Abstract
Conditions were established in which Legionella pneumophila, an intracellular bacterial pathogen, could replicate within the unicellular organism Dictyostelium discoideum. By several criteria, L. pneumophila grew by the same mechanism within D. discoideum as it does in amoebae and macrophages. Bacteria grew within membrane-bound vesicles associated with rough endoplasmic reticulum, and L. pneumophila dot/icm mutants, blocked for growth in macrophages and amoebae, also did not grow in D. discoideum. Internalized L. pneumophila avoided degradation by D. discoideum and showed evidence of reduced fusion with endocytic compartments. The ability of L. pneumophila to grow within D. discoideum depended on the growth state of the cells. D. discoideum grown as adherent monolayers was susceptible to L. pneumophila infection and to contact-dependent cytotoxicity during high-multiplicity infections, whereas D. discoideum grown in suspension was relatively resistant to cytotoxicity and did not support intracellular growth. Some known D. discoideum mutants were examined for their effect on growth of L. pneumophila. The coronin mutant and the myoA/B double myosin I mutant were more permissive than wild-type strains for intracellular growth. Growth of L. pneumophila in a Gβ mutant was slightly reduced compared to the parent strain. This work demonstrates the usefulness of the L. pneumophila-D. discoideum system for genetic analysis of host-pathogen interactions.
Bacterial pathogenesis involves the interaction of a bacterium with a complex host. Elaborate mechanisms have evolved in microorganisms to manipulate and interfere with host cell functions, and numerous host defenses have arisen to keep pathogens at bay. Recently, there has been interest in studying host-pathogen interactions by using simple, genetically manipulatable hosts. It is hoped that the bacterial factors and host genes involved in causing pathogenic effects in these simple organisms will be relevant to mammalian disease processes. Studies of the expression of antimicrobial peptides in Drosophila melanogaster led to the discovery of Toll receptors, critical components of innate immunity that have been recently recognized in mammals (26, 27). Studies of the extracellular bacterial pathogen Pseudomonas aeruginosa and its interaction with the worm Caenorhabditis elegans indicate that many of the bacterial factors that affect pathogenesis of the worm also affect pathogenesis in the mammalian mouse model and in plants (47). In this report, we introduce the free-living unicellular organism Dictyostelium discoideum as a genetically manipulatable host for the intracellular bacterial pathogen Legionella pneumophila.
L. pneumophila, the causative agent of Legionnaires' disease, is a gram-negative bacterium that exists as an intracellular parasite of freshwater amoebae (16). Pathogenesis of the bacterium within mammalian hosts and its ability to grow within amoebae are closely linked. In human pneumonia, the microorganism grows in alveolar macrophages, cells that are phagocytic and motile like amoebae (8, 23). Furthermore, L. pneumophila mutants defective for growth in macrophages also show defective growth in amoebae (18, 43).
After phagocytosis, L. pneumophila is found in a membrane-bound phagosome that avoids fusion with endocytic and lysosomal compartments and is not acidified (21, 22). Examination of markers from the endocytic pathway on the L. pneumophila phagosome indicate that avoidance of the endocytic pathway occurs within 10 min of uptake (40, 56). A defining feature of the L. pneumophila phagosome in macrophages is its association with ribosomes thought to be derived from rough endoplasmic reticulum (RER) (20, 45). As the infection proceeds, the bacterium-laden phagosome grows until it nearly fills the cell (20, 45). Cell lysis or apoptotic death releases the bacteria to initiate another round of infection (34). L. pneumophila can also kill cells by a different mechanism called contact-dependent cytotoxicity (25). At relatively high multiplicities of infection (MOI), contact between the bacteria and cells can cause osmotic lysis of the cells. No internalization of the bacteria is necessary for cytotoxicity, and the link between intracellular growth and cytotoxicity remains unclear.
Genetic hunts have identified approximately 24 L. pneumophila genes required for intracellular growth, many of which are also required for contact-dependent cytotoxicity. These genes have been named dot/icm genes (1, 3, 5, 38, 41, 42, 53). Many of the dot/icm genes are homologous to genes required for mobilization of conjugal plasmids, and indeed the dot/icm gene products are required for conjugal transfer of RSF1010 plasmids from L. pneumophila (41, 53). This has led to the hypothesis that the dot/icm gene products form a transport system that is thought to aid pathogenesis not by transferring DNA but by transporting an as yet unidentified effector protein(s) into the host cell. Determining the exact functions of the dot/icm genes and identifying the transported effector molecules remains a major challenge.
D. discoideum is a unicellular, free-living organism that lives in soil and feeds on bacteria (4). In the amoebal form, the cells are highly motile and are very active in phagocytosis. A body of literature describes the endolysosomal and phagosomal pathways in D. discoideum (references 6, 31, and 39 and references therein). During starvation, the organism undergoes a complex developmental cycle in which the normally free-living single cells aggregate to form a multicellular organism, a motile, phototactic slug. The slug further develops into a fruiting body containing D. discoideum spores and a stalk (4, 28, 49). The axenic strains of D. discoideum that are routinely used are easily maintained and can grow in pure culture in a rich medium in the absence of bacteria (44, 57).
The availability of genetic tools makes D. discoideum a genetically tractable host organism for analysis of host-pathogen interactions. The organism is haploid and has a relatively small genome of 34 Mb (10, 30). It is possible to transform the cells by electroporation and to knock out genes by homologous recombination and marker replacement (12, 32). There are plasmids that replicate in D. discoideum that can be used for complementation or ectopic expression (32). There is extensive DNA sequence information available (http://dicty.cmb.nwu.edu/dicty/dicyostelium_genomics.htm); and the complete genome sequence should be finished by 2002.
This report describes the establishment of conditions for the intracellular growth of L. pneumophila in D. discoideum. Genetic and cell biological analyses indicate that the mechanism of growth in D. discoideum is similar to that observed in macrophages and amoebae.
MATERIALS AND METHODS
Cells, strains, and routine maintenance.
D. discoideum AX3 was a kind gift from D. Knecht (University of Connecticut, Storrs) and was used in all experiments except for analysis of growth of L. pneumophila in D. discoideum mutants (29). The Gβ mutant (strain LW6) and its parent strain DH1 (58) were a kind gift from P. Devreotes (Johns Hopkins University, Baltimore, Md.). The coronin mutant (strain HG1569) and its parent strain AX2-214 (11) were kind gifts from M. Maniak (MRC-LMCB, London, England). The myosin I myoA (clone HTD2-4), myoB (clone HTD4-3), and myoA/B (clone HTD5-4) (36, 50, 55) mutants and their parent strain KAX3 were kind gifts from M. Titus (University of Minnesota, Minneapolis). Other strains examined include the strain overexpressing constitutively active rab7 (AX4 with pRab7) and the control strain carrying the vector alone (AX4 with pDA80-HA) (6) and the ΔDdpik1 ΔDdpik2 phosphatidylinositol double 3-kinase mutant strain (59).
Cells grown axenically were cultured in HL-5 liquid medium (44) supplemented with penicillin and streptomycin (100 U/ml; GibcoBRL) and other supplements (Curacil [20 μg/ml] and G418 [7.5 to 20 μg/ml]) as needed. D. discoideum was also grown as plaques on a lawn of Klebsiella aerogenes plated on SM/5 agar medium (44).
The L. pneumophila Benidorm (030E) strain was a kind gift from B. Fields (Centers for Disease Control and Prevention, Atlanta, Ga.); the L. pneumophila Philadelphia-1 strain was also obtained from the Centers for Disease Control and Prevention. All genetically manipulated strains are derived from L. pneumophila Philadelphia-1. Strain Lp01 is proficient for intracellular growth, streptomycin resistant, and restriction defective (2). Lp01 is the parent of strain HL056, which contains an in-frame deletion of dotI, and of strains HL1400 (dotO) and HL1700 (dotH), which have ethyl methanesulfonate-induced mutations in dotO and dotH, respectively (1). Strains MW49 and MW50 are derivatives of strains Lp01 and HL056 that express the green fluorescent protein (GFP; expressed by plasmid GFPmut3) from the Ptac isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter. The strains carry plasmid pAM239, which was constructed by moving the 750-bp XbaI-PstI fragment from the GFPmut3 plasmid (9) into the XbaI and PstI-digested pMMB207 backbone (33). Strain Lp03 carries a spontaneous mutation in dotA (3), strain JV302 carries a spontaneous mutation in dotB (54), strain JV328 carries a spontaneous mutation dotE (J. Vogel, unpublished results), strain 25D carries a spontaneous mutation in icmVWX (5), and strain JV573 carries a spontaneous mutation in dotG (53). L. pneumophila was grown on plates containing charcoal yeast extract agar (CYE) (14) buffered with ACES [N-(2-acetamido)-2-aminoethanesulfonic acid; Sigma] to pH 6.9. The same medium without agar and charcoal (AYE) was used for growth of L. pneumophila in liquid culture. K. aerogenes, a kind gift from D. Knecht, was routinely cultured in Luria broth (LB).
Growth of L. pneumophila in D. discoideum in liquid culture.
D. discoideum grown exponentially in shaken flasks in axenic medium was harvested and washed in phosphate-buffered saline (PBS) as follows. Cells were pelleted by a 5 min spin at 600 × g, the medium was aspirated and replaced with an equal volume of PBS, the cells were pelleted again by a 5-min spin at 600 × g, and the PBS was aspirated. The cells were resuspended in MB medium {20 mM MES [2(N-morpholino)ethanesulfonic acid) (pH 6.9), 0.7% yeast extract, 1.4% BBL thiotone E peptone} at 106 cells/ml. MB medium is modified HL-5 axenic medium, with glucose omitted and the buffer changed to 20 mM MES, pH 6.9. L. pneumophila was harvested after 48 h growth on CYE plates, resuspended in water or PBS, and used to infect D. discoideum at an MOI of approximately 1:1. The approximate concentration of bacteria was determined by assuming that A600 = 1.0 is equivalent to 109 bacteria/ml. Infected cells were distributed to 24-well tissue culture dishes, wrapped in Parafilm to prevent desiccation, and incubated at 25.5°C.
The number of viable L. pneumophila or D. discoideum on each day was determined by measuring CFU or PFU, respectively. A dilution series of harvested D. discoideum was prepared in PBS and plated on lawns of K. aerogenes spread on SM/5 medium. The plates were incubated at 21°C, and plaques were counted 3 to 4 days after plating. For quantitation of L. pneumophila, a dilution series was prepared from infected D. discoideum and plated on CYE plates. D. discoideum was lysed to release the intracellular bacteria, either by vigorous vortexing in water or by addition of 0.02% saponin (Sigma S-4521) to the well before harvesting. CYE plates were then incubated at 37°C for 3 or 4 days before colonies were counted.
Transmission electron microscopy.
D. discoideum was infected with L. pneumophila Philadelphia-1 at an MOI of 1:1 in liquid culture as described above. On day 3, infected cells were fixed for electron microscopy using a protocol modified from one previously described (13). Cells were harvested by pipetting up and down, pelleted for 5 min at 600 × g at 4°C, resuspended in fixative containing Triton X-100 (0.1 M sodium cacodylate buffer [pH 7.4], 0.01% Triton X-100, 0.5% glutaraldehyde), and incubated for 15 min on ice. Cells were pelleted for 5 min at 600 × g, washed in 0.1 M sodium cacodylate buffer (pH 7.4), pelleted again for 5 min at 600 × g, and resuspended to single cells in osmium fix (1% OsO4, 0.1 M sodium cacodylate buffer, pH 7.4) for 30 min at room temperature. The cells were washed twice more with sodium cacodylate (pH 7.4), pelleted, dehydrated in a graded series of alcohols, infused with propylene oxide, embedded in Epon 812, and sectioned into 90-nm slices. Samples were analyzed on a Philips CM-10 transmission electron microscope.
Quantitation of viable bacteria internalized by D. discoideum.
D. discoideum grown exponentially in HL-5 medium in shaken flasks was harvested, washed in PBS as described above, resuspended in MB medium at 2 × 106 cells/ml, plated in 24-well tissue culture dishes, and incubated at 25.5°C. L. pneumophila was grown at 37°C in AYE liquid medium to an A600 of 3 to 3.5, to allow maximal infectivity (7), and K. aerogenes was grown in LB until stationary phase. Prior to infections, bacterial strains were pelleted for 5 min at 16,000 × g in a microcentrifuge and resuspended in MB medium.
D. discoideum was infected with bacteria at an MOI of 5:1, and the infection was initiated by a 5-min spin at 200 × g. Thirty minutes after the initiation of infection, gentamicin was added to the medium to a final concentration of 50 μg/ml, to kill noninternalized bacteria, and was maintained in the medium for the duration of the experiment. At various times after infection, cells were washed two times with PBS to remove gentamicin and lysed with 0.02% saponin. A dilution series of the harvested bacteria was prepared in PBS prior to plating for CFU on CYE medium (L. pneumophila) or LB agar (K. aerogenes).
Immunofluoresence.
D. discoideum grown exponentially in axenic medium in shaken flasks was harvested, washed in PBS as described above, and resuspended in MB with 2 mM IPTG at 106 cells/ml. Cells were plated on poly-l-lysine-coated coverslips in tissue culture wells and allowed to adhere and equilibrate at 25.5°C for at least 1 h before bacteria were added. L. pneumophila dot+ and dotI strains expressing GFP were grown to optimal infectivity in AYE–2 mM IPTG (A600 = 3 to 3.5), pelleted, and resuspended in MB–2 mM IPTG medium. D. discoideum was infected with bacteria at an MOI of 10:1, and the infection was initiated with a 5 min spin at 200 × g.
Thirty minutes after infection, cells were fixed in 2% paraformaldehyde in PBS with one-third-strength HL-5 medium and 0.1% dimethyl sulfoxide for 5 min at room temperature (6). Samples were permeabilized by incubation in methanol containing 1% paraformaldehyde for 5 min at −20°C, washed at least five times in PBS, and blocked in 4% goat serum (Gibco-BRL)–PBS for 1 h. Antibodies for staining were diluted in PBS containing 2.5 mg of bovine serum albumin per ml and 0.1% saponin. The V-ATPase antigen was visualized using a 1:20 dilution of a monoclonal antibody against the 100-kDa subunit (17), and lysosomal membranes were visualized using a 1:1,000 dilution of a polyclonal antibody generated against purified lysosomal membrane proteins from D. discoideum. After 1-h incubations with the primary antibodies, the samples were washed 5 times in PBS and secondary antibodies coupled to Texas red were added at a 1:500 dilution (Molecular Probes). Fixed samples were analyzed using a Nikon TE300 microscope, and images were captured by a Princeton Micromax slow-scan cooled charge-coupled device camera. To raise serum against lysosomal membrane proteins, the fraction was purified as described elsewhere (48) and injected intramuscularly without adjuvant into rabbits. The primary injection of 100 μg was followed by two boosts of 100 μg each, and serum was collected after the second boost.
Cytotoxicity assay.
D. discoideum grown exponentially in axenic medium in shaken flasks was harvested, washed in PBS as described above, and resuspended in MB medium at 106 cells/ml. Cells were incubated either as adherent monolayers in tissue culture wells or in suspension in silanized 50-ml conical tubes shaken in a water bath. Cells were equilibrated for at least 2 h at 25.5°C before bacteria were added. L. pneumophila Philadelphia-1 was grown to optimal infectivity in AYE (A600 = 3 to 3.5), pelleted, and resuspended in MB medium.
D. discoideum was infected with bacteria at an MOI of 375:1 by adding bacteria in 1/10 the volume of the D. discoideum culture and incubating at 25.5°C for approximately 24 h. At the end of the infection, a dilution series of harvested cells was prepared in PBS and plated for PFU on K. aerogenes.
Survival of internalized bacteria in adherent or suspended D. discoideum.
D. discoideum grown exponentially in axenic medium in shaken flasks was harvested, washed in PBS as described above, and resuspended in MB medium. One group of cells was plated in tissue culture wells at 2 × 106 cells/ml to form adherent monolayers, while a second group was incubated with shaking in silanized 50-ml conical tubes at 5 × 106 cells/ml. All cells were incubated at 25.5°C and were allowed to equilibrate for approximately 4 h before addition of bacteria. L. pneumophila was grown to optimal infectivity in AYE to an A600 of 3 to 3.5, and K. aerogenes was grown to stationary phase in LB.
Adherent cells were infected with bacteria at an MOI of 1:1, and the infection was initiated with a 5-min spin at 200 × g. Cells in suspension were infected at an MOI of 20:1. Thirty minutes after the initiation of infection, gentamicin was added to the medium to a final concentration of 50 μg/ml to kill noninternalized bacteria. The gentamicin remained in the medium for the duration of the experiment. One hour and 19 h after infection, the number of viable intracellular bacteria was determined.
Infected cells were harvested, pelleted for 5 min at 5,000 × g, washed in PBS to remove gentamicin, pelleted again for 5 min at 5,000 × g, resuspended in PBS, and lysed with 0.02% saponin. A dilution series of the harvested bacteria was prepared in PBS and plated for CFU.
Growth of D. discoideum on lawns of L. pneumophila.
Lawns of L. pneumophila were prepared by spreading Lp01 (dot+) or HL056 (ΔdotI) on CYE plates containing reduced levels of l-cysteine (0.05 g/liter) and Fe(NO3)3 · 9H2O (0.034 g/liter). Levels of cysteine normally found in CYE plates inhibit growth of D. discoideum (data not shown). Bacterial lawns were grown at 37°C for 2 days prior to inoculation of D. discoideum. D. discoideum cells growing exponentially in axenic medium were harvested, washed, and resuspended in PBS at 106 cells/ml; 105 washed cells (100 μl) were spotted onto the lawns, and plates were incubated at 21°C for several days.
RESULTS
Growth of L. pneumophila in D. discoideum in liquid culture.
A system was established in which L. pneumophila could grow in D. discoideum in liquid culture in a manner analogous to intracellular growth of L. pneumophila within macrophages. D. discoideum will not survive above 27°C, and most clinical isolates of L. pneumophila grow best at 37°C. For this reason, initial experiments were performed with L. pneumophila Benidorm-1 (030E), which grows well at 25°C. The temperature of incubation, growth medium, and MOI were adjusted. Once optimal growth conditions were established, all further experiments were performed with the better-characterized L. pneumophila Philadelphia-1 strain.
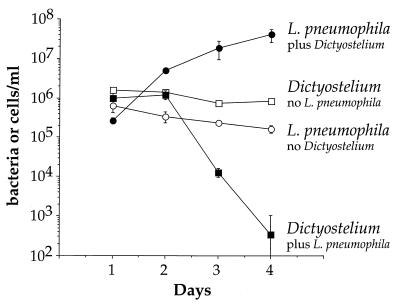
D. discoideum was plated as an adherent monolayer in tissue culture dishes and infected by adding L. pneumophila to the medium. Over a 4-day period, L. pneumophila grew more than 100-fold in the presence of D. discoideum (Fig. 1). The rate of growth between days 1 and 2 was rapid, with a doubling time of approximately 6 h, but slowed on days 2 to 4 post infection to a doubling time of approximately 16 h. The growth of L. pneumophila depended on the presence of D. discoideum in the medium. L. pneumophila plated in the medium alone, without D. discoideum, did not grow and viability usually decreased over the course of the experiment (Fig. 1). L. pneumophila growth in the presence of D. discoideum was not caused by feeding on D. discoideum corpses because heat-killed D. discoideum did not support growth of L. pneumophila (data not shown). It is also clear that live D. discoideum was not cross-feeding L. pneumophila because if bacteria and live cells were separated by a 0.4-μm-pore-size filter, there was no growth of L. pneumophila (data not shown). D. discoideum plated in the absence of bacteria remained viable over the course of the experiment but did not grow using these assay conditions (Fig. 1). In the presence of L. pneumophila, the number of viable D. discoideum remained unchanged until day 2 but then dropped rapidly on days 3 and 4 postinfection (Fig. 1). One likely explanation is that only a small fraction of the D. discoideum organisms are initially infected, and so no detectable drop in viability of the D. discoideum was seen. Two days postinfection, the titer of bacteria had increased sufficiently due to intracellular growth to allow killing of D. discoideum by a combination of cytotoxicity and continued intracellular growth.
FIG. 1.
Growth of L. pneumophila in the presence of D. discoideum in liquid culture. D. discoideum was plated into tissue culture wells in MB medium. Cells were infected with wild-type L. pneumophila Philadelphia-1 at an MOI of 1:1. L. pneumophila and D. discoideum were counted by measuring CFU and PFU, respectively. The experiment was performed twice, each point in the experiment was done in triplicate, and the error bars indicate n − 1 weighted sample standard deviation.
Growth of L. pneumophila in D. discoideum is intracellular.
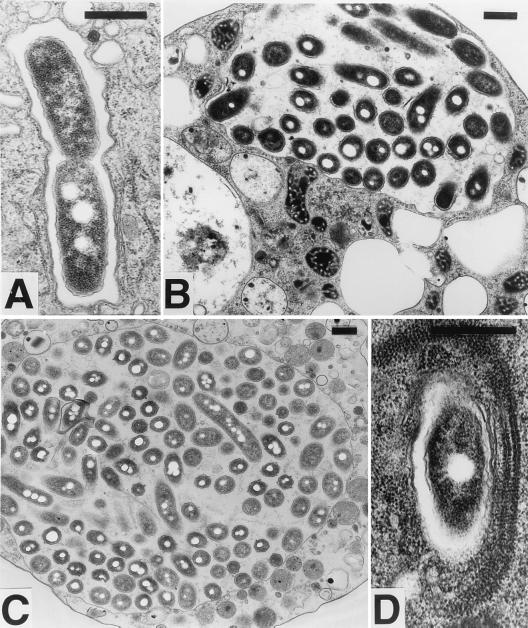
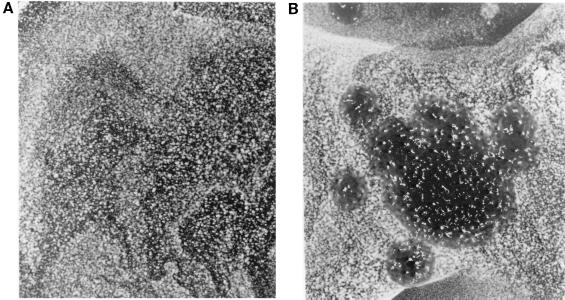
To determine whether the growth of L. pneumophila in the presence of D. discoideum was occurring intracellularly, a culture infected for 72 h was examined by electron microscopy (Fig. 2). Bacteria were uniformly found in membrane-bound vacuoles within D. discoideum. Every stage of intracellular growth could be found, including phagosomes having single cells (Fig. 2D), a bacterium apparently in the process of dividing within a phagosome (Fig. 2A), vacuoles containing a few bacteria (Fig. 2B), and cells nearly taken over by their bacterium-filled phagosomes (Fig. 2C). Association with RER is a defining feature of the L. pneumophila phagosome in macrophages (20, 45). In D. discoideum, RER can be seen associated with the phagosomes either in one layer (Fig. 2A) or multiple layers (Fig. 2D). In macrophages, the L. pneumophila phagosomal membrane was sometimes lined with ribosomes, a phenomenon that can also be seen in D. discoideum (Fig. 2B). These micrographs show that the L. pneumophila phagosomes in D. discoideum have the same characteristic association with ribosomes as seen in macrophages.
FIG. 2.
Transmission electron microscopy of D. discoideum infected with L. pneumophila. D. discoideum was infected with L. pneumophila Philadelphia-1 as in Fig. 1. On day 3, cells were harvested and prepared for electron microscopy. (A) Phagosome containing a bacterium apparently in the process of dividing; (B) vacuole containing a few bacteria; (C) a cell nearly taken over by a bacterium-filled phagosome; (D) multiple layers of RER associated with an L. pneumophila phagosome. Association of ribosomes with phagosomes can also be seen in panels A and B. In all panels, the bar equals 0.5 μm.
Growth of L. pneumophila in D. discoideum depends on dot gene functions.
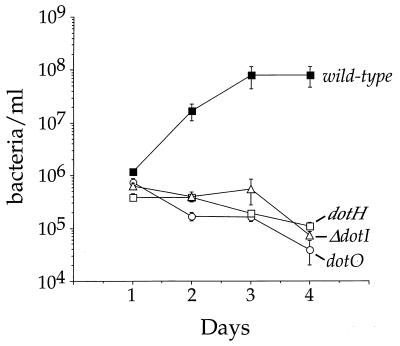
The dot genes of L. pneumophila are essential for establishing intracellular growth of L. pneumophila in macrophages and amoebae (18, 43). The analysis of growth of wild-type and three isogenic dot mutant strains of L. pneumophila in D. discoideum indicates that intracellular growth similarly requires the products of multiple dot loci (Fig. 3). The wild-type bacteria showed a characteristic 100-fold growth, while dotH, dotI, and dotO mutants all failed to grow and lost viability over the course of 4 days (Fig. 3).
FIG. 3.
Growth of wild-type and isogenic dot mutant L. pneumophila in D. discoideum. D. discoideum was plated in tissue culture wells in MB medium and infected with L. pneumophila at an MOI of 1:1. The number of viable bacteria was determined by counting CFU. The experiment was performed three times, each point in the experiment was done in triplicate, and the error bars indicate the n − 1 weighted sample standard deviation.
Wild-type L. pneumophila avoids killing within D. discoideum.
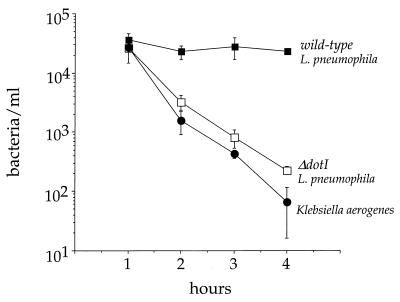
The fate of bacteria internalized by D. discoideum was followed carefully in the first few hours after infection (Fig. 4). Cells were infected with bacteria; after 30 min, gentamicin was added to the medium to kill all extracellular bacteria and remained in the medium for the duration of the experiment. Internalized K. aerogenes was rapidly killed, with the number of viable bacteria dropping over 2 logs in 3 h; the ΔdotI mutant bacteria exhibited a similar fate (Fig. 4). Interestingly, intracellular wild-type L. pneumophila persisted at the same level of viability over the course of the experiment, indicating that the bacteria resisted digestion (Fig. 4). This experiment, however, does not distinguish whether the internalized wild-type L. pneumophila avoids fusion with endocytic compartments or survives within a fused phagolysosome.
FIG. 4.
Viability of bacteria internalized by D. discoideum 1 to 4 h after infection. D. discoideum was plated in tissue culture wells in MB medium and infected with bacteria at an MOI of 5:1. After 30 min, gentamicin was added to kill extracellular bacteria. The number of viable intracellular bacteria remaining was counted by measuring CFU extracted from washed cells. The experiment was performed two times, each point was done in duplicate, and the error bars indicate the n − 1 weighted sample standard deviation.
Colocalization of internalized L. pneumophila and lysosomal membrane proteins.
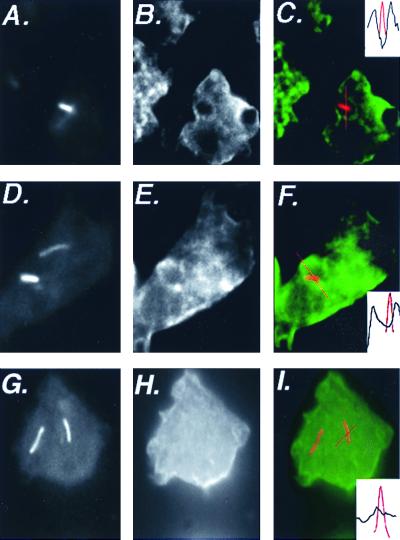
The association of internalized L. pneumophila with endosomes and lysosomes was examined by immunofluorescence. After 30 min of infection, L. pneumophila internalized by D. discoideum did not colocalize with a monoclonal antibody staining the V-ATPase (17) (data not shown). An antibody was generated against purified lysosomal membrane proteins (Materials and Methods). The antibody stained the plasma membrane and faintly stained numerous vesicles within the cell. Thirty minutes after infection, 6% (12 of 202) of dotI mutant bacteria were colocalized with anti-lysosomal membrane protein staining. Positive scoring was based on seeing large rings of lysosomal membrane protein staining around these dotI mutant bacteria (Fig. 5C and F). In contrast, none of 62 L. pneumophila dot+ bacteria colocalized with the lysosomal membrane protein staining (Fig. 5I).
FIG. 5.
Association of lysosomal membrane proteins with L. pneumophila-containing phagosomes in D. discoideum. D. discoideum was plated on coverslips in tissue culture wells in MB medium and infected with L. pneumophila at an MOI of 10:1. After 30 min of infection, cells were fixed for immunofluoresence analysis. Cells in panels A to C and D to F were infected by dotI mutant L. pneumophila; cells in panels G to I were infected with dot+ L. pneumophila. Panels A, D, and G show staining of the bacteria; panels B, E, and H show staining of the anti-lysosomal membrane protein antibody. In panels C, F, and I, the two images are superimposed, with bacterial staining shown in red and lysosomal membrane protein staining shown in green. The inset indicates the bacterial fluorescence (red) or the lysosomal membrane protein fluorescence (black) along the line drawn in each panel. Images were processed with IP Lab Spectrum version 3.2.
D. discoideum grown in suspension is resistant to L. pneumophila cytotoxicity and intracellular growth.
In all of the growth experiments described above, D. discoideum was infected as adherent cells in a monolayer. Traditionally, phagocytosis has been measured in D. discoideum suspended in shaking culture because shaking is thought to reduce the nonspecific interactions between particles and cells (52). In attempts to measure phagocytosis of L. pneumophila by D. discoideum in suspension, we observed that the amoebae were resistant to L. pneumophila.
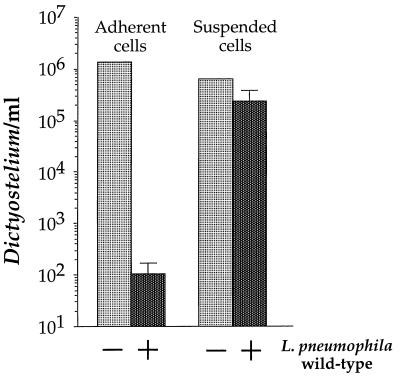
Contact-dependent cytotoxicity was greatly reduced in D. discoideum grown in suspension (Fig. 6). If adherent D. discoideum cells in MB medium were exposed to a high MOI (375:1) of wild-type L. pneumophila, the number of viable D. discoideum was reduced by 104 in 24 h (Fig. 6, adherent cells). We attribute this rapid decline in viability to contact-dependent cytotoxicity, as there was no detectable growth of L. pneumophila (data not shown). In contrast, suspended D. discoideum cells in MB medium exposed to the same multiplicity of wild-type L. pneumophila lost only fivefold viability after 24 h (Fig. 6, suspended cells). This fivefold drop in viability was larger than the sample standard deviations and is therefore statistically significant.
FIG. 6.
Susceptibility of adherent and suspended D. discoideum to a high-MOI infection of L. pneumophila. D. discoideum was incubated in MB medium either as adherent monolayers in tissue culture wells or as suspended cells shaken in tubes. Cells were infected (or not) with L. pneumophila Philadelphia-1 at an MOI of 375:1 for approximately 24 h. Viable D. discoideum cells were counted by measuring PFU. The experiment was performed twice, each condition was done in duplicate, and the error bars indicate the n − 1 weighted sample standard deviation.
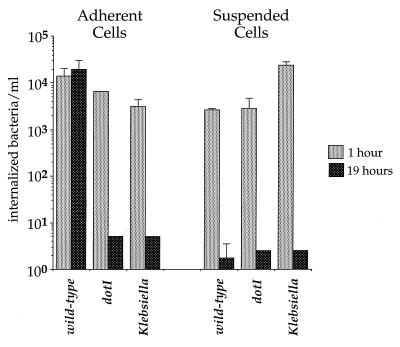
A potential explanation for this phenomenon is that D. discoideum and L. pneumophila are unable to interact in shaking culture. However, shortly (1 h) after infection of suspended D. discoideum, abundant intracellular L. pneumophila bacteria were observed by fluorescence microscopy after differential staining of extracellular and intracellular bacteria (data not shown). Bacteria incubated with suspended cells showed significant protection from gentamicin killing, further indicating the bacteria were intracellular (Fig. 7).
FIG. 7.
Survival of internalized bacteria in adherent and suspended D. discoideum. D. discoideum was incubated in MB medium either as adherent monolayers in tissue culture wells or as suspended cells shaken in tubes. Cells were infected with bacteria for 30 min, at which point gentamicin was added to kill extracellular bacteria. Viable, intracellular bacteria remaining were counted by measuring CFU extracted from washed cells. The experiment was performed twice, each condition was done in duplicate, and the error bars indicate the n − 1 weighted sample standard deviation.
Based on gentamicin protection, wild-type L. pneumophila was internalized but failed to survive in D. discoideum suspended cells (Fig. 7). Adherent cells and suspended cells were infected with L. pneumophila dotI and dot+ strains, as well as with K. aerogenes, and extracellular bacteria were killed 30 min after infection by addition of the antibiotic gentamicin to the medium. Both adherent and suspended D. discoideum internalized all three strains of bacteria, as indicated by gentamicin protection 60 min postinfection. As expected, the number of viable internalized L. pneumophila dotI and K. aerogenes was below the limit of detection after 19 h of infection in both adherent and suspended D. discoideum. In contrast, the L. pneumophila (dot+) in adherent cells were viable at 19 h postinfection. A very different result was observed with suspended cells. L. pneumophila dot+ was internalized by suspended cells and killed by 19 h post infection (Fig. 7). Thus, under conditions used in this assay system, suspended D. discoideum was not permissive for L. pneumophila growth.
Growth of D. discoideum on lawns of L. pneumophila.
D. discoideum is routinely grown on lawns of bacteria, usually K. aerogenes (44). Colonies of amoebae form plaques on the lawn after several days. Altered plaque phenotypes have been useful in genetic screens for D. discoideum mutants, and so the growth of D. discoideum on lawns of L. pneumophila (dot+ and dot mutant) was examined.
D. discoideum was unable to grow on a lawn of L. pneumophila (dot+) but was able to grow on a lawn of an isogenic dotI mutant (Fig. 8). D. discoideum spotted onto the lawn of ΔdotI L. pneumophila made a large clearing in the lawn, and fruiting bodies developed as determined by visual inspection (Fig. 8B). Several dot mutants were tested, including strains having mutations in dotA, dotB, icmVWX, dotE, dotG, dotH, and dotO, and all were able to support D. discoideum growth (data not shown). These results suggest that D. discoideum can utilize a variety of L. pneumophila dot strains as a food source and that the failure of D. discoideum to grow on wild-type L. pneumophila is due to functions supplied by the products of multiple dot genes.
FIG. 8.
Growth of D. discoideum on lawns of L. pneumophila 105 D. discoideum were spotted onto bacterial lawns grown on CYE plates made with reduced concentrations of cysteine and iron. (A) Lawn of L. pneumophila strain Lp01 (dot+); (B) lawn of L. pneumophila strain HL056 (ΔdotI).
In contrast, D. discoideum cells on the lawn of wild-type L. pneumophila were killed rapidly. Microscopic examination indicated that many more D. discoideum cells were recovered 20 h postinfection from the lawn of dotI mutant than from the dot+ lawn. The D. discoideum recovered from the dotI lawns showed normal morphology with nuclear material and clustered vesicles surrounded by a clear zone of cytoplasm. The D. discoideum cells recovered from the L. pneumophila (dot+) lawns were round, had no obvious internal organization, and were filled with small vesicles (data not shown). The rapid rate of killing, disturbed cell morphology, and the presence of a high MOI suggested that the D. discoideum was killed by contact-dependent cytotoxicity, a process that depends on dot gene functions.
Effect of known D. discoideum mutants on intracellular growth of L. pneumophila.
To begin to take advantage of D. discoideum genetics, a variety of previously characterized mutants showing defects in phagocytosis or membrane trafficking were plated in adherent monolayers and analyzed for the ability to support growth of L. pneumophila. A large number of such mutants exist, most of which contain defined lesions in single genes.
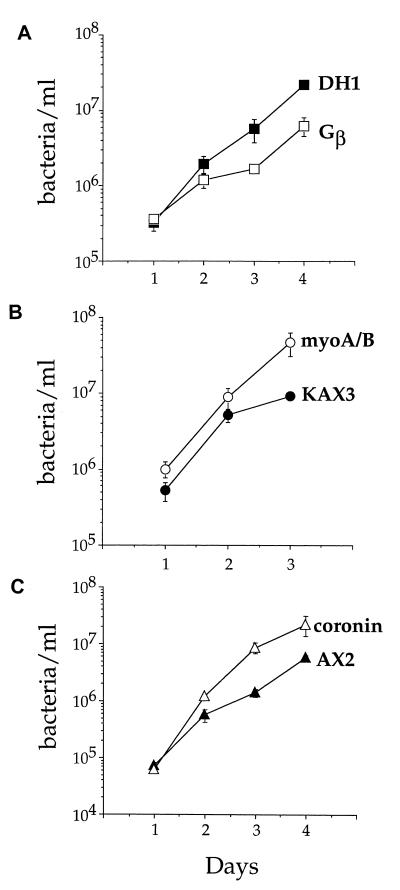
A D. discoideum strain having an insertion in Gβ, a subunit of trimeric G proteins, supported growth of L. pneumophila but at a slightly reduced rate (Fig. 9A). D. discoideum has only one Gβ subunit, and the null mutation analyzed here theoretically eliminates all trimeric G-protein signaling in the cells (58). The D. discoideum myoA/B double myosin I mutant and the coronin mutant, on the other hand, supported growth of L. pneumophila as well as, if not better, than the wild-type controls (Fig. 9B and C). The myosin I isoforms in D. discoideum play important roles in cell motility and endocytosis (51). Coronin is a WD repeat protein that localizes to the moving portions of the cell, and coronin null mutants show defects in motility, cytokinesis, and phagocytosis and pinocytosis in suspension (11, 19, 31). The enhanced growth of wild-type L. pneumophila in the coronin mutant was particularly striking, in that 3 days after infection there was routinely a 10-fold-higher yield of L. pneumophila than in wild-type D. discoideum (Fig. 9C). We tested additional D. discoideum mutants that had no effect on L. pneumophila growth, including myoA and myoB single mutants (50, 55), cells overexpressing constitutively active rab7 (6), and a double phosphatidylinositol 3-kinase mutant (59) (data not shown).
FIG. 9.
Growth of L. pneumophila in D. discoideum mutants. Various D. discoideum mutants and their parent strains were plated in tissue culture wells in MB medium and infected with L. pneumophila Philadelphia-1 at an MOI of 1:1. The number of viable bacteria was determined by counting CFU. The experiments were performed two to four times depending on the strain, each point in the experiment was done in triplicate, and the error bars indicate the n − 1 weighted sample standard deviation.
DISCUSSION
The results indicate that growth of L. pneumophila in D. discoideum occurs by a mechanism that is similar to its growth in amoebae and macrophages. In macrophages at 37°C, the titer of L. pneumophila increases 3 to 4 logs in 3 days (2, 5, 53). In adherent D. discoideum, using conditions described here, the titer of L. pneumophila increased at least 100-fold over 3 days, which is impressive given that the growth temperature was reduced to 25.5°C. L. pneumophila did not grow in D. discoideum at 21°C (data not shown), indicating that 25.5°C may be close to the lowest temperature that supports L. pneumophila intracellular growth.
Growth of L. pneumophila occurred within D. discoideum in membrane-bound vesicles associated with ribosomes and RER. Under our conditions of fixation, RER associated with L. pneumophila phagosomes was more easily detectable in D. discoideum compared to macrophages (R. Isberg, personal observation). In some circumstances, such as Fig. 2C, large bacteria-filled vacuoles could be found devoid of ribosomes. This absence of localization may be either a result of extraction of the sample by the detergent present in the fixative used here or because RER sequestration about the vacuole dissipates as the intracellular growth cycle proceeds.
Growth of L. pneumophila in D. discoideum is dependent on functions provided by multiple dot gene products. Three representative dot mutants were tested in our experiments, and all were blocked for intracellular growth. A similar phenotype is observed for these same strains in macrophages (1). This result is strong supporting evidence that the intracellular growth observed is initiated in a fashion similar to that seen in macrophages.
L. pneumophila dot+ persisted after internalization by D. discoideum, whereas a ΔdotI mutant and a K. aerogenes control strain were efficiently killed by 4 h (Fig. 4). In cultured macrophages, internalized dot mutants fail to grow but remain viable for several days in spite of fusion with lysosomes (46). This difference likely reflects the more effective digestive capabilities of D. discoideum relative to cultured macrophages.
We have attempted to directly examine whether phagosomes containing wild-type L. pneumophila avoid endosomal fusion in D. discoideum, using immunofluoresence to localize the vacuolar ATPase. Thirty minutes after infection, the visualized vacuolar ATPase, which marks the contractile vacuole, did not colocalize with either wild-type or dotI mutant L. pneumophila-containing phagosomes. The speed at which D. discoideum internalizes and digests microorganisms may make it difficult to observe the transient colocalization of V-ATPase with the phagosome. Biochemical examination of early phagosomes in D. discoideum showed that the V-ATPase is present in these membranes (39). L. pneumophila can efficiently replicate in mammalian cells that lack a contractile vacuole, and so it is not surprising that L. pneumophila is not found in that organelle.
The colocalization of intact L. pneumophila and lysosomal membrane proteins was also examined. The frequency of clear colocalization was far greater with the dotI mutant strain than with dot+ bacteria, suggesting that dot+ L. pneumophila successfully evaded lysosomal fusion in D. discoideum. One hour after infection, there was clear microscopic evidence of degradation of a dot+ strain, suggesting that evasion of the endocytic pathway may be less efficient in D. discoideum than in macrophages (data not shown).
Interestingly, D. discoideum grown in suspension was both resistant to cytotoxicity induced by high-MOI infection of L. pneumophila and unable to support intracellular growth. The explanation for these observations could be due to either bacterial or host factors. It is possible that tight adherence of bacteria to the target cell is needed for effector proteins to be transferred through the Dot-Icm complex and promote intracellular growth. Shaking of the D. discoideum culture itself could disrupt this process in a fashion similar to what is observed when conjugal DNA transfer is disrupted in shaking cultures (24). Increasing the adherence of the bacteria may overcome this block. Alternatively, D. discoideum grown in suspension could be limiting for some crucial cellular protein that is a target for a translocated L. pneumophila protein, or the suspended amoebae could lack a particular uptake pathway that L. pneumophila requires to establish its replicative compartment. Furthermore, D. discoideum in suspension may be in an altered state, similar to macrophages activated by treatment with gamma interferon, which results in resistance to L. pneumophila infection (35).
All of the D. discoideum mutants tested were capable of supporting L. pneumophila growth. L. pneumophila grew well in both independently derived axenic strains, AX3 and AX2, indicating that L. pneumophila growth does not depend on a particular strain of D. discoideum. The Gβ mutant alone showed a slight reduction in growth of L. pneumophila. Previously it had been shown that adherent Gβ mutant cells are impaired in phagocytosis as determined by uptake of yeast particles (37) and the fact that they form small plaques on lawns of K. aerogenes (58). In this strain, particle attachment is normal, but fewer attached particles are engulfed compared to wild-type cells (37). The slightly reduced growth of L. pneumophila in this mutant could be explained by a reduced efficiency of uptake, both for the initial infection and when bacteria reinfect cells after completing a round of intracellular infection.
A recent report suggests that phagosomes bearing Mycobacterium tuberculosis are blocked for entry into the endocytic pathway by failing to release a coronin homologue that coats its surface (15). In this model, the presence of this molecule, called TACO, interferes with the ability of this phagosome to traffic into a degradative pathway. Clearly coronin does not play this role for L. pneumophila in D. discoideum, as the absence of coronin does not prevent L. pneumophila growth.
The myoA/B myosin I double mutant and the coronin mutant allowed better growth of L. pneumophila than the parental controls. As adherent cells, the myoA/B mutant shows no defect in pinocytosis or phagocytosis (36). The coronin mutant shows both pinocytosis and phagocytosis defects in suspension but has not been tested in adherent cells (19, 31). Both of these mutants are defective for amoebal motility. Coronin mutants move at speeds roughly one-third that of wild-type cells (11), and myoA/B double mutants move at speeds roughly one-half that of wild-type cells (51). Perhaps this gives the L. pneumophila growth pathway a kinetic advantage relative to the digestive pathway. By this model, establishment of the L. pneumophila replication vacuole is dependent on successful competition of factors produced by the organism that support intracellular growth relative to host cell factors that target the phagosome into a route that prevents replication of the bacterium.
Using the L. pneumophila-D. discoideum system, we have begun a genetic analysis of bacterial and host functions involved in this host-pathogen interaction. In theory, any intracellular pathogen that can grow at 25°C may be capable of growth in D. discoideum. If such systems can be established, it should be possible to identify host mutants that no longer support growth of almost any pathogen. The products of these host genes may be ideal candidates for drug therapy as it may be possible to mimic the effect of the mutations with small molecules that block intracellular growth of pathogens.
ACKNOWLEDGMENTS
This work would not have been possible without the help and enthusiasm of Dave Knecht. We thank Meg Titus, Markus Maniak, and Peter Devreotes for helpful discussions and strains, Masa Watarai for providing the GFP-expressing L. pneumophila strains, and Andrea Marra for the pAM239 plasmid. Excellent technical assistance was provided by Liz Benecchi and Cathy Linsenmayer at the Tufts University Medical School electron microscopy lab. We thank Dorothy Fallows and Guillame Duménil for careful reading of the manuscript.
J. M. Solomon receives support from an NRSA award from the NIH, J. A. Cardelli is supported by grant DK39232 from the NIH, and R. R. Isberg is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Andrews H L, Vogel J P, Isberg R R. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 3.Berger K H, Merriam J J, Isberg R R. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonner J T. A descriptive study of the development of the slime mold Dictyostelium discoideum. Am J Bot. 1944;31:175–182. [Google Scholar]
- 5.Brand B C, Sadosky A B, Shuman H A. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol Microbiol. 1994;14:797–808. doi: 10.1111/j.1365-2958.1994.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 6.Buczynski G, Bush J, Zhang L, Rodriguez-Paris J, Cardelli J. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol Biol Cell. 1997;8:1343–1360. doi: 10.1091/mbc.8.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandler F W, Hicklin M D, Blackmon J A. Demonstration of the agent of Legionnaires' disease in tissue. N Engl J Med. 1977;297:1218–1220. doi: 10.1056/NEJM197712012972206. [DOI] [PubMed] [Google Scholar]
- 9.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 10.Cox E C, Vocke C D, Walter S, Gregg K Y, Bain E S. Electrophoretic karyotype for Dictyostelium discoideum. Proc Natl Acad Sci USA. 1990;87:8247–8251. doi: 10.1073/pnas.87.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Hostos E L, Rehfuess C, Bradtke B, Waddell D R, Albrecht R, Murphy J, Gerisch G. Dictyostelium mutants lacking the cytoskeletal protein coronin are defective in cytokinesis and cell motility. J Cell Biol. 1993;120:163–173. doi: 10.1083/jcb.120.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Lozanne A, Spudich J A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236:1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- 13.De Priester W. Techniques for the visualisation of cytoskeletal components in Dictyostelium discoideum. Electron Microsc Rev. 1991;4:343–376. doi: 10.1016/0892-0354(91)90009-2. [DOI] [PubMed] [Google Scholar]
- 14.Feeley J C, Gibson R J, Gorman G W, Langford N C, Rasheed J K, Mackel D C, Baine W B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979;10:437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97:435–447. doi: 10.1016/s0092-8674(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 16.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 17.Fok A K, Clarke M, Ma L, Allen R D. Vacuolar H(+)-ATPase of Dictyostelium discoideum. A monoclonal antibody study. J Cell Sci. 1993;106:1103–1113. doi: 10.1242/jcs.106.4.1103. [DOI] [PubMed] [Google Scholar]
- 18.Gao L Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages, and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110:105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz M A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horwitz M A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz M A, Maxfield F R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984;99:1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz M A, Silverstein S C. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Investig. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob F, Wollman E L. Sexuality and the genetics of bacteria. New York, N.Y: Academic Press Inc.; 1961. [Google Scholar]
- 25.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 26.Kopp E B, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 27.Lemaitre B, Nicolas E, Michaut L, Reichhart J M, Hoffmann J A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 28.Loomis W F. Role of PKA in the timing of developmental events in Dictyostelium cells. Microbiol Mol Biol Rev. 1998;62:684–694. doi: 10.1128/mmbr.62.3.684-694.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loomis W F., Jr Sensitivity of Dictyostelium discoideum to nucleic acid analogues. Exp Cell Res. 1971;64:484–486. doi: 10.1016/0014-4827(71)90107-8. [DOI] [PubMed] [Google Scholar]
- 30.Loomis W F, Welker D, Hughes J, Maghakian D, Kuspa A. Integrated maps of the chromosomes in Dictyostelium discoideum. Genetics. 1995;141:147–157. doi: 10.1093/genetics/141.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maniak M, Rauchenberger R, Albrecht R, Murphy J, Gerisch G. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein Tag. Cell. 1995;83:915–924. doi: 10.1016/0092-8674(95)90207-4. [DOI] [PubMed] [Google Scholar]
- 32.Mann S K O, Devreotes P N, Elliott S, Jermyn K, Kuspa A, Fechheimer M, Furukawa R, Parent C A, Segall J, Shaulsky G, Vardy P H, Williams J, Williams K L, Firtel R A. Cell biological, molecular genetic, and biochemical methods used to examine Dictyostelium. In: Celis J E, editor. Cell biology, a laboratory handbook. Vol. 1. San Diego, Calif: Academic Press; 1998. pp. 431–465. [Google Scholar]
- 33.Morales V M, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 34.Muller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nash T W, Libby D M, Horwitz M A. IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1988;140:3978–3981. [PubMed] [Google Scholar]
- 36.Novak K D, Peterson M D, Reedy M C, Titus M A. Dictyostelium myosin I double mutants exhibit conditional defects in pinocytosis. J Cell Biol. 1995;131:1205–1221. doi: 10.1083/jcb.131.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peracino B, Borleis J, Jin T, Westphal M, Schwartz J M, Wu L, Bracco E, Gerisch G, Devreotes P, Bozzaro S. G protein beta subunit-null mutants are impaired in phagocytosis and chemotaxis due to inappropriate regulation of the actin cytoskeleton. J Cell Biol. 1998;141:1529–1537. doi: 10.1083/jcb.141.7.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purcell M, Shuman H A. The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect Immun. 1998;66:2245–2255. doi: 10.1128/iai.66.5.2245-2255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezabek B L, Rodriguez-Paris J M, Cardelli J A, Chia C P. Phagosomal proteins of Dictyostelium discoideum. J Eukaryot Microbiol. 1997;44:284–292. doi: 10.1111/j.1550-7408.1997.tb05668.x. [DOI] [PubMed] [Google Scholar]
- 40.Roy C R, Berger K H, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 41.Segal G, Purcell M, Shuman H A. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segal G, Shuman H A. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect Immun. 1997;65:5057–5066. doi: 10.1128/iai.65.12.5057-5066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segal G, Shuman H A. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun. 1999;67:2117–2124. doi: 10.1128/iai.67.5.2117-2124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- 45.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swanson M S, Isberg R R. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect Immun. 1996;64:2585–2594. doi: 10.1128/iai.64.7.2585-2594.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan M W, Mahajan-Miklos S, Ausubel F M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Temesvari L, Rodriguez-Paris J, Bush J, Steck T L, Cardelli J. Characterization of lysosomal membrane proteins of Dictyostelium discoideum. A complex population of acidic integral membrane glycoproteins, Rab GTP-binding proteins and vacuolar ATPase subunits. J Biol Chem. 1994;269:25719–25727. [PubMed] [Google Scholar]
- 49.Thomason P, Traynor D, Kay R. Taking the plunge. Terminal differentiation in Dictyostelium. Trends Genet. 1999;15:15–19. doi: 10.1016/s0168-9525(98)01635-7. [DOI] [PubMed] [Google Scholar]
- 50.Titus M A, Wessels D, Spudich J A, Soll D. The unconventional myosin encoded by the myoA gene plays a role in Dictyostelium motility. Mol Biol Cell. 1993;4:233–246. doi: 10.1091/mbc.4.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uyeda T Q P, Titus M A. Dictyostelium—a model system for cell and developmental biology. Tokyo, Japan: Universal Academy Press, Inc., and Yamada Science Foundation; 1997. pp. 43–64. [Google Scholar]
- 52.Vogel G. Endocytosis and recognition mechanisms in Dictyostelium discoideum. Methods Cell Biol. 1987;28:129–137. doi: 10.1016/s0091-679x(08)61640-4. [DOI] [PubMed] [Google Scholar]
- 53.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 54.Vogel J P, Roy C, Isberg R R. Use of salt to isolate Legionella pneumophila mutants unable to replicate in macrophages. Ann NY Acad Sci. 1996;797:271–272. doi: 10.1111/j.1749-6632.1996.tb52975.x. [DOI] [PubMed] [Google Scholar]
- 55.Wessels D, Murray J, Jung G, Hammer III J A, Soll D R. Myosin IB null mutants of Dictyostelium exhibit abnormalities in motility. Cell Motil Cytoskel. 1991;20:301–315. doi: 10.1002/cm.970200406. [DOI] [PubMed] [Google Scholar]
- 56.Wiater L A, Dunn K, Maxfield F R, Shuman H A. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect Immun. 1998;66:4450–4460. doi: 10.1128/iai.66.9.4450-4460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams K L, Kessin R H, Newell P C. Genetics of growth in axenic medium of the cellular slime mould Dictyostelium discoideum. Nature. 1974;247:142–143. doi: 10.1038/247142a0. [DOI] [PubMed] [Google Scholar]
- 58.Wu L, Valkema R, Van Haastert P J, Devreotes P N. The G protein beta subunit is essential for multiple responses to chemoattractants in Dictyostelium. J Cell Biol. 1995;129:1667–1675. doi: 10.1083/jcb.129.6.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou K, Takegawa K, Emr S D, Firtel R A. A phosphatidylinositol (PI) kinase gene family in Dictyostelium discoideum: biological roles of putative mammalian p110 and yeast Vps34p PI 3-kinase homologs during growth and development. Mol Cell Biol. 1995;15:5645–5656. doi: 10.1128/mcb.15.10.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]