OBJECTIVES:
Here, we report the management of a catastrophic COVID-19 Delta variant surge, which overloaded ICU capacity, using crisis standards of care (CSC) based on a multiapproach protocol.
DESIGN:
Retrospective observational study.
SETTING:
University Hospital of Guadeloupe.
PATIENTS:
This study retrospectively included all patients who were hospitalized for COVID-19 pneumonia between August 11, 2021, and September 10, 2021, and were eligible for ICU admission.
Intervention:
Based on age, comorbidities, and disease severity, patients were assigned to three groups: Green (ICU admission as soon as possible), Orange (ICU admission after the admission of all patients in the Green group), and Red (no ICU admission).
MEASUREMENTS AND MAIN RESULTS:
Among the 328 patients eligible for ICU admission, 100 (30%) were assigned to the Green group, 116 (35%) to the Orange group, and 112 (34%) to the Red group. No patient in the Green group died while waiting for an ICU bed, whereas 14 patients (12%) in the Orange group died while waiting for an ICU bed. The 90-day mortality rates were 24%, 37%, and 78% in the Green, Orange, and Red groups, respectively. A total of 130 patients were transferred to the ICU, including 79 from the Green group, 51 from the Orange group, and none from the Red group. Multivariate analysis revealed that among patients admitted to the ICU, death was independently associated with a longer time between ICU referral and ICU admission, the Sequential Organ Failure Assessment score, and the number of comorbidities, but not with triage group.
CONCLUSIONS:
CSC based on a multiapproach protocol allowed admission of all patients with a good prognosis. Higher mortality was associated with late admission, rather than triage group.
Keywords: crisis standards of care, intensive care unit, mechanical ventilation, triage, withholding
KEY POINTS.
Question: What factors affected mortality when crisis standards of care (CSC) were applied, using a multiapproach triage protocol, to manage healthcare saturation during a catastrophic COVID-19 surge?
Findings: Under CSC, higher mortality seemed to be independently associated with late ICU admission, rather than triage group.
Meanings: Experimental models of CSC, particularly using a multiapproach triage protocol, can be helpful during a catastrophic surge in a pandemic.
Since late 2019, the COVID-19 pandemic has included repeated surges around the world. Some of these surges have been considered “catastrophic” because a massive influx of critically ill patients has overwhelmed the healthcare system, such as in Eastern France and Northern Italy during the first wave of the pandemic from February to April 2020 (1, 2). In these settings, complete saturation of ICUs was prevented by transferring patients to other regions of the country with lower ICU strain, which protected the healthcare system from collapse (3, 4).
Guadeloupe is a French West Indies island with a high standard of care, which is isolated from the mainland by the Atlantic Ocean. Oversea air transfer of critically ill COVID-19 patients requires an 8-hour flight and can only be conducted for highly selected patients. In July 2021, the fourth COVID-19 surge began in Guadeloupe, mainly caused by the severe acute respiratory syndrome coronavirus 2 Delta variant (5), and it appeared that the ICUs would be rapidly overwhelmed despite a dramatically increased ICU bed capacity. It became obvious that instead of using a “first come, first served” policy, a stringent triage policy for ICU beds was necessary to try to admit all patients with a potential good prognosis in the ICU and to avoid excess mortality. A task force was rapidly created to develop local guidelines and to define the allocation of ICU resources, with the goal of ensuring appropriate and fair decision-making regarding ICU transfer during the surge. This kind of strategy has previously been described as crisis standards of care (CSC) (6). Numerous CSC have been described (2, 7), and the best model is still heavily debated.
Here, we report the management of a catastrophic COVID-19 surge using CSC. Our specific aims were to describe the characteristics and outcomes of patients according to triage group and to examine the factors associated with mortality among patients who were eventually admitted to the ICU, particularly whether delayed ICU transfer was associated with higher mortality.
METHODS
This study was approved by the ethical research committee of the University Hospital of Guadeloupe, on July 16, 2021, under number n. A64_21_09_13 (for the COVIGWAD-ICU study). The ethical committee waived the requirement for individual consent for anonymous data collection, in accordance with French law. Procedures were conducted following the ethical standards of the national responsible committee on human experimentation and the Helsinki Declaration of 1975.
Study Design and Patient Selection
We conducted a retrospective observational study. The CSC were applied during the surge peak, between August 11 and September 10, 2021. This study included all patients hospitalized at University Hospital of Guadeloupe, who fulfilled the following criteria: over 18 years old, COVID-19 diagnosis confirmed by polymerase chain reaction (PCR), abnormalities on chest radiograph or pulmonary CT, and acute respiratory failure (oxygen flow ≥ 10 L/min to maintain peripheral capillary oxygen saturation [Spo2] > 92%). Notably, during this period, patients were only hospitalized at University Hospital of Guadeloupe if they had severe COVID-19 pneumonia, requiring oxygen flow greater than 5 L/min to maintain Spo2 greater than 92%.
Description of the Hospital and ICU Management
Guadeloupe is a French West Indies island with 440,000 inhabitants. University Hospital of Guadeloupe is the main and major hospital, with 440 beds. In its usual operating mode, there is one closed ICU with 22 intensive care beds and eight intermediate care beds. During the study period, the eight intermediate care beds were converted to ICU beds, and 28 additional ICU beds (expansion ICU) were created, with the same standards in terms of staff and equipment, such that there were a total of 58 ICU beds. To avoid cross contamination, 12 ICU beds set up in the postoperating room were dedicated to non–COVID-19 patients. Back-up nurses were brought in from the mainland to help staff the expansion ICU. An additional 18 intermediate care beds were set up in the medical ward for patients requiring noninvasive oxygenation. During the entire study period, lockdown measures were applied, and all scheduled medical and surgical admissions were cancelled. In addition to the ICU beds, 240 non-ICU beds were dedicated to COVID-19 patients in the ward.
CSC Protocol
Anticipating a massive influx of patients, guidelines for ICU admission and triage were developed prior to the surge. These CSC guidelines were developed by the local intensivists and the Ethic Committee of Guadeloupe. Elaboration of the CSC was guided by the principles of being fair and equitable, and the objective of maximizing the healthy life years saved. Resource allocation was based on a multiapproach strategy, which has been described as more efficient than other strategies based only on age cut-off or clinical severity (8, 9). For both COVID-19 and non–COVID-19 patients, prognosis was estimated based on the evaluation of acute illness severity (i.e., number of organ dysfunctions), severe and moderate life-limiting comorbidities, and the patient’s age.
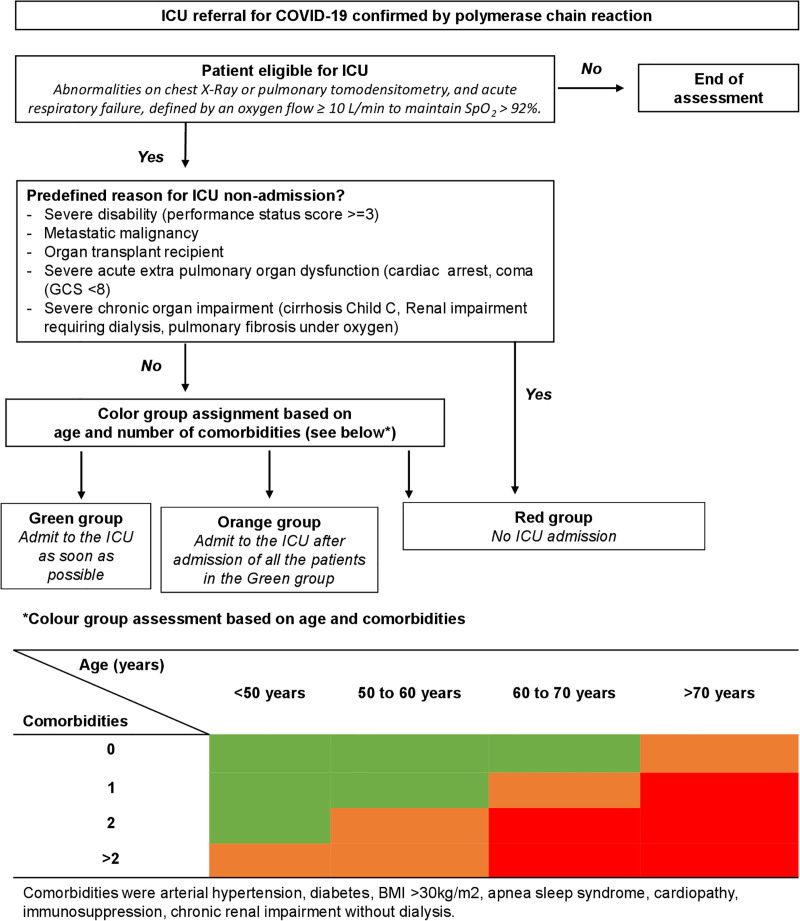
Decisions regarding the potential ICU admission of non–COVID-19 patients (Supplemental Fig. S1, http://links.lww.com/CCM/H228) were based on national criteria established by the French Society of Anaesthesia and Critical Care and Perioperative Medicine (SFAR) and the French Intensive Care Society (SRLF) (10, 11). To guide decisions regarding the potential ICU admission of COVID-19 patients (Fig. 1), three priority groups were designed based on survival rates under invasive mechanical ventilation observed in large cohorts (12–15) and in our institution (16). The Green group included patients with a higher likelihood of survival (over 50% in our cohort, unpublished data), who should be referred to ICU as soon as possible. The Orange group included patients with an intermediate probability of survival (between 30% and 50% in our cohort), who should be referred to the ICU after the admission of all patients in the Green group. Finally, the Red group included patients with a high mortality rate (above 70%) (17–19), who were not referred to the ICU during this high strain period and who should receive medical and/or palliative care.
Figure 1.
Guidelines for determining ICU admission of COVID-19 patients. BMI = body mass index, GCS = Glasgow Coma Scale, Spo2 = peripheral capillary oxygen saturation.
These CSC were validated by an expert committee including the SRLF and the SFAR. Finally, they were approved by the national health administration of France. The whole medical community of Guadeloupe was informed of the CSC, and the population was informed of the need of a triage policy due to the high number of patients referred to the hospital.
Triage Procedure and ICU Admission
All patients eligible for ICU transfer were evaluated by two senior intensivists and the ward physician in charge of the patient. Together, these three physicians assigned patients to one of the three predefined groups (Green, Orange, or Red). When the patients assigned to the Green group for ICU admission exceeded the number of available ICU beds, local and national ethics committees were consulted for advice by hotline. Several criteria were then assessed, including the number of dependent persons (children and persons with handicap) living with the patient. The triage decision was recorded in each patient’s chart.
ICU Management
For patients admitted to the ICU, COVID-19 pneumonia was treated according to World Health Organization guidelines (12) and CSC applied. No patient received prolonged cardiopulmonary resuscitation. Renal replacement therapy was discussed on a case-by-case basis. Mechanically ventilated patients were screened daily. Patients with a Pao2/Fio2 ratio above 150, lasting over 48 hours, without requiring a prone position or norepinephrine above 0.5 µg/kg/min, were considered for oversea transfer, and the decision was made after family approval. Based on previous reports (20, 21), late ICU admission was defined as admission at least 2 days after the first ICU referral, and early ICU admission as admission less than 2 days after the first ICU referral.
Data Collection
For all patients, the following variables were collected: age; gender; comorbidities (e.g., arterial hypertension, obesity, diabetes, malignancy, and immunosuppression); ratio of oxygen saturation as measured by pulse oximetry Fio2 to respiratory rate (ROX) index (22); noninvasive oxygenation strategy, such as high-flow nasal oxygen (HFNO); specific COVID-19 treatments received, such as steroids and tocilizumab; and 90-day outcome. Among patients transferred to the ICU, we also recorded the time to transfer, Simplified Acute Physiologic Score (SAPS) 2 and Sequential Organ Failure Assessment (SOFA) scores upon admission, and the use of invasive mechanical ventilation.
Statistical Analysis
All analyses were performed using R 4.0.4 (23). Variables were reported as median and interquartile range (IQR, 25–75%) for continuous data and as number and percentage for categorical data. Variables were univariately tested for association with the group attribution Green (vs Orange vs Red) and with 90-day mortality (vs alive at 90 d), using Fisher exact tests for categorical variables and Wilcoxon rank-sum test for continuous variables. The probability of 90-day mortality was further analyzed among patients admitted to the ICU using multivariate logistic regression. All variables with p value of less than 0.2 in univariate analysis were included in the multivariate analysis, and we performed backward selection on the model, stopping when the Akaike information criterion reached its minimum. No imputation was performed for missing values. To evaluate the impact of delayed admission to the ICU due to ICU strain, Kaplan-Meier overall survival curves up to day 90 were separately computed for patients with delayed ICU admission versus early ICU admission. A log-rank test was used to measure the difference.
RESULTS
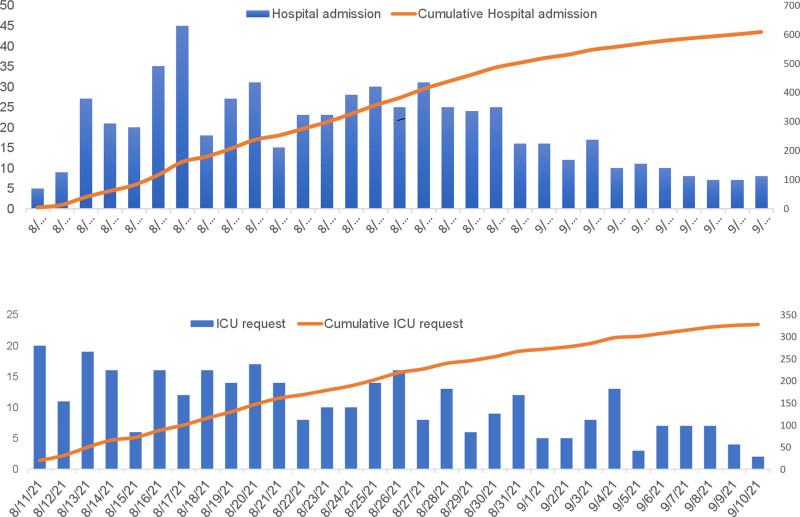
During the study period, 609 patients with PCR-confirmed COVID-19 pneumonia were hospitalized. Figure 2 shows the daily numbers of COVID-19 patients admitted and the daily numbers of new COVID-19 patients with ICU referral. Among the hospitalized patients, 328 (54%) fulfilled the criteria for ICU eligibility, of whom 130 (40%) were admitted to the ICU. Starting on August 11, 2021, weekly trans-Atlantic patient transfers occurred, and 48 of 328 ICU patients (15%) were transferred to ICUs on mainland France during the study period.
Figure 2.
Daily numbers of admissions (A) and of new ICU referrals (B) of patients with severe confirmed COVID-19 pneumonia at University Hospital of Guadeloupe during the study period.
During the surge, ICU referrals for non–COVID-19 patients (Table S1, http://links.lww.com/CCM/H229) were sharply reduced compared with usual ICU activity (data not shown). A total of 52 non–COVID-19 patients were admitted to the ICU, with a median age of 50 years (IQR, 35–65 yr). Table S1 (http://links.lww.com/CCM/H229) shows the main characteristics of these non–COVID-19 patients and the reasons for ICU admission.
Group Description
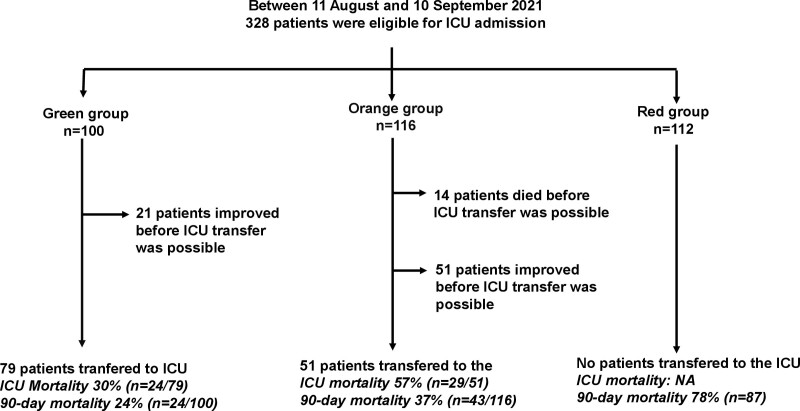
Among the 328 patients eligible for ICU admission, 100 (30%) were assigned to the Green group, 116 (35%) to the Orange group, and 112 (34%) to the Red group (Fig. 3). In the Red group, the reasons for ICU denial were secondary to severe disability (32 patients; 29%/112), metastatic cancer (11 patients; 10%), solid organ transplant (5 patients; 4%), severe extrapulmonary organ failure at hospital admission (14 patients; 13%) (including cardiac arrest in three patients, severe renal failure in eight patients, and coma in three patients), and multiple comorbidities (50 patients; 45%).
Figure 3.
Flowchart of the study. NA = not available.
Table 1 presents the patients’ characteristics and outcomes according to triage group. Briefly, patients in the Orange group were older and had more comorbidities than patients in the Green group. Patients in the Red group were older and had more comorbidities than patients in the other two groups. With regard to management, compared with patients in the Red group, patients in the Green and Orange groups were more likely to receive tocilizumab and HFNO. Notably, these treatments were predominantly given to patients who were actually candidates for ICU admission.
TABLE 1.
Characteristics and Outcomes of the Patients According to Triage Group
| All, N = 328 | Green Group, N = 100 | Orange Group, N = 116 | Red Group, N = 112 | p | |
|---|---|---|---|---|---|
| Patient’s characteristics | |||||
| Age (yr), median (interquartile range) | 59 (52–66) | 49 (42–57) | 59 (54–63) | 68 (62–73) | <0.001 |
| Gender, female, n (%) | 170 (52) | 53 (53) | 58 (50) | 59 (53) | 0.898 |
| Comorbidities, n (%) | |||||
| Arterial hypertension | 150 (57) | 26 (26) | 73 (69) | 51 (89) | <0.001 |
| Diabetes | 106 (40) | 21 (21) | 49 (47) | 36 (63) | <0.001 |
| Body mass index > 30 kg/m2 | 139 (53) | 48 (48) | 61 (58) | 30 (54) | 0.001 |
| Malignancy | 15 (5) | 1 (1) | 0 (0) | 14 (12) | <0.001 |
| Organ transplant recipient | 5 (2) | 0 (0) | 0 (0) | 5 (5) | 1 |
| Management, n (%) | |||||
| Steroids | 328 (100) | 100 (100) | 116 (100) | 112 (100) | 1.0 |
| Tocilizumab | 74 (36) | 31 (31) | 43 (40) | 0 (0) | 0.281 |
| High-flow nasal O2 administration | 140 (43) | 55 (56) | 69 (59) | 16 (14) | <0.001 |
| ROX score (day of hospital admission), median (interquartile range) | 3.7 (2.8–5.7) | 3.2 (2.7–4.7) | 4.4 (3.2–6.3) | NA | 0.007 |
| ICU transfer and management, n (%) | |||||
| ICU transfer | 130 (40) | 79 (79) | 51 (44) | 0 (0) | <0.001 |
| Time to ICU transfer, d, median (interquartile range) | 1 (0–2) | 0 (0–0) | 2 (2–3) | NA | <0.001 |
| Late ICU transfer | 44/130 (34) | 5/79 (6) | 39/51 (76) | NA | <0.001 |
| Simplified Acute Physiologic Score on ICU admission, median (interquartile range) | 29 (23–40) | 29 (22–41) | 31 (24–37) | NA | 0.639 |
| Sequential Organ Failure Assessment on ICU admission, median (interquartile range) | 5 (3–7) | 5 (3–8) | 5 (3–7) | NA | 0.585 |
| Invasive mechanical ventilation | 109 (33) | 61 (61) | 48 (41) | 0 (0) | 0.021 |
| Outcome, n (%) | |||||
| 90-day mortality | 154 (47) | 24 (24) | 43 (37) | 87 (78) | <0.001 |
| 90-d mortality in ICU | 53 (41) | 24 (30) | 29 (57) | NA | <0.001 |
NA = not available, ROX = ratio of oxygen saturation as measured by pulse oximetry Fio2 to respiratory rate.
Late ICU transfer was defined as a transfer occurring at least 2 d after the patient met the criteria for ICU eligibility.
ICU Transfer
In the Green group, 79% of patients (n = 79/100) were transferred to the ICU, whereas 21% (n = 21/100) improved to the extent that they no longer required ICU transfer. In the Orange group, 44% of patients (n = 51/116) were transferred to the ICU, 44% improved (n = 51), and 12% (n = 14) died before an ICU bed was available. No patient in the Red group was transferred to the ICU.
The time between eligibility for ICU transfer and actual ICU transfer was shorter in the Green group than in the Orange group (p < 0.001) (Table 1). Patients in the Green and Orange groups did not differ in severity, as assessed by the SOFA and SAPS2. Patients in the Orange group more often required invasive mechanical ventilation compared with patients in the Green group (94% vs 77%; p = 0.021). Intensivists did not report conflictual situations during the surge, and no legal procedures related to “non-ICU admission” decisions have been recorded.
Outcome
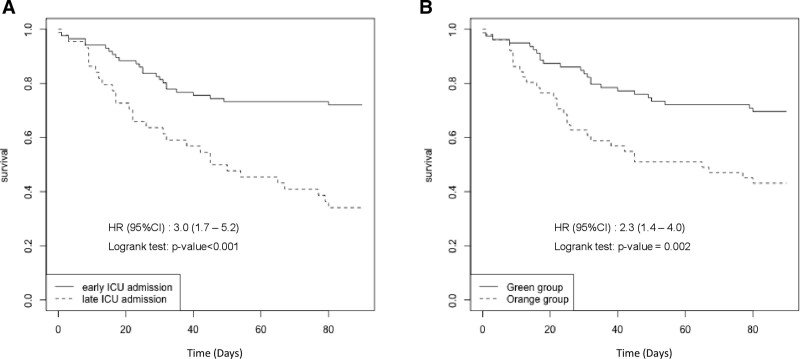
The 90-day mortality rate was 47% (n = 154/328) among all patients, 24% (n = 24/100) in the Green group, 37% (n = 43/116) in the Orange group, and 78% (n = 87/112) in the Red group (p < 0.001). Among the patients who were transferred to the ICU, 90-day mortality was 41% (n = 53) among all 130 patients, 30% (n = 24/79) in the Green group and 57% (n = 29/51) in the Orange group (p = 0.003). Table 2 shows factors associated with 90-day mortality among the 130 patients transferred to the ICU. Multivariate analysis revealed that three variables were associated with mortality: a high number of comorbidities (odds ratio [OR], 1.9; 95% CI, 1.2–3.0 per comorbidity; p = 0.007), delay between ICU referral and ICU admission (OR, 1.6; 95% CI, 1.2–2.2 per day; p = 0.001), and SOFA score (OR, 1.3; 95% CI, 1.2–1.5; p < 0.001). Among patients admitted to the ICU, the cumulative occurrence rate of death was higher among those admitted late (n = 29/44; 66%) than those admitted early (n = 24/86; 28%) (log rank, p < 0.01) and was higher among patients in the Orange group compared with those in the Green group (log rank, p = 0.02) (Fig. 4). The 90-day mortality rate among patients admitted late to the ICU was 80% (4/5) in the Green group and 64% (25/39) in the Orange group. Notably, the mortality rate among patients admitted to ICU expansion beds (29/82; 35%) was similar to among patients admitted to standard ICU beds (22/48; 46%) (p = 0.26).
TABLE 2.
Factors Associated With 90-Day Mortality in the 130 Patients Eventually Transferred to the ICU: Univariate and Multivariate Analysis
| 90-d Survivors, N = 77 | 90-d Nonsurvivors, N = 53 | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |||
| Patients characteristics | ||||||
| Age, yr, median (interquartile range) | 52.0 (46–59) | 58 (51–61) | 1.0 (1.0–1.1) | 0.024 | ||
| Gender female, n (%) | 46 (60) | 24 (45) | 0.6 (0.3–1.1) | 0.148 | 0.4 (0.2–1.0) | 0.06 |
| ROX score (at ICU referral), median (interquartile range) | 3.2 (2.7–4.7) | 3.5 (2.6–4.4) | 0.9 (0.7–1.2) | 0.860 | ||
| Simplified Acute Physiologic Score 2 score, median (interquartile range) | 26 (21–37) | 32 (28–44) | 1.03 (1.01–1.06) | 0.001 | ||
| Sequential Organ Failure Assessment score, median (interquartile range) | 4 (3–6) | 6 (4–8) | 1.3 (1.1–1.4) | < 0.001 | 1.3 (1.2–1.5) | < 0.001 |
| Comorbidities | ||||||
| Arterial hypertension, n (%) | 22 (30) | 31 (58) | 3.3 (1.6–7.0) | 0.002 | ||
| Diabetes, n (%) | 18 (25) | 18 (34) | 1.6 (0.7–3.4) | 0.346 | ||
| Body mass index > 30 kg/m2, n (%) | 35 (48) | 32 (60) | 1.7 (0.8–3.4) | 0.230 | ||
| Number of comorbidities, median (interquartile range) | 1 (0–2) | 2 (1–3) | 1.7 (1.2–2.5) | 0.008 | 1.9 (1.2–3.0) | 0.007 |
| ICU management | ||||||
| Time to ICU transfer, d, median (interquartile range) | 0 (0–1) | 2 (0–3) | 1.6 (1.2–2.0) | < 0.001 | 1.6 (1.2–2.2) | 0.001 |
| Late ICU transfer, n (%) | 15 (20) | 29 (55) | 4.9 (2.3–11.0) | < 0.001 | ||
| Tocilizumab, n (%) | 22 (30) | 13 (25) | 0.8 (0.3–1.7) | 0.67 | ||
| High-flow nasal O2 administration, n (%) | 49 (64) | 32 (61) | 0.9 (0.5–2.0) | 1 | ||
| Orange group, n (%) | 22 (29) | 29 (55) | 3.0 (1.4–6.3) | 0.003 | ||
The univariate and multivariate p values were obtained respectively from univariate logistic regression and multivariate logistic regression with backward selection. All variables which reached p < 0.2 were entered in the multivariable logistic regression model simultaneously.
ROX = ratio of oxygen saturation as measured by pulse oximetry Fio2 to respiratory rate.
Figure 4.
Cumulative prevalence of death among patients admitted to the ICU according to the delay of ICU admission (A) and according to triage group (B). HR = hazard ratio.
Among non–COVID-19 patients, the mortality rate was 12% (n = 6), and the median length of stay was 3 days.
DISCUSSION
The COVID-19 Delta surge has been dramatic in Guadeloupe, with an aftermath of 800 dead (data not shown). To our knowledge, no previous publication describes such a catastrophic surge overwhelming a modern healthcare system. To ensure that lessons are learned from these painful ordeals, we decided to analyze our management of this surge.
Our data analysis confirmed that no patient with COVID-19 pneumonia and with the best estimated prognosis in the ICU (“Green group”) died while waiting for an ICU bed. The CSC also resulted in a homogenous distribution of the patients in the three groups and helped physicians with triage. Additionally, the wide diffusion of this visual tool helped caregivers understand and accept decisions regarding non-ICU admission. However, it must be emphasized that five patients (6%) in the Green group were admitted late to the ICU, and 14 patients (12%) in the Orange group died before being admitted to an ICU bed. Non–COVID-19 ICU activity was sharply reduced, and all patients without nonadmission criteria could be admitted to the ICU.
The process of developing our CSC was difficult. CSC are usually designed for a specific situation in a given setting, and synthetic analysis of previous CSC (24) is not helpful for physicians due to the extreme heterogeneity of studied situations. In anticipation of future pandemics, multiple mathematical models have been applied using data derived from previous COVID waves (6–8, 25) but only retrospectively. With the goal of promoting simplicity, we were mainly inspired by CSC used by military physicians.
The overall mortality rate was 47%, and the overall ICU mortality was 41% higher than the usual reported death rates (12, 14) but was consistent with mortality in our ICU during previous surges (16). However, this mortality rate in the ICU cannot be directly compared with that during previous surges, because the higher frequency of invasive mechanical ventilation in admitted patients (84%) was balanced by the high selection of patients with a good prognosis.
Among the ICU-admitted patients, 90-day mortality was independently associated with the number of comorbidities, SOFA score, and time from ICU referral to ICU admission. The burden of comorbidities has previously been described (12). Interestingly, 90-day mortality among ICU-admitted patients was not independently associated with the initial triage group. Two hypotheses can be raised to explain this result. First, the analysis included only patients admitted to the ICU and thus excluded the 14 patients from the Orange group who died before ICU admission. Second—besides the SOFA score, which was similar between the two groups, and the higher number of comorbidities in the Orange group, which is already known to be associated with higher mortality (12, 14, 16)—the longer delay between ICU referral and ICU admission could have influenced ICU prognosis (26). This is reinforced by the grim mortality rate among late ICU-admitted patients (66%), which was consistent between the Orange and Green groups. Our CSC strategy reduced late ICU admissions in the Green group (6%). The association between late ICU admission and mortality has been previously reported in cases of severe community-associated pneumonia (27). This result highlights that, even with a high standard of care in non-ICU wards, which performed HFNO with awake prone positioning, delayed ICU admission for COVID-19 pneumonia seems to be associated with higher mortality (21). The results of strategies for lowering mortality among COVID-19–infected patients remain unclear (28, 29). Due to a massive influx of patients during the pandemic, HFNO has been used outside the ICU to save resources (28), with seemingly good results (30). However many studies have reported that HFNO and awake prone positioning only delay the time to mechanical ventilation (29–32), without reducing the rate of oxygenation failure. The increased mortality rate among late ICU admission patients in our study should serve as a warning to physicians to reconsider ICU admission when possible for HNFO patients managed outside the ICU.
Mortality did not differ between patients admitted to expansion ICU beds versus normal ICU beds in our study, confirming recent data (33). All the back-up staff (particularly from the mainland) had ICU qualifications and were mixed with local ICU staff in each unit. Standard functioning of the ICU was preserved. Notably, the oversea transfers of stable acute respiratory distress syndrome COVID-19 patients by plane from Guadeloupe to mainland France—intermittently, with a transfer of seven to12 patients every 4–5 days—probably allowed us to avoid ICU saturation (1, 4).
Decision-making in crisis situations necessitates difficult choices. In our study, CSC directly resulted in non-ICU admission of 101 patients (31%/328) patients who died (87 in the Red group and 14 in the Orange group) “at the ICU gates.” Triage was difficult both for the physicians and for the next of kin. Unfortunately, we have not recorded data regarding potential conflicts with families when they were informed that their relative would not be immediately or not at all transferred in the ICU. However, with a step-back of 8 months, there was no ongoing legal procedures for nonadmission to the ICU. This may result from public information in advance of the limited numbers of ICU beds and the guiding principles of the triage during the massive surge (“choice of greater remaining lifespan”). As previously described (34), empathy seems to be the predominant feeling during the aftermath. The use of an ethics committee helped with difficult decisions and eased the burden of these decisions.
Our study has many limitations, mainly its monocentric design. As Guadeloupe is an isolated island, a multicentric design was not possible. Due to its design and the strain during the study period (Fig. 2), the study is not prospective, and thus conclusions should be interpreted cautiously. We did not thoroughly study the families’ feelings about “non-ICU admission” decisions after the surge, thus limiting our knowledge regarding the real public perception of CSC applications. The main strength of our study is the precise description of a CSC, along with its implications in terms of patient management and outcomes during a catastrophic surge.
CONCLUSIONS
During the fourth COVID-19 surge in Guadeloupe, caused by the Delta variant, a massive influx of patients resulted in healthcare system overload, especially in the ICU. The use of a CSC designed based on a multiapproach protocol, and regular overseas patient transfers, avoided complete ICU saturation and allowed the ICU admission of all patients with a high probability of survival. However, many patients could not be admitted to the ICU as soon as they should have been, and delayed ICU admission was associated with higher mortality risk.
ACKNOWLEDGMENTS
The authors thank the local ethics committee who helped to design the CSC and support us during this catastrophic surge. They thank all the staff of the ICU who allowed the increase of the number of beds. Additionally, they thank all the staff of University Hospital of Guadeloupe who worked very hard during this surge and sometimes endorsed ICU denial.
Supplementary Material
Footnotes
*See also p. 148.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. Demoule’s institution received funding from Respinor; he received funding from Fisher and Paykel, Respinor, Philips, Baxter, the French Ministry of Health, Getinge, Lungpacer, Lowenstein, and Gilead. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Grasselli G, Pesenti A, Cecconi M: Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA. 2020; 323:1545–1546 [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum L: Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020; 382:1873–1875 [DOI] [PubMed] [Google Scholar]
- 3.Painvin B, Messet H, Rodriguez M, et al. : Inter-hospital transport of critically ill patients to manage the intensive care unit surge during the COVID-19 pandemic in France. Ann Intensive Care. 2021; 11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turc J, Dupré H-L, Beaussac M, et al. : Collective aeromedical transport of COVID-19 critically ill patients in Europe: A retrospective study. Anaesth Crit Care Pain Med. 2021; 40:100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaidyanathan G: Coronavirus variants are spreading in India—What scientists know so far. Nature. 2021; 593:321–322 [DOI] [PubMed] [Google Scholar]
- 6.Nadjarian A, LeClair J, Mahoney TF, et al. : Validation of a crisis standards of care model for prioritization of limited resources during the coronavirus disease 2019 crisis in an urban, safety-net, Academic Medical Center*. Crit Care Med. 2021; 49:1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daugherty Biddison EL, Faden R, Gwon HS, et al. : Too many patients…A framework to guide statewide allocation of scarce mechanical ventilation during disasters. Chest. 2019; 155:848–854 [DOI] [PubMed] [Google Scholar]
- 8.Bhavani SV, Luo Y, Miller WD, et al. : Simulation of ventilator allocation in critically ill patients with COVID-19. Am J Respir Crit Care Med. 2021; 204:1224–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piscitello GM, Kapania EM, Miller WD, et al. : Variation in ventilator allocation guidelines by US State during the coronavirus disease 2019 pandemic: A systematic review. JAMA Netw Open. 2020; 3:e2012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SFAR: Recommandations de la SFAR sur la priorisation des traitements de réanimation pour les patients en état critique en période pandémique. Available at: https://sfar.org/priorisation-des-traitements-de-reanimation-pour-les-patients-en-etat-critique-en-situation-depidemie-de-covid-19-avec-capacites-limitees/. Accessed October 25, 2022.
- 11.Lesieur O, Quenot JP, Cohen-Solal Z, et al. : Admission criteria and management of critical care patients in a pandemic context: Position of the Ethics Commission of the French Intensive Care Society, update of April 2021. Ann Intensive Care. 2021; 11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators: Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021; 47:60–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis JM, McGovern AP, Thomas NJ, et al. : Trends in 28-day mortality of critical care patients with coronavirus disease 2019 in the United Kingdom: A national cohort study, March 2020 to January 2021*. Crit Care Med. 2021; 49:1895–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dongelmans DA, Termorshuizen F, Brinkman S, et al. : Characteristics and outcome of COVID-19 patients admitted to the ICU: A nationwide cohort study on the comparison between the first and the consecutive upsurges of the second wave of the COVID-19 pandemic in the Netherlands. Ann Intensive Care. 2022; 12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reilev M, Kristensen KB, Pottegård A, et al. : Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: A nationwide cohort. Int J Epidemiol. 2020; 49:1468–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pommier JD, Martino F, Bleakley K, et al. : A tale of a two waves epidemic: Characteristics and mortality risk factors for COVID-19 ICU patients in the French West Indies [Internet]. 2021. In Review. Available at: https://www.researchsquare.com/article/rs-200243/v1. Accessed October 26, 2021
- 17.Mehta V, Goel S, Kabarriti R, et al. : Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020; 10:935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JY, Kim HA, Huh K, et al. : Risk factors for mortality and respiratory support in elderly patients hospitalized with COVID-19 in Korea. J Korean Med Sci. 2020; 35:e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Requião-Moura LR, de Sandes-Freitas TV, Viana LA, et al. : High mortality among kidney transplant recipients diagnosed with coronavirus disease 2019: Results from the Brazilian multicenter cohort study. PLoS One. 2021; 16:e0254822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vera M, Kattan E, Born P, et al. : Intubation timing as determinant of outcome in patients with acute respiratory distress syndrome by SARS-CoV-2 infection. J Crit Care. 2021; 65:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González J, Benítez ID, de Gonzalo-Calvo D, et al. : Impact of time to intubation on mortality and pulmonary sequelae in critically ill patients with COVID-19: A prospective cohort study. Crit Care. 2022; 26:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vega ML, Dongilli R, Olaizola G, et al. : COVID-19 Pneumonia and ROX index: Time to set a new threshold for patients admitted outside the ICU. Pulmonology. 2022; 28:13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team: R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2018. Available at: https://www.R-project.org/ [Google Scholar]
- 24.Timbie JW, Ringel JS, Fox DS, et al. : Systematic review of strategies to manage and allocate scarce resources during mass casualty events. Ann Emerg Med. 2013; 61:677–689.e101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprung CL, Joynt GM, Christian MD, et al. : Adult ICU triage during the coronavirus disease 2019 pandemic: Who will live and who will die? Recommendations to improve survival*. Crit Care Med. 2020; 48:1196–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peigne V, Rusinová K, Karlin L, et al. : Continued survival gains in recent years among critically ill myeloma patients. Intensive Care Med. 2009; 35:512–518 [DOI] [PubMed] [Google Scholar]
- 27.Restrepo MI, Mortensen EM, Rello J, et al. : Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest. 2010; 137:552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnet N, Martin O, Boubaya M, et al. : High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: A retrospective study. Ann Intensive Care. 2021; 11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demoule A, Vieillard Baron A, Darmon M, et al. : High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020; 202:1039–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Issa I, Söderberg M: High-flow nasal oxygen (HFNO) for patients with Covid-19 outside intensive care units. Respir Med. 2021; 187:106554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrando C, Mellado-Artigas R, Gea A, et al. : Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: A multicenter, adjusted cohort study. Crit Care. 2020; 24:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosén J, von Oelreich E, Fors D, et al. : Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: The PROFLO multicenter randomized clinical trial. Crit Care. 2021; 25:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greco M, De Corte T, Ercole A, et al. : Clinical and organizational factors associated with mortality during the peak of first COVID-19 wave: The global UNITE-COVID study. Intensive Care Med. 2022; 48:690–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaki J: Catastrophe compassion: Understanding and extending prosociality under crisis. Trends Cogn Sci. 2020; 24:587–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.