Supplemental Digital Content is Available in the Text.
Key words: fluorophotometry, sub-retinal injection, sub-macular surgery, tissue plasminogen activator
Purpose:
There is renewed interest in subretinal drug delivery as the result of novel and emerging treatments for retinal diseases, including retinal gene therapy. However, our knowledge of the distribution of subretinally delivered drugs is incomplete; herein, we describe a qualitative and quantitative means of surveying the early intraocular distribution of subretinally delivered drugs using dilute sodium fluorescein (NaFl).
Methods:
Sodium fluorescein 10% was serially diluted and mixed with a solution containing tissue plasminogen activator (tPA) at a final concentration of 0.1 mg/mL NaFl and 0.5 mg/mL of tPA. Because this solution was to be used in the context of subretinal tPA injection in the treatment of subretinal hemorrhage, fluorophotometry with, and without, the presence of human whole blood was performed to derive a formula to calculate the concentration of NaFl based on the fluorescence of aspirated intraocular fluid. Videos of subretinal tissue plasminogen activator surgery in a case are presented as a qualitative demonstration of the technique and vitreous cavity fluid collected at case completion underwent fluorophotometry to estimate the loss of therapeutic solution.
Results:
Although the presence of hemoglobin in blood suppresses fluorescence of NaFl, we demonstrate that centrifuging admixtures of blood with NaFl negates the optical effects of blood and yields identical fluorescence versus concentration plots to those of NaFl solution alone. We also demonstrate that NaFl at 0.1 mg/mL can be readily used to qualitatively assess drug losses before, during, and after subretinal injection. Furthermore, we describe how it may be used to quantitatively estimate the total loss of therapeutic solution during subretinal injection using fluorophotometry on aspirated fluid from the vitreous cavity (loss estimated as 4% in the case presented).
Conclusion:
Sodium fluorescein at a concentration of 0.1 mg/mL can be used to quantitatively and qualitatively assess the fate of subretinally injected drugs during subretinal injection surgery.
Subretinal injection as a route for therapy has garnered increasing attention over the past decade as a direct result of emerging treatments for retinal disease that necessitate this approach, such as gene therapy.1 Furthermore, it is an established technique for the management of submacular hemorrhage.2 However, there are limited data regarding the intraocular distribution and loss of therapeutic solution during subretinal injection. Furthermore, there remains some debate regarding the best approach for performing subretinal injections, and two alternative approaches are described: one-step3 and two-step4 subretinal injections. Khan et al5 have previously described the use of sodium fluorescein (NaFl) at an unknown concentration to qualitatively track the flow of tissue plasminogen activator (tPA) during subretinal injection. This approach is attractive because dilute vital dyes, including NaFl 0.1 mg/mL, appear to be safe in vitro6,7 and 50% to 70% retinal detachments using this concentration of NaFl do not significantly alter electroretinogram responses over sham procedures in murine models in vivo.8 Here, we describe the use of 0.1 mg/mL NaFl to optically “label” a modified tPA (alteplase) during subretinal injection. We anticipated that this would enable the qualitative assessment of the flow of tPA during surgery. We additionally sought to use a fluorophotometric protocol to quantify drug loss during subretinal injection in an exemplar patient undergoing vitrectomy with subretinal tPA injection and gas (SF6) for the management of submacular hemorrhage secondary to age-related macular degeneration.
Methods
The aim of this investigation was to develop a technique that could be used to qualitatively and quantitatively assess the wastage of therapeutic substances intended for delivery into the subretinal space. The research described herein uses alteplase and sodium fluorescein for “off-label” indications and was approved by the institution review board (HREC/18/POWH/562).
We first evaluated the use of fluorophotometry to quantify the concentration of serial dilutions of NaFl in phosphate buffered saline (between 0.2 and 100 ng/mL). Normalized fluorescence was plotted against concentration and the data fitted with a straight line using standard linear regression techniques: goodness of fit was evaluated using the coefficient of determination for a linear regression model (r2). To simulate the effects of mixture of this solution with variable concentrations of blood and NaFl, we mixed NaFl (700µL at a concentration of 100 ng/mL) with 10 µL of human blood and performed serial 2x dilutions of the mixture. In addition, we assessed the effects of centrifuging (Heraeus Biofuge 13; Baxter Scientific, Deerfield IL) admixtures of blood and dilute NaFl to assess its ability to remove unwanted blood products before fluorophotometry. After spinning of the mixed solution twice at the rate of 13,000 rpm for 10 minutes, the supernatant was eluted. All fluorophotometric measurements were performed with a Safire II UV-Vis fluorophotometer (Tecan Group Ltd, Männedorf, Switzerland) using an excitation wavelength of 481 nm and a wavelength of 522 nm for the analysis of emission. The techniques described were developed as part of a randomized clinical trial aimed at comparing one-step to two-step subretinal injections in the context of delivering subretinal tPA in patients undergoing so-called vitrectomy, subretinal tPA, and “pneumatic displacement”2 for the management of subretinal hemorrhage (Australian and New Zealand Clinical Trials Registry identifier ACTRN12619001121156).
Surgical Technique
We also performed a qualitative and quantitative analysis of subretinal drug delivery and wastage in the index patient of our trial. The patient, a 75-year-old woman, presented with a 4-day history of sudden vision loss in the context of underlying age-related macular degeneration. Treatment followed on from a standard 25 g vitrectomy (Constellation System; Alcon, Fort Worth TX), which consisted of core vitrectomy, induction of posterior vitreous detachment and peripheral trim (see Video, Supplemental Digital Content 1, http://links.lww.com/IAE/B213). 0.1 mL of a solution containing NaFl at a concentration of 0.1 mg/mL (half of the minimum concentration for in vitro toxicity)6,7 and tPA (alteplase) at a concentration of 0.5 mg/mL (total dose in 0.1 mL proven to be safe in animal studies)8 was injected subretinally. The solution was delivered using the foot-actuated viscous fluid control mechanism of the unit: the drug was loaded into a specially developed 1 mL syringe that can be coupled to the VFC tubing (MedOne Inc, Sarasota FL). The subretinal space was accessed using a 25/38 g Teflon tipped cannula (MedOne Inc), which was used to penetrate the neurosensory retina before the commencement of injection. Once injection was completed, an indented peripheral retinal search for breaks was performed before an air–fluid exchange and insertion of 20% SF6. All extruded fluid from the vitreous cavity was kept for fluorophotometric measurements (to ascertain the concentration of NaFl) following centrifuging, as outlined above. The total volume of aspirated fluid was also measured so that the quantity of regurgitated NaFl (and therefore the percentage loss of therapeutic solution) could be calculated. The surgery itself was recorded onto an external hard drive to facilitate the objective assessment of drug wastage.
Results
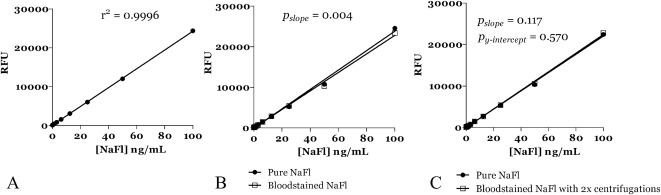
Fluorophotometric plots of relative fluorescence versus fluorescein concentration are plotted in Figure 1A. The data were well-described by a linear fit (r2 = 0.99). Contamination with known concentrations of blood resulted in a small, but statistically significant decreased fluorescence (Figure 1B; F(1, 62) = 8.96, P = 0.004) because of its absorption of the excitation and emission wavelengths of NaFl. However, centrifuging admixtures of blood and NaFl with extraction of the supernatant resulted in fluorophotometric results that were both well-described by a linear fit (r2 = 0.99) and indistinguishable from fluorescence versus concentration plots for “pure” NaFl (no significant difference in slope (F(1, 62) = 2.53, P = 0.12) or Y-intercept (F(1, 63) = 0.33, P = 0.57) (Figure 1C).
Fig. 1.
A. Fluorescence (relative fluorescence units; RFU) versus concentration (ng/mL) curves for pure sodium fluorescein in solution. Excitation wavelength 481 nm; analysis/emission wavelength 522 nm. B. Fluorescence (relative fluorescence units; RFU) versus concentration (ng/mL) curves for pure sodium fluorescein and bloodstained sodium fluorescein in solution. Excitation wavelength 481 nm; analysis/emission wavelength 522 nm. The addition of blood results in a significant decrease in fluorescence (P = 0.004). C. Fluorescence (relative fluorescence units; RFU) versus concentration (ng/mL) curves for pure sodium fluorescein and bloodstained sodium fluorescein in solution following centrifuging (see text for details). Excitation wavelength 481 nm; analysis/emission wavelength 522 nm. Note the curves are near-identical.
The surgical procedure in the index patient was uneventful (see Video, Supplemental Digital Content 1, http://links.lww.com/IAE/B213). A small quantity of NaFl-labelled solution could be seen passively mixing with balanced salt solution in the vitreous cavity after the introduction of the needle into the eye, despite no pressure being placed on the foot-pedal which controls the VFC syringe (see Video, Supplemental Digital Content 1, http://links.lww.com/IAE/B213). This is presumably a consequence of positive pressure within the syringe relative to the vitreous cavity as the result of the Constellation System's “IOP compensation mode” making adjustments to infusion pressure following the introduction of the cannula into the vitreous cavity. After the cannula is correctly inserted into the subretinal space and the injection commenced, the dye can also be readily observed filling the subretinal bleb (see Video, Supplemental Digital Content 1, http://links.lww.com/IAE/B213). At the conclusion of the injection, a further small plume of fluorescein is observed emanating from the retinotomy and the cannula tip; this is again presumed to be because of the pressure differentials between the subretinal space, syringe, and vitreous cavity. Despite these observed losses, the calculated % wastage of drug based on fluorophotometric measurements was estimated to be only 4%.
Discussion
Our aim was to develop and use a technique to study both qualitatively and quantitatively the intraocular distribution of therapeutic substances in the context of subretinal injection of tPA for the management of subretinal hemorrhage (ANZCTR identifier ACTRN12619001121156). We first derived fluorescence versus concentration data for NaFl solutions at different dilutions: these data, when plotted as fluorescence versus concentration, were well-described by a linear fit (r2 = 0.99). Furthermore, we thereafter demonstrated that contamination of dilute NaFl solution with blood results in artefactual decreases in measured autofluorescence as a fixed “filter effect,” but that this may be negated by centrifuging samples before measurement: fluorescence versus concentration plots following the latter were indistinguishable from values for pure fluorescein solutions. NaFl at the concentration used in the injected solution (0.1 mg/mL) has previously been demonstrated to be nontoxic when exposed to sprouting neurites7 and human embryonic kidney cells 6; furthermore, this concentration does not seem to be deleterious in murine models of limited iatrogenic detachment.9 We demonstrate that fluorescein at 0.1 mg/mL is also readily visualized during surgery (see Video, Supplemental Digital Content 1, http://links.lww.com/IAE/B213) and concentrations down to 0.2 ng/mL can be detected using fluorophotometry (Figure 1).
Although NaFl has been proposed as a means of qualitatively monitoring subretinal drug delivery,5,6 and has been used in murine models to track subretinal injection of gene therapy solutions,10 its use in humans in vivo has been restricted to the qualitative assessment of unknown concentrations.5 Alternative methods of estimating subretinal bleb volume using newly developed intraoperative swept-source optical coherence tomography also exist,11 although they have only been reported in porcine models of subretinal injection of a target dose of 0.05 mL (triamcinolone). In this porcine model, undetected losses of at least 12% occur during subretinal injection.11 Using our technique, we were readily able to visualize lower volumes of drug loss that are presumably undetectable when using triamcinolone.11 It should also be noted that the most commonly available microscope-integrated spectral domain-optical coherence tomography microscopes are limited by several factors.12 First, measurement and analysis is time-consuming11,12; second, current generation intraoperative spectral domain-optical coherence tomography is limited to measuring sagittal depths (i.e., bleb heights) of less than about 2.5 mm 12; third (in its present guise), intraoperative optical coherence tomography may only be used to assess retained volume when balanced salt solution is used as the vitreous substitute. Our technique is rapid, can be applied regardless of bleb height and is suitable where air–fluid exchange is necessary. The disadvantages of this technique include the fact that quantification of retained subretinal drug volume is indirect, and that estimates of wastage implicitly assume that loss of fluorescein into other ocular compartments (e.g., the anterior chamber, the residual vitreous base), and diffusion from the bleb into the vitreous cavity, are negligible. Furthermore, it assumes a complete air–fluid exchange.
In summary, the use of NaFl at a previously demonstrated nontoxic dose (0.1 mg/mL)6,7 is effective as an optical “label” to qualitatively assess the flow of subretinally injected solutions. Furthermore, when combined with fluorophotometry, it provides a quantitative estimate of drug loss.
Footnotes
Foundation Fighting Blindness USA (CD-CL-0816-0710-SYD).
None of the authors has any conflicting interests to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
References
- 1.Simunovic MP, Xue K, Jolly JK, et al. Structural and functional recovery following limited iatrogenic macular detachment for retinal gene therapy. JAMA Ophthalmol 2017;135:234–241. [DOI] [PubMed] [Google Scholar]
- 2.Stanescu-Segall D, Balta F, Jackson TL. Submacular hemorrhage in neovascular age-related macular degeneration: a synthesis of the literature. Surv Ophthalmol 2016;61:18–32. [DOI] [PubMed] [Google Scholar]
- 3.Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 2017;390:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards TL, Jolly JK, Groppe M, et al. Visual acuity after retinal gene therapy for choroideremia. N Engl J Med 2016;374:1996–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MA, Shahlaee A, Bansal AS, et al. Fluorescein-assisted subretinal tissue plasminogen activator (tPA) delivery for submacular hemorrhage. Retina 2017;37:1203–1206. [DOI] [PubMed] [Google Scholar]
- 6.Salvetti AP, Patricio MI, Barnard AR, et al. Impact of vital dyes on cell viability and transduction efficiency of AAV vectors used in retinal gene therapy surgery: an in vitro and in vivo analysis. Transl Vis Sci Technol 2017;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato S, Madachi-Yamamoto S, Hayashi Y, et al. Effect of sodium fluorescein on neurite outgrowth from the retinal explant culture: an in vitro model for retinal toxicity. Brain Res 1983;313:143–147. [DOI] [PubMed] [Google Scholar]
- 8.Lewis H, Resnick SC, Flannery JG, et al. Tissue plasminogen activator treatment of experimental subretinal hemorrhage. Am J Ophthalmol 1991;111:197–204. [DOI] [PubMed] [Google Scholar]
- 9.Qi Y, Dai X, Zhang H, et al. Trans-corneal subretinal injection in mice and its effect on the function and morphology of the retina. PLoS One 2015;10:e0136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai X, Zhang H, Han J, et al. Effects of subretinal gene transfer at different time points in a mouse model of retinal degeneration. PLoS One 2016;11:e0156542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu ST, Gabr H, Viehland C, et al. Volumetric measurement of subretinal blebs using microscope-integrated optical coherence tomography. Transl Vis Sci Technol 2018;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehlers JP, Tao YK, Farsiu S, et al. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci 2011;52:3153–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]