Background.
Fungal infections are a recognized complication of immunosuppression in solid organ transplant recipients. Phaeohyphomycoses are fungal infections caused by a diverse group of dematiaceous fungi.
Methods.
We share the learning points from 2 Australian cases of phaeohyphomycosis secondary to Phaeacreomonium species (spp). A literature review was performed using Medline, Embase, and Google Scholar to identify this condition among kidney transplant recipients.
Results.
With the 2 cases reported in this article, a total of 17 cases were identified in the literature. Phaeacremonium spp is ubiquitous in humid and temperate flora, including Australia. Minor trauma is likely the source of inoculation in most cases and diagnosis is often delayed. Presently, no guidelines for management exist given the rarity of this condition. Most known cases have been treated with surgical debulking combined with long-course antifungal therapy.
Conclusion.
This paper describes 2 Australian cases of phaeohyphomycosis in kidney transplant recipients. A high index of suspicion, especially in the immunosuppressed, is essential for timely diagnosis in kidney transplant recipients. There are several diagnostic and therapeutic challenges that remain with this condition.
Fungal infections are a recognized complication of immunosuppression in solid organ transplant recipients. They are typically associated with high patient morbidity and mortality.1 Candida species (spp) and Aspergillus spp are most frequently encountered, and manifestations may range from localized skin and soft tissue infections to disseminated infection, which can involve pulmonary, cardiac, osseous, and intracranial sites.2 Phaeohyphomycoses are fungal infections caused by a diverse group of dematiaceous fungi.3 We report 2 Australian cases of phaeohyphomycosis caused by Phaeoacremonium spp in kidney transplant recipients, presenting years after successful transplantation. Both the patients presented on the background of gardening injuries to the site of infection with presumed cutaneous inoculation. We highlight the presentation and clinical history and discuss the surgical management and clinical relevance in the context of the existing literature.
CASE 1
A 65-y-old male of Tongan descent presented with a 10-mo history of an enlarging tender lump on his left hand. This occurred on the background of successful kidney transplantation 2 y ago in the context of non–steroidal anti-inflammatory drug–induced glomerulonephritis. He had been stable on a maintenance regime for immunosuppression comprising oral tacrolimus, mycophenolate mofetil, and prednisolone since his transplant. His medical history was also notable for obstructive sleep apnea, hypertension, and an 80 pack/ smoking history. He was recently screened for diabetes with a normal hemoglobin A1c of 5%. Additionally, 1 mo before his presentation, he was noted to have had coronavirus disease 2019–related respiratory illness, requiring further immunosuppression with oral baricitinib, a single dose of tocilizumab, and a 10-d course of oral dexamethasone.
On further history, the patient was found to be an avid gardener, and he described previous minor trauma sustained to the volar aspect of his left thumb base 12 mo before his presentation while pruning in his vegetable patch. Physical examination revealed only mild tenderness over the site of injury but was otherwise unremarkable. A magnetic resonance imaging (MRI) scan of the left hand showed the presence of a multilocular cyst with internal septations. It appeared to be arising from the deep fascia, encasing the thenar eminence, but was separate from the flexor tendon sheath (Figure 1A). Differential diagnoses at the time of his presentation included ganglion cyst, bacterial abscess, and foreign body.
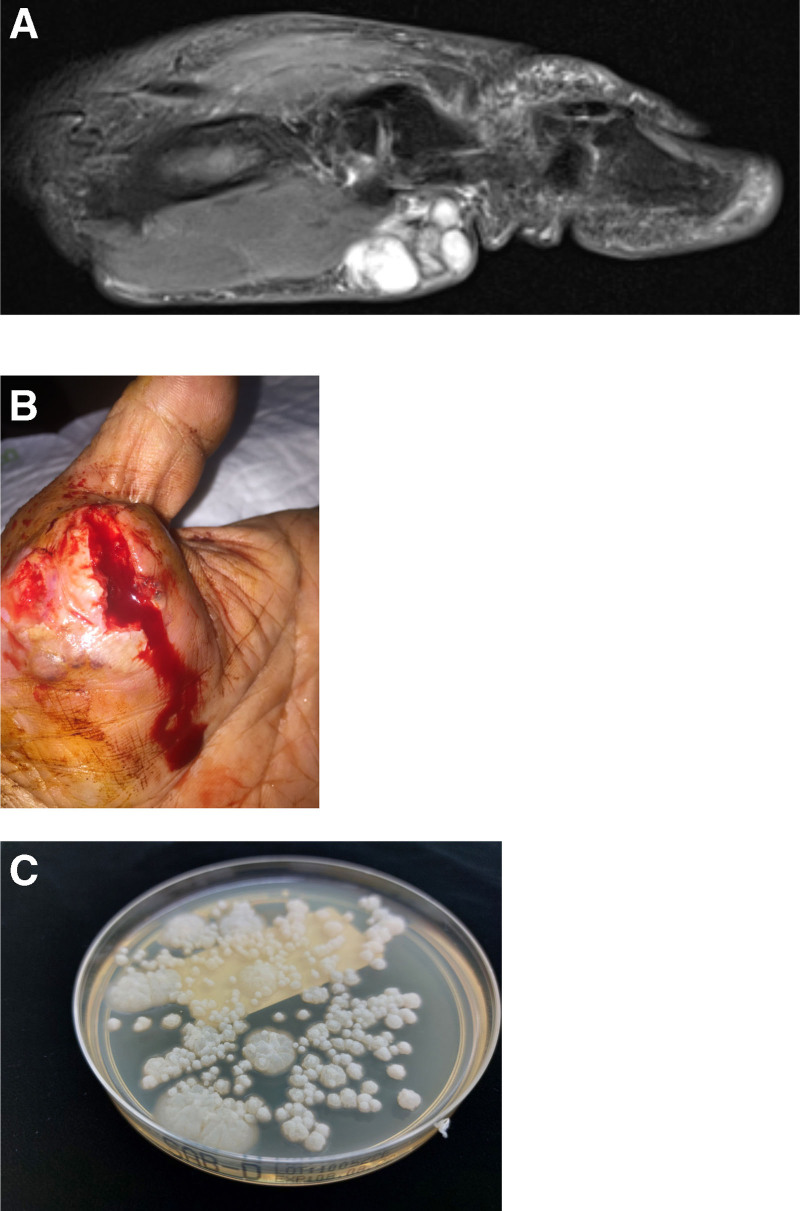
FIGURE 1.
Case 1 images. A, T2 MRI sagittal view demonstrating cystic collection over thenar eminence for case 1. B, Fungal mycetoma in case 1. C, Microbiology of case 1: growth of fungus (mold) on plate. The whitish-gray colony morphology reflects quite young colonies. MRI, magnetic resonance imaging.
Several days later, the cutaneous lesion spontaneously ruptured with copious purulent discharge and was treated with bedside saline gauze packing to the wound (Figure 1B). Microscopy performed using Gram stain on the specimen demonstrated the presence of fungal hyphae, suggestive of invasive fungal disease. Histopathology was consistent with necrotizing granulomatous inflammation. Further staining with periodic acid–Schiff and methenamine silver identified oval spores and septate fungal organisms. The specimen was eventually cultured on routine bacterial, mycobacterial, and mycological solid media, including Sabouraud dextrose agar and potato dextrose agar. A fungus was isolated on day 7 of incubation (Figure 1C). Although a lactophenol cotton blue stain was nondiagnostic, panfungal polymerase chain reaction targeting the fungal genome confirmed the presence of Phaeoacremonium parasiticum. Susceptibility testing was performed using broth microdilution according to Clinical and Laboratory Standards Institute methodology. The minimal inhibitory concentrations (MICs) for voriconazole, itraconazole, and posaconazole were 0.12, 2, and 0.5 µg/mL, respectively. The MIC for amphotericin B was 1 µg/mL.
Following the diagnosis, the patient was assessed for the possibility of disseminated invasive fungal infection in the context of his immunosuppression and notable symptoms of a dry cough, persistent headache, and upper thoracic back pain. This included a high-resolution chest computed tomography (CT), brain CT, CT spine, transthoracic echocardiogram, and panfungal nucleic acid testing on cerebrospinal fluid. These results were all negative.
The patient was commenced on voriconazole as preliminary microbiology suggested an elevated itraconazole MIC. The final regimen was eventually streamlined to weight-based dosing of regular oral voriconazole 200 mg twice daily. A voriconazole trough was performed at 1 and 3 mo, which was 1.5 mg/L. At 1-mo follow-up, a dramatic improvement had been noted in the clinical appearance of the patient’s hand. An MRI scan of the hand demonstrated significant regression of the thenar eminence cystic lesion; however, it raised the possibility of underlying carpal bone involvement. Given concerns for possible osteomyelitis and ongoing pain, a decision was made to pursue formal surgical debridement alongside oral voriconazole therapy. Surgical debridement revealed no discrete drainable collection. Intraoperative specimens revealed generalized inflammatory tissue. On follow-up at 3 mo, his symptoms had resolved, and the wound was healing well. He remains on oral voriconazole therapy, with an ongoing duration dependent on clinical and radiological progress.
CASE 2
A 41-y-old male of Southeast Asian descent presented with worsening pain in the plantar aspect of his left foot. This was on a background of kidney transplantation for focal segmental glomerulosclerosis 6 mo before and type 2 diabetes. He was on a maintenance immunosuppressive regime of tacrolimus, mycophenolate mofetil, and prednisolone. He reported a minor splinter injury while mowing his lawn in uncovered footwear 3 mo before his presentation. This subsequently developed into a presumed abscess, which was treated in a peripheral hospital with attempted drainage. The intraoperative specimen yielded negative microscopy and bacterial culture. Unfortunately, no further testing for fungal pathology was undertaken with this specimen.
The patient’s symptoms persisted postoperatively, and he presented several months later to a tertiary center with enlargement of the collection and purulent discharge (Figure 2A). Physical examination revealed complete peripheral pulses in his lower limbs, including palpable dorsalis pedis and posterior tibialis pulses. Differentials included diabetic foot infection, retained foreign body, or fungal mycetoma. Despite the presence of pulses, critical limb ischemia because of microvascular dysfunction was additionally of concern.
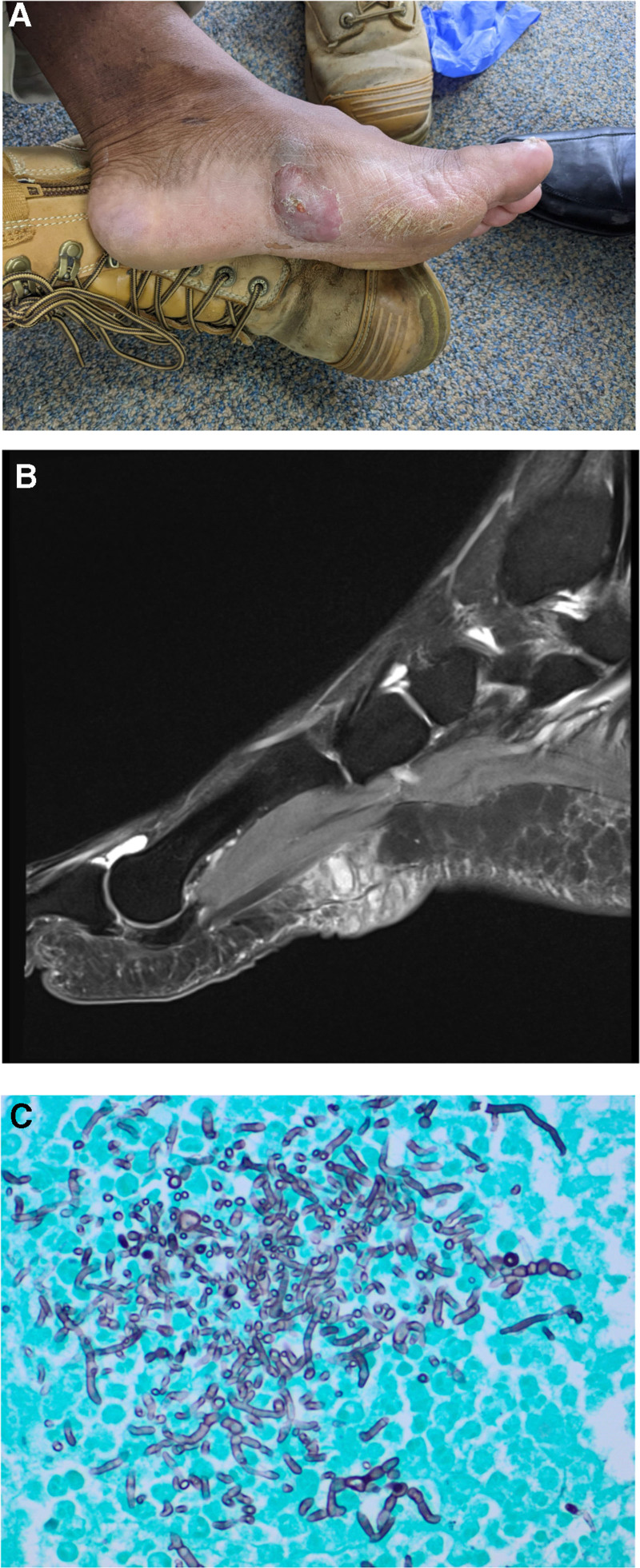
FIGURE 2.
Case 2 images. A, Fungal mycetoma for case 2. B, T2 MRI sagittal view demonstrating cystic collection in subcutaneous tissue reaching the plantar fascia for case 2. C, Microbiology for case 2: Fontana-Masson typifying fungal elements with numerous pigmented hyphae (×200 magnification). MRI, magnetic resonance imaging.
Microscopy and wound culture of the forefoot wound did not reveal any suspicious findings or bacterial growth. Initial ultrasound imaging showed a subcutaneous cystic structure, which was related to the plantar fascia and surrounding foot muscles. A lower limb MRI scan was performed, which revealed a multiloculated cystic lesion that was predominantly localized to the subcutaneous space (Figure 2B). Following assessment by a specialist transplant unit, the potential for underlying invasive fungal infection was immediately recognized. He was booked for an urgent surgical reexploration and debridement. A limited debridement, to minimize intrinsic damage to the architecture of the foot, of the subcutaneous lesion was performed. No obvious signs of infection were visible on the curette, but samples were sent for fungal analysis.
No organisms or polymorphonuclear cells were visible on a direct specimen Gram stain. Histopathology demonstrated granulomatous inflammation, whereas specific fungal staining (Fontana-Masson) demonstrated numerous fungal hyphae (Figure 2C). The specimen was cultured on routine bacterial, mycobacterial, and mycological solid media. Fungal growth was detected on day 28 of incubation. A lactophenol cotton blue stain was nondiagnostic, and the panfungal polymerase chain reaction returned a match for Phaeoacremonium spp. The MIC of voriconazole was 0.5 µg/mL. The MIC for itraconazole, posaconazole and amphotericin B were all 1 µg/mL.
Following surgical debridement, the patient was treated with oral voriconazole 200 mg on a twice daily dosing regime for a duration of 6 wk, with symptomatic resolution. He was maintained on regular therapeutic voriconazole drug levels during this time, with a trough level after 1 wk of therapy of 1.0 mg/L. Following the diagnosis of phaeohyphomycosis, the patient was screened for disseminated infection. A high-resolution CT of his chest was performed, which did not show any abnormality. On follow-up at 3 mo, the patient was symptom-free without recurrence of the disease.
DISCUSSION
Phaeohyphomycosis is a collective term used to describe fungal infections secondary to dematiaceous fungi, which primarily affect the skin and soft tissue structures, but occasionally cause disseminated disease.4 Phaeoacremonium spp are an uncommon group of dematiaceous fungi that are being increasingly recognized to cause complications in some immunocompetent individuals but mainly in the immunosuppressed, including kidney transplant recipients.5 Alongside solid organ transplant recipients, these infections also impact patients with hematological malignancies, including those with bone marrow transplants. The mold is typified by its presence of melanin or melanin-like pigments. It is ubiquitously found in tropic regions, within soil and vegetation. It is widespread in the Australian ecological system, with thermo-tolerance allowing it to survive in woody environments (particularly on Australian sandalwood trees) and soils.6 It is also hyperendemic in other tropical zones, including the Middle-East, India, and sub-Saharan Africa, which comprise “the mycetoma belt.”7 Other dematiaceous fungi that can lead to similar presentations include Alternaria and Exophiala spp.3,4 As a result, inoculation can occur from relatively minor trauma, including injuries such as splinters, scratch marks, or gardening injuries.
Within the literature, only 15 previous cases have been published describing phaeohyphomycoses in kidney transplant recipients (Table 1). Most of these have been in recipients from tropical and subcontinental locations, owing to their thermotolerant properties. From Australian ecological studies, their maximum growth occurs at a temperature of 37 °C.6 Only 1 patient had a history of definitive trauma to the affected region.8 This contrasts with the 2 patients we describe in this series of phaeohyphomycoses, who had both reported a history of minor trauma several months before presentation. In most cases, it is likely that traumatic inoculation does occur, but the injury is too trivial or remote to be recalled by the patient. In their review of literature, Colombier et al9 suggested that only 24% of immunocompromised patients diagnosed with phaeohyphomycosis in the literature had a notable history of cutaneous trauma.
Table 1.
Previous case reports of Phaeohyphomycosis in kidney transplant recipientsa
| Author | Years posttransplant (immunosuppression) | Location | Prior trauma | Microbiology | Treatment | Follow-up postdiagnosis (recurrence) |
|---|---|---|---|---|---|---|
| Choi et al8 | 5 y, (Tac/Pred/MMF) | Hand, subcutaneous | Yes | Phaeoacremonium aleophilum | -Surgical debridement-Short course of antifungal | 5 y (no) |
| Farina et al5 | 4 yb | Forefinger, subcutaneous | No | Phaeoacremonium parasiticum | -Surgical debridement ×2-No antifungal | 6 mo (no) |
| Larsen et al3 | >10 yb | Right lower leg | No | Alternaria spp | -No surgical debridement-Voriconazole | 5 mo (no) |
| de Oliveira et al4 | 4 y (azathioprine/Tac/Pred) | Subcutaneous: 2 cases: right leg, right thigh | No | Exophiala spp | Surgical excisionantifungal: case 1—voriconazole, changed to itraconazoleCase 2—intraconazole | Both cases: 5 mo (no) |
| Monaganti et al12 | 6 y (Tac/Pred) | Pulmonary | No, hematogenous | Phaeoacremonium sp. | Posaconazole, 4 mo | 4 mo (no) |
| Larbcharoensub et al10 | 14 y (Tac/Pred) | Cerebral abscess | No, hematogenous | Phaeoacremonium parasiticum (mixed)c | Voriconazole,6 mo | 5 y (no) |
| Haridasan et al11 | Median 6 mo 7 casesb |
Subcutaneous | No | Various fungal spp | Surgical debridement, extended itraconazole treatment | No recurrence |
| Colombier et al9 | 12 mo (Tac/Pred/MMF) | Subcutaneous | No | Phaeoacremonium parasiticum | Surgical debridement, voriconazole + liposomal amphotericin B | b |
aA Medline, Embase, and Cochrane search was performed to retrieve all cases with search terms “phaeohyphomycosis” and “kidney transplant recipients.”
bDetails not provided.
cPhaeoacromonium parasiticum and Scedosporium apiospermum both isolated in specimen.
MMF, mycophenolate mofetil; Pred, prednisolone; Tac, tacrolimus.
Diagnosis
This condition represents a diagnostic challenge requiring a high index of suspicion in those with a history of active immunosuppression or previous transplantation. Not only are typical features of infection absent, but they can mimic signs and symptoms of other common medical or surgical presentations. Localized and systemic signs of inflammation may be absent in these patients because of immunosuppression. Obtaining an accurate history of prior trauma and appropriate ecological risk factors are important considerations. In addition, the slow-growing nature of fungal infections may contribute to the delay in diagnosis.
Based on the existing literature, this condition may arise years to decades following transplantation but typically presents within the first year.10,11 Currently, diagnosis relies on undertaking fungal testing with a combination of histopathology, immunohistochemistry, and NAT.13 Given the increasing understanding of phaeohyphomycosis, the integration of molecular diagnostics has become crucial in identifying the species type.
Physical signs described in this series are consistent with those previously reported in the literature. Most cases seem to describe patients developing an indolent, round swelling.11 Of the existing cases, there were 2 cases of systemic spread with pulmonary and cerebral complications, with the remainder remaining localized to the soft tissue.10,12 These lesions are best appreciated on an MRI scan and demonstrate a cystic structure with T2 avidity. However, they can mimic other surgical pathologies depending on location, including ganglion cysts, lipomas, or abscesses.
A high index of suspicion needs to be maintained in all cases of solid organ transplant recipients. A biopsy and or surgical excision of all lumps/masses should occur in these cases to ensure that phaeohyphomycoses are not missed. Combining a thorough clinical history, radiological evaluation, and appropriate microbiological techniques, including immunohistochemistry, will allow for timely diagnosis.
Treatment
For the eradication of Phaeoacremonium spp without disseminated disease, surgical debridement alongside oral antifungal therapy is recommended.8,14 Most cases of kidney transplant recipients report a reduction or attempt to reduce immunosuppression to aid curative intent.10 Only 1 case in a kidney transplant recipient had successful management with surgical debridement alone, albeit after an initial recurrence.5 In contrast, triazole antifungal agents alone were the treatment of choice for 2 cases of disseminated disease, achieving cure with no recurrence.10,12 Cases that report follow-up show a very low incidence of recurrence, but the optimal duration of treatment has yet to be determined. Furthermore, whether this results in complete clearance or dormancy of the fungus in the immunocompromised host is unknown. Case 1 in our series was subjected to extended antifungal therapy and planned for definitive surgical debridement. Similarly, case 2 in our series demonstrated that incomplete source control could also be attributed to omitting antifungal treatment following debridement.
Currently, there is no consensus on antifungal of choice for Phaeoacreomonium spp or duration of antifungal treatment. As such, broth microdilution is performed to define minimum inhibitory concentrations for a variety of triazoles to select the most appropriate agent. Several different triazole antifungal agents have been reported to show good outcomes, including itraconazole, voriconazole, and posaconazole.11,12 Intralesional amphotericin B has also been described to treat Phaeoacromonium spp.9
Voriconazole has often been cited as an appropriate agent, with an oral bioavailability of 95%.15 However, triazoles antifungal agents are inhibitors of the cytochrome P450 3A4 pathway, which is involved in metabolizing common immunosuppressive agents. As such, these cases require careful monitoring of drug levels to avoid toxic side effects, particularly in organ transplant recipients who are concurrently treated with immunosuppressive agents.16
CONCLUSION
The diagnosis of fungal infections in kidney transplant recipients requires a high index of suspicion. The presence of immunosuppression should always mandate culture for fungal cause, including patients with no prior trauma. Phaeoacreomonium is rare but increasingly recognized as a cause of opportunistic infection. Definitive treatment involves appropriate surgical debridement with concurrent antifungal therapy to achieve satisfactory source control. Early involvement with the original transplant unit is highly recommended to aid in timely diagnosis and avoid issues relating to drug–drug interactions with concurrent immunosuppressive agents.
Footnotes
The authors declare no funding or conflicts of interest.
A.A.S., D.P., J.N.C., P.G., C.L.H., Z.J.W.-H., R.D.S., R.K.N., A.H., and T.L. participated in research design, writing of paper, performance of research, draft editing, and final revision. G.W. and H.P. participated in research design, draft editing, and final revision.
REFERENCES
- 1.Rajan STM, Bhaskaran S, George M, et al. Phaecremonium parasiticum hand infection in renal transplant patient. J Acad Clin Microbiol. 2019;21:40–43. [Google Scholar]
- 2.Khan A, El-Charabaty E, El-Sayegh S. Fungal infections in renal transplant patients. J Clin Med Res. 2015;7:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen CG, Arendrup MC, Krarup E, et al. Subcutaneous phaeohyphomycosis in a renal transplant recipient successfully treated with voriconazole. Acta Derm Venerol. 2009;89:657–658. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira WRP, Borsato MFL, Dabronzo MLD, et al. Phaeohyphomycosis in renal transplantation: report of two cases. An Bras Dermatol. 2016;91:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farina C, Gotti E, Mouniee D, et al. Phaeoacremonium parasiticum subcutaneous infection in a kidney-transplanted patient successfully treated by surgery. Transpl Infect Dis. 2007;9:253–255. [DOI] [PubMed] [Google Scholar]
- 6.Gramaje D, Leon M, Perez-Sierra A, et al. New Phaeoacremonium species isolated from sandalwood trees in Western Australia. IMA Fungus. 2014;5:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas PG, Kontoyiannis DP. Mycetoma and dematiaceous fungal infections. In: Goldman L, Schafer AI, eds. Goldman-Cecil Medicine. 26th ed. Elsevier; 2020:2068–2071. [Google Scholar]
- 8.Choi J, Lee Y, Chung HS, et al. Subcutaneous phaeohyphomycosis caused by phaeoacremonium species in a kidney transplant patient: the first case in Korea. Korean J Lab Med. 2011;31:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombier MA, Alanio A, Denis B, et al. Dual invasive infection with Phaeoacremonium parasiticum and Paraconiothyrium cyclothyrioides in a renal transplant recipient: case report and comprehensive review of the literature of Phaeoacremonium phaeohyphomycosis. J Clin Microbiol. 2015;53:2084–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larbcharoensub N, Chongtrakool P, Wirojtananugoon C, et al. Treatment of a brain abscess caused by Scedosporium apiospermum and Phaeoacremonium parasiticum in a renal transplant recipient. Southeast Asian J Trop Med Public Health. 2013;44:484–489. [PubMed] [Google Scholar]
- 11.Haridasan S, Parameswaran S, Bheemanathi SH, et al. Subcutaneous phaeohyphomycosis in kidney transplant recipients: a series of seven cases. Transpl Infect Dis. 2017;19:e12788. [DOI] [PubMed] [Google Scholar]
- 12.Monaganti S, Santos CAQ, Markwardt A, et al. Pulmonary Phaeohyphomycosis caused by Phaeoacremonium in a kidney transplant recipient: successful treatment with posaconazole. Case Rep Med. 2014;2014:902818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Pittol MJ, Alejo-Cancho I, Rubio E, et al. Cutaneous infection by Phaeoacremonium parasiticum. Rev Iberoam Micol. 2019;36:90–92. [DOI] [PubMed] [Google Scholar]
- 14.El-Herte RI, Schouweiler KE, Farah RS, et al. Phaeoacremonium parasiticum phaeohyphomycosis in a patient with systemic lupus erythematosus treated successfully with surgical debridement and voriconazole: a case report and review of the literature. IDCases. 2014;1:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greer ND. Voriconazole: the newest triazole antifungal agent. Proc (Bayl Univ Med Cent). 2003;16:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groll AH, Townsend R, Desai A, et al. Drug-drug interactions between triazole antifungal agents used to treat invasive aspergillosis and immunosuppressants metabolized by cytochrome P450 3A4. Transpl Infec Dis. 2017;19:1–11. [DOI] [PubMed] [Google Scholar]