Abstract
Forkhead transcription factor O1 (FOXO1) methylation is associated with inflammation. Diabetic kidney disease (DKD) is characterized with increased inflammatory markers such as uric acid, hemogram indices, C-reactive protein derived markers, omentin and neuregulin. This study aimed to investigate the effect of DNA methylation in FOXO1 gene promoter, blood glucose and lipids in the process of type 2 DKD. Bisulfite genomic sequencing was used to monitor DNA methylation in the promoter region (+1021, +1193) of FOXO1 gene. The detections were taken in glycosylated hemoglobin A1c, fasting plasma glucose and blood lipid. 81 participants were divided into the control group, the preliminary diabetes mellitus group, the pure diabetes mellitus group, and the DKD group. The other groups displayed higher fasting plasma glucose than the control group (all P value < .05). The fasting plasma glucose level was higher in the pure diabetes mellitus group than the preliminary diabetes mellitus group (P = .004). The levels of HbA1c were higher in other groups than control group and preliminary diabetes mellitus groups (all P values < .01). The high-density lipoprotein level was lower in the DKD group (P = .021, P = .022) than control and pure diabetes mellitus group. The levels of low-density lipoprotein were statistically lower in preliminary diabetes mellitus and DKD groups than control group (all P value < .02). Along with the progress of DKD, a down trend was observed in the total methylation rate of FOXO1 gene (P = .025), which contains 5 CpG sites (1021, +1193) in the promoter. Hypomethylation in the promoter of FOXO1 gene, hyperglycemia and low level of serum lipid might be associated with the pathogenesis of type 2 DKD.
Keywords: diabetic kidney disease, DNA methylation, forkhead box protein O1, glycosylated hemoglobin A1c
1. Introduction
China’s economy has grown rapidly since the reform and opening up. At the same time, unhealthy eating patterns become more and more popular such as western diet and overeating, as well as sedentary and staying up-to-night lifestyles, which directly leads to a sharp increase in the prevalence of diabetes in China these years, from 0.67% in 1980 to 10.4% in 2013.[1,2] Type 2 diabetes is the most common type of diabetes, accounting for about 90% of diabetes cases.[3] Diabetes itself and its complications have seriously affected the quality of life.[4] As the most common and harmful microvascular complication in the late stage of diabetes, the pathogenesis of diabetic kidney disease (DKD) is not yet fully understood.[5,6]
Forkhead transcription factor O1 (forkhead box protein O1, FOXO1) belongs to one of the FOXO family members.[7] Previous studies have shown that FOXO1 plays an important role in the pathogenesis of diabetes and DKD.[7,8] FOXO1 methylation is associated with inflammation.[9] On the other hand, DKD is characterized with increased inflammatory markers such as uric acid,[10] hemogram indices,[11] C-reative protein derived markers,[12] omentin,[13] and neuregulin.[14] Previous literatures also found that DNA methylation can regulate the expression of FOXO1 gene during the onset of diabetes.[15,16] Thus, studying FOXO1 in DKD is logical and prospective. We plan to observe the changes in blood glucose, blood lipids and FOXO1 gene methylation during the pathogenesis of DKD in type 2 diabetes, aiming to explore the potential epigenetic pathogenesis of DKD and provide new perspectives for its diagnosis and treatment.
2. Methods
2.1. Study subjects
A total of 81 healthy people and type 2 diabetes patients were selected as the study subjects, who completed physical examinations at Longgang People’s Hospital from July 2015 to December 2016. The classification of the subjects was based on the “Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2019 Edition).” We divided the subjects with fasting blood-glucose less than 6.1 mmol/L and postprandial blood glucose less than 7.8 mmol/L into the control group, and the others with 6.1~7.0 mmol/L fasting blood glucose or 7.8~11.1 mmol/L postprandial blood glucose into preliminary diabetes mellitus group. Based on the “Expert Consensus on the Prevention and Treatment of Diabetic Nephropathy (2014 Edition),” the cases were further classified into the pure diabetes mellitus group (Urinary Albumin Excretion Rate < 30 mg/24 hour) and the DKD group (Urinary Albumin Excretion Rate ≥ 30 mg/24 hour), taking confirmed medical records into account simultaneously.[17] Exclusion criteria referred to previous literature.[17]
2.2. Equipment and reagents
Genomic DNA extraction kit, DNA gel recovery kit and Bisulfite conversion kit (Beijing Tiangen Biological Co., Ltd.); Taq enzyme (Nanjing Vazyme Biotechnology Co., Ltd.); FOXO1 gene methylation polymerase chain reaction primers (Wuhan Tianyi Huiyuan Biotechnology Co., Ltd.); PMD19 vector and Escherichia coli DH5α competent cells (Dalian Bao Biological Engineering Co., Ltd.); Automatic biochemical analyzer and supporting reagents (Beckman AU5811); Hemoglobin test system and supporting reagents (BD VARI).
2.3. Experimental procedures
2.3.1. Sample collection.
Five mL venous blood was collected using blood collection tube (EDTA anticoagulation) for detection of glycosylated hemoglobin and the remaining blood for methylation test. Synchronously, 3 mL venous blood was collected and placed in a procoagulant blood collection tube, coagulated at room temperature for 30 minutes, and centrifuged at 1000 g × 15 minutes to detect fasting plasma glucose (FPG), blood lipids and other biochemical indicators.
2.3.2. Bisulfite genome sequencing.
(1) Design of methylation primer
The base sequence of human FOXO1 gene promoter was found according to NCBI database and ensembl database. Then we use the methylation primer design software (MethPrimer online software) to confirm the existence of CpG islands in the gene promoter region, so as to design the methylation primers for bisulfite genome sequencing. See Table 1 for specific information of primers.
Table 1.
The information of the methylation primer sequence of FOXO1 gene.
| Gene | Primer sequence | Primer length (bp) | Annealing temperature (◦C) |
|---|---|---|---|
| FOXO1 | F: 5’- AAGGATAAG GGTGATAGTAATAGTT -3’ R: 5’- ACAACTCTTC TCCTAAAAAATTTCC -3’ |
173 | 50 |
FOXO1 = Forkhead transcription factor O1.
(2)Bisulfite sequencing:
Human genomic DNA was extracted from whole blood. DNA concentration, purity and integrity was determined by 1.0% agarose gel electrophoresis. The genomic DNA in each group was mixed and the bisulfite conversion was completed. 2 μL transformed sample was taken to carry out methylation Polymerase Chain Reaction test: preheat at 95°C for 5 minutes; denature at 95°C for 20 seconds, annealing at 50°C for 20 seconds, extension at 72°C for 30 seconds; repeat for 30 cycles. Keep at 72°C for 5 minutes and store at 4°C. See Table 2. Then we use 2.0% agarose gel electrophoresis to separate and purify the methylated bands under UV light. PMD19 vector was connected to the 5 μL purified and recovered product, and was transformed into E coli DH5α competent cells to complete the blue and white clonal spot screening test. Then we select the positive cloned bacteria and culture them at 37℃, 200 rpm/min with constant temperature shaking for 8 hours, and then take 10 to 12 turbid bacterial solutions from each group as soon as possible to send to Wuhan Tianyi Huiyuan Biotechnology Co., Ltd. for sequencing. Biochemical Indicators: FPG, blood lipids and other biochemical indicators were detected using automatic biochemical analyzer (Beckman AU5811) and supporting reagents. HbA1c: HbA1c was detected by high pressure liquid chromatography with hemoglobin test system (BD VARI) and supporting reagents.
Table 2.
The PCR trial system.
| The component | The application amount (μL) |
|---|---|
| 2 × Taq Master Mix (Dye Plus) | 25 |
| Forward primer | 1 |
| Reverse primer | 1 |
| DNA template | 2 |
| ddH2O | 21 |
| Total volume | 50 |
PCR = polymerase chain reaction.
2.4. Statistical analysis
A normal distribution of our data was shown using the normality test. Difference between measurement data, which was represented as Mean ± Stand Error (S.E.), was compared through 1-way analysis of variance and that between groups with SNK test on the condition that equal variance assumed. Tamhane T2 method was applied in the case of heterogeneity of variance. Chi-squared test was applied for analysis of categorical variable. The data was entered into SPSS13.0 software for analysis. The test level is set as α = 0.05 for 2-tailed test. P < .05 is regarded as a statistically significant difference.
3. Results
3.1. General information
The general information of the subjects is shown in Table 3. There was no statistically significant difference in gender between different groups (χ² = 1.488, P = .685), but there was a statistically significant difference in age (χ² = 2.955, P = .038).
Table 3.
The basic information of different groups.
| Items | Control group | Pre-diabetes mellitus group | Pure diabetes mellitus group | DKD group |
|---|---|---|---|---|
| Gender (male/female) | 20 (12/8) | 11 (8/3) | 29 (15/14) | 21 (12/9) |
| Age (yrs) | 48.15 ± 13.99 | 41.36 ± 17.65 | 49.48 ± 17.11 | 57.81 ± 13.89b |
DKD = diabetic kidney disease.
Compared with the control group, aP < .05. Compared with the preliminary diabetes mellitus group, bP < .05. Compared with the pure diabetes mellitus group, cP < .05.
3.2. Biochemical indicators
There was no statistically significant difference in triglyceride and total cholesterol among the 4 groups of study subjects (χ² = 2.955, all P values > .05). FPG, HbA1c, high-density lipoprotein and low-density lipoprotein were with statistically difference in 4 groups (all P values < .05). Among them, compared with the control group, the FPG of the other 3 groups was significantly increased (all P values < .05). Compared with the preliminary diabetes mellitus group, the FPG of the pure diabetes mellitus group increased significantly (P = .004). Compared with the control group and the preliminary diabetes mellitus group, glycosylated hemoglobin in the other 2 groups was significantly increased (all P values < .01). Compared with the control group and the pure diabetes mellitus group, the high-density lipoprotein of the DKD group was significantly lower (P = .021, P = .022). Both the preliminary diabetes mellitus group and the DKD group were lower in low-density lipoprotein than the control group (P = .018, P = .002). The biochemical indicators of each group are shown in Table 4.
Table 4.
The basic information of blood biochemistry.
| Items | The control group | Preliminary diabetes mellitus group | Pure diabetes mellitus group | DKD group |
|---|---|---|---|---|
| FPG (mmol/L) | 4.56 ± 0.52 | 6.19 ± 1.37 a | 10.32 ± 5.54 ab | 8.46 ± 3.11 a |
| HbA1c (%) | 5.55 ± 0.35 | 6.64 ± 1.21 | 9.70 ± 2.76 ab | 9.34 ± 2.80 ab |
| Triglyceride(mmol/L) | 1.36 ± 0.56 | 1.86 ± 1.00 | 2.29 ± 2.06 | 2.06 ± 1.02 |
| Total cholesterol (mmol/L) | 5.44 ± 1.16 | 4.22 ± 0.62 | 6.14 ± 7.31 | 4.31 ± 0.96 |
| High density lipoprotein (mmol/L) |
1.49 ± 0.30 | 1.48 ± 0.43 | 1.45 ± 0.73 | 1.11 ± 0.30 ac |
| Low density lipoprotein (mmol/L) | 3.27 ± 0.97 | 2.44 ± 0.60 a | 2.78 ± 1.06 | 2.38 ± 0.76 a |
DKD = diabetic kidney disease, FPG = fasting plasma glucose, HbAlc = glycosylated hemoglobin.
Compared with the control group, aP < .05. Compared with the preliminary diabetes mellitus group, bP < .05. Compared with the pure diabetes mellitus group, cP < .05.
3.3. DNA methylation results
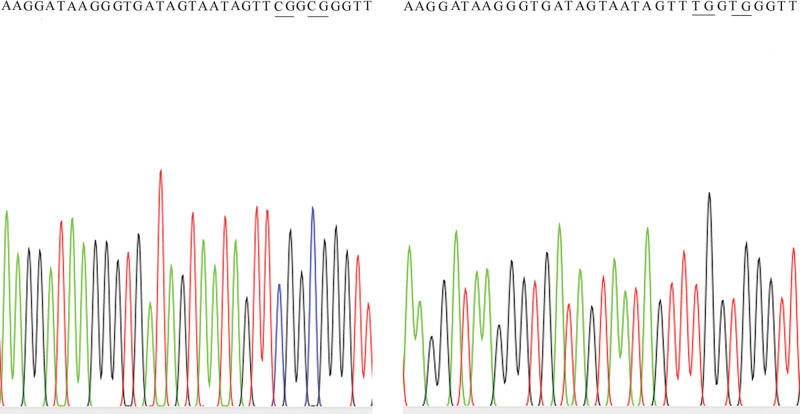
It is estimated that the promoter region (+1021, +1193) of the human FOXO1 gene contains a total of 5 CpG sites. As diabetes progresses, the overall methylation ratio of the human FOXO1 gene promoter region (+1021, +1193) gradually decreases, and the difference is statistically significant (χ² = 9.319, P = .025). See Table 5 and Figure 1.
Table 5.
The data of DNA methylation on CpG sites of FOXO1 gene.
| Sites | Control group | Preliminary diabetes mellitus group |
Pure diabetes mellitus group |
DKD group |
|---|---|---|---|---|
| CpG (%) | 12 (24%) | 6 (13.33%) | 3 (6.67%) | 3 (6%) |
| TpG (%) | 38 (76%) | 39 (86.67%) | 42 (93.33%) | 47 (94%) |
DKD = diabetic kidney disease, FOXO1 = Forkhead transcription factor O1.
Figure 1.
Graphic representation of DNA methylation of FOXO1 gene (Left: The control Group; Right: The DKD group). DKD = diabetic kidney disease, FOXO1 = Forkhead transcription factor O1.
4. Discussion
FOXO1 transcription factor participates in the metabolism, growth and development and tumor formation by regulating various physiological and pathological processes such as oxidative stress, cell proliferation and apoptosis.[18] In particular, FOXO1 plays an important role in lipid metabolism disorders and microvascular diseases, which affect the pathogenesis of kidney disease to varying degrees.[7,19] DKD may be closely associated with the changes in epigenetic modification of type 2 diabetes. Epigenetic modification is a heritable phenotypic variation other than base sequence changes. The most widely known one is DNA methylation.[20]
This is a reaction in which a methyl group is added to the 5’ position of cytosine under the catalysis of an enzyme to form 5-methylcytosine, which mainly occurs in CpG islands of guanine and cytosine-enriched genes. The hypermethylation of CpG islands in the promoter region of certain genes can lead to a decline in gene expression or even silencing.[17,18] Studies have found that the DNA methylation level of FOXO1 gene in vascular smooth muscle cells decreases, and the expression of FOXO1 protein increases, which is closely associated with lipid metabolism.[15] This suggests that FOXO1 gene methylation may play an important role in the pathogenesis of DKD.
In this study, as the course of diabetes progressed, FPG and HbA1c increased significantly, while high-density lipoprotein and low-density lipoprotein decreased significantly. This shows that the pure diabetes mellitus group and DKD group have different degrees of blood glucose and lipid metabolism disorders. This is consistent with previous reports of glucose metabolism disorders in diabetic patients characterized by increased FPG and HbA1c.[21–24] Our study suggested that the high-density lipoprotein of the DKD group was significantly decreased, compared with the control group (P = .021) and the pure diabetes mellitus group (P = .022). Compared with the control group, low density lipoprotein levels in the preliminary diabetes mellitus group (P = .018) and DKD group (P = .002) decreased significantly. This is inconsistent with the decrease of high-density lipoprotein and/or the increase of low-density lipoprotein in patients with diabetes or DKD in previous studies. It is supposed that patients with diabetes or DKD may have taken statins.
This study also found that the overall methylation ratio of the human FOXO1 gene promoter region (+1021, +1193) gradually decreased (χ² = 9.319, P = .025) as the disease progressed, suggesting that the overall methylation of the FOXO1 gene promoter region may play an crucial part in the pathogenesis of DKD. This is consistent with the results that a significant decrease in FOXO1 gene methylation level in patients with diabetic retinopathy, compared with diabetic patients, observed by Gao.[10] Other studies have shown that the reduction of methylation in the FOXO1 promoter region of smooth muscle cells is affected by oxidized low-density lipoprotein. Furthermore, it promotes lipid deposition and cell transformation in smooth muscle cells, which may result in abnormal lipid metabolism.[15]
In this study, the simultaneous occurrence of abnormal glucose and lipid metabolism and FOXO1 gene hypomethylation suggests that during the pathogenesis of DKD, hypomethylation in the promoter region of the FOXO1 gene may associated with increased gene expression, which is closely related to abnormal glucose metabolism and lipid metabolism. However, the causal relationship between them is still unclear. Moreover, several other aspects in which further research work should be intensified are put forward, such as FOXO1 gene methylation and mRNA and protein expression during the pathogenesis of DKD, temporality between metabolic abnormalities and FOXO1 gene epigenetic modification regulation through animal models.
In conclusion, by preliminarily exploring the relationship between FOXO1 gene DNA methylation, abnormal metabolism and the onset of DKD in patients with type 2 diabetes, we speculates that hypomethylation in the promoter region of FOXO1 gene promotes gene transcription, relating to increased FOXO1 protein expression, which in turn associates with abnormal sugar metabolism and lipid metabolism, eventually may lead to DKD. During the early stages of DKD, detection of epigenetic events could be valuable for timely diagnosis and prompt treatment to prevent end-stage renal disease’s progression. Identification of DKD’s epigenetic signatures might also inform precision medicine approaches.
Acknowledgments
We would like to acknowledge all participants of this project and investigators for collecting data.
Authors contributions
J.L. lead the study. X.L. performed the data analysis, implemented the methodology; X.L. and Z.G. prepared the original draft; J.L. reviewed and edited the final manuscript.
Conceptualization: Jing Liao.
Data curation: Xiaofeng Li.
Formal analysis: Xiaofeng Li.
Funding acquisition: Jing Liao.
Methodology: Xiaofeng Li, Jing Liao.
Writing – original draft: Jing Liao, Zhongqiu Guo.
Writing – review & editing: Jing Liao.
Abbreviations:
- DKD =
- diabetic kidney disease
- FOXO1 =
- forkhead transcription factor O1
- FPG =
- fasting plasma glucose
The study was approved by the Longgang People’s Hospital, Shenzhen. All procedures performed in this study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments. Participants were informed that the survey was totally voluntary. The subjects’ confidentiality was protected by ensuring that the data were addressed in anonymous mode with personal information appropriately de-identified.
This work was financially supported by the Science and Technology Project of Shenzhen [grant number JCYJ20180228164129711], Scientific Research Project of Science and Technology Innovation Bureau of Longgang District, Shenzhen [grant number LGKCYLWS2018000012, LGKCYLWS2018000004]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. J.L. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no competing interests.
How to cite this article: Li X, Liao J, Guo Z. Detection value of FOXO1 gene methylation, blood glucose and lipids in patients with type 2 diabetic kidney disease. Medicine 2022;101:49(e31663).
Contributor Information
Xiaofeng Li, Email: lixiaofeng198302@163.com.
Zhongqiu Guo, Email: guozhongqiu@163.com.
References
- [1].Liu M. Study on the regional distribution of diabetes in my country and its disease burden. Chinese Center for Disease Control and Prevention; 2019. [Google Scholar]
- [2].Diabetes Branch of Chinese Medical Association. Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2017 Edition). Chin J Prac Int Med. 2018;38:292–344. [Google Scholar]
- [3].Xu G, Liu B, Sun Y, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang WY. Epidemic characteristics and changing trends of diabetes in China. Sci China. 2018;48:812–9. [Google Scholar]
- [5].Chen M. Systematic review and meta-analysis of the treatment of early diabetic nephropathy with integrated traditional Chinese and western medicine. Beijing University of Chinese Medicine; 2017. [Google Scholar]
- [6].Li JX, Ma TT, Nan Y. Research progress on the pathogenesis of diabetic nephropathy. J Clin Nephrol. 2019;19:860–4. [Google Scholar]
- [7].Chen JW, Wang ZF, Tie L. Study on the role of transcription factor FOXO1 in diabetes. Adv Physiol Sci. 2014;45:55–60. [PubMed] [Google Scholar]
- [8].Zhu YH, Zhou HN, Yang B. Research progress of FOXO1 gene. Chin J Prac Diagn Ther. 2014;28:423–5. [Google Scholar]
- [9].Jian D, Wang Y, Jian L, et al. METTL14 aggravates endothelial inflammation and atherosclerosis by increasing FOXO1 N6-methyladeosine modifications. Theranostics. 2020;10:8939–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kocak MZ, Aktas G, Duman TT, et al. Is uric acid elevation a random finding or a causative agent of diabetic nephropathy? Rev Assoc Med Bras (1992). 2019;65:1155–60. [DOI] [PubMed] [Google Scholar]
- [11].Kocak MZ, Aktas G, Erkus E, et al. Mean platelet volume to lymphocyte ratio as a novel marker for diabetic nephropathy. J Coll Physicians Surg Pak. 2018;28:844–7. [DOI] [PubMed] [Google Scholar]
- [12].Bilgin S, Kurtkulagi O, Atak TB, et al. Does C-reactive protein to serum Albumin Ratio correlate with diabetic nephropathy in patients with Type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diab. 2021;15:1071–4. [DOI] [PubMed] [Google Scholar]
- [13].Tekce H, Tekce BK, Aktas G, et al. Serum omentin-1 levels in diabetic and nondiabetic patients with chronic kidney disease. Exp Clin Endocrinol Diabetes. 2014;122:451–6. [DOI] [PubMed] [Google Scholar]
- [14].Kocak MZ, Aktas G, Atak BM, et al. Is Neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur J Clin Invest. 2020;50:e13206. [DOI] [PubMed] [Google Scholar]
- [15].Hou MM. Study on the mechanism of ox-LDL regulating lipid deposition and phenotype transformation of smooth muscle cells via DNA methylation of the transcription factor FoxO1. Ningxia Medical University; 2018. [Google Scholar]
- [16].Gao CL, Zhang H, Zou XY. Study on the relationship between serum homocysteine level and FOXO1 DNA methylation in peripheral blood in diabetic retinopathy. J Ningxia Med Univ. 2016;38:1366–70. [Google Scholar]
- [17].Li XF, Liao J. The role of FOXP3 gene methylation in type 2 diabetic nephropathy. Chin Trop Med. 2017;17:664–7. [Google Scholar]
- [18].Yi Z, Shi Y, Zhao P, et al. Overexpression of miR-217-5p protects against oxygen-glucose deprivation/reperfusion-induced neuronal injury via inhibition of PTEN. Hum Cell. 2020;33:1026–35. [DOI] [PubMed] [Google Scholar]
- [19].Shi WX, Li N, Sui MS. Research progress on the role of forkhead box transcription factor O in kidney disease. Chin J Prac Diagn Ther. 2018;32:205–8. [Google Scholar]
- [20].Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 2019;15:327–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yu AP, Sun J. Diagnostic value of combined detection of homocysteine, glycosylated hemoglobin and urine microalbumin in early diabetic kidney injury. Mod Chin Doctor. 2015;53:100–102,112. [Google Scholar]
- [22].Xi WR. The value of glycosylated hemoglobin detection in the diagnosis of diabetes. J Prac Diab. 2015;11:12–3. [Google Scholar]
- [23].Lai MS, Jiang Y, Yu F. Application value analysis of combined detection of blood glucose and glycosylated hemoglobin in the diagnosis of diabetes. Chin Prac Med. 2020;15:97–8. [Google Scholar]
- [24].Cui W, Jiang HY, Li J. The clinical significance of glycosylated albumin in the diagnosis of diabetes and diabetic nephropathy. Chin Prac Med. 2020;15:1–4. [Google Scholar]