Abstract
The data on the effects of tofacitinib on soluble proteins in patients with rheumatoid arthritis (RA) is currently very limited. We analyzed how tofacitinib treatment and thus inhibition of the Janus kinase—signal transducer and activation of transcription pathway affects the in vivo levels of inflammation-related plasma proteins in RA patients. In this study, 16 patients with active RA [28-joint disease activity score (DAS28) >3.2] despite treatment with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) started tofacitinib treatment 5 mg twice daily. Levels of 92 inflammation-related plasma proteins were determined by proximity extension assay at baseline and at 3 months. Tofacitinib treatment for 3 months, in csDMARD background, decreased the mean DAS28 from 4.4 to 2.6 (P < 0.001). Marked (>20%) and statistically significant (P < 0.05) changes were found in the levels of 21 proteins, 18 of which decreased and 3 increased. Of these proteins, 17 are directly involved in inflammatory responses or in the cellular response to cytokines. The highest (>50%) decrease was observed for interleukin-6 (IL-6), C-X-C motif chemokine ligand 1, matrix metalloproteinase-1, and AXIN1. Higher baseline levels of IL-6 and lower levels of C-C motif chemokine 11 and Delta and Notch-like epidermal growth factor-related receptors were associated with DAS28 improvement. Our results indicate that tofacitinib downregulates several proinflammatory plasma proteins that may contribute to the clinical efficacy of tofacitinib. In addition, soluble biomarkers may predict the treatment response to tofacitinib.
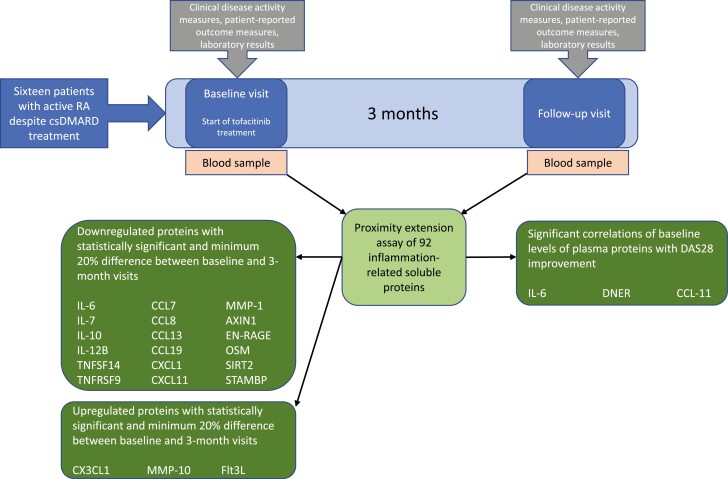
Sixteen patients with active rheumatoid arthritis, despite conventional synthetic disease-modifying antirheumatic drugs, started additional tofacitinib treatment. Blood samples were drawn at baseline and at 3-month follow-up visits, and plasma levels of 92 inflammation-related proteins were measured with proximity extension assay. Levels of 21 proteins changed substantially (>20%) and statistically significantly upon tofacitinib treatment, and the baseline levels of interleukin-6, C-C motif chemokine 11 and Delta and Notch-like epidermal growth factor-related receptor associated with the treatment response.
Graphical Abstract
Graphical Abstract.
Introduction
In rheumatoid arthritis (RA) various immune cells accumulate in the joints, a process partly driven by chemokines. Activated immune cells in the synovium communicate with each other and with synovial cells by direct cell-cell contact, as well as by secreting soluble mediators such as cytokines. In addition, matrix-degrading enzymes, such as matrix metalloproteinases (MMPs), are secreted, ultimately leading to the degradation of articular cartilage and bone [1]. Central cytokines regulating immune responses in RA include interferons (IFNs), Interleukin-1 (IL-1), IL-6, IL-7, IL-12, IL-15, IL-17, IL-23, tumor necrosis factor-α (TNF) and granulocyte-macrophage colony-stimulating factor [2].
The majority of the cytokines involved in RA act through the JAK-STAT signaling pathway that consists of Janus kinases (JAKs) and signal transducers and activators of transcription (STAT) [2]. A cytokine initiates the signaling pathway by binding to its receptor, resulting in JAK phosphorylation. The activated JAKs then induce STAT phosphorylation and dimerization, and subsequent translocation into the nucleus to regulate the expression of target genes [3]. We and others have shown that JAK-STAT pathways are constitutively active in circulating leukocytes of patients with RA [4–7]. Tofacitinib, the first JAK inhibitor developed for the treatment of RA, inhibits JAK1, JAK3, and to a slightly lesser extent JAK2 [8, 9]. Tofacitinib in a methotrexate background was shown to have similar efficacy compared to TNF inhibitor adalimumab in RA patients with active disease despite conventional synthetic disease-modifying drug (csDMARD) treatment [10].
Although the clinical effects of tofacitinib in RA have been well documented, the cellular effects of tofacitinib in RA patients in vivo have been less studied. For example, the knowledge of the effects of tofacitinib on cytokine or chemokine expression is mainly based on in vitro studies or animal models of arthritis. Tofacitinib has previously been shown to inhibit IL-17 and IFN-γ production and T cell activation in vitro by synovial and peripheral blood T cells of RA patients [11, 12]. In addition, tofacitinib suppressed the expression of IL-6, C-X-C motif chemokine ligand 9 (CXCL9), CXCL10, and CXCL11 in TNF-stimulated RA synovial macrophages [13], and expression of IL-6 in oncostatin-stimulated and the expression of C–C motif chemokine ligand 2 (CCL2), CCL5, and CXCL10 in TNF-stimulated RA fibroblast-like synoviocytes [14, 15]. Studies using immunodeficient mice with RA patient synovium and cartilage implants demonstrated that tofacitinib treatment resulted in decreased serum levels of human IL-6 and IL-8 [11]. Also, administration of tofacitinib downregulated the plasma levels of IL-6, granulocyte colony-stimulating factor, CCL2, and CXCL10 in mice immunized to develop collagen-induced arthritis [16].
Data regarding the effects of tofacitinib treatment on the levels of soluble proteins in RA is currently limited, and effects on only selected proteins have been reported. Tofacitinib treatment in RA patients in vivo has been shown to decrease the levels of circulating IL-6, CXCL10, MMP-3, and gliostatin [17–19]. Tofacitinib also reduced the expression of MMP-1, MMP-3, CCL2, CXCL10, and CXCL13 in synovial biopsies of RA patients with an inadequate response to methotrexate [19]. While tofacitinib has usually been observed to decrease the levels of immunological markers, an increase has been described, for example, for circulating levels of osteoprotegerin, osteocalcin, and vitamin D3 during six and 12 months of tofacitinib use in patients with RA [20]. The finding was assumed to reflect stabilization of bone density and positive balance of bone turnover by tofacitinib. Owing to the rarity of in vivo studies on tofacitinib use, novel markers upregulated by tofacitinib may be unidentified yet.
Studying the in vivo effects of tofacitinib helps us to understand the mechanisms of action of tofacitinib in RA patients. We have previously shown that tofacitinib inhibits multiple JAK-STAT pathways in a cytokine and cell population specific manner, and downregulates the expression of several JAK-STAT pathway components, in circulating leukocytes of RA patients [21]. In the current study, we have extended the analysis of tofacitinib treatment on the expression of a broad range of soluble inflammation-associated proteins in RA patients. The association between the levels of these proteins and tofacitinib treatment response was additionally studied.
Patients and methods
Patients
Patients were recruited from two rheumatology outpatient clinics (Tampere and Helsinki University Hospitals) between 2018 and 2020. Eligible patients were required to fulfill the 2010 ACR/EULAR classification criteria for RA and have an active disease at baseline visit: the Disease Activity Score for 28 joints based on C-reactive protein level (DAS28-4[CRP]) >3.2 despite treatment with csDMARDs. Key exclusion criteria were prior or current treatment with biologics or JAK inhibitors, a current infection, malignancy, severe hepatic impairment, pregnancy or lactation, hemoglobin <90 mg/dl, a neutrophil count of <1.9 × 109/l, or a lymphocyte count <0.75 × 109/l.
The study was approved by the National Committee on Medical Research Ethics (TUKIJA) and the Finnish Medicines Agency Fimea. The study was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients gave written informed consent for the study.
Study design
The study consisted of three visits: a screening visit 0–3 months before the baseline visit, the baseline visit, and a follow-up visit after 3 months of tofacitinib treatment. From the baseline visit on, the patients were treated with tofacitinib 5 mg twice daily throughout the study time. Ongoing csDMARD therapy and prednisolone (0–10 mg/day) were continued at stable doses. Patient-reported outcomes and clinical assessments were recorded at study visits. Treatment response was determined by the change in DAS28-4[CRP] between baseline and 3-month visits. At baseline and follow-up visits, whole blood samples were drawn into EDTA (Ethylene Diamine Tetra Acetic acid) tubes and kept on ice. Within an hour from sampling, plasma was isolated by centrifugation at 2000 g for 10 min at +4°C and subsequently stored at −80°C.
Proximity extension assay for plasma proteins
Plasma samples from the baseline and follow-up visits were sent to Olink Bioscience AB (Uppsala, Sweden) for high-throughput analysis of 92 inflammation-associated proteins in proximity extension assay in 96-well plate format [22, 23].
The proximity extension assay is a dual-recognition immunoassay, where two matched antibodies labeled with unique DNA oligonucleotides simultaneously bind to a target protein in solution. This allows the two antibodies to converge and their DNA oligonucleotides to hybridize, serving as a template for a DNA polymerase-dependent extension step. This creates a double-stranded DNA “barcode” which is unique for the specific protein. The hybridization and extension are followed by PCR amplification. Finally, the concentration of the PCR product is directly proportional to the initial concentration of the target protein.
Relative levels of proteins were reported on an arbitrary Log2-based NPX (normalized protein expression) scale that does not allow absolute protein quantities to be compared across different proteins. The logarithmic scale was converted into a linear scale for further analysis and proteins were categorized according to the public access bioinformatics databases Uniprot, Human Protein Atlas, Gene Ontology, and DisGeNET.
Statistical methods
Plasma proteins with baseline levels below the limit of detection (LOD) in more than 25% of the samples were discarded from further analysis. For the remaining proteins, the values below LOD were changed to equal LOD, as suggested by Olink. Wilcoxon signed-rank test was used to assess significance in DAS28 improvement and levels of soluble plasma proteins before and after tofacitinib treatment. P-values < 0.05 were considered statistically significant. Multiple comparisons were taken into account by considering only proteins that changed by more than 20% to be markedly affected by tofacitinib treatment. Spearman rank correlation was used to evaluate correlations between protein levels and clinical improvement. Confidence intervals were calculated with bootstrapping. Statistical analysis was carried out using SPSS (version 26; IBM, Armonk, NY, USA).
Results
Patients and clinical response to tofacitinib
Patient characteristics and the clinical response to tofacitinib treatment in the patient population have been previously reported in detail [21]. The study population consisted of 16 RA patients whose demographic and clinical characteristics are described in Table 1. Twelve (75%) patients received methotrexate (MTX) as part of their csDMARD regimen.
Table 1:
Demographic and clinical characteristics of the patients (n = 16)
| Age, years, mean (range) | 58.4 (36.4–72.9) |
| Sex, female, n (%) | 11 (69%) |
| Disease duration, years, mean (range) | 9.6 (0.5–48.0) |
| Erosive disease, n (%) | 7 (44%) |
| Rheumatoid factor positive, n (%) | 11 (69%) |
| CCP-antibody positive, n (%) | 12 (75%) |
| Disease activity (DAS28-4 [CRP]), median (IQR) | 4.4 (3.6–4.9) |
| Swollen joint count, 0–46, median (IQR) | 7 (6–9) |
| Tender joint count, 0–46, median (IQR) | 11 (5–17) |
| Patient’s global assessment, VAS, 0–100 mm, median (IQR) | 35 (31–46) |
| General health, VAS, 0–100 mm, median (IQR) | 51 (37–65) |
| Pain, VAS, 0-100 mm, median (IQR) | 43 (22–64) |
| HAQ disability index, 0–3, median (IQR) | 0.813 (0.625–1.253) |
| csDMARD treatment, n (%) | |
| Single | 3 (19%) |
| Double | 7 (44%) |
| Triple | 6 (37%) |
| Low-dose prednisolone, n (%) | 8 (50%) |
Cyclic citrullinated peptide (CCP); disease activity score for 28 joints (DAS28); interquartile range (IQR); visual analogue scale (VAS); health assessment questionnaire (HAQ); conventional synthetic disease-modifying antirheumatic drug (csDMARD).
According to DAS28-4 [CRP], 13 (81%) patients had moderate disease activity and 3 (19%) had high disease activity before treatment. During tofacitinib treatment the median DAS28 decreased from 4.4 to 2.6 (P < 0.001). At the follow-up visit, nine patients (56%) were in DAS28 remission, and four (25%) patients had low, two (13%) had moderate and one (6%) had high disease activity.
Effects of tofacitinib treatment on inflammation-associated plasma proteins
Levels of 92 soluble plasma proteins were evaluated before and after 3-month tofacitinib treatment in csDMARD background. Proteins for which a significant proportion (>25%) of samples were under LOD at baseline were discarded from further analysis (Supplementary Table S1). Of the remaining 75 proteins, 22 were significantly downregulated and 10 were upregulated during tofacitinib treatment (Supplementary Table S1).
To gain better insight into clinically meaningful changes in protein levels during tofacitinib treatment, the percentage difference between protein levels before and after treatment were calculated. Table 2 shows levels of soluble plasma proteins before and after tofacitinib treatment for the 21 proteins that demonstrated both substantial (>20%) and statistically significant change during the treatment. Of these, the levels of 18 (86%) proteins decreased and 3 (14%) increased (Table 2, Supplementary Fig. S1). IL-6, CXCL1, MMP-1, and AXIN1 were most strongly affected and demonstrated a more than a 50% decrease between baseline and 3-month values.
Table 2:
The levels of soluble plasma proteins in patients with rheumatoid arthritis before and after 3-month treatment with tofacitinib and csDMARDs. Proteins with statistically significant and minimum 20% difference between baseline and 3-month visits are shown. Results with at least 50% difference are shown in bold.
| Protein | Before, mean (CI) | After 3 months, mean (CI) | Percentage difference (CI) | P |
|---|---|---|---|---|
| IL-6 | 34.2 (15.3–63.5) | 11.2 (5.7–20.2) | −67 (−1.2 to −159) | 0.004 |
| IL-7 | 3.5 (3–4.1) | 2.7 (2.3–3.2) | −24 (−10 to −40) | 0.008 |
| IL-10 | 21.7 (16.9–26.9) | 16.1 (13.1–19.5) | −26 (−3 to −45) | 0.025 |
| IL-12B | 73.6 (58.1–88.9) | 57.4 (47.3–68.5) | −22 (−4.4 to −38) | 0.039 |
| TNFSF14 | 21.9 (18.9–25.2) | 16.6 (14.2–19.2) | −24 (−11 to −36) | 0.002 |
| TNFRSF9 | 114 (91–140) | 89.3 (73.2–106) | −22 (−9.8 to −35) | 0.006 |
| CCL7 | 4.2 (3.2–5.7) | 2.8 (2.5–3.3) | −33 (−9.6 to −64) | 0.016 |
| CCL8 | 280 (205–374) | 210 (167–256) | −25 (−9.9 to −44) | 0.003 |
| CCL13 | 11 755 (8664–15544) | 7159 (6198–8198) | −39 (−16 to −66) | 0.002 |
| CCL19 | 581 (480–688) | 377 (315–440) | −35 (−22 to −50) | <0.001 |
| CXCL1 | 239 (158–325) | 113 (93.4–134) | −53 (−22 to −85) | 0.002 |
| CXCL11 | 162 (115–215) | 109 (76.8-153) | −33 (5.5 to −70) | 0.005 |
| CX3CL1 | 16.4 (14.8–18.1) | 19.7 (17.3–22.6) | 20 (7.2 to 35) | 0.011 |
| MMP-1 | 25 120 (15 059–37423) | 9175 (6053–13,413) | −63 (−24 to −111) | 0.013 |
| MMP-10 | 483 (403–576) | 645 (482–873) | 34 (10 to 61) | 0.018 |
| AXIN1 | 14.6 (9.4–20.8) | 6.6 (4.9–8.2) | −55 (−20 to −98) | 0.006 |
| EN-RAGE | 19.4 (12.8–26.6) | 11.9 (8.1–16.2) | −38 (−14 to −64) | 0.005 |
| Flt3L | 663 (583–734) | 832 (717–945) | 26 (9.3 to 42) | 0.003 |
| OSM | 27.9 (18.5–41.2) | 17.3 (12.5–23.6) | −38 (−16 to −66) | 0.002 |
| SIRT2 | 16 (11.1–21.9) | 9.5 (7.6–11.7) | −41 (−15 to −74) | 0.006 |
| STAMBP | 32.4 (25.5–40.8) | 21.4 (18.5–24.8) | −34 (−14 to −59) | 0.003 |
Wilcoxon signed-rank test (P). Confidence intervals (CI) were calculated with bootstrapping. Conventional synthetic disease-modifying antirheumatic drug (csDMARD); interleukin (IL); Tumor necrosis factor superfamily member 14 (TNFSF14); tumor necrosis factor receptor superfamily member 9 (TNFRSF9); C–C motif chemokine (CCL); C-X-C motif chemokine (CXCL); C-X3-C motif chemokine (CX3CL); matrix metalloproteinase (MMP); S100 calcium-binding protein A12 (EN-RAGE); FMS-like tyrosine kinase 3 ligand (Flt3L); oncostatin M (OSM); NAD-dependent deacetylase sirtuin 2 (SIRT2); STAM-binding protein (STAMBP).
The proteins that were significantly affected by tofacitinib treatment included several cytokines and chemokines as well as two matrix metalloproteinases (MMP-1 and MMP-10). To gain better insight into the function of these proteins, they were grouped based on their involvement in biological processes (Table 3). The tofacitinib-affected proteins most frequently had a role in processes of cellular response to cytokine stimulus (n = 15) and inflammatory response (n = 14), and the least frequently in processes of extracellular matrix organization (n = 2) and response to hypoxia (n = 1). Additionally, the protein–protein interaction network was visually analyzed by STRING (Search Tool for the Retrieval of Interacting Genes/Proteins; Supplementary Supplementary Fig. S2). Strongest evidence for interactions (confidence score > 0.9) was demonstrated for proteins IL-6, IL-10, IL-12B, CCL19, CXCL1, MMP-1, MMP-10, and oncostatin M (OSM).
Table 3:
The involvement of soluble plasma proteins in different biological processes categorized according to the public access bioinformatics databases Uniprot, human protein atlas, gene ontology, and DisGeNET. Proteins with statistically significant and minimum 20% difference during tofacitinib treatment are shown. Eighteen proteins that decreased during tofacitinib treatment are shown on the left, and three proteins that increased on the right.
| Biological process | IL-6 | IL-7 | IL-10 | IL-12B | TNFSF14 | TNFRSF9 | CCL7 | CCL8 | CCL13 | CCL19 | CXCL1 | CXCL11 | MMP-1 | AXIN1 | EN-RAGE | OSM | SIRT2 | STAMBP | CX3CL1 | MMP-10 | Flt3L | Count |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellular response to cytokine stimulus | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 15 | ||||||
| Inflammatory response | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 14 | |||||||
| Apoptotic process | X | X | X | X | X | X | X | X | X | X | X | X | 12 | |||||||||
| Chemotaxis | X | X | X | X | X | X | X | X | X | X | X | 11 | ||||||||||
| MAPK cascade | X | X | X | X | X | X | X | X | X | 9 | ||||||||||||
| Secretion | X | X | X | X | X | X | X | X | X | 9 | ||||||||||||
| Cell adhesion | X | X | X | X | X | X | X | X | 8 | |||||||||||||
| Cell activation in immune response | X | X | X | X | X | X | 6 | |||||||||||||||
| Regulation of immune response | X | X | X | X | 4 | |||||||||||||||||
| Extracellular matrix organization | X | X | 2 | |||||||||||||||||||
| Response to hypoxia | X | 1 |
Mitogen-activated protein kinase (MAPK); interleukin (IL); tumor necrosis factor superfamily member 14 (TNFSF14); tumor necrosis factor receptor superfamily member 9 (TNFRSF9); C–C motif chemokine (CCL); C-X-C motif chemokine ligand (CXCL); C-X3-C motif chemokine ligand (CX3CL); matrix metalloproteinase (MMP);S100 calcium-binding protein A12 (EN-RAGE); Oncostatin M (OSM); NAD-dependent deacetylase sirtuin 2 (SIRT2); STAM-binding protein (STAMBP); FMS-like tyrosine kinase 3 ligand (Flt3L).
We also investigated whether the changes in protein levels during tofacitinib treatment were associated with treatment response. Of the 21 proteins studied, changes in IL-6 and tumor necrosis factor superfamily member 14 (TNFSF14) levels significantly correlated with treatment response (Table 4, right panel).
Table 4:
Significant correlations of baseline levels of plasma proteins (left panel) or changes in plasma protein levels (right panel) with improvement in DAS28-4[CRP] during 3-month treatment with tofacitinib and csDMARDs. Correlations for a change in protein levels were analyzed only for proteins that showed statistically significant (P < 0.05) and minimum 20% difference by tofacitinib treatment.
| Protein | ||||
|---|---|---|---|---|
| Level at baseline | Decrease during treatment | |||
| r | P | r | P | |
| IL-6 | 0.612 (0.141–0.899) | 0.012 | 0.639 (0.106–0.918) | 0.008 |
| TNFSF14 | 0.524 (0.027–0.867) | 0.037 | ||
| CCL-11 | −0.553 (−0.845 to 0.066) | 0.026 | ||
| DNER | −0.514 (−0.847 to 0.063) | 0.042 | ||
Spearman correlation coefficients (r) and confidence intervals (CI) calculated with bootstrapping are shown. Empty spaces denote correlations that were not significant (P > 0.05). Composite disease activity score for 28 joints based on C-reactive protein level (DAS28-4[CRP]); conventional systemic disease-modifying antirheumatic drug (csDMARD); interleukin (IL); tumor necrosis factor superfamily member 14 (TNFSF14); C-C motif chemokine 11 (CCL-11); delta and Notch-like epidermal growth factor-related receptor (DNER).
Finally, we also investigated whether the levels of proteins that were markedly affected by tofacitinib treatment correlated with disease activity at baseline. We found no significant associations between disease activity and proteins levels at baseline or the change in protein levels during tofacitinib treatment (Supplementary Table S2)
Baseline levels of plasma proteins correlate with treatment response
To investigate whether baseline levels of soluble proteins are associated with treatment response to tofacitinib, correlation coefficients were calculated between protein levels at baseline and DAS28 improvement during tofacitinib treatment. The baseline level of IL-6 correlated positively with DAS28 improvement, whereas CCL11 and Delta and Notch-like epidermal growth factor-related receptor (DNER) showed a negative correlation (Table 4, left panel).
Discussion
The results show that plasma levels of several inflammation-associated proteins are altered by the JAK inhibitor tofacitinib in vivo, as determined after 3 months of tofacitinib treatment in patients with csDMARD-irresponsive RA. Of 92 proteins determined, 18 showed a decrease of over 20%, and four of these (IL-6, CXCL1, MMP-1, and AXIN1) showed a >50% decrease. Only three proteins were increased (>20%) during tofacitinib treatment: C-X3-C motif chemokine ligand 1 (CX3CL1), MMP-10, and FMS-like tyrosine kinase 3 ligand (Flt3L). Treatment response correlated positively with baseline IL-6 levels, and negatively with baseline CCL11 and DNER levels.
To the best of our knowledge, the current study is the first to investigate the effects of tofacitinib on such a large range of inflammation-associated plasma proteins in RA patients in vivo. Previous in vitro studies have shown tofacitinib to suppress the production of IL-6, IL-17, IFN-γ, CCL2, CXCL9, CXCL10, and CXCL11 by RA T cells, macrophages, and fibroblasts [11–15]. Also, in newly discovered pathogenic dendritic cells in the circulation of RA patients, tofacitinib suppressed the production of IL-1β, IL-8, and IL-13 significantly, and that of IL-6 non-significantly [24]. In animal models of arthritis tofacitinib treatment has led to decreased levels of circulating IL-6, IL-8, CCL2, and CXCL10 [11, 16]. Our in vivo results confirmed that IL-6 and CXCL11 are significantly downregulated in RA patients treated with tofacitinib, while IL-8, IL-17, IFN-γ, CCL2, CXCL9, and CXCL10 were not affected. These differences highlight the importance of studying the biochemical effects of therapies such as JAK inhibitors in RA patients in vivo.
A recent in vivo study shows that tofacitinib 10 mg twice daily in MTX background downregulates the expression of MMP-1, MMP-3, CCL2, CXCL10, and CXCL13 in the synovium and the plasma levels of CXCL10 in RA patients during 28-day treatment [19]. We demonstrate here that while plasma MMP-1 levels were downregulated, levels of CCL2 or CXCL10 were not affected by 3-month tofacitinib 5mg twice daily plus csDMARD treatment in vivo. It is plausible that the effects of tofacitinib on the expression of chemokines in synovial tissue are not directly comparable to the effects of tofacitinib on circulating chemokine levels. The different result concerning plasma CXCL10 levels may be due to the differences in the treatment duration (1 month versus 3 months), tofacitinib dose (10 versus 5 mg twice daily) or patient characteristics between the study of Boyle et al. and the current study.
In accordance with our finding, it has been previously observed that tofacitinib treatment leads to a marked decrease in circulating IL-6 levels in RA patients [17] and in animal models of arthritis [11, 16]. Therefore, tofacitinib seems to have profound suppressive effect on IL-6 production in arthritis. A decrease in plasma IL-6 levels during treatment of active RA has also been described for MTX [25] and for the combination of MTX and TNF inhibitor [26]. On the contrary, the anti-IL-6 receptor antibody tocilizumab significantly increases plasma IL-6 levels, but on the other hand, it also induces a huge increase in soluble IL-6 receptor levels which counteracts the biological effect of IL-6 [27]. Taken together, reducing biologically available IL-6 seems to be a common effect of several antirheumatic drugs.
Altogether 18 circulating proteins were downregulated in patients treated with tofacitinib. Of these proteins, 14 are involved in the cellular responses to cytokine stimulation and 13 in the inflammatory responses (Table 3). In addition to IL-6, the function of which in the pathogenesis of RA is well documented [28], the majority of these proteins, such as IL-7, IL-12B, TNFSF14, chemokines, and MMP-1, are likely to promote the inflammatory and destructive processes in RA [1, 2]. Hence, their decreased expression may contribute to the clinical efficacy of tofacitinib. However, also the prototypic anti-inflammatory cytokine IL-10 [29] was decreased during tofacitinib treatment. It may be noted that the percentages of IL-10+ B cells after collagen epitope stimulation were shown to be reduced in RA patients and that tofacitinib treatment partially prevented this IL-10 downmodulation [30], indicating that tofacitinib may also function to promote IL-10 expression in certain situations. In healthy controls, instead, the percentages of IL-10+ B cells following collagen epitope stimulation slightly increased [30], highlighting the fact that studying the effects of tofacitinib in healthy controls does not necessarily stand for RA patients. Likewise, it has been observed that JAK inhibition using tofacitinib or baricitinib prevents reactive oxygen species (ROS) release from in vitro primed healthy control neutrophils but had no effect on ROS production by RA neutrophils [31].
The finding that plasma levels of three proteins, i.e. CX3CL1, MMP-10, and Flt3L, increased along with tofacitinib use may seem surprising. Indeed, contradicting our current results, a decline in CX3CL1 plasma levels has been seen in RA patients as a response to treatment with TNF inhibitors or a biologic drug abatacept, a fusion protein preventing T cell activation [32, 33]. Notably, the membrane-bound form of CX3CL1 is an adhesion molecule enhancing leukocyte adhesion and migration to the inflammatory foci, whereas its cleaved, soluble form acts as a chemokine for leukocytes [34]. Thus, an increase in the cleaved form at the expense of the intact molecule may indicate less effective adhesion but still possess proinflammatory potential. MMP-10 is present in synovial fluid and joint tissues of arthritis patients and potentiates cartilage collagenolysis [35]. However, MMP-10 may also be involved in the repair or healing of several tissue types [36, 37]. Flt3L acts as a differentiation factor for dendritic cells and is enriched in the serum and synovial fluid of RA patients, and has been suggested to progress arthritis [38, 39]. On the other hand, Flt3L signaling seems capable to control the formation of osteoclasts [40] and helps in maintaining the balance between protective regulatory T cells and pathogenic Th17 cells [39, 40]. Taken together, the effects of CX3CL1, MMP-10, and Flt3L can be diverse, and it may depend on the immunological context if a decrease or increase in their level is more favorable in arthritis.
There are several possible mechanisms on how tofacitinib may modulate plasma protein levels. First, the self-evident action of tofacitinib is the inhibition of JAK1 and JAK3, and to a slightly lesser extent JAK2, thus restraining cytokine-induced signaling to STATs and subsequent gene transcription [8, 9]. By studying circulating leukocytes of the same patients as in the current study, we recently demonstrated that tofacitinib suppresses both basal and cytokine-induced phosphorylation of STATs in vivo [21]. IL-6 expression is conventionally considered to be mediated via non-JAK/STAT signaling pathways, the foremost nuclear factor kappa light chain enhancer of activated B cells [28], and hence, the effect of tofacitinib on IL-6 production may be indirect. However, according to the novel data, IL-6 production can also be mediated via JAK/STAT3 [41], suggesting that tofacitinib may also directly contribute to the >60% decrease in IL-6 levels we observed.
Second, recent findings indicate that tofacitinib can also regulate cellular functions by affecting the levels of certain microRNAs [42]. Furthermore, novel results suggest that the effects of tofacitinib are associated with the functions of at least some histone deacetylases [43]. Disordered epigenetic mechanisms have been described in RA, and different epigenetic regulation patterns for various MMPs have been found in RA synovial fibroblasts [44]. Whether epigenetic mechanisms connecting tofacitinib and plasma protein expression exist, their exact molecular nature is to be elucidated.
Third, it should be noted that the production of the four plasma proteins that were most strongly reduced by tofacitinib in the current study is partly intertwined. For example, CXCL1 is one factor promoting IL-6 expression in RA synovial fibroblasts [45], and IL-6 increases MMP-1 expression in human chondrocytes [46]. In addition, in epithelial cells knockdown of AXIN1 significantly reduces lipopolysaccharide-induced IL-6 production [47]. Moreover, those 21 proteins that showed statistically significant and marked change by tofacitinib also have either known or predicted protein–protein interactions with each other as we illustrated using STRING analysis. These interactions can be direct physical or indirect functional associations. The possible multiplicative effects on the (near-by) members of the interaction network have to be considered, for example, when designing novel molecularly targeted treatments for RA.
Furthermore, it is possible that the decrease in RA disease activity itself during tofacitinib treatment is associated with the downregulation of inflammation-associated plasma proteins. In fact, when studying the levels of the 21 proteins markedly affected by tofacitinib use, we found that DAS28 improvement correlated with the decrease in the levels of two proteins, namely IL-6 and TNFSF14. Regarding that IL-6 is the crucial cytokine causing phosphorylation of the transcription factor signal transducer and activator of transcription (STAT3), the current finding is also well in line with what we recently found in the same patient population: the decrease in constitutive STAT3 phosphorylation levels in monocytes and CD4+ T cells correlated with DAS28 improvement during 3-month tofacitinib treatment [21]. Interestingly, TNFSF14 is emerging to be a potent contributor to joint injury, as it both promotes survival and activation of synovial fibroblasts in RA [48] and induces osteoclast formation and bone loss [49]. Hence, reduction of TNFSF14 levels might be involved in the structural protection of joints that accompany tofacitinib use.
We also examined if baseline DAS28 correlated with the levels of the 21 proteins markedly affected by tofacitinib use, and vice versa, if the plasma protein levels at baseline could predict treatment response to tofacitinib (determined by DAS28 improvement in 3 months). There were no associations between DAS28 at baseline and the baseline protein levels or the changes in the protein levels, suggesting that whereas DAS28 describes the extent of joint inflammation, the levels of circulating proteins may primarily reflect the systemic inflammatory processes. On the contrary, we found that high baseline IL-6 levels are associated with good treatment response, which is again in accordance with our previous findings demonstrating that constitutive STAT3 phosphorylation also associates with treatment response to tofacitinib [21]. Interestingly, while we previously noticed that STAT3 phosphorylation of circulating leukocytes was also associated with a good response to csDMARDs in recent-onset RA, plasma IL-6 levels were not [4]. Likewise, it has been reported that IL-6 levels cannot predict the treatment efficacy of MTX and prednisone in RA patients [25]. In contrast, a weak association between serum IL-6 levels and treatment response to tocilizumab has been demonstrated [50]. Nevertheless, it seems plausible that by suppressing both IL-6 signaling and production, tofacitinib counteracts this cytokine very effectively, which may be most discernible when the IL-6 levels are initially high.
DNER and CCL11 levels showed a negative correlation to treatment response. In patients with osteoarthritis, the gene of DNER was up-regulated in the affected cartilage [51]. However, little is known about the role of DNER in RA. CCL11 promotes leukocyte migration in RA, and serum and synovial fluid levels of CCL11 are elevated in RA patients [52]. OSM was among the proteins downregulated by tofacitinib use, but its plasma levels were not associated with the treatment response. Interestingly, high levels of circulating OSM have repeatedly been reported in patients with inflammatory bowel disease that does not respond to TNF inhibitors [53, 54]. It remains to be elucidated whether OSM could be a potential biomarker for predicting response to anti-TNF therapy also in RA. In general, a worthy approach, implemented also on the basis of our current results, would be using a small panel of potential markers for predicting treatment response to tofacitinib or other medications in RA.
There are several strengths in the current study: well-characterized RA patients from a prospective study are included, the in vivo setting is likely to reveal the real physiological effects of tofacitinib, and the number of simultaneously studied proteins is high, which enables the discovery of the effects of tofacitinib on novel proteins. A limitation is the relatively small size of the cohort, which allows only predictive conclusions on the association between plasma proteins and treatment response to tofacitinib. In particular, stringent adjustments for multiple testing and analysis of the data with respect to EULAR response criteria were not feasible in this setting.
To conclude, the current study revealed a comprehensive pattern of how tofacitinib treatment affects the levels of plasma proteins in patients with active csDMARD-irresponsive RA. These effects of tofacitinib were somewhat different from those previously described in in vitro studies or in animal models, highlighting the importance of analyzing protein expression in RA patients treated with tofacitinib. Most of the circulating proteins were downregulated, which may contribute to the clinical efficacy of tofacitinib since these proteins predominantly possess proinflammatory characteristics. Finally, due to the lack of biomarkers to predict treatment response to tofacitinib or other JAK inhibitors, the association of certain plasma proteins, such as IL-6, and treatment response to tofacitinib warrants further studies.
Supplementary Material
Acknowledgements
We thank Merja Peltomaa and Antti Kurttila for excellent technical assistance and Tuula Reini, Suvi Mäkinen, and Arja Kaarto for their assistance in patient recruitment and follow-up.
Glossary
Abbreviations
- CCL2
C-C motif chemokine ligand 2
- CX3CL1
C-X3-C motif chemokine ligand 1
- CXCL9
C-X-C motif chemokine ligand 9
- csDMARD
conventional synthetic disease-modifying antirheumatic drug
- DAS28-CRP(4)
disease activity score for 28 joints based on C-reactive protein level
- DAS28
28-joint disease activity score
- DNER
delta and notch-like epidermal growth factor-related Receptor
- EDTA
ethylene diamine tetra acetic acid
- Flt3L
FMS-like tyrosine kinase 3 ligand
- IFN
interferon
- IL-1
interleukin-1
- IL-6
interleukin-6
- JAK
Janus Kinase
- LOD
limit of detection
- MMP
matrix metalloproteinase
- MTX
methotrexate
- NPX
normalized protein expression
- OSM
oncostatin M
- RA
rheumatoid arthritis
- STAT
signal transducer and activator of transcription
- STRING
search tool for the retrieval of interacting genes/proteins
- TNF
tumor necrosis factor-α
- TNFSF14
tumor necrosis factor superfamily member 14.
Contributor Information
Atte Valli, Molecular Immunology Group, Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland.
Krista Kuuliala, Department of Bacteriology and Immunology, Faculty of Medicine, University of Helsinki, Helsinki, Finland.
Anniina Virtanen, Molecular Immunology Group, Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland.
Antti Kuuliala, Department of Bacteriology and Immunology, Faculty of Medicine, University of Helsinki, Helsinki, Finland.
Maaria Palmroth, Molecular Immunology Group, Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland.
Ritva Peltomaa, Inflammation Center, Department of Rheumatology, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Krista-Liisa Vidqvist, Centre for Rheumatic Diseases, Tampere University Hospital, Tampere, Finland.
Marjatta Leirisalo-Repo, Department of Bacteriology and Immunology, Faculty of Medicine, University of Helsinki, Helsinki, Finland; Inflammation Center, Department of Rheumatology, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Olli Silvennoinen, Molecular Immunology Group, Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland; Fimlab Laboratories, Pirkanmaa Hospital District, Tampere, Tampere, Finland; Institute of Biotechnology, HiLIFE Helsinki Institute of Life Sciences, University of Helsinki, Helsinki, Finland.
Pia Isomäki, Molecular Immunology Group, Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland; Centre for Rheumatic Diseases, Tampere University Hospital, Tampere, Finland.
Ethical Approval
This study involving human participants was reviewed and approved by the National Committee on Medical Research Ethics (TUKIJA) and Finnish Medicines Agency Fimea.
Conflict of Interest
KK has received a grant from Pfizer. MP has received a personal fee from Pfizer and is currently a full-time employee of MedEngine. RP has received personal fees from Pfizer, Eli Lilly and Company, and Janssen. K-LV has received personal fee from Abbvie, Boehringer-Ingelheim, Janssen Cilag, Novartis, Pfizer, and UCB Pharma. OS has received a personal fees from Pfizer and Abbvie and holds patents on JAK kinases, US Patent no. 5,728,563, US patent no. 8,841,078, AU 2011214254, CAN 2789186, and EPO 11741946.5. OS is employed by Fimlab Laboratories Ltd. PI has received a grant from Pfizer and personal fees from Pfizer, Eli Lilly and Company, Abbvie, Roche, and ViforPharma and non-financial support from Abbvie and Roche. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Patient Consent
All patients provided their written informed consent to participate in this study.
Clinical Trial Registration
This clinical trial has been registered in the EudraCT database, EudraCT number 2017-002753-11.
Funding
This study was supported by an investigator-initiated award from Pfizer. The molecular immunology laboratory in Tampere University is supported by Academy of Finland, Sigrid Jusélius Foundation, Finnish Cancer Foundation, Jane and Aatos Erkko Foundation, Tampere Tuberculosis Foundation and Pirkanmaa Hospital district competitive research funding.
Data Availability
Data is available from the authors on reasonable request.
Author Contributions
AVal, KK, AVir, AK, MP, ML-R, OS and PI planned the study. RP, KL-V and PI recruited the patients. AVal, AVir, AK and PI analyzed the results. AVal, KK, AK and PI wrote the manuscript and all authors gave valuable comments to the manuscript. All authors contributed to the article and approved the submitted version.
References
- 1. Firestein GS, McInnes IB.. Immunopathogenesis of rheumatoid arthritis. Immunity 2017, 46, 183–96. doi: 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwartz DM, Bonelli M, Gadina M, O’Shea JJ.. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 2016, 12, 25–36. doi: 10.1038/nrrheum.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gadina M, Le MT, Schwartz DM, Silvennoinen O, Nakayamada S, Yamaoka K, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology 2019, 58, i4–i16. doi: 10.1093/rheumatology/key432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuuliala K, Kuuliala A, Koivuniemi R, Oksanen S, Hämäläinen M, Moilanen E, et al. Constitutive STAT3 phosphorylation in circulating CD4+ T lymphocytes associates with disease activity and treatment response in recent-onset rheumatoid arthritis. PLoS One 2015, 10, e0137385. doi: 10.1371/journal.pone.0137385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Isomäki P, Junttila I, Vidqvist KL, Korpela M, Silvennoinen O.. The activity of JAK-STAT pathways in rheumatoid arthritis: constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology 2015, 54, 1103–13. [DOI] [PubMed] [Google Scholar]
- 6. Anderson AE, Pratt AG, Sedhom MAK, Doran JP, Routledge C, Hargreaves B, et al. IL-6-driven STAT signalling in circulating CD4+ lymphocytes is a marker for early anticitrullinated peptide antibody-negative rheumatoid arthritis. Ann Rheum Dis 2016, 75, 466–73. doi: 10.1136/annrheumdis-2014-205850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pertovaara M, Silvennoinen O, Isomäki P.. STAT-5 is activated constitutively in T cells, B cells and monocytes from patients with primary Sjögren’s syndrome. Clin Exp Immunol 2015, 181, 29–38. doi: 10.1111/cei.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Shang-Poa C, et al. Discovery of CP-690,550: a potent and selective Janus Kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem 2010, 53, 8468–84. doi: 10.1021/jm1004286. [DOI] [PubMed] [Google Scholar]
- 9. Haan C, Rolvering C, Raulf F, Kapp M, Drückes P, Thoma G, et al. Jak1 has a dominant role over Jak3 in signal transduction through γc-containing cytokine receptors. Chem Biol 2011, 18, 314–23. doi: 10.1016/j.chembiol.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 10. Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017, 390, 457–68. [DOI] [PubMed] [Google Scholar]
- 11. Maeshima K, Yamaoka K, Kubo S, Nakano K, Iwata S, Saito K, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-γ and interleukin-17 production by human CD4+ T cells. Arthritis Rheum 2012, 64, 1790–8. doi: 10.1002/art.34329. [DOI] [PubMed] [Google Scholar]
- 12. Almanzar G, Kienle F, Schmalzing M, Maas A, Tony HP, Prelog M.. Tofacitinib modulates the VZV-specific CD4+ T cell immune response in vitro in lymphocytes of patients with rheumatoid arthritis. Rheumatology 2019, 58, 2051–60. doi: 10.1093/rheumatology/kez175. [DOI] [PubMed] [Google Scholar]
- 13. Yarilina A, Xu K, Chan C, Ivashkiv LB.. Regulation of inflammatory responses in tumor necrosis factor-activated and rheumatoid arthritis synovial macrophages by JAK inhibitors. Arthritis Rheum 2012, 64, 3856–66. doi: 10.1002/art.37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosengren S, Corr M, Firestein GS, Boyle DL.. The JAK inhibitor CP-690,550 (tofacitinib) inhibits TNF-induced chemokine expression in fibroblast-like synoviocytes: autocrine role of type I interferon. Ann Rheum Dis 2012, 71, 440–7. doi: 10.1136/ard.2011.150284. [DOI] [PubMed] [Google Scholar]
- 15. Migita K, Miyashita T, Izumi Y, Koga T, Komori A, Maeda Y, et al. Inhibitory effects of the JAK inhibitor CP690,550 on human CD4+ T lymphocyte cytokine production. BMC Immunol 2011, 12, 51. doi: 10.1186/1471-2172-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 2011, 186, 4234–43. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodge JA, Kawabata TT, Krishnaswami S, Clark JD, Dowty ME, Menon S, et al. The mechanism of action of tofacitinib – an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 2016, 34, 318–28. [PubMed] [Google Scholar]
- 18. Kawaguchi Y, Waguri-Nagaya Y, Tatematsu N, Oguri Y, Kobayashi M, Nozaki M, et al. The Janus kinase inhibitor tofacitinib inhibits TNF-α-induced gliostatin expression in rheumatoid fibroblast-like synoviocytes. Clin Exp Rheumatol 2018, 36, 559–67. [PubMed] [Google Scholar]
- 19. Boyle DL, Soma K, Hodge J, Kavanaugh A, Mandel D, Mease P, et al. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann Rheum Dis 2015, 74, 1311–6. doi: 10.1136/annrheumdis-2014-206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamar A, Szekanecz Z, Pusztai A, Czókolyová M, Végh E, Pethő Z, et al. Effects of one-year tofacitinib therapy on bone metabolism in rheumatoid arthritis. Osteoporos Int 2021, 32, 1621–9. doi: 10.1007/s00198-021-05871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palmroth M, Kuuliala K, Peltomaa R, Virtanen A, Kuuliala A, Kurttila A, et al. Tofacitinib suppresses several JAK-STAT pathways in rheumatoid arthritis in vivo and baseline signaling profile associates with treatment response. Front Immunol 2021, 12, 738481. doi: 10.3389/fimmu.2021.738481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Assarsson E, Lundberg M, Holmquist G, Björkesten J, Bucht Thorsen S, Ekman D, et al. Homogenous 96-Plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014, 9, e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Validation Data Documents—Target 96 Inflammation [Internet]. olink-inflammation-validation-data-v3.0.pdf. [cited 2022 Jun 1]. Available from: https://www.olink.com/resources-support/document-download-center/
- 24. Marzaioli V, Canavan M, Floudas A, Flynn K, Mullan R, Veale DJ, et al. CD209/CD14+ dendritic cells characterization in rheumatoid and psoriatic arthritis patients: activation, synovial infiltration, and therapeutic targeting. Front Immunol 2022, 12, 722349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nowak D, Lewandowicz J, Dbkowska B, Marczak J.. Combination of methotrexate and prednizone decreases circulating concentrations of interleukin 1 beta and interleukin 6 in patients with rheumatoid arthritis. Poor correlation of cytokine suppression with clinical improvement. Int J Immunopathol Pharmacol 1999, 12, 13–21. [PubMed] [Google Scholar]
- 26. Knudsen LS, Hetland ML, Johansen JS, Skjødt H, Peters ND, Colic A, et al. Changes in plasma IL-6, plasma VEGF and serum YKL-40 during treatment with etanercept and methotrexate or etanercept alone in patients with active rheumatoid arthritis despite methotrexate therapy. Biomark Insights. 2009, 4, 91–5. doi: 10.4137/bmi.s2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jouve T, Laheurte C, Noble J, Weinhard J, Daligault M, Renaudin A, et al. Immune responses following tocilizumab therapy to desensitize HLA-sensitized kidney transplant candidates. Am J Transplant 2022, 22, 71–84. doi: 10.1111/ajt.16709. [DOI] [PubMed] [Google Scholar]
- 28. Pandolfi F, Franza L, Carusi V, Altamura S, Andriollo G, Nucera E.. Interleukin-6 in Rheumatoid Arthritis. Int J Mol Sci 2020, 21, 5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev 2010, 21, 331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 30. Isailovic N, Ceribelli A, Cincinelli G, Vecellio M, Guidelli G, Caprioli M, et al. Lymphocyte modulation by tofacitinib in patients with rheumatoid arthritis. Clin Exp Immunol 2021, 205, 142–9. doi: 10.1111/cei.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mitchell TS, Moots RJ, Wright HL.. Janus kinase inhibitors prevent migration of rheumatoid arthritis neutrophils towards interleukin-8, but do not inhibit priming of the respiratory burst or reactive oxygen species production. Clin Exp Immunol 2017, 189, 250–8. doi: 10.1111/cei.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Umemura M, Isozaki T, Ishii S, Seki S, Oguro N, Miura Y, et al. Reduction of serum ADAM17 level accompanied with decreased cytokines after abatacept therapy in patients with rheumatoid arthritis. Int J Biomed Res 2014, 10, 229–35. [PMC free article] [PubMed] [Google Scholar]
- 33. Sato M, Kasama T, Ohtsuka K, Takahashi R, Wakabayashi K, Odai T, et al. Involvement of CX3CL1/CX3CR1 axis in etanercept therapy for patients with active rheumatoid arthritis. Open Access Rheumatol Res Rev 2011, 3, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 2003, 102, 1186–95. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- 35. Barksby HE, Milner JM, Patterson AM, Peake NJ, Hui W, Robson T, et al. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: Implications for cartilage degradation in arthritis. Arthritis Rheum 2006, 54, 3244–53. doi: 10.1002/art.22167. [DOI] [PubMed] [Google Scholar]
- 36. Gomez-Rodriguez V, Orbe J, Martinez-Aguilar E, Rodriguez JA, Fernandez-Alonso L, Serneels J, et al. Functional MMP-10 is required for efficient tissue repair after experimental hind limb ischemia. FASEB J 2015, 29, 960–72. [DOI] [PubMed] [Google Scholar]
- 37. Valdés-Fernández J, López-Martínez T, Ripalda-Cemboráin P, Calvo IA, Sáez B, Romero-Torrecilla JA, et al. Molecular and cellular mechanisms of delayed fracture healing in Mmp10 (Stromelysin 2) knockout mice. J Bone Miner Res 2021, 36, 2203–13. [DOI] [PubMed] [Google Scholar]
- 38. Dehlin M, Bokarewa M, Rottapel R, Foster SJ, Magnusson M, Dahlberg LE, et al. Intra-articular Fms-like tyrosine kinase 3 ligand expression is a driving force in induction and progression of arthritis. PLoS One 2008, 3, e3633. doi: 10.1371/journal.pone.0003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramos MI, Perez S, Aarrass S, Helder B, Broekstra P, Gerlag DM, et al. FMS-related tyrosine kinase 3 ligand (Flt3L)/CD135 axis in rheumatoid arthritis. Arthritis Res Ther 2013, 15, R209. doi: 10.1186/ar4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Svensson MND, Erlandsson MC, Jonsson IM, Andersson KME, Bokarewa MI.. Impaired signaling through the FMS-like tyrosine kinase 3 receptor increases osteoclast formation and bone damage in arthritis. J Leukoc Biol 2016, 99, 413–23. doi: 10.1189/jlb.3HI1114-572RR. [DOI] [PubMed] [Google Scholar]
- 41. Li J, Cao C, Xiang Y, Hong Z, He D, Zhong H, et al. TLT2 suppresses Th1 response by promoting IL-6 production in monocyte through JAK/STAT3 signal pathway in tuberculosis. Front Immunol 2020, 11, 2031. doi: 10.3389/fimmu.2020.02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chiu YS, Bamodu OA, Fong IH, Lee WH, Lin CC, Lu CH, et al. The JAK inhibitor tofacitinib inhibits structural damage in osteoarthritis by modulating JAK1/TNF-alpha/IL-6 signaling through Mir-149-5p. Bone 2021, 151, 116024. doi: 10.1016/j.bone.2021.116024. [DOI] [PubMed] [Google Scholar]
- 43. Gonneaud A, Turgeon N, Boisvert FM, Boudreau F, Asselin C.. JAK-STAT pathway inhibition partially restores intestinal homeostasis in Hdac1- and Hdac2-intestinal epithelial cell-deficient mice. Cells 2021, 10, 224. doi: 10.3390/cells10020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Araki Y, Mimura T.. Matrix metalloproteinase gene activation resulting from disordered epigenetic mechanisms in rheumatoid arthritis. Int J Mol Sci 2017, 18, 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hou SM, Chen PC, Lin CM, Fang ML, Chi MC, Liu JF.. CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res Ther 2020, 22, 251. doi: 10.1186/s13075-020-02331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aida Y, Honda K, Tanigawa S, Nakayama G, Matsumura H, Suzuki N, et al. IL-6 and soluble IL-6 receptor stimulate the production of MMPs and their inhibitors via JAK–STAT and ERK–MAPK signalling in human chondrocytes. Cell Biol Int 2012, 36, 367–76. doi: 10.1042/CBI20110150. [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y, Luo H, Lv X, Liu J, Chen X, Li Y, et al. Axin-1 binds to caveolin-1 to regulate the LPS-induced inflammatory response in AT-I cells. Biochem Biophys Res Commun 2019, 513, 261–8. doi: 10.1016/j.bbrc.2019.03.153. [DOI] [PubMed] [Google Scholar]
- 48. Pierer M, Brentano F, Rethage J, Wagner U, Hantzschel H, Gay RE, et al. The TNF superfamily member LIGHT contributes to survival and activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology 2007, 46, 1063–70. doi: 10.1093/rheumatology/kem063. [DOI] [PubMed] [Google Scholar]
- 49. Edwards JR, Sun SG, Locklin R, Shipman CM, Adamopoulos IE, Athanasou NA, et al. LIGHT (TNFSF14), a novel mediator of bone resorption, is elevated in rheumatoid arthritis. Arthritis Rheum 2006, 54, 1451–62. doi: 10.1002/art.21821. [DOI] [PubMed] [Google Scholar]
- 50. Wang J, Platt A, Upmanyu R, Germer S, Lei G, Rabe C, et al. IL-6 pathway-driven investigation of response to IL-6 receptor inhibition in rheumatoid arthritis. BMJ Open 2013, 3, e003199. doi: 10.1136/bmjopen-2013-003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Geyer M, Grässel S, Straub RH, Schett G, Dinser R, Grifka J, et al. Differential transcriptome analysis of intraarticular lesional vs intact cartilage reveals new candidate genes in osteoarthritis pathophysiology. Osteoarthritis Cartilage 2009, 17, 328–35. doi: 10.1016/j.joca.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 52. Wakabayashi K, Isozaki T, Tsubokura Y, Fukuse S, Kasama T.. Eotaxin-1/CCL11 is involved in cell migration in rheumatoid arthritis. Sci Rep 2021, 11, 7937. doi: 10.1038/s41598-021-87199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor–neutralizing therapy in patients with inflammatory bowel disease. Nat Med 2017, 23, 579–89. doi: 10.1038/nm.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Verstockt S, Verstockt B, Machiels K, Vancamelbeke M, Ferrante M, Cleynen I, et al. Oncostatin M is a biomarker of diagnosis, worse disease prognosis, and therapeutic nonresponse in inflammatory bowel disease. Inflamm Bowel Dis 2021, 27, 1564–75. doi: 10.1093/ibd/izab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the authors on reasonable request.