Abstract
Background
Despite a growing interest among men in cosmetic procedures such as botulinum toxin, comparator clinical trial data in this population are limited.
Objectives
The authors sought to compare the efficacy and safety of prabotulinumtoxinA and onabotulinumtoxinA for the treatment of males with moderate to severe glabellar lines.
Methods
Post-hoc analyses were performed on the subpopulation of male patients treated with either a single dose of 20 U prabotulinumtoxinA (n = 25) or 20 U onabotulinumtoxinA (n = 31) in the EVB-003 Phase III glabellar line clinical study. One key efficacy endpoint was the proportion of responders with a ≥1-point improvement from baseline at maximum frown on the 4-point Glabellar Line Scale.
Results
Compared with onabotulinumtoxinA-treated males, the percentages of responders who had a ≥1-point improvement on the Glabellar Line Scale at maximum frown were higher at all postbaseline time points for prabotulinumtoxinA-treated males (P > 0.05 at all visits) by an absolute overall mean difference of 10.1% across all visits. Similar trends were observed for efficacy endpoints based on global aesthetic improvement and subject satisfaction. PrabotulinumtoxinA-treated males had a higher incidence of treatment-related headache and eyelid ptosis.
Conclusions
The percentages of patients who met the definition of a responder were higher at almost all time points examined for prabotulinumtoxinA-treated males. Despite the high level of consistency across all measures, differences between the 2 treatment groups did not reach statistical significance. Further study is warranted to establish if these post-hoc analyses observations are reproducible in a larger male patient population.
Level of Evidence: 1

Botulinum toxin injections are not only increasingly popular, but they also continue to be the most common nonsurgical cosmetic procedure performed in both males and females.1,2 Among males, a dramatic 381% increase in the number of botulinum toxin injections performed for cosmetic purposes was observed between 2000 and 2018 in the United States.3 Based on a 2019 global survey, male patients accounted for 13.4% of all botulinum toxin procedures performed by aesthetic plastic surgeons in that year.1 By a large margin, the top 2 motivating factors among aesthetically oriented men considering a facial aesthetic injectable treatment were the desire to look good for their age, followed by the desire to look more youthful.4 Glabellar lines were selected by 60% of these male respondents as the facial area of concern most likely to be treated first.
Given that women have long dominated this market, instructions for use of botulinum toxins for cosmetic indications have been based on clinical trial efficacy and safety data collected primarily from female patients. Consequently, when deciding the best approach to botulinum toxin applications in men, it is important to consider the facial anatomic differences between males and females. Briefly, compared with women, men typically exhibit greater skeletal muscle mass, including facial muscles that serve as the target for cosmetic botulinum toxin injections.5 In tandem with larger facial muscle mass, men exhibit greater facial movement and more severe facial lines/rhytids—rhytids that only become more pronounced with age.5,6 Other distinctive male facial features include a higher density of facial blood vessels; greater cranial size; greater forehead height, width, and slope; a more pronounced supraorbital ridge; a greater glabellar projection; and a prominent protruding mandible.5 In keeping with these differences—particularly those related to the facial musculature, botulinum toxins have proven in clinical studies for aesthetic indications to be somewhat less effective in men than in women7-12; higher doses may be required for male patients and are typically recommended by expert consensus panels to achieve optimal outcomes.13-16
Clinical experience in treating males with various botulinum toxins is growing, and numerous publications have been written within the last 10 years to guide clinicians in the aesthetic treatment of men.5,17-21 Still, there is a paucity of any direct comparative efficacy and safety data between toxins in this population. To the best of the authors’ knowledge, only 1 comparator study of this type has been published—a pilot study conducted in 12 male patients to compare 4 toxins for the treatment of forehead lines.22 The absence of comparative data in males may be due to the fact that few clinical studies have been conducted solely in male patients and that only a limited number of direct comparator studies have been published in this field regardless of gender. This is compounded by the knowledge that, historically, the overall number of male participants in aesthetic clinical studies of botulinum toxins has been relatively small. In a systematic review published in 2020, Roman and Zampella reported that men on average represented only 13.9% of all participants in the randomized controlled clinical trials conducted of botulinumtoxinA for cosmetic indications.23
The 900-kDa botulinum toxin type A, prabotulinumtoxinA (Jeuveau, Evolus, Inc., Newport Beach, CA), is the most recent neuromodulator approved for utilization in the United States for the treatment of glabellar lines; it is marketed as Nuceiva in Europe and Canada. Results from the multicenter, double-blind, randomized, active- and placebo-controlled Phase III clinical trial (EVB-003, n = 540) conducted in the United Kingdom, Germany, France, Sweden, and Canada confirmed that a single dose of 20 U prabotulinumtoxinA was both well-tolerated and non-inferior to 20 U onabotulinumtoxinA (Botox Cosmetic, Allergan Inc., Irvine, CA) for the treatment of moderate to severe glabellar lines in adult patients who also felt their glabellar lines had an important psychological impact.24 Males represented 11.9% (64/540) of the total study population. The current post-hoc analyses were undertaken to investigate the comparative efficacy and safety of single 20-U doses of prabotulinumtoxinA and onabotulinumtoxinA among males who participated in the EVB-003 study.
METHODS
Conduct of the Original Study
The EVB-003 study was conducted between June 2015 and April 2016. All 540 participants were adults, at least 18 years of age, who had moderate to severe glabellar lines at maximum frown; patients also had to have felt that their glabellar lines had an important psychological impact.24 They had not received a botulinum toxin in the forehead within the previous 6 months, nor had they received any facial aesthetic procedure in the glabellar area within the previous 12 months. Patients were randomly allocated 5:5:1 (employing a block randomization scheme with no stratification) to a single treatment of 20 U prabotulinumtoxinA, 20 U onabotulinumtoxinA, or placebo. No attempt was made to match patients across groups for rhytid severity, by sex, or other baseline criteria. Patients were then followed for 150 days at each of Days 2, 14, 30, 90, and 120 and at study end (Day 150). Efficacy measures included glabellar lines at maximum frown on the 4-point Glabellar Line Scale (GLS; 0 = no lines, 1 = mild, 2 = moderate, 3 = severe); aesthetics on the 5-point Global Aesthetic Improvement Scale (GAIS, 2 = much improved, 1 = improved, 0 = no change, −1 = worse, −2 = much worse); and, satisfaction on the 5-point Subject Satisfaction Scale (SSS; 2 = very satisfied, 1 = satisfied, 0 = indifferent, −1 = unsatisfied, −2 = very unsatisfied). Key safety measures included investigator assessment of adverse events.24 Refer to the original publication for additional exclusion criteria and all information regarding the ethical conduct of the study, including the names of all independent ethics committees and IRBs in each country that approved the study protocol and each of its amendments.
Statistical Methods of Post-hoc Analyses
Data from the male patients who had been randomized to treatment with a botulinum toxin were extracted from the single-dose EVB-003 glabellar line study. Analyses were primarily descriptive in nature; data were summarized by numbers and percentages of prabotulinumtoxinA- and onabotulinumtoxinA-treated male patients. For efficacy endpoints, the Fisher exact test was employed to compare the proportion of responders between groups at each posttreatment assessment visit. Two-sided exact 95% confidence intervals and associated P values were calculated for the absolute differences in the proportions of responders at each visit based on inversion of 2 one-sided tests. Key efficacy endpoints, based on the GLS at maximum frown by investigator assessment, included the percentage of responders with a ≥1-point improvement from baseline and those with a ≥2-point improvement from baseline. Other efficacy endpoints included the percentage of positive responders with a postbaseline score of improved or much improved on the GAIS, and those with a postbaseline score of satisfied or very satisfied on the SSS. For each of these efficacy endpoints, mean durability of response with standard error and median durability with 95% confidence intervals are reported; for these estimates, patients were considered responders if the response criteria were met by Day 30—that is, at either Day 2, Day 14, or Day 30. All treatment-related adverse events (all events assessed by the investigator as possibly, probably, or definitely treatment related) were summarized, including those of particular interest for this type of treatment and indication—that is, headache and ptosis. No statistical analyses of adverse event data were performed.
RESULTS
Patient Disposition and Demographics
Of the 491 patients who were randomized to treatment with a botulinum toxin in the EVB-003 study, 56 (11.4%) were males: 25 received 20 U prabotulinumtoxinA and 31 received 20 U onabotulinumtoxinA. A further 8 males had been randomized to placebo. All males attended the Day 150 visit and completed the study. Botulinum toxin–treated male patients were similar in age: the overall average was 49.3 years (range, 23-74 years), with means of 49.8 years (range, 23-71 years) and 48.8 years (range, 29-74 years) for prabotulinumtoxinA and onabotulinumtoxinA males, respectively (Table 1). Most self-identified as White (76.0% and 71.0% of prabotulinumtoxinA and onabotulinumtoxinA males, respectively). Similarly, most (92.0% and 83.9%, respectively) had Fitzpatrick skin types of I, II, or III. More prabotulinumtoxinA-treated males had severe glabellar lines at maximum frown at baseline (92.0% vs 67.7% of onabotulinumtoxinA-treated males).
Table 1.
Baseline Demographic and Glabellar Line Characteristics: EVB-003 Study, BotulinumtoxinA-Treated Males Only
| Characteristic | PRA (N = 25) | ONA (N = 31) | ||
|---|---|---|---|---|
| Age, mean (range), y | 49.8 ± 10.9 (23-71) | 48.8 ± 9.8 (29-74) | ||
| Race, n (%) | ||||
| White | 19 | (76.0) | 22 | (71.0) |
| Black/African American | 0 | (0.0) | 0 | (0.0) |
| Asian, multiple, or other | 6 | (24.0) | 9 | (29.0) |
| Fitzpatrick skin types, n (%) | ||||
| I + II + III | 23 | (92.0) | 26 | (83.9) |
| IV + V + VI | 2 | (8.0) | 5 | (16.1) |
| GLS score at maximum frown, n (%) | ||||
| Moderate by investigator assessment | 2 | (8.0) | 10 | (32.3) |
| Severe by investigator assessment | 23 | (92.0) | 21 | (67.7) |
GLS, Glabellar Line Scale; PRA, prabotulinumtoxinA; ONA, onabotulinumtoxinA.
Efficacy
Responders on the GLS
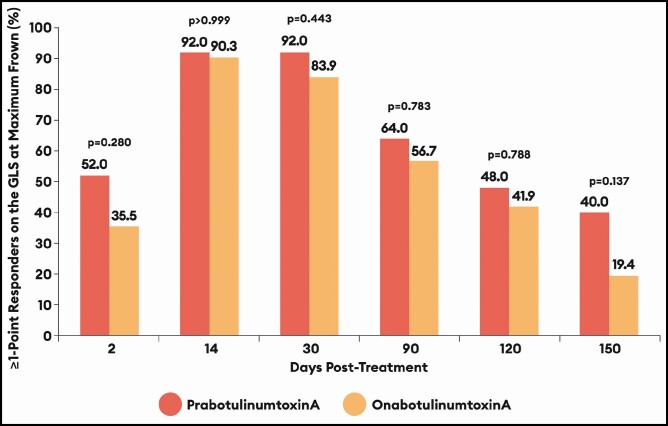
Compared with onabotulinumtoxinA-treated males, the percentages of responders who had a ≥1-point improvement on the GLS at maximum frown by investigator assessment were higher at all time points for prabotulinumtoxinA-treated males by an absolute overall mean difference of 10.1% across all visits (Table 2; Figure 1). The percentages of responders by this measure were most similar at Day 14 (absolute mean difference of 1.7%); differences were most pronounced at Day 2 (absolute mean difference of 16.5%) and at Day 150 (absolute mean difference of 20.6%). With few exceptions, similar patterns of response were observed for all efficacy measures assessed, although none of the differences observed between the 2 toxins reached statistical significance.
Table 2.
Responders Based on a ≥1-Point Improvement on the GLS at Maximum Frown: EVB-003 Study, BotulinumtoxinA-Treated Males Only
| Day | PRA (N = 25) | ONA (N = 31) | % Difference [95% CI] | P |
|---|---|---|---|---|
| 2 | 52.0% | 35.5% | 16.5 [−10.1 to 41.5] | 0.280 |
| 14 | 92.0% | 90.3% | 1.7 [−24.3 to 27.4] | >0.999 |
| 30 | 92.0% | 83.9% | 8.1 [−18.2 to 33.4] | 0.443 |
| 90 | 64.0% | 56.7% | 7.3 [−19.3 to 33.1] | 0.783 |
| 120 | 48.0% | 41.9% | 6.1 [−20.3 to 31.9] | 0.788 |
| 150 | 40.0% | 19.4% | 20.6 [−5.5 to 45.2] | 0.137 |
GLS, Glabellar Line Scale; PRA, prabotulinumtoxinA; ONA, onabotulinumtoxinA.
Figure 1.
Percentage of responders based on a ≥1-point improvement on the Glabellar Line Scale (GLS) at maximum frown from day 0 by investigator assessment: EVB-003 study, botulinumtoxinA-treated males only. See Table 2 for absolute percentage differences with 95% confidence intervals by visit.
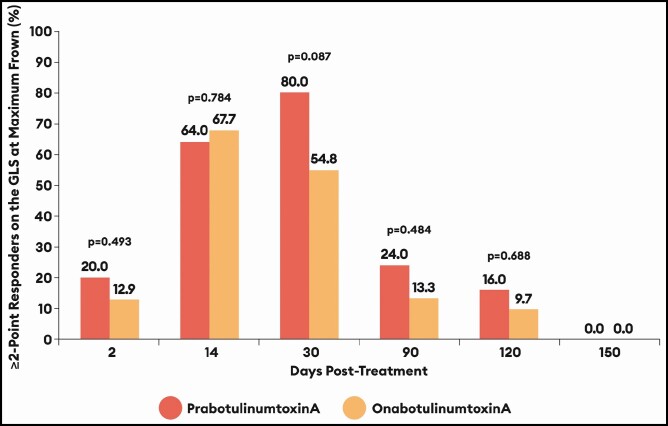
At 4 of the 5 time points from Day 2 through to Day 120, the percentages of responders who achieved a ≥2-point improvement on the GLS at maximum frown by investigator assessment were higher for prabotulinumtoxinA-treated males than for onabotulinumtoxinA-treated males by absolute differences of 7.1% at Day 2, 25.2% at Day 30, 10.7% at Day 90, and 6.3% at Day 120 (Figure 2). Differences favored onabotulinumtoxinA only at Day 14 (by an absolute difference of 3.7%). By Day 150, no males in either treatment group still retained a ≥2-point improvement on the GLS at maximum frown by investigator assessment.
Figure 2.
Percentage of responders based on a ≥2-point improvement on the Glabellar Line Scale (GLS) at maximum frown from Day 0 by investigator assessment: EVB-003 study, botulinumtoxinA-treated males only. Absolute percentage differences with 95% confidence intervals (CI) by visit were as follows:
Day Difference prabotulinumtoxinA - onabotulinumtoxinA (95% CI)
2 7.1% (−19.0 to 32.7)
14 −3.7% (−29.7 to 22.1)
30 25.2% (−1.5 to 48.8)
90 10.7% (−16.0 to 36.3)
120 6.3% (−19.7 to 31.9)
150 Not applicable
Responders on the GAIS
Data on the percentages of positive responders based on the GAIS (those assessed as either improved or much improved) paralleled those of ≥1-point responders on the GLS (Figures 3, 4). That is, by investigator assessment (Figure 3), compared with onabotulinumtoxinA-treated males, the percentages of positive responders on the GAIS were higher at all time points by an absolute overall mean difference of 13.5% (absolute mean differences ranged from a low of 2.5% at Day 14 to a high of 24.0% at Day 90) for prabotulinumtoxinA-treated males. Similarly, by patient assessment (Figure 4), compared with onabotulinumtoxinA-treated males, the percentages of positive responders on the GAIS were higher at all time points by an absolute overall mean difference of 11.9% (absolute mean differences ranged from a low of 4.9% at Day 14 to a high of 20.7% at Day 90) for prabotulinumtoxinA-treated males.
Figure 3.
Percentage of positive responders (improved + much improved) on the Global Aesthetic Improvement Scale (GAIS) by investigator assessment (IA): EVB-003 study, botulinumtoxinA-treated males only. Absolute percentage differences with 95% confidence intervals by visit were as follows:
Day Difference prabotulinumtoxinA - onabotulinumtoxinA (95% CI)
2 16.5% (−10.1 to 41.5)
14 2.5% (−23.6 to 28.1)
30 8.9% (−17.4 to 34.1)
90 24.0% (−3.0 to 48.5)
120 12.4% (−14.2 to 37.5)
150 16.6 % (−9.5 to 41.6)
Figure 4.
Percentage of positive responders (improved + much improved) on the Global Aesthetic Improvement Scale (GAIS) by patient assessment (PA): EVB-003 study, botulinumtoxinA-treated males only. Absolute percentage differences with 95% confidence intervals (CI) by visit were as follows:
Day Difference prabotulinumtoxinA - onabotulinumtoxinA (95% CI)
2 14.1% (−12.6 to 39.3)
14 4.9% (−21.2 to 30.4)
30 5.7% (−20.5 to 31.2)
90 20.7% (−6.3 to 45.5)
120 16.4% (−10.3 to 41.1)
150 9.3% (−17.1 to 34.9)
Responders on the SSS
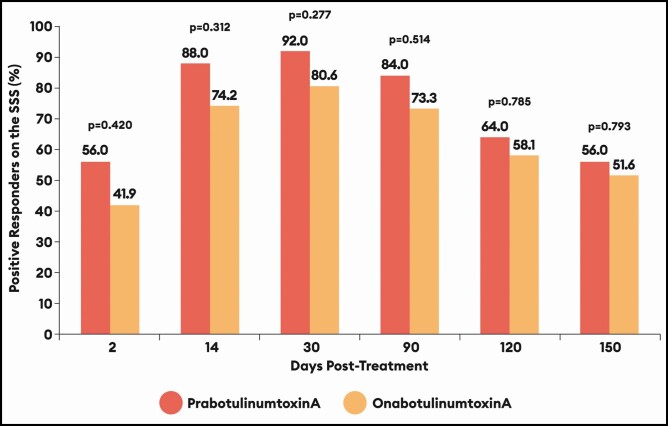
Except for Day 2, >50% of male patients remained satisfied or very satisfied with their treatment regardless of treatment allocation (Figure 5). Still, compared with onabotulinumtoxinA-treated males, the percentages of positive responders on the SSS were higher at all time points by an absolute overall mean difference of 10.1% (absolute mean differences ranged from a low of 4.4% at Day 150 to a high of 14.1% at Day 2) for prabotulinumtoxinA-treated males.
Figure 5.
Percentage of positive responders (satisfied + very satisfied) on the Subject Satisfaction Scale (SSS): EVB-003 study, botulinumtoxinA-treated males only. Absolute percentage differences with 95% confidence intervals (CI) by visit were as follows:
Day Difference prabotulinumtoxinA - onabotulinumtoxinA (95% CI)
2 14.1% (−12.6 to 39.3)
14 13.8% (−12.7 to 38.6)
30 11.4% (−15.0 to 36.4)
90 10.7% (−16.0 to 36.3)
120 5.9% (−20.5 to 31.4)
150 4.4% (−22.0 to 30.3)
Durability of Response
For each of these 5 efficacy endpoints presented, where patients were considered responders if the response criteria were met by Day 30, estimates of the mean and median durations of response were greater for prabotulinumtoxinA-treated males than for onabotulinumtoxinA-treated males (Table 3). For example, the mean durations of response based on a ≥1-point improvement on the GLS at maximum frown from Day 0 by investigator assessment were 118.6 days and 107.3 days for prabotulinumtoxinA-treated and onabotulinumtoxinA-treated males, respectively—that is, an absolute mean difference of 11.3 days.
Table 3.
Durability of Response in Days: EVB-003 Study, BotulinumtoxinA-Treated Males Only
| Parameter | PRA (N = 25) | ONA (N = 31) |
|---|---|---|
| ≥ 1-Point improvement on GLS at maximum frown from Day 0 by investigator assessment | ||
| Mean duration (standard error) | 118.6 (9.1) | 107.3 (8.8) |
| Median duration (95% CI) | 120.0 (94.0 to 158.0) | 118.0 (94.0 to 146.0) |
| ≥ 2-Point improvement on GLS at maximum frown from Day 0 by investigator assessment | ||
| Mean duration (standard error) | 84.9 (9.1) | 61.8 (9.2) |
| Median duration (95% CI) | 92.0 (85.0 to 98.0) | 86.0 (0.5 to 94.0)a |
| Improved/much improved on GAIS by investigator assessment | ||
| Mean duration (standard error) | 133.5 (7.3) | 114.3 (8.5) |
| Median duration (95% CI) | 149.0 (128.0 to 158.0) | 121.0 (96.0 to 155.0) |
| Improved/much improved on GAIS by patient assessment | ||
| Mean duration (standard error) | 132.1 (7.2) | 119.6 (8.1) |
| Median duration (95% CI) | 150.0b | 121.0 (103.0 to 155.0) |
| Satisfied/very satisfied on SSS | ||
| Mean duration (standard error) | 126.0 (9.0) | 110.6 (9.9) |
| Median duration (95% CI) | 153.0b | 127.0b |
Patients were considered responders if the response criteria were met by Day 30 (ie, at either Day 2, Day 14, or Day 30). If all 3 of these visits showed nonresponse, the patient was censored as a failure at Day 0. A patient was a failure at Day 30 if either of the prior visits showed a response, but Day 30 showed nonresponse. Otherwise, the analysis showed the time to the first failure after a response within the first 3 visits.
CI, confidence interval; GAIS, Global Aesthetic Improvement Scale; GLS, Glabellar Line Scale; PRA, prabotulinumtoxinA; ONA, onabotulinumtoxinA; SSS, Subject Satisfaction Scale.
aThe small sample size at later time points created a very wide interval for the 95% CI of median duration;
bBecause of the small sample size at later time points, the 95% CI of median duration could not be calculated.
Safety
In the EVB-003 study, the overall incidence of treatment-related adverse events was higher for prabotulinumtoxinA-treated males than for onabotulinumtoxinA-treated males: 28.0% vs 12.9% (Table 4). A higher percentage of prabotulinumtoxinA-treated males experienced treatment-related headache: 20.0% vs 3.2% of onabotulinumtoxinA-treated males. They also experienced a higher rate of treatment-related eyelid ptosis: 8.0% vs 0.0% of onabotulinumtoxinA-treated males. This ptosis rate is a result of 2 out of the 25 prabotulinumtoxinA-treated males experiencing this type of event. Of note, both events were mild in severity and assessed as possibly related to treatment; only one of the events was observed by the investigator. See the Discussion for ptosis data from all prabotulinumtoxinA-treated males in the clinical development program. In addition, 1 prabotulinumtoxinA-treated male who experienced headache also experienced treatment-related eyelid edema. Among other onabotulinumtoxinA-treated males, 1 experienced treatment-related brow ptosis, 1 experienced treatment-related muscle tone disorder, and 1 experienced treatment-related skin wrinkling—described as transverse nasal lines (ie, bunny lines). No serious adverse events that were reported in this study were assessed as related to either toxin.
Table 4.
Summary of Treatment-Related Adverse Events: EVB-003 Study, BotulinumtoxinA-Treated Males Only
| PRA (N = 25) | ONA (N = 31) | |||
|---|---|---|---|---|
| Adverse event parameter | n (%) | Events | n (%) | Events |
| Any treatment-related event | 7 (28.0) | 8a | 4 (12.9) | 4 |
| Headache | 5 (20.0) | 5 | 1 (3.2) | 1 |
| Eyelid ptosis | 2 (8.0) | 2 | 0 (0.0) | 0 |
| Brow ptosis | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Muscle tone disorder | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Skin wrinkling | 0 (0.0) | 0 | 1 (3.2) | 1 |
| Eyelid oedema | 1 (4.0) | 1 | 0 (0.0) | 0 |
PRA, prabotulinumtoxinA; ONA, onabotulinumtoxinA.
aOne prabotulinumtoxinA-treated male participant experienced both headache and eyelid oedema.
DISCUSSION
Over the last 20 years, there has been dramatic growth in the utilization of botulinum toxin injections for cosmetic indications in men.3 Yet, direct comparator clinical trial efficacy and safety data between commercially available toxins are limited in this population. To the best of the authors’ knowledge, none have been published for the treatment of glabellar lines. Only a single 2013 publication was found, which investigated the comparative onset and duration of effect of 3 botulinum toxins for the treatment of glabellar lines and included data for a subset of 27 males (9 per treatment arm), yet efficacy throughout the 180 days of study was not reported.25 In an effort to begin to fill this gap, the authors undertook a post-hoc analysis of data derived from the Phase III single-dose EVB-003 clinical study in which 540 patients had been randomized 5:5:1 to receive 20 U prabotulinumtoxinA, 20 U onabotulinumtoxinA, or placebo for the treatment of glabellar lines. In this study, 25 males had received treatment with prabotulinumtoxinA, and 31 males had received onabotulinumtoxinA.
The limited sample size and the slightly skewed numbers of 25 and 31 patients per group are acknowledged limitations of this type of post-hoc comparison. Nonetheless, the 2 groups of males were fairly well matched in terms of age, race, and skin type. Where the 2 groups of males differed noticeably was in the percentages of those with severe glabellar lines at maximum frown at baseline by investigator assessment: there were more severe patients in the prabotulinumtoxinA-treated arm with 92.0% vs 67.7% in the onabotulinumtoxinA-treated arm (difference of 24.3%). As such, this represents a further limitation of the post-hoc analysis—albeit one, it could be argued, that would more likely favor onabotulinumtoxinA-treated patients.
In any case, with few exceptions, the percentage of responders was higher at most time points for the prabotulinumtoxinA-treated males. In the case of those achieving a 1-point or greater improvement on the GLS at maximum frown by investigator assessment, the percentage of prabotulinumtoxinA-treated responders was on average a mean of 10.1% higher than the percentage of onabotulinumtoxinA-treated responders across all visits from Day 2 through to Day 150. In the case of those achieving a ≥2-point improvement on the GLS at maximum frown by investigator assessment, the percentage of prabotulinumtoxinA-treated responders was on average a mean of 12.3% higher than the percentage of onabotulinumtoxinA-treated responders across Days 2, 30, 90, and 120; differences only favored onabotulinumtoxinA-treated responders at Day 14 (by 3.7%). In the case of those achieving a positive response on the GAIS, the percentage of prabotulinumtoxinA-treated responders was on average a mean of 13.5% higher by investigator assessment (and 11.9% higher by patient assessment) than the percentage of onabotulinumtoxinA-treated responders across all visits from Day 2 through to Day 150. Finally, in the case of those achieving a positive response on the SSS, the percentage of prabotulinumtoxinA-treated responders was on average a mean of 10.1% higher than the percentage of onabotulinumtoxinA-treated responders.
Despite this apparent trend, as displayed in footnotes to Figures 1 to 5, 95% confidence intervals for the absolute differences between treatments were sufficiently wide that none of the differences observed between the 2 treatment groups reached statistical significance for any efficacy outcome. This may have been a result of the relatively small patient numbers, 25 and 31 males per group, available for inclusion in these post-hoc analyses. Of note, although differences again were not statistically significant, a similar trend was observed in the overall population, which consisted of approximately 90% females.24 Furthermore, for the 245 prabotulinumtoxinA- and 246 onabotulinumtoxinA-treated patients (both males and females) who participated in the original study, responder rates favored prabotulinumtoxinA for 26 out of the 30 exploratory efficacy endpoints examined, ranging from Day to 2 to Day 150 (data on file, Evolus Inc.). Similarly, although differences again were not statistically significant, the primary efficacy endpoint responder rates were also consistently slightly higher for prabotulinumtoxinA-treated patients compared with onabotulinumtoxinA-treated patients in the 2 Korean double-blind Phase III studies—one conducted for the treatment of glabellar lines and the other conducted for the treatment of lateral canthal lines.26,27
For each of the efficacy measures presented, prabotulinumtoxinA-treated males also appeared to experience a more sustained response than onabotulinumtoxinA-treated males. The absolute mean differences favoring prabotulinumtoxinA in the estimated duration of response ranged from 11.3 days longer based on a ≥1-point improvement on the GLS at maximum frown to 23.1 days longer based on a ≥2-point improvement on the GLS at maximum frown. Further study would be needed to determine whether differences in efficacy responder rates and durability of responses observed in these post-hoc analyses of botulinum toxin-treated male patients who participated in the EVB-003 study were reproducible in a larger male patient population.
As has been seen with other toxins,7-12 the responder rates among male botulinum toxin-treated patients observed in these post-hoc analyses were lower than those reported for the population of all botulinum toxin–treated patients who participated in the original study, 88.6% (435/491) of whom were female.24 For example, based on the numbers reported above for the outcome of a ≥1-point improvement on the GLS at maximum frown by investigator assessment (and compared with those reported in Table 2), the percentage of responders among all prabotulinumtoxinA-treated patients was higher by an overall absolute mean of 5.7% across all visits compared with the subset of male prabotulinumtoxinA-treated patients.24 The percentage of responders among all onabotulinumtoxinA-treated patients was higher by an absolute overall mean of 13.1% higher across all visits compared with the subset of male onabotulinumtoxinA-treated patients. It may simply be that, due to the greater glabellar muscle mass typical of males,28 males tend to exhibit more dynamic and severe glabellar lines than females and accordingly require higher doses of botulinum toxin to achieve a satisfactory response.7,9,28,29
As was observed in the original study for all toxin-treated patients,24 satisfaction among males remained high throughout the study. By Day 14, 88.0% of prabotulinumtoxinA-treated males and 74.2% of onabotulinumtoxinA-treated males reported feeling satisfied or very satisfied with their treatment; 50% or more reported feeling satisfied or very satisfied throughout the remainder of the study. This is an important observation given that the botulinum toxin-treated men in this study tended to have a somewhat less robust response compared with that reported for all patients in the original study. This degree of male patient satisfaction may be reflective of the fact that many men may be content with a softening of their glabellar lines and do not necessarily require elimination of glabellar lines to achieve their aesthetic goals of looking good for their age and/or looking more youthful.
The overall incidence of treatment-related adverse events proved to be higher for prabotulinumtoxinA-treated males than for onabotulinumtoxinA-treated males: 28.0% vs 12.9%, respectively. In comparison, at 15.5% and 14.6%, respectively, this type of difference was not evident in the larger population of 245 prabotulinumtoxinA- and 246 onabotulinumtoxinA-treated male and female patients who participated in the original study.24 In the authors’ opinion, the differences observed based on these post-hoc analyses were not indicative of an excessive dose of prabotulinumtoxinA or that males are inherently more sensitive than females to treatment-related adverse events. Rather, when viewed in context, it becomes apparent that the observed differences in treatment-related events in the post-hoc analyses may simply be an anomaly related to the relatively small sample size under investigation. For example, although the percentages of treatment-related headache were 20% and 3.2% for prabotulinumtoxinA- and onabotulinumtoxinA-treated male patients, respectively, these percentages represent a difference of only 4 patients—that is, just 5 prabotulinumtoxinA-treated males and 1 onabotulinumtoxinA-treated male, over a small denominator, who participated in the Phase III EVB-003 study experienced treatment-related headache. Furthermore, at an incidence rate of 8%, the 2 treatment-related ptosis events experienced by prabotulinumtoxinA-treated males in this single-dose study represent one-half of all treatment-related ptosis events experienced by prabotulinumtoxinA-treated males in the entire clinical development program. Of the 326 prabotulinumtoxinA treatments received by males in the 3 single-dose and 2 repeat-dose studies, only 4 (a rate of 1.2%) were associated with a treatment-related ptosis event (data on file, Evolus, Inc.).
Further study in a much larger sample of male patients may be warranted to establish whether differences in efficacy and safety outcomes observed in these post-hoc analyses between prabotulinumtoxinA and onabotulinumtoxinA treatments are reproducible and widely applicable.
CONCLUSIONS
In these post-hoc analyses of data extracted from botulinum toxin–treated male patients who participated in the multicenter, randomized, double-blind, single-dose Phase III EVB-003 study, the percentages of patients who met the definition of a responder were higher at most time points examined for prabotulinumtoxinA-treated males than for onabotulinumtoxinA-treated males. This was true for efficacy endpoints based on the glabellar line severity scale, the GAIS, and the SSS. There was a high level of consistency across measures, yet none of the differences observed between the 2 treatment groups reached statistical significance. Further study in a larger population of men is warranted to establish whether these observations are reproducible and, if so, whether the differences observed prove to be statistically significant.
Acknowledgments
The authors gratefully acknowledge the contributions of Teri Yurik, senior biostatistician with NAMSA (Minneapolis, MN, USA), who served as the statistical consultant for the analyses presented, and Anneke Jonker, MSc, of Medical Writing Associates of West Vancouver, BC, Canada, who provided technical assistance with manuscript preparation.
Contributor Information
Nowell Solish, University of Toronto, Toronto, ON, Canada.
Benjamin Ascher, Paris Academy, Paris, France.
Rui L Avelar, Evolus, Inc., Newport Beach, CA, USA.
Vince Bertucci, Division of Dermatology, University of Toronto, Toronto, ON, Canada.
Isaac Bodokh, Practicien Hospitalier, Service de Dermatologie, Cannes Hospital Simone Veil, Cannes, France.
Jean Carruthers, Department of Ophthalmology, University of British Columbia, Vancouver, BC, Canada.
Hugues Cartier, Centre Médical Saint-Jean, Arras, France.
Henry Delmar, private practice in Cap d’Antibes, France.
Ralf Denfeld, private practice in Stuttgart, Germany.
Marc Heckmann, Ludwig Maximilian Universität, Munich, Germany.
Per Hedén, Karolinska Institute, Art Clinic, Stockholm, Sweden.
Said Hilton, private practice in Düsseldorf, Germany.
Christopher Inglefield, private practice in London, UK.
Patricia Ogilvie, private practice in Munich, Germany.
Berthold-Josef Rzany, private practice in Vienna, Austria.
Gerhard Sattler, Rosenpark Research, Darmstadt, Germany.
Michael Sebastian, private practice in Mahlow, Germany.
Arthur Swift, McGill University, Montreal, PQ, Canada.
Patrick Trévidic, private practice in Paris, France.
Disclosures
Dr Avelar is a current employee of Evolus, Inc. (Newport Beach, CA) and receives compensation in salary, stock, and stock options. The remaining authors served as clinical trial investigators for the EVB-003 clinical study. Drs Bodokh, Delmar, Inglefield, Swift, and Trévidic have indicated no other conflicts of interest that have supported their work within the past 36 months. The remaining investigators have disclosed potential conflicts as follows: Dr Solish has served as an investigator and/or speaker for Allergan (Irvine, CA), Evolus, Galderma (Lausanne, Switzerland), Merz (Frankfurt am Main, Germany), and Revance (Nashville, TN). Dr Ascher has served as an investigator for Advanced Aesthetic Technologies (Brookline, MA), Ipsen (Paris, France), Galderma, and Symatese (Bornel, France). Dr Bertucci has served as an investigator for Allergan, Galderma, Evolus, Merz and Revance as a speaker for Allergan, Galderma, Merz, and Revance and a consultant for Allergan, Galderma, Prollenium (Aurora, ON, Canada), Revance, and Teoxane (Geneva, Switzerland). Dr Carruthers has served as a consultant and research with Evolus, Allergan, Merz, and Revance. Dr Cartier has served on the advisory boards of Sinclair (London, UK), Galderma, and Laboratoires Fillmed (Paris, France); Dr Denfeld has served as an investigator and speaker for Novartis Pharma GmbH (Nürnberg, Germany), an investigator for Infecto Pharm Arzneimittel und Consilium GmbH (Heppenheim, Germany), and as a consultant to AbbVie Deutschland GmbH (Wiesbaden, Germany) and Stallergenes Greer GmbH (Kamp-Linfort, Germany); Dr Heckmann has received honoraria for scientific presentations on the use of botulinum toxin A from Allergan/AbbVie, Galderma, and Croma (Leobendorf, Austria), and honoraria as an advisory board member from Allergan/AbbVie and Evolus, and has participated as an investigator in other clinical trials using botulinum toxin A; Dr Hedén holds stock in Strathspey Crown Holdings LLC (which has an indirect interest in Evolus, Inc.); Dr Hilton has served as an investigator and/or speaker for AbbVie, Allergan, Galderma, Hallura (Yoqneam, Israel), Merz Aesthetics North America (Raleigh, NC), Ipsen, Cassiopea (Milan, Italy), Johnson & Johnson (New Brunswick, NJ), Novartis Pharma GmbH (Nürnberg, Germany), L’Oreal (Paris, France), and Almirall (Barcelona, Spain); Dr Ogilvie has received honoraria from Evolus for advisory services; Dr Rzany has served as an advisor and/or speaker for Allergan, Croma, Evolus, Galderma, and Ipsen; Dr Sattler has served as an investigator for Advanced Aesthetics Technologies (Brookline, MA), Allergan, Hallura (Yoqneam, Israel), LG Chem (Seoul, South Korea), and Merz; Dr Sebastien has received grants or support from AbbVie, Novartis (Basel, Switzerland), Jannsen-Cilag (Madrid, Spain), Elli Lilly (Indianapolis, IN), Leo Pharma (Ballerup, Denmark), Galderma, UCB (Brussels, Belgium), Pfizer (New York, NY), Dermira (Menlo Park, CA), Dr. August Wolff Pharma (Bielefeld, Germany), Merck Pharma (Rahway, NJ), Almirall (Barcelona, Spain), Affibody (Stockholm, Sweden), Menlo, Genentech (South San Francisco, CA), Regeneron (Tarrytown, NY), Sanofi-Synthelabo (New York, NY), and Boehringer-Ingelheim (Ingelheim am Rhein, Germany), Dr. Reddys (Hyderabad, India), and Accovion (Eschborn, Germany).
Funding
The original EVB-003 Phase III study on which these post-hoc analyses were based was funded by Evolus, Inc. (Newport Beach, CA). Evolus also provided funding to the contract organizations that performed these additional analyses and assisted in manuscript preparation.
REFERENCES
- 1. International Society of Aesthetic Plastic Surgery . ISAPS international survey on aesthetic/cosmetic procedures performed in 2019. Accessed October 13, 2021. https://www.isaps.org/wp-content/uploads/2020/12/Global-Survey-2019.pdf
- 2.The Aesthetic Society’s Cosmetic Surgery National Data Bank: Statistics 2020. Aesthet Surg J. 2021;41(Supplement_2):1-16. doi: 10.1093/asj/sjab178. Accessed July 4, 2022. https://cdn.surgery.org/media/statistics/aestheticplasticsurgerynationaldatabank-2020stats.pdf
- 3. American Society of Plastic Surgeons . Plastic Surgery Statistics Report 2018. Accessed October 13, 2021. https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf
- 4. Jagdeo J, Keaney T, Narurkar V, Kolodziejczyk J, Gallagher CJ. Facial treatment preferences among aesthetically oriented men. Dermatol Surg. 2016;42(10):1155–1163. doi: 10.1097/dss.0000000000000876 [DOI] [PubMed] [Google Scholar]
- 5. Keaney TC, Alster TS. Botulinum toxin in men: review of relevant anatomy and clinical trial data. Dermatol Surg. 2013;39(10):1434–1443. doi: 10.1111/dsu.12302 [DOI] [PubMed] [Google Scholar]
- 6. Rossi A, Eviatar J, Green JB, et al. Signs of facial aging in men in a diverse, multinational study: timing and preventive behaviors. Dermatol Surg. 2017;43:S210–S220. doi: 10.1097/dss.0000000000001293 [DOI] [PubMed] [Google Scholar]
- 7. Carruthers A, Carruthers J. Prospective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type A in men with glabellar rhytids. Dermatol Surg. 2005;31(10):1297–1303. doi: 10.1111/j.1524-4725.2005.31206 [DOI] [PubMed] [Google Scholar]
- 8. Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of new botulinum toxin type A for treatment of glabellar lines: efficacy and safety. Dermatol Surg. 2009;35(12):1893–1901. doi: 10.1111/j.1524-4725.2009.01235.x [DOI] [PubMed] [Google Scholar]
- 9. Kane MAC, Brandt F, Rohrich RJ, Narins RS, Monheit GD, Huber MB; Reloxin Investigational Group . Evaluation of variable-dose treatment with a new U.S. botulinum toxin type A (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg. 2009;124(5):1619–1629. doi: 10.1097/prs.0b013e3181b5641b [DOI] [PubMed] [Google Scholar]
- 10. Baumann L, Brandt FS, Kane MAC, Donofrio LM. An analysis of efficacy data from four phase III studies of botulinum neurotoxin type A – ABO for the treatment of glabellar lines. Aesthet Surg J. 2009;29(6 Suppl):S57–S65. doi: 10.1016/j.asj.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 11. Jones DH, Kerscher M, Geister T, Hast MA, Weissenberger P. Efficacy of incobotulinumtoxinA for the treatment of glabellar frown lines in male subjects: post-hoc analyses from randomized, double-blind pivotal studies. Dermatol Surg. 2017;43(Suppl 2):S235–S241. doi: 10.1097/dss.0000000000001295 [DOI] [PubMed] [Google Scholar]
- 12. Keaney TC, Cavallini M, Leys C, et al. Efficacy, patient-reported outcomes, and safety in male subjects treated with onabotulinumtoxinA for improvement of moderate to severe horizontal forehead lines. Dermatol Surg. 2020;46(2):229–239. doi: 10.1097/dss.0000000000002047 [DOI] [PubMed] [Google Scholar]
- 13. Flynn TC. Botox in men. Dermatol Ther. 2007;20(6):407–413. doi: 10.1111/j.1529-8019.2007.00156.x [DOI] [PubMed] [Google Scholar]
- 14. Carruthers A, Kane MAC, Flynn TC, et al. The convergence of medicine and neurotoxins: A focus on botulinum toxin type A and its application in aesthetic medicine – a global, evidence-based botulinum toxin consensus education initiative. Part I: botulinum toxin in clinical and cosmetic practice. Dermatol Surg. 2013;39(3 Pt 2):493–509. doi: 10.1111/dsu.12147 [DOI] [PubMed] [Google Scholar]
- 15. Lorenc ZP, Smith S, Nestor M, Nelson D, Moradi A. Understanding the functional anatomy of the frontalis and glabellar complex for optimal aesthetic botulinum toxin type A therapy. Aesthet Plast Surg. 2013;37(5):975–983. doi: 10.1007/s00266-013-0178-1 [DOI] [PubMed] [Google Scholar]
- 16. Kaminer MS, Cox SE, Fagien S, Kaufman J, Lupo MP, Shamban A. Re-examining the optimal use of neuromodulators and the changing landscape: a consensus panel update. J Drugs Dermatol. 2020;19(Suppl 1):s5–s15. [PubMed] [Google Scholar]
- 17. De Maio M. Ethnic and gender considerations in the use of facial injectables: male patients. Plast Reconstr Surg. 2015;136(Suppl 5):40S–43S. doi: 10.1097/prs.0000000000001729 [DOI] [PubMed] [Google Scholar]
- 18. Scherer M-A. Specific aspects of a combined approach to male face correction: botulinum toxin A and volumetric fillers. J Cosmet Dermatol. 2016;15(4):566–574. doi: 10.1111/jocd.12247 [DOI] [PubMed] [Google Scholar]
- 19. Green JB, Keaney TC. Aesthetic treatment with botulinum toxin: approaches specific to men. Dermatol Surg. 2017;43(Suppl 2):S153–S156. doi: 10.1097/dss.0000000000001375 [DOI] [PubMed] [Google Scholar]
- 20. Jones IT, Fabi SG. The use of neurotoxins in the male face. Dermatol Clin. 2018;36(1):29–42. doi: 10.1016/j.det.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 21. Keaney TC, Anolik R, Braz A, et al. The male aesthetic patient: facial anatomy, concepts of attractiveness, and treatment patterns. J Drugs Dermatol. 2018;17(1):19–28. [PubMed] [Google Scholar]
- 22. Oliveira de Morais O, Reis-Filho EM, Pereira LV, Gomes CM, Alves G. Comparison of four botulinum neurotoxin type A preparations in the treatment of hyperdynamic forehead lines in men: a pilot study. J Drugs Dermatol. 2012;11(2):216–219. [PubMed] [Google Scholar]
- 23. Roman J, Zampella JG. Demographics of men and minorities in cosmetic clinical trials of botulinum toxin and hyaluronic acid fillers. Dermatol Surg. 2020;46(9):1164–1168. doi: 10.1097/dss.0000000000002294 [DOI] [PubMed] [Google Scholar]
- 24. Rzany BJ, Ascher B, Avelar RL, et al. A multicenter, randomized, double-blind, placebo-controlled, single-dose, Phase III, non-inferiority study comparing prabotulinumtoxinA and onabotulinumtoxinA for the treatment of moderate to severe glabellar lines in adult subjects. Aesthet Surg J. 2020;40(4):413–429. doi: 10.1093/asj/sjz110 [DOI] [PubMed] [Google Scholar]
- 25. Rappl T, Parvizi D, Friedl H, et al. Onset and duration of effect of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA in the treatment of glabellar frown lines: a randomized, double-blind study. Clin Cosmet Investigational Dermatol. 2013;6:211–219. doi: 10.2147/ccid.S41537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Won CH, Kim HK, Kim BJ, et al. Comparative trial of a novel botulinum neurotoxin type A versus onabotulinumtoxinA in the treatment of glabellar lines: a multicenter, randomized, double-blind, active-controlled study. Int J Dermatol. 2015;54(2):227–234. doi: 10.1111/ijd.12627 [DOI] [PubMed] [Google Scholar]
- 27. Cheon H, Jung N, Won CH, et al. Efficacy and safety of prabotulinumtoxin A and onabotulinumtoxin A for crow’s feet: a Phase 3, multicenter, randomized, double-blind, split-face study. Derm Surg. 2019;45(12):1610–1619. doi: 10.1097/DSS.0000000000001920 [DOI] [PubMed] [Google Scholar]
- 28. Monheit G, Lin X, Nelson D, Kane M. Consideration of muscle mass in glabellar line treatment with botulinum toxin type A. J Drugs Dermatol. 2012;11(9):1041–1045. [PubMed] [Google Scholar]
- 29. Fabi SG, Carruthers J, Joseph J, et al. High-dose neuromodulators: a roundtable on making sense of the data in real-world clinical practice. Aesthet Surg J Open Forum. 2021;3(4):ojab036. doi: 10.1093/asjof/ojab036 [DOI] [PMC free article] [PubMed] [Google Scholar]