Abstract
Pseudomonas aeruginosa, an opportunistic pathogen responsible most notably for severe infections in cystic fibrosis (CF) patients, utilizes the type III secretion system for eukaryotic cell intoxication. The CF clinical isolate CHA shows toxicity towards human polymorphonuclear neutrophils (PMNs) which is dependent on the type III secretion system but independent of the cytotoxin ExoU. In the present study, the cytotoxicity of this strain toward human and murine macrophages was demonstrated. In low-multiplicity infections (multiplicity of infection, 10), approximately 40% of the cells die within 60 min. Analysis of CHA-infected cells by transmission electron microscopy, DNA fragmentation assay, and Hoechst staining revealed the hallmarks of oncosis: cellular and nuclear swelling, disintegration of the plasma membrane, and absence of DNA fragmentation. A panel of 29 P. aeruginosa CF isolates was screened for type III system genotype, protein secretion profile, and cytotoxicity toward PMNs and macrophages. This study showed that six CF isolates were able to induce rapid ExoU-independent oncosis on phagocyte cells.
Pseudomonas aeruginosa is a major opportunistic pathogen causing nosocomial pneumonia, infections in immunocompromised patients, and severe pulmonary damage in cystic fibrosis (CF) patients (28). Among numerous virulence determinants, P. aeruginosa clinical isolates use the so-called type III secretion system as a specialized mechanism to provoke eukaryotic cell intoxication.
Type III secretion systems, which are conserved in various plant and animal pathogens, require close contact with the eukaryotic cell in order to deliver toxic bacterial proteins directly into the cytoplasm of the cell. The phenotypic effects induced by type III systems differ from one bacterial species to another but may be classified according to the major cell functions that are modified (12). For example, Shigella spp. (20) and Salmonella spp. (11) modulate actin organization to induce their own uptake by nonphagocytic cells. The main targets of type III secretion effectors include cells involved in innate immunity. Yersinia spp. and P. aeruginosa synthesize type III system effectors that can alter normal actin structures in macrophages to inhibit phagocytosis (2, 10). Furthermore, Yersinia (21), Shigella (15), and Salmonella (22), via the activity of type III effectors, are able to induce apoptosis in infected macrophages.
Several groups have investigated the interaction between P. aeruginosa and eukaryotic cells, using different ex vivo infection models. These studies have made it possible to elucidate the contribution of certain type III secreted proteins to P. aeruginosa cytotoxicity. To date, four type III effectors have been identified: ExoS, ExoT, ExoU, and ExoY. ExoS and ExoT have ADP-ribosylating activity toward low-molecular-weight GTP-binding proteins of the Ras family (18). Expression of ExoS is correlated with multiple effects on cellular processes, including inhibition of DNA synthesis (24), alteration in actin cytoskeletal structure (10, 26), and interference with cell matrix adherence (25). ExoY is a recently discovered adenylate cyclase whose activity is associated with profound morphological changes in epithelial cells (35). Finally, a type III secreted effector, ExoU (PepA), with unknown activity, is responsible for the acute cytotoxicity of P. aeruginosa toward epithelial cells (7, 14) and macrophages (29).
We have reported recently that a CF clinical isolate, CHA, is able to induce cell death in human polymorphonuclear neutrophils (PMNs) in an ExoU-independent manner. The cytotoxic phenotype of CHA, however, requires the functional type III secretion system (3). In the present work, we have further investigated the cytotoxicity of CHA and of 28 other CF isolates of P. aeruginosa and shown that the type III secretion-dependent, ExoU-independent cell death of phagocytes occurs by rapid oncosis, involving swelling of the cell and nucleus and disintegration of the plasma membrane. This type of eukaryotic cell death, which is distinct from apoptosis (16), has been recently associated with the cytotoxicity of some Shigella (6, 23) and enteroaggregative Escherichia coli (5) strains.
MATERIALS AND METHODS
Bacterial strains.
The P. aeruginosa strains used in this study included CHA, a bronchopulmonary isolate from a CF patient (31), and CHA-D1, an isogenic mutant of CHA in which the exsA gene, encoding the ExsA transcriptional factor necessary for type III system synthesis (9), has been inactivated (3). Further P. aeruginosa strains isolated from different CF patients were designated CF1, CF2, CF3, CF4, CF5, CF6, CF7, CF8, CF9, CF10, CF11, CF12, CF13, CF14, CF15, CF16, CF17, CF18, CF19, CF20, RIE, T6, 37.11, K569, REN0, REN3, REN4, and REN7. All strains were tested for resistance to 10% pooled normal human serum. Strains CF18, CF20, T6, 37.11, K569, REN0, and REN7 were found to be serum sensitive. All strains were grown on pseudomonas isolation agar (Difco) plates or in Luria-Bertani (LB) liquid medium at 37°C. The antibiotics used were carbenicillin (300 μg/ml) and gentamicin (200 μg/ml). The strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmid used in this study
| Strain or plasmid | Relevant genotype or phenotypea | Source or reference |
|---|---|---|
| P. aeruginosa | ||
| CHA | Mucoid CF isolate | 31 |
| CHA-D1 | exsA::Gmr mutant of CHA | 3 |
| REN0 | CF isolate | This work |
| REN0-D1 | exsA::Gmr mutant of REN0 | This work |
| CF1 | CF isolate | This work |
| CF1-D1 | exsA::Gmr mutant of CF1 | This work |
| Plasmid pDD2 | pUCP20-derived plasmid containing exsA | 3 |
Gmr, gentamicin resistance.
In vitro secretion of type III system proteins.
To test the ability of P. aeruginosa isolates to secrete in vitro type III secretion system proteins, bacteria were grown in a calcium-depleted LB medium and extracellular proteins were analyzed by 0.1% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (12% polyacrylamide) as described previously (3). The protein profiles were compared to the protein profile obtained with the CHA strain, for which secreted ExoS, ExoT, PopB, and PopD were identified by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF) (3).
Genotype analysis.
The presence of exsA, exoS, exoT, exoY, and exoU genes was determined by Southern blot analysis. Chromosomal DNA was isolated from each strain, subjected to digestion with EcoRI, transferred to a nylon membrane, and hybridized with the digoxigenin-labeled probes as specified by the manufacturer (Roche Molecular Biochemicals, Meylan, France). Probes were synthesized by PCR using primers 5′GGCCCAGGATCGGCTTGCAA and 5′GATCCGCTGCCGAGCCAAGA for amplification of exoS (34), 5′GATATCCATCGGGTTCTCCG and 5′AGGCCTCCTTGCCGCCCATT for amplification of exoT (33), 5′GTCGCAGCCATCTACCTAG and 5′GCGTCGCCCAGCATATTCCG (AF061745 EMBL data bank) for amplification of exoY, and 5′GATCTTATTTCGCTGCTCGA and 5′CCTTCTGGCGAAAAGCCAC for amplification of exoU (14). The PCR probe for exsA was obtained as described previously (3).
Preparation of PMNs and cell culture.
Human PMNs were isolated from whole blood by Percoll gradient centrifugation as described previously (3). The EBV-B lymphocytes, provided by Laboratoire d'Immunochimie, CEA-Grenoble, Grenoble, France, were grown in RPMI 1640 medium containing l-glutamine (Gibco) and supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco). The macrophage cell line J774 and HeLa cells were grown in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated FCS. At 24 h before infection, the cells were seeded in 24-well culture plates at 5 × 105 cells/well. Human macrophages were a gift from J. Plumas (Etablissement de transfusion sanguine de l'Isère et de la Savoie). The cells were obtained by differentiation from peripheral blood mononuclear cells by a 7-day incubation in the presence of granulocyte-macrophage colony-stimulating factor (500 U/ml; Sandoz) and 2% autologous serum. After purification by counterflow centrifugation, morphological and phenotypic analysis indicated that >95% of the cells were macrophages. Infection was carried out in RPMI 1640–10% FCS. All incubations were performed in a 5% CO2 incubator at 37°C.
Infection conditions and cytotoxicity assay.
Unless otherwise indicated, the bacterial strains were grown in LB medium to an optical density at 600 nm (OD600) between 1 and 1.2 after dilution of overnight cultures at 0.1 OD600, washed once with phosphate-buffered saline (PBS), and resuspended in the appropriate eukaryotic cell growth medium. Infections were carried out in 24-well culture dishes in a CO2 incubator at 37°C. Samples (300 μl) contained 5 × 105 cells/well and 5 × 106 CFU of P. aeruginosa, giving a multiplicity of infection (MOI) of 10. We developed a test in 96-well plates to analyze the cytotoxicity of P. aeruginosa CF isolates on PMNs. For infection, bacteria were collected by centrifugation, washed once with modified HEPES-buffered saline (mHBS) (3), and opsonized for 5 min with pooled normal human serum. Sample (200 μl) contained 5 × 106 PMNs/ml, 5 × 107 CFU of P. aeruginosa per ml (MOI, 10), and 10% normal human serum in mHBS. At each hour of incubation, a 30-μl aliquot was taken and the cytotoxicity was determined by measuring the release of the cytosolic enzyme lactate dehydrogenase (LDH) into infection supernatants by using the cytotoxicity detection kit (Roche Molecular Biochemicals) as described previously (3).
Examination of cell morphology.
Cells were grown and infected with bacteria (MOI, 10) in Lab-Tek chambers (Nunc). Cell morphology was assessed by phase-contrast microscopy with inverted Zeiss IM. Observations were made with a 40× objective lens.
Apoptosis assays. (i) DNA fragmentation assay.
DNA was isolated from eukaryotic cells as described previously (30), quantified, and subjected to electrophoresis on a 1.5% agarose gel containing 1 μg of ethidium bromide (EtBr) per ml. DNA was visualized under UV light.
(ii) Nuclear morphology.
After infection, cells were washed once with PBS and fixed with 3.7% formaldehyde in PBS for 10 min at room temperature. After two washes with PBS, the cells were permeabilized with 0.8% Triton X-100 in PBS for 10 min at room temperature. To analyze the nuclei, cells were stained with Hoechst 33342 (Sigma) (working dilution, 1:1,000) at 37°C for 30 min and visualized by fluorescence microscopy (Axioskop 20; Zeiss). Apoptosis in PMNs was induced by a 5-h treatment with 50 μM actinomycin D (Clontech, Palo Alto, Calif.) or a 30-min UV treatment. Apoptosis in J774 macrophages was induced by UV irradiation for 15 min.
TEM.
After 1 h of infection, PMNs and J774 macrophages were pelleted by centrifugation at 1,200 × g for 5 min, fixed for 1 h using 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), and postfixed for 1 h with 1% OsO4 in 0.1 M cacodylate buffer (pH 7.4). The pellet was embedded in epoxy resin and sectioned for transmission electron microscopy (TEM) observation (JEOL JEM 1010). The sections were scored for the occurrence of different morphological appearance of PMNs and macrophages. Phagocytosis was estimated by counting more than 100 cells.
RESULTS
Cytotoxicity of the CHA strain on macrophages, B lymphocytes, and epithelial cells.
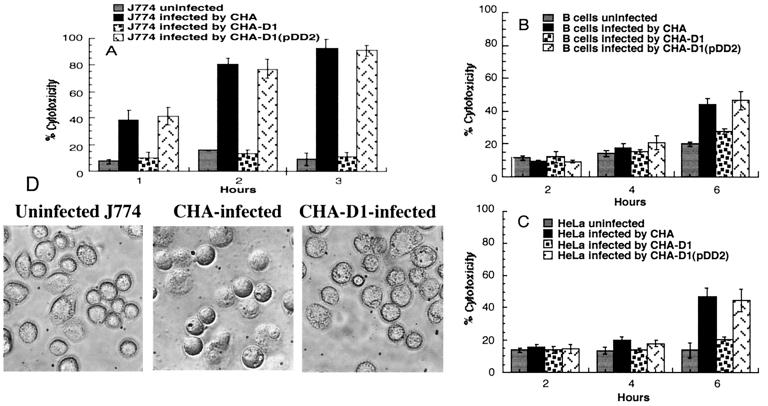
The CF clinical isolate CHA was previously shown to induce type III secretion-dependent cell death in human PMNs, yielding 80% cell lysis after 3 h of coincubation. Cell death was independent of the previously identified type III-secreted toxin ExoU, since the exoU gene is not present in the genome of the CHA strain (3). To test whether ExoU-independent cytotoxicity is exhibited toward other types of leukocytes, macrophages and B lymphocytes were infected with CHA and the CHA-D1 mutant strain at a low MOI of 10. CHA-D1 is a derivative of CHA in which the exsA gene, encoding a transcriptional activator of P. aeruginosa type III secretion system genes, has been inactivated. CHA-D1 is deficient in type III secretion and is noncytotoxic on PMNs (3). In preliminary experiments, monocyte-derived macrophages of human origin and the murine macrophage-like cell line J774 were tested in parallel. Since the results obtained with the two cell types were identical, J774 cells were used in further studies. Infected J774 cells were observed by phase-contrast microscopy, and cytotoxicity was assessed by measuring the relative release of the cytosolic enzyme LDH. CHA-infected macrophages rapidly began to round up, and a large number of cells became swollen and translucent, usually detaching from the cell dish surface (Fig. 1D). These cells were able to take up EtBr, a DNA-binding fluorescent dye, indicating a substantial loss of membrane integrity. In agreement with the microscopic observations, the LDH activity released from CHA-infected macrophages was already detectable 30 min postinfection and reached 80 to 90% within 2 h. The macrophages infected with CHA-D1 showed no release of LDH in comparison to the basal level of LDH activity measured in the supernatants of uninfected cells (Fig. 1A). In agreement with the LDH data, no important morphological changes of CHA-D1-infected cells were observed during the course of the 3-h incubation. Complementation of the mutant CHA-D1 in trans with the wild-type exsA gene restored its cytotoxicity (Fig. 1A). Unlike PMNs and macrophages, B lymphocytes infected with CHA and CHA-D1 released no LDH activity during the first 4 h of infection. If incubation was continued to 6 h, the LDH activity released by CHA-infected B cells indicated about 40% lysis (Fig. 1B). Similar results were obtained with CHA-infected epithelial cell line HeLa, in which only 40 to 50% of cells died in 6 h (Fig. 1C). This slow cytotoxic activity of CHA toward lymphocytes and epithelial cells was nevertheless dependent on the functional type III secretion system, since the ExsA mutant strain CHA-D1 was less cytotoxic and the complementation in trans restored the cytotoxicity (Fig. 1B and C). The results obtained with different cell lines suggest that there is some cell type specificity in type III secretion-mediated intoxication.
FIG. 1.
Cytotoxicity of P. aeruginosa strains CHA, CHA-D1, and CHA-D1(pDD2) toward J774 macrophages (A), B lymphocytes (B), and HeLa cells (C). The percent cytotoxicity was calculated from the release of LDH activity. Data are the means of at least three experiments. (D) J774 macrophages were infected with strain CHA or CHA-D1 (MOI, 10) and examined for morphological alterations by phase-contrast microscopy after 1 h.
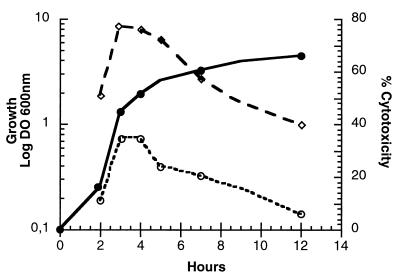
Preliminary experiments indicated that the kinetics of the CHA-induced cell death could vary significantly from one experiment to another, depending on the age of the bacterial culture. To establish the relationship between the kinetics of cytotoxicity and the bacterial growth phase, the CHA strain was grown in LB at 37°C with agitation and aliquots were taken at different time points. J774 macrophages were infected at a constant MOI of 10, and cytotoxicity was determined 1 and 2 h after infection. As shown in Fig. 2, the most rapid cell death occurred when bacteria from the late logarithmic phase were used for infection. Under these conditions, morphological changes in the infected macrophages were already noticeable 15 min after infection and 30 to 40% cell lysis was detected 1 h later. As soon as the bacterial culture entered the stationary phase, the ability of CHA to induce cell death began to decrease. The cytotoxicity of bacteria from the late stationary phase was reduced to 40% at 2 h postinfection. A similar growth phase-dependent cytotoxicity was observed for PMNs (data not shown). With the exception of the above experiments, all the infections described in this work were carried out using bacteria grown to the stage where cytotoxicity was the most efficient.
FIG. 2.
Growth-phase-dependent cytotoxicity of CHA on J774 macrophages. Bacterial culture was started at 0.1 OD600 unit (●). The percent cytotoxicity was calculated from the release of LDH activity after 1 h (○) and 2 h (◊) of infection. The experiment was repeated twice, and a representative experiment is shown. DO 600nm, optical density at 600 nm.
Characterization of cell death by DNA fragmentation and Hoechst staining.
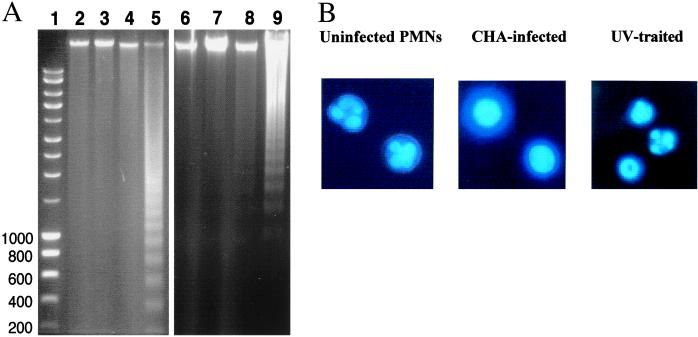
The release of LDH activity into the infected culture supernatants, and the uptake of EtBr reflect the loss of cell membrane integrity but do not provide any information about the mode of cell death. Since several animal pathogens are able to induce apoptosis of macrophages through the activity of type III secreted effectors (see the introduction), CHA-infected PMNs and macrophages were first examined for internucleosomal DNA fragmentation, one of the main indications of apoptosis. Chromosomal DNA, extracted from 3-h-infected PMNs and macrophages, showed no evidence of chromatin cleavage and was indistinguishable from DNA isolated from uninfected cells (Fig. 3A). Similarly to 3 h, at 30 min, 1 h, and 2 h, no evidence of apoptosis was detected (data not shown). As controls, apoptosis was induced in PMNs by a 5-h incubation with actinomycin D and in macrophages by 15 min of UV treatment. DNA isolated from these cells showed a clear 200-bp DNA ladder on agarose gels (Fig. 3A, lanes 5 and 9). Possible changes in nuclear morphology were also assessed by Hoechst staining. As shown in Fig. 3B, uninfected PMNs have a typical polymorphonuclear morphology while PMNs infected with CHA contain nuclei that seem to be perfectly round, uniformly stained, and swollen. No evidence of chromatin condensation, which is characteristic of apoptotic nuclei as seen in UV-treated cells, was observed in CHA-infected PMNs or macrophages. These results suggest that PMNs and macrophages killed by the CHA strain undergo cell death by a mechanism distinct from apoptosis.
FIG. 3.
DNA fragmentation assays. (A) DNA was isolated from J774 macrophages or PMNs and migrated on a 1.5% agarose gel. Lanes: 1, ladder molecular size markers; 2, uninfected J774; 3, CHA-infected J774; 4, CHA-D1-infected J774; 5, J774 treated by UV irradiation for 15 min; 6, uninfected PMNs; 7, CHA-infected PMNs; 8, CHA-D1-infected PMNs; 9, PMNs treated with 50 μM actinomycin D for 5 h. (B) Nuclear morphology of PMNs. At 2 h after infection with the CHA strain, cells were stained with the DNA-specific fluorochrome Hoechst 33342. Uninfected cells showed polymorphic nuclei typical for normal PMNs isolated from blood samples. CHA cells exhibited rounded, uniformly stained, swollen nuclei. Apoptotic cells (UV treated) showed typical highly condensed and fragmented nuclei. Observations were done using epifluorescence microscopy (magnification, ×1,000).
TEM analysis of infected PMNs and macrophages.
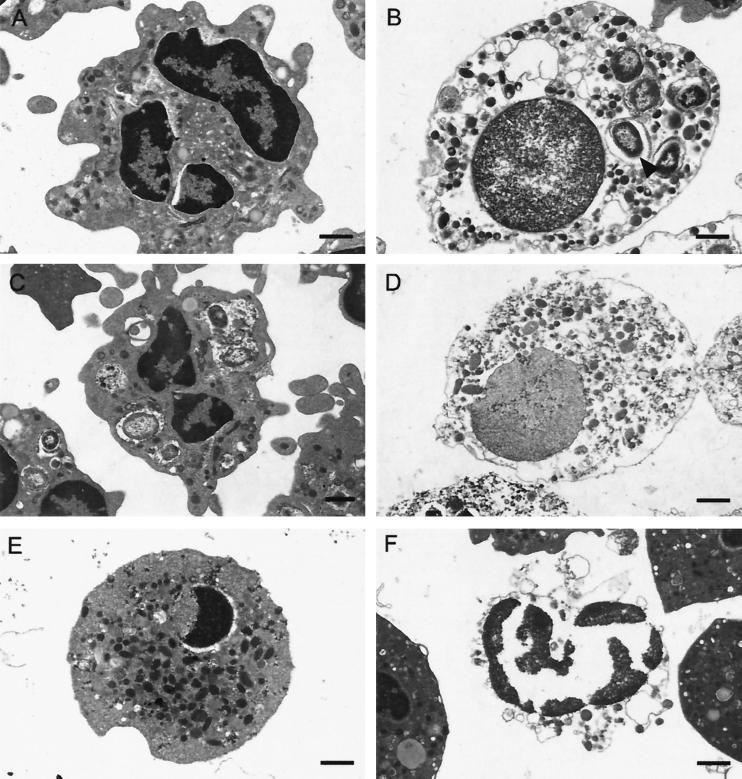
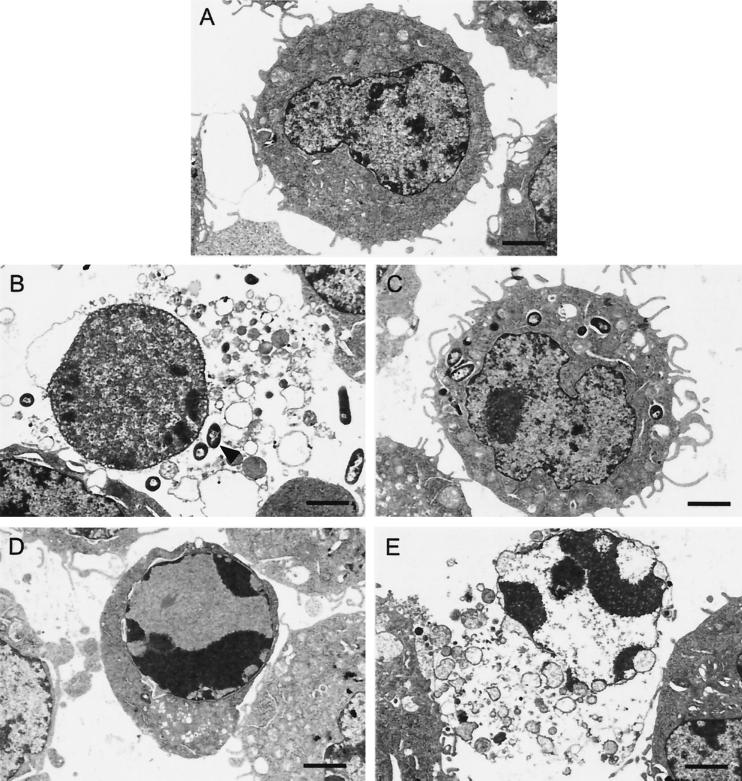
To further characterize the nature of phagocyte cell death mediated by CHA, the ultra-structure of the infected cells was examined by TEM. At 1 h after infection, when the LDH activity had reached approximately 40%, cells were recovered by centrifugation and fixed as described in Materials and Methods. Centrifugation resulted in the recovery of fewer CHA-infected cells than of control uninfected cells or even cells infected with the CHA-D1 mutant, suggesting that CHA-induced cell lysis had occurred. Indeed, preparations of PMNs infected with CHA showed a marked background of cellular debris around the cells, providing confirmation of the existence of considerable cell lysis. CHA-infected PMNs that had not been lysed contained internalized bacteria in numbers similar to those in PMNs infected with the noncytotoxic CHA-D1 mutant. However, CHA-infected PMNs showed an unusual round morphology, with many fewer pseudopodia, compared to PMNs infected with CHA-D1. In addition to the visible cellular debris, in the CHA-infected PMN preparation 3 to 4% of the cells were “ghosts,” with disintegrated plasma membranes, no visible cytoplasm, and dispersed chromatin within the swollen nuclei (Fig. 4B). These morphological changes provide additional evidence that the death of PMNs induced by infection with CHA has none of the features of apoptosis but does have those of oncosis. To further confirm the existence of this type of cell death, PMNs were heated for 30 min at 56°C to provoke accidental cell death. The heat-treated PMNs (Fig. 4D) showed the same morphological changes as did CHA-infected PMNs. In contrast, PMNs in which apoptosis was induced by actinomycin D treatment were shrunken with condensed nuclei, but the plasma membrane was intact. In this preparation, some of the cells underwent lysis but the chromatin still remained condensed.
FIG. 4.
Electron micrographs of PMNs. (A) Uninfected PMNs as a control. (B) PMNs 1 h after infection with the CHA strain, showing features of oncosis: dispersed chromatin within swollen nucleus, vacuolization, and absence of cell membrane. (C) PMNs 1 h after infection with the CHA-D1 strain. (D) PMNs heated for 30 min at 56°C, showing oncotic cell death morphology with the same profile as in the CHA-infected cells. (E and F) PMNs, treated with actinomycin D, showing either early apoptotic morphology with intense perinuclear chromatin aggregation but cytoplasm integrity (E) or late apoptotic morphology characterized by degenerated nuclei, where the chromatin is completely aggregated as in early apoptosis, but dissolution of the cytoplasm (F). The arrow indicates bacteria. Bars, 1 μm.
To confirm the type of cell death in macrophages, we also studied the morphological consequences of CHA infection (Fig. 5). As with PMNs, CHA-infected macrophages showed the characteristics of accidental cell death. The nucleus was entire with dispersed chromatin, the plasma membrane was absent, and some vacuolization could be observed (Fig. 5B). Most of the infected macrophages lacked pseudopodia, whereas these were common in the control cells. UV-treated macrophages that underwent either early or late apoptosis showed all the features of classic apoptosis, with pronounced chromatin condensation. In conclusion, the absence of DNA fragmentation, observations of Hoechst-stained nuclei, and the TEM studies have enabled us to demonstrate that CHA-induced cell death in phagocytes exhibits the features of oncosis.
FIG. 5.
Electron micrographs of J774 cells. (A) Uninfected J774 cells as a control. (B) J774 cells 1 h after infection with CHA, showing oncotic morphology: flocculation of the chromatin, dissolution of the cytoplasm, and swollen nuclei. (C) J774 cells 1 h after infection with the CHA-D1 strain. (D and E) J774 cells treated by UV-irradiation showing either early apoptotic morphology with intense perinuclear chromatin aggregation but cytoplasm integrity (D) or late apoptotic morphology with the nucleus having the same profile as in early apoptosis but dissolution of the cytoplasm (E). The arrow indicates bacteria. Bars, 2 μm.
CF clinical isolates.
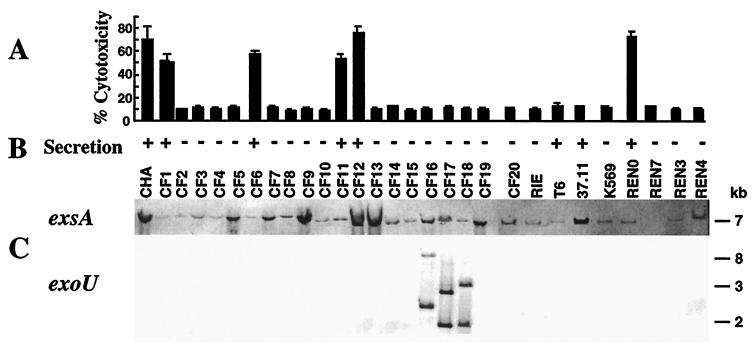
A total of 28 P. aeruginosa CF isolates collected from different patients at the Centre Hospitalier Universitaire, Grenoble, France, were obtained and tested for their ability to induce type III secretion-dependent and ExoU-independent cytotoxicity toward phagocytes. Strains were tested in parallel for the secretion of type III system proteins in vitro (3) and the presence of exsA, as well as the genes exoS, exoT, exoY, and exoU, encoding the four known type III secretion effectors (Fig. 6). The strain CHA was included in this study. Southern blot analysis of chromosomal DNA showed that all strains except one, REN7, possess genes encoding the transcriptional activator ExsA and the effectors ExoT, ExoS, and ExoY. The exoU-specific sequences were detected in only three CF isolates, CF16, CF17, and CF18, in accordance with previous studies concerning strain variability in expression of the ExoU toxin (7, 14). SDS-PAGE profile analysis of proteins secreted into culture media under inducing conditions showed that eight (27.5%) of the isolates, CF1, CF6, CF11, CF12, T6, 37.11, and REN0, as well as CHA, were able to secrete type III secretion system proteins (ExoS, ExoT, PopB, and PopD).
FIG. 6.
Distribution of the exsA and exoU genotypes, in vitro type III secretion ability, and cytotoxicity to PMNs of 29 P. aeruginosa CF isolates. (A) Percent cytotoxicity calculated from the release of LDH activity. (B) In vitro induction of the type III secretion system, measured by SDS-PAGE analysis of culture supernatants. (C) Southern blot analysis of chromosomal DNAs after digestion with EcoRI. The restriction fragment length polymorphism encountered in exoU-containing strains (CF16, CF17, and CF18) is shown. Molecular sizes markers are indicated on the right.
In the cytotoxicity test with PMNs, five isolates, CF1, CF6, CF11, CF12, and REN0, were cytotoxic. These five strains were also able to induce rapid oncosis of J774 macrophages, which was accompanied by cellular and nuclear swelling (data not shown), the morphological changes observed during infection of macrophages by the cytotoxic strain CHA. Strains CF16, CF17, and CF18, which possess the exoU gene, were unable to secrete in vitro and were noncytotoxic. To confirm that the observed cytotoxicity of CF isolates was still due to a type III secretion-mediated mechanism, as is the case for CHA, we constructed ExsA mutants in REN0 and CF1. The same construction used for inactivation of exsA in CHA was used to construct REN0-D1 and CF1-D1 (3). Both the REN0-D1 and CF1-D1 mutants were unable to induce cytotoxicity in PMNs and macrophages, with 9% ± 0.5% and 10.5% ± 0.7% cytotoxicity, respectively. The complemented strains REN0-D1(pDD2) and CF1-D1(pDD2) showed cytotoxicity values of 78% ± 0.4% and 53% ± 5%, respectively, which are similar to the values obtained with parental strains.
Taken together, the analyses of different CF isolates showed that (i) a significant proportion of CF isolates possess genes of the type III secretion system, including exsA, exoS, exoT, and exoY; (ii) 8 out of 29 strains tested were able to secrete type III secretion proteins in vitro; (iii) 6 strains were able to initiate rapid oncosis of phagocytes; and (iv) this phenotype was type III secretion dependent, but ExoU independent.
DISCUSSION
Many gram-negative pathogens use the type III secretion apparatus to deliver bacterial toxins directly to the host cell cytoplasm. It has been postulated that type III-dependent intoxication, resulting in either interruption of eukaryotic signal transduction systems or host cell death, modifies host immune responses, thus allowing pathogen survival and multiplication (2).
In this work we have shown that a P. aeruginosa bronchopulmonary isolate, CHA, from a CF patient induces rapid cell death in professional phagocytes, PMNs, and macrophages in an ExoU-independent manner (with approximately 80% LDH release after 3 h of low-multiplicity infection). This rapid cytotoxicity was dependent on the functional type III secretion system, since a CHA isogenic mutant, CHA-D1, containing the inactivated exsA gene is noncytotoxic. The rapid induction of cell death was associated with bacteria grown to the late exponential phase, and a significant delay in cytotoxicity was observed when a stationary-phase culture was used for infection. In contrast to CHA-infected macrophages and PMNs, B lymphocytes and epithelial HeLa cells start to die only after a prolonged infection of up to 6 h, indicating that there is some cell type specificity in type III system-mediated intoxication. Indeed, the differential sensitivity of epithelial cells to the action of type III-secreted ExoS has been attributed to intrinsic cellular properties (19).
Two distinct modes of eukaryotic cell death may be identified by morphological and biochemical changes. Apoptosis, also called cell death by suicide or programmed cell death, is characterized morphologically by a decrease in cellular size and by condensation of the chromatin into “half-moon” shapes within shrunken nuclei. In most but not all cases of apoptosis, the chromatin is degraded, yielding 200-bp oligosomal DNA. Cellular and nuclear swelling, blebbing, vacuolization, and disintegration of the cell membrane accompany the second type of cell death, known as “accidental” cell death. The term “oncosis” (derived from oncos, meaning swelling) has been adopted to describe this form of cell death (16).
We have clearly demonstrated that phagocytes infected with the CHA strain die by a process distinct from apoptosis. This has been confirmed not only by the absence of DNA fragmentation, but also by Hoechst staining and electron microscopy observations of infected PMNs and macrophages. The mode of cell death in both cell types is identical, with several features of oncosis, such as nuclear and cellular swelling and loss of membrane integrity, which results in dissolution of cellular cytoplasm.
The mechanism by which phagocytes are killed by the CF strain is unknown. The fact that cytotoxicity is dependent on the functional ExsA regulator suggests that a type III secreted effector(s) may be responsible. ExsA controls the synthesis of type III secretion proteins and the translocation machinery (9), as well as the expression of four genes encoding P. aeruginosa type III secreted cytotoxins. Studies of the activity of ExoS and ExoT have shown that these effectors induce actin cytoskeleton rearrangements (10) and provoke visible morphological changes in eukaryotic cells, including cell rounding and absence of microvilli (25, 32). Our observations concerning the absence of pseudopods on cells exposed to CHA indicate that ExoS and ExoT might play some role in phagocyte intoxication. However, an ExoS-deficient mutant had the same kinetics of cytotoxicity as did the parental strain, CHA (data not shown), in agreement with published data showing that ExoS and ExoT were never associated with rapid cell death (2 h of infection). Similarly, the expression of ExoY, an adenylate cyclase, leads to pronounced morphological changes in epithelial CHO and HeLa cells but not to cellular death (32, 35). The only type III effector that is synthesized by some clinical isolates and is able to provoke rapid cell death in eukaryotic cells is ExoU (7, 14), but the genome of the CHA strain does not contain exoU.
Two recent reports describe the ExoU-independent acute cytotoxicity of P. aeruginosa strains toward cultured cells. Hauser and Engel (13) showed that the exoU isogenic mutant of PA103 was capable of inducing apoptosis in macrophages and some epithelial cells at a high MOI of 160 and after a long incubation (6 h of infection). The authors suggest the presence of a novel type III secreted toxin. While the present work was in progress, Coburn and Frank (1) reported the ExoU-independent killing of bone marrow-derived macrophages from A/J mice by P. aeruginosa strain 388 at a low MOI. Similarly to the effects of CHA toward the HeLa epithelial cell line, strain 388 provoked morphological changes in the lung carcinoma-derived A549 cell line without causing significant cell death. Although the type of 388-induced cell death in macrophages has not yet been described, it is possible that strains CHA and 388 use the same mechanism of macrophage killing.
Our survey of 29 P. aeruginosa strains (including the CHA strain) isolated from different CF patients shows that the ExoU-independent rapid induction of phagocyte cell death is a phenotype associated with approximately 21% of CF isolates. The cell death of phagocytes induced by CF isolates was characterized as oncosis, with the same features as described for the CHA strain. This process seems to be, in all cases, type III secretion dependent, since cytotoxic activity was completely abolished in two exsA mutants of cytotoxic isolates (CF1-D1 and REN0-D1). Southern blot analysis showed that only 3 of 29 isolates contain the ExoU-encoding gene, suggesting that exoU is the main variable trait in CF isolates. It is important to note that ExoU is expressed in most isolates from corneal infections (7) but is present in only few strains isolated from patients with acute pneumonia (14). In contrast to corneal P. aeruginosa isolates (8), in the P. aeruginosa CF population, 28 out of 29 isolates that possess exsA are also able to encode ExoS. However, although the exsA-regulated genes encoding type III system-secreted effectors are present, only eight isolates show functional type III secretion, as indicated by in vitro secretion of ExoS, ExoT, PopB, and PopD (data not shown). Of the eight strains able to secrete type III system proteins in vitro, six are cytotoxic, suggesting that a functional type III secretion is necessary but not sufficient to induce acute injury of macrophages and PMNs. It is possible that in two intoxication-negative strains (T6 and 37.11) the translocation complex, necessary for delivery of type III effectors, is not functional in our cellular model of infection. In addition, we cannot exclude the possibility that type III secretion-dependent intoxication occurs in vivo even with strains that are secretion deficient and noncytotoxic in vitro. There may exist specific conditions during CF infections that allow the expression of type III secretion genes and hence the occurrence of the cytotoxic phenotype.
Our results, together with reports from other laboratories, show that P. aeruginosa strains have developed versatile mechanisms for host cell intoxication. This may be due to different combinations of toxin genes present in clinical isolates and/or the differential expression of certain genes, depending on the cell type and growth conditions. The variety of type III system-induced phenotypes of P. aeruginosa isolates may explain, in part, the ability of this opportunistic pathogen to cause different types of infections, including severe chronic respiratory infection in CF patients.
The rapid oncosis of professional phagocytes by cytotoxic P. aeruginosa might be an important strategy for pathogen survival. Indeed, we have shown that the cytotoxic CHA strain and isogenic noncytotoxic mutant are equally well ingested by PMNs and macrophages. However, only the cytotoxic strain is able to escape the bactericidal activity and to multiply (3). In CF patients, chronic respiratory infections and associated host inflammatory responses are the leading cause of morbidity and mortality. There are reports of an excessive influx of phagocytes (mostly PMNs) at the site of infection (17), which are unable to eliminate bacteria. In contrast, PMNs show an uncontrolled release of toxic mediators, contributing to widespread tissue destruction (4, 27). This work, which shows that CF clinical isolates are able to induce type III-dependent oncotic cell death in phagocytes, will be followed by experiments in animal models of P. aeruginosa infection to find whether this phenotype contributes to the virulence of the pathogen and the persistence of the infection.
ACKNOWLEDGMENTS
This work was supported by grants 97044 and 98033 from the Association Française de Lutte contre la Mucoviscidose (AFLM).
We thank J. Chabert and L. Quénée for technical assistance, J. M. Meyer for CF clinical isolates (T6, 37.11 and K569), J. Plumas for human macrophages, J. Garin and S. Kieffer (Laboratoire de Chimie des Proteines, DBMS, CEA, Grenoble) for mass spectrometry analysis, and A. Chapel for J774 macrophages. Thanks are due to A. Colbeau, W. Dischert, and O. Attrée for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Coburn J, Frank D W. Macrophages and epithelial cells respond differently to the Pseudomonas aeruginosa type III secretion system. Infect Immun. 1999;67:3151–3154. doi: 10.1128/iai.67.6.3151-3154.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dacheux D, Attree I, Schneider C, Toussaint B. Cell death of human polymorphonuclear neutrophils induced by a Pseudomonas aeruginosa cystic fibrosis isolate requires a functional type III secretion system. Infect Immun. 1999;67:6164–6167. doi: 10.1128/iai.67.11.6164-6167.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elborn J S, Shale D J. Lung injury in cystic fibrosis. Thorax. 1990;45:970–973. doi: 10.1136/thx.45.12.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Prada C, Tall B D, Elliott S E, Hoover D L, Nataro J P, Venkatesan M M. Hemolysin-positive enteroaggregative and cell-detaching Escherichia coli strains cause oncosis of human monocyte-derived macrophages and apoptosis of murine J774 cells. Infect Immun. 1998;66:3918–3924. doi: 10.1128/iai.66.8.3918-3924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Prada C M, Hoover D L, Tall B D, Venkatesan M M. Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytolytic event similar to oncosis but not apoptosis. Infect Immun. 1997;65:1486–1496. doi: 10.1128/iai.65.4.1486-1496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M, Wu C, Mende-Mueller L, Frank D W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 8.Fleiszig S M, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K E, Kanada D, Sawa T, Yen T S, Frank D W. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank D W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 10.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol Microbiol. 1997;25:1125–1139. doi: 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 11.Galan J E. Interaction of Salmonella with host cells through the centisome 63 type III secretion system. Curr Opin Microbiol. 1999;2:46–50. doi: 10.1016/s1369-5274(99)80008-3. [DOI] [PubMed] [Google Scholar]
- 12.Galan J E, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 13.Hauser A R, Engel J N. Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect Immun. 1999;67:5530–5537. doi: 10.1128/iai.67.10.5530-5537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauser A R, Kang P J, Engel J N. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- 15.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 17.McElvaney N G, Nakamura H, Birrer P, Hebert C A, Wong W L, Alphonso M, Baker J B, Catalano M A, Crystal R G. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Investig. 1992;90:1296–1301. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuffie E M, Frank D W, Vincent T S, Olson J C. Modification of Ras in eukaryotic cells by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1998;66:2607–2613. doi: 10.1128/iai.66.6.2607-2613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuffie E M, Fraylick J E, Hazen-Martin D J, Vincent T S, Olson J C. Differential sensitivity of human epithelial cells to Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1999;67:3494–3503. doi: 10.1128/iai.67.7.3494-3503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menard R, Dehio C, Sansonetti P J. Bacterial entry into epithelial cells: the paradigm of Shigella. Trends Microbiol. 1996;4:220–226. doi: 10.1016/0966-842X(96)10039-1. [DOI] [PubMed] [Google Scholar]
- 21.Mills S D, Boland A, Sory M P, van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nonaka T, Kuwae A, Sasakawa C, Imajoh-Ohmi S. Shigella flexneri YSH6000 induces two types of cell death, apoptosis and oncosis, in the differentiated human monoblastic cell line U937. FEMS Microbiol Lett. 1999;174:89–95. doi: 10.1111/j.1574-6968.1999.tb13553.x. [DOI] [PubMed] [Google Scholar]
- 24.Olson J C, Fraylick J E, McGuffie E M, Dolan K M, Yahr T L, Frank D W, Vincent T S. Interruption of multiple cellular processes in HT-29 epithelial cells by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1999;67:2847–2854. doi: 10.1128/iai.67.6.2847-2854.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson J C, McGuffie E M, Frank D W. Effects of differential expression of the 49-kilodalton exoenzyme S by Pseudomonas aeruginosa on cultured eukaryotic cells. Infect Immun. 1997;65:248–256. doi: 10.1128/iai.65.1.248-256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pederson K J, Vallis A J, Aktories K, Frank D W, Barbieri J T. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol Microbiol. 1999;32:393–401. doi: 10.1046/j.1365-2958.1999.01359.x. [DOI] [PubMed] [Google Scholar]
- 27.Pier G B. Pseudomonas aeruginosa: a key problem in cystic fibrosis. ASM News. 1998;64:339–347. [Google Scholar]
- 28.Salyers A A, Whitt D D. Bacterial pathogenesis: a molecular approach. Washington, D.C.: ASM Press; 1994. [Google Scholar]
- 29.Sawa T, Yahr T L, Ohara M, Kurahashi K, Gropper M A, Wiener-Kronish J P, Frank D W. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med. 1999;5:392–398. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]
- 30.Tilly J L, Hsueh A J. Microscale autoradiographic method for the qualitative and quantitative analysis of apoptotic DNA fragmentation. J Cell Physiol. 1993;154:519–526. doi: 10.1002/jcp.1041540310. [DOI] [PubMed] [Google Scholar]
- 31.Toussaint B, Delic-Attree I, Vignais P M. Pseudomonas aeruginosa contains an IHF-like protein that binds to the algD promoter. Biochem Biophys Res Commun. 1993;196:416–421. doi: 10.1006/bbrc.1993.2265. [DOI] [PubMed] [Google Scholar]
- 32.Vallis A J, Finck-Barbancon V, Yahr T L, Frank D W. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect Immun. 1999;67:2040–2044. doi: 10.1128/iai.67.4.2040-2044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yahr T L, Barbieri J T, Frank D W. Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J Bacteriol. 1996;178:1412–1419. doi: 10.1128/jb.178.5.1412-1419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yahr T L, Hovey A K, Kulich S M, Frank D W. Transcriptional analysis of the Pseudomonas aeruginosa exoenzyme S structural gene. J Bacteriol. 1995;177:1169–1178. doi: 10.1128/jb.177.5.1169-1178.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yahr T L, Vallis A J, Hancock M K, Barbieri J T, Frank D W. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]