Abstract
The study was designed to discuss the effect of stratification factors in the Mayo staging on the prognosis of hilar cholangiocarcinoma (HCCA) patients, and to evaluate the predictive value of the Mayo staging on the prognosis. The Kaplan–Meier survival curve and Log-rank test were used to perform univariate analysis on each index and obtain statistically significant influencing factors. The Kaplan–Meier survival curve and Log-rank test were used to analyze the correlation between the two staging systems and the survival period. The receiver operating characteristic (ROC) curves were used for each single staging system trend analysis, and comparison of their curve area to determine prognosis prediction ability for patients with HCCA. According to Kaplan–Meier survival curve changes and Log-rank test results, it was found that both staging systems were correlated with the survival time of the patients (P < .001). Through a pairwise comparison within the stages, it was found that the heterogeneity between the stages within the Mayo staging is very good, which was better than the TNM staging. A single trend analysis of the prognostic assessment capabilities of the two systems found that the area under the ROC curve of Mayo staging system (AUC = 0.587) was the largest and better than the TNM staging system (AUC = 0.501). Mayo staging can be used for preoperative patient prognosis assessment which can provide better stratification ability based on a single-center small sample study, and the predictive value is better than TNM staging.
Keywords: hilar cholangiocarcinoma, Mayo staging system, prognostic, TNM staging system
1. Introduction
Cholangiocarcinoma, also known as biliary malignant stenosis, refers to malignant tumors originating from biliary epithelial cells, accounting for 3% of digestive system tumors.[1–4] It is the second most common malignant tumor in primary liver tumors, and the incidence of males is higher than that of females.[5] Among them, HCCA accounts for 40% to 60% of cholangiocarcinoma. Surgical resection is an effective method for the treatment of HCCA, but the effective rate of surgery in early patients is less than 25%.[6] In early patients, the most perfect treatment is liver transplantation combined with neoadjuvant chemotherapy.[7] The current basic chemotherapy regimen is gemcitabine combined with cisplatin. However, a large amount of clinical data has proven that it is not sensitive to chemotherapy and that chemotherapy is only a means of palliative treatment, according to statistical survival only extended by 3 months.[8]
The current staging systems for HCCA are Bismuth‐Corlette typing,[9] MSKCC T staging,[10] Gazzaniga staging,[11] AJCC 8th TNM staging system (https://www.facs.org/quality-programs/cancer/ajcc/cancer-staging) and stages published by the International Cholangiocarcinoma Working Group in 2011.[12] The most commonly used are Bismuth‐Corlette typing, MSKCC T staging and AJCC 8th TNM staging system. Bismuth‐Corlette typing and MSKCC T staging are preoperative guidance for surgical patients, which can extend patient survival by increasing R0 resection rates. The definition of liver atrophy in MSKCC T staging factors is inaccurate, resulting in significantly limited practical value in clinical work. Based on histopathology results, the TNM staging system is used to evaluate the local and distant metastasis of tumor after surgery, and has low value for surgical guidance. Therefore, all three stages are surgical stages, and none is used to assess the prognosis of non-surgical patients. The International Cholangiocarcinoma Group absorbed the contents of these three stages and incorporated new variables, carried out a more comprehensive evaluation and expression. But it not only lacks the validation of large sample data, but it also cannot be layered. Non-surgical patients should be based on clinically acquired information to predict survival time rather than pathological outcomes. At present, the treatment of HCCA urgently requires a new treatment, but the clinical trial of HCCA is blocked due to the lack of a clinical stage of non-surgical treatment. Because this staging needs to be stratified for all patients in clinical experimental studies.
Currently, there is no stage that can be adapted to all patients with HCCA. In order to make up for this deficiency, Chaiteerakij et al[13] of the Mayo Clinic in the United States proposed a Mayo staging of HCCA patients based on clinical information. The staging system is based on the clinical data of 413 HCCA patients admitted to the Mayo Clinic in the past 8 years, through the ECOG (Eastern Cooperative Oncology Group) activity status score, vascular involvement, tumor metastasis, lymph node metastasis, tumor size and number, CA19-9 level and other indicators are divided into 4 periods. At the same time, compared with the imaging TNM staging, significant results were obtained. However, the single-center study was conducted in the Western population, and primary sclerosing cholangitis was considered to be a major risk factor for HCCA in this population. Therefore, we performed a retrospective analysis using the clinical data of 335 patients treated at our hospital from February 1, 2004, to January 1, 2013 and conducted a single-center study of the Mayo staging system. At the same time, we discuss the clinical use value of the Mayo staging system by comparing it with the TNM staging system of pathological standard.
2. Material and methods
2.1. Ethics statement
The research protocol was reviewed and approved by our Research Ethics Committee and performed in accordance with the Declaration of Helsinki. All participants or their guardians gave written consent for use of their clinical samples and medical information in scientific research.
2.2. Patients
We reviewed and analyzed the clinical data of 335 patients with HCCA in the Affiliated Hospital of Qingdao University during February 1, 2004 to January 1, 2013. Follow-up data were available for all patients. The two researchers combined clinical data in groups according to the TNM staging system and Mayo staging system.[13] Then they finally adjusted and compared, and listed the cases of disagreement separately. These data were judged by the chief physician who was finally a third party.
2.3. Diagnosis
We selected cases according to the following criteria: initial diagnosis, or although confirmed but did not receive any anti-tumor treatment in other medical institutions before coming to our hospital. The surgical specimen was confirmed by histopathological examination HCCA. Tissue pathology from endoscopic biopsy, confirmed by percutaneous biopsy, intraoperative biopsy. Enhanced CT or MRI cross-sectional imaging confirmed the presence of malignant biliary stricture, and had a hilar mass, and subsequent follow-up there was a malignant clinical outcome. CA19-9 > 100 U/mL, and the imaging results confirmed that malignant biliary stricture did not include bacterial cholangitis.[14] In the determination of patients without pathological results confirmed, according to their medical history, signs, imaging examination (enhanced CT, enhanced MRI, MRCP, etc), ultrasonic examination, laboratory examination, intervention and endoscopic examination, etc, need to combine the results of “Hilar cholangiocarcinoma (HCCA): expert consensus statement” of the 2015 American Hepatobiliary Pancreatic Association, “Guidelines for the Diagnosis and Treatment of Hilar cholangiocarcinoma” (2013) and “Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition” of Japanese hepato-biliary-pancreatic sciences.[15,16]
At the same time, we excluded the following patients: received anti-tumor treatment from other medical institutions before the hospital visits, including endoscopic retrograde biliary drainage (ERBD), percutaneous transhepatic cholangiography and drainage (PTCD), surgical removal, chemotherapy, etc. Combining other malignant tumors or severe chronic diseases (as chronic renal failure, cardiac insufficiency, pulmonary hypertension, etc). Patients can’t carry out Mayo staging system with incomplete clinical data. Patients who refused to participate in the study during telephone follow-up.
We used the following criteria to define baseline research: ECOG performance status, grade 0 = fully active, able to carry on all pre-disease performance without restriction, 1 = restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work, 2 = ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours, 3 = capable of only limited self-care; confined to bed or chair for more than 50% of waking hours, 4 = completely disabled; cannot carry on any self-care; totally confined to bed or chair, 5 = dead. Tumor size: the maximum diameter of the tumor is determined by imaging data for the first diagnosis (enhancement CT > MRI > ultrasound). The data is determined by imaging data from our hospital. Tumor metastasis and the number of tumors: we determine the peritoneal metastasis, liver metastasis (including intrahepatic metastasis) and other organ metastasis combined with imaging data diagnosis and determine the number of intrahepatic tumors. Lymphatic metastasis: we retrospectively analyze perihepatic hilar lymph nodes through imaging data, especially 12 groups of lymph nodes. Vascular encasement: we analyze some or all of the tumor tissue around vascular tissue including the portal vein, hepatic artery and superior mesenteric artery by imaging data.
2.4. Follow-up
Follow-up mainly consisted of telephone calls and clinical visits, with letters and household registration queries as necessary. Patients were followed up every 2 months, and every 3 months thereafter for the first year. Outpatient routine examination programs include CA19-9, liver function and hepatobiliary pancreatic ultrasound. It is necessary to carry out enhanced CT, MRCP or bile imaging examination to further clarify whether the tumor recurrence when the examination results show or consider the possibility of tumor recurrence. Then we need to record the recurrence time to develop the next treatment plan according to the patient’s physical condition and family wishes. Survival was defined as the date of operation or biliary drainage to the date of death or last follow-up, which was September 30, 2016.
2.5. Statistics
All data was managed by Microsoft Excel 2007 and subject to statistical analysis by SPSS 22.0 software (IBM Corp, Armonk, NY). The data met the continuous variable of normal distribution using standard deviation (SD), and median and interquartile spacing was used for that which did not conform to the normal distribution. According to a variety of clinical data of the patients diagnosed with HCCA before treatment, the study obtained statistically significant factors by the using of Kaplan–Meier method and Log-rank test for univariate analysis. Independent prognostic factors were analyzed by the Cox analysis method. Correlation analysis was carried out on the two staging systems and the multiple comparisons within system to evaluate the degree of differentiation and single trend of staging system. The receiver operating characteristic (ROC) curves were used for each single staging system trend analysis, and comparison of their curve area to determine prognosis prediction ability for patients with HCCA.
Combined with the TNM staging system and Mayo staging system criteria,[13] we found that the prognosis of the two staging systems is manifested in the following three aspects: accuracy: the variables that are decisive within the staging have an impact on the prognosis. Distinction degree: the survival time of patients with different stages is quite different. Single trend: the patient survival time showed a monotonous downward trend with the increment of each stage.
Based on the above content, we have the following analysis ideas: accuracy: according to clinical experience, the Kaplan–Meier survival curve was drawn using indicators that affect the prognosis of the disease as variables and analyzed by Log-rank test, P < .05 was statistically significant, thereby obtaining statistically significant influencing factors. Then the above influencing factors were introduced into the Cox regression analysis, and the risk ratio model was established for treatment, and independent factors with statistical significance for the prognosis were obtained. Distinction degree and single trend: Kaplan–Meier survival curves were drawn and Log-rank test was used to analyze the discrimination and single trend of the two staging systems. The ROC was used to evaluate the pros and cons of the two staging models by comparing the areas under the two stages.
Preoperative test and imaging data were used to classify patients, and Kaplan–Meier survival curve and Log-rank test were used to perform univariate analysis of indicators affecting prognosis, and statistically significant influencing factors were obtained. Then, the above influencing factors were imported into Cox regression analysis, and a risk ratio model was established for processing, and statistically significant prognostic factors were obtained to further understand the accuracy of the Mayo staging system. The stratification of the two staging systems was compared in pairs to evaluate the degree of distinction between the internal strata. The ROC curve was used to analyze the discrimination and single trend of the two staging systems, and the prognostic assessment ability was compared by comparing the area of the curve.
3. Results
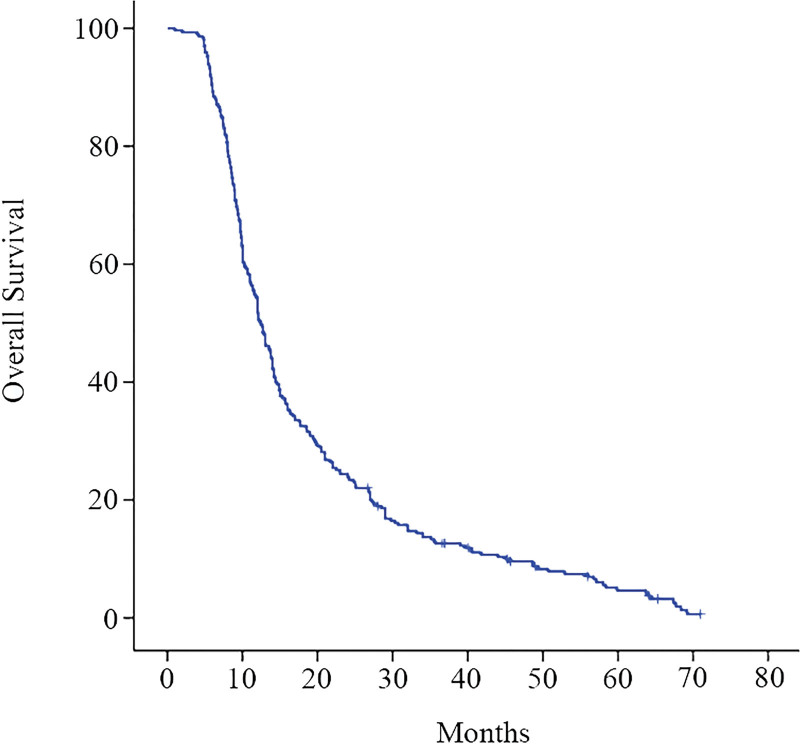
There were 335 patients with HCCA who could undergo the Mayo staging system, and 40 patients without follow-up data or who refused to participate in this study. There were a total of 295 patients who could undergo the Mayo staging system included in this study, 182 patients underwent operative treatment, of whom 96 underwent R0 resection, and 48 underwent R1 resection, and 38 underwent R2 resection. There were 113 patients with palliative biliary drainage, of whom 52 received ERBD treatment and 61 received PTCD treatment. The last follow-up was September 30, 2016. A total of 283 patients died and 12 were lost to follow-up; the rate of loss to follow-up was 4.1%. The median survival was 12.4 (11.2‐13.6) months, and the 1-, 2-, and 3-year survival rates were (51.9 ± 2.9)%, (21.1 ± 2.5)%, and (12.6 ± 1.9)% (Fig. 1).
Figure 1.
Kaplan–Meier survival estimates of 295 patients with HCCA. A total of 283 patients died and 12 lost to follow-up. HCCA = hilar cholangiocarcinoma.
Univariate analysis showed that factors affecting the prognosis of HCCA include: treatment method, albumin, prealbumin, lymph node metastasis, tumor metastasis (peritoneal and other. organs), CA19-9 > 1000 U/mL, tumor diameter > 3 cm, Bismuth‐Corlette typing, vascular encasement, the grade of ECOG performance status were prognostic indicators of HCCA (Table 1).
Table 1.
Univariable analysis of prognostic factors.
| Variable (n) | Median survival in months (95% CI) | P value |
|---|---|---|
| Gender | .914 | |
| Male (197) | 12.1 (10.9–13.2) | |
| Female (98) | 13 (10.9–15.0) | |
| Age (years) | .705 | |
| ≤60 (113) | 13 (11.4–14.6) | |
| >60 (182) | 12.1 (10.3–13.8) | |
| Blood types | .750 | |
| A (87) | 13 (11.2–14.8) | |
| B (102) | 13 (11.4–15.6) | |
| AB (36) | 12.1 (8.0–16.2) | |
| O (70) | 10 (8.0–12.0) | |
| Treatment category‡ | <.001*** | |
| R0 resection (96) | 21 (13.7–28.3) | |
| R1/R2 resection (86) | 13.8 (12.3–15.3) | |
| ERBD/PTCD (113) | 8.7 (8.0–9.4) | |
| ALB(g/L)* | <.001*** | |
| <35 (156) | 9 (8.4–9.6) | |
| ≥35 (1390) | 19.7 (17.1–22.3) | |
| PAB (g/L)* | .015* | |
| <200 (200) | 11.5 (10.2–12.8) | |
| ≥200 (95) | 14 (12.0–16.0) | |
| TBIL (μmol/L)* | .386 | |
| <200 (116) | 13.1 (11.3–14.9) | |
| ≥200 (179) | 12.0 (10.1–13.9) | |
| GGT (U/L)* | .512 | |
| <90 (21) | 10.6 (4.8–16.4) | |
| ≥90 (274) | 12.4 (11.3–13.5) | |
| TC (mmol/L)* | .922 | |
| <6.22 (160) | 12.7 (11.3–14.1) | |
| ≥6.22 (135) | 12 (10.0–14.0) | |
| CA19-9 (U/mL) | .003** | |
| <1000 (197) | 14.0 (12.6–15.4) | |
| ≥1000 (98) | 9.9 (9.1–10.7) | |
| Differentiation grade‡ | .556 | |
| Poor (77) | 14.2 (10.8–17.6) | |
| Moderate (90) | 20.4 (13.8–27.0) | |
| Well (15) | 18.6 (12.3–25.0) | |
| Distant metastasis† | <.001*** | |
| Yes (224) | 14.2 (12.6–15.8) | |
| No (71) | 7.3 (6.0–8.6) | |
| Bismuth-Corlette type | <.001*** | |
| I (36) | 29.8 (15.9–43.7) | |
| II (58) | 27.3 (25.3–29.3) | |
| IIIA (71) | 10.4 (9.6–11.2) | |
| IIIB (66) | 11.6 (9.2–14.0) | |
| IV (64) | 8.9 (7.7–10.1) | |
| Tumor diameter (cm)† | <.001*** | |
| ≤3 (176) | 14.0 (12.6–15.4) | |
| >3 (119) | 10.6 (9.6–11.6) | |
| Number of tumours† | .714 | |
| 1 (285) | 12.3 (10.9–13.7) | |
| ≥2 (10) | 14.0 (7.8–20.2) | |
| Vascular encasement † | .037* | |
| No (214) | 13.5 (12.1–14.8) | |
| Yes (81) | 10.2 (8.9–11.5) | |
| ECOG grade | <.001*** | |
| 0 (40) | 20.4 (13.4–27.4) | |
| 1/2 (240) | 12.1 (10.8–13.4) | |
| 3/4 (15) | 5.8 (5.51–6.08) |
ALB = albumin, CA19-9 = carbohydrate antigen 19-9, ECOG = Eastern Cooperative Group, ERBD = endoscopic retrograde biliary drainage, GGT = glutamyl transferase, PAB = prealbumin, PTCD = percutaneous transhepatic cholangiography and drainage, R0 = resection was defined as pathologically negative surgical margins by the naked eye, R1/R2 = resection was defined as positive microscopic resection margins (R1 resection) or positive macroscopic resection margins (R2 resection), TBIL = total bilirubin, TC = total cholesterol.
The diagnosis of the measured value.
Combining imaging data, enhancement CT > MRI > ultrasound.
Pathological examination.
P < .05.
P < .01.
P < .001.
The statistically significant factors (P > .05) obtained by univariate analysis were taken into the Cox regression analysis. Cox proportional hazard analysis showed that the treatment method (HR:2.077, 95% CI: 1.706‐2.529), albumin ≥ 35g/L (HR: 2.353, 95% CI: 1.803‐3.071), maximum diameter of tumor > 3 cm (HR: 1.335, 95% CI: 1.006‐1.772), tumor metastasis (HR: 2.596, 95% CI: 1.160‐2.197), Bismuth‐Corlette typing (HR: 1.277, 95% CI: 1.147‐1.422) and the grade of ECOG performance status (HR: 1.837, 95% CI: 1.436‐2.349) were independent factors affecting prognosis (Table 2). When conducting multi-factor analysis of the Cox proportional hazard model, interactions should be avoided between factors. Although TNM staging is also a prognostic factor, in order to avoid the impact on lymph node metastasis, tumor metastasis, and better assessment of Mayo staging, it has not been included in multivariate analysis.
Table 2.
Multivariable analysis of prognostic factors.
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| Treatment category | 2.077 (1.706–2.529) | <.001*** |
| ALB ≥ 35(g/L) | 2.353 (1.803–3.071) | <.001*** |
| PAB ≥ 200(g/L) | 1.270 (0.971–1.661) | .081 |
| Lymph node metastasis | 1.106 (0.856–1.428) | .440* |
| CA19-9 ≥ 1000(U/mL) | 1.2239 (0.943–1.587) | .129 |
| Tumor diameter > 3 cm | 1.335 (1.006–1.772) | .046* |
| Distant metastasis | 1.596 (1.160–2.197) | .004** |
| Bismuth-Corlette type | 1.277 (1.147–1.422) | <.001*** |
| ECOG grade | 1.837 (1.436–2.349) | <.001*** |
| Vascular encasement | 1.328 (0.992–1.778) | .057 |
ALB = albumin, CA19-9 = carbohydrate antigen 19-9, ECOG = Eastern Cooperative Group, PAB = prealbumin.
P < .05,
P < .01,
P < .001.
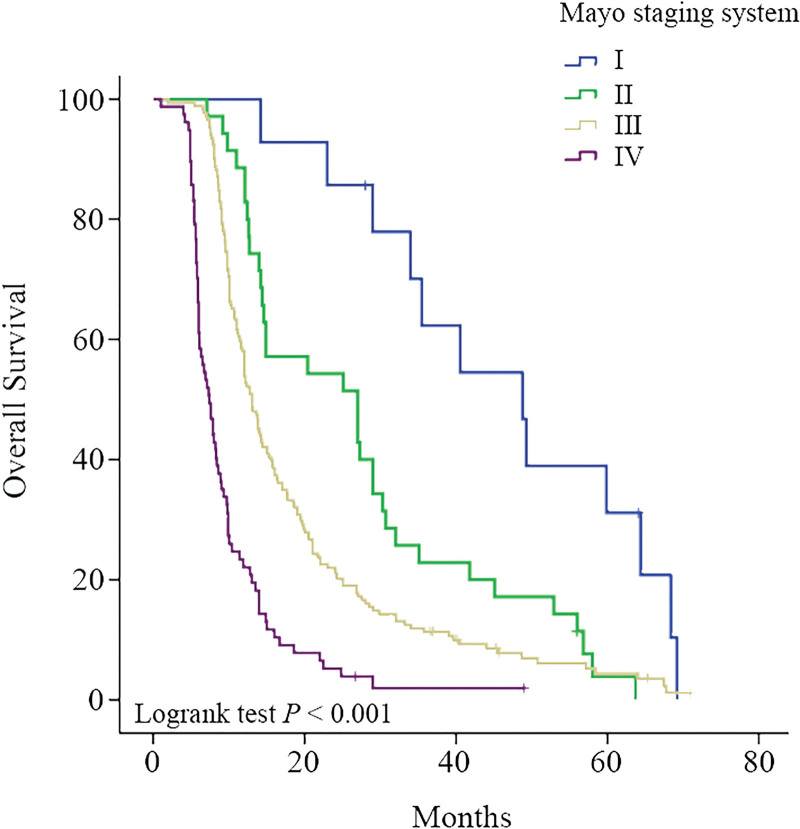
In the Mayo staging, there were 14 cases in stage I, 38 cases in stage II, 169 cases in stage III, and 77 cases in stage IV. The median survival time was 48.8 (20.7‐76.9) months, 25.1 (15.1‐35.1) months, 13.0 (11.8‐14.2) months and 7.4 (6.1‐8.7) months. The Kaplan–Meier survival curve and Log-rank test results (P < .001, Fig. 2) of Mayo staging showed that Mayo staging can be used to predict prognosis. By comparing the groups (Table 3), P < .05 showed statistical significance, indicating that the discrimination and single trend of each stage in the Mayo staging (Table 4) were statistically significant.
Figure 2.
The Kaplan–Meier survival curve and Log-rank test results (P < .001) of Mayo staging system showed that Mayo staging system can be used to predict prognosis.
Table 3.
Intergroup comparison of Mayo staging system.
| Mayo staging system | I | II | III | IV |
|---|---|---|---|---|
| Log-rank I | ||||
| Log-rank II | <0.001 | |||
| Log-rank III | <0.001 | 0.008 | ||
| Log-rank IV | <0.001 | <0.001 | <0.001 |
Table 4.
Comparison of the internal survival rate of Mayo staging system (the degree of differentiation and single trend).
| Mayo staging system (n) | Median survival in months (95% CI) | P value |
|---|---|---|
| I (14) | 48.8 (20.7–76.9) | <.001*** |
| II (38) | 25.1 (15.1–35.1) | .008** |
| III (166) | 13.0 (11.8–14.2) | <.001* |
| IV (77) | 7.4 (6.1–8.7) |
P < .05,
P < .01,
P < .001.
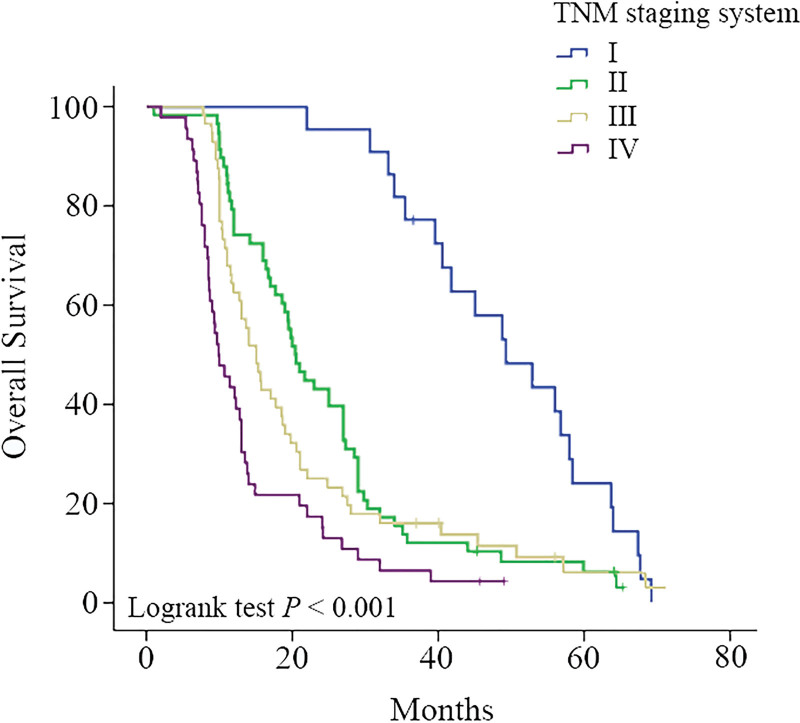
A total of 182 patients underwent surgical resection and had complete pathological data. The staging was performed according to the standard of the 8th edition of the TNM staging system, including 22 cases of stage I, 57 cases of stage II, 56 cases of stage III, and 47 cases of stage IV. The median survival time was 49.3 (37.8‐60.8) months, 20.5 (16.8‐24.2) months, 15.0 (12.6‐17.4) months, and 10.0 (7.3‐12.7) months. Kaplan–Meier survival curves and Log-rank test results of TNM staging showed that TNM staging can be used to predict prognosis (P < .001, Fig. 3). However, by comparing the groups internally (Table 5), there was no statistically significant difference between stage II and stage III (P = .173), indicating that the discrimination of TNM staging was not ideal for each stage (Table 6).
Figure 3.
The Kaplan–Meier survival curve and Log-rank test results (P < .001) of TNM staging system showed that TNM staging system can be used to predict prognosis.
Table 5.
Intergroup comparison of TNM staging system.
| TNM staging system | I | II | III | IV |
|---|---|---|---|---|
| Log-rank I | ||||
| Log-rank II | <0.001 | |||
| Log-rank III | <0.001 | 0.173 | ||
| Log-rank IV | <0.001 | <0.001 | 0.006 |
Table 6.
Comparison of the internal survival rate of TNM staging system (the degree of differentiation and single trend).
| TNM staging system (n) | Median survival in months (95% CI) | P value |
|---|---|---|
| I (22) | 49.3 (37.8–60.8) | <.001*** |
| II (57) | 20.5 (16.8–24.2) | .173 |
| III (56) | 15.0 (12.6–17.4) | .006** |
| IV (47) | 10.0 (7.3–12.7) |
P < .01,
P < .001.
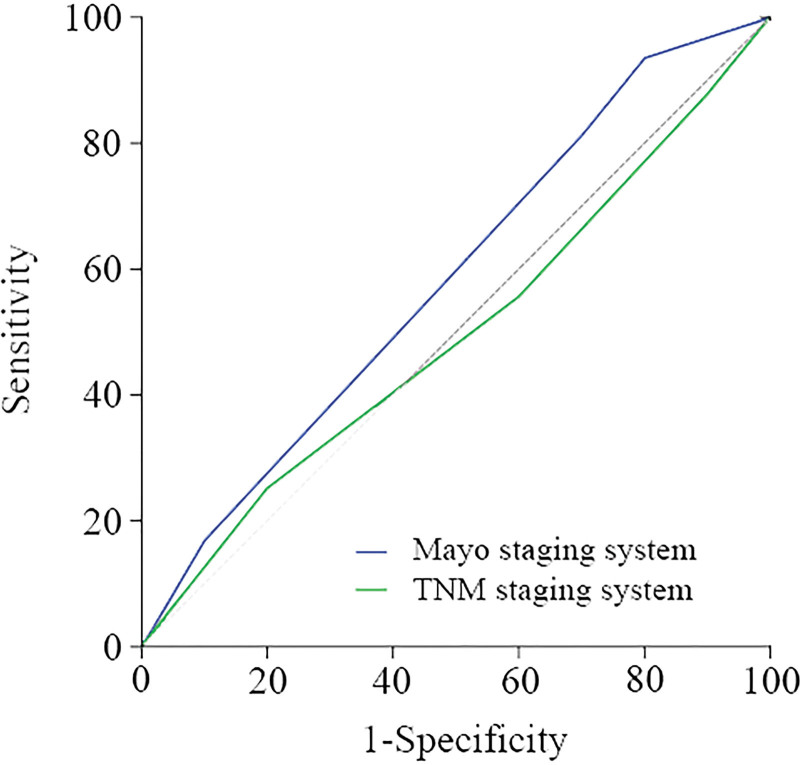
A total of 295 patients underwent Mayo staging through preoperative imaging data and laboratory results, while the TNM staging system only staged postoperative cases, a total of 182 cases. Through the two-stage survival curves, it can be found that both stages are related to survival, and the later the stage, the worse the prognosis (P < .001). In this study, there was no statistically significant difference between stage II and III (P = .173) in TNM staging. A single trend analysis of the prognostic assessment capabilities of the two staging systems (Fig. 4) revealed that the Mayo staging had the largest area under the ROC curve (AUC = 0.587), which was greater than the ROC curve area of the TNM staging (AUC = 0.501) (Table 7).
Figure 4.
A single trend analysis of the prognostic assessment capabilities of the Mayo staging system and the TNM staging system under the ROC curve. ROC = receiver operating characteristic.
Table 7.
Single trend analysis of two staging systems.
| The curve area of ROC (95% CI) | |
|---|---|
| Mayo staging system | 0.587 (0.398–0.777) |
| TNM staging system | 0.501 (0.329–0.674) |
ROC = receiver operating characteristic.
4. Discussion
There are many staging systems for HCCA, but so far there is still a lack of a widely accepted staging system. There are currently only three general clinical stages or classifications for HCCA: Bismuth‐Corlette typing,[9] MSKCC T staging[6] and TNM staging. This study found that Bismuth‐Corlette typing (P < .001) is an independent prognostic factor for the prognosis of HCCA, but the authors believe that the main cause of prognosis is surgery-related. Because HCCA is not sensitive to radiotherapy and chemotherapy,[17,18] the treatment of HCCA in China is mainly surgical resection. Among them, radical resection (21 months) was significantly better than palliative resection (13.8 months) and palliative biliary drainage (8.7 months) in median survival, which is the same as other studies.[19–21] Because Bismuth‐Corlette typing is simple to operate, it is widely used in clinical practice to guide surgical treatment, improve R0 resection rate, and achieve the purpose of prolonging survival. However, some foreign scholars used the Bismuth‐Corlette typing criteria to classify 230 patients with resectable HCCA. It was found that hepatectomy combined with caudate lobe resection can improve R0 resection rate regardless of the typing.[22] The MSKCC T staging was not included in this study. The main reason is that the definition of hepatic atrophy in the staging factors is unclear and cannot be embodied. The practical value in clinical work is obviously limited. TNM staging can be used to predict prognosis (P < .001), showing better predictive performance than previous versions,[23] but only based on postoperative pathology results. Studies have shown that TNM staging is not associated with overall survival in patients with HCCA who can be treated surgically.[24] To this end, we collected relevant data on HCCA patients who were hospitalized at the Affiliated Hospital of Qingdao University to research, which 335 patients could be used for Mayo staging, and excluded 40 patients who did not follow up the data or who refused to participate in the study. There were 295 patients in this study. Among them, 182 were treated with surgery, 96 with R0 resection, 48 with R1 resection, and 38 with R2 resection. There were 113 cases of palliative biliary drainage, including 52 cases of ERBD and 61 cases of PTCD.
We found tumors with a maximum diameter > 3 cm (HR: 1.335, 95% CI: 1.006‐1.772), tumor metastasis (HR: 2.596, 95% CI: 1.160‐2.197), and ECOG score (HR: 1.837, 95% CI): 1.436‐2.349) is an independent risk factor for prognosis in this research, which is consistent with the stratification principle of Mayo staging system. A study of 331 patients with HCCA by Regimbeau et al showed that patients with tumors larger than 3 cm in diameter died within 1 year.[25] The median survival of patients with tumor diameter > 3 cm in this study was 10.6 (9.6‐11.6) months. ECOG is a simplified activity status score table. The patient’s activity status is divided into 0 to 5 levels, a total of 6 levels, which can be used to evaluate the overall status of patients. It has been widely used in our department. Chaiteerakij et al[13] considered it to be the strongest predictor, and the predicted value of ECOG scores of 3 and 4 was higher than tumor metastasis.
Serum albumin is an important indicator used in our clinical evaluation of nutritional status. The study found that patients with albumin ≥ 35 g/L had significantly higher survival than patients with albumin < 35 g/L. Clugston et al[26] studied malnutrition in patients with obstructive jaundice and found that protein malnutrition occurred before surgery, and the postoperative complication rate and mortality rate were higher than those with good nutritional status. Obstruction of the biliary tract leads to poor bile drainage, damages to the intestinal hepatic circulation and the liver, gastrointestinal tract, brain and other substantial organs, resulting in decreased food intake and reduced energy supply. The occurrence of complications such as biliary infection further aggravates the nutritional consumption of patients. At present, the monitoring of albumin index is mostly used for postoperative patients. Intravenous albumin supplementation can reduce tissue edema, promote anastomotic healing and other functions, and achieve rapid recovery of patients. Therefore, we consider strengthening the intravenous nutrition and supplementing albumin to maintain a high level during perioperative preparation, which can improve the patient’s tolerance to surgery, shorten recovery time and avoid complications.
CA19-9 ≥ 1000 U/mL (P = .003) was found to have an impact on prognosis in our research, but it was not an independent risk factor for prognosis (P = .129). CA19-9 is a tumor marker isolated from colon cancer cells by Koprowski in 1979.[27–29] Because it specifically binds to gastric and intestinal cancer cells, it is also called gastrointestinal cancer antigen. The amount of normal human tissue cells is extremely small. It is highly expressed in cholangiocarcinoma and it is used as a tumor marker for routine examination because of its high sensitivity. Domestic studies suggest that elevated CA19-9 can be used as an independent factor in cholangiocarcinoma, but most studies are based on operable patients.[7,30–34] Domestic scholars believe that the survival rate of preoperative CA19-9 level < 150 U/mL is significantly better than that of patients with high CA19-9 level.[35] Heimbach et al[36] found that CA 19-9 > 100 U/mL can be used as a predictor of recurrence in liver transplant patients by performing orthotopic liver transplantation in 65 patients with HCCA who cannot undergo surgical resection. Based on this study, 24 patients with CA19-9 ≥ 1000 and included in Mayo stage III had a median survival time of 12.0 (6.8‐17.2) months. This study suggests that CA19-9 ≥ 1000 U/mL can be used as a qualitative factor for cholangiocarcinoma. But further research is needed to decide it as a determinant of stage III in Mayo staging.
Vascular encasement (P = .037) and lymph node metastasis (P < .001) were risk factors for predicting patient survival in our study, but through Cox risk ratio model analysis, it can be seen that lymph node metastasis and vascular involvement are not independent risk factors for prognosis. This is the same as Professor Chaiteerakij et al,[13] but it still adds the number of tumors, vascular involvement, and lymph node metastasis to the stratification. Based on previous studies to consider the correlation between individual tumor characteristics,[37–39] it has high correlation with other factors. And there were only 10 patients with tumors ≥ 2, which is inherently biased and not statistically significant.
We performed Mayo staging on patients with HCCA by preoperative imaging data and CA19-9 levels and found that it can predict prognosis better (P < .001). It has a good stratification effect through the comparison of some layers in the period (P < .05), which can be used not only for operable patients but also for non-surgical patients. It is a staging system which is better than any current staging system for overall research. At present, none of the general staging systems are suitable for liver transplantation, liver transplantation in China is only a treatment for end-stage liver disease. Studies have shown that liver transplantation is the only possible cure for HCCA patients with locally advanced but not yet diffuse, with a 5-year survival rate of 25% to 42%.[40] Early literature reports on liver transplantation for patients with unresectable HCCA, with a 5-year survival rate of only 20% to 30%.[41] However, the Mayo Clinic in the United States through multidisciplinary collaboration, the 5-year survival rate of liver transplantation for HCCA can reach 72%.[42] We need a staging system for the treatment of HCCA to study liver transplantation with the maturity of liver transplantation technology. However, Bismuth‐Corlette typing and MSKCC T staging are only limited to the evaluation of surgical respectability and only to improve the R0 resection rate. The Mayo staging is applicable to the overall staging and can be used to evaluate liver transplants. However, this study is a single-center study that lacks the treatment of liver transplantation in HCCA therapy and therefore cannot be evaluated.
The study was grouped by imaging diagnosis, and a good staging effect was obtained. But the cases were mainly concentrated in stage III (56.3%), and stage 1,2 and 4 accounted for 5%, 13.9%, and 26.1%, respectively. It is not so ideal from the perspective of the staging system. However, it is consistent with the characteristics of HCCA with the current situation of HCCA in China. There were usually no obvious clinical symptoms in the early stage, and most of them were diagnosed due to symptoms such as upper abdominal pain, jaundice and other symptoms. The diagnosis was in the middle and late stages.[43–45] Therefore, the cohort study is concentrated on stage III and is also reasonable.
We used the comparison of the area under the ROC when evaluating the two staging systems in our research, showing the discrimination and single trend of the two staging systems. The results show that the curve area of Mayo staging system is larger than the TNM staging system, indicating that Mayo staging system is superior to TNM staging system in discriminative and single trend. It can be seen from the staging characteristics that the Mayo staging system owns staged data before surgery, and the TNM staging is limited to surgical pathological staging. The Mayo staging system is superior to TNM staging system whether from surgical guidance or evaluation. The diagnosis of HCCA has also received better technical support with the advancement of imaging technology. CT examination can well show the extent of lesions and bile duct expansion, and can clearly show portal vein embolism, lymph node metastasis, and peritoneal invasion. We can also access vascular encasement and variability by means of three-dimensional reconstruction of blood vessels,[46] which provides technical support for the implementation of Mayo staging. Some scholars have pointed out that the traditional pathological TNM staging system can be replaced by imaging TNM staging system as a gold standard for comparison. However, because the tumor depth pathological information (T1) and the tumor’s extension to the surrounding tissues (T2) are indistinguishable, only stage I (T1N0M0) and stage II (T2N0M0) can be combined. Based on this, Chaiteerakij et al[13] compared the imaging TNM staging and Mayo staging in the overall, liver transplantation group, surgical resection group, and biliary drainage group, which showed that the prognosis of Mayo staging was better than the imaging TNM staging. We underwent pathological TNM staging (P < .001) with postoperative pathological findings of 182 patients, indicating a statistical significance in prognosis. In summary, the Mayo staging system can better distinguish the prognosis of all patients, while the TNM staging system is less capable of distinguishing than the Mayo staging system. At the same time, Mayo staging is significantly better than TNM staging in predicting the accuracy of patient survival.
5. Conclusion
Mayo staging can be used for preoperative patient prognosis evaluation based on a single-center small sample study, and the predicted value is better than TNM staging, which can provide better stratification ability. Tumor metastasis, maximum tumor diameter > 3 cm and ECOG score in Mayo staging system are independent factors affecting prognosis, and whether CA19-9 ≥ 1000 U/mL can be used as a stage III evaluation index requires further study. Radical surgical resection and improvement of preoperative albumin levels can significantly improve the survival of patients with prognosis.
Acknowledgements
The authors thank members of Department of Hepatobiliary and Pancreatic Surgery in The Affiliated Hospital of Qingdao University for their suggestions and critiques to the manuscript.
Author contributions
Zhaowei Sun, Xiaozhi Sun, Qinlei Wang, Xiaoliang Kang and Guanghua Cao performed the experiments and wrote the manuscript. Jingyun Guo, Xueliang Li, Maobing Wang, Haochen Zhong and HaoRan Li analyzed the data and wrote the manuscript. Bingyuan Zhang revised the manuscript and illustrated all figures. Hao Zou and Yanan Yu provided experimental guidance. Zhaowei Sun and Fangzhen Shen designed the study. Bingyuan Zhang, Yujie Feng and Kai Ma provided the funding support, analyzed the data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conceptualization: Jingyun Guo, Xiaozhi Sun.
Data curation: Jingyun Guo.
Formal analysis: Jingyun Guo.
Funding acquisition: Jingyun Guo, Yanan Yu.
Investigation: Jingyun Guo.
Methodology: Guanghua Cao, Jingyun Guo, Menshou Chen, Qinlei Wang.
Project administration: Jingyun Guo, Na Su.
Resources: Jingyun Guo, Maobing Wang.
Software: Jingyun Guo.
Supervision: Jingyun Guo, Xueliang Li.
Validation: Haoran Li, Haochen Zhong, Jingyun Guo.
Visualization: Hao Zou, Jingyun Guo, Kai Ma.
Writing – original draft: Fangzhen Shen, Jingyun Guo, Zhaowei Sun.
Writing – review & editing: Bingyuan Zhang, Jingyun Guo, Yujie Feng.
Corrections
Xiaozhi Sun has been moved to the second author in the author byline.
The corresponding author was changed from “Yujie Feng, Department of Hepatobiliary Surgery, Affliated Hospital of Qingdao University, Jiangsu 16, Qingdao 26000, China (e-mail: fengyj1943@163.com)” to “Correspondence: Zhaowei Sun, Department of Hepatobiliary Surgery, Affiliated Hospital of Qingdao University, Jiangsu 16, Qingdao 26000, China (e-mail: sunzhaowei0920@163.com).”
The author contribution statement has been changed from “ZS, JG, and XL contributed equally to this work” to “ZS, XS, JG, and XL contributed equally to this work.”
Abbreviations:
- ECOG =
- Eastern Cooperative Oncology Group
- ERBD =
- endoscopic retrograde biliary drainage
- HCCA =
- hilar cholangiocarcinoma
- PTCD =
- percutaneous transhepatic cholangiography and drainage
- ROC =
- receiver operating characteristic
ZS, XS, JG, and XL contributed equally to this work.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
Written informed consent for publication was obtained from each patient.
The authors have no conflict of interest to disclose.
The Ethics Committee of the Affiliated Hospital of Qingdao University approved this study. Written informed consent for participation was obtained from each patient.
This study was supported by the grant from ToxM1 Regulates Treg Cells to Inhibit Immune Escape of Hilar Cholangiocarcinoma (grant number 3821), the National Natural Science Foundation of China (82103545) and the Shandong Provincial Natural Science Foundation, China (ZR2020QH217).
How to cite this article: Sun Z, Sun X, Guo J, Li X, Wang Q, Su N, Chen M, Cao G, Yu Y, Wang M, Li H, Zhong H, Zou H, Ma K, Shen F, Zhang B, Feng Y. Prognostic influence for hilar cholangiocarcinoma and comparisons of prognostic values of Mayo staging and TNM staging systems. Medicine 2022;101:49(e32250).
Contributor Information
Jingyun Guo, Email: 593880577@qq.com.
Xueliang Li, Email: 1071855297@qq.com.
Qinlei Wang, Email: 283687268@qq.com.
Na Su, Email: suna19880828@163.com.
Menshou Chen, Email: 2796107760@qq.com.
Guanghua Cao, Email: CGH9210@163.com.
Yanan Yu, Email: 329879232@qq.com.
Maobing Wang, Email: 283687268@qq.com.
Haoran Li, Email: 1071855297@qq.com.
Haochen Zhong, Email: ningfan327@163.com.
Hao Zou, Email: zh37759@163.com.
Kai Ma, Email: makai1989sd@163.com.
Fangzhen Shen, Email: fangzhenshen@126.com.
Bingyuan Zhang, Email: bingyuanzhang@126.com.
Xiaozhi Sun, Email: congcongxiaozhi@126.com.
Yujie Feng, Email: fengyj1943@163.com.
References
- [1].Cai JQ, Cai SW, Cong WM, et al. Diagnosis and treatment of cholangiocarcinoma: a consensus from surgical specialists of China. J Huazhong Univ Sci Technol Med Sci. 2014;34:469–75. [DOI] [PubMed] [Google Scholar]
- [2].Fabrega-Foster K, Ghasabeh MA, Pawlik TM, et al. Multimodality imaging of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khan AS, Dageforde LA. Cholangiocarcinoma. Surg Clin North Am. 2019;99:315315-+–335.. [DOI] [PubMed] [Google Scholar]
- [4].Khan SA, Miras A, Pelling M, et al. Cholangiocarcinoma and its management. Gut. 2007;56:1755–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lauterio A, De Carlis R, Centonze L, et al. Current surgical management of peri-hilar and intra-hepatic cholangiocarcinoma. Cancers (Basel). 2021;13:3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5:824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li B, Xiong XZ, Zhou Y, et al. Prognostic value of lymphovascular invasion in Bismuth-Corlette type IV hilar cholangiocarcinoma. World J Gastroenterol. 2017;23:6685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zaydfudim VM, Clark CJ, Kendrick ML, et al. Correlation of staging systems to survival in patients with resected hilar cholangiocarcinoma. Am J Surg. 2013;206:159–65. [DOI] [PubMed] [Google Scholar]
- [11].Gazzaniga GM, Faggioni A, Filauro M. Surgical treatment of proximal bile duct tumors. Int Surg. 1985;70:45–8. [PubMed] [Google Scholar]
- [12].Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–71. [DOI] [PubMed] [Google Scholar]
- [13].Chaiteerakij R, Harmsen WS, Marrero CR, et al. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014;109:1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Azad AI, Rosen CB, Taner T, et al. Selected patients with unresectable perihilar cholangiocarcinoma (pCCA) derive long-term benefit from liver transplantation. Cancers (Basel). 2020;12:3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB. 2015;17:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Miyazaki M, Yoshitomi H, Miyakawa S, et al. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepato Biliary Pancr Sci. 2015;22:249–73. [DOI] [PubMed] [Google Scholar]
- [17].Kamarajah SK, Al-Rawashdeh W, Parente A, et al. Adjuvant chemotherapy for perihilar cholangiocarcinoma: a population-based comparative cohort study. Eur J Surg Oncol. 2021;48:1300–8. [DOI] [PubMed] [Google Scholar]
- [18].Kamarajah SK, Al-Rawashdeh W, White SA, et al. Adjuvant radiotherapy improves long-term survival after resection for gallbladder cancer A population-based cohort study. Eur J Surg Oncol. 2022;48:425–34. [DOI] [PubMed] [Google Scholar]
- [19].Cillo U, Fondevila C, Donadon M, et al. Surgery for cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Benzing C, Haiden LM, Krenzien F, et al. Textbook outcome after major hepatectomy for perihilar cholangiocarcinoma - definitions and influencing factors. Langenbecks Arch Surg. 2022;407:1561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Conci S, Vigano L, Ercolani G, et al. Outcomes of vascular resection associated with curative intent hepatectomy for intrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2020;46:1727–33. [DOI] [PubMed] [Google Scholar]
- [22].Washington K, Rocha F. Approach to resectable biliary cancers. Curr Treat Options Oncol. 2021;22:97. [DOI] [PubMed] [Google Scholar]
- [23].Kim Y, Moris DP, Zhang XF, et al. Evaluation of the 8th edition American Joint Commission on Cancer (AJCC) staging system for patients with intrahepatic cholangiocarcinoma: a surveillance, epidemiology, and end results (SEER) analysis. J Surg Oncol. 2017;116:643–50. [DOI] [PubMed] [Google Scholar]
- [24].Cheng Z, Lei Z, Shen F. Coming of a precision era of the staging systems for intrahepatic cholangiocarcinoma? Cancer Lett. 2019;460:10–7. [DOI] [PubMed] [Google Scholar]
- [25].Regimbeau JM, Fuks D, Pessaux P, et al. Tumour size over 3 cm predicts poor short-term outcomes after major liver resection for hilar cholangiocarcinoma. By the HC-AFC-2009 group. HPB. 2015;17:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Clugston A, Paterson HM, Yuill K, et al. Nutritional risk index predicts a high-risk population in patients with obstructive jaundice. Clin Nutr. 2006;25:949–54. [DOI] [PubMed] [Google Scholar]
- [27].Itzkowitz SH, Yuan M, Fukushi Y, et al. Immunohistochemical comparison of Lea, monosialosyl Lea (CA 19-9), and disialosyl Lea antigens in human colorectal and pancreatic tissues. Cancer Res. 1988;48:3834–42. [PubMed] [Google Scholar]
- [28].Iwase K, Kato K, Nagasaka A, et al. Immunohistochemical study of neuron-specific enolase and CA 19-9 in pancreatic disorders. The value of neuron-specific enolase as a marker for islet cell and nerve tissue. Gastroenterology. 1986;91:576–80. [DOI] [PubMed] [Google Scholar]
- [29].Zhang YM, Yang J, Li HJ, et al. Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med. 2015;8:11683–91. [PMC free article] [PubMed] [Google Scholar]
- [30].Deng YW, Zhong RH, Xie XY, et al. Serum CEA, CA125, CA19-9, and CA724 levels for the diagnosis and staging of cholangiocarcinoma. Biomed Res India. 2017;28:1413–8. [Google Scholar]
- [31].Fisher A, Theise ND, Min A, et al. CA19-9 does not predict cholangiocarcinoma in patients with primary sclerosing cholangitis undergoing liver transplantation. Liver Transpl Sur. 1995;1:94–8. [DOI] [PubMed] [Google Scholar]
- [32].Izquierdo-Sanchez L, Lamarca A, La Casta A, et al. Cholangiocarcinoma landscape in Europe: diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol. 2022;76:1109–21. [DOI] [PubMed] [Google Scholar]
- [33].Juntermanns B, Kaiser GM, Gutierrez SI, et al. CA19-9 in intrahepatic cholangiocarcinoma. A diagnostic and prognostic armamentarium? Chirurg. 2018;89:466–71. [DOI] [PubMed] [Google Scholar]
- [34].Li YG, Zhang N. Clinical significance of serum tumour M2-PK and CA19-9 detection in the diagnosis of cholangiocarcinoma. Dig Liver Dis. 2009;41:605–8. [DOI] [PubMed] [Google Scholar]
- [35].Lee JW, Lee JH, Park Y, et al. Prognostic impact of perioperative CA19-9 levels in patients with resected perihilar cholangiocarcinoma. J Clin Med. 2021;10:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Heimbach JK, Gores GJ, Haddock MG, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–7. [DOI] [PubMed] [Google Scholar]
- [37].Nitta N, Ohgi K, Sugiura T, et al. Prognostic impact of paraaortic lymph node metastasis in extrahepatic cholangiocarcinoma. World J Surg. 2021;45:581–9. [DOI] [PubMed] [Google Scholar]
- [38].Roy S, Banerjee P, Ekser B, et al. Targeting lymphangiogenesis and lymph node metastasis in liver cancer. Am J Pathol. 2021;191:2052–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kiriyama M, Ebata T, Aoba T, et al. Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br J Surg. 2015;102:399–406. [DOI] [PubMed] [Google Scholar]
- [40].Tan EK, Taner T, Heimbach JK, et al. Liver transplantation for peri-hilar cholangiocarcinoma. J Gastrointest Surg. 2020;24:2679–85. [DOI] [PubMed] [Google Scholar]
- [41].Sapisochin G, Fernandez de Sevilla E, Echeverri J, et al. Liver transplantation for cholangiocarcinoma: current status and new insights. World J Hepatol. 2015;7:2396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rea DJ, Rosen CB, Nagorney DM, et al. Transplantation for cholangiocarcinoma: when and for whom? Surg Oncol Clin N Am. 2009;18:325–37, ix. [DOI] [PubMed] [Google Scholar]
- [43].Grimsrud MM, Folseraas T. Pathogenesis, diagnosis and treatment of premalignant and malignant stages of cholangiocarcinoma in primary sclerosing cholangitis. Liver Int. 2019;39:2230–7. [DOI] [PubMed] [Google Scholar]
- [44].Ney A, Garcia-Sampedro A, Goodchild G, et al. Biliary strictures and cholangiocarcinoma - Untangling a diagnostic conundrum. Front Oncol. 2021;11:699401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ghidini M, Pizzo C, Botticelli A, et al. Biliary tract cancer: current challenges and future prospects. Cancer Manag .Res 2019;11:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang H, Zhu J, Ke F, et al. Radiological imaging for assessing the respectability of hilar cholangiocarcinoma: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:497942. [DOI] [PMC free article] [PubMed] [Google Scholar]