Abstract
The International Classification of Headache Disorder (ICHD) clearly guides the suspicion of intracranial vertebral artery dissection (ICVAD) in headache patients, but guidelines on how observational or imaging studies should be performed to detect dangerous progression early are unclear. Fifty-six cases with pearl and string type intracranial vertebral artery dissection were divided into 3 groups: 39 in the headache group, 6 in the infarction group, and 11 in the hemorrhagic group. Clinical and angiographic data were analyzed and compared. Most headaches resolved within 2 weeks and did not exceed 8 weeks. Of the 33 patients (84.6%) who underwent continuous follow-up imaging, 18 (54.5%) returned to normal, but 3 (9%) had deteriorated. All the patients survived without subsequent bleeding or infarction. Image changes started before 3rd month and ended after 6 to 7 months. In acute ICVADs, image changes occur at the same time as the headache resolves and continue for several months after the headache has subsided. Since the dissection is likely to worsen even after the headache disappears, the image changes continue over several months, and prediction of rupture of unruptured ICVAD is unpredictable, it is desirable to conduct continuous imaging studies regularly after the initiation of dissection until stabilization is confirmed.
Keywords: dissection, headache, vertebral artery
1. Introduction
Spontaneous intracranial vertebral artery dissections (ICVADs) usually have a benign, self-recovering nature when detected without hemorrhage or infarction.[1–3] In contrast, ICVADs, which initially cause bleeding or infarction can be aggressive and often fatal.[4–6] In general, the natural course and clinical outcome of ICVAD is highly dependent on the initial presentation pattern.[1,3] Therefore, an unruptured dissection is usually managed conservatively, whereas a ruptured ICVAD is treated immediately.[7] This type of treatment trend requires a prerequisite that an unruptured dissection with only a headache at the time of presentation should not cause any subsequent bleeding or infarction. However, there are reports supporting evidence that conservatively managed ICVADs have a potential risk of delayed hemorrhage or infarction.[8,9] Currently, owing to advances in endovascular treatment technology, more unruptured ICVADs are being treated prophylactically because of uncertainties about prognosis.[7,10] In order to prevent subsequent bleeding that may occur due to the progression of dissection, ICVADs which are thought to be prone to bleeding should be recognized early. The International Classification of Headache Disorder (ICHD) clearly guides the suspicion of intracranial artery dissection in headache patients, and although studies on angiographic properties related to spontaneous healing or bleeding are well published, guidelines on how observational or imaging studies should be performed to detect dangerous progression early are unclear.[11–13]
The angiographic pearl and string signs are features that both ruptured and unruptured ICVADs have in common when they present only with headache. Headache can be a warning sign that rupture is imminent, but neither pain intensity nor angiographic characteristics are reliable predictors of dissection progression.[9,14,15] Headache relief is not necessarily a sign of a cure; therefore, if pearl and string type ICVADs are found in patients with acute headache, a decision should be made whether to treat it immediately or simply to perform follow-up imaging studies until stabilization of the dissection is confirmed.
In this study, we observed headache progression and imaging changes during the follow-up period, determined how long a non-stroke type dissection should be followed by imaging studies, and suggested when to consider treatment options.
2. Materials and methods
This retrospective was approved by the Institutional Review Board of the Konkuk University Medical Center, Seoul, Republic of Korea (KUMC 2021-07-063). Radiologists’ reports were searched for computed tomography angiography (CTA) or magnetic resonance angiography (MRA) images covering the head and neck regions performed from 2005 to 2018 to find the keywords “dissection” and “vertebral artery” in the conclusion sections. Then, each image was analyzed by a neurosurgeon, neurologist, and neuroradiologist to find the pearl and string sign, which shows alternating combinations of dilatation and stenosis. Some cases were difficult to distinguish from mimic conditions such as reversible cerebral vasoconstriction syndrome or atherosclerotic stenosis and dilatation. Therefore, cases were enrolled only when all 3 reviewers agreed with each other’s interpretation. After the initial diagnosis, all radiographic images taken afterward were analyzed to determine when the anatomical changes of the dissection appeared and when it stopped. It was concluded that improvement was achieved when the affected vertebral artery segment was completely restored to normal or remained smoothly dilated without stenosis, and it was considered worsened when it became stenotic, tapered, or occluded.
Clinically we divided the cases into 3 subgroups; hemorrhagic group with subarachnoid hemorrhage (SAH); infarct group with acute medullary or cerebellar infarction; the remaining cases were classified into the headache group. Since the characteristics of headaches are diverse and the description methods differ from person to person, the characteristics of the patient’s headaches were simply analyzed according to their location and severity. The analysis of pain location included the hemi-cranial and occipito-nuchal regions, and the consistency with dissection location. Headache severity included abruptness and intensity assessed using a visual analog scale (VAS). To determine the duration and interval of imaging studies, the duration from the onset to the end of the headache was compared with the same duration of image changes.
One-way analysis of variance (ANOVA) was used to analyze differences in headache characteristics, such as intensity, location, and accompanying symptoms, between the 3 groups. Differences between the 2 groups (headache and infarction) of time-dependent factors, such as onset/relief of headache and onset/stop of angiographic changes, were analyzed using an independent 2-sample t-test and visualized with a Kaplan–Meier curve (IBM SPSS Statistics for Windows, Version 20. IBM Corp., Armonk).
3. Results
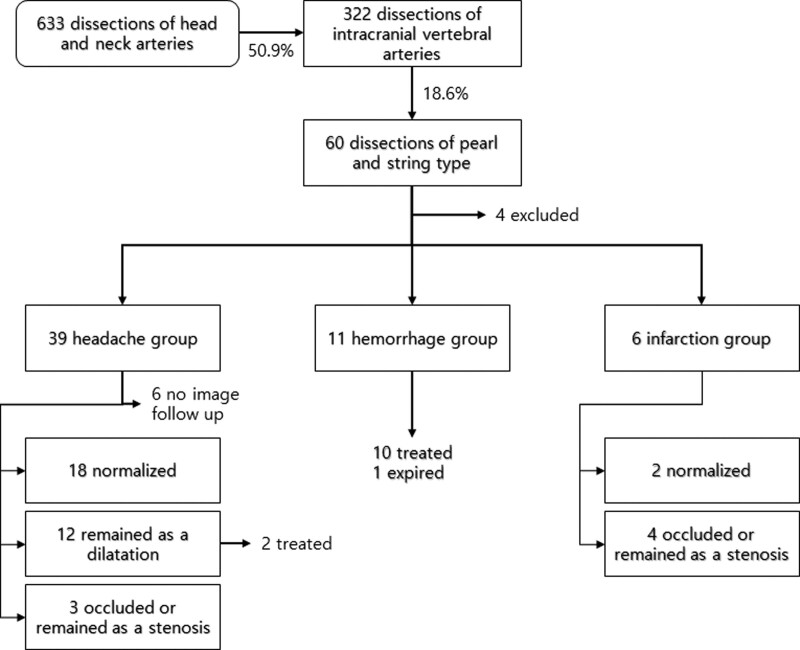
We identified 633 patients with dissection of the arterial system of the head and neck. Of these, 322 (50.9% of all head and neck dissection cases) had IVADs, showing a heterogeneous disease spectrum. Typical pearl and string type dissections were found in 60 patients (18.6% of IVADs), and medical record analysis was performed. Unfortunately, 4 patients did not appear on the next appointment and had to be excluded due to insufficient medical records. Finally, the 56 patients were divided into 3 groups: 39 in the headache group, 6 in the infarction group, and 11 in the hemorrhagic group (Fig. 1).
Figure 1.
Flow chart showing the case selection procedure and follow-up results.
3.1. Clinical and anatomical results
In the headache group, all headaches were relieved within 2 months (5–52 days, mean 14 days), and all patients survived long enough (37–5828 days, median 1498 days) without subsequent bleeding or infarction. Of the 33 patients (84.6%) who underwent continuous follow-up imaging, 18 (54.5%) returned to normal, but 3 (9%) had deteriorated into irregularly narrowed or occluded lesions. The rest showed partial improvement due to incomplete recovery of the dilated segment. Ten cases remained fusiform dilatation, and 2 cases transformed into a more saccular form and were treated using the endovascular method (Table 1).
Table 1.
Summary of clinical and angiographical analysis.
| Headache | Hemorrhage | Infarction | P | |
|---|---|---|---|---|
| Cases | 39 | 11 | 6 | |
| Age, mean (range) | 47.8 (34–67) | 46.8 (28–64) | 49.5 (32–82) | .861 |
| Sex (M:F) | 17:22 | 4:7 | 5:1 | .151 |
| Hypertension | 9 | 4 | 1 | .604 |
| Diabetes | 3 | 0 | 0 | .516 |
| Symptoms and signs | ||||
| Headache | 39 | 9 | 5 | .025 |
| Dizziness | 4 | 0 | 4 | |
| Nausea | 3 | 4 | 2 | .034 |
| Brainstem signs | 0 | 0 | 4 | |
| Seizure | 0 | 2 | 0 | |
| Unconsciousness | 0 | 1 | 0 | |
| Symptom onset–hospital visit | ||||
| Mean (range, d) | 5.8 (0–19) | 1.6 (0–7) | 1.3 9 (0–4) | .001 |
| Headache start–resolve | ||||
| Mean (range, d) | 14 (5–52) | - | 5.5 (2–13) | .033 |
| Headache onset during | ||||
| ADL | 35 | 8 | 6 | .207 |
| Sleep | 3 | 1 | 0 | .773 |
| Stressful | 15 | 2 | 3 | .632 |
| Headache characteristics | ||||
| Occipito-nuchal | 33 | 5 | 5 | .022 |
| Hemicranial | 31 | 2 | 4 | .000 |
| Ipsilateral to dissection | 27 | 1 | 4 | .001 |
| Occur acutely | 36 | 9 | 5 | .554 |
| Severe (VAS > 7) | 28 | 8 | 2 | .165 |
| Imaging changes (All through the follow up period) | ||||
| Onset–First imaging change | ||||
| Mean (range, d) | 141.0 (6–1193) | 374.7 (51–1690) | .419 | |
| Onset–End of imaging change | ||||
| Mean (range, d) | 194.9 (6–1193) | 389.2 (51–1690) | .494 | |
| Normalization | 18 | 2 | .352 | |
| Imaging changes (Follow up period restricted to 1 yr) | ||||
| Onset–First imaging change | ||||
| Mean (range, d) | 77.6 (6–196) | 111.6 (51–170) | .210 | |
| Onset–End of imaging change | ||||
| Mean (range, d) | 92.0 (6–223) | 129.0 (51–175) | .933 | |
| Duration of imaging change | ||||
| Mean (range, d) | 81.3 (0–210) | 127.6 (47–172) | .475 | |
| Normalization | 18 | 2 | .182 | |
ADL = activities of daily living, VAS = visual analog scale.
The duration of headache in the infarction group was shorter than that in the headache group (2–13, mean 5.5 days vs 5–52 days, mean 14 days, P = .033). However, anatomical improvement was less than that in the headache group. Two returned to normal (33.3%), and 4 deteriorated into stenosis or obstruction (66.7%). All patients survived long enough (51–3800 days, median 1531 days), and none of them experienced recurrent infarction or bleeding. In the hemorrhage group, 1 patient did not receive treatment due to a grave condition at the time of admission, but all others were successfully treated using the endovascular method.
3.2. Presentation patterns and characteristics of the headaches
Regardless of group, most headaches occurred during daily activities. Sometimes, headaches are accompanied by dizziness and nausea. Nausea may suggest increased intracranial pressure due to bleeding or infarction; however, its presence does not necessarily imply that such an event has occurred. Most patients in the hemorrhage and infarction groups visited the hospital earlier because they had neurological symptoms or more intense pain than those in the headache group (0–7, mean 1.6 days; 0–4, mean 1.3 days, respectively). Sometimes, in the hemorrhage group, hospital visits delayed if the pain was less intense (2, 4 and 7 days). However, in the headache group, the patient’s visit was frequently delayed (0–19, mean 5.8 days, P = .001).
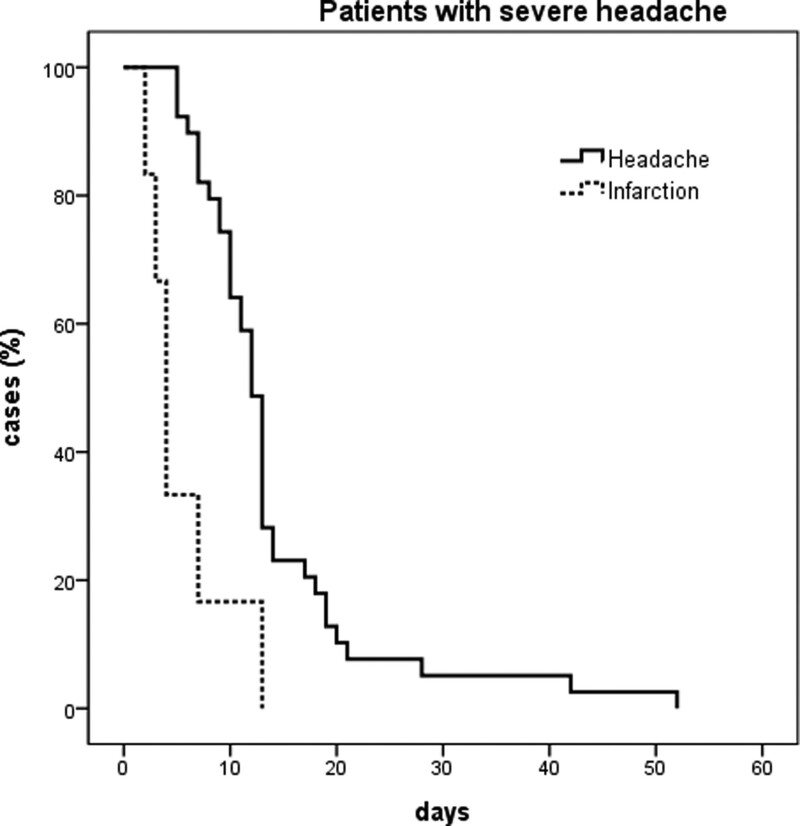
The pain was generally confined to the hemi-cranial region, and in many cases in the headache and infarction group, it was localized to the ipsilateral occipito-nuchal region (69.2% and 80%, respectively). However, in the hemorrhagic group, the pain was usually global and less localized to the side of the lesion (9%, P < .05). In contrast, although pain occurred acutely in all groups, the severity of pain was relatively lower in the infarct group than in the other 2 groups. Acute, severe pain originating in the occipito-nuchal or hemi-cranial region was the most typical headache in pearl and string type ICVAD. (Table 2). Most of these headaches resolved within 2 weeks and did not exceed 8 weeks (Fig. 2).
Table 2.
The characteristics of the headache due to unruptured vertebral artery dissections and comparison with other previous study.
Figure 2.
Kaplan–Meier analysis of the headache resolution rate. Most headaches relieved within 2 weeks in both the headache and infarction groups, and all disappeared within 8 weeks.
3.3. When does image change start and end?
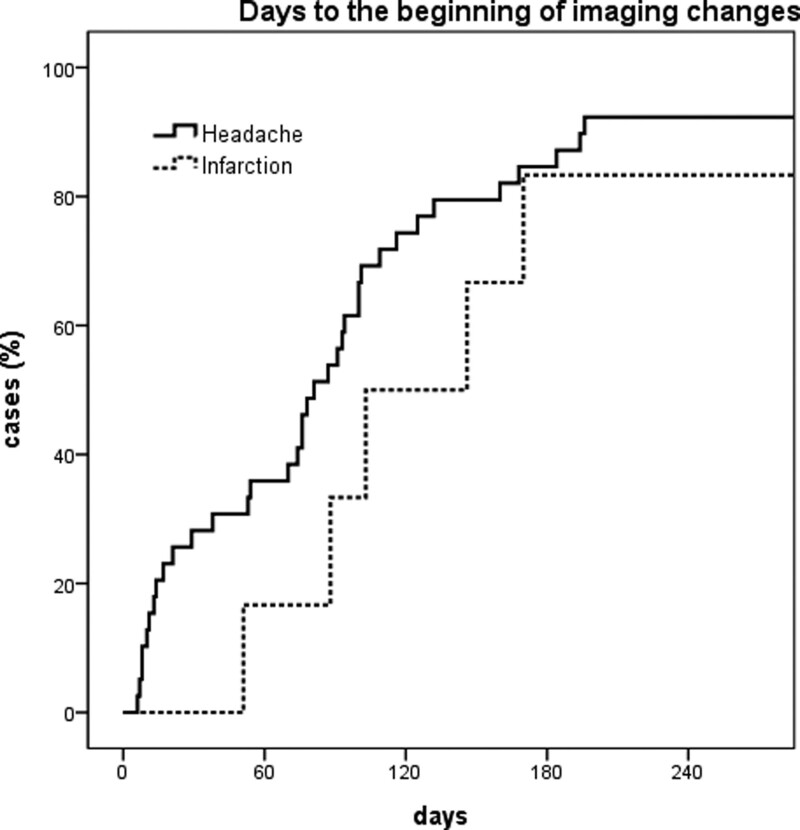
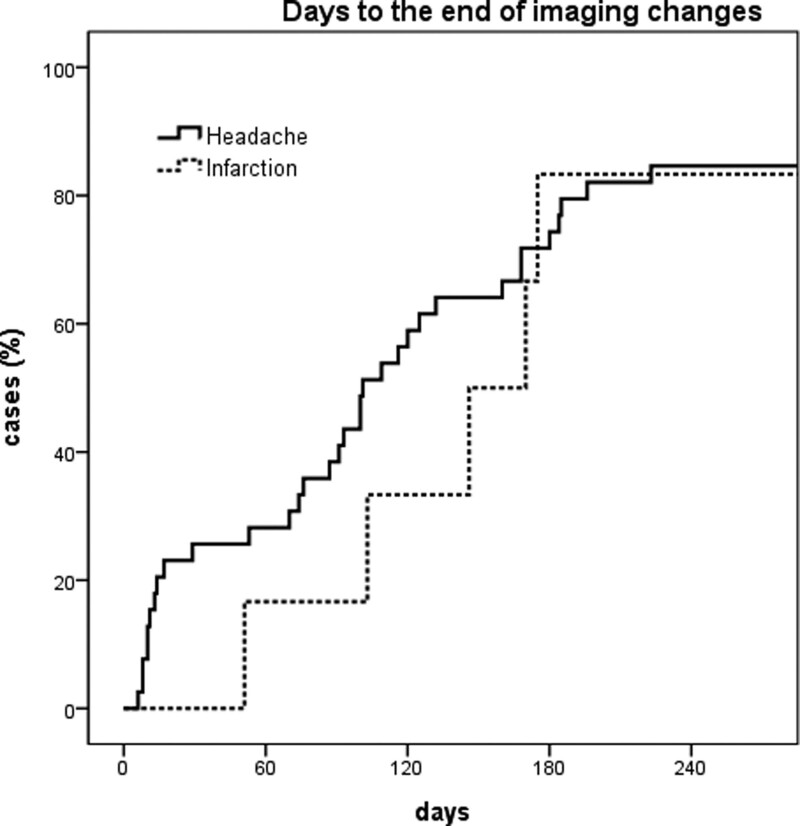
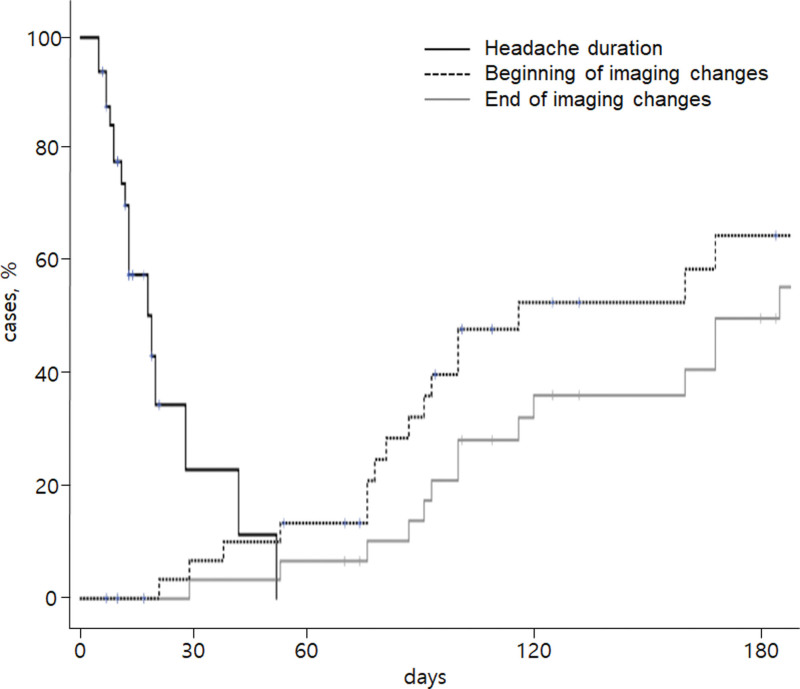
When multiple imaging studies were performed at short intervals, image changes were not a single event. Instead, a series of events continued to improve or deteriorate over time. The first images showing normalization appeared on day 6 after pain onset in the headache group, and on day 51 in the infarction group. All changes started before 3rd month and ended after 6 to 7 months when the image tracking period was limited to 1 year or less (Figs. 3 and 4). The first 2 months of the image change period overlapped with the headache resolution period (Fig. 5). However, headache resolution did not necessarily indicate ICVAD normalization. Instead, the image continued to change, even after the headache had already resolved. Regardless of improvement or deterioration, the rate of morphological change was 10.8% at 1 month, 27.0% at 3 months, and 78.4% at 6 months.
Figure 3.
Kaplan–Meier curve showing the cumulative rate of detection of the first image change. The image changes occur steadily for 7 months in both headache and infarction group, after which new imaging changes rarely begin.
Figure 4.
Kaplan–Meier curve showing cumulative rate of stabilization of image changes. Most image changes that started within a 7-month period usually end within the same period. Therefore, this period can be regarded as the period during which most pathophysiological changes occur.
Figure 5.
Relationship between the headache resolution period and the image change period. Note the 4 to 8 weeks that the 2 periods overlap. Image changes occur at the same time as the headache resolves and continue for several months after the headache has subsided.
There were no age or sex differences among the 3 groups, and a history of hypertension and diabetes was not associated with the development of ICVAD. For the management of headache and dissection, analgesics, antiplatelet agents, and statins were administered over various periods. High blood pressure and blood sugar levels were also controlled. Because there are many different ways to take these drugs, their effects on early pain relief and angiographic improvement have not been analyzed.
4. Discussion
Spontaneous intracranial arterial dissection occurs more frequently in the vertebral artery and can lead to ischemic stroke or hemorrhage.[17,18] However, while extracranial and anterior circulation dissection are more common in the non-Asian population, ICVAD is more common in the Asian population. Owing to these ethnic differences in the site of dissection, the risk of ICVAD can be overlooked or overestimated by geographic or demographic characteristics.[19]
Although headaches due to ICVAD can be typical, it is difficult to determine the presence or absence of abnormalities based on the pain pattern alone, given the variety of pain tolerances and expressions of each patient.[16] In contrast, noninvasive angiographic studies, such as CTA or MRA, can easily detect vascular abnormalities, as well as hemorrhage or infarction.[20] Hemorrhage or infarction due to ICVAD usually occurs concurrently with headache or neurological symptoms, but headache may sometimes precede vascular events.[8,9] Therefore, if some of the typical features of ICVAD, such as pain on occipito-nuchal region, unilateral, severe, acute, are accompanied by initial headache, angiographic studies using CTA or MRA can easily provide early detection of ICVAD.[16,21]
4.1. Why are pearl and string signs important?
Given the variety of morphologies, it is difficult to distinguish between acute and chronic ICVADs.[22,23] Sudden onset of headache or neck pain and chronological changes in angiographic images are strong indicators of acute vertebral artery dissection.[8] As asymptomatic ICVAD rarely shows morphological changes during the follow-up period, asymptomatic dissections without image changes over time can be considered chronic.[3]
ICVAD has been characterized according to radiological morphology, such as dilatation, stenosis, alternating stenosis and dilatation (pearl and string sign), and occlusion.[1,2,4–6,22,23] It is not clear why ICVADs have various morphologies, but the pearl and string signs that can be observed in both ruptured and unruptured lesions are more often seen in ruptured dissection.[12,24] In addition, delayed SAH has been reported to occur more often in ICVADs which shows the pearl and string sign.[8] Therefore, the pearl and sting sign with acute headache can be a good reason for further investigation to monitor the progression of ICVAD.
4.2. Pathophysiology of occurrence and spontaneous healing of the dissection
Dissection of a cerebral artery usually leads to flow disturbance by compromising its lumen with an intimal flap or tear by breaking the entire wall. Subsequent events result in a broad spectrum of diseases, characterized by mild symptoms, such as headache and dizziness, infarction, or devastating hemorrhage. Although mild symptomatic dissections usually heal spontaneously, some may develop into dangerous lesions within a short period of time.
The structural differences that lead to variations in the presentation of arterial dissections remain unclear. In several autopsy cases, it has been demonstrated that the location of the dissection plane determines the clinical presentation, indicating that subadventitial dissections are more likely to cause arterial wall rupture and subintimal dissection that causes intramural hematoma is more likely to result in luminal occlusion.[25] The former occurs more frequently in the vertebrobasilar system, whereas the latter occurs more frequently in the internal carotid system.[26] In other autopsy cases of vertebrobasilar artery dissection, the ruptured portions were located under the thin adventitia.[27,28]
However, the healing processes of IVADs have not yet been elucidated. Dissection specimens collected between 6 hours and 35 days after the onset of SAH showed initiation of the healing process with neointimal proliferation as well as extensive destruction of the internal elastic lamina and media. The neointima, composed mainly of newly synthesized smooth muscle cells and collagen fibers, extends forward from the disrupted ends of the media to the ruptured portion.[29] A series of MR vessel wall images also revealed reduction of hematomas and double lumen and wall enhancement over time.[30] These findings suggest that image changes may end up at various healing stages due to the complexity of the regeneration mechanism.
4.3. How long should we continue follow-up and when we decide to treat?
In our study, most headaches from acute pearl and string type ICVAD resolved within 2 weeks and did not last longer than 8 weeks. Meanwhile, the earliest signs of improvement appeared in imaging studies as early as 6 days to as late as 51 days after the onset of pain. Therefore, the first 2 months of the image change period overlapped with the headache resolution period. Image changes occur at the same time as the headache resolves and continue for several months after the headache has subsided. Although many ICVADs have shown improvement on follow-up imaging, some ICVADs have worsened; therefore, headache relief does not necessarily imply normalization of ICVADs. Although it is still unclear which factors are related to ICVAD enlargement, Horio et al reported that approximately 25% of ICVADs showed enlargement within 30 days.[31]
Since the dissection is likely to worsen even after the headache disappears, the image changes continue over several months and rupture of unruptured ICVAD is unpredictable; therefore, it is desirable to conduct continuous imaging studies regularly after the initiation of dissection until stabilization is confirmed. Based on our study, we propose an early follow-up imaging study at 1 week and 1 month after the initial imaging study to determine the direction of image changes and detect early exacerbation, and then at 3- or 6-month intervals to confirm healing or stabilization. Surgical or endovascular interventions should be considered when the dissection grows or extends, forms saccular dilation, and aggravates neurological symptoms.[8,22]
5. Limitations
Although this study has many biases as a retrospective observational study, the exclusion of a few cases due to lack of follow-up data may have affected the analysis. However, this is the largest homogenous subgroup consisting only of pearl and string type ICVADs and had a long-term follow-up.
6. Conclusions
In acute ICVADs, image changes occur at the same time as the headache resolves and continue for several months after the headache has subsided. It starts as early as a week and usually lasts several months. Most image changes occur within 7 months, but sometimes can last a year or more. Since the dissection is likely to worsen even after the headache disappears, the image changes continue over several months, and prediction of rupture of unruptured ICVAD is unpredictable, it is desirable to conduct continuous imaging studies regularly from the initiation of dissection until stabilization is confirmed.
Author contributions
Conceptualization: Joon Cho, Jeong-Jin Park, Hong Gee Roh, Young Il Chun.
Data curation: Yoo Sung Jeon, Jeong-Jin Park, Young Il Chun.
Formal analysis: Young Il Chun.
Investigation: Joon Cho, Jeong-Jin Park, Hong Gee Roh, Young Il Chun.
Methodology: Yoo Sung Jeon, Hong Gee Roh, Young Il Chun.
Project administration: Young Il Chun.
Resources: Joon Cho, Jeong-Jin Park, Young Il Chun.
Software: Yoo Sung Jeon, Young Il Chun.
Supervision: Joon Cho, Young Il Chun.
Validation: Hong Gee Roh, Young Il Chun.
Visualization: Yoo Sung Jeon, Young Il Chun.
Writing – original draft: Yoo Sung Jeon.
Writing – review & editing: Young Il Chun.
Abbreviations:
- CTA =
- computed tomography angiography
- ICVAD =
- intracranial vertebral artery dissection
- MRA =
- magnetic resonance angiography
- SAH =
- subarachnoid hemorrhage
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Jeon YS, Cho J, Park J-J, Roh HG, Chun YI. Acute hemicranial pain accompanied with a pearl and string type dissection of intracranial vertebral artery: Consideration for the time when to finish the medical observation. Medicine 2022;101:49(e32008).
Contributor Information
Yoo Sung Jeon, Email: 20130302@kuh.ac.kr.
Joon Cho, Email: chojoon@kuh.ac.kr.
Jeong-Jin Park, Email: parkjj@kuh.ac.kr.
Hong Gee Roh, Email: hgroh@kuh.ac.kr.
References
- [1].Nakagawa K, Touho H, Morisako T, et al. Long-term follow-up study of unruptured vertebral artery dissection: clinical outcomes and serial angiographic findings. J Neurosurg. 2000;93:19–25. [DOI] [PubMed] [Google Scholar]
- [2].Yoshimoto Y, Wakai S. Unruptured intracranial vertebral artery dissection. Clinical course and serial radiographic imagings. Stroke. 1997;28:370–4. [DOI] [PubMed] [Google Scholar]
- [3].Kobayashi N, Murayama Y, Yuki I, et al. Natural course of dissecting vertebrobasilar artery aneurysms without stroke. AJNR Am J Neuroradiol. 2014;35:1371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim CH, Son YJ, Paek SH, et al. Clinical analysis of vertebrobasilar dissection. Acta Neurochir (Wien). 2006;48:395–404. [DOI] [PubMed] [Google Scholar]
- [5].Kocaeli H, Chaalala C, Andaluz N, et al. Spontaneous intradural vertebral artery dissection: a single-center experience and review of the literature. Skull Base. 2009;19:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shin JH, Suh DC, Choi CG, et al. Vertebral artery dissection: spectrum of imaging findings with emphasis on angiography and correlation with clinical presentation. Radiographics. 2000;20:1687–96. [DOI] [PubMed] [Google Scholar]
- [7].Guan J, Li G, Kong X, et al. Endovascular treatment for ruptured and unruptured vertebral artery dissecting aneurysms: a meta-analysis. J Neurointerv Surg. 2017;9:558–63. [DOI] [PubMed] [Google Scholar]
- [8].Naito I, Iwai T, Sasaki T. Management of intracranial vertebral artery dissections initially presenting without subarachnoid hemorrhage. Neurosurgery. 2002;51:930–7; discussion 937–938. [DOI] [PubMed] [Google Scholar]
- [9].Wójtowicz K, Kunert P, Przepiórka L, et al. Warning signs in the era of unruptured intracranial aneurysms: report on 2 cases of fatal aneurysmal hemorrhage. Cerebrovasc Dis Extra. 2021;11:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim CH, Lee CH, Kim YH, et al. Flow diverter devices for the treatment of unruptured vertebral artery dissecting aneurysm. J Korean Neurosurg Soc. 2021;64:891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- [12].Shibahara T, Yasaka M, Wakugawa Y, et al. Improvement and aggravation of spontaneous unruptured vertebral artery dissection. Cerebrovasc Dis Extra. 2017;7:153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee HJ, Choi JH, Lee KS, et al. Clinical and radiological risk factors for rupture of vertebral artery dissecting aneurysm: significance of the stagnation sign. J Neurosurg 2021; 17: 1–6. [DOI] [PubMed] [Google Scholar]
- [14].Hokari M, Nakayama N, Shimoda Y, et al. Effect of headache on the pathologic findings of unruptured cerebral saccular aneurysms. World Neurosurg. 2017;103:431–41. [DOI] [PubMed] [Google Scholar]
- [15].Gilard V, Grangeon L, Guegan-Massardier E, et al. Headache changes prior to aneurysmal rupture: a symptom of unruptured aneurysm? Neurochirurgie. 2016;62:241–4. [DOI] [PubMed] [Google Scholar]
- [16].Matsumoto H, Hanayama H, Sakurai Y, et al. Investigation of the characteristics of headache due to unruptured intracranial vertebral artery dissection. Cephalalgia. 2019;39:504–14. [DOI] [PubMed] [Google Scholar]
- [17].Giannini N, Ulivi L, Maccarrone M, et al. Epidemiology and cerebrovascular events related to cervical and intracranial arteries dissection: the experience of the city of Pisa. Neurol Sci. 2017;38:1985–91. [DOI] [PubMed] [Google Scholar]
- [18].Mori S, Takahashi S, Hayakawa A, et al. Fatal intracranial aneurysms and dissections causing subarachnoid hemorrhage: an epidemiological and pathological analysis of 607 legal autopsy cases. J Stroke Cerebrovasc Dis. 2018;27:486–93. [DOI] [PubMed] [Google Scholar]
- [19].Debette S, Compter A, Labeyrie MA, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. . Lancet Neurol. 2015;14:640–54. [DOI] [PubMed] [Google Scholar]
- [20].Reynolds EL, Burke JF, Evans L, et al. Headache neuroimaging: a survey of current practice, barriers, and facilitators to optimal use. Headache. 2022;62:36–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim JG, Choi JY, Kim SU, et al. Headache characteristics of uncomplicated intracranial vertebral artery dissection and validation of ICHD-3 beta diagnostic criteria for headache attributed to intracranial artery dissection. Cephalalgia. 2015;35:516–26. [DOI] [PubMed] [Google Scholar]
- [22].Kai Y, Nishi T, Watanabe M, et al. Strategy for treating unruptured vertebral artery dissecting aneurysms. Neurosurgery. 2011;69:1085–91; discussion 1091. [DOI] [PubMed] [Google Scholar]
- [23].Nakazawa T, Takeichi Y, Yokoi T, et al. Treatment of spontaneous intradural vertebral artery dissections. Neuroradiol J. 2011;24:699–711. [DOI] [PubMed] [Google Scholar]
- [24].Matsukawa H, Shinoda M, Fujii M, et al. Differences in vertebrobasilar artery morphology between spontaneous intradural vertebral artery dissections with and without subarachnoid hemorrhage. Cerebrovasc Dis. 2012;34:393–9. [DOI] [PubMed] [Google Scholar]
- [25].Sasaki O, Ogawa H, Koike T, et al. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1991;75:874–82. [DOI] [PubMed] [Google Scholar]
- [26].Deck JH. Pathology of spontaneous dissection of intracranial arteries. Can J Neurol Sci. 1987;14:88–91. [DOI] [PubMed] [Google Scholar]
- [27].Endo S, Nishijima M, Nomura H, et al. A pathological study of intracranial posterior circulation dissecting aneurysms with subarachnoid hemorrhage: report of three autopsied cases and review of the literature. Neurosurgery. 1993;33:732–8. [DOI] [PubMed] [Google Scholar]
- [28].Mizutani T, Kojima H, Asamoto S, et al. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg. 2001;94:712–7. [DOI] [PubMed] [Google Scholar]
- [29].Mizutani T2, Kojima H, Asamoto S. Healing process for cerebral dissecting aneurysms presenting with subarachnoid hemorrhage. Neurosurgery. 2004;54:342–8. [DOI] [PubMed] [Google Scholar]
- [30].Park KJ, Jung SC, Kim HS, et al. Multi-contrast high-resolution magnetic resonance findings of spontaneous and unruptured intracranial vertebral artery dissection: qualitative and quantitative analysis according to stages. Cerebrovasc Dis. 2016;42:23–31. [DOI] [PubMed] [Google Scholar]
- [31].Horio Y, Ogata T, Abe H, et al. Factors predictive of enlargement of dissecting aneurysms in the vertebral artery. World Neurosurg. 2021;151:e935–42. [DOI] [PubMed] [Google Scholar]