Abstract
Background
Studies correlating reactogenicity and immunogenicity of COVID-19 vaccines are limited to BNT162b2, with inconsistent results. We investigated whether adverse reactions after other COVID-19 vaccines reliably predict humoral responses.
Methods
Adult volunteers were recruited for homologous or heterologous prime-boost vaccinations with adenoviral (ChAdOx1, AstraZeneca) and/or mRNA (mRNA-1273, Moderna) vaccines administered either 4 or 8 weeks apart. Adverse effects were routinely solicited and recorded by subjects in a standard diary card for up to 84 days post booster vaccination. Anti-SARS-CoV-2 IgG titers were measured pre- (visit 1), and post-booster dose at days 14 (visit 2) and 28 (visit 3).
Results
A total of 399 participants (75% women) with a median age of 41 (interquartile range, 33–48 IQR) years were included. Vaccine-induced antibody titers at days 14 and 28 were significantly higher among subjects who reported local erythema, swelling, pain, as well as systemic fever, chills, headache, myalgia, arthralgia, fatigue compared to those who did not experience local or systemic reactogenicity. Post-vaccination humoral responses did not correlate with the occurrence of skin rash and correlated weakly with gastrointestinal symptoms. A significant correlation between post-vaccination peak body temperature and anti-SARS-CoV-2 spike IgG at Day 14, independent of vaccine type and schedule, was found.
Conclusion
Specific symptoms of reactogenicity such as post-vaccination injection site pain, swelling, erythema and fever, myalgia and fatigue are significantly predictive of the magnitude of the anti-SARS-CoV-2 antibody response.
Keywords: Reactogenicity, Immunogenicity, Adverse effects, Anti-SARS-CoV-2 IgG, COVID19 vaccines
Article Summary: Whether adverse reactions after vaccination are predictive of immunogenicity is unclear. We show that specific symptoms of reactogenicity such as local pain, swelling, erythema, fever, myalgia, and fatigue correlate significantly with the magnitude of the anti-SARS-CoV-2 antibody response.
Introduction
The most important and readily measurable immunological parameter in all vaccine clinical trials is antibody levels.1 For Coronavirus Disease 2019 (COVID-19) vaccines, binding and neutralizing antibodies are thought to be potential immune correlates of protection.2 , 3 However, for the average layperson, it is common belief that adverse reactions after vaccination are predictive signs of a good immune response; yet limited data is available.4 Vaccine reactogenicity characterizes the physical manifestation of the inflammatory response to a vaccine and can result in injection site and systemic symptoms. Some symptoms can be measured objectively, others are nonspecific and subjective. There are many factors that can influence reactogenicity including host-derived factors include age, gender, autoimmune disease, and extrinsic factors such as dose number, injection technique, use of immunosuppressants or non-steroidal anti-inflammatory drugs (NSAIDs).5 , 6
We have previously reported that heterologous ChAdOx1/mRNA-1273 and homologous mRNA-1273/mRNA-1273 vaccinations provided higher immunogenicity in terms of higher SARS-CoV-2 anti-spike IgG antibody levels than two doses of ChAdOx1/ChAdOx1 vaccination (ClinicalTrials.gov number, NCT05074368).7 Our results supported the efficacy of heterologous prime-boost vaccination using the ChAdOx1 and mRNA-1273 COVID-19 vaccines.7 However, we observed higher rates of solicited adverse effects (AEs) such as local injection site pain, erythema, swelling as well as systemic AEs such as fever, chills, headache, myalgia/arthralgia, fatigue, after the second vaccine dose in heterologous ChAdOx1/mRNA-1273 vaccination and homologous mRNA-1273/mRNA-1273 vaccination groups compared with homologous ChAdOx1/ChAdOx1. Yet, we do not know if the SARS-CoV-2 anti-spike IgG antibody titers post second vaccine dose tracked consistently with all or some AEs and whether the severity of the AEs could be a simple predictor of immunogenicity. In this present study, we investigated whether there is an association between adverse reactions after vaccination and corresponding humoral immune responses.
Methods
This is a post-hoc analysis of our previous study comparing heterologous ChAdOx1/mRNA-1273 vaccination versus standard homologous ChAdOx1/ChAdOx1 and mRNA-1273/mRNA-1273 vaccination.7 The full protocol has been published previously7. Briefly, the interventional trial was conducted between July 1 to August 31, 2021, at two medical centers located in northern Taiwan (National Taiwan University Hospital and Taoyuan General Hospital), and participant were aged 20–65 years, being generally healthy or with stable pre-existing health conditions. They had received one vaccine dose of either the adenoviral vectored ChAdOx1 (AstraZeneca, UK) or messenger RNA (mRNA)-1273 (Moderna, USA) vaccine and were enrolled in the study for a second dose of homologous or heterologous vaccine. There were four groups: Group 1 - homologous ChAdOx1/ChAdOx1 (8 weeks interval), Group 2 - heterologous ChAdOx1/mRNA-1273 (8 weeks interval), Group 3 - heterologous ChAdOx1/mRNA-1273 (4 weeks interval) and Group 4 - homologous mRNA-1273/mRNA-1273 (4 weeks interval). The SARS-CoV-2 anti-spike IgG antibody titers were determined among all participants on the day before booster vaccination, and at 2 and 4 weeks after booster vaccination, with the use of Abbott SARS-CoV-2 IgG II Quant assay (06S60, Abbott, USA). This assay, designed to measure specific IgG antibodies to the receptor binding domain (RBD) of S protein, is a chemiluminescent microparticle immunoassays (CIMA) on the Architect i2000SR analyzer (Abbott, USA). Results were reported as arbitrary units (AU) per milliliter, and the cut-off value was 50.0 AU/mL. We converted AU to binding antibody units (BAU) per milliliter using the WHO international standard for SARS-CoV-2 immunoglobulin (BAU/mL = 0.142∗AU/mL). A standard diary card was designed to evaluate the safety of vaccination according to WHO guidelines.
The neutralizing antibody titers in the serum were determined by a 50% tissue culture infectious dose (TCID50)-based neutralization method. Briefly, Vero E6 cells (1 × 104 cells per well) were seeded in 96-well plates and incubated in DMEM containing 10% FBS for 18–24 h. The medium was replaced with 100 μL of fresh DMEM containing 2% FBS for 1 h before infection. Serum samples were inactivated at 56 °C for 30 min before use. Serial two-fold dilutions of sera were mixed with an equal volume of 100 TCID50 SARS-CoV-2 virus suspension. The mixture was incubated for 2 h at 37 °C. After that, the virus-antibody mixture was transferred onto a monolayer of Vero E6 cells, and the cells were incubated with the mixture for 3 days. Cells were fixed with 10% formalin (HT501128, Sigma–Aldrich, USA) and stained with 0.5% crystal violet (0528, VWR International, USA). Serum neutralization titers (NT50) were calculated and expressed as the reciprocals of the highest serum dilution that inhibits 50% of cytopathic effects. The neutralization titers for a panel of serum samples whose titers in IU/mL have been determined after comparison with the WHO IS sera (20/130, 20/136, and 20/268) was used. The results from the reference panel were used to convert NT50 to IU/mL for our test sera. Both B.1.1.7 (alpha) and the B.1.617.2 (delta) SARS-CoV-2 variants were used in the neutralizing antibody test.
All participants were instructed to record any adverse reactions in the standard diary card daily in the first week after the second homologous or heterologous vaccine dose, then weekly till 84 days after the second dose. The records of the diary card were checked at each visit by the physician investigator and the frequency and severity of adverse reactions were correlated with the antibody titers at 2 and 4 weeks after the second dose.
Antibody values were transformed to log values, and the average values were expressed as geometric means with 95% confidence interval. Mann–Whitney U Test was performed to compare the antibody responses between groups. All tests were 2-tailed and a P < 0.05 was considered statistically significant. All statistical analyses were performed using STATA software version 14.0 (Stata Corporation, College Station, TX, USA). The study was approved by the Research Ethics Committee of the National Taiwan University Hospital (202106039 MINA) and Tao Yuan General Hospital (TYGH 110027), and all study participants provided written informed consent.
Results
As previously described, 399 individuals were enrolled, and all completed the study.7 The median age of the vaccinees was 41 years (interquartile range, 33–48 years) with 75% being women. Baseline characteristics were balanced across the four groups except for a higher ratio of male to female in Group 4. However, the SARS-CoV-2 anti-spike IgG antibody titers did not differ significantly between men and women at day 14 or day 28 post 2nd vaccination dose. During the study period, neither hospitalization nor acquisition of SARS-CoV-2 infection occurred in any participants. Local pain (81.7%), fatigue (74.9%) and myalgia (73.2%) were the three most common AEs. Fever of at least 38 °C occurred in 34.0% following vaccination, whilst 39.6% experienced chills.
At 14 days after the second ChAdOx1 or mRNA-1273 vaccine dose, the SARS-CoV-2 anti-spike IgG antibody titers were significantly higher for participants who reported local injection site erythema, swelling, and pain, as well as systemic AEs, such as fever, chills, headache, myalgia, and fatigue (Table 1 ). Those who reported nausea and vomiting (n = 22/399) trended towards having higher antibody titers than those who did not report this AE, but the difference did not reach statistical significance (2652.3 vs. 1488.9, p = 0.055, BAU/mL). Overall, the anti-SARS-CoV-2 titers peaked at 14 days post 2nd dose and decreased at 28 days.
Table 1.
Anti-SARS-CoV-2 spike IgG (BAU/mL) titers at 14- and 28-days post second vaccine dose, geometric means (95%CI), n = 399.
| 14 days | p-value | 28 days | p-value | ||
|---|---|---|---|---|---|
| Gender | Male (n = 101) | 1736.9 (1344.9–2243.0) | 0.301 | 1192.3 (943.5–1506.7) | 0.189 |
| Female (n = 298) | 1474.7 (1257.3–1729.6) | 989.3 (858.0–1140.6) | |||
| Local erythema | Yes (n = 23) | 3575.0 (2713.8–4709.5) | <0.001∗ | 2126.1 (1624.7–2782.2) | <0.001∗ |
| No (n = 376) | 1459.7 (1267.8–1680.7) | 992.6 (874.5–1126.6) | |||
| Local swelling | Yes (n = 41) | 3256.6 (2591.7–4092.0) | <0.001∗ | 1939.8 (1557.2–2416.3) | <0.001∗ |
| No (n = 358) | 1410.4 (1218.8–1632.1) | 967.2 (848.4–1102.6) | |||
| Local Pain | Yes (n = 326) | 1889.5 (1647.9–2166.5) | <0.001∗ | 1236.7 (1092.7–1399.7) | <0.001∗ |
| No (n = 74) | 611.4 (430.5–868.1) | 474.0 (345.9–649.6) | |||
| Fever ≥ 38 °C | Yes (n = 136) | 2894.0 (2479.5–3377.6) | <0.001∗ | 1791.0 (1552.9–2065.7) | <0.001∗ |
| No (n = 263) | 1108.1 (928.4–1322.6) | 781.2 (666.4–915.8) | |||
| Arthralgia/arthritis | Yes (n = 31) | 3236.4 (2621.5–3995.5) | <0.001∗ | 1920.1 (1578.2–2336.2) | <0.001∗ |
| No (n = 368) | 1443.6 (1250.2–1666.9) | 984.7 (865.2–1120.7) | |||
| Nausea/vomit | Yes (n = 22) | 2652.3 (1588.9–4427.5) | 0.055 | 1599.8 (1003.1–2551.6) | 0.090 |
| No (n = 377) | 1488.9 (1294.7–1712.2) | 1011.3 (891.9–1146.6) | |||
| Chills | Yes (n = 158) | 2466.8 (2084.5–2919.1) | <0.001∗ | 1544.4 (1326.3–1798.4) | <0.001∗ |
| No (n = 241) | 1127.2 (936.5–1356.8) | 800.3 (677.1–945.9) | |||
| Headache | Yes (n = 179) | 1899.0 (1594.8–2261.2) | 0.004∗ | 1213.7 (1039.7–1416.9) | 0.019∗ |
| No (n = 220) | 1294.1 (1061.4–1577.7) | 913.4 (763.1–1093.4) | |||
| Myalgia | Yes (n = 292) | 2067.9 (1803.2–2371.4) | <0.001∗ | 1347.8 (1191.3–1524.9) | <0.001∗ |
| No (n = 107) | 684.1 (511.5–914.8) | 508.8 (391.8–660.7) | |||
| Fatigue | Yes (n = 299) | 1885.6 (1633.1–2177.1) | <0.001∗ | 1242.4 (1091.4–1414.2) | <0.001∗ |
| No (n = 100) | 834.3 (618.2–1125.8) | 605.9 (463.4–792.1) | |||
| Systemic Rash | Yes (n = 30) | 1786.4 (1065.8–2994.2) | 0.534 | 1113.1 (712.4–1739.2) | 0.745 |
| No (n = 369) | 1518.4 (1319.1–1747.7) | 1031.3 (908.5–1170.7) |
IgG: immunoglobulin G, BAU/mL: binding antibody units/mL, CI: confidence interval.
At 28 days post vaccination, anti-SARS-CoV-2 antibody titers remained significantly higher (2–2.5-fold higher) among those with solicited local erythema (2126.1 vs. 992.6 BAU/mL), swelling (1939.8 vs. 967.2 BAU/mL), pain (1236.7 vs. 474.0 BAU/mL) even though the first two AEs were reported in less than 10% of vaccinees, indicating a strong association of local reactogenicity with immunogenicity (all p < 0.001). Systemic reactogenicity such as fever, fatigue and myalgia continued to correlate with 2-fold higher antibody titers at day 28 (p < 0.001) (Table 1). Subjects reporting systemic arthralgia/arthritis and headache had statistically higher antibody titers at day 28 than subjects who did not have post-vaccination joint inflammation and headache, but the difference was <2 fold (p < 0.001) and <1.5 fold (p < 0.019), respectively. Those who experienced gastrointestinal symptoms post vaccination still had on average 1.5-fold greater antibody titers at day 28 but this trend remained insignificant due to the small numbers reporting this AE. The only AE that did not track at all with the antibody response on days 14 or 28 was “systemic rash”, with only 30/399 (7.5%) experiencing this solicited AE (Table 1).
When comparing between the four groups at day 14 post vaccination, remarkably no individual reported local injection site erythema and swelling following a second dose of homologous ChAdOx1 vaccine in Group 1, compared to between 6 and 15 individuals per group after receiving a second dose of mRNA-1273 vaccine in Groups 2–4. This lack of local erythema and swelling corresponded to a log lower antibody response in Group 1 compared to Groups 2–4 (194.1 vs. 2125.5–5368.8 BAU/mL) (Table 2 ). Local pain however was common, solicited among 62–96% of vaccinees per group. Specifically, injection site pain was more frequent when booster doses were given at a 1-month interval (Groups 3–4) compared to a 2-month interval (Groups 1–2) (94–96% vs 62–74%). Excluding Group 1 who received boosting with ChAdOx1 vaccine and the effects of different vaccine formulation on local inflammation, the higher mean antibody levels of Groups 3 and 4 with higher rates of injection site swelling compared to Group 2 (3458.2 and 5368.8 vs. 2125.5 BAU/mL) despite all receiving mRNA-1273 boosters, would support a correlation between reactogenicity and immunogenicity.
Table 2.
Sub-group analysis of anti-SARS-CoV2 spike IgG (BAU/mL) titers, geometric means (95%CI), 14 days post 2nd dose, according to vaccine group.
| Group 1 ChAdOx1/ChAdOx1 (8w) (n = 100) |
Group 2 ChAdOx1/mRNA-1273 (8w) (n = 100) |
Group 3 ChAdOx1/mRNA-1273 (4w) (n = 100) |
Group 4 mRNA/mRNA-1273 (4w) (n = 99) |
||
|---|---|---|---|---|---|
| Gender | Male | 184.8 (131.3–260.2) | 1931.0 (1400.2–2663.0) | 2921.2 (2243.9–3802.9) | 3804.1 (3207.5–4511.6) |
| Female | 196.5 (163.6–235.9) | 2436.0 (2099.8–2826.0) | 3393.9 (2949.5–3905.3) | 3783.4 (3398.4–4211.9) | |
| M: F | 20: 80 | 19: 81 | 22: 78 | 40: 59 | |
| p-value | 0.762 | 0.177 | 0.316 | 0.954 | |
| Local erythema | Yes | – | 2628.4 (1441.2–4793.4) | 3932.6 (1881.9–8217.9) | 4409.7 (3111.1–6250.3) |
| No | 194.1 (165.5–227.5) | 2306.6 (2006.6–2651.4) | 3246.2 (2863.0–3680.7) | 3734.9 (3389.9–4115.0) | |
| Y: N | 0: 100 | 8: 92 | 6: 94 | 9: 90 | |
| p-value | – | 0.601 | 0.463 | 0.306 | |
| Local swelling | Yes | – | 2125.5 (1458.8–3096.7) | 3458.2 (2312.5–5171.7) | 5368.8 (4124.4–6988.7) |
| No | 194.1 (165.5–227.5) | 2369.1 (2048.0–2740.5) | 3253.9 (2859.0–3703.3) | 3630.4 (3297.8–3996.5) | |
| Y: N | 0: 100 | 15: 85 | 15: 85 | 11: 88 | |
| p-value | – | 0.568 | 0.726 | 0.007∗ | |
| Local pain | Yes | 209.0 (169.4–257.8) | 2445.5 (2086.1–2866.8) | 3243.2 (2860.2–3677.5) | 3795.0 (3445.8–4179.7) |
| No | 172.0 (134.2–220.3) | 2033.1 (1576.8–2621.3) | 4423.9 (2006.7–9753.0) | 3729.7 (2712.3–5128.8) | |
| Y: N | 62: 38 | 74: 26 | 96: 4 | 93: 6 | |
| p-value | 0.239 | 0.231 | 0.326 | 0.935 | |
| Fever ≥ 38 °C | Yes | 155.4 (74.1–325.6) | 2711.2 (2104.7–3492.5) | 3433.0 (2920.1–4035.8) | 3959.4 (3453.1–4540.0) |
| No | 197.3 (167.3–232.9) | 2170.7 (1853.5–2542.3) | 3110.2 (2565.4–3770.6) | 3672.7 (3233.8–4171.2) | |
| Y: N | 7: 93 | 32: 68 | 55: 45 | 42: 57 | |
| p-value | 0.449 | 0.124 | 0.429 | 0.427 | |
| Arthralgia/arthritis | Yes | 667.5 (−) | 3148.1 (2380.7–4162.9) | 3345.6 (2308.0–4849.6) | 4001.0 (2486.6–6437.7) |
| No | 191.7 (163.5–224.6) | 2245.8 (1941.4–2597.9) | 3275.4 (2869.7–3738.6) | 3776.2 (3432.6–4154.3) | |
| Y: N | 1: 99 | 11: 89 | 12: 88 | 7: 92 | |
| p-value | 0.122 | 0.117 | 0.912 | 0.752 | |
| Nausea/vomit | Yes | 113.3 (5.3–2445.4) | 2636.6 (1615.6–4302.9) | 4278.6 (2614.5–7002.1) | 4392.1 (2491.3–7743.2) |
| No | 196.2 (167.0–230.6) | 2309.3 (2005.6–2658.9) | 3209.1 (2824.3–3646.3) | 3762.2 (3421.5–4136.9) | |
| Y: N | 2: 98 | 7: 93 | 8: 92 | 5: 94 | |
| p-value | 0.340 | 0.618 | 0.207 | 0.468 | |
| Chills | Yes | 190.8 (133.1–273.6) | 2515.0 (2054.2–3079.2) | 3608.9 (3038.3–4286.5) | 4074.6 (3559.7–4663.8) |
| No | 194.7 (162.7–233.0) | 2160.1 (1805.7–2584.1) | 2976.5 (2496.2–3549.1) | 3603.7 (3174.8–4090.6) | |
| Y: N | 16: 84 | 50: 50 | 51: 49 | 41: 58 | |
| p-value | 0.928 | 0.261 | 0.119 | 0.194 | |
| Headache | Yes | 207.4 (161.3–266.8) | 2237.4 (1860.2–2691.1) | 3446.8 (2803.4–3995.5) | 3658.8 (3222.6–4153.9) |
| No | 188.6 (153.9–231.2) | 2445.5 (2001.6–2987.8) | 3214.1 (2701.1–3824.5) | 3892.8 (3408.6–4445.7) | |
| Y: N | 30: 70 | 54: 46 | 53: 47 | 42: 57 | |
| p-value | 0.589 | 0.513 | 0.745 | 0.512 | |
| Myalgia | Yes | 211.5 (163.5–273.6) | 2339.0 (1970.6–2776.2) | 3356.7 (2963.6–3802.0) | 3777.6 (3419.9–4172.7) |
| No | 180.4 (147.3–220.8) | 2313.5 (1860.4–2877.0) | 2629.6 (1434.6–4820.2) | 3895.9 (2936.0–5169.8) | |
| Y: N | 46: 54 | 68: 32 | 91: 9 | 87: 12 | |
| p-value | 0.324 | 0.940 | 0.259 | 0.830 | |
| Fatigue | Yes | 197.3 (157.5–247.3) | 2410.7 (2025.6–2869.2) | 3226.8 (2849.4–3654.1) | 3774.2 (3403.1–4185.8) |
| No | 190.1 (151.0–239.5) | 2162.3 (1769.6–2642.1) | 3919.5 (2103.2–7304.3) | 3891.0 (3150.7–4805.4) | |
| Y: N | 55: 45 | 69: 31 | 92: 8 | 84: 15 | |
| p-value | 0.819 | 0.458 | 0.370 | 0.815 | |
| Systemic Rash | Yes | 170.7 (19.3–1508.3) | 2188.9 (1414.9–3386.2) | 3439.6 (1966.6–6016.0) | 3635.7 (2115.9–6247.1) |
| No | 195.4 (168.1–227.1) | 2349.0 (2035.6–2710.5) | 3270.5 (2879.0–3715.4) | 3802.0 (3457.4–4181.0) | |
| Y: N | 5: 95 | 11: 89 | 8: 92 | 6: 93 | |
| p-value | 0.872 | 0.745 | 0.826 | 0.820 |
IgG: immunoglobulin G, BAU/mL: binding antibody units/mL, CI: confidence interval.
Overall higher antibody titers were observed when the second dose mRNA-1273 vaccine was delivered at 4 weeks after the 1st dose (Groups 3 and 4) than at 8 weeks after the 1st dose (Group 2). Within mRNA-1273 vaccine boosted groups (Groups 2–4), the differences in antibody titers between those with AEs and those without did not reach statistical significance, except for local swelling in the homologous mRNA-1273 boosted subjects (Group 4), where the geometric mean titers of those with and without local swelling were 5368.8 vs. 3630.4, p = 0.007, BAU/mL, respectively.
To adjust for the difference in baseline antibody titers pre-2nd dose, due to priming by ChAdOx-1 versus mRNA-1273 vaccine, we calculated the difference in antibody gains by subtracting the baseline titers obtained at the 1st visit from the titers obtained at the 2nd (day 14) and 3rd (day 28) visit post-2nd dose (Table 3 ). This supported a correlation between immunogenicity and local and systemic reactogenicity, with a significantly larger incremental rise in anti-SARS-CoV-2 titers among those with local erythema, swelling, pain, fever, arthralgia/arthritis, chills, headache, myalgia, and fatigue (Table 3). Comparing the change in titers above baseline instead of the absolute titers at day 14, even gastrointestinal AEs became significantly associated with a greater increase in antibody response (+2443.7 vs. +1176.5, p = 0.043) (Table 3). The change in IgG titers above baseline at visits 2 and 3 were not significantly greater in those with systemic rash (e.g., urticaria) compared to those without rash (Table 3).
Table 3.
Difference in anti-SARS-CoV2 anti-spike IgG (BAU/mL), geometric means (95%CI) at days 14 and 28 from baseline, n = 399.
| Increase in titer at day 14 | p-value | Increase in titer at day 28 | p-value | ||
|---|---|---|---|---|---|
| Gender | Male (n = 101) | 1403.3 (1036.3–1900.2) | 0.337 | 905.8 (682.1–1202.8) | 0.271 |
| Female (n = 298) | 1169.6 (965.3–1417.2) | 744.4 (620.7–892.7) | |||
| Local erythema | Yes (n = 23) | 3275.7 (2493.0–4304.1) | <0.001∗ | 1828.4 (1401.7–2384.9) | <0.001∗ |
| No (n = 376) | 1153.3 (973.4–1366.3) | 742.7 (632.7–871.8) | |||
| Local swelling | Yes (n = 41) | 3015.3 (2413.0–3767.9) | <0.001∗ | 1711.6 (1389.5–2108.4) | <0.001∗ |
| No (n = 358) | 1104.6 (926.3–1317.1) | 716.5 (606.7–846.2) | |||
| Local pain | Yes (n = 326) | 1562.5 (1330.8–1834.6) | <0.001∗ | 940.6 (803.4–1101.2) | <0.001∗ |
| No (n = 74) | 406.9 (259.0–639.4) | 330.6 (219.2–498.7) | |||
| Fever ≥ 38° C | Yes (n = 136) | 2706.0 (2309.2–3171.0) | <0.001∗ | 1576.0(1353.9–1834.5) | <0.001∗ |
| No (n = 263) | 815.5 (657.0–1012.3) | 542.9 (441.8–667.1) | |||
| Arthralgia/arthritis | Yes (n = 31) | 3038.0 (2440.4–3782.0) | <0.001∗ | 1734.9 (1415.1–2127.0) | <0.001∗ |
| No (n = 368) | 1134.5 (954.5–1348.4) | 731.3 (621.0–861.2) | |||
| Nausea/vomit | Yes (n = 22) | 2443.7 (1402.1–4259.2) | 0.043∗ | 1428.8 (855.5–2386.1) | 0.060 |
| No (n = 377) | 1176.5 (994.4–1391.8) | 755.4 (644.4–885.6) | |||
| Chills | Yes (n = 158) | 2149.6 (1757.8–2628.7) | <0.001∗ | 1295.5 (1073.8–1565.5) | <0.001∗ |
| No (n = 241) | 845.9 (676.9–1057.2) | 561.7 (454.4–694.3) | |||
| Headache | Yes (n = 179) | 1617.5 (1313.9–1991.3) | 0.002∗ | 992.9 (820.8–1201.1) | 0.004∗ |
| No (n = 220) | 976.0 (769.3–1238.2) | 644.3 (512.6–809.9) | |||
| Myalgia | Yes (n = 292) | 1734.2 (1474.3–2039.9) | <0.001∗ | 1082.2 (934.1–1253.9) | <0.001∗ |
| No (n = 107) | 470.2 (328.5–672.9) | 318.4 (222.1–456.3) | |||
| Fatigue | Yes (n = 299) | 1577.4 (1330.5–1870.1) | <0.001∗ | 994.2 (849.8–1163.3) | <0.001∗ |
| No (n = 100) | 576.6 (399.9–831.4) | 381.3 (265.9–546.8) | |||
| Systemic Rash | Yes (n = 30) | 1563.3 (857.7–2849.3) | 0.399 | 947.7 (576.6–1557.7) | 0.481 |
| No (n = 369) | 1200.9 (1014.1–1422.0) | 770.6 (656.0–905.2) |
IgG: immunoglobulin G, BAU/mL: binding antibody units/mL, CI: confidence interval.
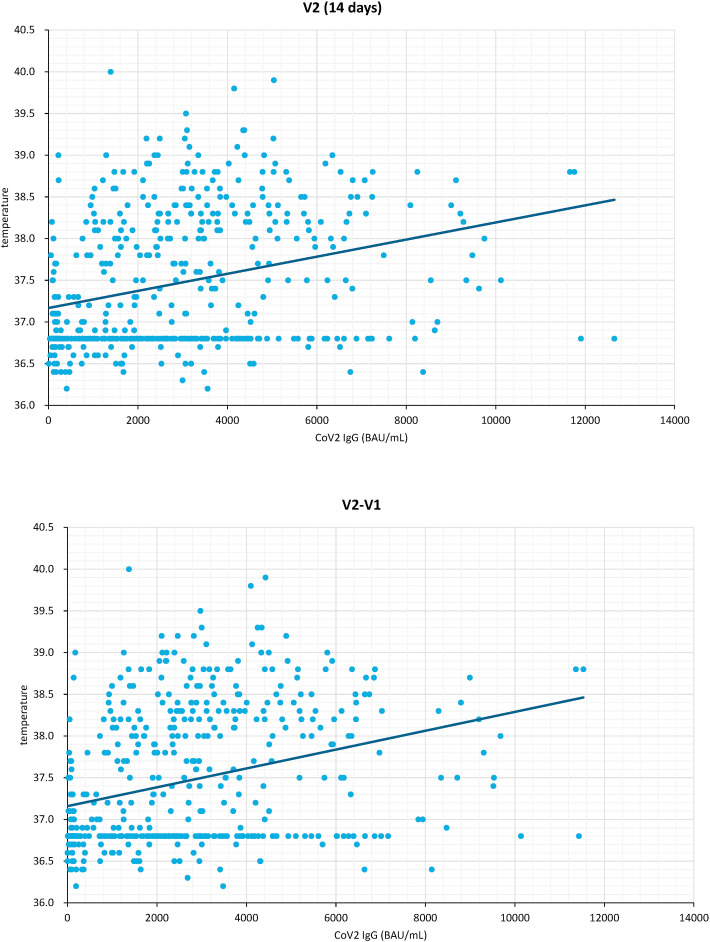
Since almost all severity gradings of AEs are subject to individual perceptions, we used fever as an objective measure. The plotted post-vaccination peak body temperatures and absolute antibody titers (Fig. 1 a, Pearson's coefficient 0.301, p < 0.001) and peak body temperatures against relative change in antibody titers at day 14 (Fig. 1b, Pearson's coefficient 0.311, p < 0.001) support a modest but significant correlation between magnitude of reactogenicity and immunogenicity independent of vaccine type and schedule.
Figure 1.
Correlation with post-vaccination peak body temperature and day 14 anti-SARS-CoV-2 spike IgG (BAU/mL). a. Antibody titers at day 14 (V2) (left). b. Difference (V2–V1) in antibody titers before (Visit 1) and 14 days after the second vaccine dose (Visit 2) (right).
Lastly, the correlations between the neutralizing antibody titers against SARS-CoV-2 alpha and delta variants, determined at day 28 from baseline in relation to AEs are shown in Table 4 . Vaccinees who experienced local pain, and systemic AEs of fever, chills, myalgia and fatigue had significantly higher neutralizing titers against both SARS-CoV-2 variants tested (Table 4).
Table 4.
Difference in anti-SARS-CoV2 neutralizing titers (NT50) against the alpha and delta variants at day 28 from baseline, n = 138.
| Neutralizing titers against the alpha variant | p-value | Neutralizing titers against the delta variant | p-value | ||
|---|---|---|---|---|---|
| Gender | Male (n = 29) | 78.49 (53.53–115.10) | 0.838 | 17.85 (12.67–25.16) | 0.578 |
| Female (n = 109) | 80.74 (66.13–98.58) | 20.18 (17.15–23.75) | |||
| Local erythema | Yes (n = 10) | 126.5 (87.01–183.90) | 0.281 | 24.68 (17.95–33.94) | 0.677 |
| No (n = 128) | 77.46 (64.34–93.26) | 19.32 (16.54–22.57) | |||
| Local swelling | Yes (n = 17) | 123.70 (98.46–155.3) | 0.233 | 25.21 (19.76–32.15) | 0.446 |
| No (n = 121) | 75.53 (62.14–91.81) | 18.99 (16.14–22.34) | |||
| Local Pain | Yes (n = 117) | 88.83 (74.22–106.3) | 0.017∗ | 21.29 (18.31–24.75) | 0.031∗ |
| No (n = 21) | 45.62 (26.55–78.41) | 12.65 (8.08–19.81) | |||
| Fever ≥ 38 °C | Yes (n = 57) | 125.50 (106.70–147.60) | <0.001∗ | 27.75 (23.92–32.21) | <0.001∗ |
| No (n = 81) | 58.60 (45.35–75.72) | 15.43 (12.49–19.08) | |||
| Arthralgia/arthritis | Yes (n = 15) | 131.40 (93.65–184.40) | 0.119 | 29.60 (21.94–39.93) | 0.102 |
| No (n = 123) | 75.58 (62.49–91.41) | 18.71 (15.97–21.92) | |||
| Nausea/vomit | Yes (n = 14) | 95.04 (51.50–175.40) | 0.482 | 23.58 (13.92–39.95) | 0.393 |
| No (n = 124) | 78.75 (65.47–94.71) | 19.27 (16.53–22.46) | |||
| Chills | Yes (n = 67) | 102.40 (82.32–127.50) | 0.014∗ | 24.03 (19.90–29.02) | 0.018∗ |
| No (n = 71) | 63.76 (48.97–83.01) | 16.28 (13.12–20.19) | |||
| Headache | Yes (n = 77) | 88.02 (70.76–109.50) | 0.432 | 21.09 (17.59–25.28) | 0.445 |
| No (n = 61) | 71.44 (53.54–95.31) | 18.01 (14.15–22.92) | |||
| Myalgia | Yes (n = 106) | 93.99 (78.33–112.80) | 0.003∗ | 22.40 (19.19–26.14) | 0.003∗ |
| No (n = 32) | 47.58 (31.20–72.55) | 12.79 (9.12–17.92) | |||
| Fatigue | Yes (n = 106) | 89.09 (73.56–107.90) | 0.038∗ | 21.50 (18.36–25.18) | 0.044∗ |
| No (n = 32) | 56.81 (37.93–85.10) | 14.64 (10.36–20.68) | |||
| Systemic Rash | Yes (n = 13) | 77.64 (42.11–143.20) | 0.691 | 18.16 (11.84–27.87) | 0.401 |
| No (n = 125) | 80.54 (66.93–96.91) | 19.83 (16.96–23.18) |
NT50 - neutralizing titers determined by a TCID50-based neutralization method, were converted into IU/mL using a WHO reference panel.
Discussion
In this study, increased reactogenicity (solicited local and systemic adverse effects) predicted higher antibody responses but not all adverse effects were equally predictive of immunogenicity. Notably, skin rash (suggestive of an idiosyncratic reaction or an atopic reaction) was not associated with antibody titers. Nausea and vomiting were also not as discerning for a robust antibody response. To our knowledge, this is the first time we have clearly shown a correlation between SARS-CoV-2 antibody titers and COVID19-vaccine adverse reactions using heterologous vaccines. Previous studies investigating immunogenicity and reactogenicity of COVID-19 vaccines found inconsistent results. One US-based study of 206 participants (64% women) found no correlation of vaccine-associated symptom severity scores and vaccine-induced antibody titers 1 month after 1st or 2nd dose vaccination with BNT162b2 (Pfizer/BioNTech).8 A smaller Croatian study (n = 64, 77% women) also studying the BNT162n2 (Pfizer/BioNTech) 2-dose vaccine showed no statistically significant difference in antibody titers among participants with or without self-reported adverse reactions.9
Two additional Japanese studies that compared vaccine-related adverse effects after the 1st and 2nd doses of homologous BNT162b2 coronavirus disease 2019 (COVID-19) mRNA vaccination (Pfizer/BioNTech), altogether including 1024 subjects (646 and 378, respectively) found systemic effects of fever and fatigue to be correlated with higher antibody titers after the 2nd dose, and with higher age-adjusted antibody titers at 3- and 6-months post 2nd-dose.4 , 10 However, they did not find the post-vaccination antibody titers to be significantly higher among those who reported local reactions although titers were numerically higher.4 Like our study, they did not find gastrointestinal symptoms to be predictive of greater immunogenicity but surprisingly, vaccinees who reported gastrointestinal symptoms after the first dose had numerically lower vaccine-induced antibody titers.4 Altogether this suggests that nausea and vomiting are “soft, less vaccine-specific symptoms” compared to fever, fatigue, and myalgia.
All previously published studies enrolled more women than men and confirmed that women were more likely than men to report adverse effects (8–11). Interestingly, a German study that looked only at the differences in antibody titers among vaccinees with the most severe adverse reactions (defined as needing to take “sick leave”) compared to those with no adverse reactions show a striking gender difference, with men with severe adverse reactions having a 1.5-fold higher SARS-CoV-2 anti-RBD antibody titer than men without adverse reactions (median 7406 versus 4793, p < 0.001, AU/mL) whereas there was no significant difference in antibody titers among women (median 5892 versus 4628, p = 0.28, AU/mL) with severe adverse reactions compared to those without.11
The strength of this study is the comparison of many log-fold difference in antibody titers, due to the large differences in immunogenicity between ChAdOx1 2nd dose (Group 1) versus mRNA 2nd dose (Groups 2–4). This may explain why we are able to observe a striking association between reactogenicity and antibody levels, whilst previous study only analyzing antibody levels following homologous BNT162n2 (Pfizer/BioNTech) struggled to show a significant difference. If we limit our analysis to only those who received booster mRNA-1273 (Groups 2,3,4) the correlation between reactogenicity and immunogenicity become less pronounced.
As previous studies, if we were to analyze within a homologous group (e.g., Group 4 receiving two doses of mRNA vaccination), we can no longer observe any difference in antibody titers among those who reported injection site pain, compared to those who did not (26,725.6 vs. 26,265.7 AU/ml). Grading of the pain, and whether there is an increase in antibody titers with increasing severity of pain, suggests this lack of difference is because only 6/99 patients did not report injection site pain. Additionally, in terms of injection site swelling, those in Group 4 who had local swelling had more augmented antibody responses at days 14 and 28 compared to those who did not report local swelling.
Limitations of our study include 1. the lack of severity grading of adverse effects to show any possible linear correlation because most adverse effects were mild, 2. lack of comparison of males and females independently given the small number of male participants 3. lack of analysis on vaccine-induced T-cell response in the context of adverse reactions.
Conclusions
Using a mix of vaccine schedules combining homologous or heterologous adenoviral or mRNA-1273 vaccines and different intervals between the 1st and 2nd dose, we were able to generate a wide range of spike protein targeted antibody responses in an adult population with relatively conserved genetic background. This study confirms a striking relationship between adverse reactions and immunogenicity. In addition, we were able to pinpoint certain local and systemic features of reactogenicity such as local pain, swelling and fever, fatigue, myalgia, that better predicted higher SARS-CoV-2 spike IgG binding and neutralizing titers than other less-dose-dependent adverse effects such as headache, gastrointestinal symptoms, and skin rash.
Funding
The funding support of this study included MOST-110-2740-B-002-006, MOST109-2327-B-002-009 from Ministry of Science and Technology Taiwan and a private donation fund to support COVID-19 studies at National Taiwan University, College of Medicine (109F004T). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
References
- 1.WHO Expert Committee on Biological Standardization . Vol. 924. WHO; Geneve, Switzerland: 2017. Guidelines on clinical evaluation of vaccines: regulatory expectations. [Google Scholar]
- 2.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 4.Uwamino Y., Kurafuji T., Sato Y., Tomita Y., Shibata A., Tanabe A., et al. Keio Donner Project Team Young age, female sex, and presence of systemic adverse reactions are associated with high post-vaccination antibody titer after two doses of BNT162b2 mRNA SARS-CoV-2 vaccination: an observational study of 646 Japanese healthcare workers and university staff. Vaccine. 2022 Feb 11;40(7):1019–1025. doi: 10.1016/j.vaccine.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hervé C., Laupèze B., Del Giudice G., Didierlaurent A.M., Da Tavares Silva F. The how's and what's of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin KY, Hsieh MJ, Chang SY, Ieong SM, Cheng CY, Sheng WH, et al. Factors affecting immunogenicity of prime-boost vaccination against SARS-CoV-2. XXXX
- 7.Sheng W.H., Chang S.Y., Lin P.H., Hsieh M.J., Chang H.H., Cheng C.Y., et al. Immune response and safety of heterologous ChAdOx1-nCoV-19/mRNA-1273 vaccination compared with homologous ChAdOx1-nCoV-19 or homologous mRNA-1273 vaccination. J Formos Med Assoc. 2022 Apr;121(4):766–777. doi: 10.1016/j.jfma.2022.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coggins S.A., Laing E.D., Olsen C.H., Goguet E., Moser M., Jackson-Thompson B.M., et al. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID-19 vaccine in a prospective study of healthcare workers. Open Forum Infect Dis. 2021 Nov 20;9(1):ofab575. doi: 10.1093/ofid/ofab575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapić I, Rogić D, Šegulja D, Zaninović L. lAntibody response and self-reported adverse reactions following vaccination with Comirnaty: a pilot study from a Croatian university hospitalJournal of Clinical Pathology Published Online First: 15 September 2021. doi: 10.1136/jclinpath-2021-207572 [DOI] [PubMed]
- 10.Koike R., Sawahata M., Nakamura Y., Nomura Y., Katsube O., Hagiwara K., et al. Systemic adverse effects induced by the BNT162b2 vaccine are associated with higher antibody titers from 3 to 6 Months after vaccination. Vaccines (Basel) 2022 Mar 15;10(3):451. doi: 10.3390/vaccines10030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauernfeind S., Salzberger B., Hitzenbichler F., Scigala K., Einhauser S., Wagner R., et al. Association between reactogenicity and immunogenicity after vaccination with BNT162b2. Vaccines. 2021;9:1089. doi: 10.3390/vaccines9101089. [DOI] [PMC free article] [PubMed] [Google Scholar]