OBJECTIVES:
Severe cases of COVID-19 pneumonia can lead to acute respiratory distress syndrome (ARDS). Release of interleukin (IL)-33, an epithelial-derived alarmin, and IL-33/ST2 pathway activation are linked with ARDS development in other viral infections. IL-22, a cytokine that modulates innate immunity through multiple regenerative and protective mechanisms in lung epithelial cells, is reduced in patients with ARDS. This study aimed to evaluate safety and efficacy of astegolimab, a human immunoglobulin G2 monoclonal antibody that selectively inhibits the IL-33 receptor, ST2, or efmarodocokin alfa, a human IL-22 fusion protein that activates IL-22 signaling, for treatment of severe COVID-19 pneumonia.
DESIGN:
Phase 2, double-blind, placebo-controlled study (COVID-astegolimab-IL).
SETTING:
Hospitals.
PATIENTS:
Hospitalized adults with severe COVID-19 pneumonia.
INTERVENTIONS:
Patients were randomized to receive IV astegolimab, efmarodocokin alfa, or placebo, plus standard of care. The primary endpoint was time to recovery, defined as time to a score of 1 or 2 on a 7-category ordinal scale by day 28.
MEASUREMENTS AND MAIN RESULTS:
The study randomized 396 patients. Median time to recovery was 11 days (hazard ratio [HR], 1.01 d; p = 0.93) and 10 days (HR, 1.15 d; p = 0.38) for astegolimab and efmarodocokin alfa, respectively, versus 10 days for placebo. Key secondary endpoints (improved recovery, mortality, or prevention of worsening) showed no treatment benefits. No new safety signals were observed and adverse events were similar across treatment arms. Biomarkers demonstrated that both drugs were pharmacologically active.
CONCLUSIONS:
Treatment with astegolimab or efmarodocokin alfa did not improve time to recovery in patients with severe COVID-19 pneumonia.
Keywords: acute respiratory distress syndrome, biomarkers, COVID-19, interleukin-22, interleukin-33
KEY POINTS.
Question: We examined whether hospitalized patients with severe COVID-19 pneumonia benefit from interleukin (IL)-33 receptor blockade to reduce hyperinflammation (astegolimab) or IL-22 pathway activation to prevent/repair lung damage (efmarodocokin alfa).
Findings: This randomized, placebo-controlled phase 2 trial demonstrated that neither drug improved time to recovery in hospitalized COVID-19 patients versus placebo (median times: astegolimab, 11 d; efmarodocokin alfa, 10 d; placebo, 10 d). Neither drug showed new safety signals; both drugs modulated pathway-specific biomarkers.
Meaning: Despite lack of clinical benefit, both drugs were safe and pharmacologically active.
In 2020, COVID-19 was the third leading cause of death in the United States. Most COVID-19–associated deaths are due to severe interstitial pneumonia, which progresses to acute respiratory distress syndrome (ARDS) and hypoxemic respiratory failure. ARDS, characterized by increased epithelial and endothelial permeability leading to alveolar edema, has been observed in 16–42% of patients with severe COVID-19 (1–5). Hyperinflammatory responses, including elevated pro-inflammatory cytokines, are associated with increased mortality in patients with COVID-19 (4). COVID-19 treatment includes direct antiviral therapeutics and anti-inflammatory agents.
Interleukin (IL)-33, an alarmin released upon epithelial injury in response to allergens, irritants, and infections in the lung (6), binds the suppression of tumorigenicity 2 (ST2) (IL-1 receptor-like 1) receptor on multiple immune cell types driving pulmonary inflammation, which triggers downstream cytokine release. Patients with ARDS have elevated serum IL-33 (7). IL-33 production increases with disease severity (8, 9) and independently predicts poor outcomes in COVID-19 (10). We hypothesized that astegolimab (MSTT1041A), a fully human immunoglobulin (Ig) G2 monoclonal antibody that binds ST2, blocks IL-33 signaling, and significantly reduces asthma exacerbations in patients with severe asthma (11), may reduce hyperinflammation during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
IL-22, an IL-10 family cytokine, acts directly on epithelial tissue. By working through regenerative and protective mechanisms, IL-22 may protect and repair lung epithelial tissue from ventilation-induced damage (12) and acute lung injury (13) following viral infections (14, 15). Patients with ARDS show reduced IL-22 levels in bronchoalveolar lavage fluid compared with mechanically ventilated patients without lung injury (14). Based on this, we hypothesized that efmarodocokin alfa (UTTR1147A), a fusion of human IL-22 and the IgG4 crystallizable fragment (16), may promote lung recovery in SARS-CoV-2 infection. The COVID-astegolimab-interleukin (COVASTIL) trial independently investigated the reduction in hyperinflammation via IL-33 blockade with astegolimab and prevention/repair of lung damage by IL-22 pathway activation with efmarodocokin alfa in patients with severe COVID-19 pneumonia.
MATERIALS AND METHODS
Trial Design and Oversight
This phase 2, randomized, double-blind, placebo-controlled, multicenter study assessed efficacy and safety of the investigational drugs, astegolimab and efmarodocokin alfa, compared with their respective placebos, in patients hospitalized with severe COVID-19 pneumonia (ClinicalTrials.gov: NCT04386616). Permitted standard-of-care (SOC) COVID-19 therapies included antiviral therapy (e.g., remdesivir), host-directed therapy (e.g., tocilizumab), systemic corticosteroids, and anticoagulants. Patients were recruited from the United States, Brazil, Mexico, and Spain.
The study consisted of a screening period followed by randomization (Fig. 1A); on day 1, patients received either one IV infusion of 700 mg astegolimab, 90 μg/kg efmarodocokin alfa, or matching placebos (2 and 5 mL vials of a sterile, clear solution to match astegolimab and efmarodocokin alfa, respectively, with diluent for efmarodocokin alfa supplied by Genentech for the matching placebo). If the patient remained hospitalized and on supplemental oxygen, a second dose of 350 mg astegolimab, 90 μg/kg efmarodocokin alfa, or matching placebo was given on day 15. The primary endpoint was evaluated through day 28; the study completion visit was day 60.
Figure 1.
COVID-astegolimab-interleukin study design and patient disposition. A, Study design. The second dose on day 15 (open triangles) was only given if the patient remained hospitalized and required supplemental oxygen. B, Patient disposition. aMatching placebo (PBO) groups for astegolimab and efmarodocokin alfa were pooled for all analyses. mITT = modified intent-to-treat, SOC = standard of care.
Genentech‚ Inc. (South San Francisco‚ CA) developed the protocol and conducted the study in full conformance with the International Council for Harmonisation (ICH) E6 guideline for Good Clinical Practice, the principles of the Declaration of Helsinki, or the laws/regulations of the country where the research occurred, whichever provided better protection to the individual. The study complied with the ICH E2A guideline. Ethics Committees for each site (Supplementary Appendix, http://links.lww.com/CCM/H235) approved the protocol (Study number: GA42469; title: A phase II, randomized, double-blind, placebo-controlled, multicenter study to evaluate the safety and efficacy of MSTT1041A or UTTR1147A in patients with severe COVID-19 pneumonia) on April 24, 2020 (United States), July 8, 2020 (Mexico; local ethics committee approved at the first site on June 29, 2020), July 13, 2020 (Spain), and July 20, 2020 (Brazil). Patients, or their legally authorized representatives, provided written, informed consent before study procedures.
Patients
Eligible patients were aged 18 years or older, hospitalized with COVID-19 pneumonia, verified by positive polymerase chain reaction assay, chest radiograph or computed tomography scan, and having peripheral capillary oxygen saturation (Spo2) less than or equal to 93% or Pao2/Fio2 less than or equal to 300 mm Hg or a requirement for supplemental oxygen to maintain Spo2 greater than 93%. Key exclusion criteria included likely progression to death within 24 hours; clinical evidence of active or unstable cardiovascular disease; and Janus kinase inhibitor use within 30 days or five drug elimination half-lives before screening (full eligibility criteria, Supplementary Appendix, http://links.lww.com/CCM/H235).
Randomization and Blinding
Patients were assigned via an interactive voice or web-based response system to treatment groups receiving astegolimab, efmarodocokin alfa, or their matching placebos at a 2:2:1:1 ratio using permuted-block randomization. Randomization was stratified by invasive mechanical ventilation use and region. The proportion of patients needing invasive mechanical ventilation was capped at ~25%. See Supplementary Appendix (http://links.lww.com/CCM/H235) for details on blinding.
Outcomes and Assessments
The primary efficacy endpoint was time to recovery, defined as time to a score of 1 or 2 on a 7-category ordinal scale (whichever occurred first): 1, discharged (or “ready for discharge” as evidenced by normal body temperature and respiratory rate, and stable oxygen saturation on ambient air or less than or equal to 2 L supplemental oxygen); 2, non-ICU hospital ward (or “ready for hospital ward”) not requiring supplemental oxygen; 3, non-ICU hospital ward (or “ready for hospital ward”) requiring supplemental oxygen; 4, ICU or non-ICU hospital ward, requiring noninvasive ventilation or high-flow oxygen; 5, ICU, requiring intubation and mechanical ventilation; 6, ICU, requiring extracorporeal membrane oxygenation (ECMO) or mechanical ventilation and additional organ support (e.g., vasopressors, renal replacement therapy); 7, death (17). During the trial, the original primary endpoint, “Clinical status assessed using a 7-category ordinal scale at day 28” was changed because prior trials for COVID-19 pneumonia indicated that time to recovery was more clinically meaningful (18). The original primary endpoint is now a secondary endpoint (Table S2, http://links.lww.com/CCM/H235). Key secondary efficacy endpoints included day 14 and day 28 mortality, time to hospital discharge or “ready for discharge,” ventilator-free days, proportion of patients alive and free of respiratory failure at day 28, rate of invasive mechanical ventilation or ECMO‚ and rate and duration of ICU stay. See Supplementary Appendix (http://links.lww.com/CCM/H235) for additional secondary endpoints. Exploratory endpoints included changes in pharmacodynamic biomarkers (circulating soluble ST2 [sST2], regenerating islet-derived protein 3A [REG3A], and C-reactive protein [CRP]) to demonstrate pharmacological activity and target engagement.
Safety assessments included frequency and severity (using Common Terminology Criteria for Adverse Events) of adverse events (AEs) and serious adverse events (SAEs), changes in vital signs, electrocardiograms, and clinical laboratory results. Pharmacokinetic assessments included serum concentration measurements of astegolimab and efmarodocokin alfa.
Statistical Methods
Efficacy analyses were conducted on the modified intent-to-treat (mITT) population (all patients who received at least one dose of study drug), with patients grouped according to treatment assigned at randomization. Placebo-treated patients were pooled for outcome analyses. Time to recovery was analyzed using the stratified log-rank test, adjusting for stratification factors. Hazard ratios (HRs) comparing astegolimab or efmarodocokin alfa with placebo, respectively, adjusting for stratification factors, were estimated using a Cox proportional hazards regression model. For time-to-recovery and time-to-improvement endpoints, patients who did not recover/improve or died before day 28 were censored at day 29.
The planned sample size of 390 patients provided ~80% power using a log-rank test to detect a 7-day difference between treatment groups (astegolimab vs placebo; efmarodocokin alfa vs placebo) in time to recovery with a minimum detectable difference of 5.3 days assuming the median time to improvement in the placebo group was 21 days (with 28 d of follow-up), using a one-sided 5% alpha.
See Supplementary Appendix (http://links.lww.com/CCM/H235) for analysis details for secondary endpoints, safety, pharmacokinetics, immunogenicity, and biomarkers. Statistical analyses were conducted using R (https://www.R-project.org/) (19).
RESULTS
Patient Disposition and Baseline Characteristics
The COVASTIL study, conducted from June 2020 to March 2021, enrolled and randomized patients at 54 investigator sites (Supplementary Appendix, http://links.lww.com/CCM/H235). Of 463 patients screened, 410 were randomized, and 396 received at least one dose of study drug or placebo (313 patients [79%] received one dose; 83 patients [21%] received two doses) and were included in the final analysis. Three-hundred thirty-two patients completed study assessments through day 28 (Fig. 1B), and 299 patients completed study assessments through day 60.
Treatment groups had similar demographic and baseline clinical characteristics (Table 1; and Table S1, http://links.lww.com/CCM/H235), including baseline ordinal scores of 3 (38.6%) or 4 (50%). This global study recruited a diverse population, including American Indian or Alaska Native (2%), Asian (4%), Black or African American (7%), Hispanic or Latino (55%), and White (68%) patients (Table S1, http://links.lww.com/CCM/H235). Time since first COVID-19 symptoms or diagnosis, hospitalization duration, and baseline use and duration of mechanical ventilation were similar between groups (Table 1). There were no significant differences between treatment groups in the proportion of patients admitted to the ICU at baseline. Mean (± sd) baseline CRP values were elevated and comparable between groups (79.7 ± 76.0 mg/L; upper limit of normal = 10 mg/L; n = 388), similar to other studies (20, 21). Concomitant medication use was similar across study groups: 381 (96%) received steroids, 227 (57%) received remdesivir, and 47 (12%) received tocilizumab.
TABLE 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | Placeboa (n = 134) | Astegolimab (n = 130) | Efmarodocokin Alfa (n = 132) | All Patients (n = 396) |
|---|---|---|---|---|
| Age | ||||
| Median (range), yr | 57 (26–89) | 58 (29–94) | 57 (27–86) | 57 (26–94) |
| Sex, n (%) | ||||
| Female | 45 (34) | 56 (43) | 52 (39) | 153 (39) |
| Male | 89 (66) | 74 (57) | 80 (61) | 243 (61) |
| Clinical statusb, n (%) | ||||
| 2 | 3 (2) | 4 (3.08) | 1 (0.76) | 8 (2.02) |
| 3 | 47 (35) | 49 (38) | 57 (43) | 153 (39) |
| 4 | 71 (53) | 64 (49) | 63 (48) | 198 (50) |
| 5 | 10 (7) | 8 (6) | 8 (6) | 26 (7) |
| 6 | 3 (2) | 4 (3) | 2 (2) | 9 (2) |
| Not available | 0 | 1 (1) | 1 (1) | 2 (1) |
| Baseline mechanical ventilation, n (%) | ||||
| No | 122 (91) | 117 (90) | 121 (92) | 360 (91) |
| Yes | 12 (9) | 13 (10) | 11 (8) | 36 (9) |
| Duration of hospitalization at randomization | ||||
| Mean (range), d | 4.14 (1–15) | 4.47 (1–16) | 4.55 (1–20) | 4.38 (1–20) |
| ICU admission, n (%) | ||||
| Admitted to ICU at randomization | 63 (47) | 58 (45) | 47 (36) | 168 (42) |
| Not admitted to ICU at randomization | 71 (53) | 72 (55) | 85 (64) | 228 (58) |
| Duration of COVID-19 diagnosis at randomization | ||||
| n | 133 | 130 | 132 | 395 |
| Mean (range), d | 5.33 (1–16) | 5.89 (1–15) | 5.9 (1–19) | 5.71 (1–19) |
| Duration of COVID-19 symptoms at randomization | ||||
| n | 133 | 129 | 132 | 394 |
| Mean (range), d | 11.59 (1–42) | 11.38 (1–24) | 11.58 (1–26) | 11.52 (1–42) |
| C-reactive protein | ||||
| n | 122 | 120 | 126 | 368 |
| mg/L, mean ± sd | 79.9 ± 64.8 | 77.3 ± 90.1 | 81.7 ± 71.7 | 79.7 ± 76.0 |
| Remdesivir treatment on study, n (%) | 78 (58.2) | 74 (56.9) | 75 (56.8) | 227 (57.3) |
| Tocilizumab treatment on study, n (%) | 16 (11.9) | 18 (13.8) | 13 (9.8) | 47 (11.9) |
| Steroid use on study, n (%) | 126 (94.0) | 129 (99.2) | 126 (95.5) | 381 (96.2) |
Matching placebo groups for astegolimab and efmarodocokin alfa were pooled for all analyses.
Clinical status was defined by the 7-category ordinal scale: 1, discharged (or “ready for discharge” as evidenced by normal body temperature and respiratory rate, and stable oxygen saturation on ambient air or ≤ 2 L supplemental oxygen); 2, non-ICU hospital ward (or “ready for hospital ward”) not requiring supplemental oxygen; 3, non-ICU hospital ward (or “ready for hospital ward”) requiring supplemental oxygen; 4, ICU or non-ICU hospital ward, requiring noninvasive ventilation or high-flow oxygen; 5, ICU, requiring intubation and mechanical ventilation; 6, ICU, requiring extracorporeal membrane oxygenation or mechanical ventilation and additional organ support (e.g., vasopressors, renal replacement therapy); 7, death.
Primary and Secondary Efficacy Endpoints
Neither astegolimab nor efmarodocokin alfa showed a significant difference from placebo in the primary endpoint, time to recovery by day 28 (HRs: astegolimab, 1.01, 95% CI: 0.75–1.36, p = 0.93; efmarodocokin alfa, 1.15, 95% CI: 0.86–1.54, p = 0.36; Table 2; Fig. 2A). Median time to recovery was 10.0, 11.0, and 10.0 days for placebo, astegolimab, and efmarodocokin alfa, respectively (Fig. 2A). Ninety-three patients (70%), 94 patients (72%), and 100 patients (76%) in the placebo, astegolimab, and efmarodocokin alfa groups, respectively, reached a clinical status of 1 or 2 (Fig. 2B). No treatment benefit was observed in key efficacy subgroups (stratification by baseline ordinal score, baseline body mass index, mechanical ventilation use, and baseline CRP) relative to placebo (Fig. S1, http://links.lww.com/CCM/H235). No significant differences occurred between placebo and either astegolimab or efmarodocokin alfa groups in secondary endpoints (Table 2; and Table S2, http://links.lww.com/CCM/H235).
TABLE 2.
Primary and Key Secondary Efficacy Endpoints
| Endpoints | Placeboa (n = 134) | Astegolimab (n = 130) | Efmarodocokin Alfa (n = 132) |
|---|---|---|---|
| Primary efficacy endpoint | |||
| Time to recovery, d (median)b | 10.0 | 11.0 | 10.0 |
| HR (95% CI) | 1.01 (0.75–1.36) | 1.15 (0.86–1.54) | |
| p | 0.93 | 0.36 | |
| Secondary efficacy endpoints | |||
| Time to hospital discharge or “ready for discharge,” d (median) | 10.0 | 11.0 | 10.0 |
| HR (95% CI) | 1.11 (0.83–1.49) | 1.16 (0.87–1.56) | |
| p | 0.47 | 0.31 | |
| Proportion of patients alive and free of respiratory failure, n (%)c | 51 (38.1) | 51 (39.2) | 53 (40.2) |
| Difference in rate (95% CI) | 1.17 (–11.34 to 13.68) | 2.09 (–10.39 to 14.57) | |
| OR (95% CI; p) | 1.05 (0.64–1.72; 0.85) | 1.09 (0.67–1.79; 0.73) | |
| Rate of invasive mechanical ventilation or extracorporeal membrane oxygenation, n (%) | 33 (24.6) | 37 (28.5) | 32 (24.2) |
| Difference in rate (95% CI) | 3.83 (–7.57 to 15.24) | –0.38 (–11.46 to 10.70) | |
| OR (95% CI; p) | 1.22 (0.70–2.10; 0.48) | 0.98 (0.56–1.71; 0.94) | |
| Ventilator-free days to day 28 (time to extubation), d (median) | 28 | 28 | 28 |
| Difference in medians | 0.0 | 0.0 | |
| p (van Elteren test) | 0.80 | 0.86 | |
| Rate of ICU stay, n (%) | 78 (58.2) | 71 (54.6) | 61 (46.2) |
| Difference in rate (95% CI) | –3.59 (–16.31 to 9.12) | –12.00 (–24.67 to 0.67) | |
| OR (95% CI; p) | 0.85 (0.53–1.41; 0.56) | 0.62 (0.38–1.00; 0.050) | |
| Duration of ICU stay (time to ICU discharge), d (median) | 3.10 | 2.62 | 0.00 |
| Difference in medians | –0.48 | –3.10 | |
| p (van Elteren test) | 0.48 | 0.057 | |
| Mortality rate at day 14, n (%) | 8 (6.0) | 11 (8.5) | 11 (8.3) |
| Difference in rate (95% CI) | 2.49 (–4.51 to 9.49) | 2.36 (–4.58 to 9.31) | |
| OR (95% CI; p) | 1.46 (0.57–3.74; 0.43) | 1.42 (0.56–3.68; 0.45) | |
| Mortality rate at day 28 (n patients), n (%) | 15 (11.2) | 19 (14.6) | 17 (12.9) |
| Difference in rate (95% CI) | 3.42 (–5.42 to 12.26) | 1.68 (–6.89 to 10.26) | |
| OR (95% CI; p) | 1.36 (0.66–2.80; 0.41) | 1.17 (0.56–2.46; 0.67) | |
HR = hazard ratio, OR = odds ratio.
Matching placebo groups for astegolimab and efmarodocokin alfa were pooled for all analyses.
bDefined as time (d) to score of 1 or 2 on the 7-category ordinal scale (whichever occurred first).
cRequiring noninvasive ventilation, high-flow oxygen, mechanical ventilation, or extracorporeal membrane oxygenation at day 28.
Figure 2.
Primary endpoint (time to recovery) and clinical status. A, Time to recovery. B, Clinical status using the 7-category ordinal scale over time. p values were generated from the log-rank test, and hazard ratios (HRs) were generated from the Cox proportional hazard model. Both parameters were stratified by country and baseline mechanical ventilation. Eight patients had an ordinal score of 2 at baseline and therefore did not contribute to the primary endpoint analysis.
Safety
During the treatment period, AE rates were comparable between groups (placebo, 87 [65%]; astegolimab, 85 [65%]; efmarodocokin alfa, 95 [72%]; Table 3). AEs reported in 5% or more of patients overall were constipation, hypokalemia, anemia, hypotension, and COVID-19 pneumonia (Table S3, http://links.lww.com/CCM/H235). SAE rates were comparable between groups (Table 3; and Supplementary Results, http://links.lww.com/CCM/H235). SAEs occurring in greater than 2% of patients were (worsening of) COVID-19 pneumonia (4.3%), septic shock (2.5%), and respiratory failure (2.8%) (Table S4, http://links.lww.com/CCM/H235). There were more related SAEs in the astegolimab (2; 2%) and in the efmarodocokin alfa (three related SAEs in two patients, 2%) groups than in the placebo group (0). The overall number of SAEs was similar between the groups, and no major imbalances AEs of special interest occurred (Table 3; Table S4 [http://links.lww.com/CCM/H235]; and Supplementary Results [http://links.lww.com/CCM/H235]).
TABLE 3.
Overview of Adverse Events
| Safety Outcomes | Placeboa (n = 134) | Astegolimab (n = 130) | Efmarodocokin Alfa (n = 132) | All Patients (n = 396) |
|---|---|---|---|---|
| Number of patients with ≥ 1 AE, n (%) | 87 (65) | 85 (65) | 95 (72) | 267 (67) |
| Number of AEs | 344 | 376 | 377 | 1,097 |
| Number of deaths, n (%) | 23 (17) | 23 (17) | 21 (16) | 67 (17) |
| Number of patients withdrawn from study due to an AE, n (%) | 1 (1) | 1 (1) | 0 | 2 (1) |
| Number of patients with ≥ 1 of the following events, n (%) | ||||
| Serious AE | 38 (28) | 38 (29) | 34 (26) | 110 (28) |
| Serious AE leading to withdrawal from treatment | 2 (2) | 2 (2) | 1 (1) | 5 (1) |
| Related serious AE | 0 | 2 (2) | 3 (2) | 5 (1) |
| AE leading to withdrawal from treatment | 4 (3) | 3 (2) | 3 (2) | 10 (3) |
| Related AE | 15 (11) | 12 (9) | 25 (19) | 52 (13) |
| Grade 3–5 AE | 43 (32) | 46 (35) | 41 (31) | 130 (33) |
AE = adverse event.
Matching placebo groups for astegolimab and efmarodocokin alfa were pooled for all analyses.
Sixty-seven deaths occurred during the study at similar rates between treatment groups (Table 3), largely attributable to progression of COVID-19 pneumonia, respiratory failure, multiple organ system failure, or sepsis. No deaths were deemed related to study drugs.
Pharmacokinetics
Because the majority of patients (astegolimab, 103 [79.2%]; efmarodocokin alfa, 107 [81.1%]) received only one dose, only pharmacokinetic parameters following the first doses of astegolimab and efmarodocokin alfa were calculated (Table S5, http://links.lww.com/CCM/H235). The cohort mean of the maximum serum concentration (Cmax) for astegolimab was 210 µg/mL and for efmarodocokin alfa was 1,286 ng/mL. The serum trough concentration (Ctrough_day 14) for astegolimab was 33.5 µg/mL and for efmarodocokin alfa was 81.8 ng/mL. Overall, pharmacokinetic exposures showed high variability (30–73%) in patients. To understand potential effects of SARS-CoV-2 infection on astegolimab and efmarodocokin alfa exposure, Cmax and area under the curve from 0 to 14 days (AUC0–14) were plotted by baseline disease severity. Patients with higher baseline disease severity (ordinal score of 4 vs 3) had lower astegolimab exposure, but baseline disease severity did not impact efmarodocokin alfa exposure (Fig. S2, http://links.lww.com/CCM/H235). The anti-drug antibody rate after treatment with either drug was low (Supplementary Results, http://links.lww.com/CCM/H235).
Biomarkers
Serum pharmacodynamic biomarkers for astegolimab and efmarodocokin alfa were measured throughout the study. Astegolimab binds sST2, a decoy receptor for ST2, allowing sST2 use as a surrogate for ST2 binding. Efmarodocokin alfa induces expression of both REG3A (a secreted antimicrobial protein produced by stomach, pancreas, and Paneth cells in the ileum) and CRP (an acute phase protein) (16, 22, 23). Sparse sample collection affected interpretability of day 15 and 21 data (samples were only collected at these timepoints from patients who remained hospitalized).
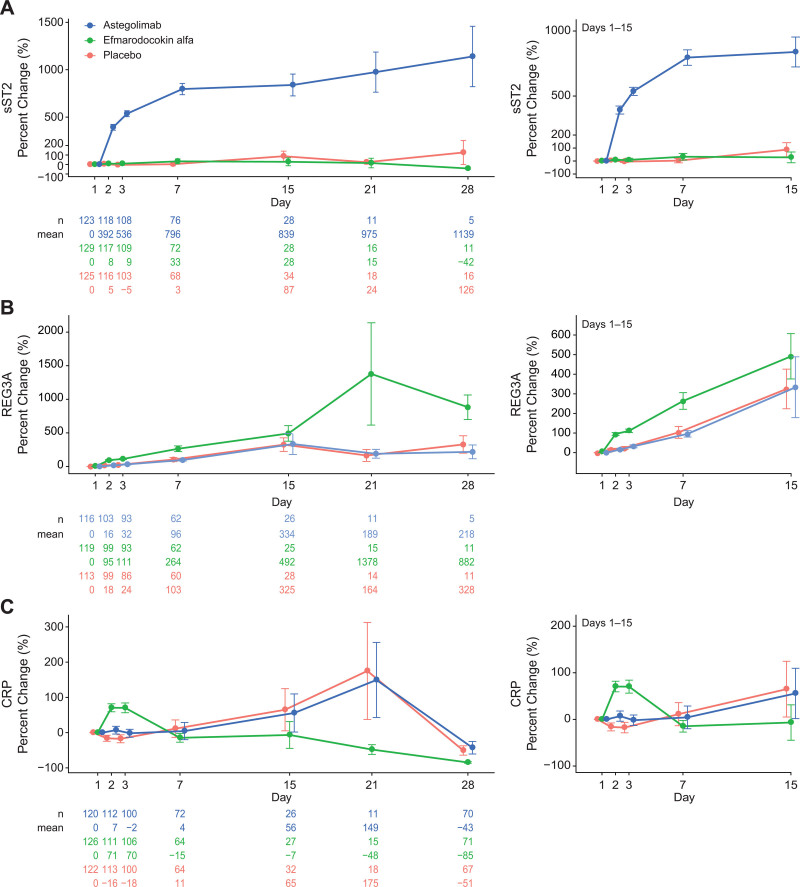
Baseline serum sST2 in COVID-19 patients was increased (13.8–1,600 ng/mL; mean, 116 ng/mL; Table S6, http://links.lww.com/CCM/H235) compared with healthy volunteers (HVs) (24). Similar to other serum proteins (25), astegolimab binding is predicted to increase the half-life of sST2, resulting in sST2 elevations. Absolute sST2 levels increased over time in astegolimab-treated patients but not in other treatment groups (Fig. 3A).
Figure 3.
Effects of astegolimab and efmarodocokin alfa on activity biomarkers. Mean percent change from baseline through day 28 in serum soluble ST2 (sST2) (A), regenerating islet-derived protein 3A (REG3A) (B), and C-reactive protein (CRP) (C). Right-hand images shows magnification of timepoints through day 15. Error bars, se.
Baseline levels of REG3A (3.15–172 ng/mL; mean, 15.0 ng/mL; Table S6, http://links.lww.com/CCM/H235) and CRP (1–670 mg/L [nmol/L]; mean, 79.7 mg/L [nmol/L]; Table 1) were increased compared with HVs (23). Normalized REG3A levels increased for efmarodocokin alfa-treated patients through day 7 (day 2: 95% ± 70%; day 7: 264% ± 335%) compared with placebo (day 2: 18% ± 45%; day 7: 103% ± 235%) (Fig. 3B). All treatment groups exhibited increases in normalized REG3A levels by day 15, but increases in efmarodocokin alfa-treated patients were more pronounced (492% ± 578%; astegolimab: 334% ± 791%; placebo: 325% ± 535%) and continued to increase through day 21 (1,378% ± 2,949%), while levels in the other groups decreased.
Normalized CRP levels peaked for efmarodocokin alfa-treated patients on day 2 (71% ± 120%), returned to baseline by day 7, and continued decreasing through day 28 (Fig. 3C). Normalized CRP increases in placebo and astegolimab groups peaked at day 21, returning to baseline by day 28 (Fig. 3C).
DISCUSSION
COVASTIL, a phase 2, double-blind, placebo-controlled trial, is the first study to examine IL-33 and IL-22 pathway modulation for treatment of hospitalized patients with COVID-19 pneumonia. Although both astegolimab and efmarodocokin alfa induced pharmacodynamic activity and were safe and well tolerated in this patient population, neither drug showed a significant difference from placebo in time to recovery, the primary endpoint, or in any of the secondary endpoints. The AEs, SAEs, and fatal events were consistent with those expected in a study of patients hospitalized with severe COVID-19 and with previous studies of these drugs (11, 23). Although there were more related SAEs in the treatment groups compared with the placebo group, the small number of events precluded any definitive conclusions. We observed no new safety signals for either study drug.
Previously identified pharmacodynamic biomarkers for astegolimab (sST2) and efmarodocokin alfa (REG3A, CRP) were measured in patients with COVID-19 pneumonia. Baseline serum levels of sST2 were elevated, possibly reflecting an association between sST2 and the presence of tissue damage, as seen in cardiac disease and ARDS (26, 27). Serum sST2 increased above baseline levels in the astegolimab arm compared with the other treatment arms‚ indicating target engagement and pharmacological activity.
Increases in REG3A following efmarodocokin alfa treatment indicated IL-22R engagement in these patients. The percent increase from baseline was lower compared with HVs (23), but elevated baseline REG3A levels may explain this finding. While REG3A has been detected in human lung fibrotic tissue (28), this is the first study demonstrating elevated levels of REG3A in the circulation of COVID-19 patients. Although the murine homolog of REG3A, REG3γ, is induced by IL-22 in lung epithelium (29) and has been shown to provide protection in lung infection models (30–33), in this study, REG3A increases were not correlated with clinical benefit.
As expected in patients with COVID-19 (20, 21, 34), baseline CRP levels were elevated. Similar to previous studies (23), efmarodocokin alfa treatment led to a significant increase in CRP that was not seen in the other groups. Due to the timing of sample collection, we did not measure the expected CRP peak after the day 15 dose. Although the early decrease of CRP as compared with the other treatment arms suggests efmarodocokin alfa treatment may promote resolution of inflammation, factors such as secondary bacterial infections and concomitant medications may have also contributed to elevated CRP levels.
There are four possible reasons for the apparent lack of efficacy of these drugs in patients with COVID-19 pneumonia, despite evidence of their pharmacological activity. First, while the IL-33 and IL-22 pathways may be involved in development of COVID-19 pneumonia, redundancies may have been sufficient to prevent measuring the effects of modulating these pathways. Second, patients were heterogeneous for disease presentation, comorbidities, and SOC treatment. During the study, COVID-19 treatment approaches were evolving and varied by region, and SOC in this study included steroids, remdesivir, and tocilizumab. In contrast to earlier studies (e.g., Adaptive COVID-19 Treatment Trial-1 [18]), most patients in this trial received dexamethasone, which may have masked the effect of astegolimab. Overall, COVID-19 treatment outcomes improved during the study, with the percentage of hospitalized patients admitted to ICUs and rates of mechanical ventilation decreasing significantly (35, 36). Third, patients who were mechanically ventilated at time of randomization comprised 10% of the patient population, and inclusion of only a small number of these severely ill patients may have limited the opportunity to see a treatment effect.
Fourth, study drug exposure (dose and frequency) or timing may have been insufficient for efficacy in COVID-19 patients. However, a dose-ranging study would be needed to address this question, and this study tested astegolimab and efmarodocokin alfa at the highest safe doses based on previous studies (11, 23). Based on efmarodocokin alfa’s mechanism of action, Cmax or AUC could be drivers of efficacy. While the observed mean pharmacokinetic exposures were expected (similar to ulcerative colitis patients but lower than HVs), the exposure distribution was highly variable in patients (Cmax, 73%; AUC0-14, 43%), possibly contributing to insufficient efficacy. For astegolimab, exposure (Cmax and AUC) was slightly lower than exposure in HVs (unpublished data). In an exploratory analysis, patients with higher disease severity (ordinal score of 4 vs 3) tended to have lower exposure. Timing of presentation with COVID-19 is heterogeneous, and it is unclear which subset of patients might benefit from either therapy with respect to timing of drug administration, number of doses of drug, and severity of COVID-19 pneumonia. In previous COVID-19 trials examining drugs that target hyperinflammation (e.g., tocilizumab and baricitinib), clinical benefit was only shown in patients with severe COVID-19 pneumonia, especially those on supplemental oxygen, who were beginning to clinically worsen (37, 38). A follow-up study measuring later clinical and imaging outcomes is ongoing to evaluate long-term effects of both interventions.
CONCLUSIONS
Although neither astegolimab nor efmarodocokin alfa showed efficacy against COVID-19 pneumonia, this study confirmed that both drugs were safe and pharmacologically active in patients with severe COVID-19 pneumonia. Findings from COVID-19 pneumonia yield important insights but may not be generalizable to ARDS. Further nonclinical and clinical studies are needed to identify the subset of COVID-19 patients who may benefit from blockade in the inflammatory cascade or from stimulation of pathways that can promote healing of damaged lung tissue.
ACKNOWLEDGMENTS
We thank the patients and their families who took part in the study, as well as the staff, research coordinators, and investigators at each participating institution.
Supplementary Material
Footnotes
*See also p. 153.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
This project has been funded in whole or in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; and Biomedical Advanced Research and Development Authority, under OT number: HHSO100201800036C and by Genentech. Writing assistance was provided by Bryan Hains and Deborah Solymar (Genentech) and was funded by Genentech.
Drs. Waters and McKinnell contributed equally as co-first authors; Drs. Mohan and Peck contributed equally as co-senior authors.
Dr. Waters has received funding from Genentech paid to his institution for the conduct of this trial. Dr. McKinnell has received funding from Genentech paid to his institution for the conduct of this trial; he reports receiving research funding paid to his institution from Gilead, Eli Lilly and Company, and Duke University; and he received funding from Pfizer and ThermoFisher. Dr. Martin has received compensation for service as a monitor of the COVID-astegolimab-interleukin trial. Dr. Buchman serves as a Senior Advisor to Biomedical Advanced Research and Development Authority (BARDA), U.S. Department of Health and Human Services under an agreement between his employer, Emory University, and that agency; he also serves as Editor-in-Chief of Critical Care Medicine, and Emory University similarly receives payment for that service from the Society of Critical Care Medicine. Drs. McKinnell, Martin, Buchman, Theess, Lekkerkerker, Staton, Rosenberger, Pappu, Wang, Zhang, Brooks, Cheung, Galanter, Chen, Mohan, and Peck disclosed the off-label product use of Astegolimab and Efmarodocokin Alfa. Dr. Theess is currently an employee of F. Hoffmann-La Roche and owns Roche stock. Drs. Staton and Wang are former employees of Genentech and own Roche stock. Dr. Chen is a former employee of Genentech. Drs. Martin, Theess, Yang, Lekkerkerker, Staton, Rosenberger, Pappu, Wang, Zhang, Cheung, Galanter, Chen, Mohan, and Peck received funding from Genentech. Drs. Theess’s, Lekkerkerker’s, Staton’s, Rosenberger’s, Wang’s, Zhang’s, Cheung’s, Chen’s, Mohan’s, and Peck’s institutions received funding from the Department of Health and Human Services, the Office of the Assistant Secretary for Preparedness and Response, and the BARDA (OT number: HHSO100201800036C). Drs. Theess, Yang, Lekkerkerker, Staton, Rosenberger, Pappu, Wang, Zhang, Brooks, Cheung, Galanter, Chen, Mohan, and Peck are employees of Genentech, a member of the Roche group, and own Roche stock. Drs. Theess and Brooks disclosed they are employed by F. Hoffman-LaRoche. Drs. Theess, Lekkerkerker, Staton, Rosenberger, Pappu, Wang, Zhang, Brooks, Cheung, Galanter, Chen, Mohan, and Peck disclosed work for hire. Dr. Brooks disclosed that he is a Principal Computational Researcher at Genentech. Dr. Kalil has disclosed that he does not have any potential conflicts of interest.
COVID-astegolimab-interleukin (IL) (COVASTIL) Study Group are listed in the Supplementary Appendix (http://links.lww.com/CCM/H235).
Contributor Information
Collaborators: Nikhil Meena, Michael Waters, Jeffrey Overcash, Forest Mealey, James McKinnell, Ivor Douglas, Theresa Buck, Luis Mendez-Mulet, Paul Boyce, Asif Saberi, Alejandro Comellas, Naseem Jaffrani, Robert Jeanfreau, Kyle Widmer, Mayur Ramesh, Patrick Perin, Jeffrey Neidhart, Scott Beegle, Vidya Menon, Andrew Wiznia, Barry Hahn, Judith Borger, Luis Jauregui-Peredo, Jason Wells, Marcelo Gareca, Gerard Criner, Raksha Jain, Raksha Jain, Arun Sanyal, Uma Malhotra, Vinay Malhotra, Estevão Nunes, Kleber Luz, Claudio Marcel Berdun Stadnik, Maria Lima, Suzana Lobo, Elie Fiss, Ludhmila Abrahão Hajjar, Juan José Morales Reyes, Roberto Mercado Longoria, José Sifuentes Osornio, Alejandra Ramírez Venegas, Samuel Navarro Álvarez, María Molina, Esther Calbo Sebastián, Enrique Míguez Rey, José Oteo, Julián Olalla, Juan Pablo Horcajada Gallego, Olga Mediano, Jesús Millán Núñez-Cortes, Miguel Marcos Martín, Carlos Dueñas Gutierrez, Melicent Peck, Divya Mohan, Hubert Chen, Wiebke Theess, Jonathan Gall, Joshua Galanter, Ajit Dash, Tiffany Wong, Xiaoying Yang, Lena Wang, Jenny Buchanan, Kristina Dokonal, Valentine Jurincic, Hilary Gray, Lixian Ma, Irina Marchenko, Holly Spoonemore, Ageliki Tzovolos, Marina Gasser-Stracca, Kit Valentine, Jessica Defreese, Mike Flanagan, Steve Hurst, Joo Park, Tasi Nelson, Priscilla Horn, Stella Costante-Hamm, Aubrey McKinney, Julie Rosseig, Sarah Roth, Jennifer Whitmore, Ha Tran, Catherine Abogado, Zara Ahmed, Jon Hilton, Eric Kum, Jennifer Pon, Daniel Sana, Elma Zannatul Ferdousy, Elaine Alexander, Tracy Staton, Annemarie Lekkerkerker, Andrea Sharp, Natasha Miley, Michael Dolton, Yehong Wang, Wenhui Zhang, Logan Brooks, Gizette Sperinde, Audrey Arjomandi, Matt Kalo, and Elisa Ciullo
REFERENCES
- 1.Guan WJ, Ni ZY, Hu Y, et al. : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM: Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323:1239–1242 [DOI] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzotzos SJ, Fischer B, Fischer H, et al. : Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit Care. 2020; 24:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cevikbas F, Steinhoff M: IL-33: A novel danger signal system in atopic dermatitis. J Invest Dermatol. 2012; 132:1326–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin SH, Fu J, Wang CJ, et al. : Inflammation elevated IL-33 originating from the lung mediates inflammation in acute lung injury. Clin Immunol. 2016; S1521-6616:30535–30536 [DOI] [PubMed] [Google Scholar]
- 8.Zizzo G, Cohen PL: Imperfect storm: Is interleukin-33 the Achilles heel of COVID-19? Lancet Rheumatol. 2020; 2:e779–e790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanczak MA, Sanin DE, Apostolova P, et al. : IL-33 expression in response to SARS-CoV-2 correlates with seropositivity in COVID-19 convalescent individuals. Nat Commun. 2021; 12:2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke H, Freeman A, Cellura DC, et al. : Inflammatory phenotyping predicts clinical outcome in COVID-19. Respir Res. 2020; 21:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelsen SG, Agache IO, Soong W, et al. : Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: A randomized clinical trial. J Allergy Clin Immunol. 2021; 148:790–798 [DOI] [PubMed] [Google Scholar]
- 12.Hoegl S, Bachmann M, Scheiermann P, et al. : Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2011; 44:369–376 [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Hu Z, Cai X, et al. : Interleukin 22 attenuated angiotensin II induced acute lung injury through inhibiting the apoptosis of pulmonary microvascular endothelial cells. Sci Rep. 2017; 7:2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whittington HA, Armstrong L, Uppington KM, et al. : Interleukin-22: A potential immunomodulatory molecule in the lung. Am J Respir Cell Mol Biol. 2004; 31:220–226 [DOI] [PubMed] [Google Scholar]
- 15.Pociask DA, Scheller EV, Mandalapu S, et al. : IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol. 2013; 182:1286–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanich EG, Rae J, Sukumaran S, et al. : Pre-clinical and translational pharmacology of a human interleukin-22 IgG fusion protein for potential treatment of infectious or inflammatory diseases. Biochem Pharmacol. 2018; 152:224–235 [DOI] [PubMed] [Google Scholar]
- 17.Rosas IO, Brau N, Waters M, et al. : Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021; 384:1503–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beigel JH, Tomashek KM, Dodd LE, et al. : Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020; 383:1813–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team: R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2020. Available at: https://www.R-project.org/. Accessed July 20, 2021 [Google Scholar]
- 20.Luo X, Zhou W, Yan X, et al. : Prognostic value of C-reactive protein in patients with coronavirus 2019. Clin Infect Dis. 2020; 71:2174–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali N: Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020; 92:2409–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DW, Zhong S, Pai R, et al. : Nonclinical safety assessment of a human interleukin-22FC IG fusion protein demonstrates in vitro to in vivo and cross-species translatability. Pharmacol Res Perspect. 2018; 6:e00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothenberg ME, Wang Y, Lekkerkerker A, et al. : Randomized phase I healthy volunteer study of UTTR1147A (IL-22Fc): A potential therapy for epithelial injury. Clin Pharmacol Ther. 2019; 105:177–189 [DOI] [PubMed] [Google Scholar]
- 24.Sánchez-Marteles M, Rubio-Gracia J, Pena-Fresneda N, et al. : Early measurement of blood sST2 is a good predictor of death and poor outcomes in patients admitted for COVID-19 infection. J Clin Med. 2021; 10:3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staton TL, Peng K, Owen R, et al. : A phase I, randomized, observer-blinded, single and multiple ascending-dose study to investigate the safety, pharmacokinetics, and immunogenicity of BITS7201A, a bispecific antibody targeting IL-13 and IL-17, in healthy volunteers. BMC Pulm Med. 2019; 19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T, Xu C, Zhao R, et al. : Diagnostic value of sST2 in cardiovascular diseases: A systematic review and meta-analysis. Front Cardiovasc Med. 2021; 8:697837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajwa EK, Volk JA, Christiani DC, et al. : Prognostic and diagnostic value of plasma soluble suppression of tumorigenicity-2 concentrations in acute respiratory distress syndrome. Crit Care Med. 2013; 41:2521–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng X, Li Q, Tian H, et al. : HIP/PAP protects against bleomycin-induced lung injury and inflammation and subsequent fibrosis in mice. J Cell Mol Med. 2020; 24:6804–6821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito T, Hirose K, Saku A, et al. : IL-22 induces Reg3γ and inhibits allergic inflammation in house dust mite-induced asthma models. J Exp Med. 2017; 214:3037–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov S, Renneson J, Fontaine J, et al. : Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J Virol. 2013; 87:6911–6924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi SM, McAleer JP, Zheng M, et al. : Innate Stat3-mediated induction of the antimicrobial protein Reg3γ is required for host defense against MRSA pneumonia. J Exp Med. 2013; 210:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue M, Zhao J, Ying L, et al. : IL-22 suppresses the infection of porcine enteric coronaviruses and rotavirus by activating STAT3 signal pathway. Antiviral Res. 2017; 142:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abood RN, McHugh KJ, Rich HE, et al. : IL-22-binding protein exacerbates influenza, bacterial super-infection. Mucosal Immunol. 2019; 12:1231–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herold T, Jurinovic V, Arnreich C, et al. : Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020; 146:128–136.e124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garg S, Patel K, Pham H, et al. : Clinical trends among U.S. adults hospitalized with COVID-19, March to December 2020: A cross-sectional study. Ann Intern Med. 2021; 174:1409–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeates EO, Nahmias J, Chinn J, et al. : Improved outcomes over time for adult COVID-19 patients with acute respiratory distress syndrome or acute respiratory failure. PLoS One. 2021; 16:e0253767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angriman F, Ferreyro BL, Burry L, et al. : Interleukin-6 receptor blockade in patients with COVID-19: Placing clinical trials into context. Lancet Respir Med. 2021; 9:655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goletti D, Cantini F: Baricitinib therapy in Covid-19 pneumonia - an unmet need fulfilled. N Engl J Med. 2021; 384:867–869 [DOI] [PMC free article] [PubMed] [Google Scholar]