Abstract
Type 1 diabetes (T1D) is an autoimmune disease resulting in the destruction of the insulin-producing pancreatic beta cells. Disease progression occurs along a trajectory from genetic risk, the development of islet autoantibodies, and autoreactive T cells ultimately progressing to clinical disease. Natural history studies and mechanistic studies linked to clinical trials have provided insight into the role of the immune system in disease pathogenesis. Here, we review our current understanding of the underlying etiology of T1D, focusing on the immune cell types that have been implicated in progression from pre-symptomatic T1D to clinical diagnosis and established disease. This knowledge has been foundational for the development of immunotherapies aimed at the prevention and treatment of T1D.
Keywords: autoimmunity, cellular immunology, diabetes, immunotherapy, autoantibodies
This review summarizes the current understanding of the underlying immunopathology of T1D, focusing on the immune cell types implicated in progression from pre-symptomatic T1D to clinical diagnosis and established disease. It also highlights how this knowledge is driving the development of immunotherapies aimed at the prevention and treatment of T1D.
Graphical Abstract
Graphical Abstract.
Introduction
Diabetes is a chronic disease characterized by high blood glucose affecting more than 422 million people worldwide and is increasing in incidence [1]. Here, we focus on the role of the immune system in beta cell damage and destruction in Type 1 diabetes (T1D). T1D represents 10–15% of all diabetes, and diagnosis occurs more often in children but can also occur in adulthood. The incidence of T1D is rising; between 2001 and 2009, there was a 21% increase in prevalence in people under the age of 20 years [2], and it is expected that 5 million people in the USA will have T1D by 2050 [2]. T1D is the result of insufficient production of insulin due to damage and destruction of the insulin-producing beta cells in the pancreatic islet by one’s own immune system. Here, we review what is currently known about immune mechanisms underlying disease pathogenesis and describe the emerging therapies for the prevention and treatment of T1D.
T1D is an autoimmune disease. Immune recognition and inflammation ultimately result in the destruction of beta cells. Yet, as in other autoimmune diseases, this process occurs over time and reflects a series of factors that result in clinical disease. Studies of the natural history of T1D have shed light on this process. From the cumulative work of many years, it is clear that T1D results from a combination of genetic risk, environmental triggers, and the development of B- and T-cell autoreactivity toward the beta cell and its products. Two factors that have been critical in advancing our understanding of disease progression have been the recognition that first-degree relatives are at high risk for T1D in part due to human leukocyte antigen (HLA) Class II-associated genes and second is the work defining the natural history of islet autoantibodies in individuals deemed to be at high risk for T1D [3–5] (Fig. 1). This was made possible by advances in assays measuring islet autoantibodies, either individually or as a composite, including those targeting insulin autoantibodies (IAA), tyrosine phosphatase (IA-2), glutamic acid decarboxylase (GADA), and zinc transporter (ZnT8) [6]. Using these assays in multiple longitudinal prospective cohorts, it has now been definitively demonstrated that the presence and number of islet autoantibodies can be used to predict the development of clinical T1D [3–5]. This knowledge has enabled the staging of pre-symptomatic T1D to time of clinical diagnosis; Stage 1 is defined by two or more islet autoantibodies with normoglycemia, Stage 2 is two or more islet autoantibodies with dysglycemia, and Stage 3 is clinical diagnosis with symptomatic T1D [7] (Fig. 1). This classification of T1D stages has been game-changing with respect to how we think about T1D diagnosis, treatment opportunities, and disease pathogenesis. However, there remain a number of key questions, in particular, we need to better understand the pathogenic mechanisms that promote progression through each of the stages of T1D, and determine whether these mechanisms are shared by all T1D subjects. We can then apply this information to guide more targeted therapy based on disease stage and patient type.
Figure 1:
Predisposition, natural history, and staging of T1D. Disease progression occurs along a trajectory from genetic risk, initiation, and progression to clinical diagnosis. During initiation and progression, there is immune activation and immune response, respectively, with the development of islet autoantibodies. Disease progression is sequential but the rate of progression is variable among individuals. There are three stages of pre-symptomatic disease as defined by JDRF, the Endocrine Society, and the American Diabetes Association [7]: Stage 1 is defined by two or more islet autoantibodies with normal blood glucose levels; Stage 2 is two or more islet autoantibodies with dysglycemia; and Stage 3 is clinical diagnosis with symptomatic T1D. Ongoing research seeks to define the environmental triggers that initiate and promote disease progression.
Immunopathology and mechanisms of T1D
There are currently many tools to study the immunopathology and mechanisms of T1D including the non-obese diabetic (NOD) mouse model of spontaneous autoimmune diabetes, humanized mouse models, and human cohorts, both cross-sectional and longitudinal. Here, we summarize conclusions from human studies, specifically cross-sectional studies and the more recent longitudinal studies, which are advancing our understanding of disease state as well as providing insight into disease trajectory over time.
Genetics, age, and antibody–antigen specificity are all risk factors for T1D and emphasize the complex nature of T1D where predisposition involves higher T- and B-cell responses to islet antigens, impaired immune regulation, and aberrant innate inflammation that impairs immune regulation and homeostasis. This innate-adaptive intersection is highlighted in the following two studies. In the first study analyzing cross-sectional and longitudinal at-risk cohorts, siblings of individuals with T1D exhibited increased early innate inflammation with progression to clinical diagnosis characterized by a loss of immunoregulatory processes [8]. In the more recent study, innate inflammation drives the activation of natural killer (NK) cells to impair regulatory T cells (Treg) [9].
Genetics is a key contributor to pathogenesis and progression of T1D
Although 85% of people with T1D have no family history of disease, it is well documented that genetics contributes to T1D susceptibility [10]. The evidence for genetics comes from family history studies and genome-wide association studies (GWAS). In families with a member with T1D, siblings have an average of 6–7% lifetime risk compared to 0.4% risk in the general population [11, 12]. Moreover, the risk of T1D in identical twins with one having T1D is >70% [13]. The HLA region on the short arm of chromosome 6 accounts for 50% of the genetic association with T1D. The HLA region corresponds to the major histocompatibility complex (MHC) in other animals and encodes cell surface receptors that present antigens to T cells. For T1D, the HLA Class II alleles, HLA-DR and HLA-DQ have the strongest association with DR3-DQ2 and DR4-DQ8, the highest risk genotypes and DQ*0602, a protective genotype. Specifically, people with T1D are carriers of either HLA-DR3, DQB1*0201 (DR3-DQ2) or DR4, DQB1*0302 (DR4-DQ8) [14, 15]. To a lesser degree, the HLA Class I alleles, HLA B38 and A24 also predispose disease, especially a younger age for onset and faster disease progression. GWAS have also identified more than 60 single-nucleotide polymorphisms (SNPs) outside the HLA region that are associated with T1D susceptibility [16–18]; of these only 10% are within the coding region of the genome with the remaining 90% located in non-coding regions. Furthermore, The Environment Determinants of Diabetes in the Young (TEDDY) study has reported an overlap between SNPs associated with T1D susceptibility and those associated with the development of the islet autoantibodies thus implicating genetics in both disease pathogenesis and progression [19].
Research investigating the functional impact of T1D-associated SNPs is ongoing. SNPs within coding regions have been implicated in a number of immune pathways including antigen presentation (HLA, INS-VNTR, CTSH), T-cell activation (PTPN22, CD25, LAT, CD226, SH2B3, UBASH3A), cytokine signaling for tuning differentiation and degree of the immune response (PTPN2, CD25, IL7RA, IL-6RA, IFIH1, TYK2), and immune regulatory pathway for dampening the response region (CTLA4, PTPN2, PTPN22, SIRPG) [20]. It should be noted, however, that the functional impact of T1D-associated SNPs is often specific to cell type and/or activation state. For example, SNPs in PTPN22 have been shown to influence the function of T cells, B cells, and monocyte populations [21, 22], and SNPs in PTPN2 and CD25 influence the IL-2 signaling pathway in effector and Treg, whereas other SNPs in the CD25 locus influence the response to IL-2 in either Treg or Teff but not the other [23–25]. Importantly, some of the associated risk alleles influence gene expression in beta cells [26, 27] and in particular the response of beta cells to inflammation [28], suggesting some cross-talk between immune cell- and beta cell-derived genetic risk. Defining the functional impact of non-coding SNPs has been more challenging with emerging evidence suggesting that these SNPs function by altering the three-dimensional structure of chromatin and thus affect the regulation of distal genes important in disease pathogenesis [29–31]. In addition, the role of the epigenome comprised of DNA methylation, histone modifications, and non-coding RNAs has only just begun to be investigated in the setting of autoimmune disease. Lastly, genetic risk scores (GRS) integrating HLA genotypes, GWAS SNPs, and in some cases autoantibody trajectories, are currently under development to predict T1D susceptibility and rates of disease progression [32, 33].
Autoantibodies, antigens, and age influence disease pathogenesis and progression
Multiple islet antigens have been defined for T1D. Importantly islet autoantibodies precede clinical disease development and now are used as a tool to predict the development of T1D [6]. Similarly, T-cell responses targeting islet antigens are well described prior to and at the time of clinical T1D onset [34]. More recently, a growing number of “neo-epitopes” have been defined including post-translationally modified islet proteins and hybrid insulin peptides [34]. These neo-epitopes are likely generated in tandem with beta cell stress, and their role in disease risk or progression is still being determined [35]. Although it is well documented that seroconversion to multiple autoantibodies ensures progression to clinical diagnosis, less is known about the contribution of epitope spreading, specifically the order and timing of islet autoantibody appearance. However, it is clear that there is heterogeneity and emerging evidence suggests that patterns of epitope spreading impact the rate of disease progression differently. The first autoantibodies to appear target either IAA or GADA and which autoantibody is first depends on HLA haplotype and age. IAA appears first in individuals who are around 1 year of age and have the HLA-DR4-DQ8 genotype whereas GADA appears first at around 2 years of age in HLA-DR3 individuals [36]. Furthermore, a recent transcriptomic analysis revealed that IAA-first individuals had different gene expression signatures than GADA-first individuals [37]. In IAA-first individuals, a NK cell-based signature was identified that increased with progression toward onset of clinical disease. In contrast, GADA-first individuals had a monocyte-based signature. Collectively, these studies highlight the importance of understanding epitope spreading in the context of pathogenesis and progression of disease.
Etiology
With the rising incidence of T1D, the contribution of high-risk HLA haplotypes has decreased [38] indicating that genetics alone is not sufficient to drive the development of T1D. To date, no single trigger has been identified and it is postulated that there is a “threshold effect” involving multiple triggers. Both enterovirus infection and rapid weight gain early in life are risk factors [39]. The gut microbiome has also been implicated as a risk factor; lack of microbial diversity and/or changes in the microbiome appear to increase the risk of T1D [40–43]. Interestingly, there is also evidence in the NOD mouse model that cross-reactivity between gut bacteria and self-antigens may trigger autoimmunity in T1D [44]. The identification of environmental triggers continues to be a challenge, but our growing ability to screen the population for T1D risk factors (genes and islet autoantibodies) holds promise as a mechanism to better define these triggers and intervene early to impede the development of T1D.
Immunopathology
Decades of research comparing the immune system in T1D to that in healthy subjects continues to emphasize the role of the adaptive immune response, including B and T cells, in disease pathology. These findings are supported by mouse models and genetic associations with T1D. In humans, key insight into T1D pathogenesis has been revealed through studying immune cells in the context of the natural history of disease and comparing fast and slow disease progression. Findings from these studies are also informative for development of immunotherapies and biomarkers of disease progression.
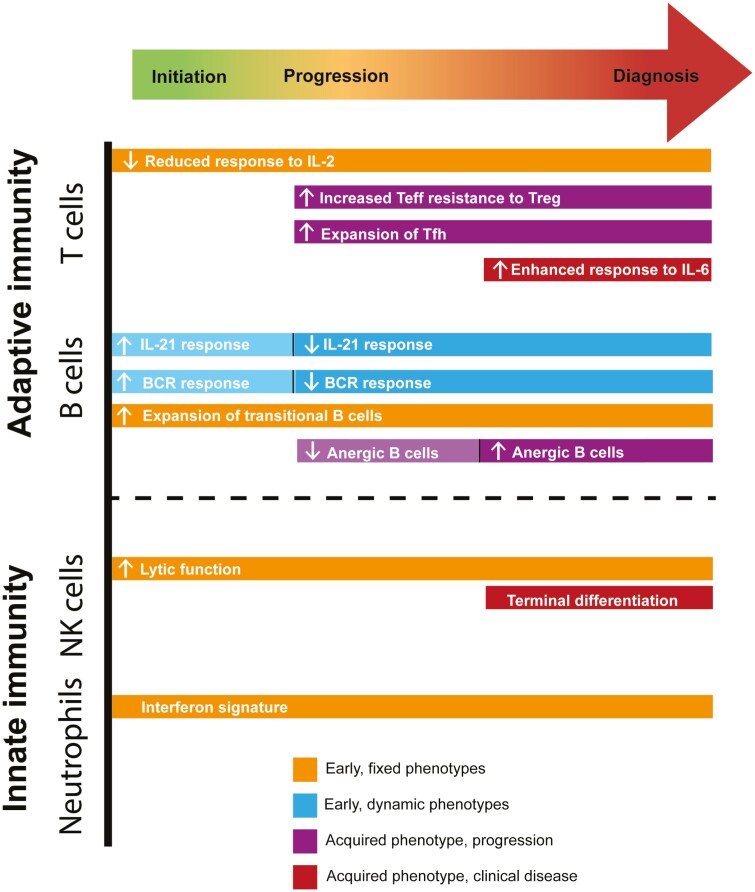
Natural history studies have shown that some immune features associated with T1D are present throughout disease development whereas others are only present at specific stages of disease (Fig. 2), suggesting that the altered immune features in T1D are either genetically fixed or acquired over time [45]. Reduced response to IL-2 by conventional CD4+ T cells, expansion of transitional B cells, and increased NK cell lytic function are examples of fixed immune features observed at time of the first appearance of a single autoantibody and present throughout progression to established disease [9, 45]. In contrast, increased frequency of both T follicular helper cells [46–48] and T peripheral helper cells [49] are acquired with disease progression following fixed immune perturbations while increased T effector resistance to Treg-mediated suppression [50, 51, 52] and more terminal NK differentiation [9] are acquired immune features, occurring with the transition to overt clinical diabetes. Similarly, the enhanced T-cell response to IL-6 [53] and the expansion of anergic B cells [54] are also acquired but only after onset of clinical disease. Interestingly, alterations in the B-cell response to either IL-21 or BCR signaling are dynamic with an enhanced response in autoantibody-positive at-risk subjects, but a diminished response in at-risk subjects that progress to clinical diagnosis and established T1D subjects [45]. The frequency of anergic B cells is also dynamic with reduced frequency prior to disease onset and an increased frequency in established T1D [55, 56]. Thus, some immune alterations may be present at or near disease initiation and influence overall disease risk.
Figure 2:
Balance of innate and adaptive fixed and acquired immune phenotypes during the trajectory of T1D. Early fixed phenotypes in orange, early dynamic phenotypes in light blue, phenotypes acquired during progression in purple, and phenotypes acquired at clinical disease onset in red.
Comparing the immune landscape of individuals with slow disease progression to individuals with fast disease progression has also been informative and complementary to the natural history studies. To date, these studies have focused on rate of disease progression in at-risk subjects as measured by time to clinical diagnosis, and in recent-onset subjects as measured by loss of C-peptide, a proxy for beta cell function. A number of immune features distinguish slow progressors from fast progressors (Fig. 3). The specificity of the first autoantibody is associated with HLA risk and rate of progression suggesting a role for antigen and antigen-presenting cells; IAA autoantibody first and DR4 are associated with fast progression while GADA autoantibody first and DR3 are associated with slower progression [3, 57]. Moreover, IA-2A as the second autoantibody is associated with higher risk as compared to all other autoantibodies [58]. Slow progressors have been characterized by a lack of islet autoantigen-specific CD8+ T cells, B cells expressing higher levels of CD95, and expansion of activated memory Tregs [59, 60]. In addition, slow progressors have alterations in Treg-mediated suppression with Tregs having impaired function and CD4+ T effector cells more responsive to Treg-mediated suppression [60]. In the setting of recent-onset T1D, slow progressors have high levels of islet-specific CD8+ T cells with features of exhaustion, and these cells are not lost over time [61, 62]. Slow progressors also have the highest frequency of CD4+CD25+CD127hi cells at diagnosis [63]. In contrast, fast progressors have the highest levels of B cells and low levels of circulating neutrophils [64, 65]. Interestingly, the levels of B cells were dependent on age and predicted of rate of progression in young subjects only [64]. More recent studies also implicate newly described cell types in rate of disease progression including lytic CD4+ T cells that are expanded in slow progressors [66] and activated islet-specific CD8+ T cells resembling pathogenic self-renewing stem-like CD8+ T cells [67–70] that are expanded in fast progressors [61]. T1D progression has also been recently associated with a loss in CD3+CD56+ Tregs [71]. Overall, these studies have advanced our understanding of pathogenic and protective immune cell types prior to clinical diagnosis as well as in established T1D. Also notable, these studies have been foundational for clinical trials investigating the immune response to therapy, helping to define the immune features that distinguish responders from non-responders.
Figure 3:
HLA, age, and immune features distinguish fast disease progression from slow disease progression. Beta cell function as measured by C-peptide loss. Fast progressors are defined by complete loss of beta cell function within 2 years of diagnosis.
Current treatment methods
Insulin replacement therapy, first administered to a human patient in 1922, remains the standard of care for treating T1D. Over the years, insulin therapy has been improved through the development of different types of insulin: short-, intermediate-, and long-acting forms and use of mixed insulin types. In recent years, there have been substantial improvements in how insulin is administered with the development of insulin delivery pens, insulin pumps, glucose sensors, and closed loop systems, sometimes known as artificial pancreas. The closed loop system integrates an insulin pump and continuous glucose monitoring, and a recent clinical trial demonstrated that this technology reduced the frequency of severe hypoglycemic events in hypoglycemic-prone adults with T1D [72]. These improvements have led to improved glucose control and enhanced the lives of the many people living with T1D.
Future of treatment
Despite the improvements in insulin therapy, the ultimate goal is for the patient to produce their own insulin by preserving their pancreatic beta cells or through replacement of beta cells. Thus, immunotherapy and stem cell-derived islet beta cells represent the future for prevention and treatment of T1D. This goal is becoming a reality as demonstrated in two recent clinical trials. The first showed that targeting T cells with the monoclonal antibody teplizumab in individuals deemed to be at-risk resulted in a delay of progression to clinical diagnosis by at least 2 years [73], indicating that immunotherapies, particularly when applied early, have the potential to delay or prevent disease. The second breakthrough is an ongoing trial testing whether transplanting stem cell-derived beta cells into people with T1D is safe and effective at producing insulin and regulating blood glucose levels (ClinicalTrials.gov Identifier: NCT04786262). These therapies in themselves are exciting, and also demonstrate that prevention and cure are possible. However, challenges remain in generating sufficient numbers of islets, preserving post-transplant survival, and preventing immunogenicity [74].
Immune therapies targeting T cells, B cells, and cytokines have been developed for a variety of autoimmune diseases and have been applied to T1D. Initial studies have been performed in new-onset T1D. Many of these trials indicate that although immune therapies are able to preserve or slow beta cell function for a short period of time, none result in long-term reversal or retention of C-peptide. Notably, this pattern has been true for therapies that target different components of the immune response, including B-cell depletion (rituximab), co-stimulation blockade (Abatacept) [75, 76], low-dose anti-thymocyte globulin (ATG) [77, 78] and inhibition of TNF-α (etanercept, golimumab) [79, 80], anti-CD3(teplizumab) [81, 82], and anti-CD2 (alefacept) [83, 84]. In contrast, other therapies targeting other components of the immune system have shown no benefit including IL-6 receptor blockade (tocilizumab) [85], and IL-1β blockade (canakinumab) [86]. In all cases, these studies have yielded insight into the concept that some individuals may respond to therapy while others may not indicating that among individuals with T1D there may be distinct endotypes that are responsive to different immune interventions [87].
A question posed by the inability to reverse or retain beta cell function in the context of new-onset T1D is whether treatment prior to clinical disease would be more effective. Not only would an individual still have adequate insulin production at this point but there may also be a window of opportunity to reinstate tolerance prior to clinical disease. In T1D, studies addressing this question are ongoing in individuals at high risk for T1D due to their genetic background and the presence of autoantibodies. Most notably among these is the teplizumab trial described above which demonstrated efficacy in this setting. A key take-home message from all these trials to date is that deciding on the appropriate immunotherapy and the appropriate window of opportunity for treatment is critical and depends on disease stage.
Development of the next generation of immunotherapies is well underway, specifically antigen-specific therapies and combination therapies. T1D presents a unique opportunity for antigen-specific therapies to be used as an intervention prior to clinical disease since the antigens have already been identified and characterized. Current approaches to delivering antigen-specific therapies include peptide immunization [88], nanoparticles [89], or antigen-specific Treg [90]. Combination therapies are also being explored. A number of these are combining immunomodulatory therapies with drugs that protect beta cell health. For example, a recent Phase 2 trial demonstrated that combining an IL-21 monoclonal antibody with the glucagon-like peptide-1 receptor (GLP-1R) agonist, liraglutide, preserved beta cell function in recently diagnosed T1D [91]. Other combination therapies are focused on enhancing immunotherapies. A recent trial combined polyclonal Tregs with low-dose IL-2 to enhance Treg survival and expansion [92]. Following immune depletion therapies with immune tolerizing therapies is also under consideration. In addition, the combination of tolerizing therapies such as chimeric antigen receptor (CAR) Treg in combination with beta cell replacement may allow re-establishment of insulin independence for individuals with established T1D. Despite the promise of therapies targeting the immune system, there remain some major challenges. First, better biomarkers of success are needed for clinical trials in order to shorten the length of the trial. We currently rely on C-peptide preservation as a measure of beta cell function as we cannot directly visualize islets or beta cell injury. Also, we need to ensure that the future therapy is better than the improved insulin replacement therapies, especially in terms of adverse events.
Conclusions
T1D is a chronic disease that we have learned to manage over the past 100 years through ever-improving delivery of insulin. In that time, through the development of strong networks of clinicians, scientists, and patients, we have developed a solid understanding of the immunopathology of T1D and now have the ability to predict who is at highest risk for disease. This gives us the opportunity to work toward disease prevention and preservation of the ability to produce insulin. Our challenges are to identify not only the best therapies, but also to whom they should be given and importantly at what point in the progression of disease. This will require the development of precision medicine-based approaches to clinical trial design and ultimately to treatment, so that we can ensure that the benefit outweighs the risk for any treatment we use.
Acknowledgements
The authors would like to thank Drs Anne Hocking, Taylor Lawson, and Virginia Green from the Benaroya Research Institute Scientific Writing Group for assistance with writing the manuscript, preparing figures, and copyediting.
Glossary
Abbreviations
- CAR
chimeric antigen receptor
- GADA
glutamic acid decarboxylase
- GLP-1R
glucagon-like peptide-1 receptor
- GWAS
genome-wide association studies
- HLA
human leukocyte antigen
- IA-2
tyrosine phosphatase
- IAA
insulin autoantibodies
- MHC
major histocompatibility complex
- NK
natural killer
- NOD
non-obese diabetic
- SNPs
single-nucleotide polymorphisms
- T1D
Type 1 diabetes
- Treg
regulatory T cells
- ZnT8
zinc transporter.
Contributor Information
S Alice Long, Center for Translational Immunology, Benaroya Research Institute at Virginia Mason, Seattle, WA, USA.
Jane H Buckner, Center for Translational Immunology, Benaroya Research Institute at Virginia Mason, Seattle, WA, USA.
Funding
This work was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust (to J.H.B.), the National Institute of Allergy and Infectious Diseases (NIH/NIAID) (R01 AI132774 to J.H.B. and R01 AI141952 to S.A.L.), and JDRF (3-SRA-2019-851-M-B to S.A.L.).
Conflict of interests
S.A.L. is a consultant for Sonoma Biotherapeutics, a member of the Type 1 Diabetes TrialNet Mechanistic Assay Group, a member of the Immune Tolerance Network Mechanistic Assay Group, and has received past research support from Caladrius Biosciences, Sonoma Biotherapeutics, and Janssen. J.H.B. is a Scientific Co-Founder and Scientific Advisory Board member of GentiBio, a consultant for Bristol-Myers Squibb and Hotspot Therapeutics, and has past and current research projects sponsored by Amgen, Bristol-Myers Squib, Janssen, Novo Nordisk, and Pfizer. She is a member of the Type 1 Diabetes TrialNet Study Group, a partner of the Allen Institute for Immunology, and a member of the Scientific Advisory Boards for the La Jolla Institute for Allergy and Immunology and BMS Immunology.
Author contributions
S.A.L. conceived, collected literature, and wrote the review. J.H.B. conceived, collected literature, and wrote the review. Both authors reviewed and approved the final manuscript.
Data availability
Not applicable.
Animal research statement
Not applicable.
Permission to reproduce (for relevant content)
Not applicable.
References
- 1. World Health Organization. Diabetes, 2022. https://www.who.int/health-topics/diabetes#tab=tab_1
- 2. Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014, 311, 1778–86. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013, 309, 2473–9. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009, 32, 2269–74. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steck AK, Vehik K, Bonifacio E, Lernmark A, Ziegler AG, Hagopian WA, et al. Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015, 38, 808–13. doi: 10.2337/dc14-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. So M, Speake C, Steck AK, Lundgren M, Colman PG, Palmer JP, et al. Advances in type 1 diabetes prediction using islet autoantibodies: beyond a simple count. Endocr Rev 2021, 42, 584–604. doi: 10.1210/endrev/bnab013. [DOI] [PubMed] [Google Scholar]
- 7. Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015, 38, 1964–74. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen YG, Cabrera SM, Jia S, Kaldunski ML, Kramer J, Cheong S, et al. Molecular signatures differentiate immune states in type 1 diabetic families. Diabetes 2014, 63, 3960–73. doi: 10.2337/db14-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dean JW, Peters LD, Fuhrman CA, Seay HR, Posgai AL, Stimpson SE, et al. Innate inflammation drives NK cell activation to impair Treg activity. J Autoimmun 2020, 108, 102417. doi: 10.1016/j.jaut.2020.102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Redondo MJ, Steck AK, Pugliese A.. Genetics of type 1 diabetes. Pediatr Diabetes 2018, 19, 346–53. doi: 10.1111/pedi.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mrena S, Virtanen SM, Laippala P, Kulmala P, Hannila ML, Akerblom HK, et al. Models for predicting type 1 diabetes in siblings of affected children. Diabetes Care 2006, 29, 662–7. doi: 10.2337/diacare.29.03.06.dc05-0774. [DOI] [PubMed] [Google Scholar]
- 12. Dorman JS, Steenkiste AR, O’Leary LA, McCarthy BJ, Lorenzen T, Foley TP.. Type 1 diabetes in offspring of parents with type 1 diabetes: the tip of an autoimmune iceberg? Pediatr Diabetes 2000, 1, 17–22. doi: 10.1034/j.1399-5448.2000.010104.x. [DOI] [PubMed] [Google Scholar]
- 13. Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T.. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 2008, 359, 2849–50. doi: 10.1056/NEJMc0805398. [DOI] [PubMed] [Google Scholar]
- 14. Morran MP, Vonberg A, Khadra A, Pietropaolo M.. Immunogenetics of type 1 diabetes mellitus. Mol Aspects Med 2015, 42, 42–60. doi: 10.1016/j.mam.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noble JA. Immunogenetics of type 1 diabetes: a comprehensive review. J Autoimmun 2015, 64, 101–12. doi: 10.1016/j.jaut.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 16. Onengut-Gumuscu S, Chen W-M, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, et al. ; Type 1 Diabetes Genetics Consortium. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet 2015, 47, 381–6. doi: 10.1038/ng.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009, 41, 703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robertson CC, Inshaw JRJ, Onengut-Gumuscu S, Chen WM, Santa Cruz DF, Yang H, et al. Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes. Nat Genet 2021, 53, 962–71. doi: 10.1038/s41588-021-00880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torn C, Hadley D, Lee HS, Hagopian W, Lernmark A, Simell O, et al. Role of type 1 diabetes-associated SNPs on risk of autoantibody positivity in the TEDDY study. Diabetes 2015, 64, 1818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shapiro MR, Thirawatananond P, Peters L, Sharp RC, Ogundare S, Posgai AL, et al. De-coding genetic risk variants in type 1 diabetes. Immunol Cell Biol 2021, 99, 496–508. doi: 10.1111/imcb.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamerman JA, Pottle J, Ni M, He Y, Zhang ZY, Buckner JH.. Negative regulation of TLR signaling in myeloid cells—implications for autoimmune diseases. Immunol Rev 2016, 269, 212–27. doi: 10.1111/imr.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson MB, Cerosaletti K, Flanagan SE, Buckner JH.. Genetic mechanisms highlight shared pathways for the pathogenesis of polygenic type 1 diabetes and monogenic autoimmune diabetes. Curr Diab Rep 2019, 19, 20. doi: 10.1007/s11892-019-1141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cerosaletti K, Schneider A, Schwedhelm K, Frank I, Tatum M, Wei S, et al. Multiple autoimmune-associated variants confer decreased IL-2R signaling in CD4+ CD25hi T cells of type 1 diabetic and multiple sclerosis patients. PLoS One 2013, 8, e83811. doi: 10.1371/journal.pone.0083811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Long SA, Cerosaletti K, Wan JY, Ho JC, Tatum M, Wei S, et al. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4+ T cells. Genes Immun 2011, 12, 116–25. doi: 10.1038/gene.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwedhelm K, Thorpe J, Murray SA, Gavin M, Speake C, Greenbaum C, et al. Attenuated IL-2R signaling in CD4 memory T cells of T1D subjects is intrinsic and dependent on activation state. Clin Immunol 2017, 181, 67–74. doi: 10.1016/j.clim.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eizirik DL, Sammeth M, Bouckenooghe T, Bottu G, Sisino G, Igoillo-Esteve M, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet 2012, 8, e1002552. doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramos-Rodríguez M, Raurell-Vila H, Colli ML, Alvelos MI, Subirana-Granés M, Juan-Mateu J, et al. The impact of proinflammatory cytokines on the β-cell regulatory landscape provides insights into the genetics of type 1 diabetes. Nat Genet 2019, 51, 1588–95. doi: 10.1038/s41588-019-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eizirik DL, Colli ML.. Revisiting the role of inflammation in the loss of pancreatic β-cells in T1DM. Nat Rev Endocrinol 2020, 16, 611–2. doi: 10.1038/s41574-020-00409-6. [DOI] [PubMed] [Google Scholar]
- 29. Mishra A, Hawkins RD.. Three-dimensional genome architecture and emerging technologies: looping in disease. Genome Med 2017, 9, 87. doi: 10.1186/s13073-017-0477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ding J, Frantzeskos A, Orozco G.. Functional genomics in autoimmune diseases. Hum Mol Genet 2020, 29, R59–65. doi: 10.1093/hmg/ddaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grant SFA, Wells AD, Rich SS.. Next steps in the identification of gene targets for type 1 diabetes. Diabetologia 2020, 63, 2260–9. doi: 10.1007/s00125-020-05248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrat LA, Vehik K, Sharp SA, Lernmark A, Rewers MJ, She JX, et al. A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat Med 2020, 26, 1247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Redondo MJ, Geyer S, Steck AK, Sharp S, Wentworth JM, Weedon MN, et al. A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care 2018, 41, 1887–94. doi: 10.2337/dc18-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. James EA, Mallone R, Kent SC, DiLorenzo TP.. T-cell epitopes and neo-epitopes in type 1 diabetes: a comprehensive update and reappraisal. Diabetes 2020, 69, 1311–35. doi: 10.2337/dbi19-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piganelli JD, Mamula MJ, James EA.. The role of β cell stress and neo-epitopes in the immunopathology of type 1 diabetes. Front Endocrinol 2020, 11, 624590. doi: 10.3389/fendo.2020.624590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giannopoulou EZ, Winkler C, Chmiel R, Matzke C, Scholz M, Beyerlein A, et al. Islet autoantibody phenotypes and incidence in children at increased risk for type 1 diabetes. Diabetologia 2015, 58, 2317–23. doi: 10.1007/s00125-015-3672-y. [DOI] [PubMed] [Google Scholar]
- 37. Xhonneux LP, Knight O, Lernmark A, Bonifacio E, Hagopian WA, Rewers MJ, et al. Transcriptional networks in at-risk individuals identify signatures of type 1 diabetes progression. Sci Transl Med 2021, 13, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 2004, 364, 1699–700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- 39. Quinn LM, Wong FS, Narendran P.. Environmental determinants of type 1 diabetes: from association to proving causality. Front Immunol 2021, 12, 737964. doi: 10.3389/fimmu.2021.737964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Goffau MC, Fuentes S, van den Bogert B, Honkanen H, de Vos WM, Welling GW, et al. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia 2014, 57, 1569–77. doi: 10.1007/s00125-014-3274-0. [DOI] [PubMed] [Google Scholar]
- 41. Davis-Richardson AG, Ardissone AN, Dias R, Simell V, Leonard MT, Kemppainen KM, et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol 2014, 5, 678. doi: 10.3389/fmicb.2014.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018, 562, 589–94. doi: 10.1038/s41586-018-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 2016, 165, 1551. doi: 10.1016/j.cell.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 44. Tai N, Peng J, Liu F, Gulden E, Hu Y, Zhang X, et al. Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. J Exp Med 2016, 213, 2129–46. doi: 10.1084/jem.20160526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Habib T, Long SA, Samuels PL, Brahmandam A, Tatum M, Funk A, et al. ; Type 1 Diabetes TrialNet Study Group. Dynamic immune phenotypes of B and T helper cells mark distinct stages of T1D progression. Diabetes 2019, 68, 1240–50. doi: 10.2337/db18-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kenefeck R, Wang CJ, Kapadi T, Wardzinski L, Attridge K, Clough LE, et al. Follicular helper T cell signature in type 1 diabetes. J Clin Invest 2015, 125, 292–303. doi: 10.1172/JCI76238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Viisanen T, Ihantola EL, Näntö-Salonen K, Hyöty H, Nurminen N, Selvenius J, et al. Circulating CXCR5+PD-1+ICOS+ follicular T helper cells are increased close to the diagnosis of type 1 diabetes in children with multiple autoantibodies. Diabetes 2017, 66, 437–47. doi: 10.2337/db16-0714. [DOI] [PubMed] [Google Scholar]
- 48. Ferreira RC, Simons HZ, Thompson WS, Cutler AJ, Dopico XC, Smyth DJ, et al. IL-21 production by CD4+ effector T cells and frequency of circulating follicular helper T cells are increased in type 1 diabetes patients. Diabetologia 2015, 58, 781–90. doi: 10.1007/s00125-015-3509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ekman I, Ihantola EL, Viisanen T, Rao DA, Näntö-Salonen K, Knip M, et al. Circulating CXCR5−PD-1hi peripheral T helper cells are associated with progression to type 1 diabetes. Diabetologia 2019, 62, 1681–8. doi: 10.1007/s00125-019-4936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH.. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol 2008, 181, 7350–5. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ihantola E-L, Viisanen T, Gazali AM, Näntö-Salonen K, Juutilainen A, Moilanen L, et al. Effector T cell resistance to suppression and STAT3 signaling during the development of human type 1 diabetes. J Immunol 2018, 201, 1144–53. doi: 10.4049/jimmunol.1701199. [DOI] [PubMed] [Google Scholar]
- 52. Lawson JM, Tremble J, Dayan C, Beyan H, Leslie RD, Peakman M, et al. Increased resistance to CD4+CD25hi regulatory T cell-mediated suppression in patients with type 1 diabetes. Clin Exp Immunol 2008, 154, 353–9. doi: 10.1111/j.1365-2249.2008.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hundhausen C, Roth A, Whalen E, Chen J, Schneider A, Long SA, et al. Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci Transl Med 2016, 8, 356ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Habib T, Funk A, Rieck M, Brahmandam A, Dai X, Panigrahi AK, et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol 2012, 188, 487–96. doi: 10.4049/jimmunol.1102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol 2005, 175, 7867–79. [DOI] [PubMed] [Google Scholar]
- 56. Smith MJ, Rihanek M, Wasserfall C, Mathews CE, Atkinson MA, Gottlieb PA, et al. Loss of B cell anergy in type 1 diabetes is associated with high risk HLA and non-HLA disease susceptibility alleles. Diabetes 2018, 67, 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Krischer JP, Lynch KF, Schatz DA, Ilonen J, Lernmark A, Hagopian WA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015, 58, 980–7. doi: 10.1007/s00125-015-3514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vehik K, Bonifacio E, Lernmark A, Yu L, Williams A, Schatz D, et al. Hierarchical order of distinct autoantibody spreading and progression to type 1 diabetes in the TEDDY Study. Diabetes Care 2020, 43, 2066–73. doi: 10.2337/dc19-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hanna SJ, Powell WE, Long AE, Robinson EJS, Davies J, Megson C, et al. Slow progressors to type 1 diabetes lose islet autoantibodies over time, have few islet antigen-specific CD8+ T cells and exhibit a distinct CD95hi B cell phenotype. Diabetologia 2020, 63, 1174–85. doi: 10.1007/s00125-020-05114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boldison J, Long AE, Aitken RJ, Wilson IV, Megson C, Hanna SJ, et al. Activated but functionally impaired memory Tregs are expanded in slow progressors to type 1 diabetes. Diabetologia 2022, 65, 343–55. doi: 10.1007/s00125-021-05595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wiedeman AE, Muir VS, Rosasco MG, DeBerg HA, Presnell S, Haas B, et al. Autoreactive CD8+ T cell exhaustion distinguishes subjects with slow type 1 diabetes progression. J Clin Invest 2020, 130, 480–90. doi: 10.1172/JCI126595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yeo L, Woodwyk A, Sood S, Lorenc A, Eichmann M, Pujol-Autonell I, et al. Autoreactive T effector memory differentiation mirrors beta-cell function in type 1 diabetes. J Clin Invest 2018, 128, 3460–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Narsale A, Lam B, Moya R, Lu T, Mandelli A, Gotuzzo I, et al. CD4+CD25+CD127hi cell frequency predicts disease progression in type 1 diabetes. JCI Insight 2021, 6, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dufort MJ, Greenbaum CJ, Speake C, Linsley PS.. Cell type-specific immune phenotypes predict loss of insulin secretion in new-onset type 1 diabetes. JCI Insight 2019, 4, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vecchio F, Lo Buono N, Stabilini A, Nigi L, Dufort MJ, Geyer S, et al. Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes. JCI Insight 2018, 3, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cerosaletti K, Barahmand-Pour-Whitman F, Yang J, DeBerg HA, Dufort MJ, Murray SA, et al. Single-cell RNA sequencing reveals expanded clones of islet antigen-reactive CD4+ T cells in peripheral blood of subjects with type 1 diabetes. J Immunol 2017, 199, 323–35. doi: 10.4049/jimmunol.1700172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gearty SV, Dündar F, Zumbo P, Espinosa-Carrasco G, Shakiba M, Sanchez-Rivera FJ, et al. An autoimmune stem-like CD8 T cell population drives type 1 diabetes. Nature 2022, 602, 156–61. doi: 10.1038/s41586-021-04248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vignali D, Cantarelli E, Bordignon C, Canu A, Citro A, Annoni A, et al. Detection and characterization of CD8+ autoreactive memory stem T cells in patients with type 1 diabetes. Diabetes 2018, 67, 936–45. doi: 10.2337/db17-1390. [DOI] [PubMed] [Google Scholar]
- 69. Abdelsamed HA, Zebley CC, Nguyen H, Rutishauser RL, Fan Y, Ghoneim HE, et al. Beta cell-specific CD8+ T cells maintain stem cell memory-associated epigenetic programs during type 1 diabetes. Nat Immunol 2020, 21, 578–87. doi: 10.1038/s41590-020-0633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ciecko AE, Schauder DM, Foda B, Petrova G, Kasmani MY, Burns R, et al. Self-renewing islet TCF1+ CD8 T cells undergo IL-27-controlled differentiation to become TCF1− terminal effectors during the progression of type 1 diabetes. J Immunol 2021, 207, 1990–2004. doi: 10.4049/jimmunol.2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Terrazzano G, Bruzzaniti S, Rubino V, Santopaolo M, Palatucci AT, Giovazzino A, et al. T1D progression is associated with loss of CD3+CD56+ regulatory T cells that control CD8+ T cell effector functions. Nat Metab 2020, 2, 142–52. doi: 10.1038/s42255-020-0173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bosi E, Choudhary P, de Valk HW, Lablanche S, Castañeda J, de Portu S, et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol 2019, 7, 462–72. doi: 10.1016/S2213-8587(19)30150-0. [DOI] [PubMed] [Google Scholar]
- 73. Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, et al. ; Type 1 Diabetes TrialNet Study Group. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019, 381, 603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. de Klerk E, Hebrok M.. Stem cell-based clinical trials for diabetes mellitus. Front Endocrinol 2021, 12, 631463. doi: 10.3389/fendo.2021.631463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. ; Type 1 Diabetes TrialNet Abatacept Study Group. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011, 378, 412–9. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Orban T, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, et al. ; Type 1 Diabetes TrialNet Abatacept Study Group. Co-Stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up one year after cessation of treatment. Diabetes Care 2013, 37, 1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Haller MJ, Long SA, Blanchfield JL, Schatz DA, Skyler JS, Krischer JP, et al. ; Type 1 Diabetes TrialNet ATG-GCSF Study Group. Low-dose anti-thymocyte globulin preserves C-peptide, reduces HbA1c, and increases regulatory to conventional T-cell ratios in new-onset type 1 diabetes: two-year clinical trial data. Diabetes 2019, 68, 1267–76. doi: 10.2337/db19-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lin A, Mack JA, Bruggeman B, Jacobsen LM, Posgai AL, Wasserfall CH, et al. Low-dose ATG/GCSF in established type 1 diabetes: a five-year follow-up report. Diabetes 2021, 70, 1123–9. doi: 10.2337/db20-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care 2009, 32, 1244–9. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, et al. Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N Engl J Med 2020, 383, 2007–17. [DOI] [PubMed] [Google Scholar]
- 81. Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr., et al. ; Protégé Trial Investigators. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet 2011, 378, 487–97. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hagopian W, Ferry RJ Jr., Sherry N, Carlin D, Bonvini E, Johnson S, et al. ; Protégé Trial Investigators. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protege trial. Diabetes 2013, 62, 3901–8. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rigby MR, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol 2013, 1, 284–94. doi: 10.1016/S2213-8587(13)70111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest 2015, 125, 3285–96. doi: 10.1172/JCI81722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Greenbaum CJ, Serti E, Lambert K, Weiner LJ, Kanaparthi S, Lord S, et al. IL-6 receptor blockade does not slow β cell loss in new-onset type 1 diabetes. JCI Insight 2021, 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 2013, 381, 1905–15. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Battaglia M, Ahmed S, Anderson MS, Atkinson MA, Becker D, Bingley PJ, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 2020, 43, 5–12. doi: 10.2337/dc19-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Smith EL, Peakman M.. Peptide immunotherapy for type 1 diabetes—clinical advances. Front Immunol 2018, 9, 392. doi: 10.3389/fimmu.2018.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang Y, Santamaria P.. Antigen-specific nanomedicines for the treatment of autoimmune disease: target cell types, mechanisms and outcomes. Curr Opin Biotechnol 2022, 74, 285–92. [DOI] [PubMed] [Google Scholar]
- 90. Serr I, Drost F, Schubert B, Daniel C.. Antigen-specific Treg therapy in type 1 diabetes—challenges and opportunities. Front Immunol 2021, 12, 712870. doi: 10.3389/fimmu.2021.712870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. von Herrath M, Bain SC, Bode B, Clausen JO, Coppieters K, Gaysina L, et al. Anti-interleukin-21 antibody and liraglutide for the preservation of β-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol 2021, 9, 212–24. doi: 10.1016/S2213-8587(21)00019-X. [DOI] [PubMed] [Google Scholar]
- 92. Dong S, Hiam-Galvez KJ, Mowery CT, Herold KC, Gitelman SE, Esensten JH, et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight 2021, 6, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.