Abstract
To elucidate the mechanism of the high incidence of lower respiratory tract infections in patients with diabetes mellitus, we investigated the kinetics of production of macrophage inflammatory protein 2 (MIP-2), an important mediator of lung neutrophil recruitment, using mice with streptozotocin-induced diabetes. Intratracheal challenge with 1 mg of lipopolysaccharide (LPS), an endotoxin, per kg of body weight resulted in a time-dependent increase in the levels of MIP-2 protein in bronchoalveolar lavage (BAL) fluid, with the peak concentration (49.4 ± 13 ng/ml) occurring at 3 h and significant neutrophil accumulation becoming apparent by 3 h in normal mice. In diabetic mice, the peak level of MIP-2 protein in BAL fluid did not occur until 6 h and was reduced to 21.9 ± 10 ng/ml. Immunohistochemical studies using anti-MIP-2 antibody confirmed that the main cellular source of MIP-2 in the lung after LPS challenge was alveolar macrophages (AMs) in normal mice. The lungs in diabetic mice, however, showed no AMs staining for MIP-2 within 3 h after LPS challenge. PCR analysis using whole-lung RNA showed a time-dependent increase in MIP-2 mRNA levels after LPS instillation. The level of MIP-2 mRNA in diabetic mice was markedly decreased compared to that in normal mice. Our results indicate that impairment of MIP-2 mRNA expression in the AMs in diabetic mice resulted in delayed neutrophil recruitment in the lungs, and this may explain the development and progression of pulmonary infection in diabetes mellitus.
Diabetes mellitus is often identified as an independent risk factor for the development of lower respiratory tract infections (13). The available literatures suggest two patterns of susceptibility to such infections in the diabetic host. First, certain types of pulmonary infections may occur with an increased frequency in diabetic patients (24, 28). Second, although certain pulmonary infections do not occur with increased frequency, they may be associated with increased morbidity and mortality in diabetic patients (18).
Effective host defense against lung bacterial infection is dependent primarily on the rapid clearance of the organism from the respiratory tract (23). Early bacterial clearance is mediated by a dual phagocytic system involving both neutrophils and macrophages. Recruitment and activation of inflammatory cells at the site of infection is closely related to a family of chemotactic cytokines (17, 30). Interleukin-8 (IL-8) appears to be a key C-X-C chemokine involved in neutrophil recruitment in a number of inflammatory conditions such as pneumonia (8). Whether IL-8 production and neutrophil recruitment are suppressed in diabetic patients with pneumonia is not yet clear.
Macrophage inflammatory protein-2 (MIP-2) is a member of the murine C-X-C chemokine subfamily, which has been considered a functional analogue of human IL-8 (22, 29). Furthermore, MIP-2 is an important mediator of lung neutrophil recruitment, bacterial clearance, and mortality in a murine model of pneumonia and severe sepsis (5, 27). Based on these observations, we hypothesized that chemokine expression might be significantly decreased during lower respiratory tract infections in diabetic mice. To test this hypothesis, we developed a murine model of diabetes and then induced lower respiratory tract inflammation in this model by intratracheal instillation of lipopolysaccharide (LPS). LPS is present in the walls of gram-negative bacteria and is a potent stimulus component for acute inflammation. Using the streptozotocin-induced diabetic mouse model, we examined the expression of MIP-2 and neutrophil counts in bronchoalveolar lavage (BAL) fluid.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free, 5-week-old male S1c:ICR mice were obtained from Charles River Agricultural Cooperative Association for Laboratory Animals, Kanagawa, Japan. The mice were provided with sterile food and water ad libitum in an environmentally controlled room. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Nagasaki University School of Medicine. Experimental diabetes was induced by a single intraperitoneal injection of streptozotocin (Sigma Chemical Co., St. Louis, Mo.) (300 mg/kg of body weight) in 0.1 M citrate buffer (pH 4.5). Control mice received an equal volume of citrate buffer without streptozotocin. At 48 h and 10 days after streptozotocin injection, the blood glucose level was measured with a Glucocard (Kyoto Daiichi Kagaku Co., Kyoto, Japan) and Glutest sensor (Sanwa Chemical Co., Nagoya, Japan). Only mice with a fasting blood glucose level of >16 mmol/liter were considered diabetic and used in the following experiments.
LPS inoculation.
Ten days after administration of streptozotocin, each group was anesthetized with sodium pentobarbital intraperitoneally (60 mg/kg), and the trachea was cannulated after tracheostomy. LPS (1.0 mg/kg of body weight) from Escherichia coli O111:B4 (Sigma) was instilled through a cannula into the trachea.
BAL.
BAL was performed at 0, 1, 3, 6, 12, and 24 h after LPS challenge in each group of five mice. Under deep anesthesia, the trachea was exposed and intubated. A 2.5-ml syringe was connected to the tracheal cannula, and the lungs were washed four times with 2 ml of Ca2+- and Mg2+-free phosphate buffered saline (PBS) at 4°C. A 1.5-ml volume of BAL fluid was recovered consistently. The cell counts in recovered BAL fluid were determined with a hemocytometer. BAL fluid was centrifuged at 150 × g for 10 min at 4°C, and the cell pellet was resuspended in 1.0 ml of PBS. Cell morphology was determined on cell monolayers prepared by Cytospin 2 (Shandon Southern Products, Astmoor, England) and stained with Diff-Quik. Recovered BAL fluid cells at 0 h were used for analysis of CD14 expression. Recovered BAL fluid supernatant was filter sterilized and stored at −80°C until used later.
Expression of CD14 in AMs.
Almost all the BAL fluid cells recovered at 0 h were alveolar macrophages (AMs) (>99%). Immunofluorescence-activated cell flow cytometric analysis was performed on collected AMs. The cells (adjusted to 5 × 104 cells) were washed twice with PBS and resuspended in 50 μl of 2% bovine serum albumin–0.5 mM EGTA–10 mM NaN3 solution into a polystyrene tube. Next, 2 μl of fluorescein isothiocyanate-conjugated rat anti-mouse CD14 monoclonal antibody (PharMingen, San Diego, Calif.) was added, and the cells were incubated for 30 min in total darkness at 4°C, washed in 1 ml of cold 0.5 mM EGTA–10 mM NaN3 solution, and resuspended in cold PBS. Stained cells were analyzed on FACScan (Becton Dickinson Co., Oxford, United Kingdom). Fluorescein isothiocyanate-conjugated rat immunoglobulin G1 (IgG1) κ isotype antibody (PharMingen) was used as a control antibody.
MIP-2 ELISA.
The concentrations of murine MIP-2 in BAL fluid supernatant were determined by a sandwich enzyme-linked immunosorbent assay (ELISA), as described previously (21). A 96-well flat-bottom microtiter plate was coated with 100 μl of rabbit anti-mouse MIP-2 IgG (2 μg/ml in 0.05 M carbonate buffer [pH 9.6]) per well for 16 h at 4°C, and the wells were washed with PBS (pH 7.5)–0.05% Tween 20 (wash buffer) three times. Nonspecific binding sites were blocked with 1% bovine serum albumin in PBS, and the plates were incubated for 120 min at 37°C. The plates were rinsed five times with a wash buffer, diluted cell-free BAL fluid samples or standards (100 μl/well) in duplicate were added, and the plates were incubated for 24 h at 4°C. The plates were washed five times, 100 μl of biotinylated rabbit anti-MIP-2 IgG (2 μg/ml in 0.5% bovine serum albumin in PBS) per well was added, and the plates were incubated for 2 h at 37°C. The plates were washed five times, 100 μl of streptavidin-alkaline phosphatase (Gibco-BAL; 1:2,000 dilution in 0.5% bovine serum albumin in PBS) per well was added, and the plates were incubated for 2 h at 37°C. The plates were washed again five times, and 100 μl of p-nitrophenyl phosphate (Sigma; 1 mg/ml in 1 M diethanolamine [pH 9.8]) per well was added. The plates were read at 405 nm after 30 min in an ELISA reader.
Lung harvesting for histologic examination.
At the designated time points, a mouse was sacrificed by deep anesthesia and both lungs were harvested for histologic examination. Once the lungs were removed, they were inflated with 0.5 ml of 4% paraformaldehyde in PBS. After 48 h, they were embedded in paraffin.
Immunohistochemical localization of antigenic MIP-2.
Paraffin-embedded specimens of whole lungs were cut into 3-μm sections, placed on silane-coated slides, dewaxed with xylene, and dehydrated through graded concentrations of ethanol. The tissue was then treated with 0.03% trypsin for 1 h. This procedure makes more antigenic sites available to the antibody. In the next step, the tissue was placed in 3% hydrogen peroxide for 5 min to reduce endogenous peroxidase activity. Non-tissue-specific binding sites were blocked with normal swine serum in PBS for 30 min. Excess serum was removed by blotting, and sections were covered overnight with a 1:5 dilution of rabbit polyclonal anti-murine MIP-2 antibody or control rabbit IgG at 4°C. After being washed with PBS, the sections were covered with the biotinylated second antibody (swine anti-rabbit IgG) for 40 min, rinsed in PBS, covered with peroxidase-anti-peroxidase (Dako Co., Santa Barbara, Calif.) reagent for 40 min at room temperature, and rinsed in PBS. Antigenic sites on sections were demonstrated by reacting these sections with a mixture of 0.05% 3,3′-diaminobenzidine tetrahydrochloride in 0.05 M Tris-HCl buffer and 0.01% hydrogen peroxide for 7 min. The sections were then counterstained with methyl green for 10 min, dehydrated in ethanol, cleaned in xylene, and mounted.
Isolation and reverse transcription-PCR amplification of whole-lung mRNA.
Whole lungs were harvested at specific times after inoculation with LPS and immediately homogenized with Isogen (Wako Pure Chemical Co., Osaka, Japan), and total cellular RNA was extracted. Purified RNA was quantitated by measuring the absorbance at 260 nm. cDNA was synthesized from 2 μg of total RNA by priming with 2.5 μmol of oligo(dT) primers, 1 mM each deoxynucleoside triphosphate, and reverse transcriptase. cDNA equivalent to 80 ng of starting RNA was used for each PCR with primers for mouse MIP-2 or β-actin. The primers used were as follows. The MIP-2 sense and antisense primers were 5′-GCTGGCCACCAACCACCAGG-3′ and 5′-AGCGAGGCACATCAGGTACG-3′, respectively, yielding an amplified product of 350 bp; and the β-actin sense and antisense primers were 5′-ATGGATGACGATATCGCTC-3′ and 5′-GATTCCATACCCAGGAAGG-3′, respectively, yielding an amplified product of 812 bp. PCR was performed with 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 1 mM deoxynucleoside triphosphates, and 2.5 U of Taq DNA polymerase (Perkin-Elmer, Branchburg, N.J.) in a final volume of 100 μl. Primers were added at a final concentration of 0.1 μmol. The reactions were carried out in a DNA thermal cycler (Perkin-Elmer), first incubated for 5 min at 94°C and then cycled 35 times under the following conditions: denaturing at 93°C for 45 s, annealing at 52°C for 45 s, and extension at 72°C for 80 s. After amplification, the sample was separated on 1% agarose gel containing 0.3 mg of ethidium bromide per ml, and bands were visualized and photographed with UV transillumination. The photographic negative was then converted to a photographic positive.
Statistical analysis.
Values are expressed as mean ± standard error of the mean (SEM). Differences between groups were examined for statistical significance using the unpaired (two-tailed) t test. A P value of <0.05 denoted the presence of a significant statistical difference.
RESULTS
Diabetic mice.
Blood glucose concentrations in diabetic and control mice were 23.7 ± 3.0 and 7.2 ± 0.6 mmol/liter, respectively, 10 days after streptozotocin treatment. The mean body weights of diabetic and control mice were 25.6 ± 2.3 and 29.2 ± 1.3 g, respectively.
Kinetics of LPS-stimulated airway leukocyte influx.
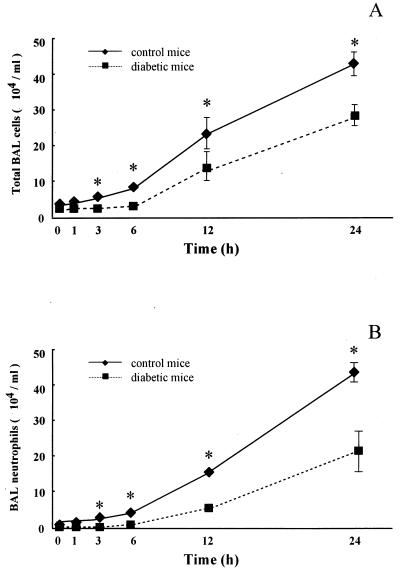
First, we investigated the kinetics of LPS-stimulated neutrophil influx into the airways. Baseline BAL fluid of control mice contained 1.63 × 104 ± 0.25 × 104 cells/ml with a differential count of 99.1% ± 1.0% AMs. In diabetic mice, the number and differential count of BAL fluid cells (2.00 × 104 ± 0.41 × 104 cells/ml with 98.6% ± 0.8% AMs) were not different at baseline from those of the control. The BAL fluid cell count increased progressively after LPS challenge. However, the counts were lower in diabetic mice at each time point than in control mice (P < 0.05) (Fig. 1A). The analysis of BAL fluid cells at each time point revealed that the increase was composed of only neutrophils, with a negligible change in the number of AMs.
FIG. 1.
Kinetics of airway leukocyte influx in response to intratracheal endotoxin challenge. LPS (1.0 mg/kg of body weight) was instilled into the trachea, and BAL was performed at 0, 1, 3, 6, 12, and 24 h after LPS challenge in control and diabetic mice. (A) The total cell number in BAL fluid was determined using a hemocytometer. (B) Absolute neutrophil recovery was calculated from a differential count obtained from Cytospin preparations stained with Diff-Quik stain. Each data point represents the mean and SEM of results from five mice. ∗, P < 0.05 between control and diabetic mice at the corresponding time intervals.
Neutrophil influx into the airway in normal mice was apparent at 3 h (0.49 × 104 ± 0.14 × 104 cells/ml) after LPS challenge and increased progressively to 82-fold (40.3 × 104 ± 3.91 × 104 cells/ml) at 24 h. However, neutrophil influx in diabetic mice was not detected for 3 h. Neutrophil influx into the airway in diabetic mice was apparent at 6 h (0.14 × 104 ± 0.04 × 104 cells/ml). Neutrophil counts were lower in diabetic than control mice at 3, 6, 12, and 24 h after instillation of LPS. Furthermore, the percent differences in neutrophil number at 6, 12, and 24 h were 5.8, 41.1, and 58.7% of the control (Fig. 1B).
LPS-stimulated airway MIP-2 production.
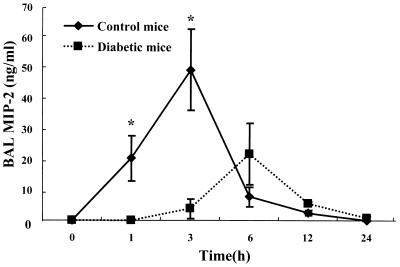
To assess the differences in neutrophil influx into the airways in control and diabetic mice, we determined the concentration of MIP-2 in BAL fluid. Immunoreactive MIP-2 levels in BAL fluid of normal mice were detected at 1 h, reached a peak concentration at 3 h (49.4 ± 13.3 ng/ml), and diminished thereafter (Fig. 2). Interestingly, the peak level of MIP-2 in BAL fluid preceded neutrophil influx. In diabetic mice, MIP-2 levels were below the detection limit in BAL fluid at 1 h and the peak concentration was shifted from 3 to 6 h relative to that of the control and was only 58% of the control peak concentration.
FIG. 2.
Kinetics of MIP-2 production in response to intratracheal endotoxin challenge. Immunoreactive MIP-2 was measured in BAL fluid at each time point by ELISA. Each data point represents the mean and SEM of results from five mice. ∗, P < 0.05 between control and diabetic mice at the corresponding time intervals. The conditions of LPS administration are the same as in Fig. 1.
Immunohistochemical studies.
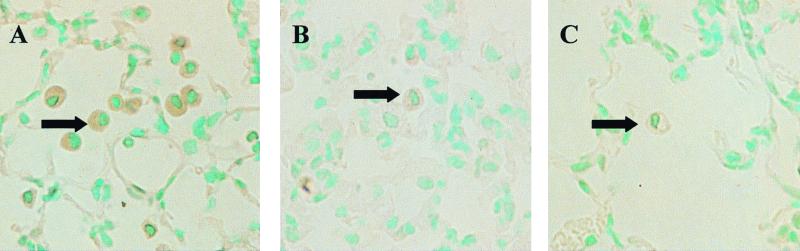
To determine the cellular source of LPS-induced MIP-2 in the murine lung, we examined the tissue by immunohistochemistry using anti-MIP-2 antibodies. In control mice, cell-associated MIP-2 was present within AMs in the lungs only at 3 h after LPS challenge (Fig. 3A). In contrast, the lungs of diabetic mice showed no staining for MIP-2 (Fig. 3B). Staining for MIP-2 was specific, since no staining was present in sections of lungs incubated with purified IgG from control serum (Fig. 3C).
FIG. 3.
(A and B) Immunohistochemical staining of murine lungs for MIP-2 antigen 3 h after intratracheal instillation of LPS in control (A) and diabetic (B) mice. (C) Control section incubated with control serum. The arrows point to AMs. Note that AMs immunoreactive with MIP-2 are present only within the lungs of control mice. The lung of the diabetic mouse showed no AMs staining for MIP-2. Staining for MIP-2 was specific, since no staining was present in sections of the lung incubated with purified IgG from control serum. Magnification, ×200. The conditions of LPS administration are the same as in Fig. 1.
Flow cytometry for CD14 expression on AMs.
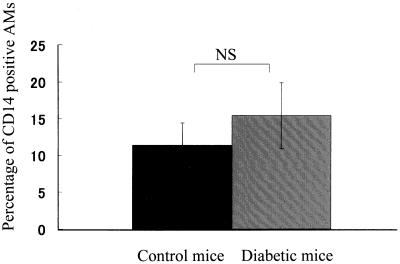
CD14 acts as a potential receptor of LPS or an LPS–LPS-binding protein (LBP) complex and is involved in MIP-2 production. Based on the LPS-induced MIP-2 production from AMs, we investigated the difference in CD14 expression on AMs between control and diabetic mice by flow cytometry (Fig. 4). The mean fluorescence intensity on AMs from BAL fluid was 11.4% ± 3.0% in normal mice and 15.4% ± 4.5% in diabetic mice. The expression of CD14 on AMs was not significantly different between the two groups. These findings suggested that the defective MIP-2 production in diabetic mice was not due to changes in LPS receptor levels.
FIG. 4.
Comparison of CD14 expression on AMs before LPS challenge between control mice and diabetic mice by using flow cytometry. Data are mean and SEM of five independent experiments. NS, not significant.
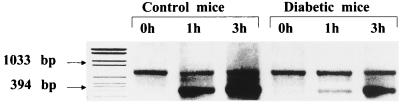
Time-dependent production of MIP-2 mRNA within the lungs after intratracheal inoculation with LPS.
To investigate whether the suppression of LPS-stimulated MIP-2 production in diabetic mice occurred at a particular mRNA level, reverse transcription-PCR was performed using mRNA extracted from whole lung. In control mice, a marked increase of MIP-2 mRNA expression in the lung homogenates was observed within 3 h after LPS challenge. In contrast, only a slight increase in MIP-2 mRNA expression at 1 h postchallenge was shown in diabetic mice (Fig. 5). The level of MIP-2 mRNA expression in diabetic mice was also decreased at 3 h postchallenge compared to control mice.
FIG. 5.
Time-dependent production of MIP-2 mRNA in lung homogenates after instillation of LPS. Total RNA obtained from the lungs before and after LPS challenge was prepared at each time point (0, 1, and 3 h). Reverse transcription-PCR analysis was performed using MIP-2 and β-actin primers. The top band shows the PCR product of β-actin, and the bottom band shows the PCR product of MIP-2. The conditions of LPS administration are the same as in Fig. 1.
DISCUSSION
The most clinically evident defect in immune system function in diabetes mellitus is increased susceptibility to infection, which is mostly evident in the frequency and severity of pulmonary infection (19). Normal resistance to lung bacterial infection requires rapid clearance of organisms by normal phagocytic and intracellular killing by neutrophils and macrophages (3, 13, 16, 20). AMs not only act as phagocytes but also function as potent initiating cells of inflammation by releasing various cytokines like tumor necrosis factor alpha (TNFα), IL-1β, and IL-8 (10). Therefore, AMs play an important role in the regulation of inflammatory reactions in the lungs. On the other hand, altered neutrophil function, including impaired chemotaxis, phagocytosis, bactericidal activity, and superoxide production, has been described in diabetic patients (2, 9). These studies on immune system cell function have been performed with peripheral blood cells, and these data may not be representative of pulmonary immune cell function. Whether the specific characteristics and function of pulmonary immune cells from patients with diabetes mellitus differ from those of cells from nondiabetic patients is not known.
The mechanism by which AMs mediate neutrophil influx and activation of these cells in response to inflammatory stimuli is not clear but is likely to be dependent on the expression of specific macrophage-derived neutrophil chemotactic and activating cytokines (1, 6). Among these, IL-8 has been recently recognized as an important neutrophil chemotactic and activating peptide (8, 17, 30). The effect of diabetes on AMs is not well characterized, and studies of the function of primary pulmonary defense cells obtained from BAL fluid from diabetic patients are limited. However, it is important to recognize the clinical effect and significance of various in vitro abnormalities described in immune system cells. In this study, we examined the kinetics of MIP-2, a member of the C-X-C chemokine family in mice, and the kinetics of neutrophil recruitment in a murine streptozotocin-induced diabetes model with intratracheal instillation of LPS. LPS is the most important inducer of lung inflammation during infection by gram-negative bacteria. Our results showed that the time-dependent expression of MIP-2 mRNA and protein within the lungs was delayed and significantly suppressed during a 24-h period after LPS instillation in diabetic mice. In addition, the suppression of MIP-2 in vivo results in a significant delay in neutrophil recruitment to the lungs. An effective antibacterial host defense requires the rapid recruitment and activation of neutrophils. Ulich et al. (25) demonstrated that antiserum to CINC (rat C-X-C chemokine) inhibited acute inflammation in a rat model of septicemia induced by intratracheal administration of endotoxin. Furthermore, Greenberger et al. (5) demonstrated that MIP-2 was an important mediator of lung neutrophil recruitment and bacterial clearance in Klebsiella pneumonia. They also showed that inhibition of MIP-2 bioactivity in vivo resulted in reduced neutrophil influx, reduced lung bacterial clearance, and reduced survival in the early period of infection in animals with Klebsiella pneumonia (5). We believe that the delayed neutrophil influx observed in our model may allow the bacteria to grow in the lungs. In support of this hypothesis, we found in a series of preliminary studies that diabetic mice were unable to recover from a sublethal dose (107 CFU/mouse) of immunotype 1 Pseudomonas aeruginosa instilled intratracheally, since 90% of diabetic mice died within 24 h compared to 0% in the control group (n = 10 per group). On the other hand, the concentrations of murine TNF-α in BAL fluid supernatant collected from LPS-exposed diabetic and control mice were determined by a sandwich ELISA. In diabetic mice, the TNF-α concentration was reduced at every time point and the peak concentration was 17.7% of the control level (diabetic mice, 0.66 ± 0.14 ng/ml; control mice, 3.73 ± 1.06 ng/ml [P < 0.05]). TNF-α enhances neutrophil and AM bacterial killing in vitro (10, 12). These factors (MIP-2 and TNF-α reduction) may contribute, at least in part, to the severity of pneumonia in the diabetic mice.
In this study, we demonstrated that the cellular source of MIP-2 was AMs, as shown in sections immunohistochemically stained for MIP-2. Production of MIP-2 by AMs was suppressed and delayed in diabetic mice after intratracheal LPS challenge. LPS activates macrophages via both CD14-dependent and CD14-independent pathways (4, 7). In our study, CD14 expression on alveolar macrophages was not significantly different between normal and diabetic mice. Therefore, any difference in stimulation by LPS of macrophages was not mediated on the CD14 receptor level. Glycation-dependent reactive oxygen species decrease the DNA binding activity of an insulin gene transcription factor, Pox-1/1PF-1/STF-1 (14). Furthermore, long-term exposure to high glucose concentrations impairs the responsive activation of NF-κB by IL-1β and TNF-α in mouse endothelial cells (26). These findings suggest that hyperglycemia may alter the regulation of some transcription factors. A crucial transcriptional factor that regulates the expression of the MIP-2 gene is NF-κB, which is part of a family of dimeric transcriptional factors. Through its dissociation from its inhibitor, IκB, it transcriptionally activates various cellular genes, including the TNF-α and IL-8 genes (11, 12, 14, 15). MIP-2 gene expression on murine AMs may be attenuated and deleted as a result of impairment of NF-κB activation by diabetes, by the same mechanism as glycation-dependent reactive oxygen species.
In summary, we have shown in the present study that diabetes mellitus is associated with reduced LPS-induced MIP-2 production by AMs at the mRNA and protein levels. These results indicate that impairment of MIP-2 gene expression in AMs results in delayed neutrophil recruitment to the lungs and may be a cause of the development and progression of pulmonary infections in patients with diabetes mellitus.
ACKNOWLEDGMENTS
We are grateful to Keiko Tagawa, Yoko Terai, and Mai Yanase for excellent technical assistance.
REFERENCES
- 1.Berg J T, Lee S T, Thepen T, Lee C Y, Tsan M F. Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphanate. J Appl Physiol. 1993;74:2812–2819. doi: 10.1152/jappl.1993.74.6.2812. [DOI] [PubMed] [Google Scholar]
- 2.Delamaire M, Maugendre D, Moreno M, Le Goff M C, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabetic Med. 1997;14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Fels A O S, Cohn Z A. The alveolar macrophage. J Appl Physiol. 1986;60:353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- 4.Fenton M J, Golenbock D T. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Greenberger M J, Strieter R M, Kunkel S L, Danforth J M, Laichalk L L, Gillicuddy D C, Standiford T J. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto S, Pittet J F, Hong K, Folkesson H, Bagby G, Kobzik L, Frevert C, Watanabe K, Tsrufuji S, Wiener-Kronish J. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol. 1996;270:L819–L824. doi: 10.1152/ajplung.1996.270.5.L819. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins H A, Monick M M, Hunninghake G W. Lipopolysaccharide upregulates surface expression of CD14 on human alveolar macrophages. Am J Physiol. 1995;269:L849–L854. doi: 10.1152/ajplung.1995.269.6.L849. [DOI] [PubMed] [Google Scholar]
- 8.Inoue H, Hara M, Massion P, Grattan K M, Lausier J A, Chan B, Kaneko K, Isono K, Jorens P G, Ueki I F, Nadel J A. Role of recruited neutrophils in interleukin-8 production in dog trachea after stimulation with Pseudomonas in vivo. Am J Respir Cell Mol Biol. 1995;13:570–577. doi: 10.1165/ajrcmb.13.5.7576693. [DOI] [PubMed] [Google Scholar]
- 9.Inoue S, Lan Y, Muran J, Tsuji M. Reduced hydrogen peroxide production in neutrophils from patients with diabetes. Diabetes Res Clin Pract. 1996;33:119–127. doi: 10.1016/0168-8227(96)01287-9. [DOI] [PubMed] [Google Scholar]
- 10.Kolls J K, Nelson S, Summer W R. Recombinant cytokines and pulmonary host defense. Am J Med Sci. 1993;306:330–335. doi: 10.1097/00000441-199311000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Kunsch C, Lang R K, Rosen C A, Shannon M F. Synergistic transcriptional activation of IL-8 gene by NF-κB p65(Rel A) and NF-IL6. J Immunol. 1994;153:153–164. [PubMed] [Google Scholar]
- 12.Le J, Vilcek J. Tumor necrosis factor and interleukin-1: cytokines with multiple overlapping biological activities. Lab Investig. 1987;56:234–248. [PubMed] [Google Scholar]
- 13.Mackowiak P, Martin R, Smith J. The role of bacterial interference in the increased prevalence of oropharyngeal gram-negative bacilli among alcoholics and diabetics. Am Rev Respir Dis. 1979;120:589–593. doi: 10.1164/arrd.1979.120.3.589. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka T, Kajimoto Y, Watada H, Kaneto H, Kishimoto M, Umayahara Y, Fujitani Y, Kamada T, Kawamori R, Yamasaki Y. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Investig. 1997;99:144–150. doi: 10.1172/JCI119126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcriptional factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan C F. Secretory products of macrophages. J Clin Investig. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene intercrine cytokine family. Annu Rev Immunol. 1991;9:617–647. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 18.Robbins S L, Tucker A W., Jr The cause of death in diabetes: a report of 307 autopsied cases. N Engl J Med. 1944;231:865. [Google Scholar]
- 19.Sasaki A. Mortality and cause of death in patients with diabetes mellitus in Japan. Diabetes Res Clin Pract. 1994;24(Suppl.):S299–S306. doi: 10.1016/0168-8227(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 20.Sibille Y, Reynolds H Y. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 21.Sonoda Y, Mukaida N, Wang J B, Shimada-Hiratsuka M, Naito M, Kasahara T, Harada A, Inoue M, Matsushima K. Physiologic regulation of postovulatory neutrophil migration into vagina in mice by a C-X-C chemokine(s) J Immunol. 1998;160:6159–6165. [PubMed] [Google Scholar]
- 22.Tekamp-Olson P, Galegos C, Bauer D, McClain J, Sherry B, Fabre M, van Deventer S, Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990;172:911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toews G B, Gross G N, Pierce A K. The relationship of inoculum size to lung bacterial clearance and phagocytic cell response in mice. Am Rev Respir Dis. 1979;120:559–566. doi: 10.1164/arrd.1979.120.3.559. [DOI] [PubMed] [Google Scholar]
- 24.Tuazon C U, Perez A, Kishaba T, Sheagren J N. Staphylococcus aureus among insulin-injecting diabetic patients. JAMA. 1975;231:1272. [PubMed] [Google Scholar]
- 25.Ulich T R, Howard S C, Remick D G, Wittwer A, Yi E S, Yin S, Guo K, Welply J K, Williams J H. Intratracheal administration of endotoxin and cytokines antiserum to CINC inhibits acute inflammation. Am J Physiol. 1995;268:L245–L250. doi: 10.1152/ajplung.1995.268.2.L245. [DOI] [PubMed] [Google Scholar]
- 26.Urata H, Yamamoto H, Goto S, Tsushima H, Akazawa S, Yamashita S, Nagataki S, Kondo T. Long exposure to high glucose concentration impairs the responsive expression of r-glutamylcysteine synthetase by interleukin-1 and tumor necrosis factor-α in mouse endothelial cells. J Biol Chem. 1996;271:15146–15152. doi: 10.1074/jbc.271.25.15146. [DOI] [PubMed] [Google Scholar]
- 27.Walley K R, Lukacs N W, Standiford T J, Streieter R M, Kunkel S L. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun. 1997;65:3847–3851. doi: 10.1128/iai.65.9.3847-3851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winterbauer R, Bedon G, Ball W. Recurrent pneumonia: predisposing illness and clinical pattern of 158 patients. Ann Intern Med. 1969;70:689. doi: 10.7326/0003-4819-70-4-689. [DOI] [PubMed] [Google Scholar]
- 29.Wolpe S, Sherry B, Juers D, Davatelis G, Yurt R W, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolpe S D, Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989;3:2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]