Abstract
Purpose of Review
Thalamic aphasia is a rare language disorder resulting from lesions to the thalamus. While most patients exhibit mild symptoms with a predominance of lexical-semantic difficulties, variations in phenotype have been described. Overall, the exact mechanisms of thalamic aphasia await empirical research. The article reviews recent findings regarding phenotypes and possible underlying mechanisms of thalamic aphasia.
Recent Findings
Variations in phenotype of thalamic aphasia may be related to different lesion locations. Overall, the thalamus’ role in language is thought to be due to its involvement in cortico-thalamic language networks with lesioning of certain nuclei resulting in the diachisis of otherwise interconnected areas. Its possible monitoring function in such a network might be due to its different cellular firing modes. However, no specific evidence has been collected to date.
Summary
While recent findings show a more distinct understanding of thalamic aphasia phenotypes and possible underlying mechanisms, further research is needed. Additionally, as standard language testing might oftentimes not pick up on its subtle symptoms, thalamic aphasia might be underdiagnosed.
Keywords: Thalamic aphasia, Thalamo-cortical language networks, Subcortical aphasia, Lexical-semantic deficits
Introduction
Thalamic aphasia describes aphasic syndromes stemming from lesions to the thalamus. Aphasia is a clinical syndrome of acquired speech impairments that typically result from lesions to the left hemispheric cortico-subcortical language network [1, 2]. Aphasia is often characterized by the language domains that are primarily affected, and well-established aphasia screening tools use cutoffs in its diagnosis [3]. For example, the “Boston” classification divides aphasia based on deficits in fluency, naming, repetition, comprehension, reading, and writing—with global aphasia describing a loss of all six functional domains [4]. Historically, aphasia was thought to only present after fronto-temporo-parietal cortical lesions and was separated into “Broca’s” and “Wernicke’s” type, referring to a predominant affection of “motor,” or “sensory,” aspects of language [5, 6]. Today, however, it is widely accepted that aphasia may also result from lesions to white matter tracts and subcortical areas [7–12]. Here, aphasia can be due to ischemic or hemorrhagic strokes, as well as tumors, or infections of, e.g., basal ganglia or the thalamus [13–15]. Additionally, language disturbances can occur as a result of stereotactic thalamic and subthalamic deep brain stimulation (DBS) surgery in, e.g., Parkinson’s or Essential Tremor patients, or during thalamotomy [16, 17, 18••, 19, 20]. However, the exact roles of the thalamus and other subcortical structures in language functions are still unclear [7, 18••]. The thalamus is mainly known for its relay function, conducting and modulating afferent signals to the cortex and between different cortical areas—thereby acting as a “gate-keeper” to what we feel and experience [21, 22]. Thus, lesions to the thalamus can result in sensory deficits and neglect, but also in visuo-spatial deficits, pain-syndromes, or even hemiparesis [23]. Additionally, thalamic lesions can lead to a multitude of attentional and cognitive deficits such as amnesia, vigilance impairment, executive dysfunction, apraxia, anosognosia, behavioral and mood alterations, as well as aphasia [24–26]. Similar to its role in sensory processing, the role of the thalamus in language is suggested to be that of a moderator conducting the transfer of lexical information to cortical areas [27]. Thalamic lesions may therefore result in a metabolically or functional decoupling of remote, but interconnected areas that are critical for language function [28]. Such a disconnection, where lesioning of an area, e.g., the thalamus, leads to a dysfunction in another non-lesioned area, for example left cortical structures, or in fact, in a network of brain regions, such as the cortico-thalamic language network, has also been termed diachisis [29].
Here, we review the existing literature on thalamic aphasia. Specifically, we (a) introduce its clinical presentation and phenotypes, (b) describe the involvement of specific, anatomic thalamic subregions, and (c) present its putative underlying mechanisms.
Clinical Presentation and Phenotypes of Thalamic Aphasia
Thalamic aphasia is less rare than one might expect. With thalamic stroke amounting to 2–4% of all ischemic lesions, and estimation of aphasia after thalamic stroke varying between 12 and 80%, we can expect about 0.25–3.2% of all stroke patients to be affected [23, 30••, 31]. Variation in the estimation of frequency seems to depend largely on clinical language assessments used—with more detailed testing (for example, Aachener Aphasie Test, AAT, compared to National Institute of Health Stroke Scale, NIHSS) leading to higher detection rates, which, in turn might bias results [25, 30••]. Recovery from thalamic aphasia is often quick, with patients showing little to no symptoms after days or weeks [32–34]. Nevertheless, some case series have also described patients to suffer from persistent aphasic symptoms [35•]. Research suggests that speed and success of aphasia recovery might depend on lesion location and size, although it is unclear whether the same factors are important in thalamic lesions [36].
A first description of a thalamic aphasia phenotype came from Crosson in 1984, who reviewed the literature of the time, comprising thalamic aphasia after ischemic or hemorrhagic lesions to be consisting of “[1] word substitutions in spoken language, primarily semantically in nature, [2] jargon in narrative discourse, consisting of words from the patient’s native language, [3] comprehension less impaired than spoken output, and [4] minimally impaired repetition” [37]. Several studies have since evaluated thalamic aphasia using different language assessment tools, and to date, several features of what may characterize it have been discussed. While clinical descriptions differ, and no specific phenotype has been singled out, the literature points towards certain language domains that seem specifically affected, reminiscent of the clinical phenotype initially suggested by Crosson [38]. Overall, aphasia after thalamic lesions has mostly been described as mild, with predominantly lexical-semantic deficits [35•, 39••]. This means that patients exhibit anomia, or naming deficits, in speaking and writing while substituting with semantic paraphasias, i.e., word substitutions with a semantic relationship to the target word, as well as perseverations [25, 30••, 40–43]. In other words, patients make semantically related, but incorrect, word choices and tend to repeat certain words [3]. In most patients that were severely affected, so-called “jargon” language was described, where speech is fluent, but senseless [44]. On the other hand, repetition of words, reading aloud and comprehension, is often intact [24, 27, 30••, 45, 46]. Note, however, that studies using the “Boston” classification have also shown dysfunctional comprehension after ischemic thalamic lesions [38]. Additionally, impaired word fluency is described in some cases, while other studies also reported patients to have intact and fluent spontaneous speech [34, 47]. While these findings appear contradictory at first, it seems likely that certain thalamic areas encode for, or are involved in, different language domains—leading to different clinical presentations in aphasic syndromes, depending on lesion location (Fig. 1) [35•, 39••].
Fig. 1.
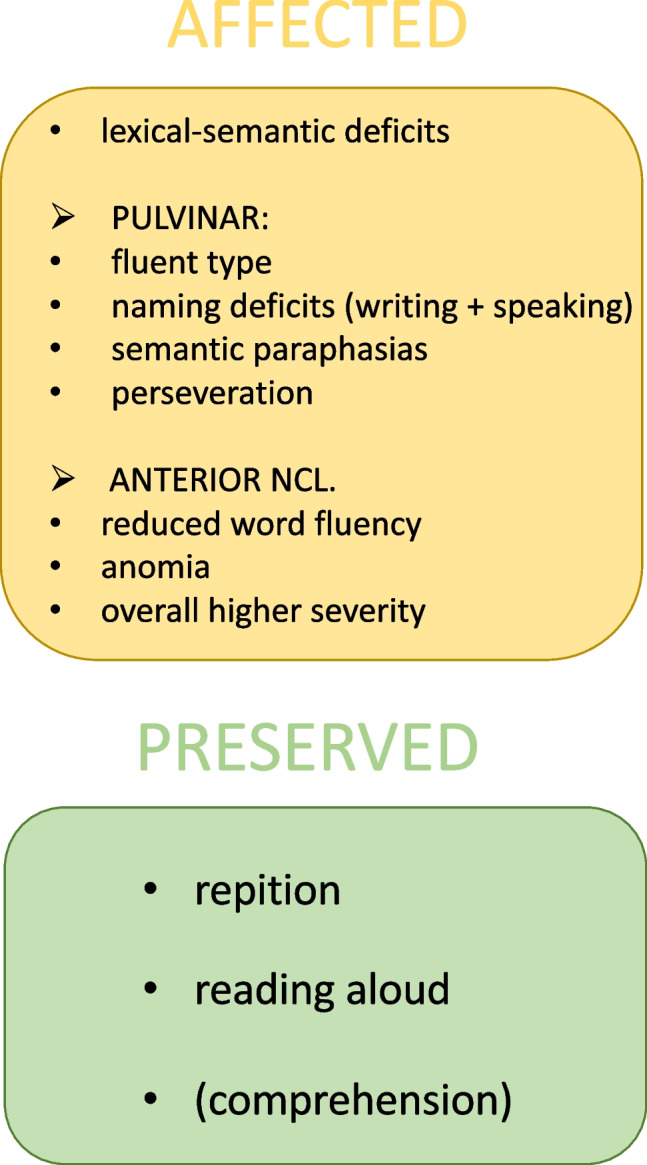
Overview of different language domains predominantly affected or preserved in thalamic aphasia as suggested by the current literature. While lexical-semantic deficits are described after lesions to almost all areas to the thalamus, some symptoms seem to be more common after lesions to specific thalamic nuclei, although more thorough analyses are needed. For example, lesions to the pulvinar are more often associated with fluent aphasia including naming deficits and semantic paraphasias, while lesions to the anterior nuclei can lead to reduced word fluency and show an overall higher severity. However, many phenotypical variations beyond this classification exist
In an early case series, categorizing deficits after thalamic ischemic lesions according to the involved vascular territories, Bogousslavsky et al. described “dysphasia” with reduced verbal output, paraphasias, and impairment of comprehension, albeit preserved repetition, in four patients with left-sided lesions in the tuberothalamic arterial territory, which typically supplies the ventral anterior and ventral lateral nucleus [47]. They additionally described impaired consciousness with subsequent dysphasia and amnestic deficits in patients with paramedian lesions, supplied by the thalamoperforating artery and involving the intralaminar and dorsomedial nuclei. While this stresses the notion of anatomically distinct phenotypes, the latter observation furthermore poses the question whether thalamic aphasia is a byproduct of vigilance impairment rather than a language disturbance in itself. For example, Mohr et al. have described a strong fluctuation in language disturbances in patients following thalamic hemorrhage, with symptoms only occurring in association with vigilance impairment or apathy, which is in line with a more recent hypothesis that thalamic language properties are mechanistically similar to its monitoring function of vigilance states [28, 48]. It must be noted that since vascular supply varies greatly, even an ischemic lesion within the same vascular territory might lead to distinct symptoms in different patients [23, 31, 49••]. On the other hand, some studies reported very similar aphasic syndromes despite patients displaying large discrepancies in thalamic lesion location [34]. Critically, however, a relevant amount of the reported data has been collected prior to MRI being routinely available, and neurocognitive assessment was rarely standardized. Thus, the variability in reported syndromes and lesion locations may be partially attributed to varying imaging techniques and quality of language and cognitive assessment.
While there is considerable variability in symptomatology, a basic concept of thalamic aphasia seems to evolve around lexical-semantic difficulties, i.e., finding exact word forms for specific semantic concepts, which is suggested to arise from impaired lexical-semantic retrieval, cf. further explanations below [37]. This notion is additionally supported by stimulation studies in patients undergoing DBS surgery for Essential Tremor or Parkinson’s [16]. Here, specific tasks, which are thought to assess the activation, retrieval, or violation of semantic representations, are associated with thalamic activity [18••, 19, 50–52]. Similarly, studies using functional MRI have reported thalamic activation specifically during object recall or verbal fluency tasks, which are also designed to evaluate lexical-semantic retrieval [1, 53, 54]. Halari et al. additionally saw greater activation during word generation than during repetition, further stressing the thalamus’ possible role in lexical-semantic retrieval and matching the proposed phenotype of thalamic aphasia with preserved repetition [55]. While a strong case can be made for the role in lexical-semantic retrieval, another recent notion has been proposed that primarily higher order language skills, such as in figurative language, synonym, and antonym generation, or in ambiguous sentences are impaired in patients with thalamic lesions [30••, 56]. Ketteler et al. suggested that the thalamus might aid specifically in language processing, such as ambiguity resolution, where more “automated mechanisms,” such as semantic priming, are not precise enough [57, 58]. Similarly, some imaging studies only saw thalamic activation in addition to cortical activation during tasks with higher levels of language difficulty [1, 59, 60]. In summary, current research supports the notion that the thalamus serves specifically during higher-order language functions.
This might have important implications for diagnostic tools for assessment of thalamic aphasia. For instance, patients that display significantly impaired word retrieval do not necessarily meet the cutoff criteria for “aphasia” in standard diagnostics, such as the “standard language test of aphasia” (SLTA) [32, 61]. This underlines the idea that typical routine language assessments might underestimate the subtle impairments in patients with thalamic aphasia [24, 32]. So even when standard diagnostic tools are administered, thalamic aphasia might be underdiagnosed, a concern that could be addressed by more sensitive aphasia examinations, or a stronger focus on separate language domains in the assessment of thalamic aphasia.
Anatomy of Thalamic Aphasia
As noted above, differences in clinical phenotype of thalamic aphasia may be related to the extent and location of thalamic lesions. While it remains unclear whether specific thalamic nuclei or areas are important for language function “on their own,” or if it is rather their involvement in thalamo-cortical language networks, certain nuclei are thought to have more predominant language properties than others [35•].
Involved Thalamic Nuclei
With regard to thalamic nuclei, it has been proven difficult to assign distinct thalamic areas to specific language functions, as converging inputs from all neocortical areas to the thalamus, as a whole, exist [62]. It is, however, likely that a nucleus’ function is closely related to the cortical area, with which it is directly connected [63]. Numerous accounts have been made for the thalamic nuclei most likely involved in language, and studies have further tried to assign specific aphasia phenotypes to lesioned areas.
Due to their extensive projections to frontal or temporo-parietal cortical areas, the anterior, ventral, and centromedian nuclei, as well as the lateral posterior thalamus and the pulvinar, are thought to be critical for language function [27, 64]. Accordingly, lesions studies have shown aphasia primarily after lesions to the left anterior and inferolateral nuclei, and in lesions to the, overall much more infrequently affected, pulvinar [24, 25, 30••, 40, 42, 65–67]. Additionally, lesions to the right, left, or both paramedian thalamic areas can lead to aphasic as well as complex neurocognitive symptoms [34, 42, 68]. Additional evidence for the importance of the anterior thalamic nuclei in language comes from functional MRI studies showing left and bilateral anterior thalamic activation during a semantic object activation task [69]. Moreover, a systematic pattern of event-related potentials is consistently reported in the vicinity of the ventral intermediate nucleus (VIM) during tasks testing semantic and syntactic rules, which authors have suggested to originate from the anterior or centromedian nuclei [19, 70]. Furthermore, verbal fluency, which has been associated with lexical-semantic retrieval, was reduced in bilateral stimulation of VIM in DBS treatment of Essential Tremor, with the effect being correlated to the frequency of stimulation further pointing to a frequency-modulated informational transfer via the thalamus [16, 17]. It has furthermore been suggested that the anterior thalamic nuclei’s role in the access of lexical-semantic representations is conducted via theta-band activity, which has been shown in electroencephalographic recordings during word retrieval [71–73]. Regarding the ventral anterior thalamus (VA), functional imaging studies provided further insight by showing direct connectivity of the VA to the pre-supplementary motor area (pre-SMA) and Broca’s area [74]. The rostrally located pulvinar holds equally strong projections to temporoparietal cortices and Broca’s area [75]. As resting state functional connectivity of the left pulvinar with left temporo-parietal cortices has been shown to correlate with picture naming, the pulvinar is similarly suggested to be important for lexical-semantical retrieval [76, 77]. It is furthermore hypothesized that the pulvinar may be important in lexical discrimination, as it holds similar properties in visual discrimination; however, no evidence towards this notion has been collected to date [35•, 78].
As mentioned above, when comparing aphasic phenotypes between different thalamic nuclei, slight differences are reported in the literature. While lesions to the pulvinar are described to rather result in a fluent aphasia with semantic paraphasias and naming deficits, lesions to the anterior and ventrolateral nucleus may lead to a non-fluent aphasia type with anomia and more severe overall language deficits [30••, 35•, 41, 67, 76, 77, 79]. Different phenotypes have also been attributed to a difference in involvement of the mentioned nuclei in specific brain networks, an aspect that will be discussed in detail below [39••, 49••].
Again, some limitations need to be mentioned. Most of the aforementioned studies are based on small samples with differing quality of language assessment and imaging techniques, rendering specific differentiations of phenotypes difficult. Additionally, as not all areas of the thalamus are equally likely to be affected by ischemic stroke due to its vascular supply and stimulation studies are mostly reporting on nuclei that are of interest in the pathology and treatment of Parkinson’s and Essential Tremor, a selection bias must be expected [16, 23]. Therefore, existing literature might not give a sufficiently accurate representation of thalamic areas relevant for language processing, with the question, which nuclei are specifically involved to what degree, remaining. Ultimately, evidence suggests that lesion location and size do not predict severity and phenotype of aphasia, both in subcortical and cortical stroke [80].
Lateralization
Another key question remaining to date is, whether language is lateralized in the thalamus, paralleling its cortical organization, where in right-handed, and most left-handed, subjects language areas are typically located in the left hemisphere [3]. Here, studies of patients with thalamic strokes, or following DBS placement, have reported aphasia to be predominantly associated with lesions, or electrode placement, in left thalamic areas [16–20, 24, 81•]. For instance, in a study with 52 thalamic stroke patients, aphasia, as defined by the utilized aphasia check list (ACL), was associated with lesions in nearly all thalamic regions in the acute-stroke-phase. Intriguingly, however, left anterior lesion location resulted in the most severe deficits, with a predominance of naming difficulties and reduced verbal fluency [30••]. This finding is corroborated by fMRI studies showing predominantly left-sided thalamic activity in language tasks [62]. The notion of such a lateralization of language in the thalamus is challenged by studies showing bilateral [54], or even only right-thalamic activity during active or passive language tasks [55]. Unfortunately, handedness was often not tested, and language impairment was often assessed in patients with left-sided thalamic lesions only, limiting these results [24, 38]. Additionally, selection criteria are not always precisely reported, and such results might partially be due to a selection bias, as more patients with left sided (thalamic as well as cortical) lesions are being detected and hospitalized overall [82].
Mechanisms of Thalamic Aphasia
So far, most concepts on thalamic aphasia have suggested the role of the thalamus in language to be that of a monitor or conductor in a thalamo-cortical language network, with lesions of the thalamus leading to a disconnection between network structures and thereby to aphasia [45, 49••, 83].
Studies of Functional and Structural Connectivity and Diachisis
Many of the studies already mentioned in this review have evaluated structural and functional connectivity between thalamic nuclei and cortical areas in association with language functions, thereby stressing the idea of thalamo-cortical networks in language [1, 53, 54, 59, 74]. Furthermore, studies specifically showing anatomic, metabolic, or functional disruption within networks after thalamic lesions bring even further insight. This so-called diachisis, originally describing an anatomical disconnection, is today understood as a multi-factorial deterioration of a non-lesioned area due to lesioning in another, remote location based on changes in their network communication [29, 84].
One hypotheses suggests that subcortical lesioning leads to temporary cortical hypoperfusion and/or hypometabolism and thereby to aphasia [85, 86]. This could explain the short-lived nature of thalamic aphasia, as duration of this disruption may be temporary [85]. Using positron emission tomography (PET) and tractography in six patients, Nishio et al. were able to show that left anterior thalamic infarctions lead to a disruption of metabolic and fiber connections between the anterior thalamus and fronto-temporal cortical areas, which was associated with lexical-semantic deficits [43, 65]. Stenset et al. described a similar hypometabolism with reduced left fronto-parieto-temporal cortical glucose uptake in PET as well as reduced fractional anisotropy (FA) after a left anterior thalamic lesion corresponding to disturbances in naming as well as in semantic and working memory which persisted over at least 6 months in one patient [87]. Baron et al. reported a correlation of aphasia, though not specified, and other neurocognitive impairments with ipsilateral, cortical hypometabolism, as seen in PET, after thalamic lesions with improvement in symptoms paralleling changes in glucose uptake [88]. In a longitudinal study using PET in a small cohort of seven patients with subcortical strokes and aphasia (predominantly anomia), De Boissezon showed a reduction of regional cerebral blood flow (rCBF) in the left fronto-temporal cortex following stroke [89]. While not all patients had exclusive lesions in the thalamus, the authors suggested the observed recovery in clinical and imaging parameters being associated with a functional diachisis phenomenon. It has further been hypothesized that (transient) aphasia after thalamic lesions might be due to transient cortical hypoperfusion associated with cerebral artery stenosis [90–92]. While Hillis et al. were able to associate specific language impairments with cortical hypoperfusion patterns in perfusion MRI in patients with non-specific subcortical lesions, Sebastian et al. only reported regional, cortical hypoperfusion in one of five patients with thalamic aphasia (showing fluent speech with naming errors) [90, 92]. Furthermore, in a single photon emission computed tomography (SPECT) and near infrared spectroscopy (NIRS) study, Obayashi et al. (2022) reported that thalamic lesions led to hypoperfusion of language-related left cortical areas such as the supplementary motor area (SMA) and the frontal cortex that would normally be connected by the frontal aslant track (FAT). Interestingly, as low SMA activity correlated with poor word retrieval, high SMA activity was observed in recovery of the same patients during follow-up [32]. While this study is limited by its small sample size, the results suggest a critical role of the SMA frontal cortex loop via FAT in aphasia after thalamic lesions. Notably, also cortical lesions have been reported to relate to changes in the thalamo-cortical language network. In post-stroke aphasic patients with left cortical lesions, increased intra-thalamic functional connectivity in the right thalamus was associated with more severe symptoms, specifically naming deficits [93]. Furthermore, Keser et al. were able to show that even non-lesioned left thalamo-cortical pathways degenerated in patients with aphasia after left-cortical strokes which, again, correlated with severity of naming deficits [94•]. Similarly, Guo et al. described aphasic patients to have an increase in functional connectivity of the right thalamus in a thalamo-cortical language network, possibly correlated to semantic processing [95]. This stresses the previously mentioned evidence on a thalamo-cortical language network being involved in naming and lexical-semantic properties and overall suggests that thalamo-cortical connections play a critical role in the pathology and recovery of aphasia.
Mechanistic Models
While a network of cortical and thalamic areas in language and other cognitive tasks seems evident as such, the underlying mechanisms have not been fully understood and remain a challenge for ongoing research. Many considerations regarding possible models have proposed similar ideas of a modulating or orchestrating effect on cortical activity. Since a specific aspect of thalamic aphasia seems to be lexical-semantic disturbances, a focus often lies on explaining the role of the thalamus in lexical-semantic retrieval. In general models of language pathology, lexical-semantic errors are thought to result from damage to the access, or storage, of preformed lexical-semantic representations, which can follow ischemic or other lesions to fronto-temporo-parietal cortical areas [96]. Even early concepts of thalamic aphasia have considered similar aspects. Ojemann et al., Wallesch and Papagno, or Fristen et al. proposed that thalamic activity would not just trigger unspecific arousal, but might activate specific, relevant cortical circuits needed for language functions and support in the selection of one of multiple lexical alternatives that were previously generated in the cortex [12, 45, 97, 98].
The idea that the thalamus helps in “choosing the right word” has also been discussed recently. DBS stimulation of VIM in Essential Tremor patients can lead to a shift towards more frequent words, similar to observations made in dementia, which is considered a marker for a “lexical simplification” of language—possibly due to dysfunctional lexical retrieval [18••]. As missing words are then substituted by semantically similar, but more frequent words, this may lead to the evolution of semantic paraphasias often observed in thalamic aphasia [49••]. Correspondingly, Nadeau and Crosson (1997) suggested that the thalamus “selectively engages” cortical areas critical for operations during lexical access [27]. As part of two proposed cortico-subcortical loops (“frontal lobe – inferior thalamic peduncle – reticular nucleus – centromedian nucleus” and “cortex – pre-SMA – Brodmann’s area 32 – dorsal caudate nucleus – ventral anterior thalamus”), thalamic nuclei may specifically access neuronal networks, leading to the release of cortically generated language segments—an execution that becomes dysfunctional in thalamic lesions [27, 49••]. The authors refined this idea in the “Response Release Semantic Feedback” Model, suggesting that the thalamus, and other basal ganglia, scale, and sequence language options before speech, is then actually produced [64]. A possible computational and mechanistic explanation for such a scaling and selection processes in language is provided in the “Lexical Selection Model” by Norris et al. They describe lexical, auditory recognition as a Bayesian inferential process in which word recognition is based on a comparative evaluation of multiple, lexical hypotheses—a process that could be based on a computational information transfer between cortex and subcortical regions [99, 100]. Relatedly, Crosson proposed a feedforward and feedback mechanism of “word selection” between cortico-cortical and cortico-thalamic networks, in which higher-order thalamic relays maintain a stable representation of known lexical information, which are perpetually compared with possible semantic solutions for a given object, proposed by the cortex. Mismatches of the object with proposed lexical forms may then lead to error signals and to suppression of possible incorrect choices. In a step-wise process of iterations of this kind, the most likely word choice would be refined and emerge [101]. The thalamus could herby aid in enhancing resolution especially in lexical ambiguity in more difficult language tasks by suppressing incorrect choices for words [57]. Taking a similar computational approach to language, the “Declarative/Procedural” model proposes an additional separation of language functions into memorized lexical formations and the underlying, grammatical rules needed to form larger words or sentences [39••, 102]. Similar to the memory system, both aspects are thought to be encoded by different neural circuits: the temporal cortex holding representation for the declarative system, and a frontal cortex-basal ganglia loop encoding for procedural memory [39••, 102]. It remains unclear, however, whether the thalamus acts merely as part of a basal-ganglia loop in procedural memory aspects of language (i.e., grammar) or whether it is also involved in declarative-lexical processes [39••]. Crosson recently proposed that the pulvinar might be involved in declarative processes while the dorsal-medial nucleus could be involved in grammatical operations. He further suggests that the ventral anterior and lateral nuclei could be involved in lexical search by suppressing competing word alternatives [39••]. This could again also explain why damage to different thalamic nuclei might lead to different forms of aphasia (as mentioned above). In summary, different hypothetical and computational models suggest possible involvements of the thalamus in language. However, an exact mode of action has yet to be discovered. Therefore, further research is needed to show the exact role of the thalamus in language processing, and ultimately, in aphasia.
The Role of Different Cell Types
A possible underlying mechanism, conducting the aforementioned selective recruitment of cortical areas in a language network, might be the specific neuronal organization and firing modes within the thalamus. Here, thalamic nuclei can be categorized into first-order relay nuclei, receiving input mainly from subcortical sources, and transferring sensory information to the cortex, and higher-order relay nuclei that conduct informational transfer from one cortical area to another [63, 103]. First-order relay cells are known to exhibit different firing modes, which in turn have an important impact on the sleep–wake-cycle [104]. While rhythmic, oscillatory activity in the delta band range stabilizes sleep and shields the organism from incoming sensory information, a tonic spiking mode is adopted in awake states to promote precise and linear information transfer to the cortex [105]. Higher-order thalamic relays make up most of the thalamus’ volume and are thought critical in transthalamic informational transfer to the cortex in cognitive tasks and memory [103, 106]. Such informational transfer, possibly via different firing modes, may be similarly driving thalamic recruitment of cortical areas, such as pars triangularis of Broca’s area, for language [49••, 107]. This notion is supported by studies suggesting an importance of specifically higher-order thalamic relay activity in other cognitive tasks such a learning, decision-making, and goal-directed behavior [108, 109]. While similar processes in language functions seem plausible, no direct evidence has been collected to date as data on different cell types or firing modes in the specific setting of thalamic aphasia are scarce and further research is needed.
Conclusions
Thalamic aphasia is relatively rare, with 0.25 to 3% of ischemic stroke patients being affected. While phenotype and severity can vary greatly, most patients typically exhibit mild symptoms, with a predominance of lexical-semantic deficits, such as anomia and paraphasias, while comprehension and repetition are mostly spared. Prognosis is overall good with most patients recovering quickly. Anatomically, lateralization of language function to the left thalamus has been widely suggested, especially in lesion studies, but some indication of additional right-sided and bilateral thalamic activity during language tasks prevails. While aphasia has been seen after lesions in almost all thalamic regions, the anterior nuclei and the pulvinar have been specifically singled out as important in language function and might have specific importance for certain language domains. Most mechanistic models on thalamic aphasia suggest these thalamic areas to be part of cortico-thalamic language networks with lesions to the thalamus leading, i.e., to a diachisis of the interconnected areas and thereby to deficits in lexical-semantic retrieval. The thalamus’ role in activating specific cortical areas needed for language could be explained due to its different cellular firing modes, with so-called higher-order thalamic nuclei, possibly driving this recruitment of areas involved in the language network. Of clinical importance, thalamic aphasia is probably underdiagnosed, as symptoms are often mild and might not be picked up by standard language testing. To submit the affected patients to the best possible care, more precise and differentiated language assessment is needed in clinical routine and in future studies examining thalamic aphasia.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of Interest
Dr. Fritsch and Dr. Rangus have no conflicts of interest. Dr. Nolte reports personal fees from Boehringer Ingelheim, personal fees from Bayer Pharma, personal fees from Daichii Sankyo, personal fees from Alexion, personal fees from Bristol-Myers Squibb, personal fees from Abbott, personal fees from Takeda, personal fees from Pfizer, grants from German Ministry of Research and Education, grants from German Center for cardiovascular Research, grants from German Center for Neurodegenerative Diseases, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Behavior
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Assaf M, Calhoun VD, Kuzu CH, Kraut MA, Rivkin PR, Hart J, et al. Neural correlates of the object-recall process in semantic memory. Psychiatry Res Neuroimaging. 2006;147(2–3):115–126. doi: 10.1016/j.pscychresns.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Crosson B. Thalamic mechanisms in language: a reconsideration based on recent findings and concepts. Brain Lang. 2013;126(1):73–88. doi: 10.1016/j.bandl.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirshner HS. Aphasia. Encyclopedia of human behavior: second edition. 2012;177–86.
- 4.Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia: Lea & Febiger; 1972. [Google Scholar]
- 5.Broca PP. Loss of speech, chronic softening, and partial destruction of the anterior left lobe of the brain. Bulletin de la Société Anthropologique. 1861;2:235–8. [Google Scholar]
- 6.Wernicke C. Der aphasische Symptomencomplex; eine psychologische Studie auf anatomischer Basis. Breslau: Cohn & Weigert; 1874.
- 7.Poeppel D, Emmorey K, Hickok G, Pylkkänen L. Towards a new neurobiology of language. J Neurosci [Internet]. 2012 Oct 10 [cited 2022 Jun 30];32(41):14125–31. Available from: https://pubmed.ncbi.nlm.nih.gov/23055482/ [DOI] [PMC free article] [PubMed]
- 8.Murdoch BE. Subcortical brain mechanisms in speech and language. Folia Phoniatr Logop. 2001;53(5):233–251. doi: 10.1159/000052679. [DOI] [PubMed] [Google Scholar]
- 9.Dick AS, Bernal B, Tremblay P (2014) The language connectome: new pathways, new concepts. Neuroscientist [Internet]. 2014 Oct 1 [cited 2022 Jun 30];20(5):453–67. Available from: https://journals.sagepub.com/doi/10.1177/1073858413513502?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed [DOI] [PubMed]
- 10.Hickok G, Poeppel D (2022) Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition [Internet]. 2004 [cited 2022 Jul 9];92(1–2):67–99. Available from: https://pubmed.ncbi.nlm.nih.gov/15037127/ [DOI] [PubMed]
- 11.Saura D, Kreher BW, Schnell S, Kümmerera D, Kellmeyera P, Vrya MS, et al. (2022) Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A [Internet]. 2008 Nov 11 [cited 2022 Jul 9];105(46):18035. Available from: https://pmc.articles/PMC2584675/ [DOI] [PMC free article] [PubMed]
- 12.Wallesch CW, Papagno C. Subcortical aphasia. London: Whurr Publishers; 1988. pp. 256–287. [Google Scholar]
- 13.Osawa A, Maeshima S. Aphasia and unilateral spatial neglect due to acute thalamic hemorrhage: clinical correlations and outcomes. [DOI] [PubMed]
- 14.Smyth GE, Stern K. Tumours of the thalamus—a clinico-pathological study. Brain [Internet]. 1938 Dec 1 [cited 2022 Jul 14];61(4):339–74. Available from: https://academic.oup.com/brain/article/61/4/339/278607
- 15.Hernandez Jimenez JM, Vahdat K, Serrano Santiago IA, Morales Hernandez M del M, Isache CL, Sands M. Thalamic bacterial abscess presenting with hemiparesis and expressive aphasia. IDCases [Internet]. 2018 Jan 1 [cited 2022 Jul 14];13. Available from: https://pubmed.ncbi.nlm.nih.gov/30101064/ [DOI] [PMC free article] [PubMed]
- 16.Klostermann F, Ehlen F, Tiedt HO. Effects of thalamic and basal ganglia deep brain stimulation on language-related functions—conceptual and clinical considerations. Eur J Paediatr Neurol. 2022;37:75–81. doi: 10.1016/j.ejpn.2022.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Ehlen F, Schoenecker T, Kühn AA, Klostermann F. Differential effects of deep brain stimulation on verbal fluency. Brain Lang [Internet]. 2014;134:23–33. Available from: 10.1016/j.bandl.2014.04.002 [DOI] [PubMed]
- 18.•• Tiedt HO, Ehlen F, Wyrobnik M, Klostermann F. Thalamic but not subthalamic neuromodulation simplifies word use in spontaneous language. Front Hum Neurosci. 2021;15:1–11. In this paper, the authors show how specifically stimulation of the thalamic VIM nucleus leads to lexical simplification, meaning a shift towards more frequently used words, which they associate with a dysfunction in lexical-semantic word retrieval, possibly underlying thalamic aphasia. [DOI] [PMC free article] [PubMed]
- 19.Wahl M, Marzinzik F, Friederici AD, Hahne A, Kupsch A, Schneider GH, et al. The human thalamus processes syntactic and semantic language violations. Neuron. 2008;59(5):695–707. doi: 10.1016/j.neuron.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Ojemann GA. Language and the thalamus: object naming and recall during and after thalamic stimulation. Brain Lang. 1975;2(C):101–20. doi: 10.1016/S0093-934X(75)80057-5. [DOI] [PubMed] [Google Scholar]
- 21.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science (1979). 1993; [DOI] [PubMed]
- 22.Sherman SM. Thalamic relays and cortical functioning. Prog Brain Res. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- 23.Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34(9):2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]
- 24.de Witte L, Brouns R, Kavadias D, Engelborghs S, de Deyn PP, Mariën P. Cognitive, affective and behavioural disturbances following vascular thalamic lesions: a review. Cortex. 2011;47(3):273–319. doi: 10.1016/j.cortex.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Fritsch M, Krause T, Klostermann F, Villringer K, Ihrke M, Nolte CH. “Thalamic aphasia” after stroke is associated with left anterior lesion location. J Neurol. 2020;267(1):106–112. doi: 10.1007/s00415-019-09560-1. [DOI] [PubMed] [Google Scholar]
- 26.Fritsch M, Villringer K, Ganeshan R, Rangus I, Nolte CH. Frequency, clinical presentation and outcome of vigilance impairment in patients with uni- and bilateral ischemic infarction of the paramedian thalamus. J Neurol [Internet]. 2021 Nov 1 [cited 2022 Jul 9];268(11):4340. Available from: https://pmc.articles/PMC8505279/ [DOI] [PMC free article] [PubMed]
- 27.Nadeau SE, Crosson B. Subcortical aphasia. Brain Lang. 1997;58(3):355–402. doi: 10.1006/brln.1997.1707. [DOI] [PubMed] [Google Scholar]
- 28.Klostermann F, Krugel LK, Ehlen F. Functional roles of the thalamus for language capacities. Front Syst Neurosci [Internet]. 2013 Jun 25 [cited 2022 Jun 30];7(JUNE). Available from: https://pmc.articles/PMC3712252/ [DOI] [PMC free article] [PubMed]
- 29.Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014;137(9):2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- 30.•• Rangus I, Fritsch M, Endres M, Udke B, Nolte CH. Frequency and phenotype of thalamic aphasia. J Neurol. 2022 Jan 1;269(1):368–76. Available from: https://pubmed.ncbi.nlm.nih.gov/34100990/. Here, the authors were able to show correlations between aphasic symptoms and lesion locations, thereby stressing phenotype variability due to differences in lesion locations. [DOI] [PMC free article] [PubMed]
- 31.Nolte CH, Endres M, Jungehülsing GJ. Vaskuläre Syndrome des Thalamus. Nervenarzt. 2011;82(2):231–241. doi: 10.1007/s00115-010-3197-z. [DOI] [PubMed] [Google Scholar]
- 32.Obayashi S. Cognitive and linguistic dysfunction after thalamic stroke and recovery process: possible mechanism. AIMS Neurosci. 2022;9(1):1–11. doi: 10.3934/Neuroscience.2022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahiri D, Ardila A, Dubey S, Ray BK. A longitudinal study of aphasia due to pure sub-cortical strokes. Ann Indian Acad Neurol. 2020;23(8):S109–S115. doi: 10.4103/aian.AIAN_475_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raymer AM, Moberg P, Crosson B, Nadeau S, Gonzalez Rothi LJ. Lexical-semantic deficits in two patients with dominant thalamic infarction. Neuropsychologia. 1996;35(2):211–219. doi: 10.1016/S0028-3932(96)00069-3. [DOI] [PubMed] [Google Scholar]
- 35.• Radanovic M, Almeida VN. Subcortical aphasia. Curr Neurol Neurosci Rep. 2021;21(12). Available from: 10.1007/s11910-021-01156-5. A very detailed review on subcortical (thalamic and non-thalamic) aphasia, evaluating literature from the past 40 years with regards to association between aphasia phenotype and lesion location. [DOI] [PubMed]
- 36.Watila MM, Balarabe B. Factors predicting post-stroke aphasia recovery. J Neurol Sci [Internet]. 2015 May 15 [cited 2022 Aug 14];352(1–2):12–8. Available from: https://pubmed.ncbi.nlm.nih.gov/25888529/ [DOI] [PubMed]
- 37.Crosson B. Role of the dominant thalamus in language: a review. Vol. 96, Psychological Bulletin. US: American Psychological Association; 1984. p. 491–517. [PubMed]
- 38.Kuljic-Obradovic DC. Subcortical aphasia: three different language disorder syndromes? Eur J Neurol. 2003;10(4):445–448. doi: 10.1046/j.1468-1331.2003.00604.x. [DOI] [PubMed] [Google Scholar]
- 39.•• Crosson B. The role of the thalamus in declarative and procedural linguistic memory processes. Front Psychol. 2021;12(September). The author implements the idea of a mechanistic model in which different thalamic areas might be involved in more lexical vs. more grammatical language operations that furthermore differ on their neuronal encodings (similar to the memory system). [DOI] [PMC free article] [PubMed]
- 40.Ghika-Schmid F, Bogousslavsky J. The acute behavioral syndrome of anterior thalamic infarction: a prospective study of 12 cases. Ann Neurol. 2000;48(2):220–227. doi: 10.1002/1531-8249(200008)48:2<220::AID-ANA12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 41.Radanovic M, Azambuja M, Mansur LL, Sellitto Porto C, Scaff M. Thalamus and language: interface with attention, memory and executive functions. Arq Neuropsiquiatr [Internet]. 2003 [cited 2022 Jul 20];61(1):34–42. Available from: http://www.scielo.br/j/anp/a/KPcPM5BwBmHGnXJLm7DLLBB/?lang=en [DOI] [PubMed]
- 42.Mori E, Yamadori A, Mitani Y. Left thalamic infarction and disturbance of verbal memory: a clinicoanatomical study with a new method of computed tomographic stereotaxic lesion localization. Ann Neurol [Internet]. 1986 [cited 2022 Jul 7];20(6):671–6. Available from: https://pubmed.ncbi.nlm.nih.gov/3545050/ [DOI] [PubMed]
- 43.Nishio Y, Hashimoto M, Ishii K, Mori E. Neuroanatomy of a neurobehavioral disturbance in the left anterior thalamic infarction. J Neurol Neurosurg Psychiatry [Internet]. 2011 Nov 1 [cited 2022 Aug 10];82(11):1195–200. Available from: https://jnnp.bmj.com/content/82/11/1195 [DOI] [PMC free article] [PubMed]
- 44.Gorelick PB, Amico LL, Ganellen R, Benevento LA. Transient global amnesia and thalamic infarction. Neurology [Internet]. 1988 [cited 2022 Aug 14];38(3):496–9. Available from: https://pubmed.ncbi.nlm.nih.gov/3347358/ [DOI] [PubMed]
- 45.Hebb AO, Ojemann GA. The thalamus and language revisited. Brain Lang. 2013;126(1):99–108. doi: 10.1016/j.bandl.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Levin N, Ben-Hur T, Biran I, Wertman E. Category specific dysnomia after thalamic infarction: a case-control study. Neuropsychologia. 2005;43(9):1385–1390. doi: 10.1016/j.neuropsychologia.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Bogousslavsky J, Regli F, Uske A. Thalarnic infarcts: clinical syndromes, etiology, and prognosis. Neurology. 1988;38(6):837–848. doi: 10.1212/WNL.38.6.837. [DOI] [PubMed] [Google Scholar]
- 48.Mohr JP, Watters WC, Duncan GW. Thalamic hemorrhage and aphasia. Brain Lang. 1975;2(C):3–17. doi: 10.1016/S0093-934X(75)80050-2. [DOI] [PubMed] [Google Scholar]
- 49.•• Crosson B. The role of cortico-thalamo-cortical circuits in language: recurrent circuits revisited. Neuropsychol Rev. 2021;31(3):516–33. Here, Crosson stresses the possible importance of cortico-thalamic networks in lexical-semantic retrieval, thereby adding to his extensive work on thalamo-cortical language networks in thalamic aphasia. [DOI] [PMC free article] [PubMed]
- 50.Alomar S, King NKK, Tam J, Bari AA, Hamani C, Lozano AM. Speech and language adverse effects after thalamotomy and deep brain stimulation in patients with movement disorders: a meta-analysis. Movement Disorders [Internet]. 2017 Jan 1 [cited 2022 Jul 18];32(1):53–63. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/mds.26924 [DOI] [PubMed]
- 51.Ojemann GA. The neurobiology of language and verbal memory: observations from awake neurosurgery. Int J Psychophysiol. 2003;48(2):141–146. doi: 10.1016/S0167-8760(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 52.Johnson MD, Ojemann GA. The role of the human thalamus in language and memory: evidence from electrophysiological studies. Brain Cogn. 2000;42(2):218–230. doi: 10.1006/brcg.1999.1101. [DOI] [PubMed] [Google Scholar]
- 53.Fiebach CJ, Friederici AD, Müller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci. 2002;14(1):11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- 54.Kraut MA, Kremen S, Segal JB, Calhoun V, Moo LR, Hart J. Object activation from features in the semantic system. J Cogn Neurosci. 2002;14(1):24–36. doi: 10.1162/089892902317205294. [DOI] [PubMed] [Google Scholar]
- 55.Halari R, Sharma T, Hines M, Andrew C, Simmons A, Kumari V. Comparable fMRI activity with differential behavioural performance on mental rotation and overt verbal fluency tasks in healthy men and women. Exp Brain Res. 2006;169(1):1–14. doi: 10.1007/s00221-005-0118-7. [DOI] [PubMed] [Google Scholar]
- 56.Whelan BM, Murdoch BE, Theodoros DG, Silburn P, Hall B. A role for the dominant thalamus in language? A linguistic comparison of two cases subsequent to unilateral thalamotomy procedures in the dominant and non-dominant hemispheres. 2010; Available from: https://www.tandfonline.com/action/journalInformation?journalCode=paph20
- 57.Ketteler D, Kastrau F, Vohn R, Huber W. The subcortical role of language processing. High level linguistic features such as ambiguity-resolution and the human brain; an fMRI study. Neuroimage [Internet]. 2008 Feb 15 [cited 2022 Jul 9];39(4):2002–9. Available from: https://pubmed.ncbi.nlm.nih.gov/18061483/ [DOI] [PubMed]
- 58.Friederici AD. What’s in control of language? Nat Neurosci. 2006;9(8):991–992. doi: 10.1038/nn0806-991. [DOI] [PubMed] [Google Scholar]
- 59.Alain C, Reinke K, McDonald KL, Chau W, Tam F, Pacurar A, et al. Left thalamo-cortical network implicated in successful speech separation and identification. Neuroimage. 2005;26(2):592–599. doi: 10.1016/j.neuroimage.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Seidman LJ, Goldstein JM, O’Craven K, Savoy R, Breiter HC, Goodman JM, et al. A functional magnetic resonance imaging study of auditory vigilance with low and high information processing demands. Neuropsychology [Internet]. 1998 Oct [cited 2022 Jul 7];12(4):505–18. Available from: /record/1998–12313–002 [DOI] [PubMed]
- 61.Obayashi S. The supplementary motor area responsible for word retrieval decline after acute thalamic stroke revealed by coupled spect and near-infrared spectroscopy. Brain Sci. 2020;10(4). [DOI] [PMC free article] [PubMed]
- 62.Llano DA. Functional imaging of the thalamus in language. Brain Lang [Internet]. 2013 Jul 1 [cited 2022 Jul 7];126(1):62. Available from: /pmc/articles/PMC4836874/ [DOI] [PMC free article] [PubMed]
- 63.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33(2):163–175. doi: 10.1016/S0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 64.Crosson B. Subcortical functions in language: a working model. Brain Lang. 1985;25(2):257–292. doi: 10.1016/0093-934X(85)90085-9. [DOI] [PubMed] [Google Scholar]
- 65.Nishio Y, Hashimoto M, Ishii K, Ito D, Mugikura S, Takahashi S, et al. Multiple thalamo-cortical disconnections in anterior thalamic infarction: implications for thalamic mechanisms of memory and language. Neuropsychologia [Internet]. 2014;53(1):264–73. Available from: 10.1016/j.neuropsychologia.2013.11.025 [DOI] [PubMed]
- 66.Rai M, Okazaki Y, Inoue N, Araki K, Fukunaga R, Sawada T. Object use impairment associated with left anterior thalamic infarction. Eur Neurol [Internet]. 2004 [cited 2022 Jul 7];52(4):252–3. Available from: https://pubmed.ncbi.nlm.nih.gov/15583460/ [DOI] [PubMed]
- 67.Millichap JJ, Bruzzone MJ, Gill R, Ruland S. RESIDENT & FELLOW SECTION Section Editor Teaching NeuroImages: aphasia after infarction of the left pulvinar nucleus DISCLOSURE. 2016; Available from: http://n.neurology.org/cgi/collection/mri [DOI] [PubMed]
- 68.Carrera E, Michel P, Bogousslavsky J. Anteromedian, central, and posterolateral infarcts of the thalamus three variant types. 2004 [cited 2022 Oct 11]; Available from: http://ahajournals.org [DOI] [PubMed]
- 69.Kraut MA, Kremen S, Moo LR, Segal JB, Calhoun V, Hart J. Object activation in semantic memory from visual multimodal feature input. J Cogn Neurosci. 2002;14(1):37–47. doi: 10.1162/089892902317205302. [DOI] [PubMed] [Google Scholar]
- 70.Tiedt HO, Ehlen F, Krugel LK, Horn A, K€ AA, Klostermann F, et al. Subcortical roles in lexical task processing: inferences from thalamic and subthalamic event-related potentials. Hum Brain Mapp. 2017;38:370–83. doi: 10.1002/hbm.23366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bastiaansen MCM, Oostenveld R, Jensen O, Hagoort P. I see what you mean: theta power increases are involved in the retrieval of lexical semantic information. Brain Lang [Internet]. 2008 Jul [cited 2022 Jul 7];106(1):15–28. Available from: https://pubmed.ncbi.nlm.nih.gov/18262262/ [DOI] [PubMed]
- 72.Mousavi N, Nazari MA, Babapour J, Jahan A. Electroencephalographic characteristics of word finding during phonological and semantic verbal fluency tasks. Neuropsychopharmacol Rep [Internet]. 2020 Sep 1 [cited 2022 Jul 7];40(3):254–61. Available from: https://pubmed.ncbi.nlm.nih.gov/32757253/ [DOI] [PMC free article] [PubMed]
- 73.Jankowski MM, Ronnqvist KC, Tsanov M, Vann SD, Wright NF, Erichsen JT, et al. The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front Syst Neurosci [Internet]. 2013 Aug 1 [cited 2022 Jul 7];7. Available from: https://pubmed.ncbi.nlm.nih.gov/24009563/ [DOI] [PMC free article] [PubMed]
- 74.Ford AA, Triplett W, Sudhyadhom A, Gullett J, McGregor K, FitzGerald DB, et al. Broca’s area and its striatal and thalamic connections: a diffusion-MRI tractography study. Front Neuroanat. 2013;7(APR):1–12. [DOI] [PMC free article] [PubMed]
- 75.Bohsali AA, Triplett W, Sudhyadhom A, Gullett JM, McGregor K, FitzGerald DB, et al. Broca’s area-thalamic connectivity. Brain Lang. 2015;141:80–88. doi: 10.1016/j.bandl.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hart J, Maguire MJ, Motes M, Mudar RA, Chiang HS, Womack KB, et al. Semantic memory retrieval circuit: role of pre-SMA, caudate, and thalamus. Brain Lang [Internet]. 2013 Jul 1 [cited 2022 Jul 7];126(1):89–98. Available from: https://pubmed.ncbi.nlm.nih.gov/22964132/ [DOI] [PubMed]
- 77.Liu J, Cui Z, Li L. Local and whole-network topologies reveal that pulvinar and semantic hub interactions correlate with picture vocabulary. Neuroreport [Internet]. 2020 [cited 2022 Jul 7];31(8):590–6. Available from: https://pubmed.ncbi.nlm.nih.gov/32366811/ [DOI] [PubMed]
- 78.Fang Q, Chou X lin, Peng B, Zhong W, Zhang LI, Tao HW. A differential circuit via retino-colliculo-pulvinar pathway enhances feature selectivity in visual cortex through surround suppression. Neuron. 2020 Jan 22;105(2):355–369.e6. [DOI] [PMC free article] [PubMed]
- 79.Ojemann GA, Fedio P, van Buren JM. Anomia from pulvinar and subcortical parietal stimulation. Brain [Internet]. 1968 Mar 1 [cited 2022 Jul 20];91(1):99–116. Available from: https://academic.oup.com/brain/article/91/1/99/361703 [DOI] [PubMed]
- 80.Kang EK, Sohn HM, Han MK, Paik NJ. Subcortical aphasia after stroke. Ann Rehabil Med [Internet]. 2017 Oct 31 [cited 2022 Jul 20];41(5):725–33. Available from: http://www.e-arm.org/journal/view.php?doi=10.5535/arm.2017.41.5.725 [DOI] [PMC free article] [PubMed]
- 81.• Wang D, Lipski WJ, Bush A, Chrabaszcz A, Dastolfo-Hromack CA, Dickey M, et al. Lateralized and region-specific thalamic processing of lexical status during reading aloud. J Neurosci. 2022 Apr 13;42(15):3228–40. Available from: https://www.jneurosci.org/content/42/15/3228. In this paper, the authors observed bilateral, but also left-lateralized thalamic activity in DBS electrode recordings during reading aloud, suggestive of an active role of the thalamus in lexical status processing. This further stresses a, possibly lateralized, monitoring function of the thalamus in language. [DOI] [PMC free article] [PubMed]
- 82.Schaller-Paule MA, Oeckel AM, Schüre JR, Keil F, Hattingen E, Foerch C, et al. Isolated thalamic stroke—analysis of clinical characteristics and asymmetry of lesion distribution in a retrospective cohort study. Neurol Res Pract. 2021;3(1). [DOI] [PMC free article] [PubMed]
- 83.Nadeau SE. Basal ganglia and thalamic contributions to language function: insights from a parallel distributed processing perspective. Neuropsychol Rev. 2021;31(3):495–515. doi: 10.1007/s11065-020-09466-0. [DOI] [PubMed] [Google Scholar]
- 84.von Monakow C. Die Lokalisation im Grosshirn und der Abbau der Funktion durch Kortikale Herde. J Am Med Assoc [Internet]. 1914 [cited 2022 Aug 5];LXIII(9):797. Available from: https://wellcomecollection.org/works/sv9frejr
- 85.Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, et al. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain [Internet]. 2002 [cited 2022 Aug 13];125(Pt 5):1094–104. Available from: https://pubmed.ncbi.nlm.nih.gov/11960898/ [DOI] [PubMed]
- 86.Perani D, Vallar G, Cappa S, Messa C, Fazio F. Aphasia and neglect after subcortical stroke. A clinical/cerebral perfusion correlation study. Brain [Internet]. 1987 Oct [cited 2022 Aug 13];110 ( Pt 5)(5):1211–29. Available from: https://pubmed.ncbi.nlm.nih.gov/3499949/ [DOI] [PubMed]
- 87.Stenset V, Grambaite R, Reinvang I, Hessen E, Cappelen T, Bjørnerud A, et al. Diaschisis after thalamic stroke: a comparison of metabolic and structural changes in a patient with amnesic syndrome. Acta Neurol Scand. 2007;115(SUPPL187):68–71. doi: 10.1111/j.1600-0404.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- 88.Baron JC, Levasseur M, Mazoyer B, Legault-Demare F, Pappata S, Cambon H, et al. Thalamocortical diaschisis: positron emission tomography in humans. J Neurol Neurosurg Psychiatry [Internet]. 1992 Oct 1 [cited 2022 Aug 9];55(10):935–42. Available from: https://jnnp.bmj.com/content/55/10/935 [DOI] [PMC free article] [PubMed]
- 89.de Boissezon X, Démonet JF, Puel M, Marie N, Raboyeau G, Albucher JF, et al. Subcortical aphasia: a longitudinal PET study. Stroke [Internet]. 2005 Jul [cited 2022 Jul 7];36(7):1467–73. Available from: https://pubmed.ncbi.nlm.nih.gov/15933252/ [DOI] [PubMed]
- 90.Sebastian R, Schein MG, Davis C, Gomez Y, Newhart M, Oishi K, et al. Aphasia or neglect after thalamic stroke: the various ways they may be related to cortical hypoperfusion. Front Neurol. 2014;5(November):1–8. doi: 10.3389/fneur.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hillis AE, Kane A, Tuffiash E, Ulatowski JA, Barker PB, Beauchamp NJ, et al. Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain Lang. 2001;79(3):495–510. doi: 10.1006/brln.2001.2563. [DOI] [PubMed] [Google Scholar]
- 92.Hillis AE, Barker PB, Wityk RJ, Aldrich EM, Restrepo L, Breese EL, et al. Variability in subcortical aphasia is due to variable sites of cortical hypoperfusion. Brain Lang. 2004;89(3):524–530. doi: 10.1016/j.bandl.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 93.Zhang J, Zhou Z, Li L, Ye J, Shang D, Zhong S, et al. Cerebral perfusion mediated by thalamo-cortical functional connectivity in non-dominant thalamus affects naming ability in aphasia. Hum Brain Mapp [Internet]. 2022 Feb 15 [cited 2022 Jul 7];43(3):940–54. Available from: https://pubmed.ncbi.nlm.nih.gov/34698418/ [DOI] [PMC free article] [PubMed]
- 94.• Keser Z, Meier EL, Stockbridge MD, Breining BL, Sebastian R, Hillis AE. Thalamic nuclei and thalamocortical pathways after left hemispheric stroke and their association with picture naming. Brain Connect. 2021;11(7):553–65. The authors show degeneration of left thalamo-cortical pathways after singular left cortical ischemic lesions which correlated with aphasia severity. This further stresses the importance of the thalamus in a cortico-subcortical language network. [DOI] [PMC free article] [PubMed]
- 95.Guo J, Yang M, Biswal BB, Yang P, Liao W, Chen · Huafu. Abnormal functional connectivity density in post-stroke aphasia. 2019 [cited 2022 Aug 13];32:271–82. Available from: 10.1007/s10548-018-0681-4 [DOI] [PubMed]
- 96.Mirman D, Britt AE. What we talk about when we talk about access deficits Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;19(369):1634. doi: 10.1098/rstb.2012.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fristen KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RSJ. Functional topography: multidimensional scaling and functional connectivity in the brain. Cereb Cortex. 1996;6(2):156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- 98.Klostermann F, Krugel LK, Ehlen F, Saalmann YB, Hebb AO. Functional roles of the thalamus for language capacities. 2013; Available from: www.frontiersin.org [DOI] [PMC free article] [PubMed]
- 99.Norris D, McQueen JM. Shortlist B: A Bayesian model of continuous speech recognition. Psychol Rev. 2008;115(2):357–395. doi: 10.1037/0033-295X.115.2.357. [DOI] [PubMed] [Google Scholar]
- 100.Bogacz R, Gurney K. The basal ganglia and cortex implement optimal decision making between alternative actions. Neural Comput [Internet]. 2007 Feb 1 [cited 2022 Jun 30];19(2):442–77. Available from: https://direct.mit.edu/neco/article/19/2/442/7152/The-Basal-Ganglia-and-Cortex-Implement-Optimal [DOI] [PubMed]
- 101.Crosson B. The role of cortico-thalamo-cortical circuits in language: recurrent circuits revisited [Internet]. Vol. 31, Neuropsychology Review. 2021 [cited 2022 Jul 17]. p. 516–33. Available from: 10.1007/s11065-019-09421-8 [DOI] [PMC free article] [PubMed]
- 102.Ullman MT. The declarative/procedural model of lexicon and grammar. J Psycholinguist Res. 2001;30(1):37–69. doi: 10.1023/A:1005204207369. [DOI] [PubMed] [Google Scholar]
- 103.Usrey WM, Sherman SM. Corticofugal circuits: communication lines from the cortex to the rest of the brain. J Comp Neurol [Internet]. 2019 Feb 2 [cited 2022 Jul 9];527(3):640. Available from: https://pmc.articles/PMC6131091/ [DOI] [PMC free article] [PubMed]
- 104.McCormick DA, Feeser HR. Functional implications of burst firing and single spike activity in lateral geniculate relay neurons. Neuroscience. 1990;39(1):103–113. doi: 10.1016/0306-4522(90)90225-S. [DOI] [PubMed] [Google Scholar]
- 105.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997; [DOI] [PubMed]
- 106.Belén Pardi M, Vogenstahl J, Dalmay T, Spanò T, Pu DL, Naumann LB, et al. A thalamocortical top-down circuit for associative memory. Science (1979) [Internet]. 2020 Nov 13 [cited 2022 Jul 9];370(6518):844–8. Available from: https://www.science.org/doi/10.1126/science.abc2399 [DOI] [PubMed]
- 107.Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proc Natl Acad Sci U S A [Internet]. 2005 Aug 23 [cited 2022 Jun 30];102(34):12236–41. Available from: https://www.pnas.org/doi/pdf/10.1073/pnas.0502843102 [DOI] [PMC free article] [PubMed]
- 108.Mitchell AS. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci Biobehav Rev [Internet]. 2015 Jul 1 [cited 2022 Jun 30];54:76–88. Available from: https://pubmed.ncbi.nlm.nih.gov/25757689/ [DOI] [PubMed]
- 109.la Terra D, Bjerre AS, Rosier M, Masuda R, Ryan TJ, Palmer LM. The role of higher-order thalamus during learning and correct performance in goal-directed behavior. Elife. 2022;1:11. doi: 10.7554/eLife.77177. [DOI] [PMC free article] [PubMed] [Google Scholar]


