Abstract
Background
For decades, facial fat grafting has been used in clinical practice for volume restoration. The main challenge of this technique is variable volume retention. The addition of supplements to augment fat grafts and increase volume retention has been reported in recent years.
Objectives
The aim of this systematic review was to investigate which supplements increase volume retention in facial fat grafting as assessed by volumetric outcomes and patient satisfaction.
Methods
Embase, Medline, Ovid, Web of Science Core Collection, Cochrane Central Register of Controlled Trials, and Google Scholar were searched up to November 30, 2020. Only studies assessing volume after facial fat grafting with supplementation in human subjects were included. Outcomes of interest were volume or patient satisfaction. The quality of the studies was assessed with the Effective Public Health Practice Project tool.
Results
After duplicates were removed 3724 studies were screened by title and abstract. After reading 95 full-text articles, 27 studies were eligible and included for comparison. Supplementation comprised of platelet-rich plasma, platelet-rich fibrin, adipose tissue–derived stromal cells or bone marrow–derived stromal cells, cellular or tissue stromal vascular fraction, or nanofat. In 13 out of 22 studies the supplemented group showed improved volumetric retention and 5 out of 16 studies showed greater satisfaction. The scientific quality of the studies was rated as weak for 20 of 27 studies, moderate for 6 of 27 studies, and strong for 1 study.
Conclusions
It remains unclear if additives contribute to facial fat graft retention and there is a need to standardize methodology.
Level of Evidence: 4

See the Commentary on this article here.
Fat grafting has been performed in clinical practice since the end of the 19th century.1 It has been used to restore volume loss due to trauma, aging, congenital defects, or for aesthetic reasons, predominantly in the face, breasts, and buttock. Facial fat grafting can be performed easily, safely, and with minimum donor-site morbidity and complications.2 However, not all transplanted tissue is retained at the acceptor site. Long-term volume retention rates vary widely between 25% and 80%.3-5 Additionally, multiple surgical procedures are often required to obtain the desired volume.
Lipografting is a form of tissue transplantation, albeit of fragmented adipose tissue. These fragments consist of multiple large lipid-laden adipocytes that are structurally supported by connective tissue and perfused with a highly developed microvasculature. Adipocytes are about 4-fold less numerous than stromal vascular cells, yet comprise about 90% of the total volume of fat.6 Upon transplantation (ie, fat grafting), the survival and regeneration of adipocytes are pivotal to retaining the grafted volume.7 Ischemia may cause apoptotic loss of adipocytes, and thus suppression of apoptosis in fat grafts might improve graft volume retention. For graft survival, it is essential to form a rapid connection between local vasculature and capillaries that literally stick out from the tissue clumps in the fat graft. Thus angiogenic stimulation by fat graft supplements is warranted. Any adipocytes lost from ischemic insult require replenishment through proliferation of pre-adipocytes (adipose tissue–derived stromal cells [ASCs]) and their differentiation and maturation into adipocytes, which would be supported by promitogenic factors in supplements. Finally, metabolic maintenance is important because adipocyte volume, ie, the storage of the high-energy triglycerides, varies with the body’s metabolic demand. Weight loss is associated with loss of adipocyte volume and consequently with reduced graft volume.8 Although repeated fat grafting does build up sufficient volume, this is an undesirable burden for the patient. Therefore, supplements that augment suppression of apoptosis, stimulate proliferation, and enhance angiogenesis are desired. In clinical applications, the cellular fate of grafted fat is usually not assessed, yet this does not preclude investigation of the influence of supplements on graft volume.
To increase graft retention, supplementation with several autologous components has been investigated. Blood-derived products, eg, platelet-rich plasma (PRP) and platelet-rich fibrin (PRF),9,10 are a source of concentrated platelets, growth factors, and cytokines which could induce better graft retention by promoting angiogenesis and reducing apoptosis.11 Adipose tissue–derived components, eg, ASCs, cellular and tissue stromal vascular fraction (cSVF, tSVF),12,13 and nano- and microfat,14-16 have shown proangiogenic action through paracrine factors which could induce better graft vascularization, reduce apoptosis, and increase proliferation.17,18 Furthermore, enzymatic cell-assisted lipografting made addition of cSVF or cultured ASC to fat grafting popular.4, 19-20 However, because cell-assisted lipografting requires enzymatic digestion of SVF and the use of animal-derived enzymes such as collagenase it is restricted by legislation in many countries,21 and hence new nonenzymatic, fast, intraoperative, mechanical dissociation procedures have been developed to produce tSVF.22,23 PRP or PRF are also easily obtained by centrifugation of blood with or without anticoagulant.24 These supplementations are believed to improve retention through either increased survival of the grafted cells by reducing/preventing cell apoptosis, or by restoring hypertrophy or increasing vascularization at the injection site.
Currently, the number of clinical studies investigating supplemented fat grafting is increasing rapidly, and multiple new supplementation therapies are being developed.25 These developments warrant systematic evaluation of the clinical available evidence. The current systematic reviews on fat graft supplementation are heterogeneous because they include human and animal studies for various indications.26,27 The aim of this systematic review was therefore to investigate the efficacy of human facial fat grafting based on quantitative volumetric outcome measures and patient satisfaction assessments.
METHODS
Protocol and Registration
This manuscript follows the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.28 The study was registered in Prospero (register code: CRD42020179975).
Search Strategy and Information Sources
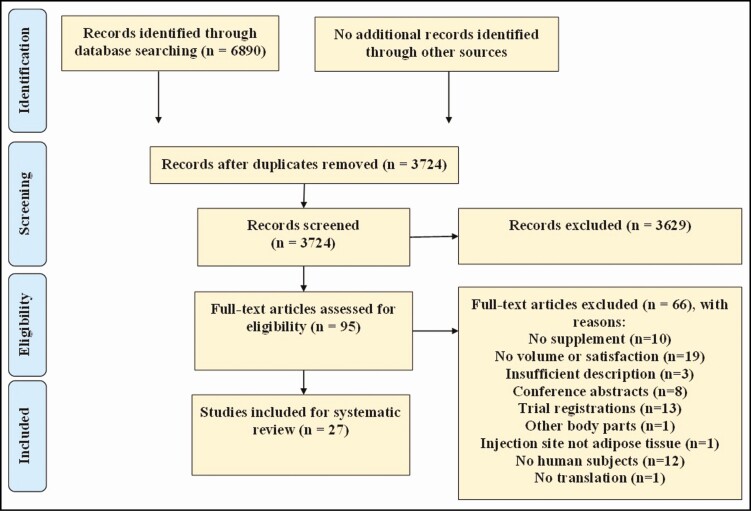
A systematic literature search was conducted in the electronic medical databases Embase (Elsevier, Amsterdam, the Netherlands), MEDLINE (National Library of Medicine, Bethesda, MD), Ovid (Wolters Kluwer, Alphen aan den Rijn, the Netherlands), Web of Science Core Collection (Clarivate Analytics, London, UK), Cochrane Central Register of Controlled Trials (CENTER; London, UK), and Google Scholar (Google, Mountain View, CA) from inception to November 30, 2020. Search strategy was based on the PICO (population, intervention, comparison, outcome) framework and combined terms related to fat graft transplantation (ie, lipofilling, fat transplantation, adipose tissue transplantation, adipose tissue grafting, volume retention) plus a supplementation therapy (ie, PRP, ASCs, SVF, nanofat, microfat).29 In databases where a thesaurus was available (Embase and MEDLINE), papers were searched by thesaurus terms and by title and/or abstract. The searches were adapted corresponding to each database (Supplemental Table 1, available online at www.aestheticsurgeryjournal.com, and Figure 1). Reference lists of included studies were analyzed to identify relevant studies missed in the searches.
Figure 1.
Flow diagram of study selection.
Eligibility Criteria
Studies were included if they clinically evaluated the effects of fat grafting in combination with a supplement, for instance the addition of PRP, ASCs, or SVF, on volume restoration in the face or patient satisfaction. Only studies injecting in the adipose tissue plane in the face were included. Studies were excluded when no volumetric outcome was reported, as were studies reporting on other body parts than the face only. If studies described multiple body parts and data of the face were separately described, the study was included. (Systematic) reviews, case studies, conference abstracts, letters to the editor, and animal and in vitro studies were also excluded. No publication date restriction was applied.
Study Selection and Data Collection Process
Two reviewers (J.S., L.V.) independently assessed titles, abstracts, and full texts. Disagreement between reviewers was discussed until consensus was reached. In the case of persistent disagreement, a senior author (M.H.) gave a binding verdict.
Data Extraction
All data were extracted by the same 2 reviewers and consisted of 5 categories: study characteristics, treatment characteristics, complications, volumetric assessment of fat graft (retention), and patient satisfaction.
Complications were categorized as minor (erythema, mild edema, hematoma, local pain at incision site, and oily cyst) and major complications (infection, tissue loss, skin necrosis, fibrosis, severe edema, pain spreading beyond injection site, cellulitis, fat embolus, and embolus causing blindness). For supplemented fat grafting therapy, data outcomes of interest were time between harvesting and injection, injected volumes, supplement dosing, cell yield or PRP concentration, isolation procedures, repeated treatments, and characterization of supplementation therapy. For volumetric outcomes, data from objective and subjective volume measurement tools and follow-up points were extracted. When studies reported fat graft resorption as an outcome measure, retention was calculated as inverse resorption (100% – x%). For each volume retention reported, a fold-change was calculated (% supplemented fat divided by % fat). A difference was reported when there was a statistically significant difference (P < 0.05).
Risk of Bias in Individual Studies
The 2 reviewers independently assessed risk of bias with the Effective Public Health Practice Project tool (EPHPP).30 This tool enables quality assessment of different types of study design. Studies were given an overall final rating as strong, moderate, or weak based on ratings of study design, selection bias, confounders, data blinding, data collection, and dropouts. According to the EPHPP tool, “strong studies” had no weak rating, “moderate studies” had 1 weak rating and “weak studies” had 2 or more weak ratings.
RESULTS
Study Selection
In total, 3724 studies were identified. After title and abstract screening, 95 studies remained for full-text assessment of eligibility criteria (Figure 1). A total of 27 studies were included; 68 studies were excluded for the following reasons: 10 studies were excluded because the fat graft was not supplemented; 23,31-39 19 studies were excluded because fat graft retention was not assessed by volumetric or patient satisfaction measurements; 40-59 3 studies were excluded because the study methods or intervention were not evaluable due to insufficient description; 60-62 8 studies were excluded because these were conference abstracts; 63-69 13 studies were excluded because these concerned trial registrations; 70-82 1 study was excluded because the supplementation therapy was not carried out in the face, but in other body parts; 83 in 1 study the injection site was not adipose tissue but product was injected within the muscles of the face (facial muscular plane); 84 12 studies were excluded because they did not describe human subjects or clinical results; 85-95 no translation of 1 study was available (Russian).96
Study Characteristics
The studies included were published between 2008 and 2020 (Table 1). Follow up ranged from 6 to 60 months and a total of 1117 participants were described in the studies (range, 6 to 236 per study). The mean age of the participants ranged between 6 and 61.5 years old and 73% of all patients were female (range, 33%-100%). Five studies had a female sex bias due to the inclusion of female participants only.97-101 Eight studies reported mean BMI, which ranged from 17 to 32 kg/m². Indications for supplemented fat grafting were with underlying pathology (48%) or cosmetic (without underlying pathology) with (19%) or without facelift (33%). Indications with underlying pathology were craniofacial deformity (31%), scars (23%), (hemi)facial lipoatrophy (15%), Parry-Romberg syndrome (15%), or a combination of these indications (15%). The majority (13/27) of the studies supplemented fat grafts with PRP/PRF. Three studies reported multiple supplements.102-104 Studies were categorized by type of supplement (PRP, SVF, and cellular components) for analysis of supplement characteristics, volumetric, and patient satisfaction outcomes (Supplemental Table 2, available online at www.aestheticsurgeryjournal.com, and Tables 2, 3).
Table 1.
Study Characteristics
| Author (year) | Design | Follow up (months) | Indication | Pathologya | Injection site | Total (n) | Ageb (years) | Female (n) | BMI (kg/m2) | Comorbidities | Intervention (fat +) | Intervention (n) | Control | Control (n) | Minor complications | Major complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bashir et al 2019 | C2G | 6 | UP | Hemifacial atrophy, craniofacial microsomia, posttraumatic and postinfective deformity | NR | 37 | 24.9 [8.1] | 28 | NR | NR | cASC | 16 | Fat | 21 | “Most patients” | Both in control and intervention group: 6% cellulitis |
| Bernardini et al 2015 | C1G | 12 | C + FL | Brows, upper sulcus, inferior orbit hollow, tear trough, perioral area, malar and zygomatic areas, lips, chin, temporal fossa | 98 | 51 | 92 | NR | NR | PRP | 98 | — | — | 4% | 3% of patients oil cyst, 1 case requiring surgical removal | |
| Castro-Govea et al 2018 | C1G | 18 | UP | Craniosynostosis | Forehead | 12 | 6 | 8 | NR | NR | cSVF | 12 | — | — | 0 | 0 |
| Cervelli et al 2009 | RC | 18 | UP | Scars, Parry-Romberg, hemifacial atrophy, mandibular cyst | Zygomatic region, cheek, buccal rim, upper and lower eyelid, temporal area, orbital area | 25 | NR | NR | NR | Diabetes, hypertension, nasal polypus, neurologic disease, arteriopathy, cardiologic disease, dislipidemy, trauma | PRP | 19 | Fat | 10 | 0 | 0 |
| Chang et al 2013 | CCT | 18 | UP | Hemifacial atrophy | NR | 20 | 27.5 | 12 | NR | — | cSVF | 10 | Fat | 10 | 0 | 0 |
| Fontdevila et al 2014 | RCT | 12 | UP | HIV lipoatrophy | Cheeks | 49 | 46.3 [7.4] | 16 | 24.3 [3.2] | Diabetes, hypertension, hypercholesterolemia, hypertriglyceridemia, fibrates/statin/ antidepressant/ anxiolytic/ antidiabetic drug use | PRP | 20 | Fat | 29 | 0 | 0 |
| Gennai et al 2017 | C1G | 6 | C + FL | Periocular, perioral area | 65 | 49.7 | 58 | NR | NR | PRP | 65 | — | — | 0 | 0 | |
| Gentile et al 2014 | C2G | 12 | UP | Burns, posttraumatic scars | NR | 20 | NR | 10 | NR | NR | cSVFPRP | 1010 | Fat | 10 | 0 | 0 |
| Gentile et al 2020 | C2G | 60 | C | Zygomatic/cheek region, lower orbital area, nasolabial fold, lips | 63 | 42.1d | 63 | 27 (21-33.16) | — | tSVF | 33 | Fat | 30 | Intervention group: 9%; control group: 13% | 0 | |
| Gu et al 2018 | C1G | 6 | UP | Scars | NR | 20 (25 scars) | 38.3 | 14 | NR | — | tSVF | 25 | — | — | NR | NR |
| Hesamirostami et al 2019 | C1G | 30 | C | Forehead | 56 | 40.2 | 52 | NR | NR | PRP | 56 | — | — | 0 | 0 | |
| Jianhui et al 2014 | C2G | 12 | UP | Parry-Romberg | NR | 36 | 24.3 [6.6]d | 25 | NR | NR | Intraoperative BMSC | 10 | Fat | 26 | 0 | 0 |
| Keyhan et al 2013 | RCT c | 12 | C | Cheek, cheekbone area | 25 | 45 | 17 | NR | — | PRP | 25c | Fat/PRF | 25c | 0 | 0 | |
| Koh et al 2012 | CCT | 15 | UP | Parry-Romberg | NR | 10 | 28 | 5 | NR | — | cASC | 5 | Fat | 5 | 0 | 0 |
| Lee et al 2012 | C1G c | 11 | C + FL | Malar eminence, infraorbital region, nasolabial fold | 9 | 43.3 | 6 | NR | NR | cSVF | 9c | Fat | 9c | 0 | 0 | |
| Li et al 2013 | C2G | 6 | NRG | Temporal, cheek, facial asymmetry | 38 | 29.4 [6.6]d | 38 | NR | NR | cSVF | 26 | Fat | 12 | “Most patients” | 0 | |
| Ozer et al 2019 | C1G | 9 | C | NR | 14 | 44.9 [11.9] | 14 | NR | NR | PRP | 14 | — | — | 0 | 0 | |
| Sasaki et al 2015 | C2G | 12 | C + FL | Midface | 236 | 61.5d | 227 | 22.2 (16.9-32.3)d | — | PRP cSVF cSVF/PRP |
106 929 |
Fat | 92 | “All patients” | 0 | |
| Sasaki et al 2019 | RCT c | 12 | C + FL | Midface | 10 | 54.4 | 10 | 22.4 (20.5-24.6) | — | PRP | 10c | Fat/saline | 10c | “All patients” | 0 | |
| Schendel et al 2015 | C1G | 17 | C | Temples, malar areas, forehead/glabella, eyelid area, lips, chin | 10 | 51.6 [9.6] | 10 | NR | NR | cSVF | 10 | — | — | 0 | 0 | |
| Sterodimas et al 2011 | CCT | 18 | UP | Several congenital or acquired facial tissue defects | NR | 20 | 45.1d | 10 | 21.6d | Smoking, hypertension, diabetes, COPD | cSVF | 10 | Fat | 10 | “Most patients” | Control group: 10% infection |
| Tanikawa et al 2013 | RCT | 6 | UP | Craniofacial microsomia | NR | 14 | 15.4 [5.6]d | 9 | <25 | NR | cSVF | 7 | Fat | 7 | “All patients” | 0 |
| Tenna 2017 | CCT | 6 | UP | Acne scars | Cheeks | 30 | NR | NR | NR | — | Fat/PRP/laser | 15 | Fat/PRP | 15 | NR | NR |
| Wei et al 2017 | CCT | 24 | C | Tempora, geisoma, frontal part, palpebra sup inf, lacrimal groove, zygoma, cheeks, nasolabial groove, chin, marionette lines, submaxilla | 139 | 28.5 | NR | NR | NR | Nanofat/PRF | 62 | Fat | 77 | 0 | 0 | |
| Willemsen et al 2018 | RCT | 12 | C | Temporal, midface, nasolabial fold, marionette lines, prejowling, chin | 25 | 52.1 [6.8] | 32 | (20-25) | NR | PRP | 13 | Fat/saline | 12 | 0 | 0 | |
| Yin et al 2020 | RCT | 50 | C | Forehead, temporal, cheek/zygomatic, nasolabial fold | 50 | 35.4 [8.2]d | 50 | 21.4 [1.9]d | — | cSVF | 25 | Fat | 25 | 0 | 0 | |
| Yoshimura et al 2008 | CCT | 13 | UP | Parry-Romberg and lupus lipoatrophy | NR | 6 | 42.5 [8.0]d | 4 | NR | NR | cSVF | 3 | Fat | 3 | “All patients” | Control group: 33% necrotized tissue requiring surgical removal |
Where indicated, values are mean [standard deviation] or mean (range); NR, not reported; COPD, chronic obstructive pulmonary disease. Study design: RCT, randomized controlled trial; CCT, controlled clinical trial; Cohort 2G, cohort study (2 groups, pre- + postoperative); Cohort 1G, cohort study (1 group pre- + postoperative); Retrospective, retrospective study. Indication: UP, underlying pathology, meaning with underlying disease, trauma, or congenital volume loss; C, cosmetic, meaning with no underlying pathology or facial disease, such as fat grafting for facial rejuvenation; C + FL, cosmetic with concomitant facelift. Enrichments, PRP, platelet-rich plasma; PRF, platelet-rich fibrin; cSVF, cellular stromal vascular fraction; tSVF, tissue stromal vascular fraction; cASC, cultured adipose-derived stromal cell; BMSC, bone marrow–derived stromal cell.
aIn the cosmetic group, there is no pathology present (ie, facial rejuvenation).
bWhen decimals are reported, these are rounded to 1 decimal place.
cSplit-face design: in the studies using a split-face design: the patients themselves serve as both intervention group (one half of the face) and control group (the other half of the face).
dWhen data are presented per group, the pooled value is calculated. —, not present in the study reported (eg, no control group was present or no complications occurred in the study).
Table 2.
Volume Outcomes
| Author (year) | Outcome assessment | Follow up (months) | Intervention % retention |
Control % retention |
Fold change | Difference in retentionb (intervention compared with control) |
|---|---|---|---|---|---|---|
| PRP/PRF | ||||||
| Bernardini et al 2015 | VA of volume | 6 | Good result (63%), excellent result (37%) | — | – | |
| Cervelli et al 2009 | VA of volume | 18 | 65% | 26% | 2.5 | — |
| Fontdevila et al 2014 | CT | 12 | NR | NR | –0.3 mL (−1.1 to –0.5 mL)a (NS) | |
| Gentile et al 2014 | MRI | 12 | 69% | 39% | 1.8 | - |
| Keyhan et al 2013 | Linear measurements of photographs | 12 | 82% (PRP) | 87% (PRF) | ND | 5% ↑ (PRF) (P < 0.05) |
| Sasaki et al 2015 | 3D SI | 12 | 69% [40%] | 38% [13%] | 1.8 | 31% ↑ (P < 0.01) |
| Sasaki et al 2019 | 3D SI | 12 | 24% [10%] | 21% [1%] | 1.1 | 3% ↑ NS |
| Tenna et al | US | 12 | 0.7 cm improvement | 0.6 cm improvement | 1.1 | 0.1 cm ↑ NS |
| Willemsen et al 2018 | VA of nasolabial fold | 12 | NR | NR | ND | NS |
| ASCs | ||||||
| Bashir et al 2019 | US | 6 | 95% [4%] | 31% [13%] | 3.1 | 64% ↑ (P < 0.001) |
| Koh et al 2012 | 3D SI | 6 | 79% | 53% | 1.5 | 26% ↑ (P = 0.002) |
| cSVF | ||||||
| Chang et al 2013 | CT | 6 | 68% [2%] | 59% [1%] | 1.2 | 10% ↑ (P < 0.001) |
| Gentile et al 2014 | MRI | 12 | 63% | 39% | 1.6 | 24% ↑ (P < 0.0001) |
| Lee et al 2012 | NRS (1-10) | 3 | Malar eminence 7 (6-8) Infraorbital region 7 (6-9) Nasolabial fold 8 (7-9)c |
Malar eminence 6 (5-7) Infraorbital region 6 (5-6) Nasolabial fold 6 (5-8)c |
Malar 1.2 Infraorbital 1.2l Nasolabial 1.3 |
Malar eminence 1 ↑ (P = 0.015) Infraorbital region 1 ↑ (P = 0.010) Nasolabial fold 2 ↑ (P = 0.017) |
| Li et al 2013 | CT | 6 | 65% [10%] | 46% [9%] | 1.4 | 18% ↑ (P < 0.01) |
| Sasaki et al 2015 | 3D SI | 12 | 73% [50%] | 38% [13%] | 1.9 | 35% ↑ (P < 0.01) |
| Schendel et al 2015 | 3D SI | 12 | 68% | — | ND | — |
| Tanikawa et al 2013 | CT | 6 | 88% [13%] | 54% [20%] | 1.6 | 34% ↑ (P = 0.002) |
| Yin et al 2020 | 3D SI (handheld) | 6 | 78% [12%] | 56% [10%] | 1.4 | 21% ↑ (P < 0.001) |
| Yoshimura et al 2008 | LS (1-4) | 12 | NR | NR | ND | NS |
| tSVF | ||||||
| Gentile et al 2020 | MRI | 36 | 61% [5%] | 31% [5%] | 2 | 30% ↑ (P < 0.0001) |
| PRP + cSVF | ||||||
| Sasaki et al 2015 | 3D SI | 12 | 70% [35%] | 38% [13%] | 1.8 | 31% ↑ (P < 0.01) |
Where indicated, values are mean [standard deviation] or (range). —, no test was performed, or no quantification was described; NR, not reported; NS, not significant. Outcome assessment: NRS, numeric rating scale; US, ultrasound; CT, computed tomography; LS, Likert scale; VA, visual assessment; SI, surface imaging. Supplements: PRP, platelet-rich plasma; PRF, platelet-rich fibrin; cSVF, cellular stromal vascular fraction; tSVF, tissue stromal vascular fraction; ASC, adipose-derived stromal cell; BMSC, bone marrow–derived stromal cell.
aFontdevila et al described no separate intervention or control volume. Only a difference between groups with a range was described.
bDifference is described in absolute percentage points; however, for the readibility of this table we have used the percentage sign %. Differences are based on the original (not rounded) data, which means rounding errors can be present.
cLee et al described surgeon-rated volume consistency based on a numeric rating scale.
dGu et al described the thickness using the POSAS questionnaire. The specific question about thickness is extracted.
Table 3.
Patient Satisfaction Outcomes
| Author (publication year) | Outcome assessment | Follow up (months) | Comparisonc | Comparison with preoperative photographs | Satisfaction intervention | Satisfaction control | Difference in satisfaction (intervention compared with control or postoperative compared with preoperative) |
|---|---|---|---|---|---|---|---|
| PRP/PRF | |||||||
| Gennai et al 2017 | LS (1-4) | 6 | Within-group outcome | Yes | Fair to good effect (2.6) | — | — |
| Gentile et al 2014 | LS (1-6) | 12 | Within-group outcome | Yes | nr | — | — |
| Hesamirostami et al 2019 | GAIS | 12 (6-30) | Within-group outcome | Yes | Moderate to excellent improvement, 7% poor improvement. | — | — |
| Ozer et al 2019 | FACE-Q | 9 | Within-group change | — | Improved from 28.4 [23.3] to 90.3 [17.5] | — | 61.9 ↑ (P < 0.001) |
| Tenna et al 2017 | FACE-Q | 6 | Between-group outcome | No | 84%b | 81%b | NS |
| Willemsen et al 2018 | VAS (1-10) | 6 | Between-group outcome | No | NR | NR | NS |
| ASCs/BMSCs | |||||||
| Bashir et al 2019 | LS (1-5) | 6 | Between-group outcome | Yes | 4.3 [0.7] | 2.5 [0.5] | 1.8 ↑ NSR |
| Jianhui et al | LS (1-3) | NR | Between-group outcome | No | NR | NR | — |
| Koh et al 2012 | VAS (1-5) | NR | Between-group outcome | No | 4.5 | 3.1 | 1.4 ↑ NSR |
| cSVF | |||||||
| Castro-Govea et al 2018 | LS of parents (1-5) | 18 | Within-group outcome | No | 67% of the parents were satisfied and 33% were slightly satisfied | — | — |
| Lee et al 2012 | NRS (1-10) | 3 | Between-group outcome | Yes | Malar eminence 7 (6-8) Infraorbital fold 8 (7-9) Nasolabial fold 8 (7-9)a |
Malar eminence 6 (5-8) Infraorbital fold 6 (5-7) Nasolabial fold 7 (5-8)a |
Malar eminence 1 ↑ (P = 0.008) Infraorbital fold 2 ↑ (P = 0.010) Nasolabial fold 1 ↑ (P = 0.011) |
| Sterodimas et al 2011 | LS (1-5) | 18 | Between-group outcome | No | 4.0 b | 4.0 b | 0 NS |
| Yin et al 2020 | LS (1-5) | 6 | NR | No | — | — | — |
| tSVF | |||||||
| Gentile et al 2020 | LS (1-6) | NR | Between-group outcome | No | 91% fully satisfied and 9% not fully satisfied | 37% fully satisfied and 63% not fully satisfied | (P = 0.031) |
| Gu et al 2018 | POSAS | 12 | Within-group change | Yes | Preoperative 28.8 [1.0] vs postoperative 12.2 [0.8] | — | 16.6 ↓ (P < 0.001)d |
| Wei et al 2017 | nr | 24 | Between group outcome | No | 90% | 70% | 20% ↑ (P < 0.01) |
Where indicated, values are mean [standard deviation] or (range). NR, not reported; NS, not significant; NSR, no significance reported, no statistical test was performed/reported; —, no quantification, no intervention or control group present or no statistical test reported. Outcome assessment: NRS, numeric rating scale, with a higher number meaning a better score; LS, Likert scale, each number represents an outcome, such as unsatisfactory-slightly satisfactory, satisfactory; VAS, visual analog scale; FACE-Q, a validated questionnaire using a combination of Likert scales and visual analog scales; POSAS, a validated questionnaire specifically designed for scars (the overall patient-reported POSAS score is reported in this table; a lower score means a greater satisfaction); GAIS, Global Aesthetic Improvement Scale is a Likert scale, 0-4. Supplements: PRP, platelet-rich plasma; PRF, platelet-rich fibrin; cSVF, cellular stromal vascular fraction; tSVF, tissue stromal vascular fraction; ASC, adipose-derived stromal cell; BMSC, bone marrow–derived stromal cell.
aOverall patient satisfaction was noted from the patient satisfaction scores.
bData were manually calculated from the tables in the article.
cWithin-group outcome means that no comparison to baseline or comparison to a control group was made. Participants were asked to evaluate the outcome after surgery without evaluating the preoperative situation.
dA lower score of the POSAS questionnaire means a greater satisfaction.
Study Design and Quality
Study designs included randomized controlled trials (n = 6),67,99,101,105-107 controlled trials (n = 6),4,19,108-111 cohort studies (2 groups with pre- and posttreatment evaluation, n = 6),34,103,104,108,112,113 cohort studies (1 group with pre- and posttreatment evaluation, n = 8)98,100,114-119 and a retrospective study (n = 1)45 (Table 4). Confounding factors were not controlled for in 8 studies.42,44,108,110,114,116-118 The reliability and validity of outcome measurements were weak in 12 studies.4,19,44,67,105,108,110,113-116,119 Four studies reported dropouts and reported the number of participants who completed the follow up.101,105,107,118 Based on the EPHPP guidelines, 20 studies had an overall final rating of weak, 6 studies were rated as moderate, and only 1 study was rated as strong (4%).106 Data pooling and meta-analysis was not possible due to heterogeneity across studies in terms of clinical features, eg, population characteristics, indications, supplementation strategies, and additional interventions (facelift, additional injections), and methodologic characteristics, eg, assessment tools, study design, and follow up.
Table 4.
Quality of the Included Studies Based on the Effective Public Health Practice Project Tool
| Reference | Selection bias | Study Design | Confounders | Blinding | Data Collection | Dropouts | Global rating |
|---|---|---|---|---|---|---|---|
| Bashir et al 2019 | 0 | 0 | – | 0 | + | – | – |
| Bernardini et al 2015 | – | 0 | – | 0 | – | – | – |
| Castro-Govea et al 2018 | – | 0 | – | 0 | – | – | – |
| Cervelli et al 2009 | – | 0 | – | – | – | – | – |
| Chang et al 2013 | – | + | – | 0 | – | – | – |
| Fontdevila et al 2014 | 0 | + | + | + | – | + | 0 |
| Gennai et al 2017 | – | 0 | – | – | – | NA | – |
| Gentile et al 2014 | – | 0 | + | 0 | 0 | – | – |
| Gentile et al 2020 | – | 0 | – | – | 0 | – | – |
| Gu et al 2018 | – | 0 | – | – | + | – | – |
| Hesamirostami et al 2019 | 0 | 0 | – | – | + | + | – |
| Jianhui et al 2014 | – | 0 | – | – | – | – | – |
| Keyhan et al 2013 | – | + | + | 0 | – | – | – |
| Koh et al 2012 | – | + | + | 0 | + | – | – |
| Lee et al 2012 | – | 0 | + | 0 | – | – | – |
| Li et al 2013 | – | 0 | + | 0 | 0 | NA | 0 |
| Ozer et al 2019 | – | 0 | + | 0 | + | NA | 0 |
| Sasaki et al 2015 | – | 0 | – | – | 0 | – | – |
| Sasaki et al 2019 | – | + | + | 0 | 0 | – | – |
| Schendel et al 2015 | – | 0 | + | 0 | 0 | + | 0 |
| Sterodimas et al 2011 | – | + | 0 | 0 | – | – | – |
| Tanikawa et al 2013 | 0 | + | + | 0 | 0 | 0 | + |
| Tenna et al 2017 | – | + | – | 0 | + | – | – |
| Wei et al 2017 | – | + | – | 0 | – | – | – |
| Willemsen et al 2018 | – | + | + | 0 | + | 0 | 0 |
| Yin et al 2020 | – | + | + | + | 0 | + | 0 |
| Yoshimura et al 2008 | – | + | + | 0 | – | – | – |
| Totals | |||||||
| Weak, n (%) | 23 (85%) | 0 (0%) | 13 (48%) | 7 (26%) | 12 (44%) | 18 (67%) | 20 (74%) |
| Moderate, n (%) | 4 (15%) | 15 (56%) | 1 (4%) | 18 (67%) | 8 (30%) | 2 (7%) | 6 (22%) |
| Strong, n (%) | 0 (0%) | 12 (44%) | 13 (48%) | 2 (7%) | 7 (26%) | 4 (15%) | 1 (4%) |
The totals at the bottom represent the distribution of how weak, moderate and strong each criterion is. Ref, reference, +, strong, 0, moderate, –, weak.
Characteristics of Supplementation Strategies
The mean injected total volume ranged from 6.8 to 100 mL. The volume-to-volume ratio of PRP-to-fat ranged from 1:2 to 1:9. In SVF-supplemented therapies, 2 out of 14 studies reported the ratio of supplementation. Repeated supplemented fat graft injections were performed in 11 studies and concerned merely supplementation with SVF and cellular components.19,34,108-113,115,117,118 A minority of studies reported supplement concentrations: platelet concentration in PRP or PRF was 0.8 × 109 to 3.6 × 109/mL (mean [standard deviation], 2.6 [1.3] × 109/mL), and the number of nucleated cells in cSVF or tSVF was 0.3 × 105 to 100 × 105 cells.112,107,109 Some studies reported a concentration range of fat-supplemented therapy and the single addition of bone marrow–derived stromal cells (BMSCs) ranged from 3 to 86 × 108 at a volume ratio of 2:1 of BMSC:fat graft.113 Most studies performed intraoperative isolation procedures of the supplementation therapy. Two studies cultured ASCs for 14 days109 and 14 to 28 days34 before supplementing fat grafts but the volume ratio was not reported.34,109 Only 4 studies reported the lag time between preparation of supplements to administration to the patient or the time (range) to prepare the supplements.106,108-110 Three studies described the characterization of supplements; nanofat plus PRF, ASCs, cSVF.19,106,109 The shared joint analyzed markers included expression of mesenchymal cell markers (CD73, CD90, and CD105) albeit that these are not restricted to mesenchyme, integrin β1 (CD29), and the absence of leukocyte markers (CD45).
Influence of Supplementation Therapies on Fat Graft Retention
Twenty studies assessed volume retention of supplemented fat grafts after 3 to 36 months, of which 3 studies reported multiple supplementation groups (PRP/cSVF, PRP/cSVF/PRP + cSVF, PRP/tSVF).102-104 Volume was measured by computed tomography (CT), MRI, 3-dimensional (3D) surface imaging, ultrasound, visual 2D photograph assessment, numeric rating scale (NRS), or Likert scale. Seven studies used 3D surface imaging to assess fat graft retention. Volume measurement methods were often not validated and details of volumetric measurements were often not described, or described too briefly to allow for reproduction of studies.
Out of the 9 studies in which the graft was supplemented with PRP, 2 showed a difference between groups.103,120 One study showed a 30% increase of volume compared to the control, ie, conventional fat grafting.103 The other study showed a difference of 5% less retention in the PRP group compared to the control group, in which PRF was used as a control.120 In 4 out of 9 PRP studies there was no difference between groups.99,101,105,111 In the other 3 studies no statistical tests were performed or could not be performed due to the absence of controls.45,104,114
The 2 studies investigating supplementation of culture-expanded ASCs both showed a difference when compared with the conventional fat graft.34,109 The volume retention varied from 1.5-fold higher109 to 3-fold higher.34 Seven out of 9 studies with cSVF as supplement showed a statistically significant increased volume compared with the conventional fat graft (1.2- to 1.9-fold).97,103,104,106-108,119 Two studies showed no increased volume. One study without a control group reported a retention of 68%.100 In 1 study no difference between groups was found. Only 6 patients were included in that study and surgeons assessed volume visually from 2D photographs.4
One study investigating tSVF supplementation showed improved outcomes.112 A 2-fold increase in volume in the supplemented group was found, but the measurement methods on MRI were not described.112 The only study describing PRP and cSVF mixed as a supplement showed significantly increased volume retention (70%).103 However, the mix of PRP and cSVF did not result in additional volume increase compared with cSVF (73%) or PRP (69%) alone.
Influence of Supplementation Therapies on Patient Satisfaction
Patient satisfaction or patient-reported outcome measures (PROMs) are considered the key outcome measurement of facial aesthetic procedures.121 Validated and reliable outcome measures, eg, the FACE-Q questionnaire, are readily available.122,123 Sixteen studies assessed patient satisfaction with the FACE-Q, the Patient and Observer Assessment Scale (POSAS), the Global Aesthetic Improvement Scale (GAIS), a visual analog scale (VAS), the Likert scale, or an NRS. The FACE-Q, GAIS, and POSAS are the only validated outcome measures and were used in 4 studies only.
To evaluate the differences in patient satisfaction between procedures in a controlled trial, the satisfaction for both the intervention and the control group should be evaluated and statistically tested for differences. Nine studies performed these “between-group” comparisons, but only 6 of these 9 studies performed statistical testing. Patient satisfaction assessment was sometimes performed together (or in the same room) with the operating surgeon,116 which may have induced interviewer bias and social desirability bias. Other studies omitted to describe the conditions under which measurements were performed and how results were obtained.102,104,112 Follow up was sometimes not reported or ranged considerably within studies (3-30 months).109,112,113,118
Overall, patients reported high satisfaction rates after both conventional and supplemented facial fat grafting. Statistical tests were performed in 8 out of 16 studies.98,101,102,110-112,117,119 Six of these 8 studies statistically tested for differences between the intervention and control group, of which 3 reported improved satisfaction in the intervention group.101,102,110-112,117,119 Two PRP studies showed no significant improvement, of which the study of Tenna et al compared PRP with or without laser.101,111 One cSVF study showed significant improvement and 1 cSVF showed no significant improvement.110,119 Two tSVF studies showed significant improvement.102,112
Complications
Minor complications occurred in 9 out of 27 studies. Three out of these 9 studies also reported major complications occurring in the acceptor site. Major complications were reported in the groups supplemented with ASCs and PRP and were also reported in the control groups (conventional fat grafting). Overall, bruising and swelling were the most common minor complications reported. Of the studies that reported major complications Bernardini et al reported 3 cases of oily cysts that required surgical removal.114 Bashir et al reported 2 cases of cellulitis: 1 in the intervention and 1 in the control group.34 Yoshimura et al reported a case of necrotized tissue in the control group that was surgically removed.4
DISCUSSION
Our study systematically reviewed the current literature to assess the efficacy of supplemented clinical facial fat grafting on volume retention. Our main results are that: (1) few studies include volumetric data or patient satisfaction; (2) these studies are heterogeneous with respect to (a) age, (b) injection frequency, (c) injection volume, (d) type of supplement, (e) mixing ratio of fat and supplement, (f) imaging, (g) concomitant interventions, (h) follow-up time, (i) outcome parameters, and (j) use of controls; and therefore (3) the low number of studies and their high heterogeneity did not allow for a proper meta-analysis.
The results showed that of all supplements, culture-derived ASCs were most effective at retaining injected fat volume, whereas addition of PRP, or mixtures of PRP and cSVF did not affect volume retention. Some of our reviewed papers assessed complications and found virtually none, irrespective of supplements. This corroborates previous studies that show fat grafting to be safe.2,124
A major shortcoming in virtually all analyzed papers is the near lack of properly described standardized procedures, and the reporting of interassay and intraassay variation. This causes several of the studies to be subjective rather than objective and unfortunately reduces the value of the outcomes.
Volume retention is the goal of facial fat grafting but is also a highly challenging parameter to measure and monitor. Several studies used validated imaging methods, including CT and MRI scanning or 3D surface imaging. It was surprising to note that none of the papers disclosed the unbiased reliability, ie, inter- and intrameasurement variation, as well as inter- and intraobserver variation. This reduces the value of the measurements as these are prone to subjective bias.
With regard to the use of validated inquiries to measure patient satisfaction and outcome, the FACE-Q, GAIS, and POSAS have been available for several years.121-123 Unfortunately, no more than a quarter of the papers report utilizing these instruments. Again, comparisons with these instruments were not reported, but we included these in our results. In general, statistical testing of outcomes was neglected in more than half of the studies. We consider this a major flaw that reduces the value of potentially relevant clinical trials to a minimum. Journal editorial boards and peer-review processes should continue to improve their standards for statistics.
Our quest was to find published papers that reported the benefit of supplemented fat grafting on volume retention. However, we could neither corroborate nor dispute these findings based on our current systematic literature analyses on supplemented clinical facial fat grafting. This study focused on supplemented fat grafting in the facial area, which might be a strength or a limitation. A systematic review on fat graft supplementation in other body parts would be interesting because it may elucidate whether supplementation therapies are effective and the influence of body location on fat graft viability. One Russian study was excluded because no translation was available in the medical library. However, it is doubtful whether inclusion of this study would have changed the general message of this systematic review. Future studies should focus on conducting well-designed randomized controlled clinical trials to be able to establish a higher level of evidence and to minimize inter- and intrastudy variation. Volume outcome measurement should be performed with valid imaging modalities and reliable volume measurement methods. Inter- and intrameasurement variation should be measured and reported. Imaging modalities based on ionizing radiation, such as CT, for follow up should be avoided. Validated patient-reported outcome questionnaires should be used and recorded both pre- and postoperatively to minimize potential recall bias. Procedures for harvesting and processing should be standardized.12,125,126 No concomitant procedures such as a facelift or blepharoplasty should be performed during these studies because these influence volume outcome and patient satisfaction. We have established a summary of recommendations for the design of future trials in Table 5.
Table 5.
Recommendations for the Study Design of New Trials
| Recommendations for new studies | |
|---|---|
| Quality of the study | 1. Controlled design (comparison with standard treatment or placebo) 2. Randomized 3. Minimal follow-up duration of 12 months 4. Following CONSORTa statement for reporting 5. Statistically testing for differences between groups |
| Standardization of the procedure | 1. Standardized harvesting, processing and injection technique 2. Standardized injection volume and volume-to-volume ratio of supplement-to-fat graft 3. No concomitant procedures (eg, facelift) that can influence volume or satisfaction outcomes 4. Single injections, no repeated procedures |
| Measurement of volume retention | 1. Clear definition of how retention is measured, based on injected volume or based on first volume measurement after surgery 2. Using valid imaging modalities (without ionizing radiation) 3. Using a reliable method of volume measurement, by either reporting reliability or using a validated method of volume measurement |
| Measurement of patient satisfaction | 1. Using a validated PROMb 2. Measuring change of PROM, including a preoperative (baseline) measurement 3. Statistically testing for difference of PROM between intervention and control group 4. Observer/surgeon should not be present when PROM is recorded, to exclude interviewer/social desirability bias |
aConsolidated Standards of Reporting Trials.
bPatient-reported outcome measures.
CONCLUSIONS
Despite multiple studies showing improved volume retention and increased patient satisfaction, no clinical superiority of supplementations could be objectified. Future well-designed clinical trials may elucidate whether supplementation therapies enhance fat graft retention and may increase patient satisfaction.
Supplementary Material
Acknowledgments
Drs Schipper and Vriend made an equal contribution to this work as co-first authors.
Contributor Information
Jan Aart M Schipper, Department of Pathology and Medical Biology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands.
Linda Vriend, Department of Plastic and Reconstructive Surgery, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Aartje J Tuin, Department of Oral and Maxillofacial Surgery, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Pieter U Dijkstra, Department of Rehabilitation Medicine, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Rutger H Schepers, Department of Oral and Maxillofacial Surgery, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Berend van der Lei, Department of Plastic and Reconstructive Surgery, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Johan Jansma, Department of Oral and Maxillofacial Surgery, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Martin C Harmsen, Department of Pathology and Medical Biology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
This study was funded by the Departments of Oral and Maxillofacial Surgery, Plastic and Reconstructive Surgery, and Pathology and Medical Biology, University and Medical Center Groningen, Groningen, the Netherlands.
REFERENCES
- 1. Neuber F. Verhandlungen der Deutschen Gesellschaftfür. Chirurgie. 1893;1:66. [Google Scholar]
- 2. Krastev TK, Beugels J, Hommes J, et al. Efficacy and safety of autologous fat transfer in facial reconstructive surgery: a systematic review and meta-analysis. JAMA Facial Plast Surg. 2018;20(5):351–360. doi: 10.1001/jamafacial.2018.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kølle S-FT, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382(9898):1113–1120. doi: 10.1016/S0140-6736(13)61410-5 [DOI] [PubMed] [Google Scholar]
- 4. Yoshimura K, Sato K, Aoi N, Kurita M. Cell‐assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose‐derived stem cells. Dermatol Surg. 2008;34:1178–85. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 5. Lv Q, Li X, Qi Y, et al. Volume retention after facial fat grafting and relevant factors: a systematic review and meta-analysis. Aesthetic Plast Surg. 2021;45(2):506–520. doi: 10.1007/s00266-020-01612-6 [DOI] [PubMed] [Google Scholar]
- 6. Ye RZ, Richard G, Gévry N, et al. Fat cell size: measurement methods, pathophysiological origins, and relationships with metabolic dysregulations. Endocr Rev. 2022;43(1):35–60. doi: 10.1210/endrev/bnab018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eto H, Kato H, Suga H, et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg. 2012;129(5):1081–1092. doi: 10.1097/PRS.0b013e31824a2b19 [DOI] [PubMed] [Google Scholar]
- 8. Maclean PS, Higgins JA, Giles ED, et al. The role for adipose tissue in weight regain after weight loss. Obes Rev. 2015;16(Suppl 1):45–54. doi: 10.1111/obr.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Serra-Mestre JM, Serra-Renom JM, Martinez L, et al. Platelet-rich plasma mixed-fat grafting: a reasonable prosurvival strategy for fat grafts? Aesthetic Plast Surg. 2014;38(5):1041–1049. doi: 10.1007/s00266-014-0374-7 [DOI] [PubMed] [Google Scholar]
- 10. Smith OJ, Kanapathy M, Khajuria A, et al. Protocol for a systematic review of the efficacy of fat grafting and platelet-rich plasma for wound healing. Syst Rev. 2017;6(1):111. doi: 10.1186/s13643-017-0505-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luck J, Smith OJ, Mosahebi A. A systematic review of autologous platelet-rich plasma and fat graft preparation methods. Plast Reconstr Surg Glob Open. 2017;5(12):1–14. doi: 10.1097/GOX.0000000000001596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuin AJ, Domerchie PN, Schepers RH, et al. What is the current optimal fat grafting processing technique? A systematic review. J Cranio-Maxillofacial Surg. 2016;44(1):45–55. doi: 10.1016/j.jcms.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 13. van Dongen JA, Tuin AJ, Spiekman M, et al. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: a systematic review. J Tissue Eng Regen Med. 2018;12(1): e261–e274. doi: 10.1002/term.2407 [DOI] [PubMed] [Google Scholar]
- 14. Tonnard P, Verpaele A, Carvas M. Fat grafting for facial rejuvenation with nanofat grafts. Clin Plast Surg. 2020;47(1):53–62. doi: 10.1016/j.cps.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 15. Rihani J. Microfat and nanofat: when and where these treatments work. Facial Plast Surg Clin North Am. 2019;27(3):321–330. doi: 10.1016/j.fsc.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 16. van Dongen JA, Tuin AJ, Harmsen MC, et al. The difference between stromal vascular fraction isolation and fat emulsification: a crucial role for centrifugation. Plast Reconstr Surg. 2020;145(1):232e–233e. [DOI] [PubMed] [Google Scholar]
- 17. Guo J, Nguyen A, Banyard DA, et al. Stromal vascular fraction: a regenerative reality? Part 2: mechanisms of regenerative action. J Plast Reconstr Aesthetic Surg. 2016;69(2):180–188. doi: 10.1016/j.bjps.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 18. Suga H, Glotzbach JP, Sorkin M, et al. Paracrine mechanism of angiogenesis in adipose-derived stem cell transplantation. Ann Plast Surg. 2014;72(2):234–241. doi: 10.1097/SAP.0b013e318264fd6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cai W, Yu LD, Tang X, Shen G. The stromal vascular fraction improves maintenance of the fat graft volume: a systematic review. Ann Plast Surg. 2018;81(3):367–371. doi: 10.1097/SAP.0000000000001589 [DOI] [PubMed] [Google Scholar]
- 20. Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12(12):3375–3382. doi: 10.1089/ten.2006.12.3375 [DOI] [PubMed] [Google Scholar]
- 21. Eisenstein M. Regulation: rewriting the regenerative rulebook. Nature. 2016;540(7632):S64–S67. doi: 10.1038/540S63a [DOI] [PubMed] [Google Scholar]
- 22. van Dongen JA, Stevens HP, Parvizi M, et al. The fractionation of adipose tissue procedure to obtain stromal vascular fractions for regenerative purposes. Wound Repair Regen. 2016;24(6):994–1003. doi: 10.1111/wrr.12482 [DOI] [PubMed] [Google Scholar]
- 23. Yao Y, Cai J, Zhang P, et al. Adipose stromal vascular fraction gel grafting: a new method for tissue volumization and rejuvenation. Dermatol Surg. 2018;44(10):1278–1286. doi: 10.1097/dss.0000000000001556 [DOI] [PubMed] [Google Scholar]
- 24. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009 [DOI] [PubMed] [Google Scholar]
- 25. Xiong S, Yi C, Pu LLQ. An overview of principles and new techniques for facial fat grafting. Clin Plast Surg. 2020;47(1):7–17. doi: 10.1016/j.cps.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 26. Vyas KS, Vasconez HC, Morrison S, et al. Fat graft enrichment strategies: a systematic review. Plast Reconstr Surg. 2020;145(3):827–841. doi: 10.1097/PRS.0000000000006557 [DOI] [PubMed] [Google Scholar]
- 27. Trojahn Kølle S-FF, Oliveri RS, Glovinski PV, et al. Importance of mesenchymal stem cells in autologous fat grafting: a systematic review of existing studies. J Plast Surg Hand Surg. 2012;46(2):59–68. doi: 10.3109/2000656X.2012.668326 [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 29. Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson N, Waters E. Criteria for the systematic review of health promotion and public health interventions. Health Promot Int. 2005;20(4):367–374. doi: 10.1093/heapro/dai022 [DOI] [PubMed] [Google Scholar]
- 31. Almadori A, Griffin M, Ryan CM, et al. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS ONE. 2019;14(7):e0218068. doi: 10.1371/journal.pone.0218068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amar RE, Fox DM. The facial autologous muscular injection (FAMI) procedure: an anatomically targeted deep multiplane autologous fat-grafting technique using principles of facial fat injection. Aesthetic Plast Surg. 2011;35(4):502–510. doi: 10.1007/s00266-010-9645-0 [DOI] [PubMed] [Google Scholar]
- 33. Bae YC, Park TS, Kang GB, et al. Usefulness of microfat grafting in patients with repaired cleft lip. J Craniofac Surg. 2016;27(7):1722–1726. [DOI] [PubMed] [Google Scholar]
- 34. Bashir MM, Sohail M, Ahmad FJ, et al. Preenrichment with adipose tissue-derived stem cells improves fat graft retention in patients with contour deformities of the face. Stem Cells Intl. 2019;2019:5146594. doi: 10.1155/2019/5146594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhooshan LS, Geetha Devi M, Aniraj R, et al. Autologous emulsified fat injection for rejuvenation of scars: a prospective observational study. Indian J Plast Surg. 2018;51(1):77–83. doi: 10.4103/ijps.IJPS_86_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Onesti MG, Fioramonti P, Carella S, et al. Improvement of mouth functional disability in systemic sclerosis patients over one year in a trial of fat transplantation versus adipose-derived stromal cells. Stem Cells Intl. 2016;2016: 2416192. doi: 10.1155/2016/2416192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Virzì F, Bianca P, Giammona A, et al. Combined platelet-rich plasma and lipofilling treatment provides great improvement in facial skin-induced lesion regeneration for scleroderma patients. Stem Cell Res Ther. 2017;8(1):236. doi: 10.1186/s13287-017-0690-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gontijo-de-Amorim NF, Charles-de-Sá L. Fat grafting for facial contouring using mechanically stromal vascular fraction-enriched lipotransfer. Clin Plast. 2019;47(1):99–109. doi: 10.1016/j.cps.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 39. Charles-de-Sa L, Gontijo-de-Amorim N, Sbarbati A, et al. Photoaging skin therapy with PRP and ADSC: a comparative study. Stem Cells Int. 2020;2020:2032359. doi: 10.1155/2020/2032359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aronowitz JA, Lockhart RA, Hakakian CS, Hicok KC. Clinical safety of stromal vascular fraction separation at the point of care. Ann Plast Surg. 2015;75(6):666–71. doi: 10.1097/sap.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 41. Braccini F, Chignon-Sicard B, Volpei C, Choukroun J. Modern lipostructure: the use of platelet rich fibrin (PRF). Rev Laryngol Otol Rhinol. 2013;134(4-5):231–235. [PubMed] [Google Scholar]
- 42. Castro-Govea Y, Garza-Pineda OL, Lara-Arias J, et al. Cell-assisted lipotransfer for the treatment of Parry-Romberg syndrome. Arch Plast Surg. 2012;39(6):659–662. doi: 10.5999/aps.2012.39.6.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cervelli V, Nicoli F, Spallone D. Treatment of traumatic scars using fat grafts mixed with platelet‐rich plasma, and resurfacing of skin with the 1540 nm nonablative laser. Clin Exp Dermatol. 2012;37(1):55–61. doi: 10.1111/j.1365-2230.2011.04199.x [DOI] [PubMed] [Google Scholar]
- 44. Cervelli V, Gentile P, Scioli MG, et al. Application of platelet-rich plasma in plastic surgery: clinical and in vitro evaluation. Tissue Eng Part C Methods 2009;15(4):625–634. doi: 10.1089/ten.tec.2008.0518 [DOI] [PubMed] [Google Scholar]
- 45. Cervelli V, Palla L, Pascali M, et al. Autologous platelet-rich plasma mixed with purified fat graft in aesthetic plastic surgery. Aesthet Plast Surg. 2009;33(5):716–721. doi: 10.1007/s00266-009-9386-0 [DOI] [PubMed] [Google Scholar]
- 46. Charles-de-Sá L, Gontijo-de-Amorim NF, Maeda Takiya C, et al. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast Reconstr Surg. 2015;135(4):999–1009. doi: 10.1097/prs.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 47. Clauser L, Lucchi A, Tocco-Tussardi I, et al. Autologous fat transfer for facial augmentation and regeneration: role of mesenchymal stem cells. Atlas Oral Maxillofac Surg Clin North Am. 2018;26(1):25–32. doi: 10.1016/j.cxom.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 48. Gontijo-de-Amorim NF, Charles-de-Sá L, Rigotti G. Fat grafting for facial contouring using mechanically stromal vascular fraction–enriched lipotransfer. Clin Plast Surg. 2020;47(1):99–109. doi: 10.1016/j.cps.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 49. Majani U, Majani A. Correction of scars by autologous fat graft and platelet rich plasma (PRP). Acta Med Mediterr. 2012;28:99–100. [Google Scholar]
- 50. Nita AC, Orzan OA, Filipescu M, Jianu D. Fat graft, laser CO₂ and platelet-rich-plasma synergy in scars treatment. J Med Life. 2013;6(4):430–433. [PMC free article] [PubMed] [Google Scholar]
- 51. Ortega VG, Sastoque D. New and successful technique for the management of Parry-Romberg syndrome’s soft tissue atrophy. J Craniofac Surg. 2015;26(6):e507–e510. doi: 10.1097/scs.0000000000002023 [DOI] [PubMed] [Google Scholar]
- 52. Pallua N, Kim BS. Microfat and lipoconcentrate for the treatment of facial scars. Clin Plast Surg. 2020;47(1):139–145. doi: 10.1016/j.cps.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 53. Park KY, Kim IS, Kim BJ, Kim MN. Letter: Autologous fat grafting and platelet-rich plasma for treatment of facial contour defects. Dermatol Surg. 2012;38(9):1572–1574. doi: 10.1111/j.1524-4725.2012.02515.x [DOI] [PubMed] [Google Scholar]
- 54. Philandrianos C, Magalon J, Daumas A, et al. Combined PRP and microfat graft for facial disability in systemic sclerosis. J Scleroderma Relat Disord. 2017;2(3):7–11. doi: 10.5301/jsrd.5000261 [DOI] [Google Scholar]
- 55. Rigotti G, Charles-De-Sá L, Gontijo-De-Amorim NF, et al. Expanded stem cells, stromal-vascular fraction, and platelet-rich plasma enriched fat: comparing results of different facial rejuvenation approaches in a clinical trial. Aesthet Surg J. 2016;36(3):261–270. doi: 10.1093/asj/sjv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tian YG, Liu XY, Tao K, et al. Adipose-derived stem cells assisted facial rejuvenation. Chin J Tissue Eng Res. 2013;16(49):9257–9264. doi: 10.3969/j.issn.2095-4344.2012.49.025 [DOI] [Google Scholar]
- 57. Tiryaki T, Findikli N, Tiryaki D. Staged stem cell-enriched tissue (SET) injections for soft tissue augmentation in hostile recipient areas: a preliminary report. Aesthetic Plast Surg. 2011;35(6):965–971. doi: 10.1007/s00266-011-9716-x. [DOI] [PubMed] [Google Scholar]
- 58. Willemsen JC, van der Lei B, Vermeulen KM, Stevens HP. The effects of platelet-rich plasma on recovery time and aesthetic outcome in facial rejuvenation: preliminary retrospective observations. Aesthetic Plast Surg. 2014;38(5):1057–1063. doi: 10.1007/s00266-014-0361-z [DOI] [PubMed] [Google Scholar]
- 59. Sadati KS, Corrado AC. Platelet-rich plasma (PRP) utilized to promote greater graft volume retention in autologous fat grafting. Am J Cos Surg. 2006;23(4):203–211. doi: 10.1177/074880680602300407 [DOI] [Google Scholar]
- 60. Abdali H, Hadilou M. Treatment of nasolabial fold with subdermal dissection and autologous fat injection added with platelet-rich plasma. J Res Med Sci. 2014;19(11):1110. [PMC free article] [PubMed] [Google Scholar]
- 61. Gontijo-De-Amorim NF, Charles-De-Sá L, Rigotti G. Mechanical supplementation with the stromal vascular fraction yields improved volume retention in facial lipotransfer: a 1-year comparative study. Aesthet Surg J. 2017;37(9):975–985. doi: 10.1093/asj/sjx115 [DOI] [PubMed] [Google Scholar]
- 62. Lee JW, Park SH, Lee SJ, et al. Clinical impact of highly condensed stromal vascular fraction injection in surgical management of depressed and contracted scars. Aesthetic Plast Surg. 2018;42(6):1689–1698. doi: 10.1007/s00266-018-1216-9 [DOI] [PubMed] [Google Scholar]
- 63. Annacontini L, Valente M, Rucci M, et al. “Cellular therapy” through lipostructuring; the role of adipose-derived adult stem cells in “regenerative surgery” processes: outcomes over the past 3 years. Eur Surg Res. 2010;45:238–239. doi: 10.1159/000321283 [DOI] [Google Scholar]
- 64. Annacontini L, Parisi D, Lembo F, et al. Cellular therapy through lipostructuring; the role of adipose-derived adult stem cells in regenerative surgery processes. J Tissue Eng Regen Med. 2012;6(Suppl 1):268. doi: 10.1002/term.1586 [DOI] [Google Scholar]
- 65. Branas EB, Salvatierra BG, Torres JN, Casado-Perez C. Stromal vascular fraction- enhanced autologous fat transfer versus lipofilling in facial lipoatrophy. Br J Surg. 2014;101:8.24276950 [Google Scholar]
- 66. Castana O, Alexaki V, Pallantzas A, et al. Adipose tissue-derived mesenchymal cells for the reparation of major facial traumatic deformities. J Invest Dermatol. 2012;132(Suppl 2):S54. doi: 10.1038/jid.2012.298 [DOI] [Google Scholar]
- 67. Keyhan SO. Use of platelet rich fibrin and platelet rich plasma in combination with fat graft; which is more effective during facial lipostructure? Int J Oral Maxillofac Surg. 2013;42(10):1252–1253. doi: 10.1016/j.ijom.2013.07.279 [DOI] [PubMed] [Google Scholar]
- 68. Lonskaya E, Kurakin K, Drobyshev A, et al. Simultaneous face fat-grafting to enhance the aesthetic outcome of orthognathic surgery. Int J Oral Maxillofac Surg. 2017;46(Suppl 1):162–163. doi: 10.1016/j.ijom.2017.02.558 [DOI] [Google Scholar]
- 69. Tamme T, Tiigimae-Saar J, Arak T. The evaluation of autologous adipose tissue grafting for facial contour deformities. Int J Oral Maxillofac Surg. 2019;48(Suppl 1):129. doi: 10.1016/j.ijom.2019.03.398 [DOI] [Google Scholar]
- 70. Chaohua L, Chenggang Y. A clinical comparative study of volume retention rate for repairing facial depressed deformity with different operative methods of autologous fat transplantation. 2018. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR1800017796
- 71. Drzewiecki KT. Lipofilling with MSC enriched fat, a permanent autologous filler?2010. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2010-023006-12-DK
- 72. Kamei Y, Takanari K. Augmentation of soft tissue defect in face, trunk and extremity with adipose-derived regenerative cell enriched lipotransfer. 2014. https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000012866
- 73. Shimizu Y. Clinical study of treatment for depressed lesions using cultured adipose derived stem cells. 2016. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000020530
- 74. Kim BJ. The efficiency of stromal vascular fraction cells in facial fat graft procedures. 2018. https://trialsearch.who.int/Trial2.aspx?TrialID=KCT0003402
- 75. Choi J. The effect of human adipose tissue-derived MSCs in Romberg’s disease. 2011. https://clinicaltrials.gov/show/NCT01309061
- 76. Willemsen J, Stevens J. The use of activated platelet rich plasma (PRP) in human autologous fat transfer. 2011. https://clinicaltrials.gov/show/NCT01461785
- 77. Tanikawa DY. Clinical trial of fat grafts supplemented with adipose-derived regenerative cells. 2012. https://clinicaltrials.gov/show/NCT01674439
- 78. Alonso N. Immunophenotyping of fresh stromal vascular fraction from adipose derived stem cells (ADSC) enriched fat grafts. 2013. https://clinicaltrials.gov/show/NCT01771913
- 79. Rahimian S. Adipose derived stem cells in facial fat grafting. 2015. https://clinicaltrials.gov/show/NCT02526576
- 80. Tanikawa DS. Fat grafts with adipose-derived regenerative cells for soft tissue reconstruction in children. 2019. https://clinicaltrials.gov/show/NCT03806361
- 81. Ismail A. Effect of adipose derived stem cells on survival of fat as filler. 2019. https://clinicaltrials.gov/show/NCT03965936
- 82. van Dongen J. Stromal vascular fraction (SVF) enriched lipofilling plus platelet rich plasma (PRP) for the treatment of the aging face. 2016. https://trialsearch.who.int/Trial2.aspx?TrialID=NTR5703
- 83. Herold C, Vogt PM. Radiodermatitis: with autologous stem cells enriched lipoaspirate transplantation as minimally invasive treatment option. Haut. 2011;22:262–265. [Google Scholar]
- 84. Al-Chalabi NJA, Al-Quisi AF, Abdul Lateef T. Single session facial lipostructure by using autologous fat mixed with platelet-rich fibrin injected by using facial autologous muscular injection technique. J Craniofac Surg. 2018;29(3): e267–e271. doi: 10.1097/scs.0000000000004307 [DOI] [PubMed] [Google Scholar]
- 85. Abuzeni PZ, Alexander RW. Enhancement of autologous fat transplantation with platelet rich plasma. Am J Cos Surg 2001;18(2):59–70. doi: 10.1177/074880680101800202 [DOI] [Google Scholar]
- 86. Choi JY, Lim JO. Combination of adipose-derived stem cells and oxygen microspheres for enhanced cell survival in fat transplantation. J Tissue Eng Regen Med. 2014;8(Suppl 1):276. [Google Scholar]
- 87. Modarressi A. Platlet rich plasma (PRP) improves fat grafting outcomes. World J Plast Surg. 2013;2(1):6–13. [PMC free article] [PubMed] [Google Scholar]
- 88. Sclafani AP, McCormick SA. Induction of dermal collagenesis, angiogenesis, and adipogenesis in human skin by injection of platelet-rich fibrin matrix. Arch Facial Plast Surg. 2012;14(2):132–6. doi: 10.1001/archfacial.2011.784 [DOI] [PubMed] [Google Scholar]
- 89. Swanson E. Does separating the stromal vascular fraction improve facial fat retention? Plast Reconstr Surg. 2016;137(3):637e–639e. doi: 10.1097/prs.0000000000002142 [DOI] [PubMed] [Google Scholar]
- 90. Choudhery MS. Systemic administration of adipose-derived stromal cells concurrent with fat grafting. Plast Reconstr Surg. 2020;145(2):456e–457e. doi: 10.1097/prs.0000000000006451 [DOI] [PubMed] [Google Scholar]
- 91. Kim SJ, Choi WI, Lee BI, et al. The effect of platelet-rich plasma (PRP) on the survival of the autologous fat graft. J Korean Soc Plast Reconstr Surg. 2007;34(3):291–297. [Google Scholar]
- 92. Jiang S, Quan Y, Wang J, et al. Fat grafting for facial rejuvenation using stromal vascular fraction gel injection. Clin Plast Surg. 2020;47(1):73–79. doi: 10.1016/j.cps.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 93. Nolan GS, Smith OJ, Mosahebi A. Enhancing fat graft survival with autologous growth factors: platelet-rich fibrin (PRF) vs platelet-rich plasma (PRP). Aesthet Surg J. 2021;41(5):NP241. doi: 10.1093/asj/sjaa274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Reksodiputro MH, Diandini D, Koento T, Arisanty R, Harahap AR. Autologous microlobular fat combined with platelet-rich fibrin is associated with good fat graft viability. J Phys Conf Ser. 2018;1073(3):032060. doi: 10.1088/1742-6596/1073/3/032060 [DOI] [Google Scholar]
- 95. Zhao J, Yi C, Zheng Y, et al. Enhancement of fat graft survival by bone marrow–derived mesenchymal stem cell therapy. Plast Reconstr Surg. 2013;132(5):1149–1157. doi: 10.1097/PRS.0b013e3182a48b6c. [DOI] [PubMed] [Google Scholar]
- 96. Chkadua TZ, Visaitova ZY, Strukova OO, et al. The feasibility of combined lipofilling methods in the treatment of patients with facial hemiatrophy. Stomatol. 2019;98(3):35–41. doi: 10.17116/stomat20199803135 [DOI] [PubMed] [Google Scholar]
- 97. Li J, Gao J, Cha P, et al. Supplementing fat grafts with adipose stromal cells for cosmetic facial contouring. Dermatologic Surg. 2013;39(3 Pt 1):449–456. doi: 10.1111/dsu.12058 [DOI] [PubMed] [Google Scholar]
- 98. Ozer K, Colak O. Micro-autologous fat transplantation combined with platelet-rich plasma for facial filling and regeneration: a clinical perspective in the shadow of evidence-based medicine. J Craniofac Surg. 2019;30(3):672–677. doi: 10.1097/scs.0000000000005122 [DOI] [PubMed] [Google Scholar]
- 99. Sasaki GH. A preliminary clinical trial comparing split treatments to the face and hand with autologous fat grafting and platelet-rich plasma (PRP): a 3D, IRB-approved study. Aesthet Surg J. 2019;39(6):675–686. doi: 10.1093/asj/sjy254 [DOI] [PubMed] [Google Scholar]
- 100. Schendel SA. Enriched autologous facial fat grafts in aesthetic surgery: 3D volumetric results. Aesthet Surg J. 2015;35(8):913–919. doi: 10.1093/asj/sjv140 [DOI] [PubMed] [Google Scholar]
- 101. Willemsen JCN, Van Dongen J, Spiekman M, et al. The addition of platelet-rich plasma to facial lipofilling: a double-blind, placebo-controlled, randomized trial. Plast Reconstr Surg. 2018;141(2):331–343. doi: 10.1097/prs.0000000000004081 [DOI] [PubMed] [Google Scholar]
- 102. Wei H, Gu SX, Liang YD, et al. Nanofat-derived stem cells with platelet-rich fibrin improve facial contour remodeling and skin rejuvenation after autologous structural fat transplantation. Oncotarget. 2017;8(40):68542–68556. doi: 10.18632/oncotarget.19721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sasaki GH. The safety and efficacy of cell-assisted fat grafting to traditional fat grafting in the anterior mid-face: an indirect assessment by 3D imaging. Aesthetic Plast Surg. 2015;39(6):833–846. doi: 10.1007/s00266-015-0533-5 [DOI] [PubMed] [Google Scholar]
- 104. Gentile P, De Angelis B, Pasin M, et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical evaluation for cell-based therapies in patients with scars on the face. J Craniofac Surg. 2014;25(1):267–272. doi: 10.1097/01.scs.0000436746.21031.ba [DOI] [PubMed] [Google Scholar]
- 105. Fontdevila J, Guisantes E, Martinez E, et al. Double-blind clinical trial to compare autologous fat grafts versus autologous fat grafts with PDGF: no effect of PDGF. Plast Reconstr Surg. 2014;134(2):219E–230E. doi: 10.1097/PRS.0000000000000409 [DOI] [PubMed] [Google Scholar]
- 106. Tanikawa DYS, Aguena M, Bueno DF, et al. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg. 2013;132(1):141–152. doi: 10.1097/PRS.0b013e3182910a82 [DOI] [PubMed] [Google Scholar]
- 107. Yin Y, Li J, Li Q, et al. Autologous fat graft assisted by stromal vascular fraction improves facial skin quality: a randomized controlled trial. J Plast Reconstr Aesthetic Surg. 2020;73(6):1166–1173. doi: 10.1016/j.bjps.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 108. Chang Q, Li J, Dong Z, et al. Quantitative volumetric analysis of progressive hemifacial atrophy corrected using stromal vascular fraction-supplemented autologous fat grafts. Dermatol Surg. 2013;39(10):1465–1473. doi: 10.1111/dsu.12310 [DOI] [PubMed] [Google Scholar]
- 109. Koh KS, Oh TS, Kim H, et al. Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann Plast Surg. 2012;69(3):331–337. [DOI] [PubMed] [Google Scholar]
- 110. Sterodimas A, De Faria J, Nicaretta B, Boriani F. Autologous fat transplantation versus adipose-derived stem cell-enriched lipografts: a study. Aesthet Surg J. 2011;31(6):682–693. doi: 10.1177/1090820x11415976 [DOI] [PubMed] [Google Scholar]
- 111. Tenna S, Cogliandro A, Barone M, et al. Comparative study using autologous fat grafts plus platelet-rich plasma with or without fractional CO2 laser resurfacing in treatment of acne scars: analysis of outcomes and satisfaction with FACE-Q. Aesthetic Plast Surg. 2017;41(3):661–666. doi: 10.1007/s00266-017-0777-3 [DOI] [PubMed] [Google Scholar]
- 112. Gentile P, Sterodimas A, Calabrese C, et al. Regenerative application of stromal vascular fraction cells enhanced fat graft maintenance: clinical assessment in face rejuvenation. Expert Opin Biol Ther. 2020;20(12):1503–1513. doi: 10.1080/14712598.2020.1815703 [DOI] [PubMed] [Google Scholar]
- 113. Jianhui Z, Chenggang Y, Binglun L, et al. Autologous fat graft and bone marrow-derived mesenchymal stem cells assisted fat graft for treatment of Parry-Romberg syndrome. Ann Plast Surg. 2014;73(Suppl 1):S99–S103. doi: 10.1097/sap.0000000000000238 [DOI] [PubMed] [Google Scholar]
- 114. Bernardini FP, Gennai A, Izzo L, et al. Superficial enhanced fluid fat injection (SEFFI) to correct volume defects and skin aging of the face and periocular region. Aesthetic Surg J. 2015;35(5):504–515. doi: 10.1093/asj/sjv001 [DOI] [PubMed] [Google Scholar]
- 115. Castro-Govea Y, Vela-Martinez A, Trevino-Garcia LA. Volumetric lipoinjection of the fronto-orbital and temporal complex with adipose stem cells for the aesthetic restoration of sequelae of craniosynostosis. Arch Plast Surg. 2018;45(2):128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gennai A, Zambelli A, Repaci E, et al. Skin rejuvenation and volume enhancement with the micro superficial enhanced fluid fat injection (M-SEFFI) for skin aging of the periocular and perioral regions. Aesthet Surg J. 2017;37(1):1–10. doi: 10.1093/asj/sjw084 [DOI] [PubMed] [Google Scholar]
- 117. Gu ZC, Li YR, Li H. Use of condensed nanofat combined with fat grafts to treat atrophic scars. JAMA Facial Plast Surg. 2018;20(2):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hesamirostami M, Modarressi A, Lebaschi A, Kazemi Ashtiani A. Forehead biconvexity enhancement with fat grafting. Eur J Plast Surg. 2019;42:231–234. doi: 10.1007/s00238-018-1489-x [DOI] [Google Scholar]
- 119. Lee SK, Kim DW, Dhong ES, et al. Facial soft tissue augmentation using autologous fat mixed with stromal vascular fraction. Arch Plast Surg. 2012;39(5):534–539. doi: 10.5999/aps.2012.39.5.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Keyhan SO, Hemmat S, Badri AA, et al. Use of platelet-rich fibrin and platelet-rich plasma in combination with fat graft: which is more effective during facial lipostructure? J Oral Maxillofac Surg. 2013;71(3):610–621. doi: 10.1016/j.joms.2012.06.176 [DOI] [PubMed] [Google Scholar]
- 121. Cano SJ, Klassen A, Pusic AL. The science behind quality-of-life measurement: a primer for plastic surgeons. Plast Reconstr Surg. 2009;123(3):98e–106e. doi: 10.1097/PRS.0b013e31819565c1 [DOI] [PubMed] [Google Scholar]
- 122. Klassen AF, Cano SJ, Schwitzer JA, et al. Development and psychometric validation of the FACE-Q skin, lips, and facial rhytids appearance scales and adverse effects checklists for cosmetic procedures. JAMA Dermatol. 2016;152(4):443–451. doi: 10.1001/jamadermatol.2016.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pusic AL, Klassen AF, Scott AM, Cano SJ. Development and psychometric evaluation of the FACE-Q Satisfaction with Appearance Scale: a new patient-reported outcome instrument for facial aesthetics patients. Clin Plast Surg. 2013;40(2):249–260. doi: 10.1016/j.cps.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 124. Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137(1):44e–57e. doi: 10.1097/PRS.0000000000001981 [DOI] [PubMed] [Google Scholar]
- 125. Sommer B, Sattler G. Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatologic Surg. 2000;26(12):1159–1166. [PubMed] [Google Scholar]
- 126. Gir P, Brown SA, Oni G, et al. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130(1):249–258. doi: 10.1097/PRS.0b013e318254b4d3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.