Abstract
Mycobacterium bovis bacillus Calmette-Guérin (BCG) is the only vaccine approved for prevention of tuberculosis. It has been postulated that serial passage of BCG over the years may have resulted in attenuation of its effectiveness. Because interleukin-12 (IL-12) and oligodeoxynucleotides (ODN) containing cytidine phosphate guanosine (CpG) motifs have been shown to enhance Th1 responses in vivo, they were chosen as adjuvants to increase the effectiveness of BCG vaccination. In this report, mice were vaccinated with BCG with or without IL-12 or CpG ODN and then challenged 6 weeks later via the aerosol route with the Erdman strain of M. tuberculosis. Mice vaccinated with BCG alone showed a 1- to 2-log reduction in bacterial load compared with control mice that did not receive any vaccination prior to M. tuberculosis challenge. Moreover, the bacterial loads of mice vaccinated with BCG plus IL-12 or CpG ODN were a further two- to fivefold lower than those of mice vaccinated with BCG alone. As an immune correlate, the antigen-specific production IFN-γ and mRNA expression in spleen cells prior to challenge were evaluated. Mice vaccinated with BCG plus IL-12 or CpG ODN showed enhanced production of IFN-γ compared with mice vaccinated with BCG alone. Finally, granulomas in BCG-vaccinated mice were smaller and more lymphocyte rich than those in unvaccinated mice; however, the addition of IL-12 or CpG ODN to BCG vaccination did not alter granuloma formation or result in added pulmonary damage. These observations support a role for immune adjuvants given with BCG vaccination to enhance its biologic efficacy.
Mycobacterium tuberculosis infection is one of the most significant causes of mortality worldwide. Since the onset of the human immunodeficiency virus-AIDS epidemic, awareness of M. tuberculosis infection has resurfaced, as it is now a major cause of mortality for human immunodeficiency virus-infected individuals. Furthermore, the evolution of multidrug-resistant strains represents a significant health problem among normal, nonimmunocompromised individuals. The World Health Organization has recently declared the current situation to be a global emergency and has made it a priority to develop more effective vaccines against M. tuberculosis.
The efficacy of M. bovis bacillus Calmette-Guérin (BCG) as a vaccine is dependent on many factors and determined clinically by its ability to prevent pulmonary or systemic disease following exposure to M. tuberculosis. Because the protective efficacy of BCG may range from 0 to 80% worldwide (18), it is necessary to determine the correlates of protection and exploit these issues in future vaccination attempts. For example, it was recently reported that the continued propagation of BCG over the past 60 years has lead to an evolution of BCG substrains marked by deletions in the parental M. bovis genome (1). Although the deletions do not include known bacterial virulence factors, the strain diversity may account for the attenuation or relative lack of efficacy against pulmonary disease. Identification of these missing genes may be an essential component of improvement of BCG efficacy.
Other vaccine approaches for tuberculosis prevention include vaccination of mice with plasmid DNA encoding specific M. tuberculosis antigen. These vaccines can substantially reduce the mycobacterial load following infectious challenge; however, the protection achieved by this type of vaccination has not proven any more effective than that achieved by vaccination with BCG alone. Thus, BCG must be considered a standard against which other vaccines should be tested in the murine model. Due to its longstanding safety profile in humans, it is likely that a future large-scale clinical efficacy trial for vaccination against M. tuberculosis will include BCG in some capacity as a control.
It is well established that the type 1 cytokines interleukin-12 (IL-12) and gamma interferon (IFN-γ) are essential for control of mycobacterial infection in both mice and humans (3, 5, 6). Although BCG can elicit type 1 immune responses, it is possible that the magnitude of this response has diminished over the years. To address this concern, we attempted to enhance the host immune response against M. tuberculosis by delivering immune adjuvants with a given strain of BCG. In this report, both IL-12 and synthetic oligodeoxynucleotides (ODN) containing cytidine phosphate guanosine (CpG) motifs were examined for the ability to enhance the efficacy of BCG vaccination by increasing the magnitude of the Th1 response prior to infectious challenge.
MATERIALS AND METHODS
Bacteria.
M. tuberculosis Erdman (TMC 107) and M. bovis BCG Pasteur strain (TMC 1011) were obtained from the Trudeau Mycobacterial Culture Collection. They were grown in Middlebrook 7H9 broth (Difco) enriched with 10% ADC additive (Becton Dickinson) and 0.05% Tween 80 (Sigma Chemical Co.) in roller bottles rotating at 4 rpm for 8 days at 37°C (4). The logarithmic-phase growth was enriched with 10% glycerol and stored at −70°C (12). The viability of the suspension was checked 24 h after freezing by plating on Middlebrook 7H11 agar and incubation at 37°C for 21 days in sealed plastic bags, at which time CFU were counted.
Vaccination.
BALB/c mice were vaccinated via the subcutaneous s.c., intranasal (i.n.), or intravenous i.v. route with various amounts of BCG (Pasteur strain). The method used for i.n. delivery of fluid has been previously described (16). At the same time, groups of mice were given a single intraperitoneal injection of IL-12 protein (500 ng) (Genetics Institute) i.p. or 50 μg of CpG ODN. (The route of delivery of CpG ODN varied in different experiments as outlined in the figure legends.) Mice were housed for 6 weeks until administration of the infectious challenge.
Oligonucleotides.
Two immunostimulatory CpG-containing ODN with the sequences GCTAGACGTTAGCGT and TCAACGTT, as well as control ODN from which the CpG motifs were eliminated by inversion (GCTAGAGCTTAGGCT and TCAAGCTT), were synthesized as previously described (13). All ODN were produced on the same synthesizer and purified by extraction with phenol-chloroform-isoamyl alcohol (25:24:1), followed by ethanol precipitation. All were endotoxin free. All ODN were administered at a dose of 50 μg per mouse.
Cytokine analysis by ELISA.
Spleen cells were harvested from vaccinated animals (6 weeks postvaccination) prior to infectious challenge. Cells (3 × 105/200 μl) were cultured in the presence of M. tuberculosis (H37Ra strain, 1 × 105/200 μl) or heat-killed BCG (1 × 105/200 μl) for 48 h at 37°C. Antigen-specific production of IFN-γ was measured in culture supernatants by specific enzyme-linked immunosorbent assay (ELISA) methods.
Cytokine analysis by reverse transcription-PCR.
Cytokine mRNA levels were determined by semiquantitative reverse transcription-PCR techniques. In brief, total RNA was isolated from spleen cells using Trizol methods (Gibco-BRL). Total RNA was reverse transcribed by avian myeloblastosis virus reverse transcriptase (Promega). Transcribed RNA (250 μg) was used for specific semiquantitative amplification of cytokine mRNA with Taq DNA polymerase (Promega) and specific cytokine sense and antisense primers as previously described (8). Southern transfers of PCR products were subsequently probed with internal cytokine-specific oligonucleotides and visualized using the ECL chemiluminescence detection system (Amersham Corp.).
Infectious challenge.
A frozen ampoule of M. tuberculosis (Erdman strain) was thawed and shaken on a Vortex shaker for 10 s. The suspension was diluted in 0.05% Tween saline to 106 CFU/ml, which previous studies had shown to infect mice, with 100 to 500 CFU of strain Erdman using a Middlebrook chamber (Glas-Col) as previously described (4). In some experiments, mice were challenged with 105 CFU of strain Erdman by tail vein injection.
Quantitation of M. tuberculosis.
Lungs were harvested 6 weeks following the infectious challenge with M. tuberculosis. Tissue was homogenized in cold 0.05% Tween saline in a Seward Stomacher 80 blender (Tekmar) and plated onto Middlebrook agar containing 2.0 μg of thiophene carboxamic hydrazide per ml to inhibit the grown of BCG. The plates were incubated in sealed plastic bags for 21 days at 37°C, after which the CFU were counted. In each experiment, quantitation of M. tuberculosis was performed on lung homogenates from at least six mice per vaccination group.
Pathology stains.
The left lung was inflated with 10% formalin injected through a no. 26 needle. Routine paraffin sections were prepared and stained with hematoxylin and eosin of Kinyoun's acid-fast stain. The number of granulomas per low-power (4× objective) field, granuloma size, and the qualitative appearance of granulomas were evaluated.
Error and statistical analysis.
A two-tailed, two-sample, equal-variance Student t test was applied to determine statistical significance between groups of vaccinated mice following infection with M. tuberculosis. Error bars represent the standard error of the mean.
RESULTS
Mice vaccinated with BCG plus IL-12 or CpG ODN have enhanced IFN-γ production compared with mice vaccinated with BCG alone.
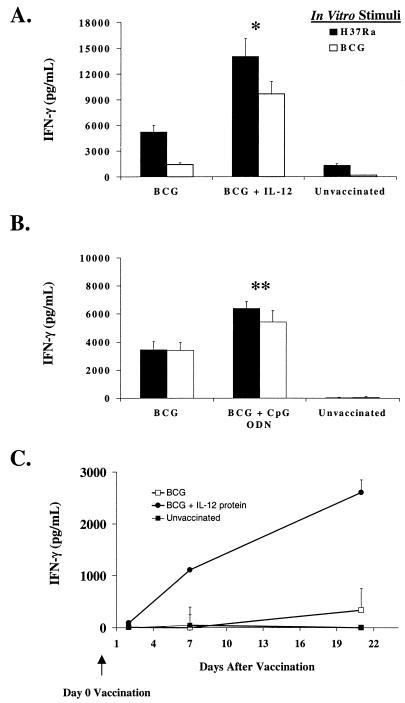
Because the magnitude of the Th1 response is critical in mediating protection against a variety of intracellular pathogens, we sought to optimize BCG vaccination by using immune adjuvants to enhance the Th1 response. BALB/c mice were vaccinated s.c. with BCG, BCG plus IL-12, or BCG plus CpG ODN. Six weeks later, antigen-specific production of IFN-γ was determined in cultured spleen cells. As shown in Fig. 1A, the addition of IL-12 to BCG caused a two- to fivefold increase in IFN-γ production following in vitro stimulation with either an avirulent strain of M. tuberculosis (H37Ra) or heat-killed BCG. Similarly, enhanced production of IFN-γ following vaccination with BCG plus IL-12 protein was seen as early as 1 week postvaccination (Fig. 1C). Finally, as seen in Fig. 1B, the addition of CpG ODN to BCG vaccination also increased the antigen-specific production of IFN-γ compared with that of mice receiving BCG alone, although this effect was less dramatic than that achieved by the addition of IL-12 protein.
FIG. 1.
IL-12 protein and CpG ODN enhance the in vitro production of IFN-γ induced by BCG vaccination. We pooled spleen cells (3 × 105/200 μl) from at least three individual mice vaccinated with BCG (s.c.) or BCG (s.c.) plus rIL-12 (i.p.) (A) or with BCG (s.c.) or BCG (s.c.) plus CpG ODNs (i.p.) (B) and cultured them in the presence of H37Ra (an avirulent strain of M. tuberculosis) or heat-killed BCG and determined the antigen-specific production of IFN-γ by a specific ELISA. (C) Spleen cells (3 × 105/200 μl) were taken from mice sacrificed at 2, 7, and 21 days postvaccination with BCG or BCG plus IL-12 and assessed for antigen-specific production of IFN-γ following in vitro stimulation with H37Ra. Data are shown after subtraction of the IFN-γ production from splenocytes cultured in medium alone (<100 pg/ml). ∗, P < 0.02; ∗∗, P < 0.05 compared with BCG alone.
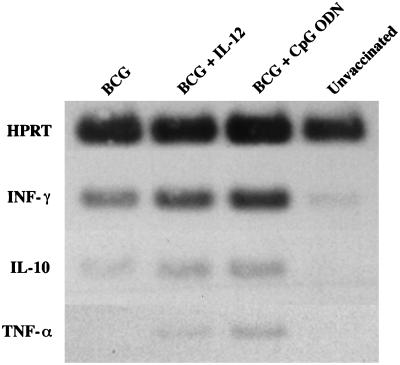
As an in vivo correlate, we also examined the effect of IL-12 and CpG ODN on the mRNA expression of various cytokines. As shown in Fig. 2, the addition of IL-12 or CpG ODN to BCG vaccination increased mRNA expression for IFN-γ but had little effect on IL-10 or tumor necrosis factor alpha. In addition, there was no difference in the expression of mRNA for IL-12 p40 (data not shown). Taken together, these data suggest that IL-12 or CpG ODN can enhance the magnitude of Th1 priming induced by BCG vaccination prior to challenge with M. tuberculosis.
FIG. 2.
Expression of mRNA for cytokines following vaccination with BCG plus IL-12 or CPG ODN. mRNA was isolated from pooled splenocytes (combined from at least three individual mice) at 6 weeks postvaccination of mice with BCG (s.c.), BCG (s.c.) plus IL-12 (i.p.), or BCG (s.c.) plus CpG ODN (i.p.). As a control, mRNA from unvaccinated mice was also prepared. Cytokine mRNA was subsequently determined for IFN-γ, IL-10, and tumor necrosis factor alpha (TNF-α) by semiquantitative reverse transcription (RT)-PCR as described in Materials and Methods. HPRT, hypoxanthine phosphoribosyltransferase.
Vaccination with BCG plus IL-12 reduced the mycobacterial burden in lungs of mice following aerosol challenge with virulent M. tuberculosis.
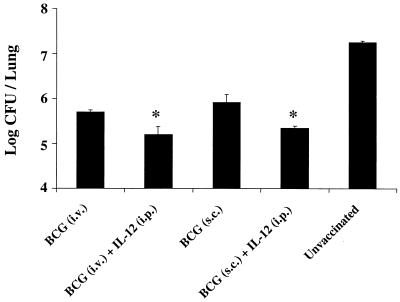
Because the magnitude of the IFN-γ response often correlates with immunity against intracellular infection, the previous figures suggest that mice vaccinated with BCG plus IL-12 or CpG ODN may have increased protection following infectious challenge. To determine the biologic relevance of increased IFN-γ production prior to infection, mice were challenged with live, virulent M. tuberculosis via the aerosol route 6 weeks after vaccination. The mycobacterial burden in the lungs 6 weeks after challenge was then assessed. Consistent with previous data (10, 21), mice vaccinated i.v. or s.c. with BCG had a 1- to 2-log bacterial load decrease compared with unvaccinated mice. Moreover, mice vaccinated with BCG plus IL-12 showed a two- to fivefold further bacterial load reduction compared with mice vaccinated with BCG alone (Fig. 3). This trend was seen in other experiments as early as 4 weeks postinfection (data not shown).
FIG. 3.
Mice vaccinated with BCG plus IL-12 protein have decreased mycobacterial loads following an infectious challenge. BALB/c mice were initially vaccinated (n = 6 to 10/group) with BCG (106) either i.v. or s.c., with or without a single i.p. injection of IL-12 (500 ng). Six weeks later, mice were challenged with 500 virulent M. tuberculosis bacteria via the aerosol route. Six weeks postinfection, lungs were harvested from individual mice (n = 6) and numbers of CFU per lung were quantitated as described in Materials and Methods. These results were obtained in at least five different experiments. ∗, P < 0.03 compared with BCG alone.
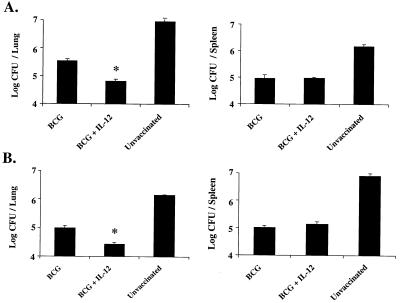
The ability of IL-12 protein to enhance immunity against M. tuberculosis when given with BCG vaccination is in contrast to the results obtained in a previous study (7). That study differed from ours in that the infectious challenge was given i.v. rather than aerogenically and repeated doses of IL-12 were given with BCG. In addition, mortality was used as a biologic endpoint. To address these apparent inconsistencies, we tested the efficacy of BCG with and without IL-12 following an infectious challenge by both aerosol and systemic routes. As shown in Fig. 4A, consistent with the data shown above, mice vaccinated with BCG plus IL-12 had a fivefold reduction (P < 0.01) in mycobacterial burden compared with mice vaccinated with BCG alone following an aerosol challenge. In contrast, there was essentially no difference in the mycobacterial burden in the spleens of these mice compared with those of mice receiving BCG alone. Similarly, there was a fivefold reduction (P < 0.01) in the mycobacterial burden in the lungs but not the spleens of mice vaccinated with BCG plus IL-12 compared with BCG alone following i.v. challenge (Fig. 4B). Taken together, these data show that a single dose of IL-12 with BCG is effective at reducing the mycobacterial load in the lungs following a systemic or aerosol challenge. In contrast, IL-12 does not affect the mycobacterial load in spleens following a systemic or aerosol challenge.
FIG. 4.
Mice vaccinated with BCG plus IL-12 have reduced mycobacterial loads in the lungs but not in the spleen following an aerosol or i.v. challenge. BALB/c mice were initially vaccinated (n = 6 to 10/group) with 106 BCG bacteria (s.c.) with or without a single i.p. injection of IL-12 (500 ng). Six weeks later, mice were challenged with 500 virulent M. tuberculosis bacteria via the aerosol route (A) or 105 virulent M. tuberculosis bacteria i.v. (B). Six weeks postinfection, spleens or lungs of individual mice (n = 6) were harvested and numbers of CFU per organ were quantitated. ∗, P < 0.01 compared with BCG alone.
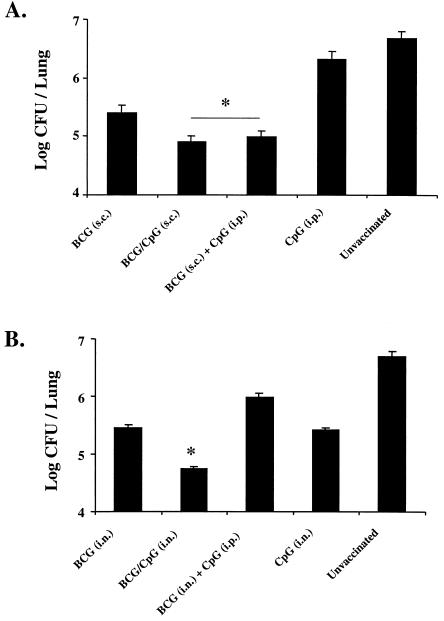
Mice vaccinated with BCG plus CpG ODN have reduced pulmonary mycobacterial burdens following aerosol challenge with virulent M. tuberculosis.
In a separate experiment, the efficacy of CpG ODN as a vaccine adjuvant with BCG was also assessed for the ability to alter the mycobacterial burden in the lungs following infection (Fig. 5). Similar to the results obtained as described above using IL-12 protein, mice vaccinated with BCG plus CpG ODN had a two- to fivefold bacterial load reduction compared with mice receiving BCG alone. This effect was similar whether the CpG ODN was given in the same s.c. injection as the BCG or given separately i.p. (Fig. 5A). As it may be necessary to direct immune cells to the site of infection for successful vaccination against M. tuberculosis, we also determined whether i.n. delivery of BCG with and without CpG ODN affected the biologic efficacy of BCG following infectious challenge. Mice receiving i.n. BCG alone showed a 1- to 2-log bacterial load reduction compared with unvaccinated mice. The bacterial loads of mice vaccinated with BCG plus CpG ODN in the same i.n. preparation were further reduced by nearly 1 log compared with those of mice vaccinated i.n. with BCG (Fig. 5B); however, vaccination with BCG i.n. plus CpG ODN at a separate site i.p. did not improve the efficacy of vaccination. Thus, CpG ODN can enhance the efficacy of BCG vaccination, provided that it is given at the same general site as BCG.
FIG. 5.
Mice vaccinated with BCG plus CpG ODN have decreased mycobacterial loads following infectious challenge. BALB/c mice were initially vaccinated (n = 6 to 10/group) with BCG (106 bacteria) s.c. with or without CpG ODN given either in the same shot or at a different site (i.p.) (∗, P < 0.05 compared with BCG alone) (A) or i.n. with or without CpG ODN given either in the same preparation i.n. with BCG or at a different site (i.p.) (∗, P < 0.01 compared with BCG alone) (B). Shown are numbers of CFU per lung calculated from lungs of six individual mice harvested 6 weeks after an aerosol challenge with a virulent strain of M. tuberculosis.
Pathologic effect of BCG vaccination.
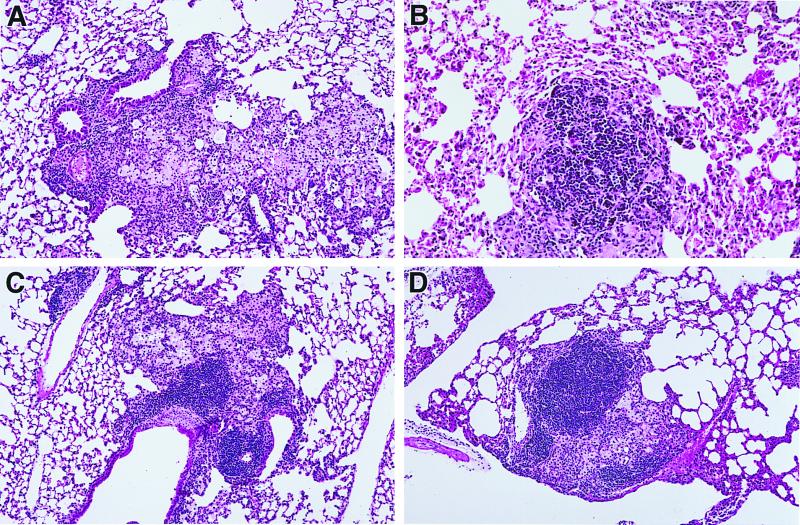
While IL-12 and CpG ODN diminished the infectious burden following challenge, the enhanced production of proinflammatory cytokines (i.e., IFN-γ) induced by these adjuvants raised the concern that this could be associated with greater lung pathology. To address this point, lungs from mice vaccinated and challenged with M. tuberculosis were harvested and processed to examine the histopathologic effects (cellular infiltrate, size and appearance of granulomas) induced by the various vaccine protocols. The numbers of granulomas were similar in all groups, but granulomas in BCG-vaccinated mice were consistently about one-half of the volume of those in unvaccinated mice (Fig. 6). Granulomas from vaccinated mice contained 20 to 40% lymphocytes, compared with approximately 15% in unvaccinated mice. The addition of IL-12 or CpG ODN to BCG vaccination did not further alter the size or appearance of granulomas, and no pulmonary damage was seen aside from the granulomas. Virtually all of the cells in the granulomas were lymphocytes or activated macrophages, and no necrosis was observed.
FIG. 6.
Addition of IL-12 or CpG ODN to BCG vaccination does not affect pulmonary granuloma formation. Mice were vaccinated, challenged with a virulent strain of M. tuberculosis, and sacrificed 6 weeks following infectious challenge. (A) Unvaccinated mouse; the granuloma is larger than that in vaccinated mice and is composed almost entirely of macrophages. (B) BCG-vaccinated mouse; the granuloma is smaller than that in the unvaccinated mouse, and small lymphocytes constitute a substantial portion of the cells. (C and D) Mice vaccinated with BCG plus IL-12 and BCG plus CpG ODN, respectively; the granulomas are similar to that shown in the BCG-vaccinated mouse.
DISCUSSION
Over the past 60 years, BCG has been widely used to control tuberculosis infection worldwide. BCG is given systemically and can confer protection against systemic or meningeal disease when exposure to M. tuberculosis occurs shortly after immunization. BCG is also inexpensive for use in underdeveloped countries. For these reasons, BCG remains useful and elimination of BCG vaccination is not feasible at this time. Of greater concern is why BCG is not universally effective at preventing adult pulmonary disease.
The increasing attenuation of BCG has been discussed in several reports. The diversity and variable efficacy of BCG were first noted by showing that clinical outcome appeared to correlate with specific strains of BCG rather than with purified protein derivative reactivity (M. A. Behr and P. M. Small, Letter, Nature 389:133–134, 1997). More recent publications suggest that gene deletions have allowed the evolution of BCG over time, so that a myriad of BCG strains are now used in different regions of the world (1). This attenuation of BCG through laboratory passaging may have limited its clinical efficacy, making it necessary to optimize this vaccine. To this end, numerous groups attempted to either enhance the effectiveness of BCG or explore other vaccine strategies, such as plasmid DNA vaccination (11, 14, 15, 22). With regard to DNA vaccination, while initial reports are promising, this approach has not proven to be more effective than BCG vaccination itself. This prompted us to evaluate whether BCG vaccination could be optimized.
The use of cytokines to enhance the efficacy of BCG vaccination in the mouse model has been previously studied. Murray et al. constructed recombinant BCG strains that were able to secrete cytokines such as IL-2, granulocyte-macrophage colony-stimulating factor, and IFN-γ (17). While this study was an elegant proof of principle, it was not demonstrated whether these strains could improve protection following challenge with M. tuberculosis. The study by Flynn et al. (7) was similar in concept to our study in using IL-12 as an adjuvant with BCG. In that study, IL-12 provided no increase in protection compared with BCG alone. The differences highlighted above (endpoint of study, systemic versus pulmonary challenge, IL-12 dosage) may help explain the contrasting results outlined in this report. Furthermore, with regard to IL-12 dosage, in the study by Flynn et al., IL-12 was given continuously with BCG (7) rather than in a single injection as reported here. It is possible that the continued use of IL-12 with BCG led to more rapid clearance of BCG, thus limiting its immunogenicity and therefore its effectiveness.
The experiments reported here were designed to enhance the effectiveness of BCG vaccination in the mouse pulmonary model of M. tuberculosis infection. In many mouse models of intracellular infection, the magnitude of the Th1 response often correlates with the subsequent control of infection. These studies focused on the use of immune adjuvants such as IL-12 or CpG ODN with BCG as a means to further increase the quantitative aspect of the Th1 response induced by BCG vaccination. While both IL-12 and CpG ODN are potent inducers of Th1 responses, CpG ODN may offer some additional advantages over IL-12. For example, CpG ODN directly induce the production of IL-12, as well as other inflammatory cytokines (tumor necrosis factor alpha, IL-6) (13, 19), and have also been shown to directly enhance T-cell activation (2). The potential advantage of CpG ODN compared to IL-12 was shown in vivo using the mouse model of Leishmania major infection. In these studies, treatment of mice with CpG ODN several days following infectious challenge enabled mice to control the infection (23, 24). This contrasted with previous reports showing that IL-12 treatment must be initiated at the time of infection for it to confer protective immunity (9, 20).
While there did not appear to be any advantage in the use of CpG ODN over that of IL-12 protein as an immune adjuvant with BCG at 6 weeks postchallenge, preliminary data suggest that CpG ODN may be more effective at later time points (12 weeks postchallenge; data not shown). Furthermore, with regard to production of IFN-γ following vaccination, mice vaccinated with BCG plus IL-12 or CpG ODN had enhanced IFN-γ production and mRNA expression in the spleen prior to infection compared with mice vaccinated with BCG alone (Fig. 1 and 2). In contrast, we could not detect any mRNA for IFN-γ in lungs of mice vaccinated with BCG s.c. with or without IL-12 or CpG ODN (data not shown). These data are consistent with our finding that, following s.c. vaccination with BCG, BCG was detectable in the spleens but not in the lungs by quantitative culture (data not shown). It is notable that despite the increase in IFN-γ production in the spleens of mice vaccinated with BCG plus IL-12- or CPG ODN, the mycobacterial load reductions in the spleens following infection were comparable among all groups of vaccinated mice. These data are compatible with the notion that while the general types of immunity required for protection against tuberculosis (i.e., Th1) are similar between systemic disease and pulmonary disease, there are likely differences in the qualitative and quantitative aspects of the immune response between the spleen and the lungs. In this regard, there is a critical threshold of Th1 cytokine production for protection in a variety of intracellular infection models. Thus, due to the greater number of immune cells (i.e., T cells) in the spleen compared with the lungs, the threshold of IFN-γ required for a significant mycobacterial load reduction in the spleen is reached by BCG alone. By contrast, due to the relative paucity of T cells in the lungs compared with the spleen, an increase in the number of IFN-γ-producing cells induced by BCG and IL-12 or CpG ODN would have a greater effect in reducing mycobacterial loads in the lungs following an infectious challenge. We postulate that following s.c. vaccination with BCG plus IL-12 or CpG ODN, an increase in the number of tuberculosis-specific IFN-γ-producing cells is generated in the spleen. These cells then migrate to the lungs following an infectious challenge, causing a reduction in mycobacterial growth.
This report shows that the addition of immune adjuvants can increase the magnitude of the Th1 response induced by BCG vaccination and reduce the pulmonary bacterial load after a challenge with M. tuberculosis. Because BCG remains the standard for protection in the mouse model, we believe that any consistent improvement over BCG alone represents a potentially significant finding. The real issue is whether this approach will offer increased protection against disease in humans. In addition, we are investigating whether the effects of addition of IL-12 or CpG ODN to BCG remain durable over a period of time. Our experiments have focused on the use of combinations of BCG, immune adjuvants, and plasmid DNA encoding antigen not present in BCG as a way to further optimize vaccination against M. tuberculosis.
ACKNOWLEDGMENTS
We thank Brenda Rae Marshall for editorial assistance.
L.S. and G.B.M. are HHMI-NIH research scholars.
REFERENCES
- 1.Behr M A, Wilson M A, Gill W P, Salamon H, Schoolnik G K, Rane S, Small P M. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 2.Bendigs S, Salzer U, Lipford G B, Wagner H, Heeg K. CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999;29:1209–1218. doi: 10.1002/(SICI)1521-4141(199904)29:04<1209::AID-IMMU1209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Biron C A, Gazzinelli R T. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 4.Collins F M. Protection to mice afforded by BCG vaccines against an aerogenic challenge by three mycobacteria of decreasing virulence. Tubercle. 1985;66:267–276. doi: 10.1016/0041-3879(85)90064-9. [DOI] [PubMed] [Google Scholar]
- 5.Cooper A M, Magram J, Ferrante J, Orme I M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn J L, Goldstein M M, Triebold K J, Sypek J, Wolf S, Bloom B R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 8.Gazzinelli R T, Hieny S, Wynn T A, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinzel F P, Schoenhaut D S, Rerko R M, Rosser L E, Gately M K. Recombinant interleukin-12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 11.Kamath A T, Feng C G, Macdonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim T H, Kubica G P. Long-term preservation and storage of mycobacteria. Appl Microbiol. 1972;24:311–317. doi: 10.1128/am.24.3.311-317.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12, and IFN-γ. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowrie D B, Silva C L, Colston M J, Ragno S, Tascon R E. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine. 1997;15:834–838. doi: 10.1016/s0264-410x(97)00073-x. [DOI] [PubMed] [Google Scholar]
- 15.Lozes E, Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Vandenbussche P, Van Vooren J P, Drowart A, Ulmer J B, Liu M A. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine. 1997;15:830–833. doi: 10.1016/s0264-410x(96)00274-5. [DOI] [PubMed] [Google Scholar]
- 16.McCluskie M J, Davis H L. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J Immunol. 1998;161:4463–4466. [PubMed] [Google Scholar]
- 17.Murray P J, Aldovini A, Young R A. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guerin strains that secrete cytokines. Proc Natl Acad Sci USA. 1996;93:934–939. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche P W, Triccas J A, Winter N. BCG vaccination against tuberculosis: past disappointments and future hopes. Trends Microbiol. 1995;3:397–401. doi: 10.1016/s0966-842x(00)88986-6. [DOI] [PubMed] [Google Scholar]
- 19.Sparwasser T, Miethke T, Lipford G, Erdmann A, Hacker H, Heeg K, Wagner H. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-α-mediated shock. Eur J Immunol. 1997;27:1671–1679. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 20.Sypek J P, Chung C L, Mayor S E H, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin-12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 22.Ulmer J B, Liu M A, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Content J, Huygen K. Expression and immunogenicity of Mycobacterium tuberculosis antigen 85 by DNA vaccination. Vaccine. 1997;15:792–794. doi: 10.1016/s0264-410x(96)00255-1. [DOI] [PubMed] [Google Scholar]
- 23.Walker P S, Scharton-Kersten T, Krieg A M, Love-Homan L, Rowton E D, Udey M C, Vogel J C. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-γ-dependent mechanisms. Proc Natl Acad Sci USA. 1999;96:6970–6975. doi: 10.1073/pnas.96.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann S, Egeter O, Hausmann S, Lipford G B, Rocken M, Wagner H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]