Abstract
Attenuated mutants of Mycobacterium tuberculosis represent potential vaccine candidates for the prevention of tuberculosis. It is known that auxotrophs of a variety of bacteria are attenuated in vivo and yet provide protection against challenge with wild-type organisms. A leucine auxotroph of M. tuberculosis was created by allelic exchange, replacing wild-type leuD (Rv2987c), encoding isopropyl malate isomerase, with a mutant copy of the gene in which 359 bp had been deleted, creating a strain requiring exogenous leucine supplementation for growth in vitro. The frequency of reversion to prototrophy was <10−11. In contrast to wild-type M. tuberculosis, the ΔleuD mutant was unable to replicate in macrophages in vitro. Its attenuation in vivo and safety as a vaccine were established by the fact that it caused no deaths in immunodeficient SCID mice. Complementation of the mutant with wild-type leuD abolished the requirement for leucine supplementation and restored the ability of the strain to grow both in macrophages and in SCID mice, thus confirming that the attenuated phenotype was due to the ΔleuD mutation. As a test of the vaccine potential of the leucine auxotroph, immunocompetent BALB/c mice, susceptible to fatal infection with wild-type M. tuberculosis, were immunized with the ΔleuD mutant and subsequently challenged with virulent M. tuberculosis by both the intravenous and aerosol routes. A comparison group of mice was immunized with conventional Mycobacterium bovis BCG vaccine. Whereas all unvaccinated mice succumbed to intravenous infection within 15 weeks, mice immunized with either BCG or the ΔleuD mutant of M. tuberculosis exhibited enhanced and statistically equivalent survival curves. However, the leuD auxotroph was less effective than live BCG in reducing organ burdens and tissue pathology of mice challenged by either route. We conclude that attenuation and protection against M. tuberculosis challenge can be achieved with a leucine auxotroph and suggest that to induce optimal protection, attenuated strains of M. tuberculosis should persist long enough and be sufficiently metabolically active to synthesize relevant antigens for an extended period of time.
Mycobacterium tuberculosis, the causative agent of human tuberculosis (TB), infects one-third of the world's population (18) and is responsible for 3 million deaths annually, sharing with human immunodeficiency virus the dubious distinction of being the leading cause of death worldwide due to an infectious agent (64). In addition, TB ranks seventh among causes of global mortality and disability, and if current predictions prove correct, it will remain among the top 10 causes of disease well into the next century (41). Directly observed treatment, short-course (DOTS), is currently the best available strategy to control the global TB crisis (42). With cure rates approaching 90% (12), DOTS has proven to be an effective strategy, yet only about 15% of countries where TB is endemic have implemented DOTS programs. Therefore, additional measures will be needed to stem the tide of TB morbidity and mortality. It has been estimated that the introduction of a new vaccine of only 50% efficacy could decrease the incidence of TB by 36 million cases, saving 9 million lives (42). Thus, by coupling efficacious vaccination for prevention with effective case treatment, greater success in global TB management can be anticipated.
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is currently the only available vaccine for the prevention of TB. In many animal models of infection, BCG has been demonstrated to induce protective immunity against M. tuberculosis challenge (8, 26, 45) and it has demonstrated protection against severe and fatal TB in children (52). However, BCG has shown itself to be of variable efficacy for protecting adults from pulmonary TB. While it imparted 77% protection in the Medical Research Council trial in the United Kingdom (25), in the single largest clinical trial that took place in India, involving more than 100,000 persons, BCG exhibited zero protective efficacy (63). Thus, the generation of an improved vaccine(s) to replace BCG and to prevent tuberculosis is urgently needed.
Compared to wild-type M. tuberculosis, 15 to 16 regions of the M. tuberculosis genome are not represented in BCG (10). It is probable that one or more of the 38 open reading frames specifically missing from BCG resulted in its attenuation. Of interest is the finding that a number of predicted transcriptional regulators identified by the H37Rv genome-sequencing project (13) are located in the region of these BCG deletions. The loss of a regulatory protein could affect multiple genetic loci and lead to deranged gene expression in vivo. Consistent with this hypothesis is the demonstration that reintroduction of one of these deleted regions into BCG results in the repression of at least 10 proteins and the upregulation of others (34). Thus, the absence of regulatory proteins might be preventing expression of a variety of antigens. It is conceivable that immunogenic and immunoprotective antigens might be missing from or inappropriately expressed in BCG, thereby compromising the immune response generated by this vaccine. It is also conceivable that if one or more of the proteins encoded within the deleted regions were present at vaccination, the immune response elicited might be more efficacious.
In both mice and guinea pigs, primary infection with M. tuberculosis induces resistance to reinfection (17, 31). A vaccine could be created by the rational deletion of genes of M. tuberculosis, promoting the attenuation but preserving the immunogenicity and protectiveness of the bacillus. Ideally, such a vaccine would provide better protection than BCG. For several other bacterial pathogens, a successful approach to creating avirulent yet immunogenic vaccine strains has been to create auxotrophic mutant strains or strains with specific requirements for growth (1, 11, 19, 24, 25, 28, 51, 56). Likewise, a leucine auxotroph of BCG, created by insertional disruption of leuD, which encodes isopropyl malate isomerase, an essential enzyme for leucine biosynthesis, failed to grow in either macrophages (9) or mice (21, 36) but demonstrated protection against a challenge with virulent M. tuberculosis that was indistinguishable from that of wild-type BCG. These findings provided the impetus to create by allelic exchange a ΔleuD strain of M. tuberculosis containing a defined deletion within leuD. This paper analyzes the effects of the leuD deletion mutation on the virulence, intracellular growth, and vaccine potential of M. tuberculosis.
MATERIALS AND METHODS
Bacterial strains and culture methods.
Liquid cultures of M. tuberculosis H37Rv, M. tuberculosis Erdman, and M. bovis BCG strains Pasteur (BCG-P), mc23034, and mc23035 were grown in Middlebrook 7H9 broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.2% glycerol, 0.05% Tween 80, and 10% Middlebrook OADC (Difco) (minimal medium). Solid cultures were grown on 7H10 agar supplemented as described above, and in addition, 100 μg of cycloheximide/ml was added to thwart fungal contamination. Strain mc23032 (H37Rv ΔleuD) was cultured on minimal medium supplemented with 50 μg of l-leucine (Sigma Chemical Co., St. Louis, Mo.)/ml (complete medium). This mutant strain is independent of a previously isolated leucine auxotroph created by Balasubramanian and colleagues (7). When necessary, hygromycin B (Boehringer Mannheim, Indianapolis, Ind.) and kanamycin (Sigma) were added at final concentrations of 50 and 20 μg/ml, respectively. Bacterial growth was monitored by measuring the optical densities of the broth cultures over time. All cultures were grown at 37°C in roller bottles.
Plasmid construction, allelic replacement, and construction of complementing strains.
To isolate the leuCD operon, primers Pleu1 (5′-TGAACACCGCCTTTGGCAAT-3′) and Pleu2 (5′-GCCTTACGCACCGATGCCTT-3′) were designed using the M. tuberculosis genome sequence database (13) to amplify a 3,342-bp DNA fragment from M. bovis BCG-P chromosomal DNA containing the leuC and leuD genes symmetrically flanked by about 600 bp of homologous DNA sequence on each side. This PCR product was cloned into the unique EcoRV site of the pBluescript II KS(−) plasmid by blunt-end ligation to generate pYUB595. A deletion of 359 bp in the leuD gene (Rv2987c) was generated by cleavage with SphI and SauI and marked with the res-hyg-res cassette (which encodes hygromycin resistance) introduced by blunt-end ligation. The res sites were included so that the hygromycin cassette could be removed if desired by supplying γδ resolvase (50), creating an unmarked mutant. The resulting plasmid, pYUB599, was digested with XbaI and HindIII to produce a 3,342-bp DNA fragment containing leuCΔD6::res-hyg-res, which was ligated to NotI-digested pMP7 by blunt-end ligation, generating pMH10.1. pMP7 is a Mycobacterium-Escherichia coli shuttle vector containing an aph gene conferring kanamycin resistance which is functional in both bacterial species, along with the counterselectable marker sacB from Bacillus subtilis, which confers lethality in the presence of sucrose (20). H37Rv was grown to an optical density at 600 nm (OD600) of 0.8, washed twice at room temperature in 10% glycerol, and resuspended in the same medium at 1/20 of the initial culture volume. Four hundred microliters of cells in a 0.2-cm-diameter cuvette were transformed with approximately 1 μg of pMH10.1 using a Gene Pulser (Bio-Rad Laboratories, Hercules, Calif.) set at 2.5 kV, 25 μF, and 1,000 Ω. Immediately following electroporation, the cells were added to tubes containing 1 ml of complete 7H9 medium and incubated overnight at 37°C. The following day, the cells were plated on leucine-supplemented 7H10 agar with 50 μg of hygromycin/ml. Hygromycin-resistant (Hygr) colonies appeared approximately 4 weeks later and were determined to be both sucrose sensitive (Sucs) (growth on complete medium with 3% sucrose agar was lethal) and kanamycin resistant (Kmr). Ten cultures of 10 individual Hygr Sucs Kmr clones were grown for a week in medium with 50 μg of hygromycin/ml and 50 μg of leucine/ml. Serial 10-fold dilutions of each individual culture were plated onto 7H10 agar supplemented with hygromycin, leucine, and 3% sucrose. A total of 334 Hygr Sucr clones (arising from the original 10 Hygr transformants) were picked into the wells of 96-well plates which contained complete 7H9 medium supplemented with leucine. Following 5 days of expansion at 37°C, the cultures were replica plated onto minimal medium (no leucine) and rich medium (leucine) with hygromycin or kanamycin. Eleven clones were found to be Hygr Kms Leu−. Southern analysis of these 11 clones confirmed that they all had undergone an allelic-replacement event (Fig. 1). To create the leuD-complementing strains mc23034 and mc23035, a leuD-containing fragment was amplified by PCR from pYUB508, a plasmid carrying leuD, using the blunt forward (5′-AAGCCTTTCACACCCACTCT-3′) and HindIII reverse (5′-GACAAGCTTTCGCCCGGTTCTACGCCT-3′) primers. The resultant 600-bp PCR fragment, which contained the coding sequence of leuD only, was digested with HindIII and ligated to both pMV261 and pMV306 (modified to contain the hsp60 promoter) previously digested with MscI/HindIII, placing leuD in frame and under the control of the mycobacterial hsp60 heat shock promoter. pMV261 (59) and pMV306 are Mycobacterium-E. coli shuttle vectors, containing an oriE and an aph gene, and they are either extrachromosomal (pMV261) or integrative (pMV306) in Mycobacterium spp. Strain mc23032 was then transformed with the episomal and integrative complementing plasmids under the conditions described above. The transformants obtained did not require exogenous leucine for growth. Strain mc23034 is complemented in multicopy, and mc23035 is complemented in single copy.
FIG. 1.
Southern blot analysis of wild-type H37Rv and H37Rv ΔleuD (mc23032). Genomic DNAs from wild-type H37Rv (lane 1) and the leucine auxotroph mc23032 (lane 2) were isolated, digested with Acc65I, and probed with the 600-bp leuD gene. Molecular size markers (in kilobases) are indicated on the left.
Southern analysis.
Genomic DNA was isolated from growing cultures of M. tuberculosis as follows. Bacteria from a 15-ml culture were pelleted, and all medium was removed. Two milliliters of a 3:1 chloroform-methanol solution was added, and the pellet was vortexed (approximately 1 min) until the bacteria were lysed, as evidenced by a clearing of the bottom layer. Two milliliters of Tris-buffered phenol (pH 8) was added, and the solution was vortexed. Then 3 ml of guanidinium thiocyanate buffer (4 M guanidinium thiocyanate, 0.1 M Tris [pH 7.5], 1% β-mercaptoethanol, 0.5% Sarkosyl) was added, the mixture was vortexed, and the solution was centrifuged at 500 × g for 15 min. Following centrifugation, the aqueous layer was removed and the DNA was precipitated by addition of an equal volume of isopropanol. Approximately 1 μg of DNA per strain was digested with Asc65I and then separated on a 0.7% agarose gel. Chemiluminescent Southern blotting was done using the ECL direct nucleic acid labeling and detection system (Amersham Pharmacia, Arlington Heights, Ill.) and was performed according to the manufacturer's recommendations. A 600-bp PCR fragment of leuD (amplified from a plasmid; described above) was used as a probe.
Reversion analysis.
A 100-ml culture of M. tuberculosis strain mc23032 was grown to an OD600 of 1.0 to 2.0 in leucine-supplemented medium without hygromycin. The titer of the culture was determined retrospectively by dilution plating on agar plates supplemented with leucine. For analysis of reversion frequency, the entire culture was pelleted and resuspended in 2 ml of leucine-free medium. The 2-ml resuspension was spread over 20 plates of minimal (leucine-free) 7H10 agar supplemented with OADC and glycerol, as described above.
Macrophages.
Primary murine bone marrow-derived macrophages were obtained by flushing the femurs of BALB/cJ mice with 5 ml of cold cation-free phosphate-buffered saline (PBS) (Gibco, Grand Island, N.Y.). The marrow was dispersed by gentle pipetting and pelleted by centrifugation, and the cells were resuspended in Dulbecco's Modified Eagle's Medium (Gibco) supplemented with 20% L-929 cell-conditioned medium, 10% heat-inactivated fetal calf serum, 2 mM glutamine, 100 U of penicillin G/ml, and 100 μg of streptomycin/ml. The cells were cultured on non-tissue culture-treated, 150-mm plastic petri dishes at 37°C and 5% CO2 for 5 to 7 days, at which time monolayers of macrophages were readily apparent. For use in assays, the cells were removed from the petri dishes using 5 mM EDTA in cation-free PBS, pelleted, and then resuspended in antibiotic-free Selectamine (Gibco) medium supplemented with 10% fetal calf serum, amino acids (with and without l-leucine), and 5% L-929 cell-conditioned medium (DL5). Approximately 2 × 105 cells were distributed per well of tissue culture-treated eight-well chamber slides (Nalge Nunc International, Naperville, Ill.).
Intracellular growth assays.
For use in intracellular growth assays, bacteria were grown in complete medium, washed and resuspended in Selectamine without leucine, and then added to macrophages at a multiplicity of infection (MOI) of 2 to 10 bacteria per macrophage. To allow optimal adherence, the bacteria were incubated with macrophages for 4 h at 37°C and 5% CO2. The monolayers were then extensively washed with warm Dulbecco's modified Eagle's medium to remove any unbound bacteria. Gentamicin (100 to 200 μg/ml) was added for 2 h to kill any remaining extracellular bacteria. The monolayers were washed thoroughly once again to remove residual antibiotic, and the medium was replaced with DL5. Eighteen hours later, the monolayers were washed once more and the medium was replaced with the same medium. At various times postinfection, the monolayers were fixed with 10% phosphate-buffered formalin, stained with rhodamine-auramine, and counterstained with neutral red (Sigma). In addition, the supernatant was plated at each time point to monitor any extracellular bacterial growth. Slides were examined by fluorescence microscopy using a DAPI (4′,6′-diamidino-2-phenylindole) filter under which bacilli appeared yellow-green. Two hundred macrophages were observed per monolayer, and the bacilli associated with those macrophages were enumerated. Any macrophage containing 10 or more bacteria was scored as having 10 organisms only. In addition, the number of bacteria per infected macrophage was recorded.
Mouse strains and in vivo infection.
Female BALB/cJ mice were obtained from Jackson Laboratories (Bar Harbor, Maine) and immunized at approximately 8 weeks of age. BALB/c SCID mice were bred in the Animal Facility of Albert Einstein College of Medicine. Both male and female SCID mice were used and were infected between 8 and 12 weeks of age. In preparation for immunization or infection of mice, titered frozen aliquots of the bacterial strains were thawed, diluted in PBS with 0.05% Tween 80 (PBS-Tween), and sonicated (10 s) with a Branson ultrasonifier to disperse clumps of bacteria. Even though the frozen bacterial aliquots had been titered previously, the titer of the inoculum was reconfirmed at the time of injection by dilution plating of the injection stock. Groups of mice were injected intravenously in the tail veins with H37Rv, BCG-P, mc23032, mc23034, or mc23035 in 100 μl of PBS-Tween. In order to monitor bacterial clearance or growth, at various times postinjection, four to five mice from each experimental group were sacrificed and their spleens, livers, and lungs were aseptically removed. Each organ was placed in PBS with 0.05% Tween 80 and disrupted using a Stomacher-80 apparatus (Tekmar, Cincinnati, Ohio). Serial 10-fold dilutions of the tissue homogenate were plated on 7H10 agar containing 0.2% glycerol, 10% OADC, and 100 μg of cycloheximide/ml and supplemented with leucine and/or antibiotic when appropriate. The plates were incubated at 37°C. CFUs were counted 3 to 4 weeks later.
In SCID mouse infection experiments, groups of mice were challenged with approximately 104 CFU of H37Rv, 104 CFU of mc23034, 104 CFU of mc23305, or 106 CFU of mc23032. To document the challenge dose, the animals were sacrificed at 18 h, their organs were removed, and CFUs were counted as described above. Thirteen mice per experimental group were then followed for survival studies. At the time of death, organs were removed from animals that succumbed to infection and CFUs were counted as described previously. SCID mice challenged with mc23032 remained healthy and were sacrificed 22 weeks postinfection, and the organ burdens were likewise assessed.
In immunization studies, BALB/cJ mice were intravenously immunized with 5 × 106 CFU of either BCG-P or mc23032. At 9 weeks postimmunization, both vaccinated and unvaccinated control mice were challenged either intravenously with 106 CFU of M. tuberculosis strain Erdman or by aerosol with approximately 300 CFU. The aerosol dose was delivered using the “nose-only” aerosolization apparatus (In-TOX Products, Albuquerque, N. Mex.) as previously described (62). Aerosols carrying M. tuberculosis were generated from a bacterial suspension consisting of 107 CFU per ml of PBS (pH 7.4) with 0.05% Tween 80. The mice were exposed to the aerosols for 20 min. At various points after infection, the tissue bacterial burden was assessed as described above. In intravenous-infection experiments, 15 mice per experimental group were monitored for survival. In order to differentiate colonies of BCG from colonies of M. tuberculosis in animals infected with both species of bacteria, homogenates were plated in duplicate on media with and without thiophene-2-carboxylic acid. This compound prevents the growth of BCG, while the growth of M. tuberculosis is unaffected (22). Thus, we could determine the proportions of the bacterial burden contributed by both species of mycobacteria.
Histopathological staining.
Tissues were removed and fixed overnight in 10% phosphate-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Alternatively, the tissues were subjected to acid-fast staining in order to visualize bacilli.
Statistical analysis.
The mean organ burdens of the various experimental groups of mice were subjected to Mann-Whitney analysis for nonparametrical data. Survival curve comparisons were analyzed by the log rank test.
RESULTS
Construction of ΔleuD mutant of M. tuberculosis H37Rv.
Until recently, creation of defined mutants of slow-growing mycobacteria (M. tuberculosis and BCG) has been difficult to achieve. However, thanks to recent advances (47, 48), it was possible for us to generate a deletion mutant of M. tuberculosis that required leucine for growth. To create this mutant, M. tuberculosis H37Rv was transformed with pMH10.1, a replicating vector that contained the ΔleuD6 allele with a deletion marked by a hygromycin cassette. It contained 2,230 bp of mycobacterial DNA 5′ and 739 bp of sequence 3′ to the hygromycin cassette. In addition, this construct contained an aph gene, encoding kanamycin resistance, and sacB, the presence of which confers sensitivity to sucrose. Hygromycin-resistant transformants were obtained and confirmed to be both kanamycin resistant and sensitive to growth on plates with 3% sucrose. Ten individual Hygr Kmr Sucs colonies were used to establish cultures growing in complete medium supplemented with leucine and hygromycin. This period of growth allowed a double homologous-recombination event followed by plasmid loss. Following plating on complete agar supplemented with leucine, hygromycin, and sucrose, 334 Hygr Sucr colonies were picked into individual wells of 96-well plates containing medium with hygromycin and leucine. The wells were subsequently replica plated onto leucine-supplemented plates containing either kanamycin or hygromycin, or they were replicated on minimal plates without leucine supplementation. Greater than 90% of the clones arising were Hygr Kms. However, 11 of 334 clones were found to be Hygr Kms Leu−. These 11 clones were derived from 4 of the original 10 Hygr Kmr Sucs clones. Southern blot analysis confirmed that these 11 clones had indeed undergone an allelic-replacement event. An example of one such Hygr Leu− clone, strain mc23032, is illustrated in Fig. 1. Wild-type genomic H37Rv DNA (lane 1) digested with Acc65I and probed with the 600-bp leuD gene yields a fragment of 2,425 bp. In contrast, mc23032 (lane 2), which has a 359-bp deletion in Leu− marked by a 1,899-bp hygromycin cassette, shows a larger band of 3,965 bp and loss of the wild-type band, thus confirming the allelic-exchange event. Strain mc22032 failed to grow on minimal agar or in minimal broth (Fig. 2). Growth could be restored with leucine supplementation (Fig. 2). The growth rate of mc23032 in broth medium supplemented with leucine was similar to that of wild-type H37Rv in leucine-free medium (Fig. 2). Notably, the density of mc23032 cultures was always slightly less than that of the wild type. As expected, complementation of mc23032 with leuD provided in trans on a multicopy plasmid (strain mc23034) or in single copy integrated at the att site (mc23035) abolished the requirement for exogenous leucine (Fig. 2). To test the stability of the phenotype, reversion analysis of mc23032 was performed on two separate occasions. The strain was grown in leucine-supplemented medium without hygromycin and then plated on minimal agar. No prototrophic clones (out of 4 × 1010 to 6 × 1010 CFU plated) arose in either experiment. Thus, the reversion frequency is calculated at <10−11).
FIG. 2.
Inactivation of leuD confers leucine auxotrophy. Bacterial growth in Middlebrook 7H9 broth with and without supplementation with 50 μg of leucine per ml. The various strains were cultured in 7H9 medium supplemented with leucine and then pelleted, washed, and resuspended in media with and without leucine supplementation. The OD600s of the broth cultures were determined daily.
The intracellular growth potential of mc23032 is impaired.
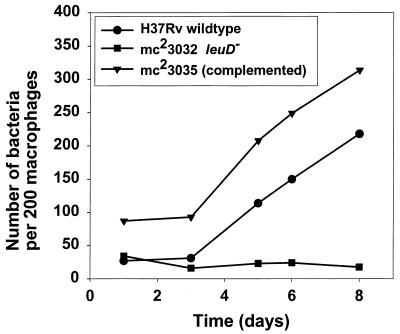
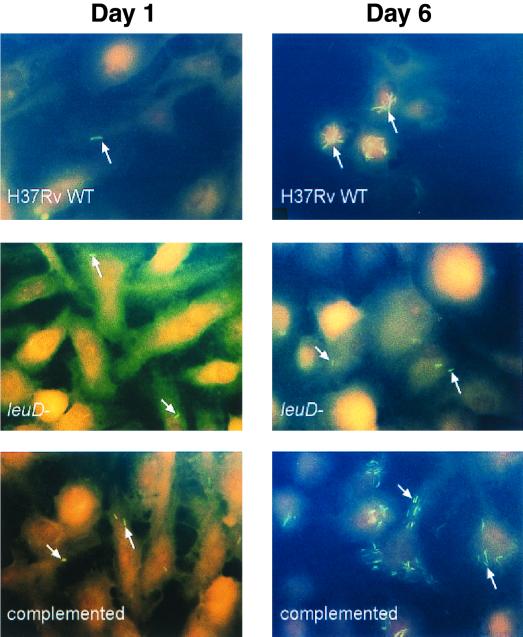
In order to determine if the requirement for exogenous leucine supplementation could affect the ability of the leuD mutant to undergo intracellular replication, we examined the growth of the leucine auxotroph in cultured macrophages in vitro. Bone marrow-derived macrophages from BALB/cJ mice were infected with either wild-type H37Rv, a ΔleuD mutant (mc23032), or mc23035, the leuD-complemented strain. Following adsorption at an MOI of 2 to 10 bacteria per macrophage, unbound bacteria were removed by extensive washing of the monolayer and gentamicin was added to kill extracellular bacteria. The MOI used resulted in approximately 15 to 30% infection of the monolayer, with each infected macrophage containing approximately 1 to 2 bacteria. Following a 72-h lag period, the numbers of both wild-type H37Rv and the complemented strain mc23035 associated with the macrophage monolayer began to increase so that at 8 days postinfection, the intracellular bacilli had expanded by approximately 10- and 7-fold, respectively (Fig. 3). The growth of these strains was also reflected in an increase in the number of bacteria per infected macrophage (data not shown). At later time points, it was difficult to accurately count large numbers of bacteria, so macrophages containing 10 or more bacteria were simply scored as containing 10 bacilli. Thus, bacterial replication was clearly underestimated. Throughout the course of the experiment, the percentage of the monolayer that was infected remained virtually unchanged, indicating that macrophage lysis and subsequent reinfection was minimal during this time (data not shown). In contrast to the wild-type strain and the leuD-complemented mutant, strain mc23032, the leucine auxotroph, failed to replicate inside macrophages, and its numbers began to decrease with time postinfection (Fig. 4). Thus, the inability of strain mc23032 to synthesize leucine rendered it incapable of intracellular growth.
FIG. 3.
Growth of M. tuberculosis H37Rv and an M. tuberculosis leucine auxotroph in murine bone marrow-derived macrophages in vitro. Bone marrow-derived macrophages were infected with wild-type H37Rv, strain mc23032 H37Rv ΔleuD, or strain mc23035 leuD+ complemented at an MOI of 2 to 10 bacteria per macrophage, as described in Materials and Methods. At various times postinfection, the macrophage monolayers were fixed and stained and examined by fluorescence microscopy. The bacteria associated with 200 macrophages were enumerated by visual inspection of the monolayers. Heavily burdened macrophages were scored as containing 10 bacteria. The data are representative of three independent experiments.
FIG. 4.
Fluorescence microscopy of murine bone marrow-derived macrophages following infection with various strains of M. tuberculosis. Murine bone marrow-derived macrophages are shown at 1 day (left panels) and 6 days (right panels) postinfection with wild-type H37Rv (upper panels), mc23032 ΔleuD (middle panels), and mc23035 leuD complemented (bottom panels). The monolayers were stained with rhodamine-auramine, counterstained with neutral red, and examined by fluorescence microscopy using a DAPI filter. Magnification, ×1,000; oil immersion. Bacilli are indicated by the arrows.
mc23032 ΔleuD is attenuated in SCID mice.
Based on the in vitro data showing that the leucine auxotroph (mc23032) cannot replicate within macrophages, it followed that strain mc23032 would be attenuated for growth in vivo. To test the effect of leucine auxotrophy on M. tuberculosis virulence, SCID mice, lacking B and T cells, were infected with wild-type H37Rv, mc23032 ΔleuD, or the complemented strain mc23034 or mc23035. SCID mice are exquisitely susceptible to M. tuberculosis infection and succumb to intravenous challenge with 104 CFU of wild-type H37Rv within a month (Fig. 5). Bacterial burdens in these animals reached 5.8 × 108 ± 3.6 × 108 CFU in the livers, 6.4 × 107 ± 3.2 × 107 CFU in the spleens, and 3.8 × 107 ± 4 × 107 CFU in the lungs at the time of death. In contrast, SCID mice receiving strain mc23032 at approximately a 100-fold-greater inoculum were able to clear the infection and remained healthy for 22 weeks, at which time the experiment was terminated (Fig. 5). No bacilli could be cultured from the lungs or spleens of these animals, but a few (14 total) colonies were recovered from the livers of five mice. These colonies failed to grow without leucine supplementation and were therefore not revertants.
FIG. 5.
Survival of SCID mice infected with various strains of M. tuberculosis. BALB/cJ SCID mice were challenged by the intravenous route with 104 CFU of wild-type H37Rv, 106 CFU of mc23032 ΔleuD, or 104 CFU of the leuD-complemented strain mc23034 (leuD in multicopy on a plasmid) or mc23035 (leuD in single copy integrated on the chromosome). Each experimental group consisted of 13 to 14 mice. This experiment was performed twice with similar results.
Infection of SCID mice with the leuD-complemented strains mc23034 and mc23035, at an inoculum equal to that of wild-type H37Rv, was lethal, and the animals died at virtually identical times which were similar but slightly delayed compared with deaths caused by the wild-type H37Rv parent (Fig. 5). Bacterial burdens in the organs at the time of death were similar to those found in animals that died as a result of infection with wild-type bacilli. Thus, restoration of virulence with wild-type leuD provided in either multicopy or single copy established that the observed attenuation of strain mc23032 was attributable to the mutation in leuD that conferred leucine auxotrophy and cannot be attributed to downstream polar effects.
Persistence and protective efficacy of strain mc23032 and comparisons with BCG.
Having established that the leucine auxotroph of M. tuberculosis was indeed attenuated in immunocompromised animals, a requirement of any new tuberculosis vaccine, we next sought to determine whether it could elicit protective immunity against a challenge with virulent organisms. Immunocompetent BALB/cJ mice, a strain relatively susceptible to M. tuberculosis, were intravenously immunized with 5 × 106 CFU of the ΔleuD strain mc23032. Similarly, a group of animals were immunized with the conventional tuberculosis vaccine, BCG-P. Following immunization, the bacterial burden in the spleens and livers of mc23032-immunized animals remained steady for a week, whereas bacterial numbers in the lung had decreased by almost 10-fold (Fig. 6). Thereafter, a steady decline ensued, so that by 13 weeks, the leucine auxotroph could not be recovered from any organs examined (Fig. 6). In contrast, immunization with BCG was followed by a slight increase in bacterial numbers in the spleen and liver. Clearance of BCG in all tissues was delayed compared to that of mc23032-immunized animals. In fact, at 16 weeks postimmunization, the splenic BCG burden had declined by only 1 log unit (Fig. 6).
FIG. 6.
Clearance of M. bovis BCG-P and M. tuberculosis H37Rv ΔleuD (mc23032) in the tissues of immunocompetent BALB/cJ mice. The mice were immunized via the lateral tail vein with 5 × 106 CFU of M. bovis BCG-P (solid symbols) or a similar number of mc23032 ΔleuD cells (open symbols). At the indicated times after immunization, the mice were sacrificed and their organs were collected and homogenized. The bacterial burdens in the lungs (triangles), spleen (squares), and liver (circles) were determined by serial dilution and plating of the organ homogenates onto leucine-supplemented medium. The error bars represent the standard deviations for the means of four to five mice per experimental group.
To compare the protective efficacy of the vaccines, 9 weeks postimmunization, the vaccinated animals and unimmunized controls were challenged intravenously with 106 CFU of virulent M. tuberculosis organisms. Bacterial burdens in unimmunized animals steadily rose in all tissues, increasing by approximately 1 log unit in the liver, 2 log units in the spleen, and 2.5 log units in the lung at 8 weeks postchallenge (Fig. 7). By 15 weeks postinfection, all unvaccinated animals had succumbed to disease (Fig. 8). Mean bacterial burdens at the time death had reached 3.8 × 108 ± 2.3 × 108 CFU in the spleen, 7.88 × 107 ± 6.5 × 107 CFU in the liver, and 7.6 × 108 ± 7 × 108 CFU in the lung. In contrast, consistent with published results, BCG vaccination slowed the growth of wild-type M. tuberculosis in all organs examined (Fig. 7) and prolonged the survival of the mice by several weeks (Fig. 8). Likewise, mice previously immunized with H37Rv ΔleuD (mc23032) also exhibited enhanced survival that was statistically equivalent (P < 0.01) to that of the BCG-vaccinated group (Fig. 8).
FIG. 7.
Growth of an intravenous inoculum of virulent M. tuberculosis in the tissues of vaccinated and unvaccinated mice. BALB/cJ mice were challenged intravenously with 106 CFU of virulent M. tuberculosis either without prior immunization or 9 weeks post-intravenous vaccination with either M. bovis BCG-P or ΔleuD mc23032. At various times following challenge, the mice were sacrificed and their organs were collected and homogenized. The bacterial burdens in the liver (A), spleen (B), and lungs (C) were determined by serial dilution and plating of the homogenates. The error bars represent the standard deviations for the means of four to five mice per experimental group.
FIG. 8.
Survival of vaccinated and unvaccinated mice subsequent to a challenge with virulent M. tuberculosis. Immunocompetent BALB/cJ mice were given 106 CFU of virulent M. tuberculosis by intravenous injection either without prior immunization or 9 weeks post-intravenous vaccination with either M. bovis BCG-P or M. tuberculosis H37Rv ΔleuD (mc23032). The survival of these animals was followed and is expressed as a percentage of the experimental group surviving over time. Each group consisted of 15 to 16 mice.
Despite the comparable efficacy of BCG and H37Rv ΔleuD (mc23032) in enhancing survival after a lethal intravenous challenge with virulent M. tuberculosis, differences between the two vaccinated groups were apparent. Specifically, at 8 weeks postchallenge, bacterial numbers in the lungs of BCG-vaccinated mice were approximately 1.5 log units lower than those in the unvaccinated controls and 1 log unit less than that of the ΔleuD mutant-vaccinated mice. In addition, BCG vaccination halted bacterial expansion in the spleen and promoted bacterial clearance in the liver. At both time points and in all organs, BCG-vaccinated animals displayed significantly lower (P < 0.05) bacterial burdens than either the unvaccinated or ΔleuD strain-vaccinated mice. Relative to the unvaccinated group, mice vaccinated with the ΔleuD M. tuberculosis most often had lower bacterial burdens which were statistically significant (P < 0.05) in the spleen at 1 and 2 months, in the liver at 1 month (P < 0.05), and in the lung at 2 months (P < 0.05).
Histologically, differences between experimental groups were apparent as well. At 2 months postchallenge, the lungs of mice vaccinated with BCG showed multifocal interstitial granulomatous pneumonia, with moderate perivascular lymphocytic infiltration and localized histiocytic and epithelioid cell inflammatory response (Fig. 9A). Acid-fast stain showed low numbers of M. tuberculosis organisms (Fig. 9D). The unvaccinated mice showed severe, diffuse granulomatous pneumonia, composed of predominately epithelioid cells, resulting in severe loss of alveolar spaces (Fig. 9B), and large numbers of M. tuberculosis organisms within macrophages (Fig. 9E). Mice vaccinated with the H37Rv ΔleuD auxotroph vaccine showed moderate perivascular and interstitial granulomatous pneumonia. The pneumonia was more extensive than in BCG-vaccinated mice but less severe than in unvaccinated mice (Fig. 9C), and there were numerous M. tuberculosis organisms. So, despite a large bacterial burden, histologically the lungs of the ΔleuD strain-vaccinated animals were more similar to those of the BCG-vaccinated animals.
FIG. 9.
Histopathology of the lungs of vaccinated and unvaccinated mice 2 months after an intravenous challenge with virulent M. tuberculosis. (A) Lung of mouse vaccinated with BCG-P. These mice developed multifocal pervascular pneumonia characterized by a moderate localized infiltration of lymphocytes accompanied by histiocytes. (B) Severe and extensive granulomatous pneumonia in an unvaccinated control mouse. (C) Lung of mouse vaccinated with the ΔleuD attenuated M. tuberculosis showing moderate perivascular and interstitial pneumonia. (D) Scattered small numbers of acid-fast organisms in the lung of a BCG-vaccinated mouse. (E) Lung of unvaccinated mouse showing large numbers of acid-fast M. tuberculosis organisms. (F) Moderate numbers of acid-fast M. tuberculosis organisms in the lung of a mouse vaccinated with the ΔleuD auxotroph of M. tuberculosis.
As a more natural route of exposure, we also challenged both the vaccinated and unvaccinated control mice by the aerosol route. At 9 weeks postvaccination with BCG-P or H37Rv ΔleuD, the mice received approximately 300 CFU of virulent M. tuberculosis by inhalation. By 4 weeks post-aerosol challenge, bacterial seeding of the liver and spleen had occurred in all groups of animals but was markedly less in BCG-P-vaccinated mice (Fig. 10A and B). Specifically, the liver burden in the BCG-P-vaccinated group was approximately 1 log unit less than in either the unvaccinated or H37Rv ΔleuD-vaccinated mice (Fig. 10A). In addition, BCG vaccination virtually halted M. tuberculosis spread to the spleen: <50 CFU were recovered from each animal in that group (Fig. 10B). Moreover, the bacterial burden in the livers and spleens of the BCG-vaccinated animals remained unchanged at 8 weeks postchallenge. Consistent with the intravenous challenge data, at 1 month post-aerosol challenge, BCG-vaccinated animals displayed significantly lower (P < 0.05) bacterial burdens in the lung than either the unvaccinated or ΔleuD strain-vaccinated mice (Fig. 10C). In no organ at any time were there significant differences between the bacterial burdens of the H37Rv ΔleuD-vaccinated animals and the unvaccinated controls.
FIG. 10.
Growth of an aerosol inoculum of virulent M. tuberculosis in the tissues of both vaccinated and unvaccinated mice. Unvaccinated BALB/cJ mice were challenged by the respiratory route with approximately 300 CFU of virulent M. tuberculosis or were challenged 9 weeks postvaccination with either BCG-P or M. tuberculosis H37Rv ΔleuD strain mc23032. At various times postchallenge, the mice were sacrificed and their organs were collected and homogenized. The bacterial burdens in the livers (A), spleens (B), and lungs (C) were determined by serial dilution and plating of the homogenates as described in Materials and Methods. The error bars represent the standard deviations for the means of four to five mice per experimental group.
Differences in severity of pneumonia among the three groups were apparent at 4 and 8 weeks post-aerosol challenge (data not shown). Unvaccinated controls showed multifocal and coalescing diffuse granulomatous pneumonia with severe lung involvement, resulting in extensive obliteration of alveolar air spaces. Aerosol challenge of BCG-vaccinated mice produced pronounced multifocal granulomatous pneumonia, located adjacent to bronchioles with perivascular infiltrates of large numbers of lymphocytes (data not shown). In comparison, mice vaccinated with the H37Rv leuD auxotroph showed expansive multifocal and, in some areas, coalescing peribronchial and interstitial granulomatous pneumonia of lesser severity than that in the control unvaccinated mice. Thus, histologically, BCG-vaccinated mice displayed partial protection against aerosol challenge with virulent M. tuberculosis, manifested as multifocal granulomatous areas of pneumonia with reduction in the extent of coalescing lung involvement. Similar to what was observed in the intravenous-challenge experiments, the leuD auxotroph also appeared to partially limit the extent of interstitial pneumonia but was less effective than BCG.
DISCUSSION
In this work, we describe the construction of a leucine auxotroph of M. tuberculosis in which the wild-type leuD gene was replaced with a copy containing a defined deletion created by allelic exchange. The defined-deletion mutant failed to show any measurable reversion to prototrophy and remained auxotrophic in vivo. Until very recently, it has been difficult to create defined mutants of M. tuberculosis, a factor that has hampered the genetic analysis of this important pathogen. Although allelic replacement was readily performed by several groups in the rapidly growing and nonpathogenic Mycobacterium smegmatis (27, 30, 46, 53), the slow-growing M. tuberculosis complex members, including M. tuberculosis, M. bovis, and M. bovis BCG, proved to be less amenable to such genetic manipulations. Early attempts at gene exchange using a nonreplicating suicide vector approach were unsuccessful and either yielded single-crossover transformants (2) or resulted in a high incidence of nonhomologous, illegitimate recombination (2, 30). Reasons given to explain the initial lack of success included low levels of transformation efficiency, a high background level of random nonhomologous integration, and the effects of an intein within the open reading frame of the recombination-influencing recA gene (37). However, in the last 4 years, several groups have achieved allelic exchange in the previously genetically refractive slow-growing species (5, 7, 49, 64). These early successes were laboriously achieved and accomplished by screening numerous erroneous clones. However, the subsequent use of the counterselectable marker, sacB, a gene from B. subtilis conferring sucrose sensitivity (20), reduced the amount of screening necessary to identify an allelic-exchange mutant (4, 47, 48). Coupling this counterselection with a replicating plasmid containing a temperature-sensitive origin of replication further enhanced the identification of double-crossover homologous recombinants, but the necessity for growth at the lower permissive temperature (32°C) greatly prolonged the time required to generate the desired mutant (48).
The approach we used to create a leuD mutant of M. tuberculosis involved the use of a standard episomal Mycobacterium-E. coli shuttle vector (pMV261) (59) carrying the ΔleuD6 allele, an oriM, the selectable marker aph, and the counterselectable marker sacB. Following electroporation, antibiotic-resistant transformants were expanded in culture to allow for both double-crossover recombination and plasmid loss. Despite the absence of a conditional replicon, we were able to obtain the desired double-homologous-deletion mutant. However, the approach was less than optimally efficient, since only 11 (3%) of the 334 hygromycin-resistant, sucrose-resistant clones screened by replica plating were leucine auxotrophs. In the vast majority of these sucrose-resistant, leu+ clones, a deletion had occurred in the plasmid backbone such that both the kanamycin-resistant marker and sacB were lost (data not shown). This finding prompted the reconstruction of the replicating vector so that sacB and aph were separated by the marked mutant gene of interest on one side of the plasmid and the origin of replication (oriM) on the other. Thus, with antibiotic (hygromycin) selection for the mutant allele, a deletion of both sacB and aph would now require two independent events. The modification improved the success of the replicating-vector approach to allelic exchange. When it was used to create a different mutant, 77% of the Hygr Kms clones were determined to have undergone the correct allelic-exchange event (M. Glickman, personal communication). Thus, this replicating, sacB-containing vector will facilitate both the production and identification of double homologous recombinants, and it should prove useful in strains of mycobacteria in which transformation efficiency is too low to achieve either a double- or single-crossover recombination event using a nonreplicating suicide vector.
Through the construction of a leucine auxotroph of M. tuberculosis, we hoped to learn something about the nature of the intracellular environment in which this organism resides. The availability of nutrients is crucial to the survival of any microbe and is particularly essential to those living in an intracellular location. Organisms must evolve mechanisms to access nutrients or face eventual elimination. For instance, virulent Listeria monocytogenes, a facultative intracellular pathogen naturally auxotrophic for seven amino acids, expresses a hemolysin that facilitates bacterial escape from the phagosome into the amino acid-rich cytosol, in which the bacterium readily replicates (34). Pathogens whose intracellular lifestyle is confined to the phagosome may modify their vacuolar environment to allow acquisition of nutrients (15, 54). Nevertheless, it would appear that host leucine is unavailable to intracellular M. tuberculosis ΔleuD, since it is incapable of replication in macrophages or in the tissues of mice. Perhaps in vivo bacterial protein synthesis is required for the bacillus to acquire intracellular nutrients and the leuD mutant is compromised in its ability to synthesize proteins de novo.
The connection between bacterial metabolism and virulence was established 50 years ago by Bacon and colleagues, who mutagenized a virulent strain of Bacterium typhosum (Salmonella enterica serovar Typhi) and showed that certain mutants, including those requiring leucine, purines, or para-amino benzoic acid for growth, were less virulent for mice (6). They attributed this loss of virulence to restricted host tissue availability of the required growth factor. An important feature of their work was the finding that the vast majority of the auxotrophic mutants they generated were fully virulent (6). Thus, without knowing the specifics of an organism's lifestyle, there is no a priori reason to assume that auxotrophy will affect virulence. Nevertheless, since these early studies, several groups have reported auxotrophic mutants of Salmonella enterica serovar Typhimurium, Legionella pneumophlia, Shigella flexneri, and Corynebacterium pseudotuberculosis to be attenuated for growth either in vitro within macrophages or in animals in vivo (1, 19, 24, 33, 40, 44, 56). We have found that a ΔleuD auxotroph of M. tuberculosis exhibits the same growth restrictions. M. tuberculosis with a deletion mutation in leuD cannot multiply in either macrophages (Fig. 3 and 4) or mice (Fig. 5 and 6). Importantly, ΔleuD M. tuberculosis is attenuated even in immunocompromised hosts, a desirable trait in a live vaccine, particularly one for which many of the vaccine recipients will be at risk for developing AIDS (18). The attenuated phenotype is attributable to the mutation in leuD, since both intracellular growth and virulence can be restored by complementation with a wild-type copy of the gene.
Once we established that strain mc23032 ΔleuD was attenuated, we questioned whether it could be useful as a vaccine, as has been described for auxotrophs of other bacterial species (11, 14, 16, 24, 32, 56, 57). In this work we have demonstrated that an attenuated leucine auxotroph of M. tuberculosis (mc23032) can induce protective immunity to a virulent strain of the same organism. Protection was manifested by both a reduction in tissue pathology and enhanced survival postchallenge; survival was statistically equivalent to that of animals immunized with the conventional BCG vaccine. However, immunization with BCG better restricted the growth of virulent bacilli in all organs examined and was associated with even less pathology. Although the ΔleuD mutant did induce a protective immune response, because of the likely inaccessibility of leucine, the strain may not have expressed all relevant antigens necessary for protection in vivo. Furthermore, relative to BCG, the in vivo clearance of the leucine auxotroph was more rapid. Such limitations might be common to other types of auxotrophs of M. tuberculosis. Recently, it has been shown that purine auxotrophs of BCG and M. tuberculosis are attenuated for growth in macrophages and survival in vivo (30) and that guinea pigs vaccinated with these strains are able to restrict the growth of virulent M. tuberculosis in their lungs. However, both of these purine mutants were less able than conventional BCG to limit the growth of M. tuberculosis in the spleen; in fact, the BCG purine auxotroph showed no protection in that organ (29). That work and ours emphasize the challenge of achieving the optimal balance of attenuation and immunogenicity. Mutations that severely cripple an organism may make it impotent as a vaccine. It is well established that live bacilli induce more effective immunity than killed bacilli (45, 58, 66). Treatment with antimicrobials that hinder the expansion of the organism in vivo will oppose the development of protective immunity (17). However, the effect of chemoprophylaxis can be overcome if the vaccination dose is large (17). Bacterial replication in vivo likely influences both the quantity and quality of antigen available to the immune system. Thus, a limitation of killed vaccines, and perhaps auxotrophs, is that certain antigens will not be represented, as they are expressed only in vivo (39). It is likely that for optimal immune priming, a vaccine strain will need to replicate briefly in vivo, to ensure that relevant antigens are expressed. Nevertheless, vaccination with a larger immunizing dose or booster immunizations may improve the immunogenicity of attenuated strains.
Most experimentation to measure protection against M. tuberculosis includes comparisons with the current standard BCG vaccine. This comparison is reasonable, as substantial evidence, both experimental (8, 26, 45) and clinical (3, 23) exists to support the notion that BCG vaccination engenders immunity to M. tuberculosis. Nonetheless, BCG has frequently faltered in safeguarding adult vaccinees against pulmonary tuberculosis, spurring the desire to create a better vaccine. Thus, the ideal antituberculosis vaccine would provide protection exceeding that afforded by BCG, making it valid to question whether we can expect to do better than BCG. Furthermore, we must ask whether the current animal model systems employed for analysis of protective efficacy would be able to discern superior protection if it was present. To date, the best protection induced (as measured by bacterial burden and survival) by any prospective M. tuberculosis vaccine candidate has been equivalent to that provided by BCG (26, 29, 61). As definitive immunological correlates of protection are lacking, the identification of protection superior to BCG may be difficult. Furthermore, most M. tuberculosis in vivo infection and protection model systems, regardless of species and route of challenge used, are acute in nature. However, in actuality, active tuberculosis is most often the result of chronic infection. Thus, it will be appropriate to evaluate any promising M. tuberculosis vaccine candidate (for example, one providing protection equivalent to BCG in the acute-infection model) by additional means, likely including a low-dose chronic-infection model. Limitations of physical space and financial constraints will make these longer-term M. tuberculosis experiments more arduous, but they may provide necessary information and display differences among vaccine candidates not observed in acute-infection studies.
ACKNOWLEDGMENTS
We thank Bing Chen, Ilona Breiterene, Keming Yu, Vellore P. Mohan, and XiaoJaun Wang for providing technical assistance with the murine infection experiments. Appreciation is extended to Martin Pavelka for contributing pMP7 and for helpful discussions regarding this work. We are grateful to Francis Lee for performing the statistical analysis of the bacterial-burden data and to Miriam Braunstein for critique of the manuscript.
These studies were supported by funds provided by the Howard Hughes Medical Research Institute and TBRU N01-AI45244.
REFERENCES
- 1.Ahmed Z U, Sarker M R, Sack D A. Protection of adult rabbits and monkeys from a lethal shigellosis by oral immunization with a thymine-requiring and temperature-sensitive mutant of Shigella flexneri Y. Vaccine. 1990;8:153–158. doi: 10.1016/0264-410x(90)90139-d. [DOI] [PubMed] [Google Scholar]
- 2.Aldovoni A, Husson R N, Young R A. The uraA locus and homologous recombination in Mycobacterium bovis BCG. J Bacteriol. 1993;175:7282–7289. doi: 10.1128/jb.175.22.7282-7289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson J D, Aronson C F, Taylor H C. A twenty-year appraisal of BCG vaccination in the control of tuberculosis. Arch Intern Med. 1958;101:881–893. doi: 10.1001/archinte.1958.00260170037006. [DOI] [PubMed] [Google Scholar]
- 4.Azad A K, Sirakova T D, Fernandes N D, Kolattukudy P E. Gene knockout reveals a novel gene cluster unique to pathogenic mycobacteria. J Biol Chem. 1997;272:16741–16745. doi: 10.1074/jbc.272.27.16741. [DOI] [PubMed] [Google Scholar]
- 5.Azad K A, Sirkova T D, Rogers L M, Kolattukudy P E. Targeted replacement of the mycocerosic acid synthase gene in Mycobacterium bovis BCG produces a mutant that lacks mycosides. Proc Natl Acad Sci USA. 1996;93:4787–4792. doi: 10.1073/pnas.93.10.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacon G A, Burrows T W, Yates M. The effects of biochemical mutation on the virulence of Bacterium typhosum: the virulence of mutants. Br J Exp Pathol. 1950;31:714–723. [PMC free article] [PubMed] [Google Scholar]
- 7.Balasubramanian, V., M. S. Pavelka, S. S. Bardarov, J. Martin, T. R. Weisbrod, R. A. Mcadam, B. R. Bloom, and W. R. Jacobs. Allelic exchange in Mycobacterium tuberculosis with long linear recombination substrates. 1996. J. Bacteriol. 178:273–279. [DOI] [PMC free article] [PubMed]
- 8.Baldwin S L, D'Souza C, Roberts A D, Kelly B, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D M, Orme I M. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bange F-C, Brown A M, Jacobs W R. Leucine auxotrophy restricts growth of Mycobacterium bovis BCG in macrophages. Infect Immun. 1996;64:1794–1799. doi: 10.1128/iai.64.5.1794-1799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behr M A, Wilson M A, Gill W P, Salamon H, Schoolnik G K, Rane S, Small P M. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 11.Bowe F, O'Gaora P, Maskell D, Cafferkey M, Dougan G. Virulence, persistence, and immunogenicity of Yersina enterocolitica O:8 aroA mutants. Infect Immun. 1989;57:3234–3236. doi: 10.1128/iai.57.10.3234-3236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.China Tuberculosis Control Collaboration. Results of directly observed short-course chemotherapy in 112,842 Chinese patients with smear-positive tuberculosis. Lancet. 1996;347:358–362. [PubMed] [Google Scholar]
- 13.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Conner R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor L K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 14.Cooper, G. L., L. M. Venables, M. J. Woodward, and C. E. Hormaeche. 1994. Vaccination of chickens with strain CVL30, a genetically defined Salmonella enteritidis aroA live oral vaccine candidate. 62:4747–4754. [DOI] [PMC free article] [PubMed]
- 15.Desai S A, Krogstad D J, McCleskey E W. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature. 1993;362:643–646. doi: 10.1038/362643a0. [DOI] [PubMed] [Google Scholar]
- 16.Dougan G, Maskell D, Pickard D, Hormaeche C. Isolation of stable aroA mutants of Salmonella typhi Ty2: properties and preliminary characterization in mice. Mol Gen Genet. 1987;207:402–405. doi: 10.1007/BF00331607. [DOI] [PubMed] [Google Scholar]
- 17.Dubos R J, Schaefer W B. Antituberculous immunity induced in mice by virulent primary infection. Am Rev Tuberculous Pulm Dis. 1956;74:541–551. doi: 10.1164/artpd.1956.74.4.541. [DOI] [PubMed] [Google Scholar]
- 18.Dye C, Scheele S, Dolin P, Pathania V, Raviglione M C. Consensus statement. global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 19.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gay P, LeCoq D, Steinmetz M, Berkelman T, Kado C I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 1985;164:918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guliera I, Tietelbaum R, McAdam R A, Kalpana G, Jacobs W R, Jr, Bloom Barry R. Auxotrophic vaccines for tuberculosis. Nat Med. 1996;2:334–337. doi: 10.1038/nm0396-334. [DOI] [PubMed] [Google Scholar]
- 22.Harrington R, Karlson A G. Differentiation between Mycobacterium tuberculosis and Mycobacterium bovis by in vitro procedures. Am J Vet Res. 1966;27:1193–1196. [Google Scholar]
- 23.Hart P D, Sutherland I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early life. Br Med J. 1977;22:293–295. doi: 10.1136/bmj.2.6082.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoiseth S K, Stocker B A D. Aromatic dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 25.Homchampa P, Strugnell R A, Adler B. Molecular analysis of the aroA gene of Pasteurella multocida and vaccine potential of constructed aroA mutants. Mol Microbiol. 1992;8:3585–3593. doi: 10.1111/j.1365-2958.1992.tb01794.x. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard R D, Flory C M, Cocito C, Collins F M. Immunization of mice with antigen A60 of Mycobacterium bovis BCG. Clin Exp Immunol. 1992;88:129–131. doi: 10.1111/j.1365-2249.1992.tb03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husson R N, James B E, Young R A. Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol. 1990;172:519–524. doi: 10.1128/jb.172.2.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivins B E, Welkos S L, Knudson G B, Little S F. Immunization against anthrax with aromatic compound-dependent (AroA−) mutants of Bacillus anthracis and with recombinant strains of Bacillus subtilis that produce anthrax protective antigen. Infect Immun. 1990;58:303–308. doi: 10.1128/iai.58.2.303-308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson M, Phalen S W, Langranderie M, Ensergueix D, Chavarot P, Marchal G, McMurray D N, Gicquel B, Guilhot C. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotrophic vaccine. Infect Immun. 1999;67:2867–2873. doi: 10.1128/iai.67.6.2867-2873.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalpana G V, Bloom B R, Jacobs W R. Insertional mutagenesis and illegitimate recombination in mycobacteria. Proc Natl Acad Sci USA. 1991;88:5433–5437. doi: 10.1073/pnas.88.12.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanai K, Yanagisawa K. Studies on the reinfection in experimental tuberculosis of guinea pigs. Jpn J Med Sci Biol. 1955;8:115–127. doi: 10.7883/yoken1952.8.115. [DOI] [PubMed] [Google Scholar]
- 32.Karnell A, Stocker B A, Katakura S, Sweiha H, Reinholt F P, Cam P D, Reach D D, Lindberg A A. An auxotrophic live oral Shigella flexneri vaccine: development and testing. Rev Infect Dis. 1991;13(Suppl. 4):S357–S361. doi: 10.1093/clinids/13.supplement_4.s357. [DOI] [PubMed] [Google Scholar]
- 33.Leung K Y, Findlay B B. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci USA. 1991;88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marhairas G G, Sabo P J, Hickey M J, Singh D C, Stover C K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marquis H, Bouwer H G A, Hinrichs D J, Portnoy D A. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect Immun. 1993;61:3756–3760. doi: 10.1128/iai.61.9.3756-3760.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAdam R A, Weisbrod T R, Martin J, Scuderi J D, Brown A M, Cirillo J D, Bloom B R, Jacobs W R., Jr In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect Immun. 1995;63:1004–1012. doi: 10.1128/iai.63.3.1004-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFadden J. Recombination in mycobacteria. Mol Microbiol. 1996;21:205–211. doi: 10.1046/j.1365-2958.1996.6271345.x. [DOI] [PubMed] [Google Scholar]
- 38.McFarland W C, Stocker B A D. Effect of different purine auxotrophic mutations on mouse virulence of a Vi-positive strain of Salmonella dublin and two strains of Salmonella typhimurium. Microb Pathog. 1987;3:129–141. doi: 10.1016/0882-4010(87)90071-4. [DOI] [PubMed] [Google Scholar]
- 39.McKenney D, Pouliot K L, Wang Y, Murthy V, Ulrich M, Doring G, Lee J C, Goldmann D, Pier G B. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 40.Mintz C S, Chen J, Shuman H A. Isolation and characterization of auxotrophic mutants of Legionella pneumophila that fail to multiply in human monocytes. Infect Immun. 1988;56:1449–1455. doi: 10.1128/iai.56.6.1449-1455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray C J L, Lopez A D. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 42.Murray C J L, Solomon J A. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci USA. 1998;95:13881–13886. doi: 10.1073/pnas.95.23.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Callaghan D, Maskell D, Liew F Y, Easmon C S F, Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attenuation, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988;56:419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada N, Saskawa C, Tobe T, Yamada M, Nagai S, Talukder K A, Komatsu K, Kanegasuki S, Yoshikawa M. Virulence-associated chromosomal loci of Shigella flexneri identified by random Tn5 insertion mutagenesis. Mol Microbiol. 1991;5:187–195. doi: 10.1111/j.1365-2958.1991.tb01839.x. [DOI] [PubMed] [Google Scholar]
- 45.Opie E L, Freund J. An experimental study of protective inoculation with heat killed tubercle bacilli. J Exp Med. 1937;66:761–788. doi: 10.1084/jem.66.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavelka M S, Jr, Jacobs W R. Biosynthesis of diaminopimelate (DAP), the precursor of lysine and a component of the peptidoglycan, is an essential function of Mycobacterium tuberculosis. J Bacteriol. 1996;178:6496–6507. doi: 10.1128/jb.178.22.6496-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pavelka M S, Jr, Jacobs W R. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis Bacillus Calmette-Guerin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J Bacteriol. 1999;181:4780–4789. doi: 10.1128/jb.181.16.4780-4789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelicic V, Jackson M, Reyrat J M, Jacobs W R, Jr, Gicquel B, Guilhot C. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1997;94:10955–10960. doi: 10.1073/pnas.94.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyrat J-M, Berthet F-X, Gicquel B. The urease locus of Mycobacterium tuberculosis and its utilization for the demonstration of allelic exchange in Mycobacterium bovis Bacillus Calmette Guerin. Proc Natl Acad Sci USA. 1995;92:8768–8772. doi: 10.1073/pnas.92.19.8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rimphanitchayakit V, Hatfull G F, Grindley N D. The 43 residue DNA binding domain of gamma delta resolvase binds adjacent major and minor grooves of DNA. Nucleic Acids Res. 1989;17:1035–1050. doi: 10.1093/nar/17.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts M, Maskell D, Novotny P, Dougan G. Construction and characterization in vivo of Bordetella pertussis aroA mutants. Infect Immun. 1990;58:732–739. doi: 10.1128/iai.58.3.732-739.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodrigues L C, Gill N, Smith P G. BCG vaccination in the first year of life protects children of Indian subcontinent ethnic origin against tuberculosis in England. J Epidemiol Community Health. 1991;45:78–80. doi: 10.1136/jech.45.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sander P, Meier A, Bottger E C. Rps1+: a dominant selectable marker for gene replacement in mycobacteria. Mol Microbiol. 1995;16:991–1000. doi: 10.1111/j.1365-2958.1995.tb02324.x. [DOI] [PubMed] [Google Scholar]
- 54.Schwab J C, Beckers C, Joiner K A. A putative pore in the parasitophorous vacuole membrane of Toxoplasma gondii identified by microinjection of fluorescent probes. Mol Biol Cell. 1992;3(Suppl.):303a. [Google Scholar]
- 55.Segall T, Linberg A A. Salmonella dublin experimental infection in calves: protection after oral immunization with an auxotrophic aroA live vaccine. J Vet Med Ser B. 1991;38:142–160. doi: 10.1111/j.1439-0450.1991.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 56.Simmons P, Hodgson A L M, Strugnell R A. Attenuation and vaccine potential of aroQ mutants of Corynebacterium pseudotuberculosis. Infect Immun. 1997;65:3048–3056. doi: 10.1128/iai.65.8.3048-3056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith B P, Reina-Guerra M, Stocker B A, Johnson E. Aromatic-dependent Salmonella dublin as a parental modified live vaccine for calves. Am J Vet Res. 1984;45:2231–2235. [PubMed] [Google Scholar]
- 58.Smith T. Certain aspects of natural and acquired resistance to tuberculosis and their bearing on preventative measures. JAMA. 1917;68:764–679. [Google Scholar]
- 59.Stover K, de la Cruz V F, Fuerst T R, Burlein J E, Benson L A, Bennett L T, Bansal G P, Young J, Lee M, Hatfull G F, Snapper S, Barletta R, Jacobs W R, Jr, Bloom B R. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 60.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddis P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russel D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 61.Tascon, R. E., M. J. Colston, S. Ragro, E. Stavropoulous, D. Gregory, and D. B. Lowrie. 1996. Vaccination against tuberculosis by DNA injection. 8:888–892. [DOI] [PubMed]
- 62.Tsenova L, Moreira A, Party E, Freedmanj V H, Kaplan G. Aerosol infection of mice with mycobacteria using a nose-only exposure device. J Am Biol Saf Assoc. 1997;2:20–31. [Google Scholar]
- 63.Tuberculosis Prevention Trial. Madras. Trial of BCG vaccines in South India for tuberculosis prevention. Indian J Med Res. 1980;72:1–74. [PubMed] [Google Scholar]
- 64.World Health Organization. The world health report 1999. Making a difference. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 65.Yaun, Y., D. D. Crane, R. M. Simpson, Y. Q. Zhu, M. J. Hickey, D. R. Sherman, and C. E. Barry III. 1998. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. 95:9578–9583. [DOI] [PMC free article] [PubMed]
- 66.Zinsser H, Ward H K, Jennings F B. The significance of bacterial allergy as a sign of resistance. J Immunol. 1925;10:719–723. [Google Scholar]