Abstract
Background
Transbronchial biopsy (TBB) with endobronchial ultrasonography and a guide sheath (EBUS‐GS) is an effective examination tool for the diagnosis of lung cancer. Factors related to making the diagnosis are still not fully understood.
Methods
A total of 367 patients who underwent EBUS‐GS and were diagnosed with lung cancer in Saga University Hospital were investigated retrospectively. Clinical characteristics were compared between 244 patients who were diagnosed with lung cancer and 123 patients who were not diagnosed by TBB with EBUS‐GS but were diagnosed by other examinations.
Results
Size of target lesion, rate of patients with target lesion size ≥20 mm, presence of the bronchus sign, and detection by EBUS imaging were significantly associated with making the diagnosis (all p < 0.01). In patients whose lesion was detected by EBUS imaging, patients with positive findings within the lesion were significantly more often diagnosed by TBB with EBUS‐GS than those with positive findings adjacent to the lesion (p < 0.01). The odds ratio (OR) of patients whose lesion was detected by EBUS imaging (OR [95% confidence interval] 14.5 [8.0–26.4]) tended to be higher compared to the ORs of size of lesion ≥20 mm (3.9 [2.2–6.8]) and the bronchus sign (7.5 [4.6–12.2]).
Conclusion
Targeted lesion diameter ≥20 mm, bronchus sign, and detection by EBUS imaging, especially within the lesion, are important factors for the diagnosis of lung cancer by TBB with EBUS‐GS.
Keywords: diagnosis, endobronchial ultrasonography, guide sheath, lung cancer, transbronchial biopsy
The present study showed that size of targeted lesion, especially diameter ≥20 mm, the bronchus sign, and detection on EBUS imaging were significant contributors to making the diagnosis of lung cancer by TBB with EBUS‐GS. In addition, the odds ratio (OR) of detection on EBUS imaging for the diagnosis of lung cancer with EBUS‐GS tended to be higher than the ORs of other factors, including targeted lesions >20 mm and the bronchus sign. According to these data, detection on EBUS imaging, especially within the lesion, is important for the diagnosis by TBB with EBUS‐GS.

INTRODUCTION
Lung cancer is a life‐threatening disease, and 1.79 million deaths due to lung cancer were reported worldwide in 2020; it is the most frequent cause of cancer‐related death. 1 For the diagnosis and selection of treatments such as surgical resection, radiation, and chemotherapies, precise collection of lung samples is necessary. 2 Moreover, the detection sensitivity of lung lesions on thin‐slice, high‐resolution computed tomography (CT) is increasing because of its easy access and improved quality, 3 , 4 contributing to providing more challenges for physicians to make the diagnosis of relatively small and peripheral lung lesions.
Bronchoscopy is the pivotal tool for making the diagnosis, 5 and the diagnostic sensitivity for peripheral lesions ≥20 mm and those <20 mm has been reported to be 66% and 34%, respectively. 5 When abnormal cells are not found on bronchoscopy, other examinations, such as CT‐guided biopsy or surgical resection, should be considered, but this leads to delay in making the diagnosis and causes additional physical invasion. 6 These data indicate that a more sensitive tool for diagnosis is needed, and factors associated with making the diagnosis of lung cancer on bronchoscopy should be clarified.
Recently, endobronchial ultrasonography with a guide sheath (EBUS‐GS) has been clinically indicated. A guide sheath has the capacity to fix the appropriate bronchus with reduced bleeding as a complication, and radial ultrasonography can detect surrounding tumor directly 7 , 8 which contributes to an increased diagnosis rate of lung cancer. 9 , 10 Ali et al. reported that the diagnostic yield of peripheral lesions by radial endobronchial ultrasound was 72.3% in their meta‐analysis of 25 prospective studies with 2920 lesions, 11 even though a recent report from Huang et al. from Taiwan did not show the superiority of using EBUS with GS compared to EBUS without GS. 12
In a Japanese, single‐center, retrospective analysis, Minami et al. showed that the diagnostic sensitivity for lung cancer in 60 patients who underwent EBUS‐GS was higher, at 83.3%, than that for 50 patients who underwent transbronchial biopsy (TBB) without EBUS‐GS, at 68%, and they concluded that EBUS‐GS is effective for diagnosing lung cancers <20 mm. 13 In addition, the bronchus sign, which is defined as the responsible bronchus clearly reaching inside the target lesion 14 and detection on EBUS imaging, within or adjacent to the lesion, were also identified as factors associated with making the diagnosis of lung cancer by TBB with EBUS‐GS. 15 These data suggested that EBUS‐GS contributes to increased sensitivity for the diagnosis of lung cancer, but factors associated with the diagnosis on TBB with EBUS‐GS are still not fully understood.
In the present study, the clinical characteristics of patients who were diagnosed with lung cancer by TBB with EBUS‐GS and those who were not diagnosed by TBB with EBUS‐GS, but were diagnosed by other means, such as CT‐guided biopsy, surgical resection, and others, were retrospectively compared. It was found that size of lesion ≥20 mm, presence of the bronchus sign, and EBUS image detection, especially within the lesion, were significant factors associated with making the diagnosis of lung cancer on TBB with EBUS‐GS.
METHODS
Study design and patients
In our institute, bronchoscopists are encouraged to use TBB with EBUS‐GS because of the expected higher diagnostic rate and fewer adverse effects, except for visible central lesions, compared to TBB without EBUS‐GS. To identify patients who underwent TBB with EBUS‐GS, 1227 cases who underwent diagnostic bronchoscopy for lung lesions at Saga University Hospital between 2015 and 2020 were retrospectively analyzed. A total of 860 patients were excluded because 805 patients were diagnosed with diseases other than lung cancer, and 55 patients who were diagnosed with lung cancer underwent bronchoscopy without EBUS‐GS. Thus, 367 patients who underwent TBB with EBUS‐GS and were diagnosed with lung cancer were identified. A total of 244 patients were diagnosed by TBB with EBUS‐GS at the initial procedure, and 123 patients were diagnosed by other examinations. Of the 123 patients who were not diagnosed by TBB with EBUS‐GS, the diagnosis was made by surgical resection in 94 patients and CT‐guided biopsy in 16 patients who underwent the procedures to make the diagnosis of lung cancer for the targeted lesion. Four patients underwent TBB with EBUS‐GS frequently and were diagnosed with lung cancer by subsequent examinations, but not the initial TBB with EBUS‐GS. Six patients were clinically diagnosed as having lung cancer based on the efficacy of subsequent anticancer treatments. Two patients were diagnosed by transbronchial needle aspiration with EBUS collected from their mediastinal lymph node, and one patient was diagnosed by collection of pleural effusion fluid; their target lesions were also clinically diagnosed as lung cancer considering the chest CT findings (Figure 1). All cases underwent thin‐section chest CT before bronchoscopy. Patient data including age, sex, smoking status, size of lesion on chest CT, and bronchus signs were evaluated at the time closest to when EBUS‐GS was performed. On thin‐section chest CT, a pulmonologist identified the bronchus sign, defined as the responsible bronchus clearly reaching the inside of the target lesion; a representative image is shown in Figure 2(a). EBUS images within (Figure 2(b)) and adjacent to (Figure 2(c)) the lesion and complications related to bronchoscopy were also evaluated by a pulmonologist. In terms of complications, fever was defined as a temperature ≥38°C 24 h after the bronchoscopy, and hemorrhage was directly observed by bronchoscopy or detected as bloody sputum after bronchoscopy. The sites of target lesions on CT imaging were categorized by lobe, referring to the previous report. 16
FIGURE 1.
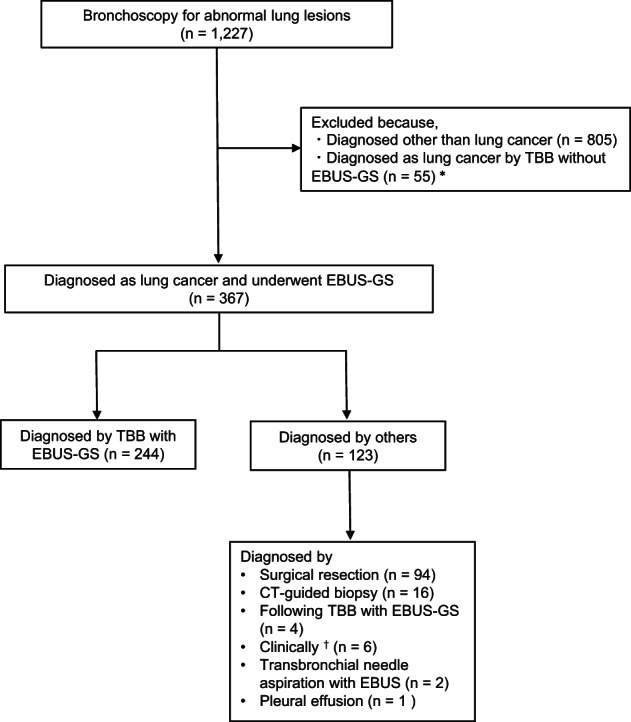
Study design. Patients diagnosed with lung cancer by TBB with EBUS‐GS. A total of 367 patients who underwent TBB with EBUS‐GS and were diagnosed with lung cancer were identified. A total of 244 patients were diagnosed by TBB with EBUS‐GS, and 123 patients were diagnosed by other examinations. *Of the 55 patients, 53 had a visible central lesion, and only two patients with a peripheral lesion in the lung underwent TBB without EBUS‐GS. †Six patients were clinically diagnosed as having lung cancer considering the efficacy of chemotherapy. Abbreviations: EBUS‐GS, endobronchial ultrasonography with a guide sheath; TBB, transbronchial biopsy
FIGURE 2.
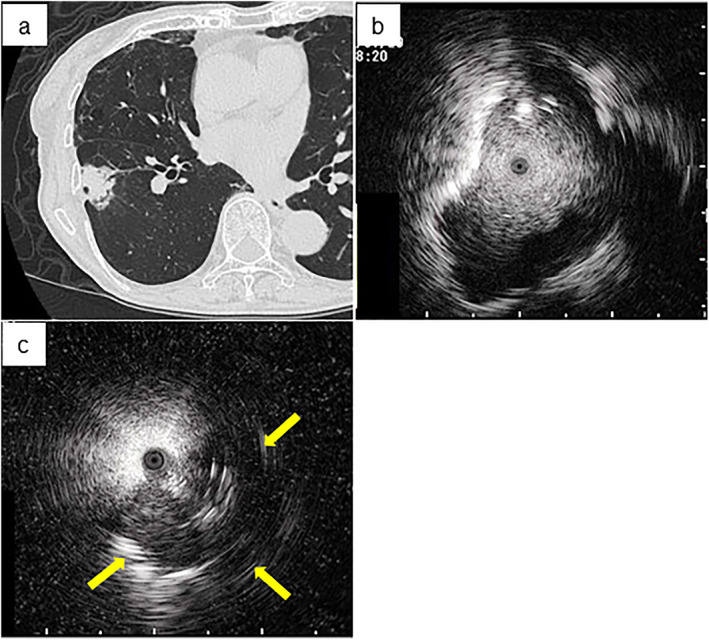
Representative images of the bronchus sign and endobronchial ultrasonography with a guide sheath (EBUS) imaging including within and adjacent to the lesion. (a) The bronchus sign is observed in the peripheral lung field on chest computed tomography with the lung window setting. EBUS imaging of (b) within and (c) adjacent to (arrow shows the detected image of the targeted lesion) the image are shown
Bronchoscopy procedure
After administration of local pharyngeal anesthesia, all patients were lightly sedated with midazolam or midazolam and fentanyl. A 20‐MHz radial type ultrasound probe with an external diameter of 1.4 mm (UM‐S20‐17S; Olympus Medical Systems) connected to an endoscopic ultrasonography system (EU‐M30S Olympus Medical Systems, Tokyo, Japan) and a guide sheath with an external diameter of 1.9 mm (K‐201 kit equipped with a biopsy forceps and cytological brush; Olympus Medical Systems) were used in all cases. The bronchoscopists chose bronchoscopes with a 2.0‐mm‐diameter working channel (BF‐P260F, BF‐P290; Olympus Medical Systems) at their own discretion. Similarly, frequencies of collected samples were also determined by each bronchoscopist.
Cytological and pathological evaluations
Diagnosis of lung cancer using histological and cytological samples was performed by a histopathologist and was based on hematoxylin–eosin staining and immunostaining of formalin‐fixed, paraffin‐embedded samples for classification of histological type in Saga University Hospital.
Statistical analysis
Quantitative data are expressed as means ± standard deviation (SD). The clinical data were analyzed by Student's t‐test for continuous variables or the chi‐squared test for categorical variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by logistic regression analysis. A p‐value less than 0.05 was considered statistically significant. Statistical analysis was performed with JMP Pro version 14.2.0 software (SAS Institute Inc.).
RESULTS
Characteristics of patients with lung cancer who were and were not diagnosed by TBB with EBUS‐GS
Of the 367 total patients with lung cancer, 244 patients (66.5%) were diagnosed, and 123 patients (33.5%) were not diagnosed by TBB with EBUS‐GS. Age, sex, and rate of current or past smoking were not significantly different between the two groups. The targeted lesions in the group of patients who were diagnosed by TBB with EBUS‐GS were significantly larger than those who were not diagnosed, with diameters of 37.5 and 30.5 mm, respectively (p < 0.01). The rates of patients whose lesion was ≥20 mm, who showed the bronchus sign, and had EBUS image detection were higher in patients who were diagnosed by TBB with EBUS‐GS than in patients who were not diagnosed (diameter ≥ 20 mm: 89.3% vs. 68.3%, p < 0.01; bronchus sign: 80.7% vs. 34.8%, p < 0.01; EBUS image detection: 92.6% vs. 46.3%, p < 0.01). The diagnostic yield of lung cancer was 72.2% in patients whose targeted lesion diameter was ≥20 mm and 40.0% in patients whose targeted lesion diameter was <20 mm. Diagnostic yields also depended on the number of bronchus signs, which were 37.3%, 76.1% and 86.7% for 0, 1, and 2 or more bronchus signs, respectively. Pathological features were not significantly different between the two groups except for non‐small cell carcinoma (NSCLC) not otherwise specified. There were also no differences in lobar distribution between the two groups (Table 1).
TABLE 1.
Characteristics of patients with lung cancer who were and were not diagnosed by TBB with EBUS‐GS
| Diagnosed by TBB with EBUS‐GS (n = 244) | Undiagnosed by TBB with EBUS‐GS (n = 123) | p‐value | ||
|---|---|---|---|---|
| Age (years) | 72.0 ± 9.8 | 72.6 ± 7.5 | 0.55 | |
| Sex (M/F) | 162/82 | 73/50 | 0.18 | |
| Current or past smoker | 177 (72.5%) | 80 (65.0%) | 0.14 | |
| Size (diameter, mm) | 37.5 ± 17.5 | 30.5 ± 17.3 | <0.01 | |
| Size ≥ 20 mm (diameter) | 218 (89.3%) | 84 (68.3%) | <0.01 | |
| Bronchus sign | 197 (80.7%) | 44 (35.8%) | <0.01 | |
| EBUS image detection | 226 (92.6%) | 57 (46.3%) | <0.01 | |
| Pathology | Adenocarcinoma | 159 (65.2%) | 75 (61.0%) | 0.52 |
| Squamous cell carcinoma | 45 (18.4%) | 29 (23.6%) | 0.25 | |
| NSCLC/NOS | 16 (6.6%) | 2 (1.6%) | <0.05 | |
| Small cell lung cancer | 9 (3.7%) | 4 (3.3%) | 0.83 | |
| Others | 15 (6.1%) | 12 (9.8%) | 0.21 | |
| Lobar distribution | Right upper lobe | 74 (30.3%) | 45 (36.6%) | 0.23 |
| Right middle lobe | 16 (6.6%) | 7 (5.7%) | 0.75 | |
| Right lower lobe | 49 (20.1%) | 32 (26.0%) | 0.20 | |
| Left upper lobe | 66 (27.0%) | 24 (19.5%) | 0.11 | |
| Left lower lobe | 39 (16.0%) | 15 (12.2%) | 0.33 |
Abbreviations: EBUS‐GS, endobronchial ultrasonography with a guide sheath; NSCLC/NOS, non‐small cell lung cancer/not otherwise specified; TBB, transbronchial biopsy.
Diagnostic yield depending on ultrasonic probe location and association with detection on EBUS imaging
Looking at the diagnostic yield of TBB with EBUS‐GS considering EBUS imaging, 21.4% (18/84) of patients with lesions were not detected on EBUS imaging, 60.3% (44/73) of patients were detected on EBUS imaging adjacent to the lesion, and 86.7% (182/210) of patients detected on EBUS imaging within the lesion were diagnosed with lung cancer. In 283 patients with lesions detected on EBUS imaging, patients with positive findings within the lesion were significantly more often diagnosed by TBB with EBUS‐GS than those with positive findings adjacent to the lesion (p < 0.01) (Table 2).
TABLE 2.
Difference in the type of EBUS image between within and adjacent to the lesion in the diagnosis of lung cancer by TBB with EBUS‐GS
| Diagnosed by TBB with EBUS‐GS | Undiagnosed by TBB with EBUS‐GS | p‐value | |
|---|---|---|---|
|
Type of EBUS image (n = 283) |
|||
| Within | 182 | 28 | <0.01 |
| Adjacent to | 44 | 29 | – |
Abbreviations: EBUS‐GS, endobronchial ultrasonography with a guide sheath; TBB, transbronchial biopsy.
Odds ratios of factors including size of lesion ≥20 mm, presence of the bronchus sign, and detection on EBUS imaging
To investigate the factors related to the diagnosis of lung cancer, ORs with 95% CIs were calculated focusing on significantly different parameters between patients who were and were not diagnosed by TBB with EBUS‐GS. Targeted lesion diameter ≥20 mm (OR [95% CI]: 3.9 [2.2–6.8]) and the bronchus sign (7.5 [4.6–12.2]) were significantly associated with the diagnosis of lung cancer by TBB with EBUS‐GS. In addition, the OR of detected on EBUS imaging tended to be higher compared to the ORs of size of lesion ≥20 mm and the bronchus sign (14.5 [8.0–26.4]) (Figure 3).
FIGURE 3.
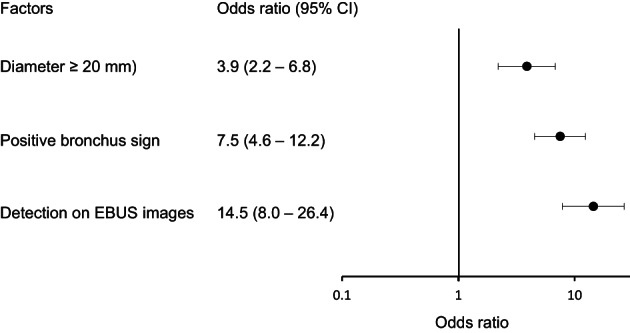
Odds ratios of factors including the rate of patients with targeted lesion diameter ≥20 mm, the bronchus sign, and detected on EBUS imaging. Abbreviations: EBUS‐GS, endobronchial ultrasonography with a guide sheath; 95% CI, 95% confidence interval
Examination of molecular markers for patients with NSCLC treated with cytotoxic chemotherapies, molecular targeted drugs, or immune checkpoint inhibitors
Because exploring molecular markers is important for treatment selection in patients with NSCLC treated with anticancer therapy, 17 the markers were evaluated to clarify whether they were useful. A total of 91 patients with NSCLC diagnosed by TBB with EBSU‐GS were treated with cytotoxic chemotherapies, molecular targeted drugs, or immune checkpoint inhibitors. Markers were evaluated in 80 patients (87.9%) for epidermal growth factor receptor (EGFR), 56 (62.5%) anaplastic lymphoma kinase (ALK), 44 (48.4%) programmed death‐ligand 1 (PD‐L1), 22 (24.2%) V‐ros UR2 sarcoma virus oncogene homolog 1 (ROS‐1), 11 (12.1%) V‐raf murine sarcoma viral oncogene homolog B1(BRAF), and two (2.2%) MET by single plex assay. Only one (1.1%) patient was evaluated by multiplex mutation assay (Table 3).
TABLE 3.
Examination of molecular markers in 91 patients with non‐small cell lung cancer who were diagnosed by TBB with EBUS‐GS and treated with cytotoxic chemotherapies, molecular targeted drugs, or immune checkpoint inhibitors
| Molecular markers | n = 91 |
|---|---|
| EGFR | 80 (87.9%) |
| ALK a | 56 (61.5%) |
| PD‐L1 b | 44 (48.4%) |
| ROS‐1 | 22 (24.2%) |
| BRAF | 3 (3.3%) |
| MET | 2 (2.2%) |
| Multiplex assay | 1 (1.1%) |
Abbreviations: ALK, anaplastic lymphoma kinase; BRAF, V‐raf murine sarcoma viral oncogene homolog B1; EBUS‐GS, endobronchial ultrasonography with a guide sheath; EGFR, epidermal growth factor receptor; PD‐L1, programmed death‐ligand 1; ROS‐1, V‐ros UR2 sarcoma virus oncogene homolog 1; TBB, transbronchial biopsy.
Evaluated by fluorescence in situ hybridization or immunohistochemistry.
Evaluated by immunohistochemistry.
Complications of TBB with EBUS‐GS
In the present study, 30 patients (8.2%) developed complications associated with TBB with EBUS‐GS. Eight patients (2.2%) had fever, five (1.4%) had hemorrhage, four (1.1%) had bacterial bronchitis, and three (1.0%) had pneumothorax, and all recovered with specific treatments. Briefly, all five patients with hemorrhage had mild bleeding and recovered without any additional intervention. In the three patients who suffered pneumothorax, one patient was treated conservatively, and the other two patients recovered with insertion of a chest tube.
DISCUSSION
In the present retrospective study, the diagnostic yield of TBB with EBUS‐GS for lung cancer was 66.5%, and several factors related to the diagnosis of lung cancer by TBB with EBUS‐GS were identified. In particular, the size of the targeted lesion, rate of patients whose targeted lesion diameter was ≥20 mm, bronchus sign, and detected on EBUS imaging were significant contributors to making the definite diagnosis by TBB with EBUS‐GS. Furthermore, detection on EBUS imaging tended to be more associated with the diagnosis than the others, and in patients detected on EBUS imaging, the finding of within the lesion was significantly more related to making the diagnosis than that adjacent to the lesion.
TBB with EBUS‐GS has been reported to contribute to increased sensitivity for the diagnosis of lung cancer, with increasing evidence compared to conventional flexible bronchoscopy. 5 , 13 The diagnostic rate of traditional transbronchial biopsy in several studies ranged from 14% to 63%, 18 , 19 , 20 which is lower than the results of the current study. In addition, a meta‐analysis of EBUS‐GS showed a diagnostic yield of 73.2%, which was the highest compared to other modalities, including electromagnetic navigation bronchoscopy, virtual bronchoscopy, and ultrathin bronchoscopy combined with radial endobronchial ultrasound. 21 According to these data, EBUS‐GS is a pivotal examination tool for making the diagnosis of lung cancer. Notably, a comparative evaluation of the diagnostic yield of lung cancer by EBUS‐GS referring to previous reports is challenging because it depends on the skill of the bronchoscopist and assistant, position and size of the lesion, and even with the condition of patients under anesthesia. Indeed, Jaing et al. performed a prospective, controlled study, and their diagnostic yield for targeting peripheral pulmonary lesions that averaged 28.7 mm was 64.5%, which was close to the current results. 22
In the present study, the size of the targeted lesion, especially diameter ≥20 mm, the bronchus sign, and detection on EBUS imaging were associated with the diagnosis of lung cancer by TBB with EBUS‐GS. A pooled analysis of a study of lung cancer diagnosis with radial probe endobronchial ultrasound showed that the diagnostic yield for lesions >20 mm was significantly higher, at 77.7%, than for lesions ≤20 mm, at 56.3%, supporting the present results. 23 Lee et al. analyzed an EBUS‐GS database of 393 patients with peripheral lung lesions, and concluded that the bronchus sign along with EBUS imaging positive within the lesion affected the diagnostic yield of EBUS‐GS in patients, particularly those with pulmonary emphysema, 15 which is also consistent with the present results.
Detection on EBUS imaging is involved in the diagnosis of lung cancer by TBB with EBUS‐GS because it reflects physical proximity between the targeted lesion and the approaching bronchus. 24 This fact was first described by Huang et al. approximately 15 years ago, who indicated that the position of the probe was an independent predictor of the diagnostic yield by TBB with EBUS, 25 which is consistent with the present results. Indeed, the present results also indicated that detection on EBUS imaging was significantly associated with the diagnosis of lung cancer by TBB with EBUS‐GS compared to patients who were not diagnosed (Table 1). Importantly, probe position including within, adjacent to, or not detected is essential to show the difference in the respective diagnostic yields in the present study. Probe positioning within, but not adjacent to, or invisible, on EBUS imaging was reported as significantly associated with the diagnostic yield of EBUS‐GS. 15 , 26 Interestingly, the finding of adjacent to the lesion might also be important for the diagnosis because the yield is higher than that of not detected, supporting the association of detection on EBUS imaging compared to nondetection, even though detection sensitivity was significantly lower compared to the finding of within the lesion (Table 2). In addition, the OR of detection on EBUS imaging for the diagnosis of lung cancer with EBUS‐GS tended to be higher than the ORs of other factors, including targeted lesions >20 mm and the bronchus sign (Figure 3). According to these data, detection on EBUS imaging, especially within the lesion, is important for the diagnosis by TBB with EBUS‐GS.
Notably, molecular marker analysis of lung cancer is also important for appropriate treatment selection along with diagnosis in patients treated by anticancer therapy. 17 TBB with EBUS‐GS is a useful examination for diagnosis targeting relatively small tumors, but only small specimens are obtained, which might not be a sufficient volume of samples for extraction of deoxyribonucleic acid and ribonucleic acid and evaluation of immunohistochemistry for molecular analysis. In the present study, a high rate of patients with NSCLC diagnosed by TBB with EBSU‐GS who were treated with cytotoxic chemotherapies, molecular targeted drugs, or ICIs, was evaluated by single plex assays, such as for EGFR, ALK, and PD‐L1 (Table 3). Only one patient (1.1%) underwent multiplex assay examination because the assay was approved only 6 months before the last registration period for the present study in May 2019 in Japan and might be related to the small volume of samples collected by TBB with EBUS‐GS. Thus, frequent sample collection using a bigger sheath and forceps might contribute to multiplex assay performance.
The present study has some limitations. First, selection of patients who underwent bronchoscopy depended on physician judgment, and skill of both the bronchoscopist and assistant which might have contributed to the present results. Second, the targeted lesion of the present cases was not assessed as central or peripheral, although previous reports indicated that it is important for the diagnosis of lung cancer by bronchoscopy. 16 , 27 We did not specify whether the lesions were located centrally or peripherally because of difficulty in the definition, which affects the diagnostic yield in the present study. Third, examination tools including biopsy forceps, cytological brushing, washing, and numbers performed were different depending on the individual, which might have contributed to the diagnostic yield. Finally, the present study involved a small number of patients at a single hospital with limited ethnic diversity. To confirm the validity of the results, multicenter, prospective studies designed with appropriate controls and larger numbers of patients should be performed.
In conclusion, the present cross‐sectional study showed that size of targeted lesion, especially diameter ≥ 20 mm, the bronchus sign, and detection on EBUS imaging were significant contributors to making the diagnosis of lung cancer by TBB with EBUS‐GS. In addition, in patients whose lesions were detected on EBUS imaging, the finding of within the lesion was more important for the diagnosis than that of adjacent to the lesion, which indicated that possible detection within the lesion but not adjacent to the lesion is necessary to increase the diagnostic sensitivity for lung cancer of TBB with EBUS‐GS.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
Kurihara Y, Tashiro H, Takahashi K, Tajiri R, Kuwahara Y, Kajiwara K, et al. Factors related to the diagnosis of lung cancer by transbronchial biopsy with endobronchial ultrasonography and a guide sheath. Thorac Cancer. 2022;13(24):3459–3466. 10.1111/1759-7714.14705
Funding information Saga University
REFERENCES
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021. https://pubmed.ncbi.nlm.nih.gov/33818764/ [DOI] [PubMed] [Google Scholar]
- 2. Pujol JL, Quantin X. Time to diagnosis of lung cancer: technical and pyschological factors that slow down diagnostic and treatment timelines. J Thorac Oncol. 2009;4(10):1192–4. [DOI] [PubMed] [Google Scholar]
- 3. de Hoop B, Schaefer‐Prokop C, Gietema HA, et al. Screening for lung cancer with digital chest radiography: sensitivity and number of secondary work‐up CT examinations. Radiology. 2010;255(2):629–37. [DOI] [PubMed] [Google Scholar]
- 4. Rubin GD. Lung nodule and cancer detection in computed tomography screening. J Thorac Imaging. 2015;30(2):130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–e65S. [DOI] [PubMed] [Google Scholar]
- 6. Vakil E, Jackson N, Sainz‐Zunega PV, et al. Optimizing diagnostic and staging pathways for suspected lung cancer: a decision analysis. Chest. 2021;160(6):2304–23. [DOI] [PubMed] [Google Scholar]
- 7. Kurimoto N, Murayama M, Yoshioka S, et al. Analysis of the internal structure of peripheral pulmonary lesions using endobronchial ultrasonography. Chest. 2002;122(6):1887–94. [DOI] [PubMed] [Google Scholar]
- 8. Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound‐guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002;20(4):972–4. [DOI] [PubMed] [Google Scholar]
- 9. Katsurada N, Tachihara M, Jimbo N, et al. Yield of tumor samples with a large guide‐sheath in endobronchial ultrasound transbronchial biopsy for non‐small cell lung cancer: a prospective study. PLoS One. 2021;16(10):e0259236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang L, Xu J, Liu C, et al. Diagnosis of peripheral pulmonary lesions with transbronchial lung cryobiopsy by guide sheath and radial endobronchial ultrasonography: a prospective control study. Can Respir J. 2021;2021:6947037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: a systematic review and meta‐analysis. Respirology. 2017;22(3):443–53. [DOI] [PubMed] [Google Scholar]
- 12. Huang CT, Chang LY, Chen CY, et al. Endobronchial ultrasound‐guided transbronchial biopsy with or without a guide sheath for peripheral pulmonary malignancy. ERJ Open Res. 2021;7(3):00267‐2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Minami D, Takigawa N, Morichika D, et al. Endobronchial ultrasound‐guided transbronchial biopsy with or without a guide sheath for diagnosis of lung cancer. Respir Investig. 2015;53(3):93–7. [DOI] [PubMed] [Google Scholar]
- 14. Minezawa T, Okamura T, Yatsuya H, et al. Bronchus sign on thin‐section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging. 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee KM, Lee G, Kim A, et al. Clinical outcomes of radial probe endobronchial ultrasound using a guide sheath for diagnosis of peripheral lung lesions in patients with pulmonary emphysema. Respir Res. 2019;20(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE registry. Am J Respir Crit Care Med. 2016;193(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sholl LM, Aisner DL, Varella‐Garcia M, et al. Multi‐institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience. J Thorac Oncol. 2015;10(5):768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence‐based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):108S–30S. [DOI] [PubMed] [Google Scholar]
- 19. Rivera MP, Mehta AC. American College of Chest P . Initial diagnosis of lung cancer: ACCP evidence‐based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):131S–48S. [DOI] [PubMed] [Google Scholar]
- 20. Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117(4):1049–54. [DOI] [PubMed] [Google Scholar]
- 21. Wang Memoli JS, Nietert PJ, Silvestri GA. Meta‐analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142(2):385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalamo M, Maenpaa J, Seppala T, et al. Descriptive study on subjective experience of genetic testing with respect to relationship, family planning and psychosocial wellbeing among women with lynch syndrome. Hered Cancer Clin Pract. 2021;19(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta‐analysis. Eur Respir J. 2011;37(4):902–10. [DOI] [PubMed] [Google Scholar]
- 24. Zhang L, Wu H, Wang G. Endobronchial ultrasonography using a guide sheath technique for diagnosis of peripheral pulmonary lesions. Endosc Ultrasound. 2017;6(5):292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang CT, Ho CC, Tsai YJ, et al. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology. 2009;14(6):859–64. [DOI] [PubMed] [Google Scholar]
- 26. Park S, Yoon HY, Han Y, et al. Diagnostic yield of additional conventional transbronchial lung biopsy following radial endobronchial ultrasound lung biopsy for peripheral pulmonary lesions. Thorac Cancer. 2020;11(6):1639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim YW, Jeon M, Song MJ, et al. Differences in detection patterns, characteristics, and outcomes of central and peripheral lung cancers in low‐dose computed tomography screening. Transl Lung Cancer Res. 2021;10(11):4185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]


