Abstract
We have purified apical merozoite antigen 1 (AMA-1) from extracts of red blood cells infected with the rodent malaria parasite Plasmodium yoelii yoelii YM. When used to immunize mice, the protein induced a strong protective response against a challenge with the parasite. Monoclonal antibodies specific for P. yoelii yoelii AMA-1 were prepared, and one was very effective against the parasite on passive immunization. A second protein that appears to be located in the apical rhoptry organelles and associated with AMA-1 was identified.
Early studies by Cohen et al. (6) and more recently by Sabchareon et al. (39) demonstrated that passive immunization with purified immunoglobulin obtained from adults living in areas holoendemic for malaria transferred clinical protection against Plasmodium falciparum, the causative agent of the most severe form of malaria. The mechanisms involved in this protective effect of antibody have been analyzed in in vivo and in vitro studies using humans and animal models. With respect to merozoites and erythrocyte (red blood cell [RBC]) invasion, it has been proposed that the protective mechanisms include merozoite neutralization by blocking ligand-receptor interactions or agglutination, the targeting of monocytes to secrete cytotoxic factors in an antibody dependent cell-mediated inhibition (4), and the specific inhibition of essential biochemical events such as the processing of merozoite surface protein 1 (MSP-1) (3).
Apical membrane antigen 1 (AMA-1) is a merozoite protein that is a target of immune effector mechanisms that most likely neutralize merozoite invasion. The protein is located in the apical rhoptry organelles of the developing and free merozoite (8) and can be relocated from here to the surface of the parasite. In immunofluorescence studies with specific antibodies, a characteristic punctate pattern is observed, together with a circumferential (merozoite surface) staining pattern (32). This protein was initially identified in the simian malaria parasite P. knowlesi and named PK66 (here called PkAMA-1) (12). Monoclonal antibodies (MAbs) and their Fab fragments specific for PkAMA-1 were inhibitory in in vitro cultures, acting at a point in the parasite's asexual blood-stage development beyond schizont maturation (9, 42). Further evidence that AMA-1 can induce a strong protective immune response has been provided by immunization of nonhuman primates against simian malaria parasites (7, 11) and of mice against P. chabaudi (1). The 83-kDa P. falciparum AMA-1 (PfAMA-1; also named PF83 [35, 44]) is well conserved at the primary sequence level compared to the simian and rodent malaria proteins, except for an N-terminal extension in PfAMA-1. The sequence conservation within the AMA-1 family, including the protein in other human (5), nonhuman primate (15, 36, 45), and rodent (25) malaria parasites, suggests that there are strong functional constraints on the structure of this protein. The protein contains a large external ectodomain followed by a transmembrane region and a short cytoplasmic tail. Analysis of the deduced amino acid sequence of PfAMA-1 in in vitro-adapted parasite lines of different geographic origin and in primary parasite isolates suggests that the number of allelic variants is large (31, 34). However, the diversity is largely restricted to within specific regions of the ectodomain (44). During P. falciparum infection in humans, antibodies to PfAMA-1 can be detected. Investigation of immune responses in populations in areas of Africa where malaria is endemic suggested that antibodies to PfAMA-1 are prevalent (43) and that the protein contains several T-cell determinants (28).
Despite the information already available, there is a clear need to develop a suitable host-parasite system to study the function of AMA-1 and its role in RBC invasion and to analyze the host's immune response to it. We have applied a rodent model, P. yoelii yoelii YM in laboratory mice, to purify parasite-derived AMA-1 and study the potential of an immune response to block AMA-1 function and merozoite infectivity. We have also developed MAbs for passive immunization studies to identify neutralizing specificities in order to map the functional region(s) of AMA-1 involved in putative ligand-receptor interactions. In this report, we show that purified P. yoelii yoelii AMA-1 (PyAMA-1) is protective when used to immunize against a virulent parasite challenge infection. Furthermore, we identify a PyAMA-1-specific MAb that is protective by passive immunization. We also identify another putative rhoptry protein of 140 kDa that may be part of a protein complex containing AMA-1.
MATERIALS AND METHODS
Parasites and metabolic labeling.
The rodent malaria parasite P. yoelii yoelii YM was a clone obtained from David Walliker, University of Edinburgh (26), and grown in BALB/c mice. To enrich for mature trophozoites and schizonts, parasitized blood was collected in phosphate-buffered saline (PBS)-heparin, diluted with 5 volumes of RPMI 1640–0.5% (wt/vol) Albumax (Gibco BRL, Life Technologies, Paisley, United Kingdom), and passed through a CF11 column to remove leukocytes (22). Parasitized RBCs were then purified on a 50% Nycodenz gradient (Nycomed, Oslo, Norway) essentially as described elsewhere (32). P. yoelii yoelii merozoites were isolated by a polycarbonate sieve method (14, 23; D. L. Narum et al., unpublished data). The human malaria parasite P. falciparum FCB-1 was maintained in vitro, and schizonts were purified on Plasmagel as described elsewhere (2). P. yoelii yoelii and P. falciparum-parasitized RBCs were metabolically radiolabeled by incubation with [35S]PROMIX (Amersham, Little Chalfont, United Kingdom) at 100 μC/ml in methionine-deficient RPMI 1640 for 4 and 2 h, respectively. Cells were harvested by centrifugation, washed, aliquoted, and stored at −70°C.
Protein purification and preparation.
Blood was collected at P. yoelii yoelii parasitemias averaging 30 to 40%; the cells were washed in RPMI 1640 and then stored at −70°C. Parasitized RBCs (2 × 1011) were extracted on ice for 1 h in at least 10 volumes of buffer containing 1% Nonidet P-40 (NP-40) (20, 33). The extract was centrifuged at 1,000 × g (20 min at 10°C), and then the supernatant was centrifuged again (10 min, 10,000 × g, 10°C), filtered though a 0.22-μm-pore-size filter (Millipore, Watford, United Kingdom), and applied to an immunoaffinity column at 6°C essentially as described elsewhere (33). The affinity column was prepared by coupling MAb 28G2dc1, specific for a C-terminal sequence in AMA-1, to cyanogen bromide-activated Q-Sepharose CL 4B (Pharmacia, Biotech, St. Albans, United Kingdom). The affinity column was washed (33), and AMA-1 was eluted with elution buffer (pH 2.8; Pierce) containing 0.1% NP-40 into vials containing 1 M Tris-HCl (pH 8.0) to neutralize the pH. The P. yoelii yoelii YM MSP-119 glutathione S-transferase (GST) fusion protein (GST–MSP-119) was produced and purified as described elsewhere (29). AMA-1 and GST–MSP-119 proteins were dialyzed in PBS and stored at −70°C.
Active and passive immunization and parasite challenge.
Individual groups of female BALB/c mice 6 to 8 weeks of age were immunized with either approximately 1 to 3 μg of AMA-1, 10 μg of GST–MSP-119, or PBS emulsified in Freund's complete adjuvant administered subcutaneously on day 0 and then in Freund's incomplete adjuvant on days 14 and 28. Serum samples were collected 12 days after priming and boosting for analysis of antibody titers. Two weeks after the third immunization, groups of mice were challenged intravenously (i.v.) with 5,000 parasitized RBCs. Thin smears of tail blood stained with Giemsa's reagent were examined daily after day 3 postchallenge, and parasitemias were scored on alternate days. Mice were killed if the parasitemia exceeded 70%. Passive immunization studies were conducted with groups of BALB/c mice 6 to 8 weeks of age by administering intraperitoneally a total of 2 mg of immunoglobulin G (IgG) in PBS on days −1, 0, and +1. On day 0, mice were challenged i.v. with 5,000 parasitized RBCs and monitored as described above.
MAbs.
The rat MAb 28G2dc1 recognizes a highly conserved C-terminal region within AMA-1 from several Plasmodium species (32), and rat MAb 58F8dc1 recognizes the amino-terminal region of P. falciparum AMA-1 (32). Additional MAbs were produced using spleen cells obtained from BALB/c mice immunized with P. yoelii yoelii AMA-1 as described above and fused with Sp2/0-Ag14 myeloma cells (18). Hybridoma culture supernatants were screened by indirect immunofluorescence assay (IFA) against methanol-fixed parasitized RBCs prepared on 15-well slides. IgG was detected using a goat anti-mouse IgG γ-chain-specific fluorescein isothiocyanate-coupled reagent (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). IFA-positive hybridoma cell lines were cloned twice by limiting dilution prior to large-scale culture (6 liters) in vitro. Supernatants from these cultures were concentrated 10-fold, and then IgG was purified by protein G column chromatography using the ImmunoPure buffer system (Pierce, Rockford, Ill.). IgG subclasses were determined by enzyme-linked immunosorbent assay (Sigma-Aldrich, Poole, Dorset, United Kingdom).
Immunoprecipitation, immunoblotting, and immunofluorescence assay.
Aliquots of approximately 2.5 × 108 parasitized RBCs that had been metabolically labeled were extracted in buffer containing 1% NP-40 (10, 32), protein aliquots were precipitated with trichloroacetic acid, and radioactivity in the proteins was measured by liquid scintillation counting. Samples containing equal amounts of radioactivity were immunoprecipitated with MAb coupled to protein G or Q-Sepharose, and the precipitates were washed as previously described using a buffer containing NP-40 (10, 32). The antigens were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and detected by fluorography essentially as previously described (13). Immunoblots were prepared essentially as described previously (33); analyses of purified P. yoelii yoelii AMA-1 used approximately 10 to 25 ng of purified protein per track. IFA as previously described (32) used fluorescein- and rhodamine-labeled secondary antibodies (Kirkegaard & Perry Laboratories). The same microscope field was photographed using excitation for both fluorescein and rhodamine. IFA with unfixed merozoites was performed as described previously (23).
RESULTS
Purification of native P. yoelii yoelii YM AMA-1.
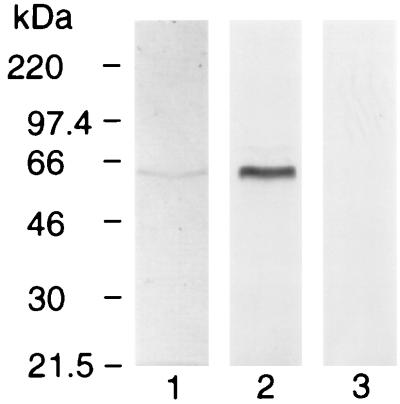
We reported previously the development of a rat MAb (28G2dc1) that recognizes a linear epitope in the C-terminal sequence of AMA-1 from all species of Plasmodium evaluated so far (32). Using affinity chromatography with MAb 28G2dc1, a 60-kDa protein was purified from extracts of P. yoelii yoelii-parasitized erythrocytes (Fig. 1, track 1). Immunoblot analysis with MAb 28G2dc1 confirmed that the 60-kDa protein band was AMA-1 (track 2). As a negative control, MAb 58F8dc1, which recognizes an N-terminal epitope present only in PfAMA-1 (32), failed to recognize PyAMA-1 (track 3). The quantity of AMA-1 purified from 2 × 1011-parasitized RBC was estimated by Coomassie blue staining to be 75 μg. Examination of the purified AMA-1 with more protein loaded onto the gel used for SDS-PAGE revealed the presence of an additional 140-kDa doublet (data not shown).
FIG. 1.
PyAMA-1. Affinity-purified native PyAMA-1 revealed by Coomassie staining (track 1) and immunoblot using MAb 28G2dc1 (track 2), MAb 58F8dc1 (specific for the N terminus of PfAMA-1) is included as a negative control (track 3).
Immunization with AMA-1, antibody specificity, and parasite challenge.
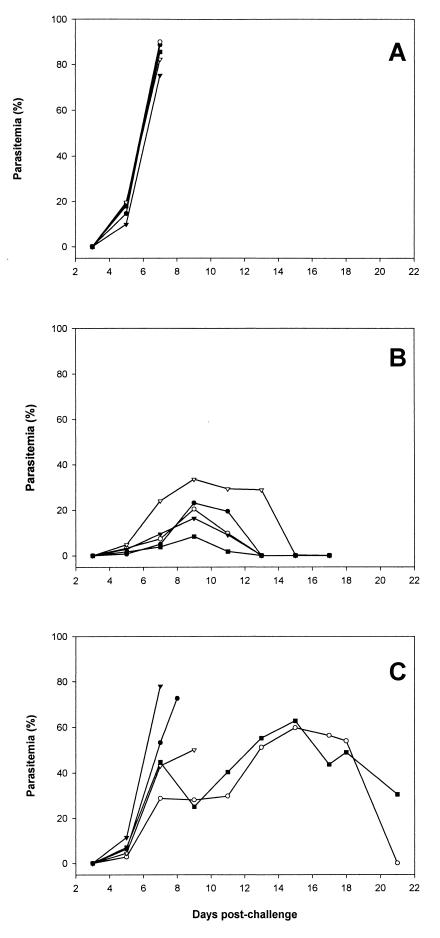
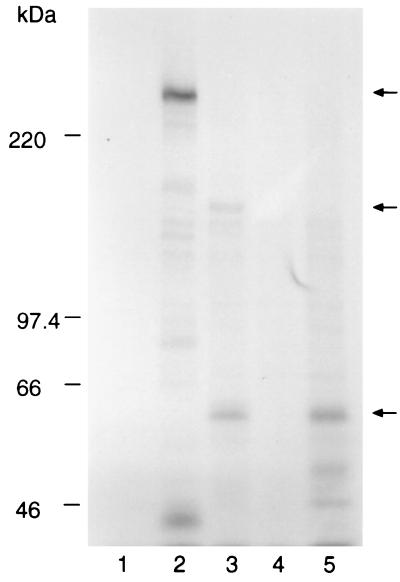
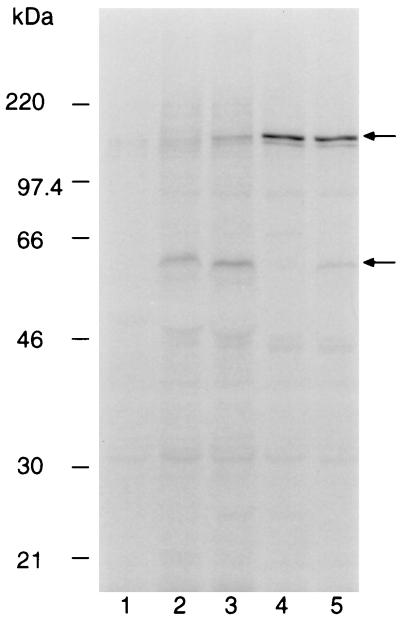
Groups of five BALB/c mice were immunized with the PyAMA-1 preparation, GST–MSP-119, or PBS, all in Freund's adjuvant. The recombinant GST–MSP-119 and PBS were included as positive and negative controls, respectively. Antibody titers of the mice were determined by IFA on samples obtained at the time of i.v. parasite challenge. The reciprocal antibody titers for the group immunized with PyAMA-1 ranged from 800 to 3,200; in the group immunized with GST–MSP-119, titers ranged from 1,600 to 12,800. The antibodies from these mice reacted with antigens that had subcellular localization on merozoites typical of AMA-1 (apical and punctate) and MSP-1 (circumferential) (data not shown). After challenge with P. yoelii yoelii YM-infected erythrocytes, parasitemias were scored on alternate days for a period of 3 weeks. The results are shown in Fig. 2. All of the control mice were sacrificed on day 7 with a parasitemia greater than 70% (Fig. 2A). In contrast, all of the mice immunized with PyAMA-1 cleared their infection (Fig. 2B). Only 40% of the mice immunized with GST–MSP-119 were able to clear their infection, one on day 21 and the other on day 25 (not shown) (Fig. 2C). Analysis of the sera for the AMA-1 and GST–MSP-119 groups showed that the level of protection was broadly correlated with antibody titers in the IFA prior to challenge (data not shown). Immunoprecipitation of 35S metabolically labeled proteins from parasite extracts, using pooled sera from each group, identified either the 230-kDa MSP-1 and fragments derived from it (Fig. 3, lane 2) (41) or the 60-kDa AMA-1 protein (lane 3). The antiserum to AMA-1 also immunoprecipitated a 140-kDa protein which was not immunoprecipitated by MAb 28G2dc1 (lane 5), suggesting that the protein sample used for immunizing the mice contained the 140-kDa protein, either as a contaminant or in a complex with AMA-1.
FIG. 2.
Time course of P. yoelii yoelii YM infection in groups of 5 BALB/c mice immunized with either PBS (A), purified AMA-1 protein (B), or recombinant GST–MSP-119 (C), together with Freund's adjuvant. Mice were challenged with 5,000 parasitized erythrocytes i.v. at day 0, and the parasitemia was monitored by microscopy every 2 days on blood films stained with Giemsa's reagent. The results for individual mice are shown.
FIG. 3.
Immunoprecipitation of [35S]methionine-labeled polypeptides from detergent extracts of P. yoelii yoelii YM-infected RBCs with pooled immune sera from groups of five BALB/c mice immunized with either the purified AMA-1 preparation, a recombinant GST–MSP-119, or PBS. Bound proteins were eluted in SDS-PAGE sample buffer and separated on a 7.5% polyacrylamide gel. Labeled proteins were detected by fluorography. Tracks: 1, pooled preimmune mouse sera; 2, sera from mice immunized with GST–MSP-119; 3, sera from mice immunized with the purified AMA-1; 4, sera from mice immunized with PBS; 5, AMA-1-specific MAb 28G2dc1. The mobilities of molecular mass markers are shown at the left; positions of the 230-kDa MSP-1, the 140-kDa species, and the 60-kDa AMA-1 are indicated by arrows on the right.
Passive immunization with MAbs.
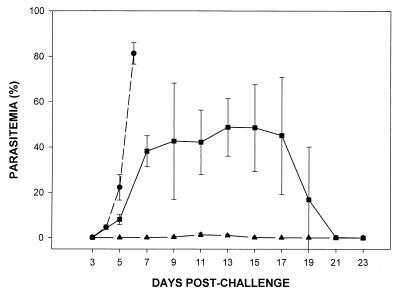
Since the AMA-1 preparation also contained small amounts of a 140-kDa protein that could have been responsible for inducing the protective immune response, there was a need to understand more fully the target of the protective response induced by immunization. Therefore, MAbs were produced and tested for the ability to passively immunize BALB/c mice. Hybridomas were produced from mice immunized with the purified PyAMA-1, and supernatants were screened by IFA against methanol-fixed parasites. Seven MAbs were produced that gave a characteristic apical punctate fluorescence pattern with the parasite: 8D2 and 45D11 (subclass IgG2b); 42A5, 48B2, and 48F8 (subclass IgG1); and 45B1 and 47B4 (subclass IgG2a). In an initial passive immunization experiment with groups of three mice, one MAb, 45B1, protected BALB/c mice against a challenge infection of P. yoelii yoelii YM (data not shown). For subsequent studies, two MAbs were selected: 45B1, the inhibitory MAb, and 48F8, a noninhibitory antibody specific for the 140-kDa contaminant (see below). The results of a passive immunization experiment are shown in Fig. 4. The mean parasitemias are shown for groups of five mice that received either MAb 45B1, MAb 48F8, or MAb 25.77, which recognizes the 235-kDa rhoptry proteins (20). MAb 48F8 had no effect on the course of infection, whereas 45B1 protected mice from challenge infection, with low parasitemias and 100% survival. Furthermore, any detectable parasites were restricted to reticulocytes (data not shown). The course of the infection in mice that received MAb 25.77 was similar to that reported previously (17).
FIG. 4.
Time course of P. yoelii yoelii YM infection in groups of 5 BALB/c mice injected intraperitoneally with MAbs 45B1 (▴), 48F8 (●), and 25.77 (■). Mice were challenged with 5,000 parasitized erythrocytes i.v. on day 0, and parasitemia was monitored by microscopy on alternate days on blood films stained with Giemsa's reagent. Error bars represent ±1 SD.
Immunoprecipitation and immunoblot analysis of the antigens recognized by the MAbs.
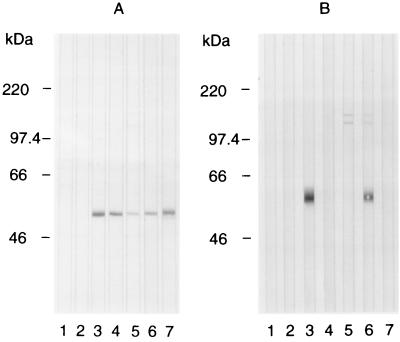
Immunoprecipitation of protein from extracts of [35S]methionine-labeled P. yoelii yoelii YM infected erythrocytes was used to investigate the specificity of the MAbs. The AMA-1 C-terminal sequence-specific MAb 28G2dc1 precipitated the 60-kDa PyAMA-1 protein (Fig. 5, track 2). MAb 45B1 recognized the 60-kDa AMA-1 protein but also precipitated a faint 140-kDa protein doublet (track 3). MAb 48F8 precipitated only the 140-kDa protein doublet (track 4), and 48B2 precipitated the 140-kDa protein doublet and AMA-1 to a lesser extent (track 5). Immunoblot analysis of extracts of purified P. yoelii yoelii YM merozoites separated by SDS-PAGE under both nonreduced and reduced conditions was also used to study antibody specificity (Fig. 6). Under nonreduced conditions (Fig. 6A), MAbs 28G2dc1 (track 3), 45B1 (track 4), 48F8 (track 5), 48B2 (track 6), and 8D2 (track 7) reacted with AMA-1. After reduction of the merozoite extract (Fig. 6B), MAb 28G2dc1 (track 3) reacted with AMA-1 at ∼60 kDa, but MAbs 45B1 (track 4), 48F8 (track 5), and 8D2 (track 7) no longer reacted with AMA-1, suggesting that these MAbs recognize a disulfide-dependent structure. With the reduced proteins, MAb 48F8 recognized a 140-kDa protein doublet (track 5), while MAb 48B2 appeared to recognize both the 140-kDa protein doublet and AMA-1 (track 6). On immunoblots of P. yoelii yoelii YM schizont extract, MAbs 48F8 and 48B2 reacted strongly with the 140-kDa protein doublet (data not shown).
FIG. 5.
Immunoprecipitation of [35S]methionine-labeled polypeptides from extracts of P. yoelii yoelii YM parasites with a panel of MAbs from hybridomas generated by immunization with purified PyAMA-1. Bound proteins were washed in a buffer containing 1% NP-40 and then washed in a buffer containing 0.1% SDS before elution in SDS-PAGE sample buffer. Bound proteins were separated by SDS-PAGE on a 10% polyacrylamide gel, and labeled proteins were detected by fluorography. The tracks contain immunoprecipitates with purified normal mouse IgG (track 1), rat MAb 28G2dc1 coupled to Q-Sepharose (track 2), mouse MAb 45B1 (track 3), mouse MAb 48F8 (track 4), and mouse MAb 48B2 (track 5). All mouse MAbs were coupled to protein G-Sepharose. The mobilities of molecular mass markers are indicated at the left; positions of the 140-kDa species and the 60-kDa AMA-1 are indicated by arrows on the right.
FIG. 6.
Immunoblot analysis using a panel of MAbs against the affinity-purified PyAMA-1 protein separated by SDS-PAGE on a 7.5% polyacrylamide gel and electroblotted onto a membrane. The proteins were fractionated under nonreduced (A) or reduced (B) conditions and then incubated with secondary anti-rat and anti-mouse antibody alone (track 1), MAb 58F8dc1 (track 2), MAb 28G2dc1 (track 3), MAb 45B1 (track 4), MAb 48F8 (track 5), MAb 48B2 (track 6), and MAb 8D2 (track 7). The mobilities of molecular mass markers are indicated at the left.
Localization by immunofluorescence of antigens recognized by MAbs.
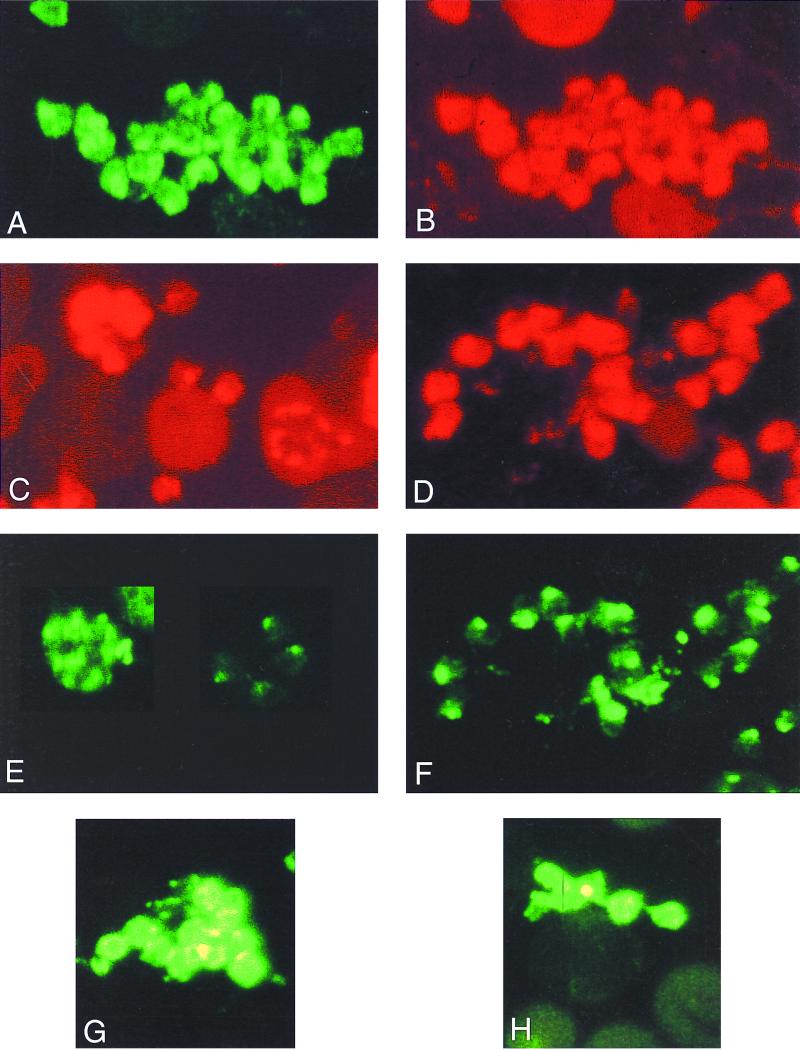
In fixed preparations of free merozoites or mature schizonts, the fluorescence pattern with both MAb 28G2dc1 and MAb 45B1 was punctate, largely restricted to the apical end of the merozoite, but also showing a pattern of circumferential staining (Fig. 7A to and C), as previously reported for the location of AMA-1 in P. falciparum parasites (32). In mature schizonts, the fluorescence pattern of an antibody reactive with the 140-kDa protein was apical. The reaction of the antibodies which recognized the 140-kDa protein suggested that this protein remained restricted to the apical region in free merozoites, even in instances when AMA-1 had been relocated to the surface (Fig. 7D [28G2dc1] and F [48F8]). With fixed mature schizonts and fixed free merozoites, the fluorescence pattern of MAb 48B2 was similar to that of MAb 28G2dc1 (Fig. 7E [48B2]. On free unfixed merozoites, both MAb 45B1 (Fig. 7G) and MAb 48F8 (Fig. 7H) stained the surface of the merozoite, although the frequency of surface staining by MAb 48F8 was markedly less than that of 45B1. MAb 48B2 and MAb 28G2dc1, which is specific for the C terminus of AMA-1, did not stain free unfixed merozoites (data not shown), suggesting that the epitopes recognized by these two MAbs are intracellular.
FIG. 7.
Single- and dual-antibody immunofluorescence analysis of the subcellular location of PyAMA-1 and the 140-kDa protein in fixed and unfixed free merozoites and in mature schizonts of P. yoelii yoelii YM. (A to C) Colocalized apical and surface immunofluorescence of PyAMA-1-specific MAb 45B1 (A) and AMA-1 C-terminal sequence-specific MAb 28G2dc1 (B) on fixed free merozoites and, for comparison, the reaction of MAb 28G2dc1 with mature schizonts is (C); (D and F) colocalized apical immunofluorescence of MAb 28G2dc1 and 140-kDa protein-specific MAb 48F8 on fixed free merozoites, respectively; (E) reaction of MAb 48B2 with mature schizonts and free merozoites; (G and H) surface reactivity of MAbs 45B1 and 48F8 with free and unfixed merozoites, respectively.
MAb cross-reactivity with P. falciparum.
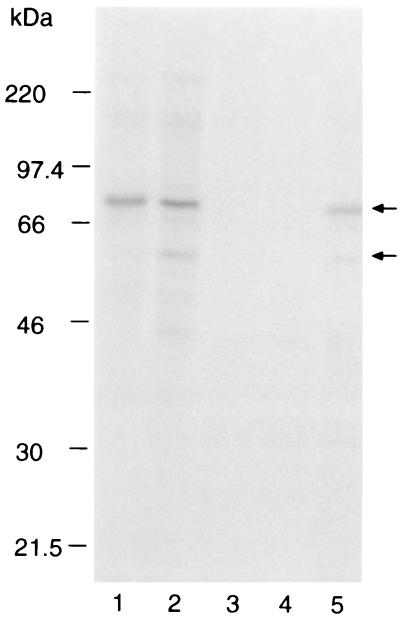
Immunoprecipitation of [35S]methionine-labeled polypeptides from extracts of P. falciparum FCB-1 was used to determine whether or not the antibodies cross-reacted with PfAMA-1 (Fig. 8). MAb 58F8dc1, specific for an N-terminal sequence in PfAMA-1, recognized the full-length 83-kDa protein (track 1), whereas MAb 28G2dc1, which recognizes a C-terminal sequence in all known AMA-1 proteins, precipitated both the 83-kDa and processed 60-kDa forms (track 2). MAb 48B2 recognized both full-length and processed forms of PfAMA-1 (track 5), while MAbs 45B1 (track 3) and 48F8 (track 4) did not cross-react.
FIG. 8.
Immunoprecipitation of [35S]methionine-labeled polypeptides from P. falciparum FCB-1 parasite extracts with MAbs. Bound proteins were washed in a buffer containing 1% NP-40 and eluted in SDS-PAGE sample buffer. Proteins were separated on by SDS-PAGE on a 7.5% polyacrylamide gel, and labeled proteins were detected by fluorography. Tracks: 1, MAb 58F8dc1; 2, 28G2dc1; 3, 45B1; 4, 48F8; 5, 48B2. Both rat MAbs were coupled to Q-Sepharose, and all mouse MAbs were coupled to protein G-Sepharose. The mobilities of molecular mass markers are noted at the left; arrows indicate positions of the 83-kDa PfAMA-1 and the processing product.
DISCUSSION
Plasmodium AMA-1 is a target for an effective host immune response against the parasite. This has been shown previously in various ways for P. knowlesi, P. fragile, P. chabaudi, and now for the first time P. yoelii yoelii. The P. yoelii yoelii YM rodent model has allowed several unique observations that further our understanding of AMA-1 and its potential as a malaria vaccine component. In the first reported AMA-1 vaccine trial in rhesus monkeys, affinity-purified native PkAMA-1 was used (11). In this nonhuman primate model, four out of six rhesus monkeys were protected against a challenge infection. The level of protection appeared to correlate with PkAMA-1 antibody titers. In the present study, small quantities of parasite-derived PyAMA-1 were sufficient to induce protective responses in BALB/c mice despite relatively low IFA titers. The contribution of the response to the 140-kDa protein to the protective effect is difficult to determine, although the results of the passive immunization studies are consistent with AMA-1 being the important component. In recent reports of AMA-1 vaccine trials conducted in nonhuman primates or mice, either a purified recombinant baculovirus-expressed product (7) or a purified refolded bacterial expression product (1) was used. Each of these recombinant proteins induced a level of protection against a challenge infection that was not as complete as that induced by the parasite-derived PyAMA-1 against P. yoelii yoelii. For P. falciparum, the only data available suggesting that AMA-1 is the target of a protective response are derived from in vitro invasion inhibition studies using antibodies and seroepidemiological studies using a recombinant full-length PfAMA-1 protein produced in insect cells. We identified a conformational epitope within the processed 66-kDa fragment of the PfAMA-1 ectodomain (27) that bound a rat MAb (4G2dc1) capable of inhibiting erythrocyte invasion in vitro. Sera from individuals living in areas where malaria is holoendemic also contain antibodies that recognized this epitope (43; Narum et al., unpublished data).
We report the production of a panel of MAbs; their properties are summarized in Table 1. One of these MAbs (45B1) is protective on passive immunization and recognizes a secondary structural epitope within the ectodomain of PyAMA-1. This observation suggests that a recombinant protein with a correct secondary structure may be suitable as an immunogen for inducing protective responses when properly presented to the immune system. Therefore, for induction of an antibody similar to 45B1 against PfAMA-1, the correct tertiary structure of the recombinant protein may not be essential, in contrast to the results of our earlier studies with PfAMA-1 (27), in which it was concluded that the recognition of a tertiary structure was significant for the antibody-mediated protection observed in vitro. This conjecture is supported by the level of sequence conservation between AMA-1 molecules (5, 15, 25, 30, 35, 45) and may be particularly important given the difficulty of expression and purification of correctly folded recombinant PfAMA-1 protein (19, 33). However, the immunogenicity of the parasite-derived material may be much higher based on our present results and those obtained with PkAMA-1. It is possible that epitopes are exposed or present in the native protein that are not present in the recombinant form. For example, immunization with purified parasite derived MSP-1 induced much more effective protection in Aotus monkeys than immunization with recombinant fragments (16). The contribution of the 140-kDa protein present in the PyAMA-1 preparation to the protection induced by immunization will need to be assessed using either a nucleic acid vaccine or recombinant or purified 140-kDa protein.
TABLE 1.
Properties of MAbs
| MAb | IgG isotype | Protection | Immunoprecipitation
|
Immunoblotting
|
IFAa
|
Cross-reaction with PfAMA-1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 60-kDa AMA-1 | 140-kDa species | AMA-1
|
140-kDa species
|
Fixed | Unfixed | ||||||
| −DTT | +DTT | −DTT | +DTT | ||||||||
| 8D2 | 2b | − | NDb | ND | + | − | − | − | A/C | ND | ND |
| 42A5 | 1 | − | ND | ND | ND | ND | ND | ND | A/C | ND | ND |
| 45B1 | 2a | + | + | ± | + | − | − | − | A/C | A/C | − |
| 45D11 | 2b | − | ND | ND | ND | ND | ND | ND | A/C | ND | ND |
| 47B4 | 2a | − | ND | ND | ND | ND | ND | ND | A/C | ND | ND |
| 48B2 | 1 | − | + | + | + | + | + | + | A/C | − | + |
| 48F8 | 1 | − | − | + | − | − | + | + | A | A/C | − |
| 28G2dc1c | ND | + | − | + | + | − | − | A/C | − | + | |
| 58F8dc1c | ND | − | − | − | − | − | − | ND | ND | + | |
A, apical fluorescence; C, circumferential (merozoite surface).
ND, not done.
Previously reported (32).
The content of the rhoptry organelles is complex, and their function poorly understood. The subcellular location of individual proteins may be differentially regulated, as we have reported previously (23, 32). These earlier reports suggested that the relocalization of PfAMA-1 and PkAMA-1 to the parasite surface was linked to an N-terminal processing event. It is unclear whether or not a similar processing event is necessary for relocalization of PyAMA-1. No processing of PyAMA-1 has been observed by immunoblot or immunoprecipitation experiments, although this question has not been specifically addressed by pulse-chase studies. The presence of the 140-kDa protein in the purified PyAMA-1 suggests that AMA-1 has the potential to interact with another putative rhoptry protein and form a noncovalent association. Two other protein complexes have been described in P. falciparum rhoptries (24): the high-molecular-mass rhoptry protein complex (21) and the low-molecular-mass rhoptry protein complex containing rhoptry-associated proteins 1 and 2 (38, 40). The association between the two P. yoelii yoelii molecules appears to be dynamic given that they may copurify using an AMA-1 specific MAb and yet may be independently distributed from the rhoptries to other cellular locations. Several rhoptry and microneme proteins have a transmembrane and putative cytoplasmic domain that may interact with the microtubular network below the merozoite plasmalemma. The relocalization of these proteins by an active process, possibly involving myosin (37), may be involved in erythrocytic invasion.
Antibodies to merozoite proteins can modify the host cell range of the malaria parasite. For example, passive immunization with MAb 25.77, which is specific for the P. yoelii yoelii 235-kDa rhoptry protein family, suppressed blood-stage parasitemia and restricted the parasites to reticulocytes (17). It has been proposed that MAb 25.77 recognizes a protein or family of proteins involved in determining virulence or, more specifically, invasion of mature erythrocytes. Passive immunization with a MAb specific for MSP-1 also showed a similar parasite restriction to reticulocytes (41). Based on these and our present observations, it appears that antibody targeting of merozoite ligands involved in erythrocyte invasion may be sufficient to restrict P. yoelii yoelii YM parasites into reticulocytes. The mechanism(s) responsible for this changed phenotype in P. yoelii yoelii YM is unknown, but the observation may have significant implications with regard to targeting P. falciparum, which also infects mature erythrocytes.
Finally, the demonstration that AMA-1 is a target for an effective immune response against blood stages of P. yoelii yoelii, by active immunization with native PyAMA-1 and by passive administration of a specific neutralizing MAb, clearly supports the idea that AMA-1 is a suitable target for immunological intervention. This may be by vaccination using DNA and/or protein delivery systems or by passive therapy with an AMA-1 neutralizing antibody. A peptidomimetic that interferes with the interaction of AMA-1 with other molecules is also a possibility. In future studies it will be of interest to evaluate the effect of combined passive therapies in the P. yoelii yoelii rodent model, which should increase our understanding of the protective mechanism(s) of antibody against the merozoite.
ACKNOWLEDGMENTS
We express our gratitude to Terry Scott-Finnigan for excellent technical help with the hybridoma development, Margaret Goggin for large-scale culture, Irene Ling for providing the recombinant GST–MSP-119, and Mike Blackman for helpful discussion of the data.
REFERENCES
- 1.Anders R F, Crewther P E, Edwards S, Margetts M, Matthew M L, Pollock B, Pye D. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine. 1998;16:240–247. doi: 10.1016/s0264-410x(97)88331-4. [DOI] [PubMed] [Google Scholar]
- 2.Blackman M J, Heidrich H G, Donachie S, McBride J S, Holder A A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackman M J, Scott-Finnigan T J, Shai S, Holder A A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Q, Saul A. Sequence analysis of the apical membrane antigen I (AMA-1) of Plasmodium vivax. Mol Biochem Parasitol. 1994;65:183–187. doi: 10.1016/0166-6851(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, McGregor I A, Carrington S C. Gamma globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 7.Collins W E, Pye D, Crewther P E, Vandenberg K L, Galland G G, Sulzer A J, Kemp D J, Edwards S J, Coppel R L, Sullivan J S, et al. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am J Trop Med Hyg. 1994;51:711–719. doi: 10.4269/ajtmh.1994.51.711. [DOI] [PubMed] [Google Scholar]
- 8.Crewther P E, Culvenor J G, Silva A, Cooper J A, Anders R F. Plasmodium falciparum: two antigens of similar size are located in different compartments of the rhoptry. Exp Parasitol. 1990;70:193–206. doi: 10.1016/0014-4894(90)90100-q. [DOI] [PubMed] [Google Scholar]
- 9.Deans J A, Alderson T, Thomas A W, Mitchell G H, Lennox E S, Cohen S. Rat monoclonal antibodies which inhibit the in vitro multiplication of Plasmodium knowlesi. Clin Exp Immunol. 1982;49:297–309. [PMC free article] [PubMed] [Google Scholar]
- 10.Deans J A, Jeans W C. Structural studies on a putative protective Plasmodium knowlesi merozoite antigen. Mol Biochem Parasitol. 1987;26:155–166. doi: 10.1016/0166-6851(87)90139-3. [DOI] [PubMed] [Google Scholar]
- 11.Deans J A, Knight A M, Jean W C, Waters A P, Cohen S, Mitchell G H. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kD merozoite antigen. Parasite Immunol. 1988;10:535–552. doi: 10.1111/j.1365-3024.1988.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 12.Deans J A, Thomas A W, Alderson T, Cohen S. Biosynthesis of a putative protective Plasmodium knowlesi merozoite antigen. Mol Biochem Parasitol. 1984;11:189–204. doi: 10.1016/0166-6851(84)90065-3. [DOI] [PubMed] [Google Scholar]
- 13.Deans J A, Thomas A W, Cohen S. Stage-specific protein synthesis by asexual blood stage parasites of Plasmodium knowlesi. Mol Biochem Parasitol. 1983;8:31–44. doi: 10.1016/0166-6851(83)90032-4. [DOI] [PubMed] [Google Scholar]
- 14.Dennis E D, Mitchell G H, Butcher G A, Cohen S. In vitro isolation of Plasmodium knowlesi merozoites using polycarbonate sieves. Parasitology. 1975;71:475–481. doi: 10.1017/s0031182000047235. [DOI] [PubMed] [Google Scholar]
- 15.Dutta S, Malhotra P, Chauhan V S. Sequence analysis of apical membrane antigen 1 (AMA-1) of Plasmodium cynomolgi bastianelli. Mol Biochem Parasitol. 1995;73:267–270. doi: 10.1016/0166-6851(95)00112-e. [DOI] [PubMed] [Google Scholar]
- 16.Etlinger H M, Caspers P, Matile H, Schoenfeld H J, Stueber D, Takacs B. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun. 1991;59:3498–3503. doi: 10.1128/iai.59.10.3498-3503.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman R R, Trejdosiewicz A J, Cross G A. Protective monoclonal antibodies recognising stage-specific merozoite antigens of a rodent malaria parasite. Nature. 1980;284:366–368. doi: 10.1038/284366a0. [DOI] [PubMed] [Google Scholar]
- 18.Harlow E, Lane D. Antibodies. A laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 19.Hodder A N, Crewther P E, Matthew M L, Reid G E, Moritz R L, Simpson R J, Anders R F. The disulfide bond structure of Plasmodium apical membrane antigen-1. J Biol Chem. 1996;271:29446–29452. doi: 10.1074/jbc.271.46.29446. [DOI] [PubMed] [Google Scholar]
- 20.Holder A A, Freeman R R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981;294:361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 21.Holder A A, Freeman R R, Uni S, Aikawa M. Isolation of a Plasmodium falciparum rhoptry protein. Mol Biochem Parasitol. 1985;14:293–303. doi: 10.1016/0166-6851(85)90057-x. [DOI] [PubMed] [Google Scholar]
- 22.Homewood C A, Neame K D. A comparison of methods used for the removal of white cells from malaria-infected blood. Ann Trop Med Parasitol. 1976;70:249–251. doi: 10.1080/00034983.1976.11687119. [DOI] [PubMed] [Google Scholar]
- 23.Howard R F, Narum D L, Blackman M J, Thurman J. Analysis of the processing of Plasmodium falciparum rhoptry-associated protein 1 and localization of Pr86 to schizont rhoptries and p67 to free merozoites. Mol Biochem Parasitol. 1998;92:111–122. doi: 10.1016/s0166-6851(97)00238-7. [DOI] [PubMed] [Google Scholar]
- 24.Howard R F, Reese R T. Plasmodium falciparum—heterooligomeric complexes of rhoptry polypeptides. Exp Parasitol. 1990;71:330–342. doi: 10.1016/0014-4894(90)90038-e. [DOI] [PubMed] [Google Scholar]
- 25.Kappe S H, Adams J H. Sequence analysis of the apical membrane antigen-1 genes (AMA-1) of Plasmodium yoelii yoelii and Plasmodium berghei. Mol Biochem Parasitol. 1996;78:279–283. doi: 10.1016/s0166-6851(96)02619-9. [DOI] [PubMed] [Google Scholar]
- 26.Knowles G, Walliker D. Variable expression of virulence in the rodent malaria parasite Plasmodium yoelii yoelii. Parasitology. 1980;81:211–219. doi: 10.1017/s0031182000055165. [DOI] [PubMed] [Google Scholar]
- 27.Kocken C H, van der Wel A M, Dubbeld M A, Narum D L, van de Rijke F M, van Gemert G J, van der Linde X, Bannister L H, Janse C, Waters A P, Thomas A W. Precise timing of expression of a Plasmodium falciparum-derived transgene in Plasmodium berghei is a critical determinant of subsequent subcellular localization. J Biol Chem. 1998;273:15119–15124. doi: 10.1074/jbc.273.24.15119. [DOI] [PubMed] [Google Scholar]
- 28.Lal A A, Hughes M A, Oliveira D A, Nelson C, Bloland P B, Oloo A J, Hawley W E, Hightower A W, Nahlen B L, Udhayakumar V. Identification of T-cell determinants in natural immune responses to the Plasmodium falciparum apical membrane antigen (AMA-1) in an adult population exposed to malaria. Infect Immun. 1996;64:1054–1059. doi: 10.1128/iai.64.3.1054-1059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling I T, Ogun S A, Holder A A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 30.Marshall V M, Peterson M G, Lew A M, Kemp D J. Structure of the apical membrane antigen I (AMA-1) of Plasmodium chabaudi. Mol Biochem Parasitol. 1989;37:281–283. doi: 10.1016/0166-6851(89)90160-6. [DOI] [PubMed] [Google Scholar]
- 31.Marshall V M, Zhang L, Anders R F, Coppel R L. Diversity of the vaccine candidate AMA-1 of Plasmodium falciparum. Mol Biochem Parasitol. 1996;77:109–113. doi: 10.1016/0166-6851(96)02583-2. [DOI] [PubMed] [Google Scholar]
- 32.Narum D L, Thomas A W. Differential localization of full-length and processed forms of PF83/AMA-1, an apical membrane antigen of Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1994;67:59–68. doi: 10.1016/0166-6851(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 33.Narum D L, Welling G W, Thomas A W. Ion-exchange-immunoaffinity purification of a recombinant baculovirus Plasmodium falciparum apical membrane antigen, PF83/AMA-1. J Chromatogr. 1993;657:357–363. doi: 10.1016/0021-9673(93)80291-F. [DOI] [PubMed] [Google Scholar]
- 34.Oliveria D A, Udhayakumar V, Bloland P, Shi Y P, Nahlen B L, Oloo A J, Hawley W E, Lal A A. Genetic conservation of the Plasmodium falciparum apical membrane antigen-1 (AMA-1) Mol Biochem Parasitol. 1996;76:333–336. doi: 10.1016/0166-6851(95)02548-0. [DOI] [PubMed] [Google Scholar]
- 35.Peterson M G, Marshall V M, Smythe J A, Crewther P E, Lew A, Silva A, Anders R F, Kemp D. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol Cell Biol. 1989;9:3151–3154. doi: 10.1128/mcb.9.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson M G, Nguyen-Dinh P, Marshall V M, Elliott J F, Collins W E, Anders R F, Kemp D J. Apical membrane antigen of Plasmodium fragile. Mol Biochem Parasitol. 1990;39:279–283. doi: 10.1016/0166-6851(90)90067-v. [DOI] [PubMed] [Google Scholar]
- 37.Pinder J C, Fowler R E, Dluzewski A R, Bannister L H, Lavin F M, Mitchell G H, Wilson R J, Gratzer W B. Actomyosin motor in the merozoite of the malaria parasite, Plasmodium falciparum: implications for red cell invasion. J Cell Sci. 1998;111:1831–1839. doi: 10.1242/jcs.111.13.1831. [DOI] [PubMed] [Google Scholar]
- 38.Ridley R G, Takacs B, Lahm H W, Delves C J, Goman M, Certa U, Matile H, Woollett G R, Scaife J G. Characterisation and sequence of a protective rhoptry antigen from Plasmodium falciparum. Mol Biochem Parasitol. 1990;41:125–134. doi: 10.1016/0166-6851(90)90103-s. [DOI] [PubMed] [Google Scholar]
- 39.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 40.Saul A, Cooper J, Hauquitz D, Irving D, Cheng Q, Stowers A, Limpaiboon T. The 42-kD rhoptry-associated protein of Plasmodium falciparum. Mol Biochem Parasitol. 1992;50:139–150. doi: 10.1016/0166-6851(92)90251-e. [DOI] [PubMed] [Google Scholar]
- 41.Spencer Valero L M, Ogun S A, Fleck S L, Ling I T, Scott-Finnigan T J, Blackman M J, Holder A A. Passive immunization with antibodies against three distinct epitopes on Plasmodium yoelii merozoite surface protein 1 suppresses parasitemia. Infect Immun. 1998;66:3925–3930. doi: 10.1128/iai.66.8.3925-3930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas A W, Deans J A, Mitchell G H, Alderson T, Cohen S. The Fab fragments of monoclonal IgG to a merozoite surface antigen inhibit Plasmodium knowlesi invasion of erythrocytes. Mol Biochem Parasitol. 1984;13:187–199. doi: 10.1016/0166-6851(84)90112-9. [DOI] [PubMed] [Google Scholar]
- 43.Thomas A W, Trape J F, Rogier C, Goncalves A, Rosario V E, Narum D L. High prevalence of natural antibodies against Plasmodium falciparum 83- kilodalton apical membrane antigen (PF83/AMA-1) as detected by capture-enzyme-linked immunosorbent assay using full-length baculovirus recombinant PF83/AMA-1. Am J Trop Med Hyg. 1994;51:730–740. doi: 10.4269/ajtmh.1994.51.730. [DOI] [PubMed] [Google Scholar]
- 44.Thomas A W, Waters A P, Carr D. Analysis of variation in Pf83, an erythrocytic merozoite vaccine candidate antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1990;42:285–288. doi: 10.1016/0166-6851(90)90172-i. [DOI] [PubMed] [Google Scholar]
- 45.Waters A P, Thomas A W, Deans J A, Mitchell G H, Hudson D E, Miller L H, McCutchan T F, Cohen S. A merozoite receptor protein from Plasmodium knowlesi is highly conserved and distributed throughout Plasmodium. J Biol Chem. 1990;265:17974–17979. [PubMed] [Google Scholar]