Background:
Despite progress in treating homozygous familial hypercholesterolemia, most patients do not achieve low-density lipoprotein cholesterol (LDL-C) targets. This study examined efficacy and safety of the PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, alirocumab, in pediatric patients (aged 8–17 years) with inadequately controlled homozygous familial hypercholesterolemia.
Methods:
In this open-label, single-arm, multinational, Phase 3 study, patients (n=18) received alirocumab 75 mg or 150 mg (bodyweight <50 kg/≥50 kg) every 2 weeks as an adjunct to background treatment. The primary endpoint was percent change in LDL-C from baseline to Week 12. Secondary endpoints included changes in LDL-C and other lipid parameters up to 48 weeks, safety/tolerability, and alirocumab pharmacokinetics.
Results:
The mean age of patients was 12.4 years; 16/18 (89%) had mutations in the low-density lipoprotein receptor gene (LDLR) and 2/18 (11%) had mutations in the LDLR adapter protein 1 gene (LDLRAP1). At baseline, mean LDL-C (standard deviation) was 373.0 (193.5) mg/dL, which decreased by 4.1% at Week 12 (primary endpoint) and 11.4%, 13.2%, and 0.4% at Weeks 4, 24, and 48, respectively. At Week 12, 9/18 (50%) patients achieved LDL-C reductions ≥15%. Mean absolute LDL-C decreases ranged from 25 to 52 mg/dL over follow-up. A post hoc analysis demonstrated heterogeneity of responses according to genotype. There were no unexpected safety/tolerability findings. Free PCSK9 was reduced to near zero for all patients at Weeks 12 and 24.
Conclusions:
The study supports the efficacy and safety of alirocumab as a potential adjunct to treatment for some pediatric patients with homozygous familial hypercholesterolemia.
Registration:
Highlights.
In pediatric patients receiving alirocumab 75 mg or 150 mg (based on bodyweight) Q2W, mean low-density lipoprotein cholesterol decreased by 11.4%, 4.1%, 13.2%, and 0.4% at Weeks 4, 12, 24, and 48 compared with baseline, respectively.
Up to ≈50% of pediatric patients achieved low-density lipoprotein cholesterol reductions ≥15%, with mean absolute low-density lipoprotein cholesterol decreases ranging from 25 to 52 mg/dL over follow-up.
Substantial variability of the response to treatment with alirocumab was observed across all functional status subgroups, highlighting the importance of measuring low-density lipoprotein receptor activity in addition to determining genotype to decide on treatment strategies.
The safety profile in the study was consistent with the known profile of alirocumab in adults.
See accompanying editorial on page 1458
Homozygous familial hypercholesterolemia (HoFH) is a life-threatening disorder characterized by extremely elevated circulating levels of LDL (low-density lipoprotein) cholesterol (LDL-C), which results in premature and progressive atherosclerotic cardiovascular disease and extensive xanthomas.1 HoFH is a rare disease with an estimated frequency of 1/160 000 to 1/320 000.2 The principal aim of treatment is to lower LDL-C as much as possible and, in order to achieve this, it is strongly recommended that treatment be started as early as possible.1 Interventions usually involve multiple lipid-modifying therapies, such as a statin, ezetimibe, a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, and/or lomitapide, as well as LDL (low-density lipoprotein) apheresis.3
HoFH is most often caused by mutations in the LDL receptor (LDLR) gene, but mutations in other genes involved in the LDL receptor (LDLR) pathway, such as apolipoprotein B (APOB), PCSK9, and the LDLRAP1 (LDLR adaptor protein 1) have also been implicated in the disease and response to treatment.1,4 Patients with complete loss of LDLR activity in both inherited genes (null/null functional status) demonstrate more severe disease, earlier development of comorbidities such as atherosclerotic vascular disease, aortic stenosis, and poorer response to therapies that upregulate LDLR function, compared with individuals who are just defective in both alleles.1,4,5 Compound heterozygous patients may be assigned null/null, null/defective, or defective/defective functional status; those assigned null/defective functional status may show a phenotype in between the null/null and defective/defective phenotypes.
Guidelines for treating adult and pediatric patients with HoFH recommend early initiation of lipid-lowering therapy (ideally at the maximally tolerated level)1,2 and initiation of treatment with a statin and ezetimibe at diagnosis has been recommended by the European Atherosclerosis Society Consensus panel.6 Visiting specialized lipid clinics for LDL apheresis is also recommended if feasible.2,6 Evolocumab (a PCSK9 inhibitor) and evinacumab (an angiopoietin-like 3 inhibitor) are licensed for use in the treatment of patients with HoFH aged ≥10 years and ≥12 years respectively in the United States and EU.7–10 The 2017 National Lipid Association and European Society of Cardiology/European Atherosclerosis Society have recommended that PCSK9 inhibitor therapy may be considered to reduce LDL-C in patients with HoFH of unknown genotype, or in those known to be LDLR defective who are on the maximum tolerated dose of statins with/without ezetimibe, and have LDL-C ≥70 mg/dL or non–high-density lipoprotein cholesterol (non–HDL-C) ≥100 mg/dL, except those with null/null LDLR mutations.11,12 Despite the considerable progress in treatment for HoFH in recent years, most patients do not achieve recommended LDL-C targets with current lipid-modifying therapies, and the unmet medical need remains high.1
Alirocumab is a fully human monoclonal antibody that targets PCSK9, a protein involved in lipoprotein homeostasis.13,14 Binding of PCSK9 to the LDLR reduces recycling of LDLR and results in fewer LDLR on cell surface, resulting in less LDL-C being removed from circulation via binding to LDLR.13 When alirocumab binds PCSK9, PCSK9 is no longer able to bind the LDLR, improving recycling of the LDLR and thus increasing removal of LDL-C from circulation.14 The ODYSSEY HoFH study, conducted in adults with HoFH, found alirocumab treatment (150 mg once every 2 weeks, administered in addition to background lipid-modifying therapies) was effective at reducing LDL-C compared with placebo (least squares mean [SE] change from baseline to Week 12: −26.9% [4.6%] and +8.6% [6.3%], respectively; between-treatment difference: 35.6% [7.8%, P<0.0001]).15 Alirocumab is indicated as an adjunct to diet and maximally tolerated statin therapy for adults who require additional lowering of LDL-C, for the treatment of primary hypercholesterolemia (heterozygous familial or non-familial), mixed dyslipidemia, or established atherosclerotic cardiovascular disease.14,16 However, it is currently not approved for use in the pediatric population.
Here, we report the results from a study that evaluated the efficacy and safety of alirocumab 75 or 150 mg (based on bodyweight) once every 2 weeks (Q2W) in pediatric patients with HoFH aged 8 to 17 years.
Methods
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.
Study Design
This was an open-label, multinational, multicenter Phase 3 study with a single arm in 10 active centers in 10 countries. For a list of study sites and investigators, please see Table S1. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. The institutional review board or independent ethics committee at each study center approved the study protocol, and written informed consent was obtained from each participant and/or their parents. The study was registered with clinicaltrials.gov, the European Union Clinical Trials Register and the European Union Drug Regulating Authorities Clinical Trials Database (NCT03510715/EFC14660/EudraCT: 2017-002297-39).
The assessment schedule consisted of a run-in period (≤4 weeks as needed), a screening period (≤2 weeks in all patients), a treatment period (48 weeks), and a follow-up period (8 weeks; Figure S1). Screening took place 2 weeks before baseline measurement.
Participants
Male and female children and adolescents aged 8 to 17 years with HoFH assessed by genotyping were eligible for the study if their HoFH was inadequately controlled despite treatment with an optimal dose of statin with or without other lipid-modifying therapies, or non-statin lipid-modifying therapies if statin-intolerant, at stable dose(s) for ≥4 weeks. Patients with a null/null genotype were excluded a priori based on investigator assessment. The diagnosis of HoFH was made based on the result of genotyping performed previously or based on results of centralized genotyping performed during the run-in period of the current study if no previous testing was available. Genotyping data were evaluated by the investigators; the presence of 2 mutated alleles in the LDLR, Apo B, PCSK9, or LDLRAP1 genes (true homozygotes, compound heterozygotes, or double heterozygotes) in addition to corresponding phenotype (eg, very high LDL-C level, presence of xanthoma) and family history were considered to support a diagnosis of HoFH. Patients continued their background therapy throughout the study.
For those patients who required LDL apheresis, they must have been undergoing stable therapy for ≥4 weeks prior to the screening visit (Week –2) and had initiated LDL apheresis treatment ≥6 months before entering the study. Patients undergoing apheresis were required to align the Day 1/Week 0, Week 4, and Week 12 visits with an apheresis procedure and alirocumab administration, with the study drug being injected immediately following apheresis completion. Following Week 12, patients were free to adapt apheresis appointments to their individual needs, with the recommendation that the Week 24 and 48 visits should coincide with an apheresis procedure.
Patients who were <8 or >17 years of age, null/null for LDLR, had LDL-C <130 mg/dL at screening, or bodyweight <25 kg were excluded. For a full list of patient inclusion/exclusion criteria please see Supplemental Material.
Intervention
Eligible patients received subcutaneous injections of alirocumab either 75 mg Q2W for patients with bodyweight <50 kg, or 150 mg Q2W for patients with bodyweight ≥50 kg. The first injection was administered at the investigation site, after which subsequent injections occurred at a patient-preferred location and were performed either by the patient (if aged 12 years or older and trained), or by a parent/carer (if patient was under 12 years of age). As such, 6 doses were administered by Week 12; of these, 5 were administered at home. After Week 12, the dose could be adjusted in cases where bodyweight increased or decreased to above or below the 50 kg dosing threshold during the study. Adherence to treatment was assessed via alirocumab administration data recorded by the investigator in the electronic case report form and by patients/parents in a patient’s diary. Also, used and unused doses were returned to site and counted by investigators. Treatment adherence to statins was tracked by interviewing the patients and parents on any changes in statins at each visit, and information on use of statins was also recorded in the electronic case report form by study investigators and in patient diaries by patients.
Endpoints and Assessments
The primary endpoint was percent change in LDL-C levels (pre-apheresis, if applicable) from baseline to Week 12. Secondary endpoints included: (1) percent change in LDL-C levels (pre-apheresis, if applicable) from baseline to Weeks 24 and 48; (2) percent change from baseline to Weeks 12, 24, and 48 in other lipid parameters, apolipoprotein B (Apo B), non–HDL-C, total cholesterol, lipoprotein a (Lp [a]), triglycerides, HDL-C, Apo A-1 (apolipoprotein A-1); (3) proportion of patients with ≥15% reduction in LDL-C levels at Weeks 12, 24, and 48; and (4) absolute change in LDL-C from baseline to Weeks 12, 24, and 48.
The safety and tolerability of alirocumab ≤48 weeks of treatment, including incidences of treatment-emergent adverse events, serious adverse events, adverse events of special interest, and deaths, were also evaluated. Adverse events of special interest included allergic events or local injection site reactions that required a separate consultation, liver enzyme elevation (ALT [alanine aminotransferase] >3× upper limit of normal, if baseline ALT <upper limit of normal, or ALT ≥2 times the baseline value, if baseline ALT ≥upper limit of normal), neurologic events that required additional examinations or procedures and/or referral to a specialist, all neurocognitive events, pregnancy, and symptomatic overdose.
Additional assessments included the pharmacokinetic parameters of alirocumab, such as the lowest concentration before administration of the next dose (Ctrough) and serum concentration of total and free PCSK9 at baseline, Week 12, Week 24, and Week 48. Samples to determine alirocumab Ctrough were taken between 11 and 17 days after previous injection of alirocumab at Week 12 and Week 48 (and between 8 and 21 days at Week 24) and may have been just prior to the next injection. The potential development of anti-alirocumab antibodies (ADA) over the study course was also assessed.
Statistical Analysis
No sample size calculation was performed. Approximately 18 patients were planned to be enrolled to have ≥15 evaluable patients, considering the recruitment constraints in this rare disease population; due to the small patient population, no sex-based stratification analyses were planned.
Efficacy was analyzed in the intent-to-treat population, which was defined as all enrolled patients who received ≥1 dose or partial dose of alirocumab. The safety population was made up of enrolled patients who had received ≥1 dose or partial dose of alirocumab, as well as patients for whom it was unclear whether they received study medication. Pharmacokinetic analyses were performed in a population consisting of the safety population with ≥1 available pharmacokinetic sample post first alirocumab injection, while ADA analyses were performed in those included in the safety population with a blood sample on Week 0 (baseline) and ≥1 evaluable blood sample for ADA post first alirocumab injection. Blood samples were collected before alirocumab injection, and before LDL apheresis if applicable.
All efficacy analyses were descriptive, with no formal statistical test, due to lack of control group in this single-arm study (please see Supplemental Material for details). Post hoc exploratory subgroup efficacy analyses were performed to better understand where the large inter-patient variability in response to alirocumab stemmed from. To facilitate this, we assigned a functional LDLR activity to each participant based on individual genotyping data, using reference to the Leiden Open Variation Database data and literature, after the end of the study. The percent changes in LDL-C from baseline to Weeks 12, 24, and 48 were evaluated according to the functional LDLR status identified by the exploratory analysis, and according to concomitant treatment with LDL apheresis.
Results
Baseline Characteristics
Of the 18 patients, the mean age was 12.4 years (range 9–17 years) and 9 (50%) were male (Table 1). Overall, 16 patients entered the study with an HoFH diagnosis based on prior genotyping information; the 2 remaining patients underwent centralized genotyping at enrollment. A total of 3 patients with prior genotyping data also underwent centralized genotyping at enrollment. After genotyping, 10 (56%) patients were confirmed as true homozygotes and the remaining 8 (44%) as compound heterozygotes. In total, 16 (89%) patients had mutations in LDLR and the remaining 2 (11%) had mutations in LDLRAP1 (see Table S2 for details of patient genotypes). Despite LDLR null/null functional status being an exclusion criterion, 6 such patients were assigned to this status post hoc, and the 2 patients with mutations in the LDLRAP1 gene were assigned to a null/null functional status. These patients were included in the intent-to-treat analysis. At baseline, mean LDL-C (SD was 373.0 [193.5] mg/dL (Table 1). An instability of LDL-C level was observed during the screening period, with individual LDL-C measurements at baseline varying from −76% to 211% of the screening value, highlighting the large intraindividual variability in this population.
Table 1.
Patient Baseline Demographic, LDL-C, and Treatment Data
Background Treatment Before and During the Study
All patients were receiving statin treatment at baseline. Most patients were not on the maximal dose of statin they could tolerate because of a regional practice guideline, AEs, or patient/parent refusal. Strict adherence to statin treatment was observed in all patients during the study. A statin dose change was reported in 1 patient around Week 44 of the study. In total, 13/18 (72.2%) patients were on ezetimibe at baseline. Two patients not on ezetimibe at baseline started ezetimibe treatment around Week 24 of the study. Six of 18 (33%) patients were on LDL apheresis treatment. Two patients received apheresis QW, 1 patient Q2W, 2 patients Q3W, and 1 patient had an irregular apheresis schedule. Three patients missed ≥1 procedure in the 4 weeks leading to the scheduled visits at Week 12 and/or 24 and/or 48.
Alirocumab Treatment
Two patients had a change of alirocumab dose during the study due to increase in bodyweight (one at Week 26, the other at Week 36). Adherence to alirocumab treatment was high with an overall mean (SD) compliance rate of 98.3% (3.2). One patient missed 4 of the scheduled injections, after Week 12.
Efficacy
LDL-C
At Week 12 of treatment, mean (SD) percent change from baseline in LDL-C was −4.1% (36.0). A decrease from baseline of −11.4% (17.6) was seen at Week 4, of −
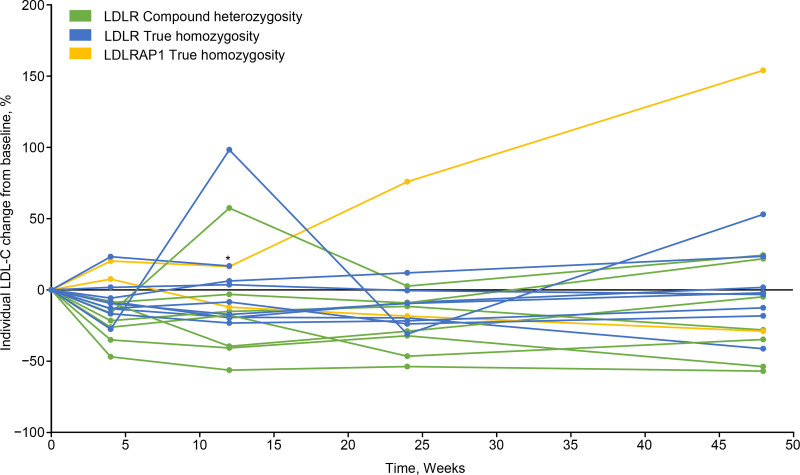
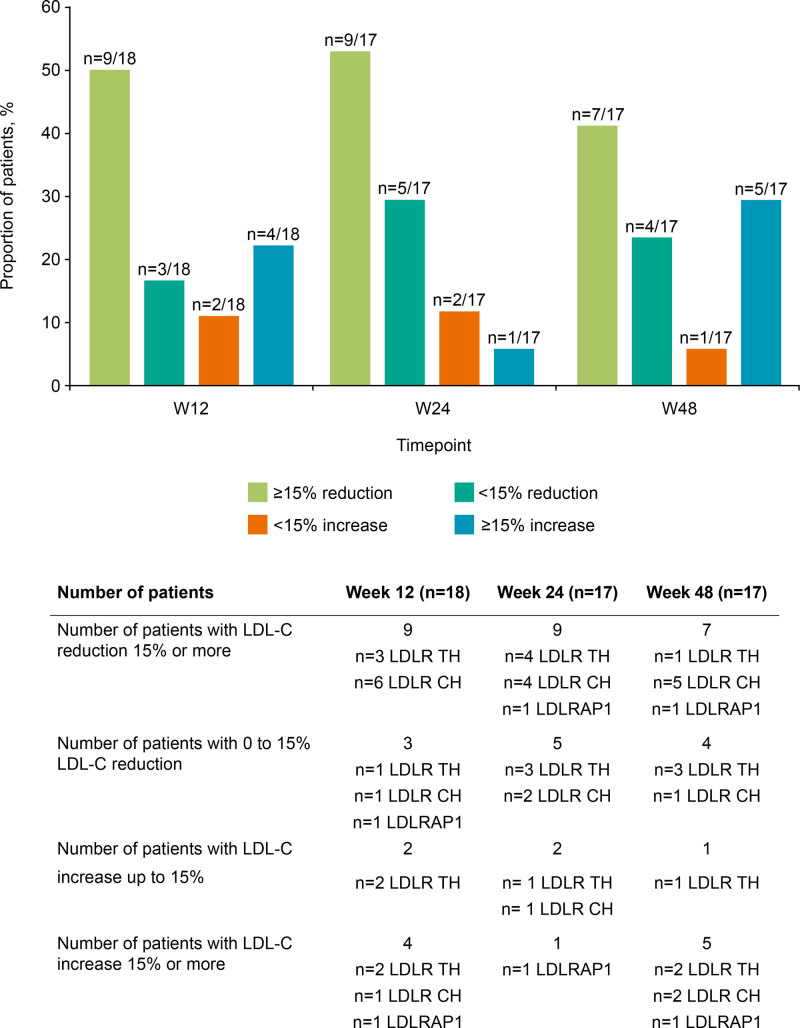
13.2% (28.5) at Week 24, and of −0.4% (49.6) at Week 48. The absolute changes in mean LDL-C (SD) from baseline were −35.5 (47.1) mg/dL at Week 4, −33.4 (83.1) mg/dL at Week 12, −52.3 (71.4) mg/dL at Week 24, and −25.3 (100.1) mg/dL at Week 48. Percent change in LDL-C by genotype is shown in Figure 1. The percentage of patients achieving ≥15% reduction from baseline in LDL-C was 50.0% by Week 12, 52.9% by Week 24, and 41.2% by Week 48 (Figure 2).
Figure 1.
Individual percent change from baseline in low-density lipoprotein cholesterol (LDL-C) from baseline over the study period, by genotype. Each line represents absolute LDL-C change from baseline for 1 individual patient. *Patient death recorded following treatment discontinuation. LDLR indicates LDL receptor; and LDLRAP1, LDLR adapter protein 1.
Figure 2.
Low-density lipoprotein cholesterol (LDL-C) change for the whole cohort at Weeks 12, 24, and 48. N=17 from Week 24 onward. CH indicates compound homozygote; LDLR, LDL receptor; TH, true homozygote; and W, Week.
One patient experienced a treatment-emergent serious adverse event (cardiac failure) unrelated to study treatment and discontinued treatment shortly after completing Week 18. The patient subsequently died 1 week after discontinuation. Available data for this patient (up to Week 12) were included in all efficacy analyses; at Week 12, this patient’s LDL-C change from baseline was +16.8%.
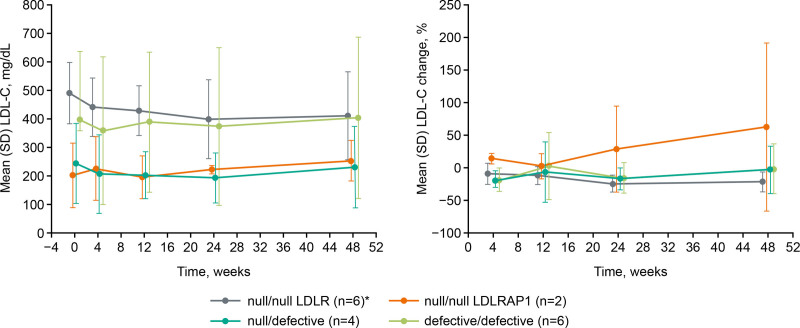
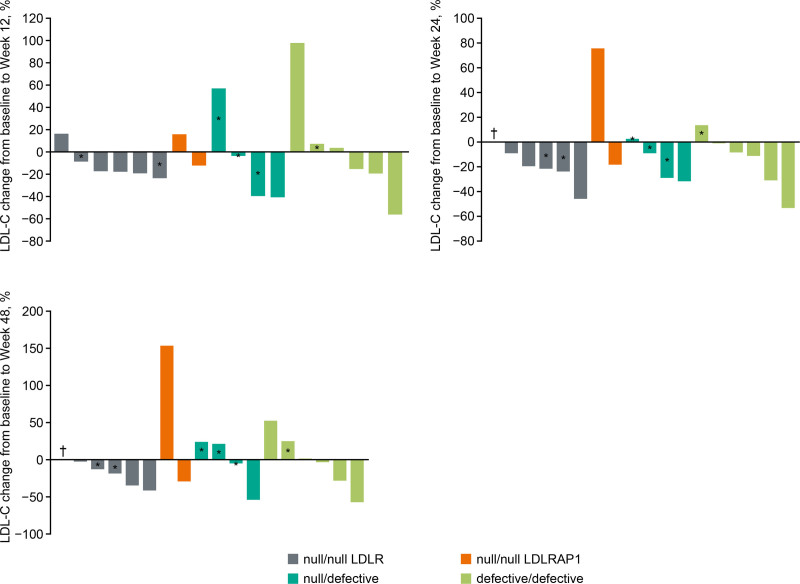
While inter-patient responses to alirocumab varied, individual responses were typically consistent between visits. To better understand the source of the large variability in response to alirocumab, we performed a post hoc analysis on LDL-C measurements for subgroups created according to assigned LDLR functional status. Patients with a null/null functional status for LDLR (n=6) showed the highest mean (SD) LDL-C value at baseline with 491.1 (107.2) mg/dL compared with 202.1 (116.0) mg/dL for those null/null in LDLRAP1 (n=2); this was 397.9 (242.0) mg/dL for patients with a defective/defective status and 243.9 (142.2) mg/dL for null/defective individuals (Table 1). The variability of these values was large, regardless of the functional status, and this persisted throughout the study (Figure 3A).
Figure 3.
Low-density lipoprotein cholesterol (LDL-C) absolute values (mg/dL) and percent (%) change from baseline to Week 48 according to assigned low-density lipoprotein receptor (LDLR) functional status. *n=5 from Week 24 onward. Measurements were performed pre-apheresis where applicable. Error bars indicate SD. LDLRAP1 indicates low-density lipoprotein receptor adapter protein 1.
In the patients who were null/null in LDLR, mean LDL-C decreased versus baseline for all timepoints, whereas patients who were null/null in LDLRAP1 showed an increase at each timepoint compared with baseline. Percent change in LDL-C over time was similar for patients who were null/defective or defective/defective (Figure 3B). Mean percent changes in LDL-C had large SDs, indicating that there was a large variability in response within the LDLR functional status subgroups (Figure 3B). Target achievement of ≥15% reduction in LDL-C at Week 12 was 4/6 (66.7%) for the patients null/null in LDLR and 0/2 (0%) for those null/null in LDLRAP1 (Table S3); in the null/defective and defective/defective groups this was 2/4 and 3/6 (50%), respectively.
We also investigated changes in LDL-C stratified by whether patients were on LDL apheresis treatment during the study. Mean (SD) baseline LDL-C was similar for patients receiving LDL apheresis (396.4 [191.0] mg/dL, pre-apheresis) compared with those who were not (360.6 [201.2] mg/dL; Table 1; Figure S2A). Mean (SD) percent change in LDL-C at Week 12 was −1.8% (33.2) pre-apheresis and −65.5% (15.0) post-apheresis, compared with −5.2% (38.7) in patients not on LDL apheresis (Figure S2B). Percent change in LDL-C from baseline at Week 24 and Week 48 were similar, regardless of apheresis status. At Week 12, 2/6 (33.3%) patients on LDL apheresis and 7/12 (58.3%) patients not on LDL apheresis achieved ≥15% reduction in LDL-C. Respective values for Week 24 were 3/6 (50.0%) and 6/11 (54.5%), and for Week 48 were 1/6 (16.7%) and 6/11 (54.5%; Table S3).
An analysis of the effect of LDLR functional status on LDL-C levels for individual patient is shown in Figure 4. Substantial variability of the response to treatment with alirocumab was observed across all functional status subgroups.
Figure 4.
Percent change in low-density lipoprotein cholesterol (LDL-C) from baseline (pre-apheresis if applicable) according to assigned functional status at Week 12, Week 24, and Week 48. *Patient on apheresis; †patient deceased. Measurements were performed pre-apheresis where applicable. LDLR indicates LDL receptor; and LDLRAP1, low-density lipoprotein receptor adapter protein 1.
Other Lipid Parameters
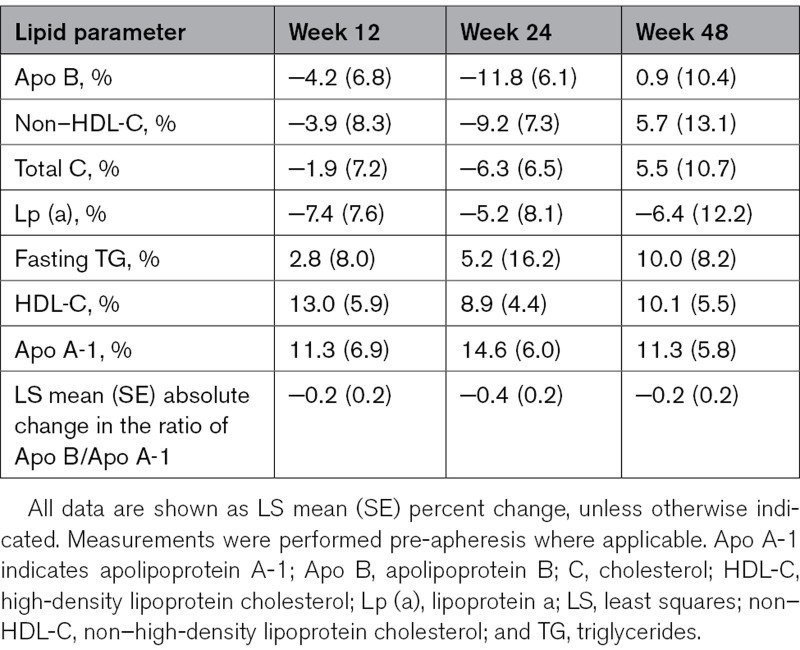
Changes in other lipid parameters are presented in Table 2. Mean decreases versus baseline in Apo B, non–HDL-C, and total C were observed at Weeks 12 and 24, with an increase observed at Week 48, albeit with high variability throughout. A small decrease in Lp (a) (Lipoprotein a) versus baseline was seen at all study timepoints. Small increases across all timepoints were observed for triglycerides, HDL-C, and Apo A-1.
Table 2.
Percent Change From Baseline in Other Lipid Parameters
Safety
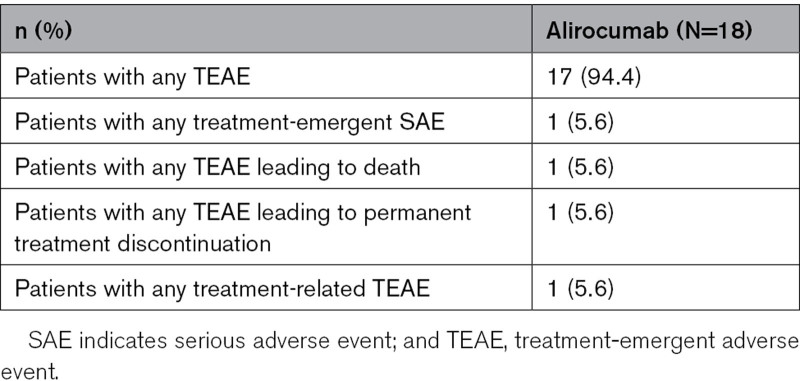
Overall, 17/18 (94%) patients experienced any treatment-emergent adverse event (Table 3). The most frequently reported treatment-emergent adverse events by primary system organ class were infections and infestations (n=7 patients), respiratory, thoracic, and mediastinal disorders (n=5), and cardiac disorders (n=4). As noted earlier, one patient death unrelated to study treatment was recorded following treatment discontinuation. Death was assessed as the result of cardiac failure secondary to severe atherosclerotic aortic stenosis. Available data for this patient (up to Week 12) were included in all safety analyses. One patient experienced a non-serious local injection site reaction that was judged to be treatment-related.
Table 3.
Safety Outcomes
Pharmacokinetic
The concentrations of total PCSK9 and free PCSK9 in patients on LDL apheresis (Figure S3A and S3B) and not on LDL apheresis (Figure S3C and S3D) were evaluated. The concentration of total PCSK9 increased ≈ 10-fold in the whole cohort on treatment compared with baseline values. The concentration of free PCSK9 reduced to near zero for all patients at Weeks 12 and 24. One patient, on LDL apheresis, had an unexplained increase at Week 24 compared with baseline.
Mean (SD) Ctrough alirocumab concentrations for patients not on LDL apheresis increased from zero at baseline to 16.4 (3.7) µg/mL for the 75 mg Q2W dose and 24.4 (8.1) µg/mL for the 150 mg Q2W dose, respectively at Week 12, which was maintained at Week 24 (Figure S4A). At Week 48, mean (SD) Ctrough increased further to 26.7 (19.6) and 28.3 (11.3) µg/mL, respectively. In patients on LDL apheresis, mean pre-apheresis Ctrough alirocumab concentration was generally similar to those of patients not on LDL apheresis: 13.9 to 24.0 µg/mL at Week 12 increasing to 22.9 to 30.9 µg/mL at Week 48, for patients who received LDL apheresis (pre-apheresis measurement; Figure S4B). However, mean post-apheresis Ctrough alirocumab concentration was ≈ 50% lower than the corresponding pre-apheresis concentration at each post-baseline timepoint (data not shown).
Anti-Alirocumab Antibodies
No positive ADA responses were observed in this study.
Discussion
This study provides evidence supporting the use of alirocumab as a prospective adjunct therapeutic option for pediatric patients with HoFH, even in those predicted to see limited benefit based on LDLR functional status. A small percent change in mean LDL-C compared with baseline was observed at Week 12 (primary endpoint); the large SD associated with the mean reflects the large variability of the inter-individual responses for this parameter. Similar observations were made at the other timepoints. Despite the small percentage changes in LDL-C, mean absolute reductions ranged from ≈33 to 52 mg/dL from Week 4 until Week 48 after baseline. Previous research has shown a decrease in major coronary events, major vascular events and all-cause mortality with similar LDL reductions, and suggests that these changes, though small, are clinically relevant.17
Interestingly, we observed that effects for individual patients could only be determined following treatment implementation. It is thought that this may be due to the genetic heterogeneity of the patient population, and differences in existing treatment (both between patients and in terms of changes over time in individual patients), such as the use of LDL apheresis. As a likely result of this heterogeneity, there was large variability in LDL-C level between patients at baseline in addition to large intra-individual variability in LDL-C during the screening period compared with baseline. This is consistent with both clinical practice and previous studies in patients with HoFH showing a high variability of LDL-C both before and in response to treatments that upregulate LDLR function, such as statins or PCSK9 inhibitors.18,19
Percent change reduction of LDL-C achieved with statins in patients with HoFH is generally estimated at 10% to 25% of the baseline value.1 Although the study was designed to limit baseline variability by requiring stable background therapy and LDL apheresis status before entering the study, the large variability in baseline LDL-C mentioned above may reflect difficulties in the optimization of treatment in the pediatric HoFH population.
The variability seen at baseline and throughout the study led to a post hoc genetic analysis to examine outcomes by LDLR functional status, revealing the inclusion of 6 patients with LDLR null/null functional status and 2 patients with null/null mutations in the LDLRAP1 gene. A numerical decrease in the mean LDL-C level from baseline was observed at all timepoints for this subgroup of patients who were null/null for LDLR, which was unexpected. Despite this apparent reduction in LDL-C, levels in patients null/null for LDLR in the present study remained far from the therapeutic goal.
Individual responses to treatment showed large variability across all 4 patient functional status subgroups. Despite investigation of various parameters that could have impacted the variability in the response of alirocumab, no clear trend or consistent explanation was identified. The limited effect of alirocumab on LDL-C reduction despite good treatment compliance and strong PCSK9 inhibition in patients with defective LDLR functional status emphasizes the unpredictability of the phenotypic expression unless treatment with a PCSK9 inhibitor has been tested in an individual. Results from other studies of PCSK9-targeting therapies have also shown a large degree of variability in treatment response.5,15,20,21
There did not appear to be a consistent effect of alirocumab on the pre-apheresis percent change in LDL-C levels from baseline in patients already treated with LDL apheresis. Patients on LDL apheresis did seem to have a smaller percent reduction in LDL-C at Week 12 compared with those not on LDL apheresis. This is in line with the trend seen in the ODYSSEY HoFH adult study, which showed mean (SD) reductions of –29.1% (35.7) for patients not on LDL apheresis, versus −8.3% (24.2) for patients on LDL apheresis.15 Similar trends were also seen at Week 12 in the TAUSSIG study of evolocumab in HoFH.21 In addition, the percentage of patients in this study who achieved ≥15% reduction in LDL-C was higher in patients not on LDL apheresis. This difference might be due to LDL apheresis already having had a large effect on LDL-C, leaving limited opportunity for further reduction by alirocumab, although mean LDL-C values at baseline were similar in patients on apheresis and patients not on apheresis. Although it was not possible to fully elucidate the reasons for the large variability in response in the present study, a high proportion of patients achieved a clinically relevant ≥15% reduction in LDL-C level over the study course, supporting the efficacy of alirocumab in a pediatric population.
Ezetimibe, which was a background therapy for 13 patients in the current study, has a modest effect on LDL-C, with a reduction of ≈ 17% to 18% seen when used as monotherapy in patients with primary hypercholesterolemia.22,23 Evinacumab is approved as an adjunct therapy for the treatment of HoFH but is likely to be most useful in patients with severe HoFH who lack any LDLR functionality.24
The dosing regimens evaluated in the present study were optimal to maximize the effect of alirocumab in this pediatric population with HoFH. Indeed, alirocumab Ctrough was similar to the concentration observed in the ODYSSEY study in adults with HoFH. In this latter study, alirocumab Ctrough reached a steady level of ≈25 µg/mL at Weeks 8 to 12,15 compared with Ctrough of 16.4 to 24.4 µg/mL at Week 12 and 26.7 to 28.3 µg/mL at Week 48 for patients not on LDL apheresis (and generally similar for pre-apheresis measurements in patients who received LDL apheresis) in the current study. Furthermore, the concentration of free PCSK9 measured before dose administration decreased to almost zero for most timepoints, suggesting that alirocumab bound PCSK9 and prevented the protein from engaging with LDLR throughout the dosing interval. The safety profile in the study was consistent with the known profile of alirocumab in adults.15
The limitations of this study include the small cohort size and the even more limited number of patients included in each LDLR functional status/LDL apheresis subgroup, and also the lack of a comparator arm; therefore, the significance of the results must be gauged carefully and in context within the wider field of research. Despite this, this post hoc characterization based on LDLR functionality assessment provides an innovative way to better understand highly variable patient populations with HoFH. However, LDLR activity was not directly measured during the study, but the post hoc analysis was based on functional LDLR status of patients, determined based on their genetic background. Assigning LDL-receptor functionality based on genetic analyses is challenging when functional studies for a specific mutation have not yet been reported; it should be noted that for some mutations, functionality is predicted based on in silico modeling only. Therefore, future studies that specifically assess LDLR activity will help fully characterize this patient population and their response to new treatments.
In conclusion, the HoFH population is highly heterogeneous and deserves well-characterized genotyping and LDLR function assessment to fully understand potential treatment effects. However, the potential for LDL-C reduction is still difficult to predict even when genetic data are available, and the effects for an individual patient can only be determined once treatment with a PCSK9 inhibitor has been tested, irrespective of genetic background. Our study adds evidence supporting alirocumab, a PCSK9 inhibitor, as potential adjunct to treatment for pediatric patients with HoFH, even in those presumed to see limited benefit based on LDLR functional status.
Article Information
Acknowledgments
We want to thank the children and adolescents and their families, as well as the investigators and their teams for participating in the study. We would also like to thank Dr J.C. Defesche for his invaluable contribution to the patient genotyping analyses. Medical writing and editorial assistance was provided by Alex van der Wateren, PhD, and Heather Shawcross, PhD, of Fishawack Communications Ltd, and was funded by Sanofi. We thank Wanda Stipek, PharmD, BCPS (Sanofi), for coordinating the development, facilitating author discussions, and critical review of this article.
Sources of Funding
This study was funded by Sanofi.
Disclosures
Dr Bruckert declares having received honoraria from AstraZeneca, AMGEN, MSD, Sanofi and Regeneron, Danone, Aegerion, Servier, Ionis-pharmaceuticals, AKCEA, Mylan, Silence Therapeutics, Alexion, and Genfit. S. Caprio, declares having received honoraria from Lilly, AstraZeneca, Takeda, and Daiichi-Sankyo. A. Wiegman, declares having received research support for pharmaceutical trial of lipid-lowering agents from Amgen, Regeneron, Novartis and Silence Therapeutics. M.T. Baccara-Dinet, is an employee of Sanofi and may hold shares and/or stock options in the company. G. Manvelian, is an employee of Regeneron Pharmaceuticals, Inc. A. Ourliac is an Aixial contractor for Sanofi. M. Scemama is an employee of Sanofi and may hold shares and/or stock options in the company. S.R. Daniels is a member of data monitoring committees for Novo Nordisk and for Regeneron.
Supplemental Material
Supplementary Information I (Detailed patient inclusion/exclusion criteria)
Supplementary information II (Detailed information on statistical analyses)
Figures S1–S4
Tables S1–S3
Major Resources Table
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ADA
- anti-alirocumab antibodies
- AESI
- adverse events of special interest
- ALT
- alanine aminotransferase
- Apo A-1
- apolipoprotein A-1
- HDL-C
- high-density lipoprotein cholesterol
- HoFH
- homozygous familial hypercholesterolemia
- LDL
- low-density lipoprotein
- LDL-C
- low-density lipoprotein cholesterol
- LDLR
- LDL receptor
- LDLRAP1
- LDLR adapter protein 1
- Lp (a)
- lipoprotein a
- PCSK9
- proprotein convertase subtilisin/kexin type 9
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.122.317793.
For Sources of Funding and Disclosures, see page 1456.
References
- 1.Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, Kuivenhoven JA, Nordestgaard BG, Descamps OS, Steinhagen-Thiessen E, et al. ; European Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia. Homozygous familial hypercholesterolaemia: New insights and guidance for clinicians to improve detection and clinical management. A position paper from the consensus panel on familial hypercholesterolaemia of the european atherosclerosis society. Eur Heart J. 2014;35:2146–2157. doi: 10.1093/eurheartj/ehu274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. ; ESC Scientific Document Group. 2019 esc/eas guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 3.Hovingh GK, Goldberg AC, Moriarty PM. Managing the challenging homozygous familial hypercholesterolemia patient: Academic insights and practical approaches for a severe dyslipidemia, a national lipid association masters summit. J Clin Lipidol. 2017;11:602–616. doi: 10.1016/j.jacl.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 4.Ajufo E, Cuchel M, Cuchel M. Recent developments in gene therapy for homozygous familial hypercholesterolemia. Curr Atheroscler Rep. 2016;18:22 10.1007/s11883-016-0579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thedrez A, Blom DJ, Ramin-Mangata S, Blanchard V, Croyal M, Chemello K, Nativel B, Pichelin M, Cariou B, Bourane S, et al. Homozygous familial hypercholesterolemia patients with identical mutations variably express the ldlr (low-density lipoprotein receptor): Implications for the efficacy of evolocumab. Arterioscler Thromb Vasc Biol. 2018;38:592–598. doi: 10.1161/ATVBAHA.117.310217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, Ose L, Averna M, Boileau C, Borén J, et al. ; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia in children and adolescents: Gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36:2425–2437. doi: 10.1093/eurheartj/ehv157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Evkeeza (evinacumab) summary of product characteristics. 2021. Accessed July 2022. https://www.ema.europa.eu/en/documents/product-information/evkeeza-epar-product-information_en.pdf
- 8.European Medicines Agency. Repatha (evolocumab) summary of product characteristics. 2018. Accessed July 2022. https://www.ema.europa.eu/en/documents/product-information/repatha-epar-product-information_en.pdf
- 9.US Food and Drug Administration. Evkeeza (evinacumab-dgnb) prescribing information. 2021. Accessed July 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
- 10.US Food and Drug Administration. Repatha (evolocumab) prescribing information. 2021. Accessed July 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125522orig2s000lbl.pdf
- 11.Landmesser U, Chapman MJ, Farnier M, Gencer B, Gielen S, Hovingh GK, Lüscher TF, Sinning D, Tokgözoglu L, Wiklund O, et al. ; European Society of Cardiology (ESC). European society of cardiology/european atherosclerosis society task force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: Practical guidance for use in patients at very high cardiovascular risk. Eur Heart J. 2017;38:2245–2255. doi: 10.1093/eurheartj/ehw480 [DOI] [PubMed] [Google Scholar]
- 12.Orringer CE, Jacobson TA, Saseen JJ, Brown AS, Gotto AM, Ross JL, Underberg JA. Update on the use of pcsk9 inhibitors in adults: Recommendations from an expert panel of the national lipid association. J Clin Lipidol. 2017;11:880–890. doi: 10.1016/j.jacl.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson B, Patil NG, Fok M, Lam CWK. Role of pcsk9 inhibitors in patients with familial hypercholesterolemia. Endocrinol Metab (Seoul). 2021;36:279–295. doi: 10.3803/EnM.2021.964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Praluent (alirocumab) prescribing information. 2017. Accessed July 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
- 15.Blom DJ, Harada-Shiba M, Rubba P, Gaudet D, Kastelein JJP, Charng MJ, Pordy R, Donahue S, Ali S, Dong Y, et al. Efficacy and safety of alirocumab in adults with homozygous familial hypercholesterolemia: The odyssey hofh trial. J Am Coll Cardiol. 2020;76:131–142. doi: 10.1016/j.jacc.2020.05.027 [DOI] [PubMed] [Google Scholar]
- 16.European Medicines Agency. Praluent (alirocumab) summary of product characteristics. 2021. Accessed July 2022. https://www.ema.europa.eu/en/documents/product-information/praluent-epar-product-information_en.pdf
- 17.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of ldl cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet (London, England). 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raal F, Panz V, Immelman A, Pilcher G. Elevated pcsk9 levels in untreated patients with heterozygous or homozygous familial hypercholesterolemia and the response to high-dose statin therapy. J Am Heart Assoc. 2013;2:e000028. doi: 10.1161/JAHA.112.000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA; TESLA Investigators. Inhibition of pcsk9 with evolocumab in homozygous familial hypercholesterolaemia (tesla part b): A randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2015;385:341–350. doi: 10.1016/S0140-6736(14)61374-X [DOI] [PubMed] [Google Scholar]
- 20.Raal F, Bruckert E, Blom D, Kurtz C, Coll B, Tang L, Somaratne R, Stein EA. Evolocumab treatment in paediatric patients with homozygous familial hypercholesterolaemia: The trial assessing long-term use of pcsk9 inhibition in subjects with genetic LDL disorders (taussig). Euro Soc Cardiol (ESC) Cong. 2017;38(suppl_1):ehx504.3105. 10.1093/eurheartj/ehx504.3105 [DOI] [Google Scholar]
- 21.Santos RD, Stein EA, Hovingh GK, Blom DJ, Soran H, Watts GF, López JAG, Bray S, Kurtz CE, Hamer AW, et al. Long-term evolocumab in patients with familial hypercholesterolemia. J Am Coll Cardiol. 2020;75:565–574. doi: 10.1016/j.jacc.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 22.Dujovne CA, Ettinger MP, McNeer JF, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP; Ezetimibe Study Group. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1092–1097. doi: 10.1016/s0002-9149(02)02798-4 [DOI] [PubMed] [Google Scholar]
- 23.Knopp RH, Gitter H, Truitt T, Bays H, Manion CV, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP; Ezetimibe Study Group. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J. 2003;24:729–741. doi: 10.1016/s0195-668x(02)00807-2 [DOI] [PubMed] [Google Scholar]
- 24.Kuehn BM. Evinacumab approval adds a new option for homozygous familial hypercholesterolemia with a hefty price tag. Circulation. 2021;143:2494–2496. doi: 10.1161/CIRCULATIONAHA.121.055463 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.