Abstract
Introduction
Chronic inflammation can remain many years after the completion of cancer treatment and is associated with cancer recurrence. The purpose of this study was to examine how a 16-week therapeutic yoga program (TYP) modulates the cytokine profile in heterogeneous cancer survivors.
Methods
Eligible participants were 18 years of age or older and clinically diagnosed with cancer. Consenting participants were asked to attend three, 75-min sessions weekly of TYP with meditation. Seventeen patients provided blood samples at baseline and end of study. Eight cytokines (interferon (IFN)-γ; interleukin (IL)-1b, IL-1ra, IL-4, IL-6, IL-8, IL-10; and tumor necrosis factor (TNF)-α), three receptors (sIL-6R, sTNFRI, sTNFRII), and C-reactive protein (CRP) were quantified.
Results
Patients were 59.6 ± 7.3 years old; over half (56%) were overweight or obese BMI ≥ 25 kg/m2); majority were female (71%) and breast cancer survivors (65%), of which 44% were Hispanic. Marked reductions were observed in all cytokines except IL-4, with significant reductions (p < 0.05) found in IL-1b (− 13%) and IL-1ra (− 13%). No significant changes were observed in soluble cytokine receptors or CRP.
Conclusions
TYP led to significant reduction in circulating cytokines associated with chronic inflammation in a heterogeneous sample of cancer survivors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-022-07536-y.
Keywords: Recurrence, Survivorship, Physical activity, Immunology, Body composition
Introduction
The American Cancer Society predicts that there will be over 1.9 million new cancer cases diagnosed and 609,360 cancer deaths in the USA in 2022 [1]. Survival rates are increasing due to earlier detection coupled with better treatments, which has shifted the paradigm of the cancer diagnosis of a fatal disease to a chronic disease [1, 2]. However, once a diagnosis of cancer occurs, even though treated and in remission, the possibility of recurrence plays heavy in the minds of cancer survivors and is a concern for the treating oncologist [3].
Multiple factors play a role in cancer recurrence, including obesity and systemic inflammation [4–6]. Elevated body mass index (BMI) is associated with increased systemic concentrations of inflammatory and pro-tumorigenic cytokines [7]. Further, the elevations of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-1β, have been targeted because of their multifactorial impact on tumor progression, as well as many secondary events, such as fatigue and sarcopenia [8–10].
Many studies have demonstrated feasibility of implementing a yoga program to improve quality of life and emotional, physical, and mental well-being in patients with cancer [11–20]. There is also evidence that yoga can attenuate systemic inflammation associated with increase risk of cancer onset and recurrence [13, 21]. There is a growing interest in studying the effects of therapeutic yoga in cancer survivors because it can offer a tailored and individualized approach.
What remains to be understood is the immunologic response to yoga in a heterogeneous sampling of cancer survivors and how it presents in an ethnically diverse sample. Furthermore, the multiple approaches to implementing a yoga program provide opportunities to better understand whether the practice of yoga can impact physiological outcomes. The purpose of this study was to determine how 16 weeks of therapeutic yoga can influence the inflammatory cytokine profile of cancer survivors using a single-arm, self-controlled, pilot, exploratory study design. We hypothesized that 16-weeks of therapeutic yoga would significantly modify cytokines associated with chronic inflammation and cancer recurrence in a diverse cancer survivor cohort.
Methods
Study design and participants
A single-arm, self-controlled, block enrollment study design was used. Participants were recruited from January 2020 to March 2020. Participants were recruited from the surrounding community using advertisements and word of mouth. Flyers were placed throughout the University of Texas Health San Antonio’s (UTHSA) Mays Cancer Center. The flyer included a contact number to request information.
Individuals that expressed interest were screened for eligibility. The study inclusion criteria were the following: at least 18 years of age, had been given a diagnosis of any cancer in their lifetime (active treatment or post-treatment), had access or use of a mobile phone or computer to complete surveys and respond to text messages, were able to speak and understand English or Spanish, and were oriented to time and place. Participants were excluded if they were currently enrolled in a competing protocol or presented with any absolute contraindication to exercise testing as detailed by the American College of Sports Medicine Guidelines on Exercise Testing and Exercise Prescription [22].
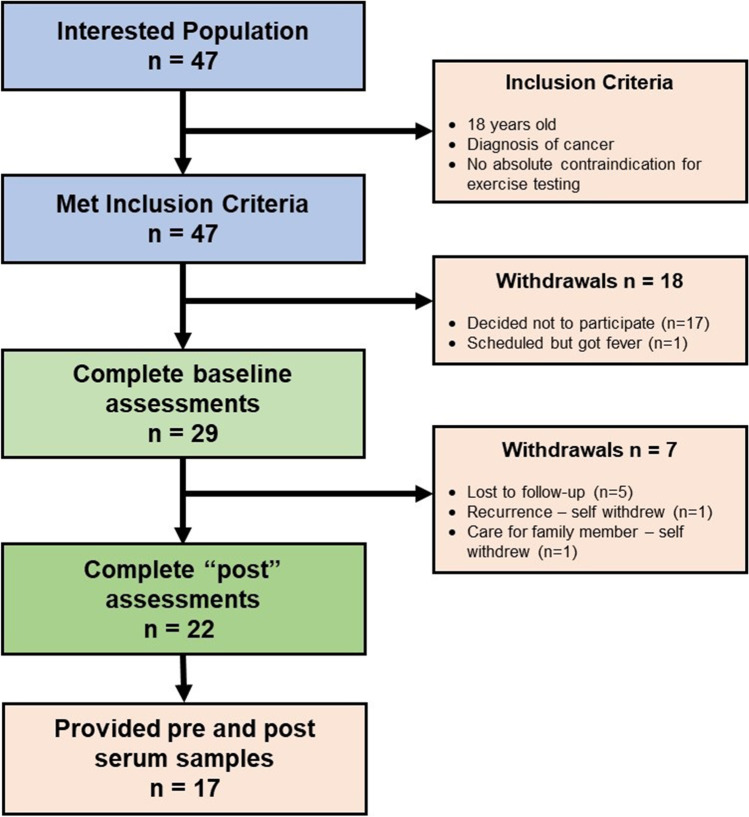
Once contact with the potential participants were made, the coordinator assigned a unique subject identification number. The participants were given a scripted brief explanation of the study in their language of choice (English or Spanish). Participants were told that after accrual reached the cohort target of thirty, the study would start. A list of 43 interested participants was collected. In numerical order, the first thirty participants who passed screening for eligibility were invited to participate in the study and were scheduled for baseline assessments at the Holistic Exercise Advancement Laboratory (HEAL) at the UTHSA Mays Cancer Center. Out of the 30 invited, 29 provided informed consent and completed baseline assessments (Fig. 1).
Fig. 1.
Therapeutic yoga program CONSORT diagram
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted approval by the Institutional Review Board of the UTHSA (date: December 17, 2019; No: 20190637HU). Informed consent was obtained from all individual participants included in the study.
Demographics and patient characteristics
After consenting to the study, height, weight, blood pressure, demographic information, and medical history were collected. BMI was calculated as weight [kg]/(height [m])2.
Yoga protocol
Study participants were asked to participate in a 16-week therapeutic yoga program three times per week on-site at the Mays Cancer Center at UTHSA. Each session lasted 75 min and included structured sequence of combined yoga postures (asanas), breathing practice (pranayama), and loving-kindness meditation (LKM), referred to as the therapeutic yoga protocol (TYP) developed by an experienced and certified yoga instructor as previously described [23]. The TYP was developed to increase mobility of extremities and torso (specifically thoracic mobility), increase overall strength and muscular endurance, improve overall function, guide participants in safe transition to the floor and back up, reduce stress, improve focus and concentration, introduce meditation with breath as a focus during the movement practices, and introduce LKM during the resting portion of the practice.
During participation in the TYP, postures were held for 3 slow and controlled breaths. The breath was used as a focus for concentration and to determine the individual participants’ tolerance to the yoga activities. The practice began with introduction, Therapeutic Sun Salutation, transition to quadruped and prone postures, transition to standing postures, transition to floor for seated postures, transition to supine postures, transition to supported modified inversion to final resting posture in supine for LKM. The Therapeutic Sun Salutation is a modified version of the traditional Sun Salutation poses that allow for individuals with balance issues, joint or mobility issues, and individuals with limited hamstring flexibility, to progress through the poses without the risk of injury.
The COVID-19 outbreak forced changes to the protocol after the first week of program initiation. The live on-site therapeutic yoga class practices were discontinued to meet the health and safety concerns for this high-risk population. The TYP protocol was transitioned to a virtual implementation. Livestream TYP coupled with video recordings were made available to study participants via the BlueJay Telehealth portal (Pleasanton, CA) in order to complete the 16-week protocol. Each participant was given an individual username and password for accessing the Telehealth portal and was able to attend live sessions of the TYP or view recorded sessions to complete the TYP asynchronously.
Cytokine, receptor, and stress hormone evaluation
Baseline and end-of-study blood samples were collected into K2-EDTA tubes and centrifuged to separate and aliquot plasma for subsequent cytokine protein analysis. The samples were analyzed in duplicate using the FlexMap 3D platform system (Luminex, Austin, TX) with the MILLIPLEX MAP Human Cytokine Panel A (HCYTA-60 K-08 with TNF-α, IL-1ra, IL-1β, IL-4, IL-6, IL-8, IL-10, and IFN-γ), Human Soluble Cytokine Receptor Panel (HSCRMAG-32 K-03 with sTNFRII, sTNFRII, and sIL-6R) and Milliplex-Human CVD Panel 3 with CRP (HCVD3MAG-67 K-01). Multiplex analysis was performed by the Bioanalytics and Single-Cell Core Laboratory in the Department of Molecular Medicine at UTHSA.
Statistical analysis
Study data were collected and managed using the REDCap electronic data capture tools hosted at UTHSA [24]. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources. Per-protocol analysis was employed, and only those who completed the study protocol were included.
All continuous variables were evaluated for normality using the Kolmogorov–Smirnov test. The effect of the intervention was evaluated by calculating changes in circulating cytokine concentrations between baseline and at the end of the intervention. Percentage changes in biomarkers concentrations from baseline to end of study were calculated as follows: [mean baseline − mean end of study]/[mean baseline] × 100. Within-group differences in cytokine concentrations were evaluated using the Wilcoxon rank-sum test. Data are presented as median (interquartile range). The significance level was set at p < 0.05. The primary outcome was changes in cytokine concentrations after the completion of the 16-week TYP. The analysis was restricted to only the individuals that provided both baseline and end-of-study samples because many individuals chose not to return for the end-of-study visits due to the onset of the COVID-19 pandemic. All analyses were conducted using GraphPad Prism version 9.3.1 (San Diego, CA).
Results
Participant demographics and clinical characteristics
Twenty-two of the twenty-nine consented participants completed the end of study visit, and 17 of those 29 participants provided both baseline and end-of-study blood samples for the analysis of circulating cytokines. These participants were 59.6 ± 7.3 years old (range: 47–69), mostly overweight/obese (56% with BMI ≥ 25 kg/m2) female (71%) breast cancer survivors (65%) with 8 of the 17 participants being Hispanic (see supplemental file for full demographics details).
For the 5 participants that did not have a complete blood set, the reasons included hemolysis of samples, short draws, or not willing to provide blood samples.
Cytokine, cytokine receptor, and CRP response to therapeutic yoga
Biomarker data is presented in Table 1. Significant decreases in concentrations were found only for IL-1β (p = 0.003) and IL-1ra (p = 0.02), with a trend observed in IL-8 (p = 0.07). No significant changes were observed in soluble cytokine receptors; however, sTNFRI was trending towards significance (p = 0.052).
Table 1.
Changes in circulating cytokines in cancer survivors who completed a 16-week online yoga intervention. Data is presented as pg/ml
| Pre | Post | △ | p-value | |
|---|---|---|---|---|
| Cytokine | Median (IQR) | Median (IQR) | ||
| IFN-γ | 2.42 (1.95, 3.35) | 2.15 (1.90, 3.07) | − 11% | 0.12 |
| IL-1b | 6.88 (6.32, 9.79) | 5.96 (5.14, 8.14) | − 13% | 0.003 |
| TNFα | 15.42 (13.76, 18.80) | 15.95 (13.76, 18.75) | 3% | 0.5 |
| IL-4 | 1.67 (1.12, 3.17) | 1.48 (1.23, 2.99) | − 11% | 0.46 |
| IL-10 | 12.28 (9.36, 25.97) | 13.07 (8.58, 24.13) | 6% | 0.13 |
| IL-1ra | 4.66 (3.18, 11.34) | 4.05 (2.98, 10.38) | − 13% | 0.02 |
| IL-6 | 1.74 (1.06, 3.42) | 1.89 (1.31, 2.23) | 9% | 0.32 |
| IL-8 | 2.56 (2.23, 3.73) | 2.16 (1.87, 2.70) | − 15% | 0.07 |
| Cytokine receptors | ||||
| sIL-6R | 5234 (4389, 5825) | 5264 (4545, 5958) | − 1% | 0.31 |
| sTNFRI | 332.2 (283.7, 412.3) | 388.7 (299.7, 449.2) | 17% | 0.052 |
| sTNFRII | 1216 (978, 1591) | 1258 (946, 1534) | 3% | 0.43 |
| Inflammation hormone | ||||
| CRP | 2.59 (1.11, 17.18) | 3.30 (0.87, 9.44) | 27% | 0.32 |
Discussion
For cancer survivors, the rumination of threat of cancer recurrence can negatively impact health-related quality of life (HR-QOL). Behavioral and lifestyle modifications are associated with reduced rates of cancer recurrence [25, 26], though mechanisms underlying these benefits are inconclusive and warrant continued investigation. Yoga is a low impact physical activity intervention that has been reported to improve function, fatigue, and quality of life in cancer survivors [11–15, 17–21, 27–30]. The primary objective of this pilot, exploratory study was to determine the effects of 16-week TYP on circulating cytokines. Results from this self-controlled study partially support our hypothesis that yoga can lead to significant reductions in concentrations of inflammatory cytokines that are associated with chronic inflammation and tumor recurrence in a heterogeneous sample of cancer survivors. Specifically, we observed significant reductions in circulating concentrations of IL-1β and IL-1ra. Trends for increased sTNFRI were also observed following the 16-week TYP.
The mixed results of our study are indicative of the complexity in studying inflammatory cytokines in older participants with cancer, who may have many other factors that may influence the concentrations of the proteins measured in our study. For example, the simple aging process is associated with increased inflammation, term inflammaging [31], that may have underlying effects on the results of our study. In our study, 55% of participants were above the age of 60 years.
Two proteins of interest in our study, CRP and TNF-α, were not significantly lower when compared to baseline, which contradicts a recent systematic review published by Koshravi et al. in which the overall effects of exercise on circulating cytokine concentrations were interpreted [32]. The studies included in this systematic review were overwhelmingly inclusive of aerobic exercise with only two papers including yoga as an exercise modality [21, 29]. Those two studies showed no effect of yoga on CRP or TNF-α, thus, suggesting that CRP and TNF-α response may be dependent on dose and type of intervention.
Our study results support several other yoga studies that have reported varying effects on circulating inflammatory cytokines [12, 13, 21, 29]. For example, a 12-week Iyengar yoga intervention also found no significant changes in CRP, sTNFRII, a marker of TNF-α activity, or IL-6 [21]. However, our results indicated a significant reduction in IL-1ra following 16 weeks of our TYP, while similar results were not observed following 12 weeks of Iyengar yoga [21].
Interestingly, the inflammatory cytokine concentrations were either stable or slightly elevated in the Iyengar yoga participants, whereas most of the measured cytokines in our study demonstrated reductions after the yoga program, though some were not statistically significant. This could be perhaps due to the inclusion of breathing practice (pranayama) and loving-kindness meditation in this study, which incorporates mindfulness-based practices which can reduce stress, thereby, reducing cytokine concentrations [33].
In another study, yoga following either mastectomy or breast reconstruction surgery in breast cancer survivors found a significant reduction in TNF-α concentrations [29], while other published studies found no significant changes in inflammatory cytokines concentrations in similar patient populations [12, 34]. Further, given that we found no significant changes in TNF-α concentrations, it should be expected that sTNFRII concentration remain unchanged as it is a marker for TNF-α activity [35].
Briefly, IL-1ra consists of three isoforms and inhibits IL-1-mediated tumor progression [36]. The reduction in IL-1ra concentration in our study may be in part due to feedback mechanisms due to reductions in IL-1β. IL-1β is a product of blood monocytes, tissue macrophages, and dendritic cells that only appears when stimulated by other cytokines, such as TNF-α [37]. Since no significant differences in TNF-α were observed, we can hypothesize that the TYP can overcome the autoinflammatory effects of TNF-α to reduce both IL-1β and IL-1ra. Further research needs to be done to determine the exact mechanism of this action.
The tendency for increased sTNFRI is intriguing. The pleiotropic nature of sTNFRI complicates our understanding of the intervention effect on this protein. sTNFRI is not only involved in inducing apoptosis [38], but can also transduce cell survival signals [39]. While the signaling pathways are well defined for sTNFRI, the regulation of life/death signaling is still poorly understood. Future research with a powered sample size will help provide insights into the role of yoga on sTNFRI concentrations.
The impact of our intervention on IFN-γ concentrations, although not statistically significant, contradicts previous studies in cancer survivors that have reported an increase in IFN-γ concentrations following structured yoga programs [13, 40]. IFN-γ has been found in different studies to have protective effects on tumor growth and suggestive of a strong immune response [41]. Specifically, low serum IFN-γ levels have been inversely associated with tumor stage [42] and tumor size [43] though the specific mechanism attributed to this benefit is still unknown.
Our continued interest in understanding the physiological response underlying the benefits of yoga on cancer survivors was formed with the premise that in healthy individuals, yoga is promoted as an anti-inflammatory intervention [27, 28, 30]. Given the significant role that cytokines, such as TNF-α [44], IFN-γ [45], IL-6 [46], and other interleukins and cytokine receptors, play in cancer onset and progression [47, 48], there is a significant need to better understand if yoga can be effectively used as an “anti-cancer exercise intervention.” However, due to the mixed results currently presented in the scientific literature, there is no established consensus on these effects.
Interpretation of our study results is made with caution given the number of limitations to our study design. First, the single group comparison limits our ability to clearly define the effects TYP may have in modulating the inflammatory cytokines measured in this study. Second, the heterogeneous sample of cancer survivors, inclusive of both males and female with varying cancer diagnoses and years of survivorship, though useful for generalizability, may have impacted the levels of inflammatory cytokines. Third, our small sample size likely impacted the non-significant differences observed in concentration of inflammatory cytokines that had marked decreases at the end of the study. Lastly, the impact of the COVID-19 pandemic impacted our studies retention and our study results. Though none of the participants tested positive for COVID-19 while the study was implemented, the stress of the social distancing protocols implemented to limit the spread of the virus may have impacts that are immeasurable.
In conclusion, the results of our pilot, exploratory, single-arm, pre-post study suggest that a 16-week TYP can reduce the concentration of cytokines associated with chronic inflammation. Despite the limitations, the results of this study have set a precedent for continued research using more rigorous study design models, such as randomized controlled studies, with powered samples to better understand the effects of yoga on mechanisms important for secondary cancer prevention.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge the study participants that generously volunteered their time to participate in this study, despite the COVID-19 pandemic. Without their unwavering sacrifice, we would not have been able to complete this study.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Darpan Patel, Gustavo Almeida, Bianca Gutierrez, Angelika Lapetoda, and Daniel Hughes. The first draft of the manuscript was written by Darpan Patel, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Funding for this project was provided by the Mays Cancer Center’s P30 Cancer Center Support Grant from the National Cancer Institute (CA054174). Funding for the REDCap database was provided to the Institute for Integration of Medicine and Science (UL1-RR024982).
Availability of data and materials
The data and materials that support the findings of this study are available from the corresponding author, DP, upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted approval by the Institutional Review Board of the UTHSA (Date: 12/17/2019; No: 20190637HU). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Cancer Society . Cancer Facts & Figures 2022. Atlanta: American Cancer Society; 2022. [Google Scholar]
- 2.Witter DC, Le Bas J (2008) Anderson cancer center: cancer as a chronic disease. Oncol Rep Physicians (53):1–5
- 3.Yang HC, Thornton LM, Shapiro CL, et al. Surviving recurrence: psychological and quality-of-life recovery. Cancer. 2008;112(5):1178–1187. doi: 10.1002/cncr.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellizzi KM, Rowland JH, Jeffery DD, et al. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23(34):8884–8893. doi: 10.1200/jco.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 5.Bhindi B, Xie WY, Kulkarni GS, et al. Influence of metabolic syndrome on prostate cancer stage, grade, and overall recurrence risk in men undergoing radical prostatectomy. Urology. 2016;93:77–85. doi: 10.1016/j.urology.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 6.Park J, Morley TS, Kim M, et al. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10(8):455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229(1):45–52. doi: 10.1111/j.1749-6632.2011.06096.x. [DOI] [PubMed] [Google Scholar]
- 8.O’Higgins C, Brady B, O’Connor B, et al. The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer. 2018;26(10):3353–3364. doi: 10.1007/s00520-018-4318-7. [DOI] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Aziz N, et al. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Aoyagi T, Terracina KP, Raza A, et al. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7(4):17–29. doi: 10.4251/wjgo.v7.i4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes DC, Darby N, Gonzalez K, et al (2015) Effect of a six month yoga exercise intervention on fitness outcomes for breast cancer survivors. Physiotherapy Research and Practice. Physiother Res Pract. In Press [DOI] [PMC free article] [PubMed]
- 12.Parma DL, Hughes DC, Ghosh S, et al. Effects of six months of Yoga on inflammatory serum markers prognostic of recurrence risk in breast cancer survivors. Springerplus. 2015;4(1):1–10. doi: 10.1186/s40064-015-0912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaushik D, Shah PK, Mukherjee N, et al. Effects of yoga in men with prostate cancer on quality of life and immune response: a pilot randomized controlled trial. Prostate Cancer Prostatic Dis. 2021 doi: 10.1038/s41391-021-00470-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramer H, Lauche R, Klose P, et al., (2017) Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 1(1): p. Cd010802 DOI: 10.1002/14651858.CD010802.pub2. [DOI] [PMC free article] [PubMed]
- 15.Hardoerfer K, Jentschke E. Effect of yoga therapy on symptoms of anxiety in cancer patients. Oncol Res Treat. 2018;41(9):526–532. doi: 10.1159/000488989. [DOI] [PubMed] [Google Scholar]
- 16.Hilfiker R, Meichtry A, Eicher M, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. 2018;52(10):651–658. doi: 10.1136/bjsports-2016-096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin PJ, Kleckner IR, Loh KP, et al. Influence of yoga on cancer-related fatigue and on mediational relationships between changes in sleep and cancer-related fatigue: a nationwide, multicenter randomized controlled trial of yoga in cancer survivors. Integr Cancer Ther. 2019;18:1534735419855134. doi: 10.1177/1534735419855134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundt A, Jentschke E. Long-term changes of symptoms of anxiety, depression, and fatigue in cancer patients 6 months after the end of yoga therapy. Integr Cancer Ther. 2019;18:1534735418822096. doi: 10.1177/1534735418822096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasyar N, Barshan Tashnizi N, Mansouri P, et al. Effect of yoga exercise on the quality of life and upper extremity volume among women with breast cancer related lymphedema: a pilot study. Eur J Oncol Nurs. 2019;42:103–109. doi: 10.1016/j.ejon.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Naderi M, Kordestani H, Sahebi Z, et al. Serum and gene expression profile of cytokines following combination of yoga training and vitamin D supplementation in breast cancer survivors: a randomized controlled trial. BMC Womens Health. 2022;22(1):90. doi: 10.1186/s12905-022-01671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bower JE, Greendale G, Crosswell AD, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–29. doi: 10.1016/j.psyneuen.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Sports Medicine . ACSM's guidelines for exercise testing and prescription. 10. Philadelphia: Lippincott Williams & Wilkins; 2018. [DOI] [PubMed] [Google Scholar]
- 23.Hughes DC, Darby N, Gonzalez K, et al. Effect of a six-month yoga exercise intervention on fitness outcomes for breast cancer survivors. Physiother Theory Pract. 2015;31(7):451–460. doi: 10.3109/09593985.2015.1037409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 27.Djalilova DM, Schulz PS, Berger AM, et al. Impact of yoga on inflammatory biomarkers: a systematic review. Biol Res Nurs. 2019;21(2):198–209. doi: 10.1177/1099800418820162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiecolt-Glaser JK, Christian L, Preston H, et al. Stress, inflammation, and yoga practice. Psychosom Med. 2010;72(2):113. doi: 10.1097/PSY.0b013e3181cb9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao RM, Nagendra H, Raghuram N, et al. Influence of yoga on postoperative outcomes and wound healing in early operable breast cancer patients undergoing surgery. Int J Yoga. 2008;1(1):33. doi: 10.4103/0973-6131.36795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shete SU, Verma A, Kulkarni DD et al (2017) Effect of yoga training on inflammatory cytokines and C-reactive protein in employees of small-scale industries. J Educ Health Promot 6 [DOI] [PMC free article] [PubMed]
- 31.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908(1):244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 32.Khosravi N, Stoner L, Farajivafa V, et al. Exercise training, circulating cytokine levels and immune function in cancer survivors: a meta-analysis. Brain Behav Immun. 2019;81:92–104. doi: 10.1016/j.bbi.2019.08.187. [DOI] [PubMed] [Google Scholar]
- 33.Antoni MH, Lechner S, Diaz A, et al. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain Behav Immun. 2009;23(5):580–591. doi: 10.1016/j.bbi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Payne JK, Held J, Thorpe J et al (2008) Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. in Oncology nursing forum [DOI] [PubMed]
- 35.Lejeune FJ, Rüegg C, Liénard D. Clinical applications of TNF-α in cancer. Curr Opin Immunol. 1998;10(5):573–580. doi: 10.1016/S0952-7915(98)80226-4. [DOI] [PubMed] [Google Scholar]
- 36.Chirivi RG, Garofalo A, Padura IM, et al. Interleukin 1 receptor antagonist inhibits the augmentation of metastasis induced by interleukin 1 or lipopolysaccharide in a human melanoma/nude mouse system. Can Res. 1993;53(20):5051–5054. [PubMed] [Google Scholar]
- 37.Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25(6):469–484. doi: 10.1016/j.smim.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 39.Peschon JJ, Torrance DS, Stocking KL, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160(2):943–952. doi: 10.4049/jimmunol.160.2.943. [DOI] [PubMed] [Google Scholar]
- 40.Gopal A, Mondal S, Gandhi A, et al. Effect of integrated yoga practices on immune responses in examination stress - a preliminary study. Int J Yoga. 2011;4(1):26–32. doi: 10.4103/0973-6131.78178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Todorović-Raković N, Milovanović J, Greenman J, et al. The prognostic significance of serum interferon-gamma (IFN-γ) in hormonally dependent breast cancer. Cytokine. 2022;152:155836. doi: 10.1016/j.cyto.2022.155836. [DOI] [PubMed] [Google Scholar]
- 42.Patel DI, Abuchowski K, Bedolla R, et al. Nexrutine and exercise similarly prevent high grade prostate tumors in transgenic mouse model. PLoS ONE. 2019;14(12):e0226187. doi: 10.1371/journal.pone.0226187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee IC, Huang YH, Chau GY, et al. Serum interferon gamma level predicts recurrence in hepatocellular carcinoma patients after curative treatments. Int J Cancer. 2013;133(12):2895–2902. doi: 10.1002/ijc.28311. [DOI] [PubMed] [Google Scholar]
- 44.Balkwill F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25(3):409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 45.Mojic M, Takeda K, Hayakawa Y. The dark side of IFN-γ: its role in promoting cancer immunoevasion. Int J Mol Sci. 2017;19(1):89. doi: 10.3390/ijms19010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heikkilä K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44(7):937–945. doi: 10.1016/j.ejca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 47.Kamangar F, Cheng C, Abnet CC, et al. Interleukin-1B polymorphisms and gastric cancer risk—a meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15(10):1920–1928. doi: 10.1158/1055-9965.EPI-06-0267. [DOI] [PubMed] [Google Scholar]
- 48.Zhou B, Shu B, Yang J, et al. C-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysis. Cancer Causes Control. 2014;25(10):1397–1405. doi: 10.1007/s10552-014-0445-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and materials that support the findings of this study are available from the corresponding author, DP, upon reasonable request.