Background:
This study aimed to systematically analyze the association between long-term use of proton pump inhibitors (PPIs) and the risk of gastric cancer (GC).
Methods:
We performed a systematic search of articles on the relationship between long-term use of PPIs and the risk of GC from PubMed and EMBASE. We calculated the pooled odds ratio of GC in PPI users compared to non-PPI users using random-effects models.
Results:
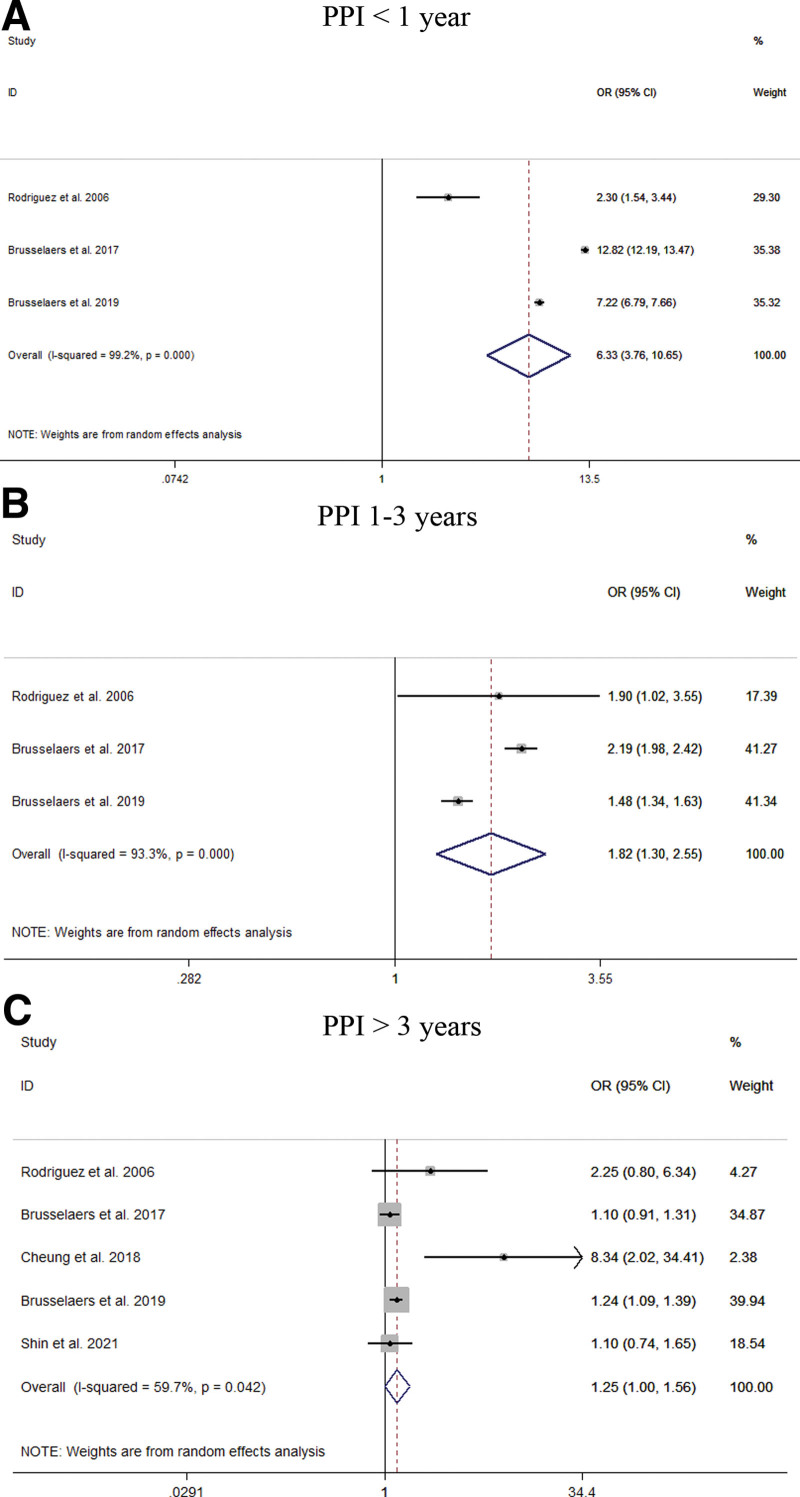
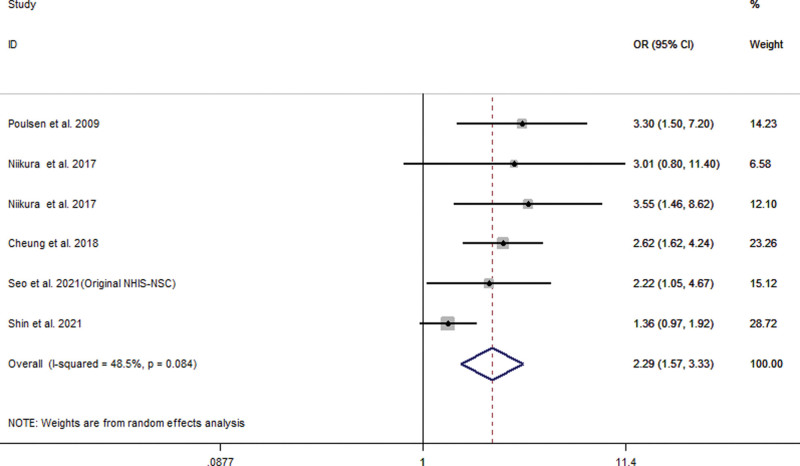
This meta-analysis included 18 studies from 20 different databases with 4348,905 patients enrolled. In the random effects model, we found that an increased risk of GC among PPI users (OR = 1.94; 95% CI [1.43, 2.64]). The long-term use of PPIs compared with histamine-2 receptor antagonist users did not increase the risk of GC (OR = 1.65; 95% CI [0.92, 2.97]). Stratified analysis showed that PPI users had a significantly increased risk of noncardia GC (OR = 2.53; 95% CI [2.03, 3.15]), but had a relatively small relationship with the risk of gastric cardia cancer. (OR = 1.79; 95% CI [1.06, 3.03]). With the extension of PPI use time, the estimated risk value decreases (<1 year: OR = 6.33, 95% CI [3.76, 10.65]; 1–3 years: OR = 1.82, 95% CI [1.30, 2.55]; >3 years: OR = 1.25, 95% CI [1.00, 1.56]). Despite Helicobacter pylori eradication, the long-term use of PPIs did not alter the increased risk of GC (OR = 2.29; 95% CI [1.57, 3.33]).
Conclusion:
Our meta-analysis found that PPI use may be associated with an increased risk of GC. Further research on the causal relationship between these factors is necessary.
Keywords: gastric cancer, histamine-2 receptor antagonist, meta-analysis, proton pump inhibitor, risk
1. Introduction
Gastric cancer (GC) is among the most prevalent cancers worldwide. According to the 2018 GLOBOCAN report, its incidence ranks fifth among all cancers and its mortality ranks third among cancers worldwide.[1] However, there are large differences in the epidemiological trends of GC among different regions. The incidence and mortality rates of GC in East Asia are the highest, whereas those in North America and Northern Europe are low.[2] Helicobacter pylori (H. pylori) has been listed as a class I carcinogen by the World Health Organization and is the most common risk factor for GC.[3] The main risk factors for cardia GC include obesity and gastroesophageal reflux disease, while the main risk factors for noncardia GC include H. pylori infection, high salt intake, and smoked food intake.[4] It is estimated that nearly 90% of noncardia GC cases (75% of all GC) can be attributed to H. pylori infection.[5] However, H. pylori eradication cannot completely prevent the development of GC. Therefore, it is necessary to identify the potential risk factors for GC.[6]
Acid-suppressing drugs include proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs). PPIs are irreversible inhibitors of gastric H+/K+/ATP enzymes in parietal cells. They are the most common prescription drugs in the world.[7] PPIs are more effective inhibitors of gastric acid secretion than H2RAs. They are widely used in the treatment of acid-related upper gastrointestinal diseases, including gastroesophageal reflux disease, peptic ulcer and H. pylori infection. However, a large number of observational studies have raised concerns about adverse events related to PPIs, which can cause several conditions, including intestinal infection, GC, overgrowth of bacteria in the upper gastrointestinal tract, and imbalance in intestinal microbiota.[8] The long-term use of PPIs can accelerate or induce the progression of gastric atrophy and lead to hypergastrinemia, thus increasing the risk of GC.[9,10] Although previous meta-analyses have elaborated on the risk relationship between PPIs and GC, the results have been controversial. In recent years, research on their relationship has been ongoing, and a large amount of new data can be used to further analyze the relationship between PPIs and GC.
2. Method
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[11]
2.1. Data source and search strategy
All studies published up to January 2022 were searched from Embase and PubMed using MeSH terms and free words. Comprehensive search terms are listed in Appendix 1, Supplemental Digital Content, http://links.lww.com/MD/I90.
2.2. Study selection
The 2 authors (HG and LL) independently selected articles to be included and conducted critical assessments. Discrepancies were resolved by reaching a consensus with the senior author (YZ). Eligible studies were selected according to the following inclusion criteria: they are studies on the relationship between PPIs and GC, the incidence of GC in the control and case groups can be extracted when exposed to or not exposed to PPIs, the odds ratio (OR) or relative risk and 95% confidence interval (95% CI) can be directly or indirectly calculated. The exclusion criteria are The literature cannot provide valid data, the repeated study of the same population sample, and the secondary data analysis literature such as review or meta-analysis.
2.3. Data extraction
Data extraction was independently completed by the 2 researchers (HQ Gao and LN Li)). First of all, after the thorough review of the title and abstract, obtain the full-text literature which meets the inclusion criteria. Two sides discuss for the controversial literature and seek the third senior researcher (YZ) to make a final decision if controversies were meet. The following data were extracted from each article: first author’s name, year of publication, study country, study type, control group, H. pylori infection status and number of control and case groups, as well as related measurement indicators (OR or relative risk and 95% CI).
2.4. Quality assessment
Two authors (HG and LL) independently evaluated the quality of the articles. Discrepancies were resolved by reaching a consensus with the senior author (YZ). The quality of the literature was evaluated using the Newcastle-Ottawa Scale.[12] The scale was evaluated from study population selection (4 points), intergroup comparability (2 points), and exposure or outcome evaluation (3 points). The total score is 9 points, with higher scores indicating better methodological quality.
2.5. Risk of bias assessment
The 2 authors (HG and LL) performed the risk assessment of the included studies. Any disputes or inconsistencies were discussed in the group to achieve a consistent result. The risk of bias was evaluated by the ROBINS-I (Risk Of Bias In Nonrandomized Studies—of Interventions) tool.[13] Based on the risk of bias of 7 different domains, the overall bias risk for each outcome and study was estimated. It is divided into 3 parts: preintervention (bias due to confounding, bias in selection of participants into the study), at intervention (bias in classification of interventions), and postintervention (bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported result).
2.6. Statistical analysis
The relationship between PPIs and GC was summarized by the binary method. OR and 95% CI were calculated for each study. To investigate the sources of heterogeneity, we carried out the following tests: heterogeneity tests, subgroup analysis, and sensitivity analysis. The heterogeneity of each study was statistically analyzed by Q test and I2 test. When I2 was 0 to 40%, it means littler no heterogeneity, 30 to 60% means moderate heterogeneity, 50 to 90% indicates substantial heterogeneity, and 75 to 100% indicates considerable heterogeneity.[14] The random effects model is used to pool the analysis,[15] and subgroup analysis is carried out according to the factors that may cause heterogeneity (study type, control group, H. pylori infection status, and GC site, etc). Sensitivity analysis verifies the stability of the results by eliminating each study one by one. Assessment of potential publication bias was made using Peters regression and funnel plots. Data collation and analysis were performed using Stata13.1 software.
3. Result
3.1. Study selection and characteristics
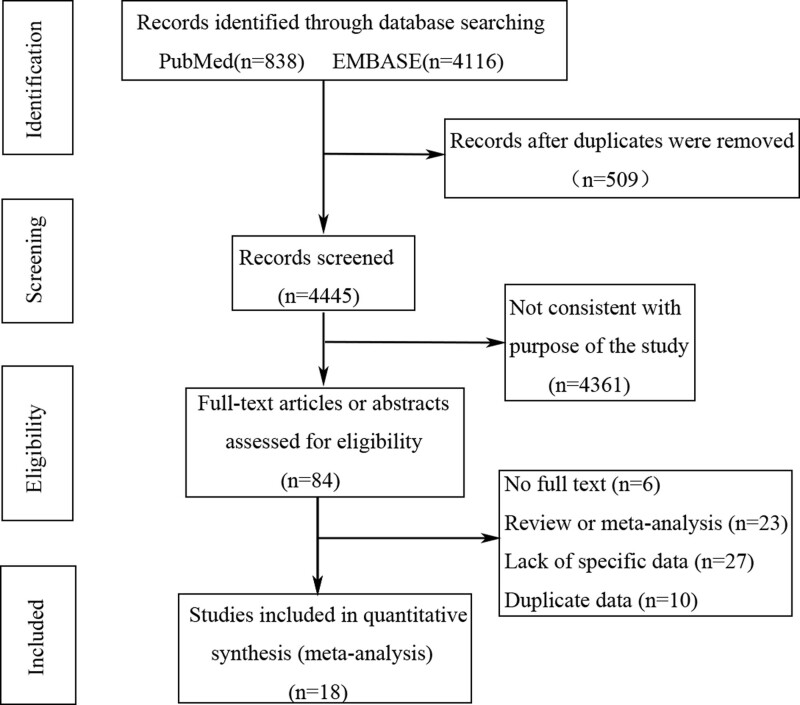
Based on the inclusion and exclusion criteria, 18 studies were included in our analysis.[16–33] The flow diagram of the systematic review is shown in Figure 1. Table 1 summarizes the baseline characteristics of the included studies. There were a total of 20 different databases. These studies were classified according to different research databases, including 8 case–control studies[21–24,28,31–33] and 12 cohort studies.[16–21,25–27,29,30] The studies were conducted in different regions: 7 from Europe (the UK, Denmark, and Sweden),[16,21,25,28–30,33] 3 from North America (the USA and Canada),[22,31,32] and 8 from Asia (Japan, China [Hong Kong], China [Taiwan], Republic of Korea, and Israel).[17–20,23,24,26,27] Nine of these studies compared the effect of H2RA users as a control group on GC risk.[16–18,23,25,26,28–30] Another 13 studies used non-PPI users as controls.[18–22,24,26,27,31–33] Most studies did not report the type and dose of PPIs. With regard to the status of H. pylori infection, 3 studies showed that it had been completely eradicated in the study population,[18,26,27] 5 studies showed partial eradication,[16–19,30] 2 studies showed partial infection of the study population,[24,29] and the other 11 studies did not report the infection status.[18,20–23,25,28,31–33] According to the Newcastle Ottawa Scale, eleven studies were ranked as very good,[16–19,21,22,24,27,29,30] 8 as good,[20,21,23,25,26,28,31,33] and one as satisfactory[32](Table S1, Supplemental Digital Content, http://links.lww.com/MD/I87).
Figure 1.
Flowchart showing selection of publications for review.
Table 1.
Characteristics of literatures included in the meta-analysis.
| First author | Area | Study type | Observation period | Control group | Exposure data(T/C) | Control data(T/C) | Hp infection status |
|---|---|---|---|---|---|---|---|
| Rodriguez et al 2006 | Britain | Case–control | 1994–2001 | Non-PPI users | 442/43 | 9851/464 | Not reported |
| Crane et al 2007 | America | Case–control | 1971–2000 | Non-PPI users | 11/2 | 231/119 | Not reported |
| Tamim et al 2008 | Canada | Case–control | 1995–2003 | Non-PPI users | 1299/234 | 6930/837 | Not reported |
| Poulsen et al 2009 | Denmark | Cohort | 1990–2003 | H2RA users | 18790/109 | 17478/52 | Partial eradication |
| Brusselaers et al 2017 |
Sweden | Cohort | 2005–2012 | H2RA users | 797067/2219 | 20210/12 | Partial infection |
| Wennerstrom et al2017 |
Denmark | Case-control | 1995–2011 | H2RA users | 1179202/1347 | 384658/703 | Not reported |
| Niikura et al 2017 |
Japan | Cohort | 1998–2017 | Non-PPI users | 118/13 | 415/8 | Total eradication |
| Niikura et al 2017 |
Japan | Cohort | 1998–2017 | H2RA users | 118/13 | 38/3 | Total eradication |
| Cheung et al 2018 |
China Hongkong | Cohort | 2003–2012 | Non-PPI users | 3271/19 | 60126/134 | Total eradication |
| Peng et al 2019 | China Taiwan | Case-control | 1996–2011 | H2RA users | 1693/- | 429/- | Not reported |
| Brusselaers et al 2019 |
Sweden | Cohort | 2005–2012 | H2RA users | 796492/- | N/A | Not reported |
| Lai et al 2019 | China Taiwan | Case-control | 2000–2013 | Non-PPI users | 539/308 | 759/341 | Partial infection |
| Lee et al 2020 | America | Case-control | 1996–2016 | Non-PPI users | 937/164 | 10839/1069 | Not reported |
| Liu et al 2020 | Britain (PCCIU) | Case-control | 1999–2011 | Non-PPI users | 1542/329 | 6513/1119 | Not reported |
| Liu et al 2020 | Britain (UK Biobank) | Cohort | 1999–2014 | Non-PPI users | 46146/44 | 425633/206 | Not reported |
| Seo et al 2021 | Korean (NHIS-CDM database) | Cohort | 2002–2013 | Non-PPI users | 11741/118 | 11741/40 | Partial eradication |
| Seo et al 2021 | Korean (NHIS-CDM database) | Cohort | 2002–2013 | H2RA users | 5067/9 | 5067/6 | Not reported |
| Seo et al 2021 | Korean (NHIS-NSC database) | Cohort | 2002–2013 | Non-PPI users | 3256/- | 3256/- | Total eradication |
| Kei-Yan Ng et al 2021 |
China Hongkong | Cohort | 2004–2017 | Non-PPI users | 6738/17 | 6738/7 | Partial eradication |
| Gingold-Belfer et al 2021 | Israel | Cohort | 2002–2016 | Non-PPI users | 11008/72 | 40397/125 | Not reported |
| Shin et al 2021 | Korean | Cohort | 2004–2015 | H2RA users | 38512/421 | 38512/396 | Partial eradication |
| Abrahami et al 2022 | Britain | Cohort | 1990–2018 | H2RA users | 973281/1166 | 198306/244 | Partial eradication |
PPI: proton pump inhibitor, H2RA: histamine 2 receptor antagonist, Hp: Helicobacter pylori, T: total number, C:cases.
3.2. Risk of bias within studies
According to the ROBINS-I tool, 2 studies had a serious risk of bias,[20,26] twelve studies had a moderate risk of bias,[19,21–25,28,30–33] and 6 of the other studies had a low risk of bias[16–18,27,29] (Table S2, Supplemental Digital Content, http://links.lww.com/MD/I88).
3.3. Relationship between PPIs and risk of GC
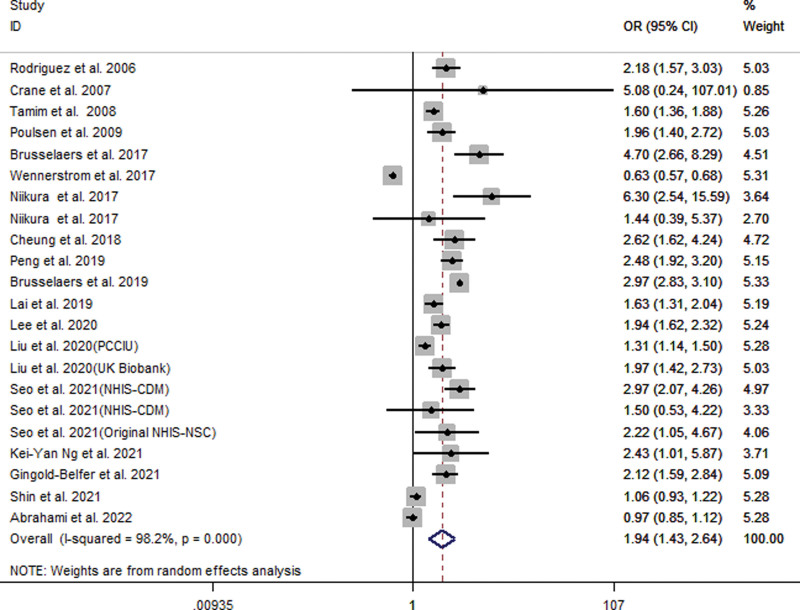
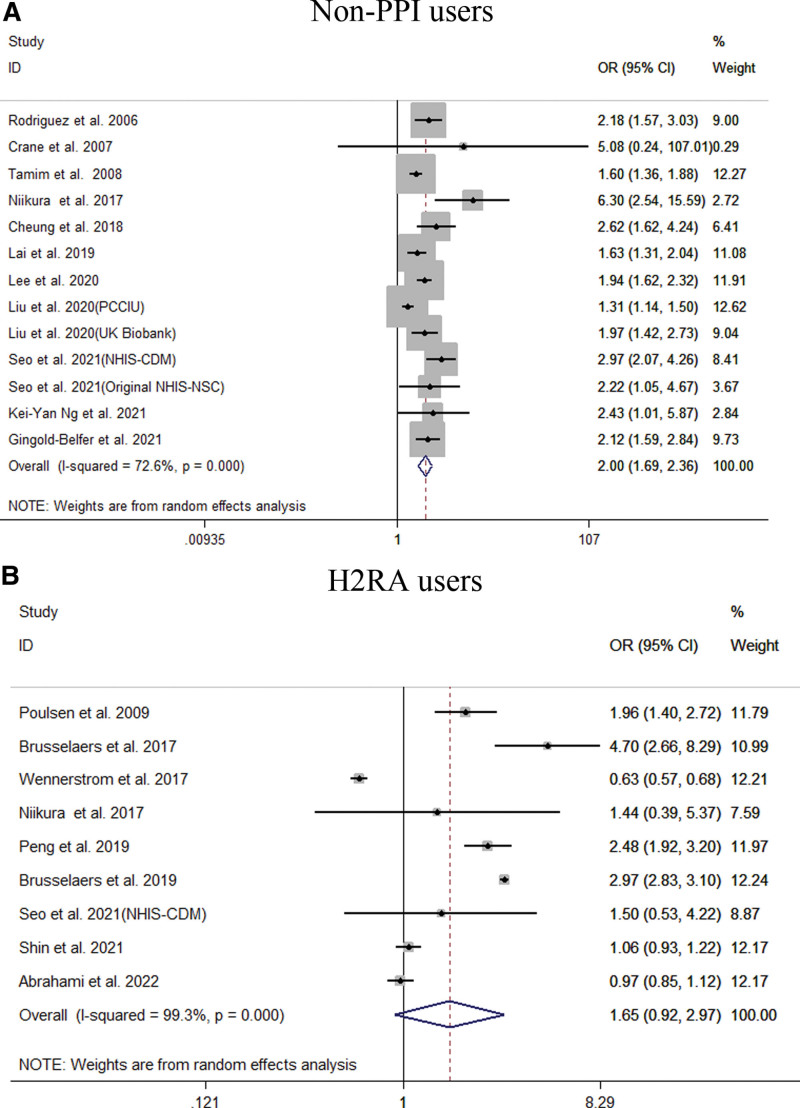
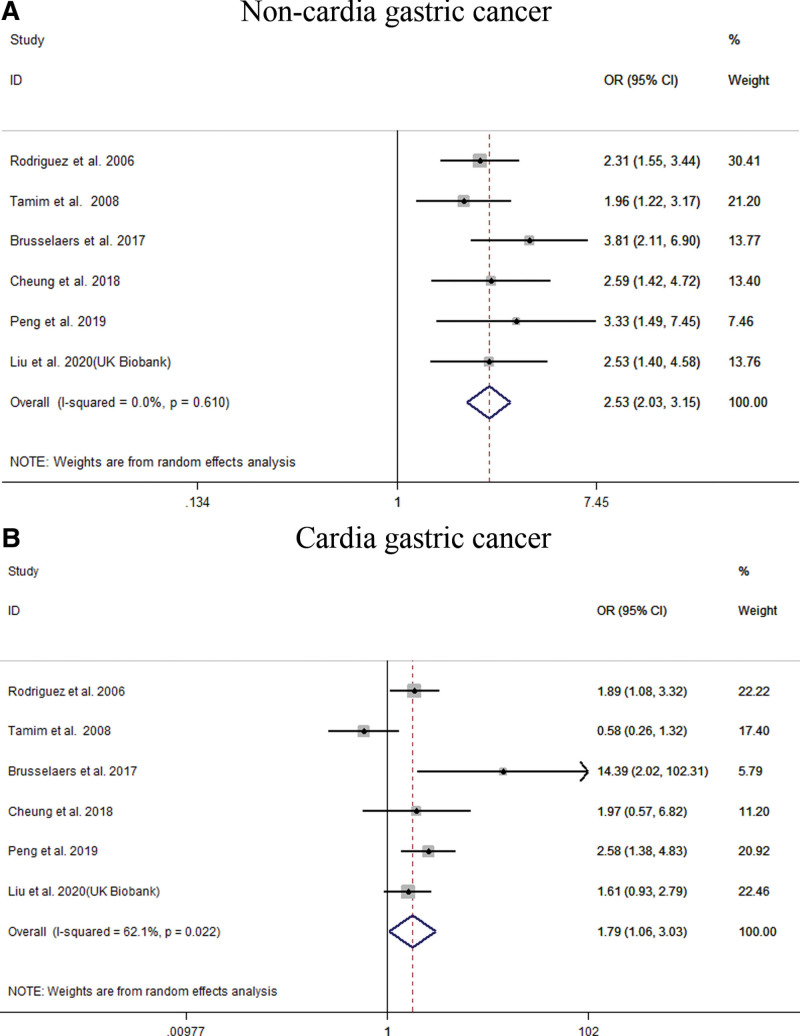
Our meta-analysis of all 22 studies showed that PPI users had an increased risk of GC (OR = 1.94; 95% CI [1.43, 2.64]), as shown in Figure 2. Subgroup analysis showed that long-term use of PPIs did not increase the risk of GC compared to H2RA (OR = 1.65; 95% CI [0.92, 2.97]). Compared with non-PPI users, PPI users had a significantly increased risk of GC (OR = 2.00; 95% CI [1.69, 2.36]), as shown in Figure 3. When grouped based on the study type, significant differences were found in the increase in GC risk among PPI users (case-control study: OR = 1.58; 95% CI [1.03, 2.43]; cohort study: OR = 2.18; 95% CI [1.53, 3.12]). In addition, there were 6 studies[21,23,27,29,31,33] that could perform a subgroup analysis of the location of GC. The results showed that the use of PPIs was more closely associated with noncardia GC (OR = 2.53; 95% CI [2.03, 3.15]), while it had a relatively weak relationship with cardia GC (OR = 1.79; 95% CI [1.06, 3.03]), as shown in Figure 4. Subgroup analysis of the duration of PPI use showed that the duration of PPI use did not show a time-dependent trend (<1 year: OR = 6.33, 95% CI [3.76, 10.65]; 1–3 years: OR = 1.82, 95% CI [1.30, 2.55]; >3 years: OR = 1.25, 95% CI [1.00, 1.56]), as shown in Figure 5. Regarding the effect of H. pylori infection status on the relationship between PPI use and GC risk, our meta-analysis found that 6 studies[17,18,26,27,30] performed a statistical analysis of the post-bacterial population. The results showed that despite H. pylori eradication, the increased risk of long-term use of PPIs for GC could not be changed (OR = 2.29; 95% CI [1.57, 3.33]), as shown in Figure 6. In addition, we used the method of eliminating each study individually to analyze the sensitivity and evaluate the impact of a research result on the overall results. The results showed that after excluding one study, the pooling effect of the remaining studies was the same as the original total pooling effect, and the OR value of each group was similar to that before excluding one study. None of the studies had a significant impact on the total pooling effect. This confirms the stability of our results.
Figure 2.
Meta-analysis of the association between long-term use of proton pump inhibitors and the risk of gastric cancer. Each horizontal bar summarizes a study. Bars represent 95% CIs. Gray squares inform on each of the studies’ weight in the meta-analysis. Diamond in the lower part of the graph depicts the pooled estimate along with 95% CIs.
Figure 3.
Meta-analysis of the association between long-term use of proton pump inhibitors and the risk of gastric cancer stratified by control group. Each horizontal bar summarizes a study. Bars represent 95% CIs. Gray squares inform on each of the studies’ weight in the meta-analysis. Diamond in the lower part of the graph depicts the pooled estimate along with 95% CIs.
Figure 4.
Meta-analysis of the association between long-term use of proton pump inhibitors and the risk of gastric cancer (GC) stratified by GC site. Each horizontal bar summarizes a study. Bars represent 95% CIs. Gray squares inform on each of the studies’ weight in the meta-analysis. Diamond in the lower part of the graph depicts the pooled estimate along with 95% CIs.
Figure 5.
Meta-analysis of the association between long-term use of proton pump inhibitors and the risk of gastric cancer stratified by duration of PPI use. Each horizontal bar summarizes a study. Bars represent 95% CIs. Gray squares inform on each of the studies’ weight in the meta-analysis. Diamond in the lower part of the graph depicts the pooled estimate along with 95% CIs.
Figure 6.
Meta-analysis of the association between long-term use of proton pump inhibitors and the risk of gastric cancer after Helicobacter pylori eradication. Each horizontal bar summarizes a study. Bars represent 95% CIs. Gray squares inform on each of the studies’ weight in the meta-analysis. Diamond in the lower part of the graph depicts the pooled estimate along with 95% CIs.
3.4. Publication bias
Publication bias was tested using Peters regression combined with funnel plots. The results of the funnel plot showed asymmetric signs (Figure S1, Supplemental Digital Content, http://links.lww.com/MD/I89), suggesting that publication bias may exist. However, when we used the trim and fill method to identify and correct the asymmetry of the funnel plot caused by publication bias, there was no possibility of performing the fill statistics, indicating that no publication bias was detected. Peters’ test showed that the P-value was 0.908, indicating that the difference was not statistically significant, and there was no significant publication bias in the whole study.
4. Discussion
We aimed to compare the risk relationship between the long-term use of PPIs and GC. This meta-analysis was based on 18 studies from 20 different databases with 4348,905 patients, which makes the current study much larger than previous studies. We found that the overall risk of GC significantly increased with the long-term use of PPIs compared to the control group. According to the stratification of the control group, there was no significant difference between the long-term use of PPIs and that of H2RAs, while the risk of GC was significantly increased compared with non-PPI users.
This may be because PPIs or H2RAs can reduce gastric acid by modulating competitive inhibitors of H+/K+/ATP enzymes or histamine-binding sites in gastric parietal cells. The resulting decrease in gastric acid levels may lead to increased bacterial colonization and an increase in the number of bacteria producing nitrosamines[34,35] and compounds associated with an increased risk of gastric adenocarcinoma.[36] In vivo and in vitro studies have shown that nitrosamine compounds promote gastric tumorigenesis by upregulating prostaglandin receptor subtypes EP2 and EP4, stimulating vascular endothelial growth factor and angiogenesis, and inhibiting apoptosis.[37] In addition, the decrease in acidity opens the positive feedback of the gastric acid cascade, triggers the release of gastrin, and stimulates enterochromaffin-like (ECL) cells to release histamine.[38] If acid secretion does not return to a level sufficient to counteract this reflex, it leads to hypergastrinemia. Hypergastrinemia occurs rapidly after PPIs use, and 80 to 100% of patients develop hypergastrinemia after long-term use of PPIs.[39–42] Gastrin also stimulates ECL cell proliferation and increases the incidence of atrophic gastritis and gastric polyps, which are precancerous lesions of GC.[43,44] Although there is current evidence that tumor-related growth effects are caused by hypergastrinemia and that gastrin plays a role in the growth or pathogenesis of human chronic hypergastrinemia, this evidence is not convincing and remains controversial. The ability of PPI use to cause chronic hypergastrinemia and changes in gastric ECL cell proliferation in humans or GC in rodents has not been demonstrated in humans with long-term use of PPIs for < 5 years. The experimental results of the effect of gastrin on the growth of many other cancers have also sparked a debate about its long-term and lifelong safety. Additionally, the National Institute of Diabetes and Digestive and Kidney Diseases argues that chronic hypergastrinemia does not increase the risk of GC.[45]
In addition, according to the stratification of the location of GC, the long-term use of PPIs significantly increased the risk of noncardia GC, but there was no significant increasing trend in the risk of cardia GC. The etiology of GC differs from that of noncardiac GC. Most noncardia GCs are related to peptic ulcers and chronic mucosal infections caused by H. pylori,[33] whereas cardia GC is mostly related to gastroesophageal reflux, obesity, smoking, alcoholism, and low intake of fruits and vegetables. PPIs are suitable for the treatment of gastroesophageal reflux disease.[46] Therefore, many patients with Barrett’s esophagus receive PPIs therapy. The incidence of dysplasia was lower in patients with Barrett’s esophagus treated with PPIs, which may weaken the effect of PPIs on cardiac carcinogenesis.[47,48] This may explain the relationship between the long-term use of PPIs and the risk of noncardia GC. However, recent data suggest that some patients with Barrett’s esophagus are more likely to develop adenocarcinoma due to the hypergastrinemia caused by PPIs use upon reaching a certain gastrin threshold level.[49] In vitro studies have shown that gastrin interacts with CCK2R and increases cyclooxygenase-2, induces apoptosis, prostaglandin E2 release, and leads to Barrett’s esophagus cell proliferation.[50] Prostaglandins can lead to the final progression of adenocarcinoma by stimulating cell division, inducing angiogenesis, and inhibiting apoptosis.[51] At present, the number of relevant studies on the relationship between the long-term use of PPIs and the risk of gastric cardia cancer is still limited, and its potential adverse consequences cannot be ignored.
As we all know, H. pylori has been listed as class I carcinogen. It is estimated that at least 50% of the world’s population is infected.[52] Meining et al[53] suggested that H. pylori may lead to the formation of gastric adenocarcinoma by causing severe gastric inflammation and complete gastric atrophy. Studies have shown that gene polymorphisms of proinflammatory mediators (IL-1B, IL-01RN, IL-10, TNF-α) increased the risk of noncardia GC in the case of H. pylori infection.[54] Our study found that the long-term use of PPIs increased the risk of GC even after H. pylori eradication. On one hand, it may lead to the overgrowth of non-H. pylori bacteria due to the acid-suppressive effect of PPIs.[55,56] In the context of a compromised gastric mucosal barrier (secondary to H. pylori infection), resident non-H. pylori bacteria develop pathogenic properties that allow them to adhere to and penetrate the mucosa. In addition, it has been suggested that non-H. pylori and its byproducts may act as a persistent antigenic stimulus to enhance the inflammatory response caused by H. pylori infection.[57,58] Cumulative damage to the gastric mucosa caused by double infection with H. pylori and non-H. pylori bacteria may lead to the development of atrophic gastritis and eventually GC in the long term. In contrast, among non-PPI users, H. pylori mainly colonizes the gastric antrum, which may easily lead to antral gastritis. Owing to the complete acid production of the remaining stomach, its persistent acidic environment makes it difficult for H. pylori to survive, making it difficult to infect. However, once gastric acid is inhibited by PPIs, H. pylori colonization spreads to the corpus, leading to corpus-dominant gastritis and accelerated progression of atrophic gastritis and pangastritis.[59] In addition, even if the infection was eradicated, there were still patients who failed to eradicate H. pylori, and we were unable to determine the infection status of H. pylori during PPI use. Therefore, some H. pylori-positive patients were included in this study. In addition, H. pylori-positive patients may require more PPIs for symptom control. We believe that comparing the long-term use of PPIs in H. pylori-negative patients with the risk of GC should not mean that there is a causal relationship because there may be deviation (subjects may still remain H. pylori-positive) and there may be an association. However, we cannot exclude the possibility of a causal relationship between long-term PPI use and GC risk. Therefore, physicians should exercise caution when prescribing long-term PPIs to these patients, even after successful H. pylori eradication. If H. pylori is eradicated, long-term use of PPIs and inhibition of gastric acid may be needed to prevent the occurrence of GC. Therefore, long-term PPI should be used with caution in high-risk areas for GC.
In addition, our study found that the risk estimate seems to be decreasing with longer PPI use. However, the main data in the study we included were from Brusselaers et al,[29] and they found that the risk of GC was highest when PPIs were used for < 1 year, the risk increased after 3 years of PPIs use, but decreased after > 5 years of PPIs use. Despite being similar to the results of our meta-analysis, these findings may be misleading because of the significantly reduced number of relevant studies included in the stratified analysis and the lack of a standardized PPI administration duration. Seo et al,[18] Lai et al[24] and Cheung et al[27] found a seemingly duration-dependent relationship between the use of PPIs and the risk of GC. In other words, the longer the duration of PPI use, the greater the risk of GC. This does not mean that the use of maintenance PPIs can be considered a potential independent risk factor for GC. The indications for a long time are not consistent (especially those irrelevant to the increased risk of GC). This may be because PPIs are beneficial to most individuals with known cancer risk factors (such as peptic ulcer and H. pylori infection); however, for those who do not have gastrointestinal indications (such as aspirin and nonsteroidal anti-inflammatory drug users), further research is needed. This poses a challenge to a wide range of maintenance PPIs treatments, especially in cases of weak indications.
Our meta-analysis showed that the long-term use of PPIs increases the risk of GC. However, we believe that the incidence rate of GC associated with PPI use may be due to a confusion with the existent indications. Patients with H. pylori infection and gastric ulcer need PPI treatment, which is also a potential risk factor related to GC, not PPIs themselves. In fact, it is necessary to clarify whether PPIs are an independent risk factor for GC, whether they have a synergistic effect with H. pylori infection, or whether they are overestimated by common H. pylori infection. Deep acid inhibition may accelerate the progression of atrophic gastritis caused by chronic H. pylori infection, but PPIs can also inhibit the growth and bacterial activity of H. pylori.
Compared to the latest meta-analysis, we analyzed 20 different databases, including 6 additional studies and more than 2500,000 additional patients. Segna et al[60] included 12 studies (13 databases), a stratified analysis of the location of GC, and the same number of studies. Although the same cutoff values were used for stratified analysis based on PPI duration, our updated meta-analysis included more studies. We additionally analyzed the effect of PPI use on the risk of GC in the state of H. pylori infection and further compared whether the long-term use of H2RA, which is also an acid suppressant, increases the risk of GC. However, Segna et al did not analyze these factors. The other 2 recent meta-analyses Lin et al[61] and Jiang et al[62] included fewer studies. Our meta-analysis investigated more research articles, thus increasing the reliability of our study.
However, our study also has some limitations. First, many confounding factors were not considered in the study design or data analysis. Risk factors beyond H. pylori infection for noncardia GC include older age, cigarette smoking, alcohol consumption, and familial predisposition. A positive association between gastro-esophageal reflux disease and cardia GC has been reported. In addition, a large prospective case-cohort study published in Lancet Public Health in 2021 clearly showed that H. pylori infection is a strong risk factor not only for noncardia cancer but also for cardia cancer in Chinese adults. H. pylori infection causes approximately 80% of noncardia cancers and more than 60% of cardia cancers in this population.[63] It can be seen that the presence or absence of H. pylori infection is the most important confounding factor to study the association between the 2. Similarly, we investigated the effect of PPIs on the risk of GC after H. pylori eradication. Our study lacked data on other important risk factors that, if adjusted for, might have weakened the association. However, we could not analyze these other risk factors of GC according to the current relevant articles. In addition, we performed an integrated meta-analysis of all the research literature, but Asian populations have a higher risk of GC than Western populations, and our findings may not apply to all ethnicities. Second, the heterogeneity of the total pooled effect was high. Although this heterogeneity can be reduced after subgroup analysis, it also reduces the number of studies, limiting the reliability of the data. Finally, there were clear dose-response and time-response trends in PPI use and GC risk. The type and dose of PPI and their time of use have not been reported in many studies. However, we could not analyze this in detail.
5. Conclusion
In summary, our meta-analysis found that PPI use may increase the risk of GC. Further research on the causal relationship between these factors is necessary. Very long-term and careful prospective research can completely solve all the problems that lead to the current controversy regarding the lifelong use of PPIs. We suggested that H. pylori eradication status should be determined before starting long-term PPI treatment to reduce the risk of severe atrophic gastritis and gastric dysplasia. In addition, we recommend the use of the lowest effective dose of PPIs in patients who need to maintain the treatment regimen. Forcing withdrawal of PPIs from their maintenance regimens may result in compromised quality of life for patients.
Author contributions
HG contributed to study design, literature search, quality assessment, data extraction and data analysis, manuscript drafting, and editing. YZ contributed to research identification and selection, data extraction, data analysis and interpretation, manuscript drafting, and editing. LL contributed to study design, literature search, quality assessment, data extraction and data analysis, manuscript drafting, and editing. KG and CT contributed to the design and discussion of the study, manuscript drafting, and editing. YC and FC contributed to manuscript drafting and editing. All authors have approved the final version of this manuscript.
Writing—original draft: Huiqin Gao, Lunan Li.
Writing—review and editing: Ke Geng, Changzheng Teng, Yuanyuan Chen, Fei Chu, Yi Zhao.
Supplementary Material
Abbreviations:
- CI =
- confidence interval
- ECL =
- enterochromaffin-like
- GC =
- gastric cancer
- H. pylori =
- Helicobacter pylori
- H2RAs =
- histamine-2 receptor antagonists
- OR =
- odds ratio
- PPIs =
- proton pump inhibitors
- ROBINS-I =
- Risk Of Bias In Nonrandomized Studies—of Interventions
HG and LL contributed equally to this work.
Supplemental Digital Content is available for this article.
Ethics and dissemination ethical approval are not required since it is secondary data analysis based on published literature.
The authors have no conflicts of interest to disclose.
The authors received no financial support for the research, authorship, and/or publication of this article.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Gao H, Li L, Geng K, Teng C, Chen Y, Chu F, Zhao Y. Use of proton pump inhibitors for the risk of gastric cancer: Use of proton pump inhibitors for the risk of gastric cancer. Medicine 2022;101:49(e32228).
Contributor Information
Huiqin Gao, Email: 2506489457@qq.com.
Lunan Li, Email: 782027214@qq.com.
Ke Geng, Email: 46049923@qq.com.
Changzheng Teng, Email: 309115597@qq.com.
Yuanyuan Chen, Email: chen77912022@163.com.
Fei Chu, Email: 13965765221@139.com.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Wong MCS, Huang J, Chan PSF, et al. Global incidence and mortality of gastric cancer, 1980-2018. JAMA Netw Open. 2021;4:e2118457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the evaluation of carcinogenic risks to humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- [4].Machlowska J, Baj J, Sitarz M, et al. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Plummer M, Franceschi S, Vignat J, et al. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–90. [DOI] [PubMed] [Google Scholar]
- [6].Cheung KS, Leung WK. Risk of gastric cancer development after eradication of Helicobacter pylori. World J Gastrointest Oncol. 2018;10:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States From 1999-2012. JAMA. 2015;314:1818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Macke L, Schulz C, Koletzko L, et al. Systematic review: the effects of proton pump inhibitors on the microbiome of the digestive tract—evidence from next-generation sequencing studies. Aliment Pharmacol Ther. 2020;51:505–26. [DOI] [PubMed] [Google Scholar]
- [9].Joo MK, Park JJ, Chun HJ. Proton pump inhibitor: the dual role in gastric cancer. World J Gastroenterol. 2019;25:2058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors-evidence and plausibility. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res Ed). 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [13].Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clinical Res Ed). 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In Higgins JP, Green S, (Eds). Cochrane handbook for systematic reviews of interventions. Hoboken, NJ, USA: Wiley; 2008:pp. 243–296. [Google Scholar]
- [15].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [16].Abrahami D, McDonald EG, Schnitzer ME, et al. Proton pump inhibitors and risk of gastric cancer: population-based cohort study. Gut. 2022;71:16–24. [DOI] [PubMed] [Google Scholar]
- [17].Shin GY, Park JM, Hong J, et al. Use of proton pump inhibitors vs histamine 2 receptor antagonists for the risk of gastric cancer: population-based cohort study. Am J Gastroenterol. 2021;116:1211–9. [DOI] [PubMed] [Google Scholar]
- [18].Seo SI, Park CH, You SC, et al. Association between proton pump inhibitor use and gastric cancer: a population-based cohort study using two different types of nationwide databases in Korea. Gut. 2021;70:2066–75. [DOI] [PubMed] [Google Scholar]
- [19].Ng AKY, Ng PY, Ip A, et al. Association between proton pump inhibitors after percutaneous coronary intervention and risk of gastric cancer. BMJ Open Gastroenterol. 2021;8:e000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gingold-Belfer R, Schmilovitz-Weiss H, Issa N, et al. Use of proton pump inhibitors is associated with increased risk of gastric cancer in community dwelling elderly. United Eur Gastroenterol J. 2021;9(suppl 8):310. [Google Scholar]
- [21].Liu P, McMenamin UC, Johnston BT, et al. Use of proton pump inhibitors and histamine-2 receptor antagonists and risk of gastric cancer in two population-based studies. Br J Cancer. 2020;123:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee JK, Merchant SA, Schneider JL, et al. Proton pump inhibitor use and risk of gastric, colorectal, liver, and pancreatic cancers in a community-based population. Am J Gastroenterol. 2020;115:706–15. [DOI] [PubMed] [Google Scholar]
- [23].Peng YC, Huang LR, Lin CL, et al. Association between proton pump inhibitors use and risk of gastric cancer in patients with GERD. Gut. 2019;68:374–6. [DOI] [PubMed] [Google Scholar]
- [24].Lai SW, Lai HC, Lin CL, et al. Proton pump inhibitors and risk of gastric cancer in a case-control study. Gut. 2019;68:765–7. [DOI] [PubMed] [Google Scholar]
- [25].Brusselaers N, Lagergren J, Engstrand L. Duration of use of proton pump inhibitors and the risk of gastric and oesophageal cancer. Cancer Epidemiol. 2019;62:101585. [DOI] [PubMed] [Google Scholar]
- [26].Niikura R, Hayakawa Y, Hirata Y, et al. Long-term proton pump inhibitor use is a risk factor of gastric cancer after treatment for Helicobacter pylori: a retrospective cohort analysis. Gut. 2018;67:1908–10. [DOI] [PubMed] [Google Scholar]
- [27].Cheung KS, Chan EW, Wong AYS, et al. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut. 2018;67:28–35. [DOI] [PubMed] [Google Scholar]
- [28].Wennerström ECM, Simonsen J, Camargo MC, et al. Acid-suppressing therapies and subsite-specific risk of stomach cancer. Br J Cancer. 2017;116:1234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brusselaers N, Wahlin K, Engstrand L, et al. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: a nationwide population-based cohort study in Sweden. BMJ Open. 2017;7:e017739e017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Poulsen AH, Christensen S, McLaughlin JK, et al. Proton pump inhibitors and risk of gastric cancer: a population-based cohort study. Br J Cancer. 2009;100:1503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tamim H, Duranceau A, Chen LQ, et al. Association between use of acid-suppressive drugs and risk of gastric cancer. A nested case-control study. Drug Saf. 2008;31:675–84. [DOI] [PubMed] [Google Scholar]
- [32].Crane SJ, Locke GR, 3rd, Harmsen WS, et al. Subsite-specific risk factors for esophageal and gastric adenocarcinoma. Am J Gastroenterol. 2007;102:1596–602. [DOI] [PubMed] [Google Scholar]
- [33].García Rodríguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut. 2006;55:1538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stockbruegger RW. Bacterial overgrowth as a consequence of reduced gastric acidity. Scand J Gastroenterol Suppl. 1985;111:7–16. [DOI] [PubMed] [Google Scholar]
- [35].Stockbrugger RW, Cotton PB, Eugenides N, et al. Intragastric nitrites, nitrosamines, and bacterial overgrowth during cimetidine treatment. Gut. 1982;23:1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rowland JR. The toxicology of N-nitroso compounds, In Hill MJ, (ed). Nitrosamines-toxicology and microbiology. London: Ellis Horwood; 1988:pp. 117–141. [Google Scholar]
- [37].Shin VY, Jin HC, Ng EK, et al. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone promoted gastric cancer growth through prostaglandin E receptor (EP2 and EP4) in vivo and in vitro. Cancer Sci. 2011;102:926–33. [DOI] [PubMed] [Google Scholar]
- [38].Laine L, Ahnen D, McClain C, et al. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:651–68. [DOI] [PubMed] [Google Scholar]
- [39].Lundell L, Vieth M, Gibson F, et al. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther. 2015;42:649–63. [DOI] [PubMed] [Google Scholar]
- [40].Raines D, Chester M, Diebold AE, et al. A prospective evaluation of the effect of chronic proton pump inhibitor use on plasma biomarker levels in humans. Pancreas. 2012;41:508–11. [DOI] [PubMed] [Google Scholar]
- [41].Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012;18:5495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep. 2010;12:448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jalving M, Koornstra JJ, Wesseling J, et al. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006;24:1341–8. [DOI] [PubMed] [Google Scholar]
- [44].Singh P, Indaram A, Greenberg R, et al. Long term omeprazole therapy for reflux esophagitis:follow-up in serum gastrin levels,EC cell hyperplasia and neoplasia. World J Gastroenterol. 2000;6:789–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lee L, Ramos-Alvarez I, Ito T, et al. Insights into effects/risks of chronic hypergastrinemia and lifelong ppi treatment in man based on studies of patients with Zollinger–Ellison syndrome. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–5. [DOI] [PubMed] [Google Scholar]
- [47].Cooper BT, Chapman W, Neumann CS, et al. Continuous treatment of Barrett’s oesophagus patients with proton pump inhibitors up to 13 years: observations on regression and cancer incidence. Aliment Pharmacol Ther. 2006;23:727–33. [DOI] [PubMed] [Google Scholar]
- [48].El-Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2004;99:1877–83. [DOI] [PubMed] [Google Scholar]
- [49].McCarthy DM. Adverse effects of proton pump inhibitor drugs: clues and conclusions. Curr Opin Gastroenterol. 2010;26:624–31. [DOI] [PubMed] [Google Scholar]
- [50].Abdalla SI, Lao-Sirieix P, Novelli MR, et al. Gastrin-induced cyclooxygenase-2 expression in Barrett’s carcinogenesis. Clin Cancer Res. 2004;10:4784–92. [DOI] [PubMed] [Google Scholar]
- [51].Chueca E, Lanas A, Piazuelo E. Role of gastrin-peptides in Barrett’s and colorectal carcinogenesis. World J Gastroenterol. 2012;18:6560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wroblewski LE PR, Jr., Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Meining A, Riedl B, Stolte M. Features of gastritis predisposing to gastric adenoma and early gastric cancer. J Clin Pathol. 2002;55:770–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–201. [DOI] [PubMed] [Google Scholar]
- [55].Sanduleanu S, Jonkers D, De Bruine A, et al. Non-Helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Aliment Pharmacol Ther. 2001;15:379–88. [DOI] [PubMed] [Google Scholar]
- [56].Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. New Engl J Med. 1996;334:1018–22. [DOI] [PubMed] [Google Scholar]
- [57].Sanduleanu S, Jonkers D, de Bruine A, et al. Does double infection of the stomach with Helicobacter pylori and non-Helicobacter pylori bacterial-ora accelerate the development of atrophic gastritis in patients treated with gastric acid inhibition. Gut. 2000;47(Suppl. III):A31. [Google Scholar]
- [58].Sanduleanu S, Jonkers D, de Bruine A, et al. Double gastric infection with Helicobacter pylori and non-Helicobacter pylori bacteria enhances the expression of circulating pro-in-ammatory cytokines and 3 parallels the development of atrophic corpus gastritis. Gut. 2000;47(Suppl. III):A110. [Google Scholar]
- [59].Kuipers EJ. Proton pump inhibitors and gastric neoplasia. Gut. 2006;55:1217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Segna D, Brusselaers N, Glaus D, et al. Association between proton-pump inhibitors and the risk of gastric cancer: a systematic review with meta-analysis. Ther Adv Gastroenterol. 2021;14:175628482110514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ju-Li L, Jian-Xian L, Chao-Hui Z, et al. Long-term proton pump inhibitor use and the incidence of gastric cancer: a systematic review and meta-analysis. J Gastr Surg. 2020;2:1–11. [Google Scholar]
- [62].Jiang K, Jiang X, Wen Y, et al. Relationship between long-term use of proton pump inhibitors and risk of gastric cancer: a systematic analysis. J Gastroenterol Hepatol. 2019;34:1898–905. [DOI] [PubMed] [Google Scholar]
- [63].Yang L, Kartsonaki C, Yao P, et al. China Kadoorie Biobank Collaborative Group. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health. 2021;6:e888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.