for clinical evaluation from real samples, some of them providing equivalent performance to gold-standard laboratory assays in considerably faster time. However, some of these devices still require qualified personnel to perform the detection assay. A lot of research is now dedicated to overcome this limitation and some progress is achieved in the development of simple, fast, easy-to-use and low-cost assays for clinical diagnosis in resource-limited areas. For translation of these devices into clinical application, user friendly software interfaces able to accurately correlate the optical signal with the CRP concentration are also needed to be developed, which could facilitate introduction of these devices in medical offices and clinics. We strongly believe that the current PNPs-based sensing strategies and portable devices for CRP detection will open new opportunities for easy, fast, accurate and real-time diagnosis and monitoring of inflammatory infections.
CRediT authorship contribution statement
Timea Nagy-Simon: Conceptualization, Writing – original draft, Writing – review & editing. Alexandru-Milentie Hada: Writing – original draft. Sorina Suarasan: Writing – original draft. Monica Potara: Conceptualization, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI – UEFISCDI, project number PN-III-P4-ID-PCE-2020-1592, within PNCDI III. This work is dedicated to honor Prof. Dr. Simion Astilean on the occasion of his birthday. The authors thank Prof. Dr. Simion Astilean for introducing them in the field of nanotechnology, for his valuable scientific guidance and constructive criticism.
References
- 1.Ansar W., Ghosh S. C-reactive protein and the biology of disease. Immunol. Res. 2013;56:131–142. doi: 10.1007/s12026-013-8384-0. [DOI] [PubMed] [Google Scholar]
- 2.Garg M., Sharma A.L., Singh S. Advancement in biosensors for inflammatory biomarkers of SARS-CoV-2 during 2019–2020. Biosens. Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ngwa D.N., Agrawal A. Structure-Function Relationships of C-Reactive Protein in Bacterial Infection. Front. Immunol. 2019:10. doi: 10.3389/fimmu.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurlimann J., Thorbecke G.J., Hochwald G.M. The liver as the site of C-reactive protein formation. J. Exp. Med. 1966;123:365–378. doi: 10.1084/jem.123.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clyne B., Olshaker J.S. The C-reactive protein. J. Emerg. Med. 1999;17:1019–1025. doi: 10.1016/s0736-4679(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 6.T.A. Pearson, G.A. Mensah, R.W. Alexander, J.L. Anderson, R.O. Cannon, M. Criqui, Y.Y. Fadl, S.P. Fortmann, Y. Hong, G.L. Myers, N. Rifai, S.C. Smith, K. Taubert, R.P. Tracy, F. Vinicor, Markers of Inflammation and Cardiovascular Disease, Circulation. 107 (2003) 499–511. https://doi.org/10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed]
- 7.Wang G., Wu C., Zhang Q., Wu F., Yu B., Lv J., Li Y., Li T., Zhang S., Wu C., Wu G., Zhong Y. C-Reactive Protein Level May Predict the Risk of COVID-19 Aggravation. Open Forum Infect. Dis. 2020;7:ofaa153. doi: 10.1093/ofid/ofaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan C., Huang Y., Shi F., Tan K., Ma Q., Chen Y., Jiang X., Li X. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J. Med. Virol. 2020;92:856–862. doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain C.M., Kailasa S.K. Elsevier; Amsterdam: 2021. Handbook of nanomaterials for sensing applications. [Google Scholar]
- 11.Anker J.N., Hall W.P., Lyandres O., Shah N.C., Zhao J., Van Duyne R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008;7:442–453. doi: 10.1038/nmat2162. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Xianyu Y., Jiang X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017;50:310–319. doi: 10.1021/acs.accounts.6b00506. [DOI] [PubMed] [Google Scholar]
- 13.Jain P.K., Lee K.S., El-Sayed I.H., El-Sayed M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B. 2006;110:7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 14.Khlebtsov N.G., Dykman L.A. Optical properties and biomedical applications of plasmonic nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2010;111:1–35. doi: 10.1016/j.jqsrt.2009.07.012. [DOI] [Google Scholar]
- 15.Petryayeva E., Krull U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta. 2011;706:8–24. doi: 10.1016/j.aca.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Mayer K.M., Hafner J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011;111:3828–3857. doi: 10.1021/cr100313v. [DOI] [PubMed] [Google Scholar]
- 17.Yu H., Peng Y., Yang Y., Li Z.-Y. Plasmon-enhanced light–matter interactions and applications. Npj Comput. Mater. 2019;5:1–14. doi: 10.1038/s41524-019-0184-1. [DOI] [Google Scholar]
- 18.Geddes C.D., Lakowicz J.R. Metal-Enhanced Fluorescence. J. Fluoresc. 2002;12:121–129. doi: 10.1023/A:1016875709579. [DOI] [Google Scholar]
- 19.Cialla D., März A., Böhme R., Theil F., Weber K., Schmitt M., Popp J. Surface-enhanced Raman spectroscopy (SERS): progress and trends. Anal. Bioanal. Chem. 2012;403:27–54. doi: 10.1007/s00216-011-5631-x. [DOI] [PubMed] [Google Scholar]
- 20.Tang L., Li J. Plasmon-Based Colorimetric Nanosensors for Ultrasensitive Molecular Diagnostics. ACS Sens. 2017;2:857–875. doi: 10.1021/acssensors.7b00282. [DOI] [PubMed] [Google Scholar]
- 21.Jazayeri M.H., Aghaie T., Avan A., Vatankhah A., Ghaffari M.R.S. Colorimetric detection based on gold nano particles (GNPs): An easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion) Sens. Bio-Sens. Res. 2018;20:1–8. doi: 10.1016/j.sbsr.2018.05.002. [DOI] [Google Scholar]
- 22.Yu L., Song Z., Peng J., Yang M., Zhi H., He H. Progress of gold nanomaterials for colorimetric sensing based on different strategies. TrAC Trends Anal. Chem. 2020;127 doi: 10.1016/j.trac.2020.115880. [DOI] [Google Scholar]
- 23.Ghosh S.K., Pal T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007;107:4797–4862. doi: 10.1021/cr0680282. [DOI] [PubMed] [Google Scholar]
- 24.Hu J., Wang Z., Li J. Gold Nanoparticles With Special Shapes: Controlled Synthesis, Surface-enhanced Raman Scattering, and The Application in Biodetection. Sensors. 2007;7:3299–3311. doi: 10.3390/s7123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howes P.D., Rana S., Stevens M.M. Plasmonic nanomaterials for biodiagnostics. Chem Soc Rev. 2014;43:3835–3853. doi: 10.1039/C3CS60346F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim W.Q., Gao Z. Plasmonic nanoparticles in biomedicine. Nano Today. 2016;11:168–188. doi: 10.1016/j.nantod.2016.02.002. [DOI] [Google Scholar]
- 27.Zhou W., Gao X., Liu D., Chen X. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015;115:10575–10636. doi: 10.1021/acs.chemrev.5b00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raj V., Sreenivasan K. Selective detection and estimation of C-reactive protein in serum using surface-functionalized gold nano-particles. Anal. Chim. Acta. 2010;662:186–192. doi: 10.1016/j.aca.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Mackiewicz M.R., Hodges H.L., Reed S.M. C-Reactive Protein Induced Rearrangement of Phosphatidylcholine on Nanoparticle Mimics of Lipoprotein Particles. J. Phys. Chem. B. 2010;114:5556–5562. doi: 10.1021/jp911617q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwasaki Y., Kimura T., Orisaka M., Kawasaki H., Goda T., Yusa S. Label-free detection of C-reactive protein using highly dispersible gold nanoparticles synthesized by reducible biomimetic block copolymers. Chem. Commun. 2014;50:5656–5658. doi: 10.1039/C4CC01855A. [DOI] [PubMed] [Google Scholar]
- 31.Byun J.-Y., Shin Y.-B., Kim D.-M., Kim M.-G. A colorimetric homogeneous immunoassay system for the C-reactive protein. Analyst. 2013;138:1538–1543. doi: 10.1039/C3AN36592A. [DOI] [PubMed] [Google Scholar]
- 32.António M., Ferreira R., Vitorino R., Daniel-da-Silva A.L. A simple aptamer-based colorimetric assay for rapid detection of C-reactive protein using gold nanoparticles. Talanta. 2020;214 doi: 10.1016/j.talanta.2020.120868. [DOI] [PubMed] [Google Scholar]
- 33.Yu R.-J., Ma W., Liu X.-Y., Jin H.-Y., Han H.-X., Wang H.-Y., Tian H., Long Y.-T. Metal-linked Immunosorbent Assay (MeLISA): the Enzyme-Free Alternative to ELISA for Biomarker Detection in Serum. Theranostics. 2016;6:1732–1739. doi: 10.7150/thno.16129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ran B., Zheng W., Dong M., Xianyu Y., Chen Y., Wu J., Qian Z., Jiang X. Peptide-Mediated Controllable Cross-Linking of Gold Nanoparticles for Immunoassays with Tunable Detection Range. Anal. Chem. 2018;90:8234–8240. doi: 10.1021/acs.analchem.8b01760. [DOI] [PubMed] [Google Scholar]
- 35.Dong H., Huang L., Liu D., Zhou L., Wu Z., Cheng Z., Liu H., Mao H. Robust and multiplexed colorimetric immunoassay for cardiovascular disease biomarkers detection in serum with high specificity. Microchem. J. 2020;152 doi: 10.1016/j.microc.2019.104334. [DOI] [Google Scholar]
- 36.Siddiqui M.F., Khan Z.A., Park S. Detection of C-Reactive Protein Using Histag-HRP Functionalized Nanoconjugate with Signal Amplified Immunoassay. Nanomaterials. 2020;10:1240. doi: 10.3390/nano10061240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong M., Wu J., Ma Z., Peretz-Soroka H., Zhang M., Komenda P., Tangri N., Liu Y., Rigatto C., Lin F. Rapid and Low-Cost CRP Measurement by Integrating a Paper-Based Microfluidic Immunoassay with Smartphone (CRP-Chip) Sensors. 2017;17:684. doi: 10.3390/s17040684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell S.M., de la Rica R. Paper transducers to detect plasmon variations in colorimetric nanoparticle biosensors. Sens. Actuators B Chem. 2018;270:327–332. doi: 10.1016/j.snb.2018.05.052. [DOI] [Google Scholar]
- 39.Chen H., Shao L., Woo K.C., Ming T., Lin H.-Q., Wang J. Shape-Dependent Refractive Index Sensitivities of Gold Nanocrystals with the Same Plasmon Resonance Wavelength. J. Phys. Chem. C. 2009;113:17691–17697. doi: 10.1021/jp907413n. [DOI] [Google Scholar]
- 40.Chen H., Kou X., Yang Z., Ni W., Wang J. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir. 2008;24:5233–5237. doi: 10.1021/la800305j. [DOI] [PubMed] [Google Scholar]
- 41.Charles D.E., Aherne D., Gara M., Ledwith D.M., Gun'ko Y.K., Kelly J.M., Blau W.J., Brennan-Fournet M.E. Versatile Solution Phase Triangular Silver Nanoplates for Highly Sensitive Plasmon Resonance Sensing. ACS Nano. 2010;4:55–64. doi: 10.1021/nn9016235. [DOI] [PubMed] [Google Scholar]
- 42.Fournet M.E.B., Ledwith D., Voisin M., Cunningham S., Fournet P., Charles D., Aherne D., Blau W.J., Kelly J.M. IEEE-NANO. 2009. High surface plasmon resonant sensitive silver nanoplates for detection of C-reactive protein, in: 2009 9th IEEE Conf. Nanotechnol; pp. 866–869. [Google Scholar]
- 43.Zhang Y., Charles D.E., Ledwith D.M., Aherne D., Cunningham S., Voisin M., Blau W.J., Gun'ko Y.K., Kelly J.M., Brennan-Fournet M.E. Wash-free highly sensitive detection of C-reactive protein using gold derivatised triangular silver nanoplates. RSC Adv. 2014;4:29022–29031. doi: 10.1039/C4RA04958F. [DOI] [Google Scholar]
- 44.Byun J.-Y., Shin Y.-B., Li T., Park J.-H., Kim D.-M., Choi D.-H., Kim M.-G. The use of an engineered single chain variable fragment in a localized surface plasmon resonance method for analysis of the C-reactive protein. Chem. Commun. Camb. Engl. 2013;49:9497–9499. doi: 10.1039/c3cc45046e. [DOI] [PubMed] [Google Scholar]
- 45.Garifullina A., Shen A.Q. Optimized Immobilization of Biomolecules on Nonspherical Gold Nanostructures for Efficient Localized Surface Plasmon Resonance Biosensing. Anal. Chem. 2019;91:15090–15098. doi: 10.1021/acs.analchem.9b03780. [DOI] [PubMed] [Google Scholar]
- 46.S.-J. Ha, J.-H. Park, J.-Y. Byun, Y.-D. Ahn, M.-G. Kim, A localized surface plasmon resonance (LSPR) immunosensor for CRP detection using 4-chloro-1-naphtol (4-CN) precipitation, in: J. Choo, S.-H. Park (Eds.), Jeju, Korea, Republic of, 2017: p. 103240E. https://doi.org/10.1117/12.2271397.
- 47.Yeom S.-H., Han M.-E., Kang B.-H., Kim K.-J., Yuan H., Eum N.-S., Kang S.-W. Enhancement of the sensitivity of LSPR-based CRP immunosensors by Au nanoparticle antibody conjugation. Sens. Actuators B Chem. 2013;177:376–383. doi: 10.1016/j.snb.2012.10.099. [DOI] [Google Scholar]
- 48.Oh S.Y., Heo N.S., Bajpai V.K., Jang S.-C., Ok G., Cho Y., Huh Y.S. Development of a Cuvette-Based LSPR Sensor Chip Using a Plasmonically Active Transparent Strip. Front. Bioeng. Biotechnol. 2019;7 doi: 10.3389/fbioe.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.James-Pemberton P., Łapińska U., Helliwell M., Olkhov R.V., Hedaux O.J., Hyde C.J., Shaw A.M. Accuracy and precision analysis for a biophotonic assay of C-reactive protein. Analyst. 2020;145:2751–2757. doi: 10.1039/C9AN02516B. [DOI] [PubMed] [Google Scholar]
- 50.Belushkin A., Yesilkoy F., González-López J.J., Ruiz-Rodríguez J.C., Ferrer R., Fàbrega A., Altug H. Rapid and Digital Detection of Inflammatory Biomarkers Enabled by a Novel Portable Nanoplasmonic Imager. Small. 2020;16 doi: 10.1002/smll.201906108. [DOI] [PubMed] [Google Scholar]
- 51.Strianese M., Staiano M., Ruggiero G., Labella T., Pellecchia C., D'Auria S. In: Fluorescence-Based Biosensors. Bujalowski W.M., editor; Bujalowski W.M., editor. Humana Press; Totowa, NJ: 2012. pp. 193–216. (Spectrosc. Methods Anal.). [DOI] [PubMed] [Google Scholar]
- 52.Jeong Y., Kook Y.-M., Lee K., Koh W.-G. Metal enhanced fluorescence (MEF) for biosensors: General approaches and a review of recent developments. Biosens. Bioelectron. 2018;111:102–116. doi: 10.1016/j.bios.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Hong B., Tang L., Ren Y., Kang K.A. In: Oxyg. Transp. Tissue XXVIII. Maguire D.J., Bruley D.F., Harrison D.K., editors. Springer US; Boston, MA: 2007. Real-Time, Automated, Fluorophore Mediated Multi-Cardiac Marker Biosensing System with Nano-Metallic Particle Reagent; pp. 23–29. [DOI] [PubMed] [Google Scholar]
- 54.Heo Yunmi, Lee Seung-Ah, Lee Sang-Won, Kang Seong Ho. Single C-Reactive Protein Molecule Detection on a Gold-Nanopatterned Chip Based on Total Internal Reflection Fluorescence. Bull. Korean Chem. Soc. 2013;34:2725–2730. doi: 10.5012/BKCS.2013.34.9.2725. [DOI] [Google Scholar]
- 55.Zhang Y., Keegan G.L., Stranik O., Brennan-Fournet M.E., McDonagh C. Highly sensitive C-reactive protein (CRP) assay using metal-enhanced fluorescence (MEF) J. Nanoparticle Res. 2015;17:326. doi: 10.1007/s11051-015-3128-9. [DOI] [Google Scholar]
- 56.Zhao L.-J., Yu R.-J., Ma W., Han H.-X., Tian H., Qian R.-C., Long Y.-T. Sensitive detection of protein biomarkers using silver nanoparticles enhanced immunofluorescence assay. Theranostics. 2017;7:876–883. doi: 10.7150/thno.17575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuura R., Tawa K., Kitayama Y., Takeuchi T. A plasmonic chip-based bio/chemical hybrid sensing system for the highly sensitive detection of C-reactive protein. Chem. Commun. 2016;52:3883–3886. doi: 10.1039/C5CC07868G. [DOI] [PubMed] [Google Scholar]
- 58.S. Ghosh, A. Metlushko, S. Chaudhry, M. Dutta, M.A. Stroscio, Detection of C-Reactive Protein using network-deployable DNA aptamer based optical nanosensor, in: 2019 IEEE EMBS Int. Conf. Biomed. Health Inform. BHI, IEEE, Chicago, IL, USA, 2019: pp. 1–4. https://doi.org/10.1109/BHI.2019.8834479.
- 59.Bravin C., Amendola V. Wide range detection of C-Reactive protein with a homogeneous immunofluorimetric assay based on cooperative fluorescence quenching assisted by gold nanoparticles. Biosens. Bioelectron. 2020;169 doi: 10.1016/j.bios.2020.112591. [DOI] [PubMed] [Google Scholar]
- 60.Xing Y., Gao Q., Zhang Y., Ma L., Loh K.Y., Peng M., Chen C., Cui Y. The improved sensitive detection of C-reactive protein based on the chemiluminescence immunoassay by employing monodispersed PAA-Au/Fe 3 O 4 nanoparticles and zwitterionic glycerophosphoryl choline. J. Mater. Chem. B. 2017;5:3919–3926. doi: 10.1039/C7TB00637C. [DOI] [PubMed] [Google Scholar]
- 61.Zong C., Zhang D., Jiang F., Yang H., Liu S., Li P. Metal-enhanced chemiluminescence detection of C-reaction protein based on silver nanoparticle hybrid probes. Talanta. 2019;199:164–169. doi: 10.1016/j.talanta.2019.02.060. [DOI] [PubMed] [Google Scholar]
- 62.Lombardi J.R., Birke R.L. A Unified View of Surface-Enhanced Raman Scattering. Acc. Chem. Res. 2009;42:734–742. doi: 10.1021/ar800249y. [DOI] [PubMed] [Google Scholar]
- 63.Kneipp K., Kneipp H., Kartha V.B., Manoharan R., Deinum G., Itzkan I., Dasari R.R., Feld M.S. Detection and identification of a single DNA base molecule using surface-enhanced Raman scattering (SERS) Phys. Rev. E. 1998;57:R6281–R6284. doi: 10.1103/PhysRevE.57.R6281. [DOI] [Google Scholar]
- 64.Campbell F.M., Ingram A., Monaghan P., Cooper J., Sattar N., Eckersall P.D., Graham D. SERRS immunoassay for quantitative human CRP analysis. Analyst. 2008;133:1355–1357. doi: 10.1039/B808087A. [DOI] [PubMed] [Google Scholar]
- 65.Guo W., Hu Y., Wei H. Enzymatically activated reduction-caged SERS reporters for versatile bioassays. Analyst. 2017;142:2322–2326. doi: 10.1039/C7AN00552K. [DOI] [PubMed] [Google Scholar]
- 66.Sloan-Dennison S., Laing S., Shand N.C., Graham D., Faulds K. A novel nanozyme assay utilising the catalytic activity of silver nanoparticles and SERRS. Analyst. 2017;142:2484–2490. doi: 10.1039/C7AN00887B. [DOI] [PubMed] [Google Scholar]
- 67.Benford M.E., Wang M., Kameoka J., Coté G.L. Detection of cardiac biomarkers exploiting surface enhanced Raman scattering (SERS) using a nanofluidic channel based biosensor towards coronary point-of-care diagnostics. Plasmon. Biol. Med. VI, International Society for Optics and Photonics. 2009 doi: 10.1117/12.809661. [DOI] [Google Scholar]
- 68.Benford M., Wang M., Kameoka J., Good T., Cote G. Functionalized nanoparticles for measurement of biomarkers using a SERS nanochannel platform. Plasmon. Biol. Med. VII, International Society for Optics and Photonics. 2010 doi: 10.1117/12.842486. [DOI] [Google Scholar]
- 69.Kim H., Kim E., Choi E., Baek C.S., Song B., Cho C.-H., Jeong S.W. Label-free C-reactive protein SERS detection with silver nanoparticle aggregates. RSC Adv. 2015;5:34720–34729. doi: 10.1039/C5RA00040H. [DOI] [Google Scholar]
- 70.Li J., Zhu Z., Zhu B., Ma Y., Lin B., Liu R., Song Y., Lin H., Tu S., Yang C. Surface-Enhanced Raman Scattering Active Plasmonic Nanoparticles with Ultrasmall Interior Nanogap for Multiplex Quantitative Detection and Cancer Cell Imaging. Anal. Chem. 2016;88:7828–7836. doi: 10.1021/acs.analchem.6b01867. [DOI] [PubMed] [Google Scholar]
- 71.Liu B., Ni H., Zhang D., Wang D., Fu D., Chen H., Gu Z., Zhao X. Ultrasensitive Detection of Protein with Wide Linear Dynamic Range Based on Core-Shell SERS Nanotags and Photonic Crystal Beads. ACS Sens. 2017;2:1035–1043. doi: 10.1021/acssensors.7b00310. [DOI] [PubMed] [Google Scholar]
- 72.Rong Z., Xiao R., Xing S., Xiong G., Yu Z., Wang L., Jia X., Wang K., Cong Y., Wang S. SERS-based lateral flow assay for quantitative detection of C-reactive protein as an early bio-indicator of a radiation-induced inflammatory response in nonhuman primates. The Analyst. 2018;143:2115–2121. doi: 10.1039/c8an00160j. [DOI] [PubMed] [Google Scholar]
- 73.Wang S., Luo J., He Y., Chai Y., Yuan R., Yang X. Combining Porous Magnetic Ni@C Nanospheres and CaCO3 Microcapsule as Surface-Enhanced Raman Spectroscopy Sensing Platform for Hypersensitive C-Reactive Protein Detection. ACS Appl. Mater. Interfaces. 2018;10:33707–33712. doi: 10.1021/acsami.8b13061. [DOI] [PubMed] [Google Scholar]
- 74.Liu X., Yang X., Li K., Liu H., Xiao R., Wang W., Wang C., Wang S. Fe3O4@Au SERS tags-based lateral flow assay for simultaneous detection of serum amyloid A and C-reactive protein in unprocessed blood sample. Sens. Actuators B Chem. 2020;320 doi: 10.1016/j.snb.2020.128350. [DOI] [Google Scholar]
- 75.Hu Z., Zhou X., Duan J., Wu X., Wu J., Zhang P., Liang W., Guo J., Cai H., Sun P., Zhou H., Jiang Z. Aptamer-based novel Ag-coated magnetic recognition and SERS nanotags with interior nanogap biosensor for ultrasensitive detection of protein biomarker. Sens. Actuators B Chem. 2021;334 doi: 10.1016/j.snb.2021.129640. [DOI] [Google Scholar]
- 76.Nguyen A.H., Shin Y., Sim S.J. Development of SERS substrate using phage-based magnetic template for triplex assay in sepsis diagnosis. Biosens. Bioelectron. 2016;85:522–528. doi: 10.1016/j.bios.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 77.Chen R., Du X., Cui Y., Zhang X., Ge Q., Dong J., Zhao X. Vertical Flow Assay for Inflammatory Biomarkers Based on Nanofluidic Channel Array and SERS Nanotags. Small. 2020;16 doi: 10.1002/smll.202002801. [DOI] [PubMed] [Google Scholar]
- 78.Hwang A., Kim E., Moon J., Lee H., Lee M., Jeong J., Lim E.-K., Jung J., Kang T., Kim B. Atomically Flat Au Nanoplate Platforms Enable Ultraspecific Attomolar Detection of Protein Biomarkers. ACS Appl. Mater. Interfaces. 2019;11:18960–18967. doi: 10.1021/acsami.9b04363. [DOI] [PubMed] [Google Scholar]