Objective:
To evaluate the impact of direct discharge home (DDH) from ICUs compared with ward transfer on safety outcomes of readmissions, emergency department (ED) visits, and mortality.
Data Sources:
We searched MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and Cumulative Index to Nursing and Allied Health Literature from inception until March 28, 2022.
Study Selection:
Randomized and nonrandomized studies of DDH patients compared with ward transfer were eligible.
Data Extraction:
We screened and extracted studies independently and in duplicate. We assessed risk of bias using the Newcastle-Ottawa Scale for observational studies. A random-effects meta-analysis model and heterogeneity assessment was performed using pooled data (inverse variance) for propensity-matched and unadjusted cohorts. We assessed the overall certainty of evidence for each outcome using the Grading Recommendations Assessment, Development and Evaluation approach.
Data Synthesis:
Of 10,228 citations identified, we included six studies. Of these, three high-quality studies, which enrolled 49,376 patients in propensity-matched cohorts, could be pooled using meta-analysis. For DDH from ICU, compared with ward transfers, there was no difference in the risk of ED visits at 30-day (22.4% vs 22.7%; relative risk [RR], 0.99; 95% CI, 0.95–1.02; p = 0.39; low certainty); hospital readmissions at 30-day (9.8% vs 9.6%; RR, 1.02; 95% CI, 0.91–1.15; p = 0.71; very low-to-low certainty); or 90-day mortality (2.8% vs 2.6%; RR, 1.06; 95% CI, 0.95–1.18; p = 0.29; very low-to-low certainty). There were no important differences in the unmatched cohorts or across subgroup analyses.
CONCLUSIONS:
Very low-to-low certainty evidence from observational studies suggests that DDH from ICU may have no difference in safety outcomes compared with ward transfer of selected ICU patients. In the future, this research question could be further examined by randomized control trials to provide higher certainty data.
Keywords: direct discharge home, home, intensive care unit, patient discharge, patient readmission, safety, survival
KEY POINTS.
Question: Are patients who are direct discharges home from ICU compared with ward transfers prior to hospital at higher risk of adverse events?, for example, emergency department visits, readmissions, and mortality?
Findings: In this systematic review/meta-analysis, there was no difference in the risk of emergency department visits and readmissions to hospital or mortality, although the certainty of evidence is very low to low.
Meaning: There may be no difference between DDH from ICU in safety outcomes compared with ward transfer of selected ICU patients.
Traditionally, patients discharged from ICU have been transferred to a hospital ward in order to facilitate recovery, rehabilitation, and organize discharge planning to the community, prior to discharge to home (1–7). Due to high hospital ward censuses and transfer delays, the practice of direct discharge home (DDH) from ICUs is increasing, ranging from 11% to 15% in various jurisdictions (1–6). This increase in DDH is likely due to strained ward capacity precluding timely transfer from blocked ward beds, with the result of these delays being increasingly expensive due to increased ICU costs (8). Prior work has demonstrated an inverse correlation between DDH and ICU census (low ICU census with higher DDH), with no correlation between DDH and ward census (4, 6). Patients who are directly discharged home are typically younger, with few comorbidities and with relatively simple discharge diagnoses (1–7).
Although previously underexamined (7), there has been a recent increase in the number of studies examining the practice of DDH compared with ward transfers from ICUs (1–7). Our prior review had insufficient data to generate specific point estimates on the impact of DDH on patient-important outcomes, hence the need to update this systematic review to also include a meta-analysis. Systematically evaluating care process issues by quantifying these point estimates for efficacy and potential harms of DDH in ICU patients is the best way to inform safety of this practice with the best available evidence.
To this end, our objective was to perform a systematic review and meta-analysis regarding safety outcomes (readmissions and emergency department [ED] visits) and mortality associated with DDH compared with ward transfer prior to hospital discharge in adult critically ill patients.
MATERIALS AND METHODS
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (9–11) and registered the protocol with International Prospective Register of Systematic Reviews CRD42020169328 (registered April 28, 2020). We have included the complete PRISMA checklist in Supplemental Table 1 (http://links.lww.com/CCM/H239).
Data Sources, Search Strategy, and Study Selection
We conducted a systematic electronic literature search in the MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials electronic databases (all via Ovid interface) and Cumulative Index to Nursing and Allied Health Literature (CINAHL) database (via EBSCO platform) from inception to March 28, 2022. The search was performed by a clinical librarian with experience in conducting electronic literature searches in consultation with the review authors. A sensitive search strategy was developed by combining synonymous searches composed of controlled vocabularies, such as medical subject headings in MEDLINE, CINAHL headings in CINAHL, or Emtree descriptors in EMBASE, and free-text terms into the search blocks of: Patient Discharge/Patient Transport + Critical/Intensive care + Home care. All languages were included. The full search strategy is outlined in Appendix Supplement 1 (http://links.lww.com/CCM/H239).
Operational definitions have been previously described in our prior narrative review (7). ICU was defined as a distinct unit in the hospital that provided invasive monitoring, invasive or noninvasive mechanical ventilation, or administration of vasoactive agents to critically ill patients. A critically ill patient was defined as any patient admitted either electively or nonelectively, requiring invasive monitoring, invasive or noninvasive mechanical ventilation, or administration of vasoactive agents. We classified high dependency units or step-down units as ICUs. Home was defined as any place of residence that was a nonhealthcare facility or a facility that did not routinely have healthcare personnel available to care for residents (e.g., complex care, rehabilitation facility, and nursing home) (7).
We included all randomized control trials (RCTs) and observational studies, which described adult ICU patients who were either DDH from ICU versus transferred to a ward prior to hospital discharge. The searches were restricted to studies in adult humans (age ≥18 yr). Studies from pediatric populations, case reports, conferences, gray literature, abstracts, and studies of patients receiving chronic mechanical ventilation or palliation were excluded. The bibliographies of identified relevant studies were reviewed to locate additional studies of interest.
In the first stage, at least two reviewers (V.I.L., R.D., S.P., E.J.S.), independently and in duplicate, assessed each of the citations for eligibility. Any citations selected by either of the reviewers were advanced to the second stage (full-text screening). Disagreements at any stage were resolved through discussion and consultation with a third reviewer (V.I.L, E.J.S.), if necessary. We used Covidence (Veritas Health Innovation, Melbourne, Australia) to manage search results, screen, and select studies (12).
Data Abstraction
Independently and in duplicate, reviewers used predeveloped abstraction forms in Microsoft Excel Version 14.0.6 (Microsoft Corporation, Redmond, WA) to extract the following data: study characteristics (title and author), patient group demographic/clinical data, interventions and comparators (DDH versus ward transfer), clinical outcome data (including ED visits, readmissions, and mortality at closest to 30 and 90 d), and the jurisdiction(s) (e.g., province and country) in which the study was performed. If two studies contained overlapping datasets, the larger of the two studies was selected for inclusion in the meta-analysis. We contacted study authors for missing data.
Study Quality Assessment and Risk of Bias
We assessed the Risk of Bias (ROB) in nonrandomized trials using the Newcastle-Ottawa Scale (NOS). Domains and scoring are listed in the footnotes (13).
Data Synthesis and Analysis
Continuous data were presented as means and sd, or medians and interquartile ranges.
We performed meta-analysis using the RevMan Version 5.3 software (Cochrane Collaboration, Copenhagen, Denmark) (12, 14). We used the method of DerSimonian and Laird and inverse variance to pool effect sizes for each outcome under a random-effects model for all outcomes of interest (15). We analyzed study results obtained from both propensity-matched and unmatched cohort analyses, depending on the approach used in primary studies. We presented summary effect estimates as relative risks (RRs) with 95% CIs (14). We assessed between-study heterogeneity using the I2 statistic, the χ2 test for homogeneity (p < 0.1 for significance of substantial heterogeneity), and visual inspection of the forest plots. We considered an I2 value greater than 50% to indicate of substantial heterogeneity (12, 14).
Although we planned to assess for publication bias using funnel plots and Egger test, we could not conduct the analysis because there were fewer than 10 studies identified per outcome.
We planned prespecified subgroup analyses (hypothesized direction of effect in parentheses) to investigate sources of heterogeneity. If subgroups effects were credible, we presented the outcomes separately for each subgroup and assessed using Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) (16).
Level 3 (highest acuity) ICUs versus others ICU types (see Appendix Supplement 1, http://links.lww.com/CCM/H239) (DDH associated with better outcomes for patients in highest acuity, level 3 ICU studies, compared with ward transfer)
High versus low ROB studies (DDH associated with better outcomes in high ROB studies, compared with ward transfer)
Observational versus randomized studies (DDH associated with better outcomes in observational studies, compared with ward transfer)
North American versus other geographical location (DDH associated with better outcomes in North American studies, compared with ward transfer)
Grading of Recommendations Assessment, Development, and Evaluation
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the certainty of evidence in pooled outcome data, assessing each of the following domains: ROB, indirectness, imprecision, inconsistency, and other considerations (e.g., publication bias, large magnitude of effect, and addressing residual confounding). We rated certainty as high, moderate, low, or very low (17, 18) with RCTs starting as high and observational studies starting as low.
RESULTS
Study Characteristics
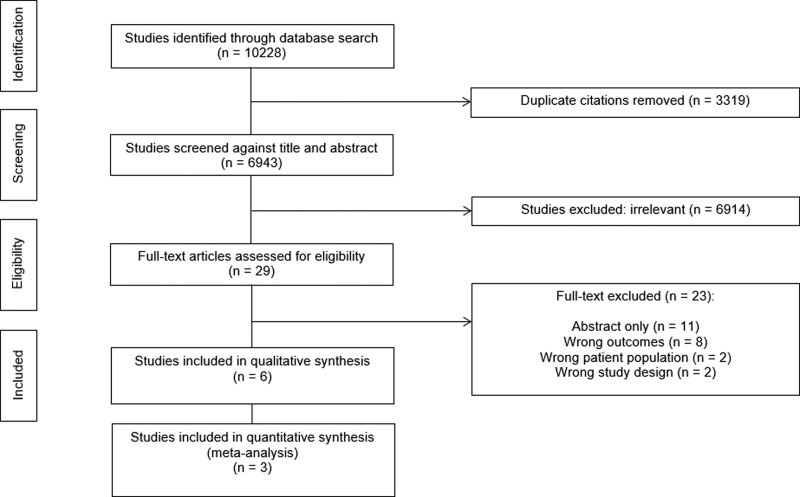
Of 10,228 citations identified, we reviewed 29 full texts and included six studies (n = 49,376 patients) (1–3, 5, 6, 19, 20) fulfilling eligibility criteria (Fig. 1).
Figure 1.
Direct From ICU Sent Home Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
A summary of all eligible studies is presented in Supplemental Table 2 (http://links.lww.com/CCM/H239). All eligible studies were observational cohort studies of adult patients from mixed (medical/surgical) ICUs, but only three had sufficient outcome data for meta-analysis (1–3). Five studies were conducted in North America (1–3, 6, 19), whereas one study was conducted in the United Kingdom (20).
Demographics and clinical characteristics of included study patients are presented in Supplemental Table 2 (http://links.lww.com/CCM/H239). Of note, DDH patients were generally younger compared with ward transfer patients (DDH, 45–63 years compared with ward transfer, 54–65 years [range]). The most common admission diagnoses in DDH patients were: diabetic complications (31–37%), overdose (20–36%), pneumonia (15%), gastrointestinal illness (14%), and respiratory failure (10%). Ward transfer patients had longer ICU LOS (DDH: 2 d vs ward: 3 d [range]) and hospital length of stay (DDH: 2–3 d compared with ward: 2–14 days [range]) compared with DDH patients. Ward occupancy rates were much higher than ICU occupancy rates (ward: 84–104% compared with ICU: 72–96% [range]) (1–3, 5, 6, 19, 20).
Risk of Bias
The assessment for ROB for observational cohort studies is shown in Supplemental Table 3 (http://links.lww.com/CCM/H239). Three studies were deemed to have low ROB for all domains (1–3). The other three studies were deemed to have high ROB (5, 19, 20). Common ROB issues among these studies were: selection (no description of nonexposed nonintervention cohort) (19, 20), comparability (no control for age, intervention/comparator exposure, or other additional factors) (5, 19, 20), and short or inadequate follow-up (19, 20).
Clinical Outcomes
Of the six studies included in this systematic review, three studies (1–3) were deemed to be high quality (full NOS scores) and were included in the meta-analysis. Two studies were excluded due to insufficient data (19, 20), and one study was excluded (5) because its dataset overlapped with another larger study (2, 6).
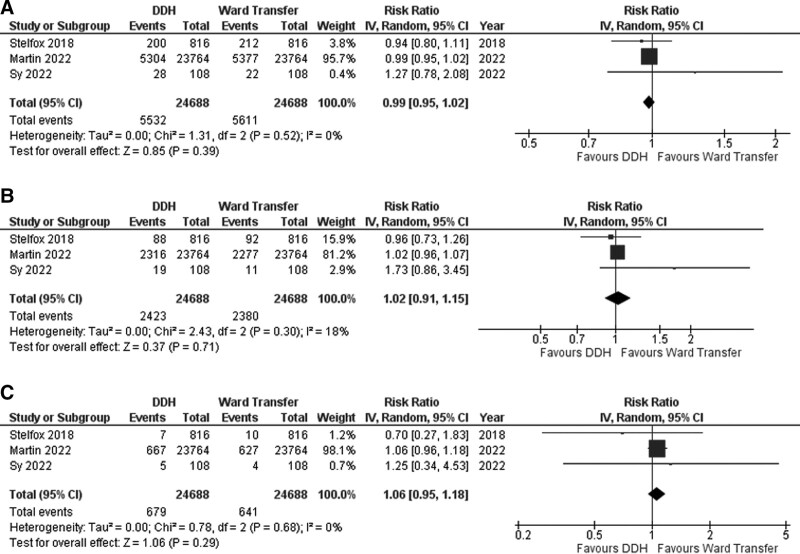
Results of the meta-analysis (forest plots) of three studies (1–3) are presented in Figure 2. We investigated three outcomes comparing DDH versus ward transfer after ICU: readmission (at 30 d), ED visits (at closest to 30 and 90 d), and mortality (both closest to 30 and 90 d).
Figure 2.
Forest plot of emergency department (ED) visits, hospital readmission, and mortality ED visits at closest to 30 d (A), hospital readmission at closest to 30 d (B), and mortality at closest to 90 d (propensity-matched cohorts) (C).
Overall, for propensity-matched patients (n = 49,376), there was no difference in ED visits at 30 days between DDH (22.4%) and ward transfer (22.7%) patients (RR, 0.99; 95% CI, 0.95–1.02; p = 0.39, low certainty) (Fig. 2A).
There was no difference in hospital readmissions at closest to 30 days between DDH (9.8%) and ward transfer (9.6%) patients (RR, 1.02; 95% CI, 0.91–1.15; p = 0.71, low certainty) (Fig. 2B).
There was no difference in mortality as measured closest to 90 days between DDH (2.8%) and ward transfer (2.6%) patients (RR, 1.06; 95% CI, 0.95–1.18; p = 0.29, low certainty) (Fig. 2C).
Heterogeneity was low for all the outcomes (I2 = 0–18%). Visual inspection of the forest plots revealed the same conclusions (Fig. 2A–C).
Visual inspection for publication bias using funnel plots was not performed as there were less than 10 trials per outcome.
Subgroup and Unadjusted Cohort Analyses
We were unable to perform any of these preplanned subgroup analyses for the following reasons: 1) there were no high ROB studies to compare to low ROB subgroup, as the high ROB studies lacked sufficient data (19, 20), 2) all studies were observational (and could not be compared to a RCT group) (1–3), 3) all included meta-analysis studies were conducted in North America (1–3), with the remaining U.K. study not having enough data for comparison (20), and 4) there was insufficient data regarding level 3 ICU versus other ICU types. Therefore, ICEMAN was also not performed as there were no credible subgroups.
Pooling of unadjusted data led to very low certainty of evidence for all outcomes of interest and an uncertain effect of DDH as compared to ward discharge.
GRADE Assessment
For all outcomes, three observational studies were assessed in our GRADE Summary of Findings (Supplemental Table 4, http://links.lww.com/CCM/H239) (1–3). There was no downgrading for ROB, inconsistency, indirectness, and imprecision.
For other considerations, we determined that there was no large magnitude of effect size or adjustment for residual confounding (no upgrades). We addressed publication bias by implementing a very comprehensive search strategy, searching references lists, and contacting authors for data. Therefore, we did not downgrade for publication bias.
Overall, we were unable substantiate these findings due to the very low-to-low level of certainty in the evidence for DDH versus ward transfer in adult critically ill post-ICU survivors for included the clinical outcomes of readmissions, ED visits, and mortality (1–3).
DISCUSSION
In our systematic review, DDH may not lead to any differences in outcomes of interest (readmissions, ED visits, and mortality) compared with ward transfer of patients prior to discharge. This is important because DDH may be a viable option to decant selected low-risk patients home (1–7), rather than wait many days for a ward bed, and incur high costs for their delay (8). However, this was based on very low-to-low certainty of evidence, and there is a selection bias for patients considered for DDH. Direct discharge to home of ICU patients is an evolving practice borne out of resource constrained healthcare infrastructure (1). However, the discharge of ICU patients can leave them vulnerable to untoward sequela that follows these transitions in care.
To our knowledge, this is the first meta-analysis that attempts to quantify the impact of direct discharge to home on hospital readmissions, ED visits, and mortality. As the practice of DDH increases (~11–16% of ICU admissions in various jurisdictions), there are several reasons why DDH may be preferable to ward transfer for ICU patients and the healthcare system for selected patients (1–7). Given that step-down and high-dependency units were also classified as ICUs, this may have influenced the results, as lower acuity patients may experience DDH from these ICUs compared with other patients (5, 6). Some hospitals may also lack proper step-down units, therefore leading to more ICU admissions to compensate, also influencing these results by potentially increasing DDH rates (5, 6).
Even for short clinical encounters (21, 22), increased handovers between care teams are associated with worse outcomes (21–24). Patients may be most vulnerable in the transition periods of care, for example, ward teams may require more time to familiarize themselves with complex ICU patients, resulting in delayed medication reconciliation, which could be prone to error. When compared with ward teams, ICUs may have additional dedicated services and personnel (e.g., allied health care and pharmacists) as well as improved healthcare provider-to-patient ratios to assist with discharge planning (5). However, operationalizing DDH may involve further activities that ICUs are less familiar with: 1) liaising with community resources to ensure a safe transition, 2) assessing patient’s safety in a home environment, 3) and ensuring adequate outpatient follow-up is arranged (3, 25). Because the potential healthcare cost-savings by reducing avoidable days waiting for a ward bed (1, 8) are substantial, scrutiny of the balance of benefits and harms is warranted. For certain groups of patients (e.g., recovered and less sick), safe convalescence at home, rather than additional time spent on a hospital ward, can lead to less capacity strain while avoiding iatrogenic harms (1–7). DDH is potentially a patient-centered strategy that may assist transitions of certain patients (2, 6, 8), result in higher patient and family satisfaction (26), and can help optimize healthcare resources use (8, 27). However, physicians have less comfort and experience with this practice (26, 28) and may need further experience before establishing guidelines around safe discharge from ICU.
Expanding on the evidence from our prior narrative scoping review (7), we performed a large meta-analysis with pooling of large population-based, propensity-matched observational cohorts in different jurisdictions examining DDH versus ward transfer. Prior authors have suggested that DDH from ICU may be feasible and safe compared with ward transfer for certain patients, albeit with very low-to-low certainty based on this evidence. Hospital and long-term care crowing likely influence these results, leading to decreased flow out of ICU (1–3). With bed capacity being strained during the coronaravirus-19 pandemic and even prior to this (1–3), DDH discharges may become more prevalent in order to accommodate increasing influxes of ICU patients, especially when bed pressure in ICU is low (1).
However, what is still unclear is which patients are best suited for safe DDH from ICU (1–6) and which patients need further outpatient interventions from the healthcare system prior to discharge (1–3). Although different from typical ICU admissions (e.g., sepsis and respiratory failure), overdose, seizures, and diabetic ketoacidosis are the most common reasons of ICU admission in DDH patients (1–5), which is evidenced in other jurisdictions outside of Canada as well (e.g., United Kingdom [17] and United States [29]). These patients still have ICU-level care needs, as evidence by nursing man power scores (1). Even though propensity-matched cohorts were primarily analyzed, there can be selection bias and confounding (e.g., lower acuity patients with better social home supports) who undergo DDH more than other patients. Therefore, the question of residual confounding is an important one. We were unable to use individual patient data to control for other potential confounders, which may still influence the underlying results. Even in this review, there are some potential signals of harm for DDH (owing to heterogeneity in patient populations, leading to inconsistency and imprecision in our meta-analysis). Differences in transition to a patient’s home (which may lack supports) versus rehabilitation hospital or nursing home (with more supports) may also influence a clinician’s decision to transfer home. Prior work suggests that DDH patients typically are not discharged to long-term care or nursing home (3), but future study is required to determine if these types of supports would influence these results.
Future research should explore a clinical prediction model, which can identify low-risk versus High-risk DDH patients and determine which patients could benefit from further follow-up posthospital discharge aside from usual care (e.g., additional support systems, home settings, and future access to healthcare resources) (1–3). Derivation of this model could be performed in one jurisdiction and validated in another, with factors that may be associated with poor DDH outcomes (1, 3, 6, 30).
The importance of our findings is that despite having high-quality, well-conducted, large population-based, propensity-matched observational cohort studies, the certainty in the pooled data remains very low-to-low. This highlights the need for rigorously conducted, well-powered RCTs in this area. The current research team is designing RCTs, examining accelerated discharge from ICU compared with usual care. Prior literature suggests that DDH patients go home with of family and friends, but fewer funded home care or private nursing (3). There is likely a role to also study how increased home supports will influence this practice, including the advent of virtual hospitals and “hospital at home” models of care (31).
Strengths of this systematic review include the comprehensiveness of our search strategy, the comprehensive meta-analysis methodology, a preregistered protocol, and the application of GRADE to assess certainty of the estimates of effect.
Limitations include: 1) low certainty in most outcomes limiting our conclusions, 2) insufficient subgroup datasets that could benefit from DDH, 3) uncertain generalizability to other jurisdictions (e.g., by country, by public, or by private system funding) not studied (given a predominantly North American distribution), and 4) few included studies in the meta-analysis. Publication bias can also not be ruled out given the few studies available, although we performed a rigorous systematic search with prespecified inclusion and exclusion criteria to mitigate this issue.
CONCLUSIONS
Very low-to-low certainty evidence from observational studies led to our inability to substantiate that DDH from ICU may have no difference on safety outcomes compared with ward transfer of patients prior to discharge. In the future, this research question could be subject to further observational studies and RCTs to provide higher certainty data.
ACKNOWLEDGMENTS
We thank Hannah Wunsch, Sandy Kassir, Jose Diego Marques Santos, and Chiraag Gupta for their support of this work. We thank Karin Dearness from McMaster University for her assistance with PRESS.
None of the funders played a role in the conception, design, conduct, oversight, analysis, interpretation, or decision to submit this manuscript for publication or in the preparation, review, or approval of the manuscript.
Supplementary Material
Footnotes
*See also p. 156.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
All authors made substantial contributions to conception and design, acquisition of data, analysis, and interpretation of data; drafted the submitted article and revised it critically for important intellectual content; and provided final approval of the version to be published. Drs. Lau and Sy helped in conception and background. Drs. Lau, Donnelly, Parvez, Gill, Iansavichene, and Sy helped in data collection. Drs. Lau, Donnelly, Parvez, Gill, Bagshaw, Ball, Basmaji, Cook, Fiest, Fowler, Mailman, Martin, Rochwerg, Scales, Stelfox, and Sy helped in data analysis.
Study methods, operations, and article generation were coordinated by the Direct From ICU Sent Home Systematic Review and Meta-analysis steering committee (V.I.L., E.J.S.).
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Dr. Parvez received funding from the University of Saskatchewan College of Medicine Dean’s Summer Student 2020 Award. Dr. Bagshaw received funding from Baxter and BioPorto. Dr. Scales’ institution received funding from the Canadian Institute for Health Research. Dr. Sy received funding from the Saskatchewan Health Research Foundation Establishment Grant. Dr. Bagshaw is supported by a Canada Research Chair in Critical Care Outcomes and Systems Evaluation. Dr. Fowler is the H. Barrie Fairley Professor of Critical Care Medicine at the University Health Network and the Interdepartmental Division of Critical Care Medicine at the University of Toronto. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Lau VI, Priestap FA, Lam JNH, et al. : Factors associated with the increasing rates of discharges directly home from intensive care units—a direct from ICU sent home study. J Intensive Care Med. 2016; 33:121–127 [DOI] [PubMed] [Google Scholar]
- 2.Stelfox HT, Soo A, Niven DJ, et al. : Assessment of the safety of discharging select patients directly home from the intensive care unit: A multicenter population-based cohort study. JAMA Intern Med. 2018; 178:1390–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau VI, Lam JNH, Basmaji J, et al. : Survival and safety outcomes of ICU patients discharged directly home—a direct from ICU sent home study*. Crit Care Med. 2018; 46:900–906 [DOI] [PubMed] [Google Scholar]
- 4.Martin CM, Lam M, Allen B, et al. : Determinants of direct discharge home from critical care units: A population-based cohort analysis. Crit Care Med. 2019; 48:475–483 [DOI] [PubMed] [Google Scholar]
- 5.Martin CM, Lam M, Le B, et al. : Outcomes after direct discharge home from critical care units: A population-based cohort analysis. Crit Care Med. 2022; 50:1256–1264 [DOI] [PubMed] [Google Scholar]
- 6.Basmaji J, Lau V, Lam J, et al. : Lessons learned and new directions regarding Discharge Direct from Adult Intensive Care Units Sent Home (DISH): A narrative review. J Intensive Care Soc. 2018; 20:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shamseer L, Moher D, Clarke M, et al. : Preferred Reporting Items for Systematic Review and Meta-Analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015; 350:g7647. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. : Preferred Reporting Items for Systematic reviews and Meta-Analyses: The PRISMA statement. J Clin Epidemiol. 2009; 62:1006–1012 [DOI] [PubMed] [Google Scholar]
- 9.Veritas Health Innovation Covidence Systematic Review Software. 2019. Available at: http://www.covidence.org. Accessed January 3, 2020
- 10.Joanna Briggs Institute: JBI Checklist for Cohort Studies. 2020. Available at: HYPERLINK https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Cohort_Studies2017_0.pdf. Accessed November 2, 2022
- 11.Higgins JP, Green S: Cochrane Handbook for Systematic Reviews of Interventions. London, UK, Cochrane Collaboration, 2011, pp 449–480 [Google Scholar]
- 12.Higgins JPT, Thompson SG, Deeks JJ, et al. : Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G, Shea B, O’Connell D, et al. : Ottawa Hospital Research Institute. 2019. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 27, 2019
- 14.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188 [DOI] [PubMed] [Google Scholar]
- 15.Schünemann H, Brożek J, Guyatt G, et al. : GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Hamilton, Canada, McMaster University, 2013 [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, et al. : What is “quality of evidence” and why is it important to clinicians? BMJ. 2008; 336:995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing S, Roshdy A, Radhakrishnan J, et al. : Discharge home from critical care: Safety assessment in a resource constrained system. J R Coll Physicians Edinb. 2019; 49:23–25 [DOI] [PubMed] [Google Scholar]
- 18.Chawla S, D’Agostino RL, Pastores SM, et al. : Homeward bound: An analysis of patients discharged home from an oncologic intensive care unit. J Crit Care. 2012; 27:681–687 [DOI] [PubMed] [Google Scholar]
- 19.Petersen LA, Brennan TA, O’Neil AC, et al. : Does housestaff discontinuity of care increase the risk for preventable adverse events? Ann Intern Med. 1994; 121:866–872 [DOI] [PubMed] [Google Scholar]
- 20.Horwitz LI, Moin T, Krumholz HM, et al. : Consequences of inadequate sign-out for patient care. Arch Intern Med. 2008; 168:1755–1760 [DOI] [PubMed] [Google Scholar]
- 21.Saager L, Hesler BD, You J, et al. : Intraoperative transitions of anesthesia care and postoperative adverse outcomes. Anesthesiology. 2014; 121:695–706 [DOI] [PubMed] [Google Scholar]
- 22.Jones PM, Cherry RA, Allen BN, et al. : Association between handover of anesthesia care and adverse postoperative outcomes among patients undergoing major surgery. JAMA. 2018; 319:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagshaw SM, Tran DT, Opgenorth D, et al. : Assessment of costs of avoidable delays in intensive care unit discharge. JAMA Netw Open. 2020; 3:e2013913–e2013913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagshaw SM, Wang X, Zygun DA, et al. : Association between strained capacity and mortality among patients admitted to intensive care: A path-analysis modeling strategy. J Crit Care. 2018; 43:81–87 [DOI] [PubMed] [Google Scholar]
- 25.Stelfox HT, Hemmelgarn BR, Bagshaw SM, et al. : Intensive care unit bed availability and outcomes for hospitalized patients with sudden clinical deterioration. Arch Intern Med. 2012; 172:467–474 [DOI] [PubMed] [Google Scholar]
- 26.Lam JNH, Lau VI, Priestap FA, et al. : Patient, family, and physician satisfaction with planning for direct discharge to home from intensive care units: Direct from ICU sent home study. J Intensive Care Med. 2017; 35:82–90 [DOI] [PubMed] [Google Scholar]
- 27.Sy E, Parvez S, Kassir S, et al. : Canadian healthcare provider perceptions of discharging patients directly home from the intensive care unit. Can J Anesth. 2021; 68:1840–1842 [DOI] [PubMed] [Google Scholar]
- 28.Opgenorth D, Stelfox HT, Gilfoyle E, et al. : Perspectives on strained intensive care unit capacity: A survey of critical care professionals. PLoS One. 2018; 13:e0201524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel PM, Fiorella MA, Zheng A, et al. : Characteristics and outcomes of patients discharged directly home from a medical intensive care unit: A retrospective cohort study. J Intensive Care Med. 2021; 36:1431–1435 [DOI] [PubMed] [Google Scholar]
- 30.Lau VI, Priestap F, Lam JNH, et al. : Clinical predictors for unsafe direct discharge home patients from intensive care units. J Intensive Care Med. 2018; 25:1067–1073 [DOI] [PubMed] [Google Scholar]
- 31.Shepperd S, Iliffe S: Hospital at home versus in-patient hospital care. Cochrane Database Syst Rev. 2005; 20:CD000356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.