Study Design.
Prospective.
Objective.
To investigate the influence of paraspinal fatty muscle infiltration (FMI) and cumulative lumbar spine degeneration as assessed by magnetic resonance imaging on long-term clinical outcome measures in patients with lumbar spinal canal stenosis (LSCS) of the Lumbar Stenosis Outcome Study (LSOS) cohort.
Summary of Background Data.
Past studies have tried to establish correlations of morphologic imaging findings in LSCS with clinical endpoints. However, the impact of FMI and overall lumbar spinal degeneration load has not been examined yet.
Materials and Methods.
Patients from the LSOS cohort with moderate to severe LSCS were included. Two radiologists assessed the degree of LSCS as well as cumulative degeneration of the lumbar spine. FMI was graded using the Goutallier scoring system. Spinal Stenosis Measure (SSM) was used to measure the severity level of symptoms and disability. European Quality of Life 5 Dimensions 3 Level Version (EQ-5D-3L) was used to measure health-related quality of life.
Results.
The nonsurgically treated group consisted of 116 patients (age 74.8±8.5 yr), whereas the surgically treated group included 300 patients (age 72.3±8.2 yr). Paraspinal FMI was significantly different between the groups (54.3% vs. 32.0% for Goutallier grade ≥2; P<0.001). Total degeneration score was comparable in both groups (9.5±2.0 vs. 9.3±2.0; P=0.418). FMI was associated with lower SSM function and lower EQ-5D-3L (all P<0.05), but not with SSM symptoms. Total degeneration of the lumbar spine was associated neither with SSM symptoms, nor with SSM function, nor with EQ-5D-3L (all P>0.05).
Conclusions.
FMI is associated with higher disability and worse health-related quality of life of LSCS patients in the LSOS cohort. There was no significant association between total cumulative lumbar spine degeneration and the outcome of either surgically or nonsurgically treated patients.
Level of Evidence.
3.
Key Words: lower spinal canal stenosis, paraspinal muscles, fatty muscle infiltration, spine, back pain
Lumbar spinal canal stenosis (LSCS) is defined as narrowing of the central spinal canal and might lead to nerve root affection, making LSCS the most frequent indication for spinal surgery in patients older than 65 years.1 Hence, due to an aging population in industrialized countries the incidence and morbidity associated with this condition may further increase in the near future.2,3
Imaging plays a key role in the diagnosis of LSCS. Especially magnetic resonance imaging (MRI) is often performed. Due to increasing image and depiction quality in recent years, many studies have tried to establish correlations of morphologic imaging findings with clinical endpoints, however, with limited success.4–6
Beyond controversially discussed morphologic criteria of LSCS and quantification techniques, there may be other imaging factors such as fatty muscle infiltration (FMI) or the extent of spinal degeneration that impact on the outcome of patients with LSCS.7,8 FMI is usually semiquantitatively assessed by using established scales, for examination, Goutallier grading.9,10 More recently, quantitative methods to assess FMI (e.g., thresholding or fat-water images) have been used to attempt standardization of measurements.11 Recent studies have also looked at the geometry of fatty infiltration by using texture analysis of MRIs and its possible impact on clinical outcome measures.12 Some studies found an association of multifidus muscle and psoas muscle morphology with functional status in LSCS patients but concluded that further studies are needed to prove a relation between prognosis/outcome and muscle atrophy.13,14
In contrast, spine degeneration is a major cause of LSCS and foraminal stenosis with ensuing radicular or nonradicular lower back pain. However, the lumbar spine of an individual is usually affected by degenerative changes at various levels, complicating symptom attribution to a single level or specific imaging feature.4,15 Most studies investigated degenerative changes of specific spine levels and imaging features, for example, maximal foraminal stenosis at a certain side and level but neglected adjacent or overall degeneration load.
Despite efforts to assess the influence of muscle quality and morphologic imaging features on clinical outcome in LSCS, there is a lack of studies that have examined the differential impact of FMI and overall spinal degeneration load on clinical outcomes.
Thus, the purpose of this study was to investigate the influence of paraspinal FMI and overall lumbar spine degeneration as assessed by MRI on long-term clinical outcome measures in LSCS patients of the Swiss “Lumbar Stenosis Outcome Study” (LSOS) cohort.
MATERIALS AND METHODS
The LSOS is a large multicenter prospective cohort study that includes patients that suffer from LSCS with neurogenic claudication and positive imaging findings.16 MRI was routinely performed at the first time point, that is, the first study visit in addition to clinical assessment. Yearly clinical follow-up (12 and 24 months) up to 36 months after study inclusion was performed. The collective data is available in the LSOS database.
The institutional review board and the local ethics committee (Ethics Committee Zurich) approved the study. Written general consent for the use of clinical data for scientific purposes was obtained from each patient.
Eligibility Criteria
For the retrospective data analysis of this study only patients from the LSOS cohort with at least one segment of the lower spine [third lumbar vertebra (L3) to first sacral vertebra (S1)] with “moderate” to “severe” spinal stenosis were included (definition see below). Exclusion criteria are listed in Figure 1. Subjects were classified either in a surgical treatment group (lumbar decompression surgery and/or spinal fusion surgery) or in a nonsurgical group. Patient characteristics are shown in Table 1.
Figure 1.
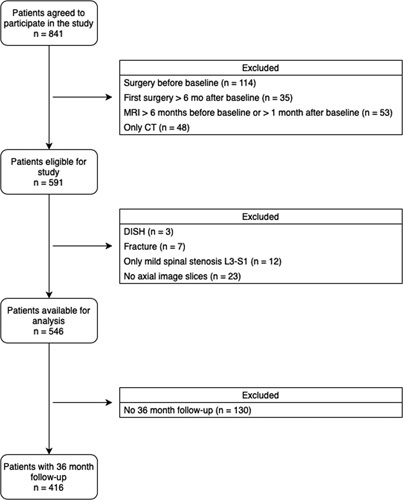
Patients’ flowchart detailing inclusion and exclusion criteria of the study. CT indicates computed tomography; DISH, diffuse idiopathic skeletal hyperostosis; MRI, magnetic resonance imaging.
TABLE 1.
Patient Demographics at the Time of Study Inclusion (t=0)
| n (%) | P | ||
|---|---|---|---|
| No OP | OP | ||
| Number of patients (n) | 116 | 300 | |
| Age [mean (SD)] | 74.8 (8.5) | 72.3 (8.2) | 0.007 |
| Female | 59 (50.9) | 149 (49.7) | 0.913 |
| BMI [mean (SD)] | 27.9 (5.9) | 27.3 (4.6) | 0.275 |
| BMI ≥25 | 78 (67.2) | 203 (67.7) | 1 |
| Compulsory education | 25 (21.6) | 70 (23.3) | 0.796 |
| CIRS [mean (SD)] | 9.3 (4.4) | 9.3 (3.9) | 0.99 |
| Diabetes | 14 (12.1) | 28 (9.3) | 0.516 |
| Smoker | 21 (18.1) | 50 (16.7) | 0.838 |
| Problem getting better or worse in the last 3 mo | <0.001 | ||
| Getting better | 28 (24.1) | 15 (5.0) | |
| Staying about the same | 26 (22.4) | 44 (14.7) | |
| Getting worse | 62 (53.4) | 239 (79.7) | |
| Don’t know | 0 (0.0) | 2 (0.7) | |
| Duration of symptoms>6 mo | 79 (68.1) | 228 (76.0) | 0.129 |
| HADS depression | 17 (14.7) | 51 (17.0) | 0.666 |
| HADS anxiety | 16 (13.8) | 59 (19.7) | 0.209 |
| Walking distance | 0.656 | ||
| >3 km | 20 (17.4) | 48 (16.1) | |
| >200m, ≤3 km | 59 (51.3) | 160 (53.5) | |
| >15 m, ≤200 m | 24 (20.9) | 70 (23.4) | |
| ≤15 m | 12 (10.4) | 21 (7.0) | |
| Walking distance>200 m | 79 (68.7) | 208 (69.6) | 0.958 |
| SSM symptoms [mean (SD)] | 3.0 (0.7) | 3.1 (0.6) | 0.038 |
| SSM function [mean (SD)] | 2.2 (0.8) | 2.3 (0.7) | 0.676 |
| EQ-5D-3L summary index [mean (SD)] | 0.6 (0.3) | 0.5 (0.3) | 0.003 |
| Muscle fat MRI Goutallier | <0.001 | ||
| Grade 0 | 18 (15.5) | 64 (21.3) | |
| Grade 1 | 35 (30.2) | 140 (46.7) | |
| Grade 2 | 47 (40.5) | 79 (26.3) | |
| Grade 3 | 15 (12.9) | 12 (4.0) | |
| Grade 4 | 1 (0.9) | 5 (1.7) | |
| Muscle fat MRI Goutallier grade 2, 3, or 4 | 63 (54.3) | 96 (32.0) | <0.001 |
| Degeneration score total [mean (SD)] | 9.5 (2.0) | 9.3 (2.0) | 0.418 |
Bold values indicate statistical significance.
BMI indicates body mass index; CIRS, Cumulative Illness Rating Scale; EQ-5D-3L, European Quality of Life 5 Dimensions 3 Level Version; HADS, Hospital Anxiety and Depression Scale; MRI, magnetic resonance imaging; OP, operation; SSM, Spinal Stenosis Measure.
Imaging and Analysis
LSOS being a multicenter study imaging was performed on different magnetic resonance (MR) scanners in seven different radiology departments (1.5 and 3 T). Scanning parameters varied but MRI protocols were adjusted beforehand to harmonize the results. Minimum requirements were high-resolution sagittal T1 weighted (w) and T2w images as well as axial T2w images.
Two radiologists with 16 years and six years of experience in spine imaging analyzed the MR scans independently. Intrareader and interreader agreements for all qualitative and quantitative parameters regarding LSCS and degenerative changes of the lumbar spine of LSOS patients have already been assessed by Winklhofer et al 17 and were therefore not included in this study.
Spinal Stenosis Assessment
In accordance with the consensus paper of Andreisek et al 18 and previously published studies from the LSOS cohort, three core parameters were used to assess the severity of LSCS: (1) compromise of the central zone19; (2) relation between fluid and cauda equina (Schizas classification)20; and (3) nerve root compression in the lateral recesses.21 (Table 2) The highest grading in one of the three core parameters on all levels subsequently defined the highest stenotic grading (i.e., “mild,” “moderate,” or “severe”).
TABLE 2.
Grading of Lumbar Spinal Canal Stenosis According to Magnetic Resonance Imaging Parameters
| Magnetic Resonance Parameters | Grade | Description |
|---|---|---|
| Compromise of central zone | Mild | Compromise of ≤1/3 of its normal size |
| Moderate | Compromise of 1/3–2/3 of its normal size | |
| Severe | Compromise of >2/3 of its normal size | |
| Relation between fluid and cauda equina (Schizas classification) | Mild | The nerve rootlets lie dorsally or centrally; CSF is well visible in the dorsal sac |
| Moderate | The nerve rootlets occupy the whole of the dural sac; some CSF is still present | |
| Severe | No nerve rootlets visible; no CSF visible | |
| Nerve root compression in the lateral recesses | Mild | Lateral recess narrowing; no nerve root compression |
| Moderate | More significant lateral recess narrowing; nerve root compression with CSF around | |
| Severe | Severe nerve root compression without CSF around |
CSF indicates cerebrospinal fluid.
Degeneration Score
To assess the degree of spinal degeneration a score based on anterior and posterior column affection was established, based on a modified three-column classification for spinal injuries by Denis.22 Each column contained three items: disk degeneration, Modic type endplate changes, and spondylolisthesis for the anterior column; facet joint degeneration, ligamentum flavum hypertrophy, and epidural lipomatosis for the posterior column.
Disk degeneration was evaluated using the Pfirrmann classification system for lumbar disk degeneration.23 Patients with grades I to III were labeled as “no disk degeneration” considered to be clinically relevant, whereas patients with grades IV and V were given a point for “disk degeneration.” Endplate changes were graded using the Modic classification for vertebral body endplate MRI signal.24 Patients with Modic type II and III changes received a point for “Modic degeneration.” Spondylolisthesis was assessed with Meyerding grades by dividing the superior endplate of the following vertebra in four parts and measuring the position of the posteroinferior corner of the vertebra above.25 Grades >0 were rated as positive for spondylolisthesis and were given a point. Presence (yes or no) of facet joint degeneration, ligamentum flavum hypertrophy, and epidural lipomatosis was rated based on morphologic criteria (Table 3).
TABLE 3.
MR Degeneration Score With Respective Items for the Anterior and Posterior Columns of the Lower Spine
| Classification/Grading | Description | Points | |
|---|---|---|---|
| Anterior column | |||
| Disk degeneration | Pfirrmann grade I | Homogeneous disk with hyperintense T2-weighted signal and normal height | 0 |
| Pfirrmann grade II | Inhomogeneous disk with hyperintense T2-weighted signal and normal height | 0 | |
| Pfirrmann grade III | Inhomogeneous disk with an intermittent gray T2-weighted signal intensity and normal or slightly decreased height | 0 | |
| Pfirrmann grade IV | Inhomogeneous disk with hypointense dark gray T2-weighted signal intensity and slightly/moderately decreased height | 1 | |
| Pfirrmann grade V | Inhomogeneous disk with a hypointense black T2-weighted signal intensity and disk space collapse | 1 | |
| Endplate changes | Modic type I | T1 low signal, T2 high signal, T1+C enhancement | 0 |
| Modic type II | T1 high signal, T2 iso to high signal | 1 | |
| Modic type III | T1 and T2 low signal | 1 | |
| Spondylolisthesis | Meyerding grade I | 0%–25% of anterior displacement | 1 |
| Meyerding grade II | 26%–50% of anterior displacement | 1 | |
| Meyerding grade III | 51%–75% of anterior displacement | 1 | |
| Meyerding grade IV | 76%–100% of anterior displacement | 1 | |
| Meyerding grade V | >100% of anterior displacement | 1 | |
| Posterior column | |||
| Facet joint degeneration | Yes | Joint effusion, irregular articular surface | 1 |
| Ligamentum flavum hypertrophy | Yes | Subjective visual thickening | 1 |
| Epidural lipomatosis | Yes | Increase of epidural fat compartment | 1 |
Every segment (from L3 to S1) was graded with 0 to 3 points for the anterior column and 0 to 3 points for the posterior column, consecutively maximizing the score to 6 points for segments that featured all criteria of degeneration. For all three segments, the maximum score was 18 points and the minimum score was zero points (no degeneration).
Lumbar Muscle Fatty Degeneration
Paraspinal FMI (right and left multifidus and erector spinae) was graded using the Goutallier scoring system (0–4; grade 0 for “normal muscle without fat,” grade 1 for “few fatty streaks within the muscle,” grade 2 for “less fat than muscle within the muscle,” grade 3 for “same amount of fat and muscle within the muscle,” and grade 4 for “more fat than muscle within the muscle”)26 on a transaxial T2w image at level L3 at the height of the vertebral body. The axial slice orientation was corrected to be perpendicular to the right and left multifidus and erector spinae combined muscle mass.
Outcome Measures
Spinal Stenosis Measure (SSM) was used to measure the severity level of symptoms (score range 1–5, best-worst) and function (score range 1–4, best-worst), a method developed by Stucki et al.27 To measure the health-related quality of life, the “European Quality of Life 5 Dimensions 3 Level Version” (EQ-5D-3L) was used. It includes mobility, self-care, usual activities, pain/discomfort and anxiety/depression.28 We worked with a summary index (SI) value (health state converted into a single value) which had a range from −0.53 to 1.0 represented a health state equivalent to being dead, 1 represented a health state of full health.29,30
Clinical Outcomes
The main outcomes of this study were changes in SSM symptoms (pain), SSM function (disability), and EQ-5D-3L SI (quality of life) between baseline and one, two, and three years of follow-up.
Statistical Analysis
Patient characteristics at baseline were summarized with means and SDs for continuous and ordinal variables, and counts and percentages of the total for categorical variables. To test for differences between the groups of patients at baseline, we used χ2 and Mann-Whitney tests and reported P values.
Linear mixed-effects regression models were used for the continuous outcomes of SSM symptoms scores, SSM function scores, and EQ-5D-3L SI scores over time (overall change from baseline to 12, 24, and 36 months) to study whether paraspinal FMI and overall lumbar spine degeneration had an effect on these outcomes. The models included the following covariates measured at baseline: paraspinal FMI (Goutallier score ≥2), treatment group (surgical), total degeneration score, age, sex, body mass index (BMI), duration of symptoms (>6 months), Cumulative Illness Rating Scale (CIRS), pain course before inclusion into study (problem getting worse in the last three months before inclusion into study), depression (Hospital Anxiety and Depression Scale depression subscale ≥8 points), and follow-up time. Further, we evaluated whether an interaction term of paraspinal FMI and one of the following covariates was necessary: treatment group, degeneration score, age, sex, or BMI. The interaction term was included in the model if the corresponding P value of the interaction was<0.05. In addition, the following covariates were centered with the mean value: total degeneration score (mean value=9.4), age (73.0), BMI (27.5), and CIRS (9.3). We presented the results as β coefficients and corresponding 95% confidence intervals (CIs). All analyses were conducted with R for Windows.31
RESULTS
Between December 2010 and December 2015, a total of 841 patients participated in the LSOS study. Of these, 425 patients did not meet the inclusion criteria (Figure 1). Patient characteristics of both LSCS patient groups (with or without ensuing operation) at the start of data collection (t=0) are presented in Table 1.
The nonsurgically treated group consisted of 116 patients, whereas the surgically treated group included 300 patients. The female proportion was similar in both groups (50.9% and 49.7%, respectively).
There were differences with regard to patient age (mean age: 74.8 and 72.3 yr, respectively; P=0.007), pain course before inclusion into study (53.4% vs. 79.7% with worsening symptoms; P<0.001), paraspinal FMI (54.3% vs. 32.0%; P<0.001), SSM symptoms (mean: 3.0 vs. 3.1; P=0.038), and EQ-5D-3L SI (mean: 0.6 vs. 0.5; P=0.003).
Fatty Muscle Infiltration
A total of 116 nonsurgically treated patients were included, 18 of which had normal paraspinal muscles (Goutallier grade 0; 15.5%), 35 had minor changes (Goutallier grade 1; 30.2%), 47 had <50% of FMI (Goutallier grade 2; 40.5%), and 15 had ∼50% of FMI (Goutallier grade 3; 12.9%). Only one patient who was nonsurgically treated had over 50% of FMI (Goutallier grade 4; 0.9%).
In contrast, 300 surgically treated patients were included, 64 of which had normal muscle condition (Goutallier grade 0; 21.3%), 140 with minor changes in muscle quality (Goutallier grade 1; 46.7%), 79 with <50% of FMI (Goutallier grade 2; 26.3%), and 12 patients with Goutallier grade 3 FMI (4%). Goutallier grade 4 FMI was diagnosed in five patients (1.7%) (Figure 2).
Figure 2.
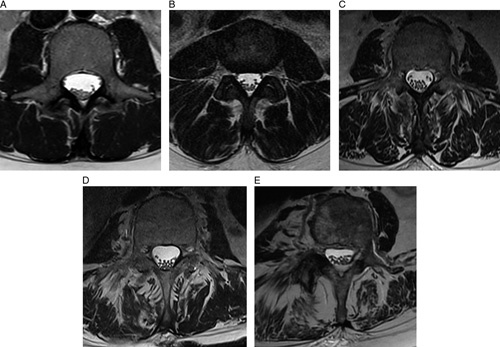
Assessment of fatty muscle infiltration of the paraspinal muscles of the lower spine using the Goutallier grading system on axial T2-weighted magnetic resonance images. Normal muscle (grade 0) (A); some fatty streaks (grade 1) (B); <50% fatty muscle atrophy (grade 2) (C); 50% fatty muscle atrophy (grade 3) (D); >50% fatty muscle atrophy (grade 4) (E).
Change in SSM Symptoms Scores From Baseline Over Time
A mixed-effects model was fitted with random intercepts for patients (Table 4). On average, patients experienced less pain/symptoms in the course of the study (t=12, 24, 36 months) with most improvement taking place in the first 12 months and stable symptoms in the months thereafter (Table 3). Paraspinal FMI and total degeneration score were not associated with SSM symptoms (β=0.089, 95% CI: −0.031 to 0.209, P=0.150; and β=0.006, 95% CI: −0.022 to 0.034, P=0.692, respectively). In addition, the nonsignificant effects were quite small compared with the score range of SSM symptoms. Further, there was no interaction between paraspinal FMI and treatment group, or total degeneration score, or age, or sex, or BMI, respectively. Therefore, we did not include them in the model.
TABLE 4.
SSM Symptoms Analysis
| Mixed-effects | Effects | P | ||
|---|---|---|---|---|
| Estimate | 2.5% | 97.5% | ||
| Intercept | 2.301 | 2.092 | 2.510 | <0.001 |
| FMI of paraspinal muscles (Goutallier grade ≥2) | 0.089 | −0.031 | 0.209 | 0.150 |
| Degeneration score total | 0.006 | −0.022 | 0.034 | 0.692 |
| Age | 0.004 | −0.003 | 0.011 | 0.278 |
| Female | 0.227 | 0.117 | 0.338 | <0.001 |
| BMI | 0.019 | 0.008 | 0.030 | 0.001 |
| Duration of symptoms>6 mo | 0.147 | 0.027 | 0.268 | 0.018 |
| CIRS | 0.025 | 0.011 | 0.038 | <0.001 |
| Problem getting worse in the last 3 mo before inclusion into study | 0.248 | 0.124 | 0.372 | <0.001 |
| HADS | 0.383 | 0.237 | 0.529 | <0.001 |
| Study visit at 12 mo | −0.502 | −0.638 | −0.366 | <0.001 |
| Study visit at 24 mo | −0.519 | −0.654 | −0.383 | <0.001 |
| Study visit at 36 mo | −0.526 | −0.661 | −0.391 | <0.001 |
| Surgery | 0.101 | −0.057 | 0.260 | 0.216 |
| Study visit at 12 mo: surgery | −0.539 | −0.700 | −0.379 | <0.001 |
| Study visit at 24 mo: surgery | −0.480 | −0.639 | −0.320 | <0.001 |
| Study visit at 36 mo: surgery | −0.485 | −0.644 | −0.326 | <0.001 |
Bold values indicate statistical significance.
SSM symptoms range 1 to 5, best to worst; minimal clinically important difference=0.48.
BMI indicates body mass index; CIRS, Cumulative Illness Rating Scale; FMI, fatty muscle infiltration; HADS, Hospital Anxiety and Depression Scale; SSM, Spinal Stenosis Measure.
Change in SSM Function Scores From Baseline Over Time
A mixed-effects model was fitted with random intercepts for patients (Table 5). On average patients experienced less disability in the course of the study (t=12, 24, 36 months). Total degeneration score was not associated with SSM function (β=−0.005, 95% CI: −0.028 to 0.018), whereas FMI proved to be associated with SSM function (β=0.162, 95% CI: 0.063–0.262, P=0.002), even though the estimate was small compared with the score range of SSM function. Further, there was no interaction between FMI and treatment group, or total degeneration score, or age, or sex, or BMI, respectively. Therefore, we did not include them in the model.
TABLE 5.
SSM Function Analysis
| Mixed-effects | Effects | P | ||
|---|---|---|---|---|
| Estimate | 2.5% | 97.5% | ||
| Intercept | 1.719 | 1.544 | 1.894 | <0.001 |
| FMI of paraspinal muscles (Goutallier grade ≥2) | 0.162 | 0.063 | 0.262 | 0.002 |
| Degeneration score total | −0.005 | −0.028 | 0.018 | 0.699 |
| Age | 0.005 | −0.001 | 0.010 | 0.136 |
| Female | 0.178 | 0.086 | 0.269 | <0.001 |
| BMI | 0.022 | 0.013 | 0.031 | <0.001 |
| Duration of symptoms>6 mo | 0.020 | −0.080 | 0.120 | 0.702 |
| CIRS | 0.012 | 0.001 | 0.024 | 0.033 |
| Problem getting worse in the last 3 mo before inclusion into study | 0.250 | 0.148 | 0.353 | <0.001 |
| HADS | 0.372 | 0.251 | 0.493 | <0.001 |
| Study visit at 12 mo | −0.413 | −0.534 | −0.292 | <0.001 |
| Study visit at 24 mo | −0.424 | −0.544 | −0.303 | <0.001 |
| Study visit at 36 mo | −0.366 | −0.486 | −0.246 | <0.001 |
| Surgery | 0.017 | −0.118 | 0.152 | 0.810 |
| Study visit at 12 mo: surgery | −0.341 | −0.484 | −0.198 | <0.001 |
| Study visit at 24 mo: surgery | −0.300 | −0.442 | −0.158 | <0.001 |
| Study visit at 36 mo: surgery | −0.323 | −0.464 | −0.181 | <0.001 |
Bold values indicate statistical significance.
SSM function ranges 1 to 4, best to worst; minimal clinically important difference=0.52.
BMI indicates body mass index; CIRS, Cumulative Illness Rating Scale; FMI, fatty muscle infiltration; HADS, Hospital Anxiety and Depression Scale; SSM, Spinal Stenosis Measure.
Change in EQ-5D-3L SI Scores From Baseline Over Time
A mixed-effects model was fitted with random intercepts for patients (Table 6). Data concerning quality of health showed that on average patients experienced an improvement in the course of the study (t=12, 24, 36 months). Total degeneration score was not associated with while FMI was associated with EQ-5D-3L SI (β=−0.003, 95% CI: −0.013 to 0.06, P=0.467; and β=−0.131, 95% CI: −0.198 to −0.063, P=0.000, respectively). For FMI, the estimate is quite considerable regarding the score range of the EQ-5D-3L SI. Further, there was an interaction between FMI and surgical therapy, therefore we included it in the model (β=0.098, 95% CI: 0.020–0.176, P=0.016). However, there was no interaction between FMI and total degeneration score, or age, or sex, or BMI, respectively. Therefore, we did not include them in the model.
TABLE 6.
EQ-5D-3L Analysis
| Mixed-effects | Effects | P | ||
|---|---|---|---|---|
| Estimate | 2.5% | 97.5% | ||
| Intercept | 0.851 | 0.775 | 0.927 | <0.001 |
| FMI of paraspinal muscles (Goutallier grade ≥2) | −0.131 | −0.198 | −0.063 | <0.001 |
| Degeneration score total | −0.003 | −0.013 | 0.006 | 0.467 |
| Age | −0.001 | −0.003 | 0.001 | 0.421 |
| Female | −0.052 | −0.089 | −0.016 | 0.005 |
| BMI | −0.008 | −0.011 | −0.004 | <0.001 |
| Duration of symptoms>6 mo | −0.031 | −0.071 | 0.008 | 0.126 |
| CIRS | −0.005 | −0.010 | −0.001 | 0.021 |
| Problem getting worse in the last 3 mo before inclusion into study | −0.113 | −0.153 | −0.072 | <0.001 |
| HADS | −0.177 | −0.224 | −0.128 | <0.001 |
| Study visit at 12 mo | 0.094 | 0.042 | 0.146 | <0.001 |
| Study visit at 24 mo | 0.096 | 0.044 | 0.147 | <0.001 |
| Study visit at 36 mo | 0.081 | 0.029 | 0.132 | 0.002 |
| Surgery | −0.129 | −0.195 | −0.063 | <0.001 |
| Study visit at 12 mo: surgery | 0.191 | 0.130 | 0.252 | <0.001 |
| Study visit at 24 mo: surgery | 0.170 | 0.109 | 0.230 | <0.001 |
| Study visit at 26 mo: surgery | 0.174 | 0.113 | 0.234 | <0.001 |
| FMI of paraspinal muscles (Goutallier grade ≥2): surgery | 0.098 | 0.020 | 0.176 | 0.016 |
Bold values indicate statistical significance.
EQ-5D-3L range −0.53 to 1, worst to best; minimal clinically important difference=0.19.
BMI indicates body mass index; CIRS, Cumulative Illness Rating Scale; EQ-5D-3L, European Quality of Life 5 Dimensions 3 Level Version; FMI, fatty muscle infiltration; HADS, Hospital Anxiety and Depression Scale; SSM, Spinal Stenosis Measure.
DISCUSSION
This study assessed the impact of paraspinal FMI and cumulative lumbar spine degeneration on long-term clinical outcome measures in 416 patients of the LSOS cohort. FMI was associated with higher SSM function and lower EQ-5D-3L SI, but not with SSM symptoms. Total degeneration of the lower spine was associated neither with SSM symptoms, nor with SSM function, nor with EQ-5D-3L SI.
FMI was associated with higher disability and worse health-related quality of life in LSCS patients but there was no significant association between FMI and pain. This is only partially consistent with a study of Teichtahl et al,32 which showed that FMI was associated not only with disability but also with low back pain in community-based adults. Similarly, a study of Fortin et al 14 showed that greater multifidus muscle fatty infiltration and lower psoas relative total cross-sectional area were associated with higher disability and also with higher pain interference scores.
To this day, the authors are not aware of any other studies that evaluated the impact of cumulative lumbar spine degeneration on the outcome of LSCS patients. We therefore attempted to quantify the cumulative degeneration load of the lumbar spine by a score that integrated degenerative changes of both, anterior and posterior elements of the spinal column. Common sense suggested that a higher overall degeneration load would be associated with a worse outcome. However, this hypothesis was proven to be wrong as the total degeneration score created for this study was not associated with a worse outcome. This may be explained by the fact that a higher overall degeneration of the spine lowers its mobility and flexibility33 and therefore may reduce pain. In addition, the sensitivity of pain receptors in individuals with more severe degenerations of the spine may be reduced as is proprioception.34
Strengths of this study include its prospective design with a high number of patients that were followed over a long period of time (i.e., at least 36 months). LSOS being a multicenter study, imaging was performed on different MR scanners in seven different radiology departments. As a result, scanning parameters varied. However, MRI protocols were adjusted beforehand with certain sequences (sagittal T1w and T2w as well as axial T2w images) as minimum requirements. Another limitation may originate in the use of the qualitative Goutallier scoring system to assess FMI. Quantitative methods (e.g., thresholding or fat-water images) might be more accurate and have better intrarater and interrater reliability; those measurements were retrospectively however not available from all patients. Another limitation is that paraspinal FMI was measured only at level L3. This level was chosen as it showed on average most fatty infiltration with least susceptibility to scoliotic changes. However, this might not be true in other cohorts. Mandelli et al 35 recently published a study demonstrating differences in paraspinal FMI between spinal levels in patients with LSCS. Therefore, the authors acknowledge that their assessment of muscle composition is limited. Furthermore, the total degeneration score that was designed specifically for this study to sum up changes of various lumbar spine elements has not been validated before. Different aspects of degenerative changes may impact differently on clinical symptoms and function and may therefore need to be weighed differently in a cumulative degeneration score. A deeper analysis of the suggested score may be warranted in additional studies.
In conclusion, this study suggests that paraspinal FMI is associated with higher disability and worse health-related quality of life. In contrast, there is no significant association between total cumulative lumbar spine degeneration and the outcome of LSCS patients. More studies are needed to confirm the results of this study and identify other prognostic indicators for better/worse outcome in patients with LSCS. Until then, radiologists and clinicians should be aware of the importance of paraspinal FMI when treating patients with LSCS and identify individuals which could benefit from additional supportive treatment to optimize long-term outcomes.
Key Points.
This study investigated the influence of paraspinal FMI and cumulative lumbar spine degeneration on clinical outcome measures in patients with LSCS of the LSOS cohort.
FMI was associated with higher disability and worse health-related quality of life of LSCS patients in the LSOS cohort.
However, there was no significant association between total cumulative lumbar spine degeneration and the outcome of LSCS patients.
Footnotes
The study was approved by the institutional review board and the local ethics committee and conducted in accordance with the principles of the Declaration of Helsinki. Written general consent for the use of clinical data for scientific purposes was obtained from each patient.
The authors report no conflicts of interest.
Contributor Information
Jonas M. Getzmann, Email: jonas.getzmann@usz.ch.
Hamidreza Ashouri, Email: hamidreza.ashouri@team-radiologie.ch.
Jakob M. Burgstaller, Email: jakob.burgstaller@usz.ch.
Fabio Valeri, Email: fabio.valeri@usz.ch.
Sebastian Winklhofer, Email: sebastian.winklhofer@usz.ch.
Nils H. Ulrich, Email: nils.ulrich@balgrist.ch.
Roman Guggenberger, Email: roman.guggenberger@usz.ch.
References
- 1.Ciol MA, Deyo RA, Howell E, et al. An assessment of surgery for spinal stenosis: time trends, geophraphic variations, complications, and reoperations. J Am Geriatr Soc. 1996;44:285–290. [DOI] [PubMed] [Google Scholar]
- 2.Christensen K, Doblhammer G, Rau R, et al. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson RP, McManus AC, Moore J. Lumbar spinal stenosis: treatment options for an aging population. Mo Med. 2012;109:466–469. [PMC free article] [PubMed] [Google Scholar]
- 4.Burgstaller JM, Schüffler PJ, Buhmann JM, et al. Is there an association between pain and magnetic resonance imaging parameters in patients with lumbar spinal stenosis? Spine (Phila Pa 1976). 2016;41:E1053–E1062. [DOI] [PubMed] [Google Scholar]
- 5.Verde E, Risso-Neto M, Mistro Neto S, et al. Correlation between lumbar spinal stenosis based on morphology of the dural sac and the quality of life. Coluna/Columna. 2019;18:28–31. [Google Scholar]
- 6.Park HJ, Kim SS, Lee YJ, et al. Clinical correlation of a new practical MRI method for assessing central lumbar spinal stenosis. Br J Radiol. 2013;86:20120180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulrich NH, Burgstaller JM, Gravestock I, et al. The influence of endplate (Modic) changes on clinical outcomes in lumbar spinal stenosis surgery: a Swiss prospective multicenter cohort study. Eur Spine J. 2020;29:2205–2214. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Park SW, Kim YB, et al. The fatty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. Spine J. 2017;17:81–87. [DOI] [PubMed] [Google Scholar]
- 9.Goutallier D, Postel JM, Bernageau J, et al. Fatty infiltration of disrupted rotator cuff muscles. Rev Rhum Engl Ed. 1995;62:415–422. [PubMed] [Google Scholar]
- 10.Chen YY, Pao JL, Liaw CK, et al. Image changes of paraspinal muscles and clinical correlations in patients with unilateral lumbar spinal stenosis. Eur Spine J. 2014;23:999–1006. [DOI] [PubMed] [Google Scholar]
- 11.Hodges PW, Bailey JF, Fortin M, et al. Paraspinal muscle imaging measurements for common spinal disorders: review and consensus-based recommendations from the ISSLS degenerative spinal phenotypes group. Eur Spine J. 2021;30:3428–3441. [DOI] [PubMed] [Google Scholar]
- 12.Mannil M, Burgstaller JM, Thanabalasingam A, et al. Texture analysis of paraspinal musculature in MRI of the lumbar spine: analysis of the Lumbar Stenosis Outcome Study (LSOS) data. Skeletal Radiol. 2018;47:947–954. [DOI] [PubMed] [Google Scholar]
- 13.Storheim K, Berg L, Hellum C, et al. Fat in the lumbar multifidus muscles—predictive value and change following disc prosthesis surgery and multidisciplinary rehabilitation in patients with chronic low back pain and degenerative disc: 2-year follow-up of a randomized trial. BMC Musculoskelet Disord. 2017;18:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortin M, Lazáry À, Varga PP, et al. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur Spine J. 2017;26:2543–2551. [DOI] [PubMed] [Google Scholar]
- 15.Ulrich NH, Burgstaller JM, Held U, et al. The influence of single-level versus multilevel decompression on the outcome in multisegmental lumbar spinal stenosis: analysis of the Lumbar Spinal Outcome Study (LSOS) data. Clin Spine Surg. 2017;30:E1367–E1375. [DOI] [PubMed] [Google Scholar]
- 16.Steurer J, Nydegger A, Held U, et al. LumbSten: The Lumbar Spinal Stenosis Outcome Study. BMC Musculoskelet Disord. 2010;11:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winklhofer S, Held U, Burgstaller JM, et al. Degenerative lumbar spinal canal stenosis: intra- and inter-reader agreement for magnetic resonance imaging parameters. Eur Spine J. 2017;26:353–361. [DOI] [PubMed] [Google Scholar]
- 18.Andreisek G, Deyo RA, Jarvik JG, et al. Consensus conference on core radiological parameters to describe lumbar stenosis: an initiative for structured reporting. Eur Radiol. 2014;24:3224–3232. [DOI] [PubMed] [Google Scholar]
- 19.Lurie JD, Tosteson AN, Tosteson TD, et al. Reliability of readings of magnetic resonance imaging features of lumbar spinal stenosis. Spine (Phila Pa 1976). 2008;33:1605–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976). 2010;35:1919–1924. [DOI] [PubMed] [Google Scholar]
- 21.Bartynski WS, Lin L. Lumbar root compression in the lateral recess: MR imaging, conventional myelography, and CT myelography comparison with surgical confirmation. AJNR Am J Neuroradiol. 2003;24:348–360. [PMC free article] [PubMed] [Google Scholar]
- 22.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983;8:817–831. [DOI] [PubMed] [Google Scholar]
- 23.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26:1873–1878. [DOI] [PubMed] [Google Scholar]
- 24.Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. [DOI] [PubMed] [Google Scholar]
- 25.Meyerding HW. Spondyloptosis. Surg Gynaecol Obstet. 1932;54:371–377. [Google Scholar]
- 26.Goutallier D, Postel JM, Bernageau J, et al. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 27.Stucki G, Liang MH, Fossel AH, et al. Relative responsiveness of condition-specific and generic health status measures in degenerative lumbar spinal stenosis. J Clin Epidemiol. 1995;48:1369–1378. [DOI] [PubMed] [Google Scholar]
- 28.Selivanova A, Buskens E, Krabbe PFM. Head-to-head comparison of EQ-5D-3L and EQ-5D-5L health values. Pharmacoeconomics. 2018;36:715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgstaller JM, Wertli MM, Ulrich NH, et al. Evaluating the minimal clinically important difference of EQ-5D-3L in patients with degenerative lumbar spinal stenosis: a Swiss Prospective Multi-Center Cohort Study. Spine (Phila Pa 1976). 2020;45:1309–1316. [DOI] [PubMed] [Google Scholar]
- 30.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 31.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 32.Teichtahl AJ, Urquhart DM, Wang Y, et al. Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community-based adults. Spine J. 2015;15:1593–1601. [DOI] [PubMed] [Google Scholar]
- 33.Lao L, Daubs MD, Scott TP, et al. Effect of disc degeneration on lumbar segmental mobility analyzed by kinetic magnetic resonance imaging. Spine (Phila Pa 1976). 2015;40:316–322. [DOI] [PubMed] [Google Scholar]
- 34.Meier ML, Vrana A, Schweinhardt P. Low back pain: the potential contribution of supraspinal motor control and proprioception. Neuroscientist. 2019;25:583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandelli F, Nüesch C, Zhang Y, et al. Assessing fatty infiltration of paraspinal muscles in patients with lumbar spinal stenosis: goutallier classification and quantitative mri measurements. Front Neurol. 2021;12:656487. [DOI] [PMC free article] [PubMed] [Google Scholar]


