Abstract
The prognosis of patients with Ewing’s sarcoma family of tumors (ESFT) relapse is poor; the 5-year overall survival (OS) is 13%. We evaluated the effectivity of high-dose therapy (HDT) with autologous stem cell transplantation (ASCT) in adult patients with ESFT relapse. Between January 2010 and January 2021, we retrospectively analyzed 20 patients with ESFT who received HDT upon relapse. A combination of busulfan with melphalan was used as a conditioning regimen before ASCT. The median follow-up from diagnosis and from first relapse was 46.08 months (range; 10.71–186.87) and 14.41 months (range; 4.34–104.11), respectively. The median of age patients was 21.2 years (range, 17.6–25.3), and 10 (50%) patients were female. The tumor originated from the bone in 13 patients and soft tissue in 7 patients. Twelve patients had early (<2 years) relapse, and 8 patients had late (>2 years) relapse. Before HDT, 13 (65%) and 7 (35%) patients had pulmonary and extrapulmonary metastasis, respectively. After induction chemotherapy, 14 patients achieved complete response. The median OS1 and OS2 were 51.6 months (95% confidence interval [CI], range: 16.2–87) and 15.7 months (95% CI, range: 10.2–21.2), respectively. The 1-, 2-, and 5-year OS rates were 50%, 30%, and 15%, respectively. One patient died (sepsis) 1 month after ASCT. In univariate analyses, a disease-free interval (DFI) of < 2 years (P = .008) and incomplete response (P = .021) before ASCT were poor prognostic factors for OS2.HDT with ASCT can result in long-term survival of patients with ESFT relapse. HDT should be considered an important treatment opt ion in patients with a DFI > 2 years and complete response before transplantation.
Keywords: autologous stem cell transplantation, chemoterapy, ESWT, Ewing sarcoma, relapse disease
1. Introduction
Ewing’s sarcoma family of tumors (ESFT) can develop in bone and soft tissue and most commonly occur in adolescents and young adults.[1] ESFT is characterized by chromosomal translocations, the most common of which is t(11;22) (q24;q12), which is associated with EWS-FL-1 fusion (85%–90%) and strongly expresses the MIC2 (CD99) antigen.[1,2] ESFT is an aggressive disease; patients with localized disease only have a 60% to 70% 5-year overall survival (OS) despite undergoing intensive multimodality treatment, including surgery, radiotherapy, and chemotherapy.[1–4]
Approximately 30% to 40% of patients with initially localized disease experience relapse, resulting in a poor prognosis.[3,4] Currently, there is no standardized treatment for ESFT relapse[4–8]; however, radical surgery may be beneficial for local relapse.[4,5] Although salvage conventional chemotherapy is the most used treatment for disease relapse, the response rates are between 30% and 60%, and there is no standardized treatment protocol.[4–8] Despite aggressive treatment, the 5-year OS of patients with ESFT relapse is < 20%[3,5,8]; hence, alternative therapies are needed.
The feasibility of high-dose therapy (HDT) with autologous stem cell transplantation (ASCT) was evaluated in patients with localized, metastatic, recurrent, and progressive ESFT.[4,7,8] Although there are no direct, comparative, and randomized studies regarding HDT and salvage conventional chemotherapy, results of first-line treatment of ESFT relapse are acceptable.[7–9] The efficacy of HDT for ESFT relapse has been demonstrated in previous studies, and the 5-year OS ranged between 23% and 42.8%.[9–16]
To our knowledge, there are few reports regarding HDT, including the busulfan–melphalan conditioning regimen for adult patients with ESFT relapse. In this single tertiary center study, we aimed to present parameters that may determine the efficacy of HDT for ESFT relapse.
2. Methods
Between January 2010 and January 2021, 20 patients with histologically proven localized ESFT that relapsed were treated with HDT with ASCT. All patients had their tumor biopsy specimens reviewed by an expert sarcoma-specific pathologist. The following data were collected from the patients: age at diagnosis, sex, previous treatments (surgery, radiotherapy, chemotherapy), origin of the tumor (bone or soft tissue), time and type of relapse, disease status at ASCT, time to progression, and OS. At initial relapse or progression, all patients underwent full restaging, including computed tomography or magnetic resonance imaging to assess localized disease and positron emission tomography computed tomography to assess systemic involvement. The type of relapse was divided into 3: localized, systemic, and localized and systemic. Early and late relapse were defined as disease recurrence within and more than 2 years, respectively.
When relapse occurred, patients were initially treated with induction chemotherapy until remission was achieved or there was no further response. The choice of induction chemotherapy was based on disease progression and time from completion of first-line chemotherapy. For induction chemotherapy, cyclophosphamide, carboplatin, and etoposide were administered in in 12 patients, and ifosfamide-based regimens were administered in 8 patients. The disease status prior to HDT was complete remission in 14 patients and incomplete response in 6 patients. Besides systemic treatment, local treatment options, including salvage surgery and radiotherapy, were administered to patients.
Patients undergoing the busulfan and melphalan conditioning regimen received busulfan 3.2 mg/kg intravenous infusion on days − 6 through − 4, melphalan 140 mg/m2/d on days − 3 and − 2, and stem cell infusion on day 0. Engraftment was defined as a stable absolute neutrophil count of at least 0.5 g/L and a stable platelet count of at least 20 g/L.
2.1. Statistical analysis
The Response Evaluation Criteria in Solid Tumors v.1.1 were used to measure the patient responses to treatment. Adverse events were reported using the Common Terminology Criteria for Adverse Events v.4.0.
SPSS version 25.0. (IBM Corp, Armonk, NY) was used for statistical analysis. For descriptive statistics, numbers and percentages are given for categorical variables, whereas mean, standard deviation, and minimum and maximum values are given for numerical variables. Proportions in independent groups were analyzed using the Chi-square test. Comparisons of numerical variables in 2 independent groups were made using the Mann–Whitney U test or independent t-test.
Both the event-free and OS were estimated using the Kaplan–Meier test. Event-free survival (EFS) was calculated from the date of HDT to the time of progression of any existing disease or recurrent disease at any site or death if this occurred without prior disease progression. OS1 was defined as the time from diagnosis to death from any cause or last known contact. OS2 was defined as the time from ASCT to death from any cause or last known contact.
3. Results
3.1. Patient characteristics
The demographics and clinical characteristics of the patients are shown in Table 1. The median age of the study participants was 21.2 years (range, 17.6–25.3), and 10 (50%) were female. The tumor originated from the bone in 13 patients and soft tissue in 7 patients. Twelve patients had early (<2 years) relapse, and 8 patients had late (>2 years) relapse. Localized, systemic, and localized and systemic relapse occurred in 5 (25%), 10 (50%), and 5 (25%) patients, respectively. Before ASCT, 13 (65%) and 7 (35%) patients had pulmonary and extrapulmonary metastasis, respectively. After induction chemotherapy, 14 patients achieved complete response before ASCT. The median time to neutrophil and platelet engraftment was 9.5 (range, 8–12) and 10.8 (range, 9–14), respectively.
Table 1.
Demographics and clinical characteristics of patients.
| n | % | |
|---|---|---|
| Age at diagnosis; median, years (range) | 21.2 (17.6–25.3) | |
| Age at transplant; median, years (range) | 24.3 (22.3–28.2) | |
| Median follow-up time from diagnosis; months (range) | 46.08 (10.71–186.87) | |
| Median follow-up time from relapse; months (range) | 14.41 (4.34–104.11) | |
| Gender | ||
| Male | 10 | 50 |
| Female | 10 | 50 |
| Primary tissue | ||
| Bone | 13 | 65 |
| Soft tissue | 7 | 35 |
| Local control at the primary site | ||
| Surgery | 19 | 95 |
| Radiotherapy | 14 | 70 |
| Time to relapse | ||
| Early (<2 years) | 12 | 60 |
| Late (>2 years) | 8 | 40 |
| Type of relapse | ||
| Locally | 5 | 25 |
| Systemic | 10 | 50 |
| Local + systemic | 5 | 25 |
| Pulmonary metastasis (before ASCT) | 13 | 65 |
| Extrapulmonary metastasis (before ASCT) | 7 | 35 |
| Disease status at ASCT | ||
| Complete response | 14 | 75 |
| Incomplete response | 6 | 25 |
3.2. Treatment outcomes
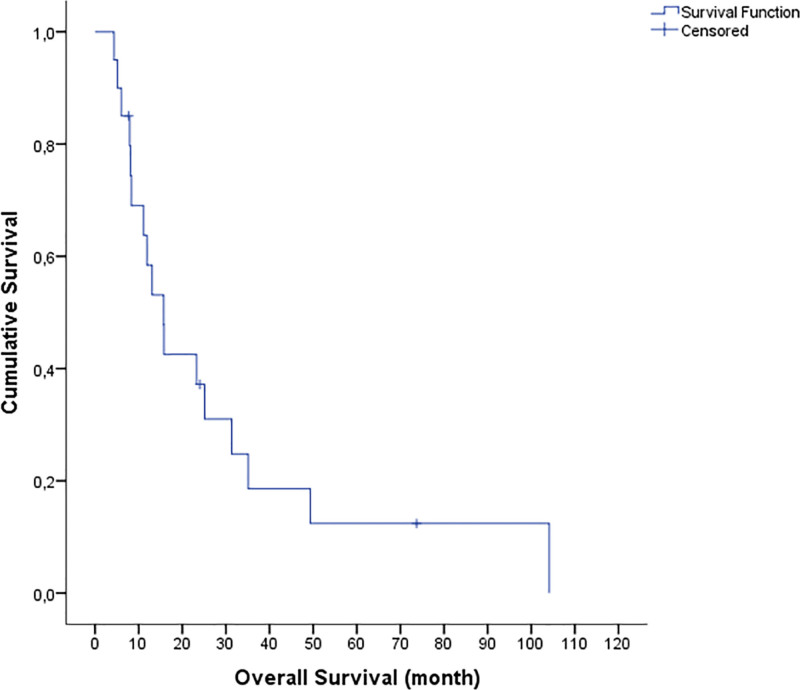
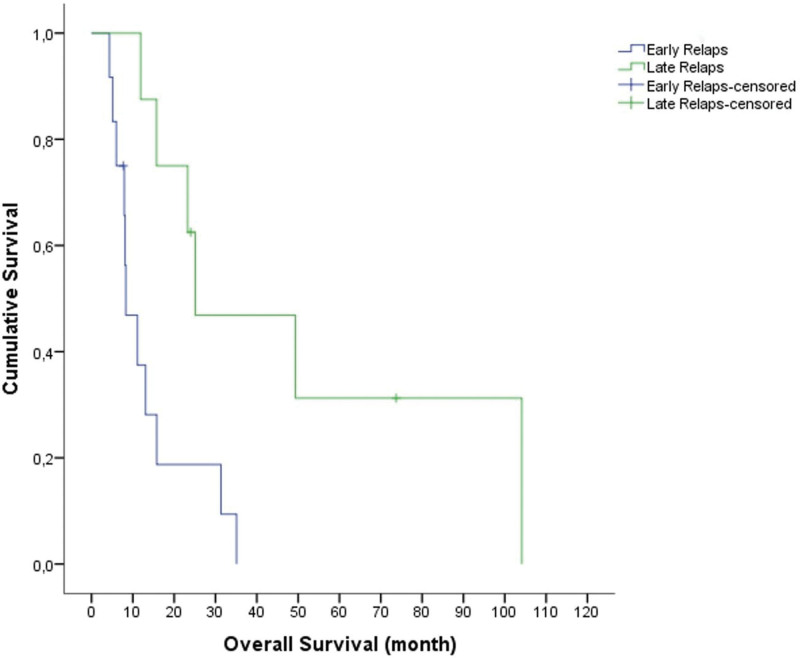
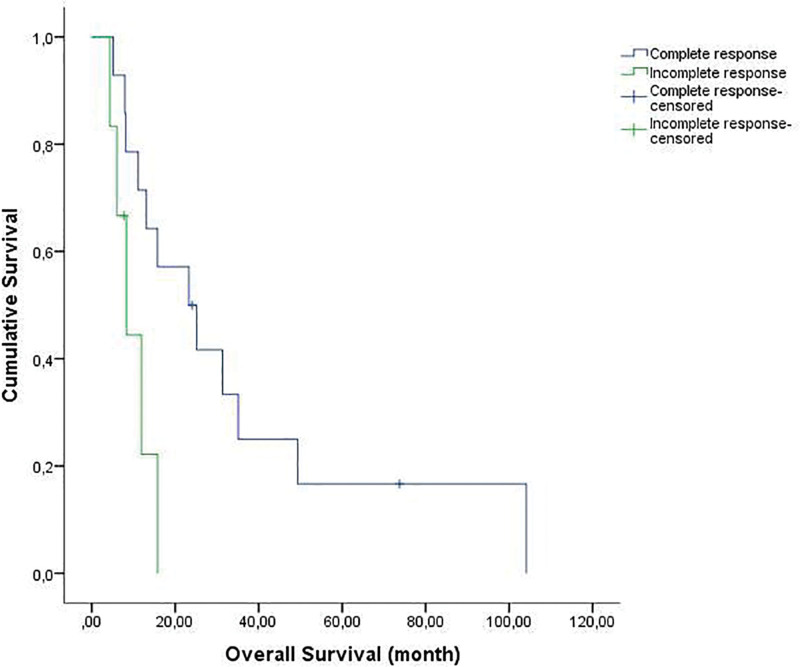
The median follow-up time from diagnosis and from initial relapse was 46.08 months (range, 10.71–186.87) and 14.41 months (range, 4.34–104.11), respectively. The OS1 was 51.6 months (95% confidence interval [CI], range: 16.2–87), and the OS2 was 15.7 months (95% CI, range: 10.2–21.2) (Fig. 1). The 1-, 2-, and 5-year OS rates were 60%, 40%, and 15% respectively. As of June 1, 2022, 3 patients were still alive after HDT. The 1- and 2-year EFS rates (from HDT) were 40% and 20%, respectively. In the univariate analysis, early relapse (P = .08) and incomplete response before ASCT (P = .02) were significantly poor prognostic factors affecting OS (Figs. 2 and 3).
Figure 1.
The median overall survival (OS) was 15.7 months (95% CI, range: 10.2–21.2). The 1-, 2-, and 5-year OS rates were 60%, 40%, and 15%, respectively.
Figure 2.
The median overall survival (OS) in patients with early relapse was 8.3 ± 2.3 months (range; 3.6–12.9 months). The median OS in patients with late relapse was 25.1 ± 15.5 months (range; 0-55.6 months) (P = .008).
Figure 3.
The median overall survival (OS) in patients with incomplete response before ASCT was 8.3 ± months (range; 3.8–12.8 months). The median OS in patients with complete response before ASCT was 15.7 ± 2.8 months (range; 10.2–21.2 months) (P = .021).
3.3. Toxicity
No death caused by ASCT occurred during HDT. However, all patients experienced grades 3–4 leukopenia, neutropenia, and thrombocytopenia after ASCT. The major grades 3–4 toxicities was summarized in Table 2. Mucositis and diarrhea were the most common grades 1–2 adverse events. One patient with pulmonary tuberculosis died one month after ASCT due to sepsis. The median duration of hospitalization after ASCT was 16.1 days (range, 12–22).
Table 2.
Grades 3 and 4 toxicities.
| Toxicity | Grades 3–4 (N = 20) | |
|---|---|---|
| No. | % | |
| Hematologic | ||
| Neutropenia, n (%) | 20 | 100 |
| Leukopenia, n (%) | 20 | 100 |
| Anemia, n (%) | 11 | 55 |
| Thrombocytopenia, n (%) | 20 | 100 |
| Nonhematologic | ||
| Mucositis/stomatitis, n (%) | 16 | 80 |
| Nausea/vomiting, n (%) | 6 | 30 |
| Diarrhea, n (%) | 6 | 30 |
| Abdominal pain, n (%) | 5 | 25 |
| Edema | 2 | 10 |
| ALT/AST increase, n (%) | 4 | 20 |
4. Discussion
In this study, we presented adult patients with ESFT relapse who were treated with ASCT. The median OS from ASCT was 15.7 months. Early relapse (<2 years) and incomplete response before ASCT were poor prognostic factors affecting OS2.
The prognosis of relapsed ESFT is poor, and there is currently no accepted standardized treatment for the disease.[4–6,8] Mono/combination therapies with different chemotherapeutic agents have been attempted, but the results have not been favorable, and relapsed ESFT appears to be chemorefractory in contrast to localized disease.[3,4,6] Therefore, effective treatment options are needed for relapsed ESFT
The effectivity of HDT with ASCT was evaluated in patients with different ESFT status. However, the most common indication of HDT in ESFT was consolidation therapy after first-line chemotherapy in high-risk localized diseases.[4,8,17,18] Its use in primary metastatic or relapse refractory disease is limited, and data are mostly from retrospective studies.5,7,10-12 HDT for relapsed ESFT has been studied, with the resulting 5-year OS between 23% and 42.8%.7,10-12 In a study by McTiernan et al, the 2- and 5-year OS rates were 51% and 43, respectively.[10] In another study by Ferrari et al, 20 patients with ESFT relapse received HDT, and the 5-year OS rate was 50%.[9] In another study by Shankar et al, the median OS was 49 months.[13] Bacci et al studied 195 patients with relapsed Ewing’s sarcoma. In their study, 33 patients received HDT, and the 5-year EFS was 21.2%.[16] In our study, the 1-, 2-, and 5-year OS rates were 60%, 40%, and 15%, respectively; 3 patients experienced long-term disease control with complete response and are still alive as of June 2022. Additionally, the 1- and 2-year EFS rates in our study were 40% and 20%, respectively, which are consistent with those in previous studies.[9–14] Notably, in our study, we found that a long 2-year DFI and a complete response before ASCT were favorable prognostic indicators of OS, although as an important limiting factor, our sample was small in number and heterogeneous. Relapse ESWT has a very aggressive course and unfortunately there is no effective treatment. Although promising results have been shown with HDT treatment in retrospective cross-sectional studies in this disease,[9–14] the lack of randomized studies is an important limitation.
In a recent study on 47 patients treated with tandem (20 patients) or single (27 patients) ASCT, Pawlowska et al reported that the 10- and 15-year OS rates were 46% (95% CI, 31%–60%) and 42% (95% CI, 27%–56%), respectively.[19] In this study, patients received an alkylating agent-based conditioning regimen that consisted of busulfan, melphalan, and topotecan. An interesting point of this study was that the disease status at ASCT (complete response) and late relapse (>2 years) were identified as good prognostic factors for OS. Previous studies have shown that HDT may be more effective in patients with ESFT relapse when they had a disease-free interval (DFI) > 2 years.[3–5,7,19] In our study, HDT was more effective in patients with DFI longer than 2 years similar to the study of Pawlowska et al In light of all these results, for patients with ESFT relapse with a DFI of > 2 years and who achieved complete remission through medical therapy and/or local treatment (surgery, radiotherapy), consolidation with HDT may be considered.
In our study, all patients experienced grades 3–4 leukopenia, neutropenia, and thrombocytopenia after ASCT. Meanwhile, mucositis and diarrhea were the most common grades 1–2 adverse effects. One patient, who had a history of pulmonary tuberculosis, died after ASCT due to sepsis; except for this event, all side effects were manageable, and the patients remained compliant to HDT.
This study has several limitations. First, its retrospective design with a small number of patients may have led to biases in the patient and treatment choices. However, it is essential to note that all patients were evaluated by the same physicians. Second, this retrospective clinical study was conducted on a heterogeneous patient population, and unlike randomized trials with strict inclusion criteria, our findings may be more representative of patients observed in routine clinical practice.
Herein, we presented real-life data from a single institution on HDT with ASCT as first-line therapy for relapsed ESFT. Based on the study findings, we recommend HDT for patients with ESFT relapse due to its effectiveness and acceptable safety profile. Despite the small number of patients in our sample, we found that a long DFI of 2 years and having achieved complete response before ASCT were positive prognostic indicators of OS.
Author contributions
Conceptualization: Nail Paksoy, Ferhat Ferhatoğlu, Ni̇jat Khanmammadov, Ayca Iribas Celik, Mert Basaran.
Data curation: Nail Paksoy, Ferhat Ferhatoğlu, Izzet doğan, Ni̇jat Khanmammadov, Ayca Iribas Celik, Mert Basaran.
Formal analysis: Nail Paksoy, Zafer Gulbas, Mert Basaran.
Investigation: Nail Paksoy, Izzet doğan, Ni̇jat Khanmammadov, Ayca Iribas Celik, Mert Basaran.
Methodology: Nail Paksoy, Ferhat Ferhatoğlu, Ni̇jat Khanmammadov, Zafer Gulbas, Mert Basaran, Ayca Iribas Celik.
Project administration: Nail Paksoy, Mert Basaran.
Resources: Nail Paksoy, Izzet doğan, Ni̇jat Khanmammadov, Ayca Iribas Celik.
Software: Nail Paksoy, Ferhat Ferhatoğlu.
Supervision: Zafer Gulbas.
Validation: Nail Paksoy, Ferhat Ferhatoğlu, Izzet doğan, Ni̇jat Khanmammadov, Ayca Iribas Celik, Zafer Gulbas, Mert Basaran.
Visualization: Zafer Gulbas, Mert Basaran.
Writing—original draft: Nail Paksoy, Zafer Gulbas, Mert Basaran.
Writing—review and editing: Nail Paksoy, Izzet doğan, Zafer Gulbas, Mert Basaran.
Abbreviations:
- ASCT =
- autologous stem cell transplantation
- CC =
- conventional chemotherapy
- DFI =
- disease-free interval
- EFS =
- event-free survival
- ESFT =
- Ewing’s sarcoma family of tumors
- HDT =
- high-dose therapy
- OS =
- overall survival
Retrospective analyses of clinical data were approved by the Academic Committee of Istanbul University (File no: 2021/2101). The committee had agreed to the retrospective analysis of routinely collected clinical data without the prior informed consent of patients.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
How to cite this article: Paksoy N, Ferhatoglu F, Dogan İ , Khanmammadov N, Iribas Celik A, Gulbas Z, Başaran M. High-dose chemotherapy followed by autologous stem cell transplantation for adult patients With first relapse of Ewing's sarcoma: A single institution experience. Medicine 2022;101:49(e32213).
Contributor Information
Ferhat Ferhatoglu, Email: drferhatoglu@gmail.com.
Nijat Khanmammadov, Email: nicatxanmemmedli@gmail.com.
Ayca Iribas Celik, Email: aycairibas@hotmail.com.
Zafer Gulbas, Email: zafer.gulbas@anadolusaglik.org.
Mert Başaran, Email: mertbasaran@yahoo.com.
References
- 1.Paulussen M, Bielack S, Jürgens H, et al.; ESMO Guidelines Working Group. Ewing’s sarcoma of the bone: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:140–2. [DOI] [PubMed] [Google Scholar]
- 2.Grünewald TGP, Cidre-Aranaz F, Surdez D, et al. Ewing sarcoma. Nat Rev Dis Primers. 2018;4:5. [DOI] [PubMed] [Google Scholar]
- 3.Stahl M, Ranft A, Paulussen M, et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer. 2011;57:549–53. [DOI] [PubMed] [Google Scholar]
- 4.Casali PG, Bielack S, Abecassis N, et al. Bone sarcomas: ESMO–PaedCan–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv79–iv95. [DOI] [PubMed] [Google Scholar]
- 5.Huang M, Lucas K. Current therapeutic approaches in metastatic and recurrent Ewing sarcoma. Sarcoma. 2011;2011:863210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Mater D, Wagner L. Management of recurrent Ewing sarcoma: challenges and approaches. Onco Targets Ther. 2019;12:2279–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenneti P, Zahid U, Iftikhar A, et al. Role of high-dose chemotherapy and autologous hematopoietic cell transplantation for children and young adults with relapsed Ewing’s sarcoma: a systematic review. Sarcoma. 2018;2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesla AC, Papakonstantinou A, Tsagkozis P. Current status of management and outcome for patients with Ewing sarcoma. Cancers. 2021;13:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari S, Luksch R, Hall KS, et al. Post‐relapse survival in patients with Ewing sarcoma. Pediatr Blood Cancer. 2015;62:994–9. [DOI] [PubMed] [Google Scholar]
- 10.McTiernan A, Driver D, Michelagnoli MP, et al. High dose chemotherapy with bone marrow or peripheral stem cell rescue is an effective treatment option for patients with relapsed or progressive Ewing’s sarcoma family of tumours. Ann Oncol. 2006;17:1301–5. [DOI] [PubMed] [Google Scholar]
- 11.Rasper M, Jabar S, Ranft A, et al. The value of high‐dose chemotherapy in patients with first relapsed Ewing sarcoma. Pediatr Blood Cancer. 2014;61:1382–6. [DOI] [PubMed] [Google Scholar]
- 12.Barker LM, Pendergrass TW, Sanders JE, et al. Survival after recurrence of Ewing’s sarcoma family of tumors. J Clin Oncol. 2005;23:4354–62. [DOI] [PubMed] [Google Scholar]
- 13.Shankar AG, Ashley S, Craft AW, et al. Outcome after relapse in an unselected cohort of children and adolescents with Ewing sarcoma. Off J SIOP. 2003;40:141–147. [DOI] [PubMed] [Google Scholar]
- 14.Laurence V, Pierga JY, Barthier S, et al. Long-term follow up of high-dose chemotherapy with autologous stem cell rescue in adults with Ewing tumor. Am J Clin Oncol. 2005;28:301–9. [DOI] [PubMed] [Google Scholar]
- 15.Lamm W, Rabitsch W, Köstler WJ, et al. Autologous stem cell transplantation in adults with metastatic sarcoma of the Ewing family: a single centre experience. Wien klin Wochenschr. 2013;125:129–33. [DOI] [PubMed] [Google Scholar]
- 16.Bacci G, Ferrari S, Longhi A, et al. Therapy and survival after recurrence of Ewing’s tumors: the Rizzoli experience in 195 patients treated with adjuvant and neoadjuvant chemotherapy from 1979 to 1997. Ann Oncol. 2003;14:1654–9. [DOI] [PubMed] [Google Scholar]
- 17.Whelan J, Le Deley MC, Dirksen U, et al. High-dose chemotherapy and blood autologous stem-cell rescue compared with standard chemotherapy in localized high-risk Ewing sarcoma: results of Euro-EWING 99 and Ewing-2008. J Clin Oncol. 2018;36:3110–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz MA, Lassaletta A, Perez A, et al. High-dose busulfan and melphalan as conditioning regimen for autologous peripheral blood progenitor cell transplantation in high-risk Ewing sarcoma patients: a long-term follow-up single-center study. Pediatr Hematol Oncol. 2010;27:272–82. [DOI] [PubMed] [Google Scholar]
- 19.Pawlowska AB, Sun V, Calvert GT, et al. Long-term follow-up of high-dose chemotherapy with autologous stem cell transplantation in children and young adults with metastatic or relapsed Ewing sarcoma: a single-institution experience. Transplantation and cellular Therapy. 2021;27:72.e172-e. 72.e7 [DOI] [PubMed] [Google Scholar]