Summary
Background.
Poor social connections (eg, small networks, infrequent interactions, and loneliness) are modifiable risk factors for cognitive decline. Existing meta-analyses are limited by reporting aggregate responses, a focus on global cognition, and combining social measures into single constructs. We aimed to investigate the association between social connection markers and the rate of annual change in cognition (ie, global and domain-specific), as well as sex differences, using an individual participant data meta-analysis.
Methods
We harmonised data from 13 longitudinal cohort studies of ageing in North America, South America, Europe, Africa, Asia, and Australia. Studies were eligible for inclusion if they had baseline data for social connection markers and at least two waves of cognitive scores. Follow-up periods ranged from 0 years to 15 years across cohorts. We included participants with cognitive data for at least two waves and social connection data for at least one wave. We then identified and excluded people with dementia at baseline. Primary outcomes were annual rates of change in global cognition and cognitive domain scores over time until final follow-up within each cohort study analysed by use of an individual participant data meta-analysis. Linear mixed models within cohorts used baseline social connection markers as predictors of the primary outcomes. Effects were pooled in two stages using random-effects meta-analyses. We assessed the primary outcomes in the main (partially adjusted) and fully adjusted models. Partially adjusted models controlled for age, sex, and education; fully adjusted models additionally controlled for diabetes, hypertension, smoking, cardiovascular risk, and depression.
Findings
Of the 40 006 participants in the 13 cohort studies, we excluded 1392 people with dementia at baseline. 38 614 individual participants were included in our analyses. For the main models, being in a relationship or married predicted slower global cognitive decline (b=0·010, 95% CI 0·000–0·019) than did being single or never married; living with others predicted slower global cognitive (b=0·007, 0·002–0·012), memory (b=0·017, 0·006–0·028), and language (b=0·008, 0·000–0·015) decline than did living alone; and weekly interactions with family and friends (b=0·016, 0·006–0·026) and weekly community group engagement (b=0·030, 0·007–0·052) predicted slower memory decline than did no interactions and no engagement. Never feeling lonely predicted slower global cognitive (b=0·047, 95% CI 0·018–0·075) and executive function (b=0·047, 0·017–0·077) decline than did often feeling lonely. Degree of social support, having a confidante, and relationship satisfaction did not predict cognitive decline across global cognition or cognitive domains. Heterogeneity was low (I2=0·00–15·11%) for all but two of the significant findings (association between slower memory decline and living with others [I2=58·33%] and community group engagement, I2=37·54–72·19%), suggesting robust results across studies.
Interpretation
Good social connections (ie, living with others, weekly community group engagement, interacting weekly with family and friends, and never feeling lonely) are associated with slower cognitive decline.
Funding
EU Joint Programme–Neurodegenerative Disease Research grant, funded by the National Health and Medical Research Council Australia, and the US National Institute on Aging of the US National Institutes of Health.
Introduction
The 2020 Lancet Commission on dementia prevention estimated that tackling social isolation could prevent 4% of dementia cases worldwide.1 Social isolation is one aspect under the umbrella term social health.2 Social connections, a key concept for social health, have components pertaining to structure (eg, social networks and living situation), function (eg, social support), and quality (eg, loneliness and relationship quality).3 Socially stimulating environments promote neuroprotective mechanisms,4 and social connections are theorised to contribute to cognitive reserve,5 whereby the brain actively copes with neuropathology by using alternative pre-existing or compensatory cognitive processes.6 Bridging (ie, loose social ties providing cognitive stimulation) and bonding (ie, close social ties buffering stress and influencing function of the neuroendocrine system and the hypothalamic–pituitary–adrenal axis) pathways have been proposed to lead from social connections to cognitive health.4
Meta-analyses show associations between poor social connections and increased risk of cognitive decline. A meta-analysis of 43 studies reported that poorer social connection indicators pertaining to both structure and function predicted greater cognitive decline.7 However, this meta-analysis combined various social connection markers and so could not reach definitive conclusions about specific markers. Another meta-analysis of 39 studies reported that social activity and social support were associated with decline in global cognition and specific cognitive domains.8 Frequent social activity was associated with improved memory, executive function, visuospatial ability, and processing speed, whereas frequent social support was associated only with improved memory.8 Existing meta-analyses are limited by the use of aggregated data from studies that adjust differently for potential confounders (eg, one study did not adjust for confounders9 and another study adjusted for only demographic variables and baseline cognition10). Furthermore, existing evidence about social connection markers and cognitive decline is mainly from North America and Europe, and these meta-analyses have not included loneliness, which is associated with increased cognitive decline in some cohort studies.11
Sex differences are important to investigate because women have faster cognitive decline than do men in global cognition and executive function.12 One study reported that increased memory decline was associated with baseline social isolation (ie, a combination of living situation, frequency of social contact, and membership of community or religious groups at baseline) for men and accumulated social isolation (ie, social isolation across multiple waves of the study) for women.13 Interaction with community groups has been associated with slower cognitive decline for men than for women.14 Scientific literature on sex differences in the associations between social connection function or quality and cognitive decline, however, is sparse. For instance, social support was reportedly protective against memory decline for only men in one study,15 whereas high baseline loneliness was associated with cognitive decline for only women in another cohort study.16
We aimed to investigate the association between various social connection markers and cognition (ie, global and domain-specific), as well as sex differences, using an individual participant data meta-analysis, which has fewer limitations than does a traditional meta-analysis. First, we hypothesised that improved social connections (eg, in terms of social structure, function, and quality) are associated with a decreased rate of global cognitive decline. Second, we hypothesised that markers of improved social connection structure are associated with slow decline across all cognitive domains, whereas markers of improved social connection function and connection quality are associated specifically with slow memory decline.8 Finally, we hypothesised that sex differences exist in the association between social connection markers and cognitive function. Specifically, improved baseline social connection structures (eg, being in a relationship and high interaction frequency) are associated with a slow rate of cognitive decline only for men, given previous research.13,17,18
Methods
Contributing studies and participants
We collected and harmonised data from 13 longitudinal cohort studies of ageing from around the world: Bambuí Cohort Study of Ageing (Bambuí, Brazil);19 China Longitudinal Aging Study (CLAS, China);20 English Longitudinal Study of Ageing (ELSA, England, UK);21 Epidemiology of Dementia in Central Africa (EPIDEMCA, Central African Republic and Republic of the Congo);22 Gothenburg H70 Birth Cohort Studies (the H70 study, Sweden);23 Hellenic Longitudinal Investigation of Aging and Diet (HELIAD, Greece);24 Korean Longitudinal Study on Cognitive Aging and Dementia (KLOSCAD, South Korea);25 Leipzig Longitudinal Study of the Aged (LEILA75+, Germany);26 Neuroprotective Model for Healthy Longevity among Malaysian Older Adults Towards Using Ageing (LRGS TUA, Malaysia);27 Sydney Memory and Ageing Study (MAS, Australia);28 Monongahela-Youghiogheny Healthy Aging Team (MYHAT, USA);29 Puerto Rican Elderly Health Conditions Study (PREHCO, Puerto Rico);30 and Singapore Longitudinal Study of Ageing (SLAS, Singapore).31 We identified studies for inclusion from the Cohort Studies of Memory in an International Consortium (COSMIC) for the Social Health and Reserve in the Dementia Patient Journey (SHARED) consortium. ELSA was identified through a literature search of the longitudinal studies with multiple social connection variables and was not part of COSMIC. Studies were eligible if they had baseline data for social connection markers and at least two waves of cognitive scores (sample characteristics of cohorts are provided in the appendix p 4). We did not apply any date restrictions. Within each study, we included participants who had social data for at least one wave and cognitive data for at least two waves. We excluded participants with dementia at baseline. We conducted an individual participant data meta-analysis to examine the associations between baseline social connections (ie, relationship status, living situation, frequency of interactions with family and friends, engagement in community groups, social support, having a confidante, relationship satisfaction, and loneliness) and change in cognition over time (ie, global cognition, memory, language, and executive function). We used the STROBE reporting checklist to describe our study (appendix pp 1–2).
The project was approved by the University of New South Wales Sydney Human Research Ethics Committee (HC200268). The contributing cohorts had previous ethics approval (appendix p 3). Written informed consent was exclusively or predominantly obtained from participants in all studies, including consent to share da other scientific research (appendix p 3). For EPIDEMCA, consent was obtained from family when the participant was unable to express their consent. For both EPIDEMCA and LRGS TUA, verbal consent and thumbprint mark was obtained from people who were illiterate. Further participant consent for this study was not deemed necessary as only fully de-identified data were shared with the analysis team.
Procedures
We completed a COSMIC studies data request to collect data from twelve cohorts, and applied for access to ELSA data separately. We requested data for participants with social data for at least one wave and cognitive data for at least two waves. We collected data for cognitive scores and diagnoses, physical health and lifestyle risk factors for cognitive decline or dementia, and any social variables. The data were shared and stored in a secure OneDrive folder with multifactor authentication. The data were queried and confirmed by SSa and GM via descriptive statistics and visual analyses and cross-checked with original studies where discrepancies, errors, or outliers were noticed.
Global cognition, memory, language, and executive function measures, including descriptive statistics and proportion of missing data, are shown in the appendix (pp 5–6). Total scores for cognitive screening tests (ie, the Mini-Mental State Examination in most cohorts) were used to assess global cognition, rather than creating a composite of domain scores. The standardisation process for global cognition and cognitive domain scores is described in the appendix (p 8).
Covariates that were included in the models were harmonised in line with previous research by COSMIC.32 The measures and harmonisation protocols used for the covariates are described in the appendix (pp 9–13). We controlled for age, sex, and education in the main (partially adjusted) models using data from all 13 cohorts. In fully adjusted models, we also controlled for previous history of diabetes, hypertension, smoking, cardiovascular disease risk (ie, previous history of angina, myocardial infarction, and any other heart disease), and depression. Fully adjusted models included ten cohorts with data available for the aforementioned covariates (ie, Bambuí,19 CLAS,20 ELSA,21 the H70 study,23 HELIAD,24 KLOSCAD,25 LEILA75+,26 LRGS TUA,27 MAS,28 and MYHAT29).
We harmonised social connection markers (ie, relationship status, living situation, engagement in community groups, interactions with family and friends, having a confidante, relationship satisfaction, and loneliness) by consensus among multiple authors (SSa, GM, DML, Y-HJ, MV-D, and HB) using as many potential questionnaire responses as possible. The measures and harmonisation protocols that were used for the social connection markers are described in the appendix (pp 14–21). Data for relationship status and living situation were derived mostly from demographic questions, and data for social interactions with family and friends, having a confidante, and relationship satisfaction were derived from single questions. Data for engagement with community groups were obtained from single questions that represented different community groups across studies (eg, whether participants played cards, games, bingo, or mahjong in the SLAS study31 and whether participants attended meetings of retirement clubs or other clubs in the H70 study23).
Loneliness frequency data were available in only four studies,21,23,26,27 of which only LRGS TUA27 used a validated loneliness scale (UCLA 3-item Loneliness Scale).33 To allow comparison across response options for loneliness, we used similar items from ELSA21 (ie, how often the respondent feels lonely: “hardly ever or never”, “some of the time”, “often”), the H70 study23 (“Feeling lonely”: “never”, “seldom/sometimes”, “often”), LEILA75+26 (“I felt lonely”: “rarely or none of the time, less than 1 day”, “some or a little, 1–2 days”, “occasionally or a moderate amount, 3–4 days”, “most or all the time, 5–7 days”), and LRGS TUA27 (“How often do you feel that you lack companionship?”; “hardly ever”, “some of the time”, “often”).
Outcomes
The primary outcomes were the annual rates of change in global cognition and cognitive domain scores over time until final follow-up within each cohort study. The secondary outcomes were the sex differences in the association between social connection structure and the annual rates of change in global cognition and cognitive domain scores. We also conducted exploratory analyses on the sex differences in the association between social connection function or quality and cognitive decline.
Statistical analysis
We used a two-stage individual participant data meta-analysis to pool effects across studies,34 which enables controlling for the same covariates across studies rather than relying on aggregate data that is adjusted for different sets of covariates. Furthermore, variables can be defined consistently across studies, thereby reducing methodological heterogeneity. The first stage used linear mixed modelling to estimate the association between social connections and cognitive outcomes within each study. We specified random intercept for participant and fixed effect for time in study (coded as years since baseline). First, we examined the overall linear effect of time in study (unstandardised regression coefficient [b]=−0·015, 95% CI −0·048 to 0·018) on cognitive function (ie, global cognition and the cognitive domains) and the quadratic effect of time in study (b=−0·013, 95% CI −0·032 to 0·005) on cognitive function. As the quadratic time-in-study effect was not significant, we used a linear effect in all analyses involving social connection markers. Model assumptions for linear mixed regression were checked and satisfied by GM. We ran each social connection marker–cognitive outcome model separately because of differences in the availability of social connection markers and cognitive variables across cohort studies. In these models, a significant interaction between a social connection marker at baseline and time in study indicates that the social connection marker was associated with annual rate of cognitive decline.
The second stage pooled the effects across studies by use of random-effects meta-analysis with a restricted maximum likelihood estimator, which is a relatively unbiased and efficient estimator of between-study variance.35 We examined heterogeneity using the I2 and τ2 statistics36 and bias using Egger’s test (appendix pp 26–27) and funnel plots (appendix pp 28–31).37 To examine sex differences in the associations between social connection markers and change in cognition over time, we repeated the analysis with additional terms for the interactions between sex, time in study, and social connection markers and then pooled the interaction effects in the meta-analysis. We present b in the meta-analyses results and forest plots. Unstandardised coefficients from the linear mixed models represent differences in the annual rate of change in cognitive Z scores comparing the target category of the social connection marker with the reference category. A negative b indicates that the predictor is associated with faster decline in cognition, whereas a positive b indicates that the predictor is associated with slower decline in cognition. We conducted sensitivity analyses with complete cases to determine whether the results obtained from multiple imputation for missing data were robust.
Missing data ranged from 0·00% to 43·36% for covariates and 0·00% to 35·08% for social connection markers. We visually inspected data to examine whether our observed variables were related to the pattern of missingness and used auxiliary variables (ie, data from predictors and covariates) in the multiple imputation to reduce the effect of non-random missing data on the pattern of results.
Multiple imputation with the Markov Chain Monte Carlo method was used to impute missing data for the demographics, covariates, and social connection markers with less than 50% missing data, incorporating information from auxiliary variables and generating 20 imputed datasets in each study.38 Estimates computed from each imputed dataset were then pooled by use of Rubin’s rules.39 Although multiple imputation is effective with up to 50% missingness,39 we conducted additional sensitivity analyses to compare the major findings when only complete cases (ie, 0% missing) were used.
We used R studio software version 4.1.2 and R packages mice for multiple imputation, lme4 for linear mixed modelling, and metafor for meta-analyses.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Of the 40 006 people in the 13 cohorts, we excluded 1392 people living with dementia at baseline (ranging from no participants to 289 participants per study; appendix p 7) and 38 614 participants were included in the analyses. Sample descriptive statistics for age, sex, education, and study timepoints are presented in appendix p 4, for covariates in appendix p 22, and for social connection markers in appendix pp 23–25. Recruitment dates (baseline and final follow-up year) for individual cohorts are provided in the appendix (p 4).
Follow-up periods ranged from 2 years to 15 years across cohorts, and the time in study for participants ranged from 0 years to 15 years, with a median of 3 years (IQR 2–6). Participants were community dwelling in all cohorts, apart from 50 (4·8%) of 1045 participants from the LEILA75+ study, who resided in assisted living facilities (50 [0·1%] of 38 614 of the overall sample).
The sample included in the main analyses (13 cohorts, n=38 614) was similar in age at baseline (mean 70·50 years, SD 8·67; female mean age 70·63 years [SD 8·87 vs male mean age 70·32 [8·38]) compared with the sample for the fully adjusted models (ten cohorts, n=29 718, mean 70·49 years, SD 8·65, t=−0·13, df=58 282, p=0·90; female mean age 70·72 years [8·90] vs male mean age 70·19 years [8·30]). In the main models, married men were slightly older (mean 69·79 [SD 7·83]) than married women (67·36 [7·59]).Furthermore, the sample for the main analyses had a similar proportion of women (22 556 [58·4%] of 38 614 vs 17 145 [57·7%] of 29 728; χ2=3·50, df=1, p=0·060) to and fewer years of education (mean 8·48 years [SD 4·88] vs 9·12 years [4·61]; df=58 282, p<0·0001) than did the sample for the fully adjusted models.
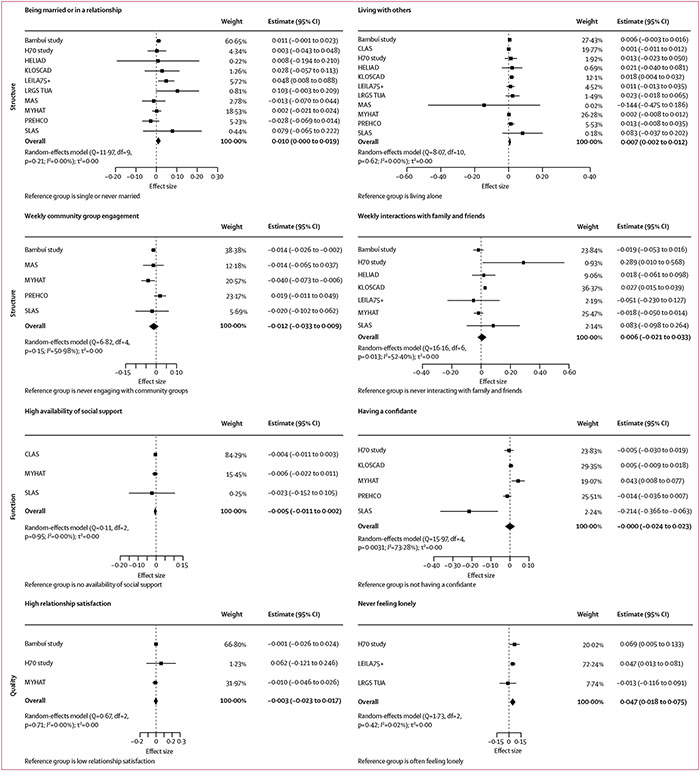
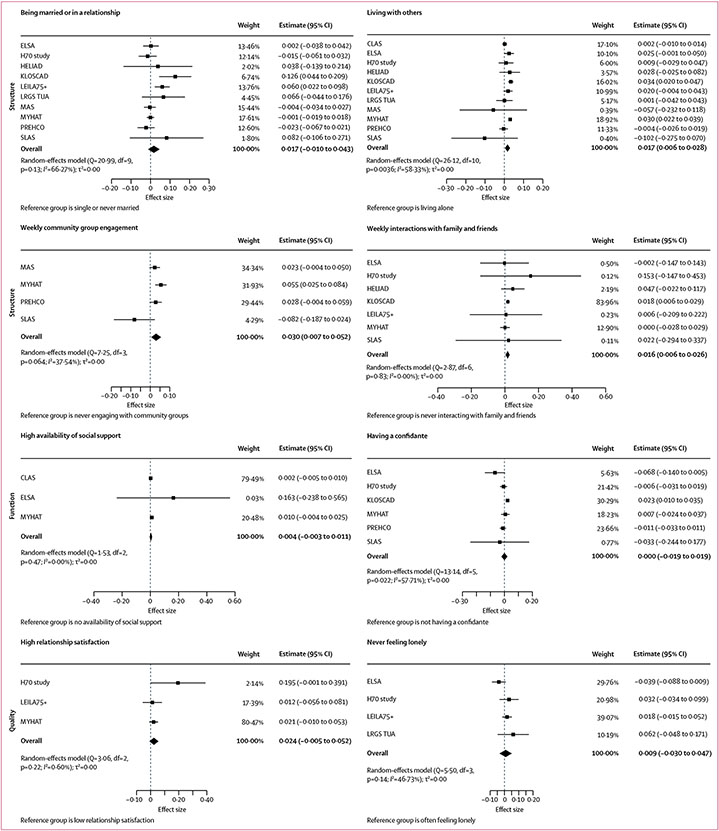
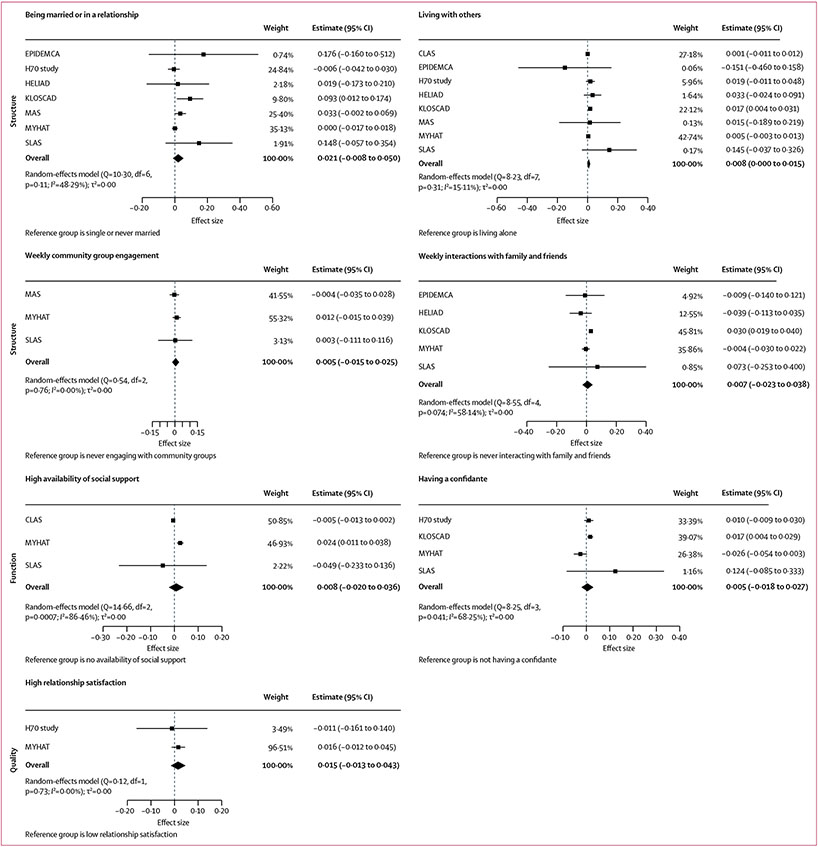
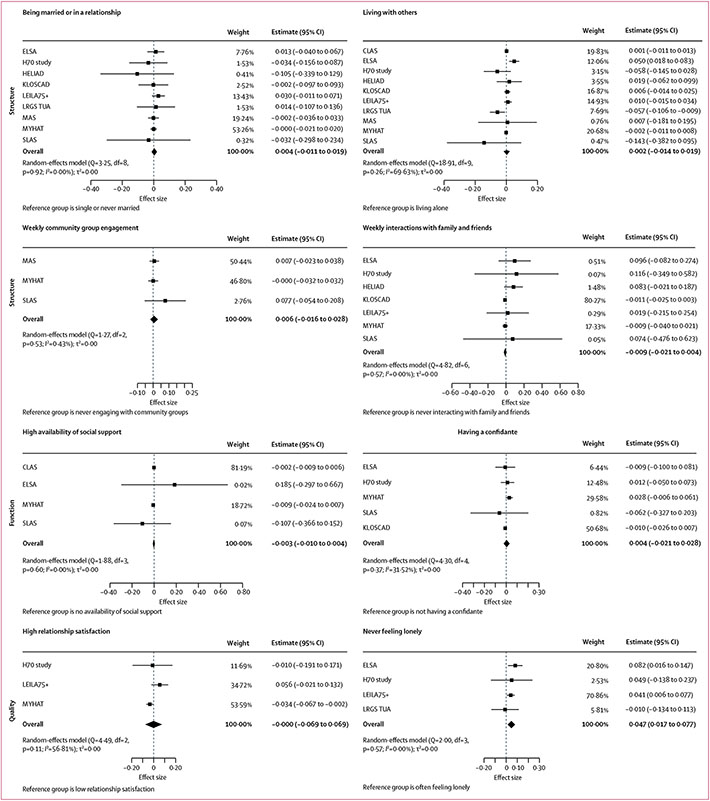
Longitudinal associations between social connection markers and each of the four cognitive outcomes for the main models are shown in Figures 1,2,3, & 4 (detailed results of the main and fully adjusted models are shown in the appendix pp 26–27). Here, we report the results for the main models that were replicated in the fully adjusted models. Being married or in a relationship was associated with slower global cognitive decline than was being single or never married. Living with others was associated with slower global cognitive, memory, and language decline than was living alone. Yearly, monthly, or weekly engagement in a community group was associated with slower annual memory decline than was never engaging with community groups. Additionally, monthly or weekly interactions with family and friends were associated with slower memory decline than was never interacting (appendix pp 26–27). Never feeling lonely was associated with slower annual decline in global cognition and executive function than was often feeling lonely. Heterogeneity estimates for the results reported here for the main models were low (I2=0·00–15·11%), indicating that the findings were largely consistent across studies. The exceptions were for the association between memory and living situation (I2=58·33%), for which only the North American and South Korean cohorts showed significant results, and for the association between memory and community group engagement (I2=37·54–72·19%), which might reflect the different activities assessed in different cohorts. These findings were all replicated in the fully adjusted models (ie, in ten of 13 cohorts).
Figure 1: Associations between social connection markers and global cognition.
Markers are categorised by structure, function, and quality. Data presented are for main (partially adjusted) models.
Figure 2: Associations between social connection markers and memory.
Markers are categorised by structure, function, and quality. Data presented are for main (partially adjusted) models.
Figure 3: Associations between social connection markers and language.
Markers are categorised by structure, function, and quality. Data presented are for main (partially adjusted) models. The meta-analytic model for the association between loneliness and language could not be run because data were available for only the H70 study.23
Figure 4: Associations between social connection markers and executive function.
Markers are categorised by structure, function, and quality. Data presented are for main (partially adjusted) models.
Several findings were inconsistent between the main and fully adjusted models. In the fully adjusted models, monthly engagement in a community group was significantly associated with faster global cognitive decline than was never engaging with community groups. In the main models, yearly interactions with family and friends were associated with slower annual decline in global cognition than was never interacting, but this association was not replicated in the fully adjusted models (appendix pp 26–27). Sensitivity analyses using complete cases for the main models suggested that the overall pattern of findings was consistent (appendix pp 38–39).
In terms of sex differences, examining the pooled interaction effects of social connection markers with sex across cohorts (table) identified a few sex differences in the associations between social connection markers and cognitive function. Men, but not women, who were in a relationship or were married had faster cognitive decline in global cognition than those not in a relationship or married. Women, but not men, who were in a relationship or were married had slower decline in memory than those not in a relationship or married.
Table:
Sex differences in the association between social health markers and annual change in cognition by domain in the main analysis
| I2 (%) | τ2 | Interaction effect of social connection marker, sex, and time in study, b (95% CI) |
Egger’s test, Z score (p value) |
Simple main effects for men and women (when b is significant; 95% CI) |
|
|---|---|---|---|---|---|
| Global cognition | |||||
| Structure | |||||
| Being married or in a relationship | 0·00% | 0·00 | 0·033 (0·009 to 0·578) | −0·42 (p=0·68) | −0·023 (−0·045 to −0·002) for men; 0·010 (−0·001 to 0·021) for women |
| Living with others | 14·96% | 0·00 | 0·004 (−0·011 to 0·018) | −0·82 (p=0·41) | |
| Yearly engagement in community group | 0·69% | 0·00 | 0·013 (−0·015 to 0·042) | −0·39 (p=0·70) | |
| Monthly engagement in community group | 15·53% | 0·00 | −0·009 (−0·038 to 0·019) | 0·03 (p=0·97) | ·· |
| Weekly engagement in community group | 0·00% | 0·00 | 0·010 (−0·013 to 0·034) | −0·30 (p=0·77) | ·· |
| Yearly interactions with family and friends | 0·00% | 0·00 | 0·039 (−0·028 to 0·107) | 0·44 (p=0·66) | ·· |
| Monthly interactions with family and friends | 0·00% | 0·00 | 0·006 (−0·019 to 0·031) | −0·08 (p=0·94) | |
| Weekly interactions with family and friends | 0·00% | 0·00 | 0·004 (−0·017 to 0·026) | 0·11 (p=0·91) | |
| Function | |||||
| High availability of social support | 0·45% | 0·00 | 0·002 (−0·013 to 0·014) | −1·66 (p=0·10) | |
| Having a confidante | 0·00% | 0·00 | −0·002 (−0·023 to 0·018) | 0·10 (p=0·92) | ·· |
| Quality | |||||
| High relationship satisfaction | 0·00% | 0·00 | −0·036 (−0·078 to 0·006) | −0·27 (p=0·80) | |
| Never feeling lonely | 0·00% | 0·00 | −0·057 (−0·140 to 0·027) | 0·45 (p=0·66) | |
| Memory | |||||
| Structure | |||||
| Being married or in a relationship | 7·14% | 0·00 | 0·042 (0·010 to 0·074) | 0·01 (p=0·99) | −0·023 (−0·047 to 0·001) for men; 0·024 (0·001 to 0·048) for women |
| Living with others | 42·70% | 0·00 | 0·009 (−0·012 to 0·031) | 0·94 (p=0·35) | ·· |
| Yearly engagement in community group | 8·33% | 0·00 | 0·001 (−0·035 to 0·054) | NR* | ·· |
| Monthly engagement in community group | 0·00% | 0·00 | 0·006 (−0·025 to 0·037) | 0·10 (p=0·92) | ·· |
| Weekly engagement in community group | 0·00% | 0·00 | 0·017 (−0·017 to 0·051) | 0·86 (p=0·39) | ·· |
| Yearly interactions with family and friends | 57·32% | 0·05 | −0·076 (−0·389 to 0·235) | −0·14 (p=0·89) | ·· |
| Monthly interactions with family and friends | 0·00% | 0·00 | 0·011 (−0·014 to 0·036) | −1·42 (p=0·16) | ·· |
| Weekly interactions with family and friends | 52·73% | 0·00 | 0·002 (−0·064 to 0·068) | −1·90 (p=0·057) | ·· |
| Function | |||||
| High availability of social support | 33·13% | 0·00 | 0·008 (−0·019 to 0·035) | 0·20 (p=0·84) | ·· |
| Having a confidante | 0·00% | 0·00 | −0·005 (−0·025 to 0·015) | −0·14 (p=0·89) | ·· |
| Quality | |||||
| High relationship satisfaction | 0·00% | 0·00 | −0·029 (−0·087 to 0·028) | −0·42 (p=0·67) | ·· |
| Never feeling lonely | 0·00% | 0·00 | −0·039 (−0·102 to 0·024) | −0·38 (p=0·71) | ·· |
| Language | |||||
| Structure | |||||
| Being married or in a relationship | 0·00% | 0·00 | 0·010 (−0·024 to 0·043) | −1·76 (p=0·079) | ·· |
| Living with others | 0·13% | 0·00 | 0·007 (−0·007 to 0·022) | 0·87 (p=0·39) | ·· |
| Yearly engagement in community group | 77·24%† | 0·00 | −0·001 (−0·094 to 0·092) | NR* | ·· |
| Monthly engagement in community group | 90·58%† | 0·02 | 0·018 (−0·135 to 0·171) | 2·61 (p=0·0069) | ·· |
| Weekly engagement in community group | 47·96% | 0·00 | −0·011 (−0·077 to 0·055) | −0·06 (p=0·95) | ·· |
| Yearly interactions with family and friends | 39·08% | 0·03 | −0·072 (−0·343 to 0·198) | −0·43 (p=0·67) | ·· |
| Monthly interactions with family and friends | 0·00% | 0·00 | −0·006 (−0·030 to 0·018) | −0·29 (p=0·80) | ·· |
| Weekly interactions with family and friends | 0·00% | 0·00 | 0·004 (−0·017 to 0·024) | −0·60 (p=0·55) | ·· |
| Function | |||||
| High availability of social support | 0·00% | 0·00 | 0·002 (−0·011 to 0·016) | 0·65 (p=0·52) | ·· |
| Having a confidante | 0·00% | 0·00 | −0·001 (−0·022 to 0·019) | 0·25 (p=0·81) | ·· |
| Quality | |||||
| High relationship satisfaction | 0·00% | 0·00 | −0·000 (−0·057 to 0·056) | NR* | ·· |
| Never feeling lonely | NR‡ | NR‡ | NR‡ | NR* | ·· |
| Executive function | |||||
| Structure | |||||
| Being married or in a relationship | 0·00% | 0·00 | 0·046 (0·011 to 0·082) | −0·38 (p=0·71) | −0·027 (−0·054 to 0·000) for men; 0·017 (−0·002 to 0·036) for women |
| Living with others | 0·00% | 0·00 | 0·016 (0·001 to 0·031) | 0·25 (p=0·80) | −0·009 (−0·022 to 0·003) for men; 0·005 (−0·002 to 0·013) for women |
| Yearly engagement in community group | 61·34% | 0·00 | −0·033 (−0·110 to 0·045) | NR* | ·· |
| Monthly engagement in community group | 73·11%† | 0·01 | −0·034 (−0·138 to 0·070) | −1·59 (p=0·11) | ·· |
| Weekly engagement in community group | 0·00% | 0·00 | −0·035 (−0·079 to 0·010) | −0·76 (p=0·45) | ·· |
| Yearly interactions with family and friends | 57·15% | 0·06 | 0·133 (−0·246 to 0·513) | −0·11 (p=0·91) | ·· |
| Monthly interactions with family and friends | 0·01% | 0·00 | 0·008 (−0·022 to 0·038) | −0·08 (p=0·93) | ·· |
| Weekly interactions with family and friends | 0·00% | 0·00 | 0·023 (−0·002 to 0·049) | −0·39 (p=0·70) | ·· |
| Function | |||||
| High availability of social support | 0·00% | 0·00 | 0·005 (−0·008 to 0·019) | 0·54 (p=0·59) | ·· |
| Having a confidante | 0·00% | 0·00 | −0·024 (−0·081 to 0·033) | 0·23 (p=0·82) | ·· |
| Quality | |||||
| High relationship satisfaction | 11·29% | 0·00 | −0·014 (−0·093 to 0·065) | 1·37 (p=0·17) | ·· |
| Never feeling lonely | 42·24% | 0·00 | 0·042 (−0·078 to 0·162) | 0·81 (p=0·42) | ·· |
The model results reflect the annual change in cognition (b [95% CI]) for women compared with men and are controlled for age at baseline, education, and sex. Positive interaction effect b indicates slower annual decline in cognition for women than for men; negative interaction effect b indicates slower annual decline in cognition for men than for women. For sex-stratified analyses, simple main effects positive b indicates slower annual decline in cognition than for the reference group for the specific social connection marker. NR=not reported.
Some models had less than three cohort studies, and we could not compute Egger’s test for them.
p<0·05.
Not enough data points to run analysis.
When examining the whole sample, there was a significant sex interaction for living with others and being in a relationship or married, with both factors predicting slower decline in executive function than living alone or being single or never married. Sex differences in the association between social connection markers and annual rate of change in cognition for individual cohorts are shown in the appendix (pp 32–37). Sensitivity analyses using complete cases replicated the pattern of results (appendix pp 40–41).
Discussion
In line with our hypothesis that good social connections would be associated with slower cognitive decline over time than would poor social connections, we identified that being married or in a relationship, living with one or more person, and never feeling lonely were associated with slower annual decline in global cognition than were being single or never married, living alone, and often feeling lonely. Our findings regarding relationship status are consistent with a previous meta-analysis, which showed that being married is protective against dementia due to slower cognitive decline.8 We also identified an association between never feeling lonely and a slower rate of cognitive decline, similar to previous findings.11 Overall, our findings support previous research and suggest that improved social connection structures (eg, relationship status, living situation, and interaction frequency), quality (eg, loneliness), but not necessarily function (eg, social support), are associated with decreased rates of cognitive decline. Social connections and cognitive function can also have a bidirectional relationship, where each promotes the other, such that having social connections promotes cognitive skills and vice versa.40 Declining cognition can also limit social connections and vice versa. Our results support both the bridging (ie, cognitive stimulation via doing activities with loose ties) and bonding (ie, stress buffering via close relationships) pathways that have been proposed to lead from social connections to cognitive health.4
In terms of cognitive domains, our hypothesis that good social connection structure markers are associated with slow decline across all cognitive domains was partly supported. Living with others was associated with slower decline in memory and language, but not executive function, than was living alone. We also identified that yearly, monthly, and weekly engagement in a community group and monthly and weekly interactions with family and friends were associated with slower memory decline than were no engagement or interactions, although the association of yearly interactions with family and friends and memory was not replicated in fully adjusted models. Our results are in line with another meta-analysis that identified that social connection structure was linked specifically with memory, although the previous meta-analysis did find associations with executive function, which we did not.8 We also identified an association between social health structure and language, a relationship that was not examined in the previous meta-analysis.8 Living with others and interacting regularly with the community and with family and friends could provide frequent opportunities to use memory or language skills.41 Our hypothesis was good social connection function and quality markers are associated with slower decline in memory was not supported. However, never feeling lonely was associated with slower executive function decline than was often feeling lonely. We identified that good social connections structure (ie, being in a relationship and living with others) and quality (ie, never feeling lonely) were associated with slower global cognitive decline than were being single or never married, living alone, and feeling lonely. Importantly, associations between specific social connection markers and specific cognitive domains might be overlooked by focusing solely on global cognition. Feeling connected to others (vs feeling lonely) might increase cognitive reserve by reducing stress and slowing down memory and executive function decline, whereas interactions with family and friends and community members might provide cognitive stimulation and additional opportunities to practise a variety of cognitive functions, such as memory and language, which is in line with the theorised bridging and bonding pathways and other previous findings.4,8
As per our hypothesis, we identified a few sex differences in the associations between social connection markers and cognition, but we did not identify support for our hypothesis that good social health structure markers are associated with a decreased rate of cognitive decline only for men. We identified slower rates of cognitive decline (ie, global cognition and memory) over time for women who were in a relationship or married than for women who were single. These findings are contrary to previous research showing that being in a relationship or married is associated with slower cognitive decline for men only.18 Although older women might have few social interactions beyond their spouse due to increased house-hold responsibilities, interactions with family and friends outside of the home can provide opportunities for cognitive stimulation.15 Additionally, women in a relationship or who are married might have greater financial stability and better general and cognitive function. Across the 13 cohorts in our study, the mean age of married men in the main models was 2·43 years greater than was the mean age of married women. This difference in age might explain slower rates of cognitive decline for married women than for men, as married men are older and might have cognitive decline during marriage, whereas married women might have cognitive decline later in life during widowhood. Sex differences in the association between social connection and cognitive decline are inconclusive; some previous studies have reported no differences,7 whereas others have reported evidence for women benefiting from interactions with family and friends more than men do when newly diagnosed with dementia.42 Our exploratory analyses on the sex differences between social connection function and quality markers and cognitive decline showed no significant results.
Poor social connections have been proposed to affect cognitive function via multiple pathways. Strong social ties promote cognitive health via cognitive stimulation and affect the function of the neuroendocrine system and activation of the hypothalamic–pituitary–adrenal axis via psychosocial processes (eg, stress buffering).4 Being in a relationship or married, living with others, and frequent social interactions might provide opportunities for cognitive stimulation and reduce cognitive decline via the stress buffering pathway. Although our effect sizes represented a 1–2% reduction in the rate of cognitive decline per year, these results might accumulate over decades. Nevertheless, having poor social connections is one contributing factor for cognitive decline and should be considered alongside other risk factors when trying to reduce the risk of cognitive decline.
Our study had several strengths. Previous meta-analyses or collaborative studies have relied on data from North America and Europe, used combined or conflated social connection markers, used aggregated statistics from studies with diverse sets of covariates, and focused solely on global cognition as the outcome. We harmonised data from 13 longitudinal cohorts, including culturally diverse and low-income and middle-income countries (ie, Brazil, China, and Central African Republic). Our sample was well powered and more representative than were many previous studies examining social connections and cognitive outcomes in older adults. Using an individual participant data meta-analysis, we controlled for the same covariates across studies. Additionally, harmonisation allowed us to examine multiple markers of social connections as risk factors for cognitive decline. We have strictly delineated between structural, functional, and quality markers of social connections.3 Previous studies have emphasised the need to measure and assess distinct markers of social connections as they might have varying effects on different cognitive domains.8 We went beyond previous studies by examining associations with memory, executive function, and language, rather than only global cognition. We also examined previously underinvestigated sex differences across multiple social connection markers and change in cognitive function for global cognition and specific cognitive domains.
There were some limitations to our study. Harmonising data from multiple studies entailed a loss of granularity and precluded analyses involving detailed categories of social connection markers. Our results might have been affected by the contributing cohort studies not using validated measures of social connections. For instance, most cohort studies used single questions for concepts such as loneliness instead of validated scales. Few studies provided data for relationship satisfaction (ie, four studies) or loneliness frequency (ie, three studies), emphasising the need for improved quality of data for social connection quality. Disparities in the number of social connection markers between cohort studies meant that we could not compute composite scores for social connection domains (ie, structure, quality, and function) or control for other social connection markers when examining the association between specific social connection markers and cognitive outcomes. It is possible that other social connection markers (eg, loneliness) might confound the relationship between a specific social connection marker and a cognitive outcome (eg, being in a relationship and global cognition). In our study, the meta-analyses models had to be run with different groupings of studies for each social connection predictor–cognitive outcome pair. Future research should investigate the interaction of social connection markers with each other and cognitive outcomes by use of data from studies that included the same set of social connection markers. Reverse causality might affect our results, as cognitive skills are required to maintain social connections, although we excluded people living with dementia at baseline to minimise the risk. Finally, due to the absence of data for social connection markers at follow-up, we examined only baseline differences in social connection markers and were unable to examine changes in social connection markers over time. Future research could investigate if changes in social connections affect the rate of cognitive decline over the course of dementia. The biopsychosocial pathways leading from having good social connection structure and function to stable cognition function (or vice versa) have not been explored.
Although most cohort studies include measures of only social connections, social health has been expanded to include the individual’s ability to adapt to and manage challenges that influence their social participation and activities.43 More theory and measures of this ability to adapt are needed to get an understanding of the dynamic nature of social health.
To summarise, harmonised individual participant data from 13 longitudinal cohort studies of ageing were meta-analysed at the participant level to examine the association between social connections and risk of cognitive decline. Better social connection structure (eg, relationship status, living situation, and interaction frequency) and quality (eg, never lonely), but not function (eg, social support and having a confidante), were associated with slower rates of cognitive decline. Additionally, being in a relationship or married was associated with slower decline in memory, but only for women.
Data sharing
All aggregate participant data are presented either in the manuscript or appendix. Individual participant data cannot be made publicly available because they are protected by a confidentiality agreement. Data were provided by the contributing studies to COSMIC on the understanding and proviso that the relevant study leaders be contacted for further use of their data and additional formal data sharing agreements be made. Researchers can apply to use COSMIC data by completing a COSMIC Research Proposal Form available from https://cheba.unsw.edu.au/consortia/cosmic/research-proposals.
Supplementary Material
Research in context.
Evidence before this study
To identify gaps in the literature, we searched Web of Science and PsycINFO for meta-analyses published in English between Jan 1, 2012, and May 5, 2022, with the keywords “(dementia OR alzheimer* OR cognitive dysfunction OR cognitive decline)” AND “(social OR connection* OR relationship* OR friend* OR family OR network OR activit* OR interaction* OR health OR behav* OR support OR participat* OR isolat*)”. Social isolation is a modifiable risk factor for dementia, accounting for about 4% of preventable cases worldwide. Recent meta-analyses have examined several facets of social health. One of these studies reported that poor social health structure and function predicted greater cognitive decline. Another study examined individual social connection markers separately and found that low social participation, infrequent social contact, and loneliness were associated with increased risk of dementia. However, these meta-analyses used aggregated (not individual-level) data from studies with different covariates. Furthermore, most studies focused on global cognition, ignoring potential associations between social connections and specific cognitive domains. Sex differences in the link between social connections and domain-specific cognitive changes are also underexplored. Finally, existing meta-analyses have primarily included data from North America and Europe.
Added value of this study
To our knowledge, this study is the largest individual participant data meta-analysis of the association between social connections and cognition. We showed that good social connections slow cognitive decline not only in global cognition, as previous studies have shown, but also specifically for memory and language. Additionally, we showed that being in a relationship or married was associated with slower memory decline than being single or never married, but only for women. Results from this study are consistent with previous research from Europe and North America, but we included studies from South America, Africa, Asia, and Australia, including low-income and middle-income countries.
Implications of all the available evidence
Good social health structure and quality appear to slow the annual rate of cognitive decline. There should be greater emphasis on preserving or enhancing older adults’ social relationships. We showed effects for specific cognitive domains, and future research could further explore the bidirectional pathways between cognitive domains and social connections.
Acknowledgments
The head of COSMIC is PSS, and the study coordinator is DML. The research scientific committee leads the scientific agenda of COSMIC and provides ongoing support and governance; it is comprised of member study leaders. The COSMIC research scientific committee and additional principal investigators are listed at https://cheba.unsw.edu.au/consortia/cosmic/scientific-committee. We thank the participants and their informants for their time and generosity in contributing to this research. We also thank the research teams for the contributing cohort studies. This work was supported by the JPND project SHARED. In Australia, the project is funded by a National Health and Medical Research Council grant (grant number APP1169489), which is awarded and governed by the JPND. Funding for COSMIC comes from the NIA–NIH (award number 1RF1AG057531–01). The EPIDEMCA study was funded by the French National Research Agency (ANR-09-MNPS-009–01), the AXA Research Fund (grant 2012–Project Public Health Institute [Inserm]–PREUX Pierre-Marie), and the Limoges University Hospital through its Appel à Projet des Equipes Émergentes et Labellisées scheme. The Bambuí Cohort Study of Ageing was funded by Financiadora de Estudos e Projetos, Conselho Nacional de Desenvolvimento Científico e Tecnológicos, and Fundação de Amparo Pesquisa do Estado de Minas Gerais. The HELIAD cohort was funded by the Alzheimer’s Association, European Social Fund, and Greek Ministry of Health. The LEILA75+ study was funded by the Interdisciplinary Centre for Clinical Research at the University of Leipzig (grant 01KS9504). The LRGS TUA study was funded by Long-Term Research Grant Scheme at the Ministry of Higher Education (LRGS/BU/2012/UKM-UKM/K/01). The MAS study was funded by the National Health and Medical Research Council (grant numbers APP350833, APP568969, and APP1093083) in Australia. The MYHAT study was funded by a grant made by NIA to the University of Pittsburgh (grant number R37AG023651). Funding for the CLAS study was from the Ministry of Science and Technology, National Pillar Program 2009BAI77B03 and the National Key Clinical Disciplines at Shanghai Mental Health Center (Office of Medical Affairs, Ministry of Health, 2011-873). Funding for ELSA was provided by the National Institute of Aging (grants 2RO1AG7644-01A1 and 2RO1AG017644) and a consortium of UK Government departments coordinated by the Office for National Statistics. The H70 study was supported by AgeCap-Center for Aging and Health, Riksbankens Jubileumsfond, FORTE, and the Swedish Brain Power. The H70 study data collection was supported by The Swedish Research Council, Swedish Research Council for Health, Working Life and Welfare, Epilife, Swedish Brain Power, The Alzheimer’s Association Zenith Award, The Alzheimer’s Association Stephanie B Overstreet Scholars, The Bank of Sweden Tercentenary Foundation, Stiftelsen Söderström-Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Handlanden Hjalmar Svenssons Forskningsfond, and Stiftelsen Professor Bror Gadelius’ Minnesfond. KLOSCAD was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, South Korea (grant number HI09C1379 [A092077]). PREHCO was supported in part by the NIA (grants R21 AG045722 and P30AG022838). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA or the NIH. SLAS was supported by a research grant (number 03/1/21/17/214) from the Biomedical Research Council, Agency for Science, Technology and Research, Singapore.
Footnotes
Declaration of interests
HB declares consulting fees from Biogen; advisory board fees from Nutricia, Roche, Skin2Neuron, and Cranbrook Care; and grant funding through the EU Joint Programme–Neurodegenerative Disease Research (JPND) from the National Health and Medical Research Council Australia. DML declares funding to their institution by the US National Institute on Aging–National Institutes of Health (NIA–NIH) award (number RF1AG05753 1RF1AG057531–01). RM declares funding from the JPND. HW declares a research grant from the JPND for the SHARED project (grant number HESOCARE-329–109). PSS declares payments for advisory board meetings for Biogen Australia and Roche Australia, and funding to the university for another cohort study (OATS), unrelated to the submitted work. SSa declares payments for lectures from New York University Sydney and University of Sydney; grant funding from the Dementia Australia Research Foundation, unrelated to the submitted work; and grant funding from the JPND and National Health and Medical Research Council Australia. NS declares that they are the chair of the data safety monitoring board for a study funded by the NIH at Albert Einstein College of Medicine. DS declares funding under the aegis of the JPND (National Center for Research and Development in Poland, project number JPND/06/2020). JM declares funding as part of the SHARED consortium, a JPND that is supported by the Alzheimer’s Society (reference 469) in the UK. All other authors declare no competing interests.
Contributor Information
Suraj Samtani, Centre for Healthy Brain Ageing, Discipline of Psychiatry, Faculty of Medicine and Health, UNSW Sydney, Sydney, NSW, Australia.
Gowsaly Mahalingam, Centre for Healthy Brain Ageing, Discipline of Psychiatry, Faculty of Medicine and Health, UNSW Sydney, Sydney, NSW, Australia.
Ben Chun Pan Lam, Centre for Healthy Brain Ageing, Discipline of Psychiatry, Faculty of Medicine and Health, UNSW Sydney, Sydney, NSW, Australia.
Darren M Lipnicki, Centre for Healthy Brain Ageing, Discipline of Psychiatry, Faculty of Medicine and Health, UNSW Sydney, Sydney, NSW, Australia.
Maria Fernanda Lima-Cost, Center for Studies in Public Health and Aging, René Rachou Research Center, Oswaldo Cruz Foundation, Belo Horizonte, Brazil.
Sergio Luís Blay, Department of Psychiatry, Federal University of São Paulo, São Paulo, Brazil.
Erico Castro-Costa, Center for Studies in Public Health and Aging, René Rachou Research Center, Oswaldo Cruz Foundation, Belo Horizonte, Brazil.
Xiao Shifu, Department of Geriatric Psychiatry, Shanghai Mental Health Center, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Maëlenn Guerchet, Inserm U1094, IRD UMR270, University of Limoges, CHU Limoges, Epidemiology of Chronic Diseases in Tropical Zone, Institute of Epidemiology and Tropical Neurology, OmegaHealth, Limoges, France.
Pierre-Marie Preux, Inserm U1094, IRD UMR270, University of Limoges, CHU Limoges, Epidemiology of Chronic Diseases in Tropical Zone, Institute of Epidemiology and Tropical Neurology, OmegaHealth, Limoges, France.
Antoine Gbessemehlan, Inserm U1094, IRD UMR270, University of Limoges, CHU Limoges, Epidemiology of Chronic Diseases in Tropical Zone, Institute of Epidemiology and Tropical Neurology, OmegaHealth, Limoges, France.
Ingmar Skoog, Department of Psychiatry and Neurochemistry, Neuropsychiatric Epidemiology Unit, Institute of Neuroscience and Physiology, Sahlgrenska Academy, Centre for Ageing and Health, University of Gothenburg, Mölndal, Sweden; Psychiatry, Cognition and Old Age Psychiatry Clinic, Sahlgrenska University Hospital, Gothenburg, Sweden.
Jenna Najar, Department of Psychiatry and Neurochemistry, Neuropsychiatric Epidemiology Unit, Institute of Neuroscience and Physiology, Sahlgrenska Academy, Centre for Ageing and Health, University of Gothenburg, Mölndal, Sweden; Psychiatry, Cognition and Old Age Psychiatry Clinic, Sahlgrenska University Hospital, Gothenburg, Sweden.
Therese Rydberg Sterner, Department of Psychiatry and Neurochemistry, Neuropsychiatric Epidemiology Unit, Institute of Neuroscience and Physiology, Sahlgrenska Academy, Centre for Ageing and Health, University of Gothenburg, Mölndal, Sweden.
Nikolaos Scarmeas, 1st Department of Neurology, Aiginition University Hospital of Athens, National and Kapodistrian University of Athens, Athens, Greece; Taub Institute for Research in Alzheimer’s Disease and the Aging Brain, The Gertrude H Sergievsky Center, Department of Neurology, Columbia University, New York, NY, USA.
Ki-Woong Kim, Department of Neuropsychiatry, Seoul National University Bundang Hospital, Seongnam, South Korea; Department of Psychiatry, Seoul National University College of Medicine and Department of Brain and Cognitive Science, College of Natural Sciences, Seoul National University, Seoul, South Korea.
Steffi Riedel-Heller, Institute of Social Medicine, Occupational Health and Public Health, Faculty of Medicine, University of Leipzig, Leipzig, Germany.
Susanne Röhr, Institute of Social Medicine, Occupational Health and Public Health, Faculty of Medicine, University of Leipzig, Leipzig, Germany; Global Brain Health Institute, Trinity College Dublin, Dublin, Ireland.
Alexander Pabst, Institute of Social Medicine, Occupational Health and Public Health, Faculty of Medicine, University of Leipzig, Leipzig, Germany.
Suzana Shahar, Centre for Healthy Aging and Wellness, Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia.
Katya Numbers, Centre for Healthy Brain Ageing, Discipline of Psychiatry, Faculty of Medicine and Health, UNSW Sydney, Sydney, NSW, Australia.
Mary Ganguli, Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Department of Epidemiology, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Department of Neurology, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Erin Jacobsen, Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Tiffany F Hughes, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Department of Sociology, Anthropology, and Gerontology, Youngstown State University, Youngstown, OH, USA.
Michael Crowe, Department of Psychology, University of Alabama at Birmingham, Birmingham, AL, USA.
Tze Pin Ng, Department of Psychological Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Jane Maddock, MRC Unit for Lifelong Health and Ageing, University College London, London, UK.
Anna Marseglia, Division of Clinical Geriatrics, Centre for Alzheimer Research, Department of Neurobiology, Care Sciences, and Society, Karolinska Institutet, Stockholm, Sweden.
René Mélis, Department of Geriatrics, Radboud University Medical Centre, Nijmegen, Netherlands.
Dorota Szcześniak, Department of Psychiatry, Wroclaw Medical University, Wroclaw, Poland.
Henrik Wiegelmann, Department of Health Care Research, Institute of Public Health and Nursing Research, University of Bremen, Bremen, Germany.
Myrra Vernooij-Dassen, Faculty of Medical Sciences, Radboud University, Nijmegen, Netherlands.
Yun-Hee Jeon, Susan Wakil School of Nursing and Midwifery, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia.
Perminder S Sachdev, Centre for Healthy Brain Ageing, Discipline of Psychiatry, Faculty of Medicine and Health, UNSW Sydney, Sydney, NSW, Australia.
Henry Brodaty, Centre for Healthy Brain Ageing, Discipline of Psychiatry, Faculty of Medicine and Health, UNSW Sydney, Sydney, NSW, Australia.
References
- 1.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396: 413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huber M, Knottnerus JA, Green L, et al. How should we define health? BMJ 2011; 343: d4163. [DOI] [PubMed] [Google Scholar]
- 3.Holt-Lunstad J, Steptoe A. Social isolation: an underappreciated determinant of physical health. Curr Opin Psychol 2022; 43: 232–37 [DOI] [PubMed] [Google Scholar]
- 4.Perry BL, McConnell WR, Coleman ME, Roth AR, Peng SY, Apostolova LG. Why the cognitive ‘fountain of youth’ may be upstream: pathways to dementia risk and resilience through social connectedness. Alzheimers Dement 2022; 18: 934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernooij-Dassen M, Moniz-Cook E, Verhey F, et al. Bridging the divide between biomedical and psychosocial approaches in dementia research: the 2019 INTERDEM manifesto. Aging Ment Health 2021; 25: 206–12. [DOI] [PubMed] [Google Scholar]
- 6.Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012; 11: 1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuiper JS, Zuidersma M, Zuidema SU, et al. Social relationships and cognitive decline: a systematic review and meta-analysis of longitudinal cohort studies. Int J Epidemiol 2016; 45: 1169–206. [DOI] [PubMed] [Google Scholar]
- 8.Kelly ME, Duff H, Kelly S, et al. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Syst Rev 2017; 6: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle PA, Buchman AS, Barnes LL, Bennett DA. Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Arch Gen Psychiatry 2010; 67: 304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosma H, van Boxtel MPJ, Ponds RWHM, et al. Engaged lifestyle and cognitive function in middle and old-aged, non-demented persons: a reciprocal association? Z Gerontol Geriatr 2002; 35: 575–81. [DOI] [PubMed] [Google Scholar]
- 11.Freak-Poli R, Wagemaker N, Wang R, et al. Loneliness, not social support, is associated with cognitive decline and dementia across two longitudinal population-based cohorts. J Alzheimers Dis 2022; 85: 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine DA, Gross AL, Briceño EM, et al. Sex differences in cognitive decline among US adults. JAMA Netw Open 2021; 4: e210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Read S, Comas-Herrera A, Grundy E. Social isolation and memory decline in later-life. J Gerontol B Psychol Sci Soc Sci 2020; 75: 367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, Jean Yeung W-J. Gender matters: productive social engagement and the subsequent cognitive changes among older adults. Soc Sci Med 2019; 229: 87–95. [DOI] [PubMed] [Google Scholar]
- 15.Liao J, Scholes S. Association of social support and cognitive aging modified by sex and relationship type: a prospective investigation in the English Longitudinal Study of Ageing. Am J Epidemiol 2017; 186: 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z, Mao F, Zhang W, Towne SD Jr, Wang P, Fang Y. The association between loneliness and cognitive impairment among older men and women in China: a nationwide longitudinal study. Int J Environ Res Public Health 2019; 16: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anstey KJ, Peters R, Mortby ME, et al. Association of sex differences in dementia risk factors with sex differences in memory decline in a population-based cohort spanning 20-76 years. Sci Rep 2021; 11: 7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murata C, Saito T, Saito M, Kondo K. The association between social support and incident dementia: a 10-year follow-up study in Japan. Int J Environ Res Public Health 2019; 16: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima-Costa MF, Firmo JOA, Uchoa E. Cohort profile: the Bambuí (Brazil) Cohort Study of Ageing. Int J Epidemiol 2011; 40: 862–67 [DOI] [PubMed] [Google Scholar]
- 20.Xiao S, Li J, Tang M, et al. Methodology of China’s national study on the evaluation, early recognition, and treatment of psychological problems in the elderly: the China Longitudinal Aging Study (CLAS). Shanghai Arch Psychiatry 2013; 25: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English Longitudinal Study of Ageing. Int J Epidemiol 2013; 42: 1640–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerchet M, Mbelesso P, Ndamba-Bandzouzi B, et al. Epidemiology of Dementia in Central Africa (EPIDEMCA): protocol for a multicentre population-based study in rural and urban areas of the Central African Republic and the Republic of Congo. Springerplus 2014; 3: 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorvaldsson V, Karlsson P, Skoog J, Skoog I, Johansson B. Better cognition in new birth cohorts of 70 year olds, but greater decline thereafter. J Gerontol B Psychol Sci Soc Sci 2017; 72: 16–24. [DOI] [PubMed] [Google Scholar]
- 24.Dardiotis E, Kosmidis MH, Yannakoulia M, Hadjigeorgiou GM, Scarmeas N. The Hellenic Longitudinal Investigation of Aging and Diet (HELIAD): rationale, study design, and cohort description. Neuroepidemiology 2014; 43: 9–14. [DOI] [PubMed] [Google Scholar]
- 25.Han JW, Kim TH, Kwak KP, et al. Overview of the Korean Longitudinal Study on Cognitive Aging and Dementia. Psychiatry Investig 2018; 15: 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedel-Heller SG, Busse A, Aurich C, Matschinger H, Angermeyer MC. Prevalence of dementia according to DSM–III–R and ICD–10: results of the Leipzig Longitudinal Study of the Aged (LEILA75+) part 1. Br J Psychiatry 2001; 179: 250–54. [DOI] [PubMed] [Google Scholar]
- 27.Shahar S, Omar A, Vanoh D, et al. Approaches in methodology for population-based longitudinal study on Neuroprotective Model for Healthy Longevity (TUA) among Malaysian Older Adults. Aging Clin Exp Res 2016; 28: 1089–104. [DOI] [PubMed] [Google Scholar]
- 28.Sachdev PS, Brodaty H, Reppermund S, et al. The Sydney Memory and Ageing Study (MAS): methodology and baseline medical and neuropsychiatric characteristics of an elderly epidemiological non-demented cohort of Australians aged 70–90 years. Int Psychogeriatr 2010; 22: 1248–64. [DOI] [PubMed] [Google Scholar]
- 29.Ganguli M, Snitz B, Vander Bilt J, Chang CH. How much do depressive symptoms affect cognition at the population level? The Monongahela–Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry 2009; 24: 1277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell T, Dávila AL, Clay O, Markides KS, Andel R, Crowe M. The association between cognitive decline and incident depressive symptoms in a sample of older Puerto Rican adults with diabetes. Int Psychogeriatr 2017; 29: 1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niti M, Yap K-B, Kua E-H, Tan C-H, Ng T-P. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-epsilon 4 genotype in Chinese older adults. Int Psychogeriatr 2008; 20: 237–51. [DOI] [PubMed] [Google Scholar]
- 32.Lipnicki DM, Crawford JD, Dutta R, et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study. PLoS Med 2017; 14: e1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys: results from two population-based studies. Res Aging 2004; 26: 655–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340: c221. [DOI] [PubMed] [Google Scholar]
- 35.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat 2005; 30: 261–93. [Google Scholar]
- 36.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med 2012; 31: 3805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Little RJA, Rubin DB. Statistical analysis with missing data. Hoboken, NJ: John Wiley & Sons, 2019. [Google Scholar]
- 39.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods 2002; 7: 147–77. [PubMed] [Google Scholar]
- 40.Casey A-NS, Liu Z, Kochan NA, Sachdev PS, Brodaty H. Cross-lagged modeling of cognition and social network size in the Sydney Memory and Ageing Study. J Gerontol B Psychol Sci Soc Sci 2021; 76: 1716–25. [DOI] [PubMed] [Google Scholar]
- 41.Ybarra O, Burnstein E, Winkielman P, et al. Mental exercising through simple socializing: social interaction promotes general cognitive functioning. Pers Soc Psychol Bull 2008; 34: 248–59. [DOI] [PubMed] [Google Scholar]
- 42.Donato KM, León-Párez G, Wallston KA, Kripalani S. Something old, something new: when gender matters in the relationship between social support and health. J Health Soc Behav 2018; 59: 352–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vernooij-Dassen M, Jeon Y-H. Social health and dementia: the power of human capabilities. Int Psychogeriatr 2016; 28: 701–03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.