Abstract
Purpose of Review
Probiotics intake may be considered beneficial by prospective and pregnant mothers, but their effects on offspring development are incompletely understood. The purpose of this review was to examine recent pre-clinical and clinical studies to understand how maternal probiotics exposure affects offspring health outcomes.
Recent Findings
Effects were investigated in the context of supporting offspring growth, intestinal health, and gut microbiota, preventing allergic diseases, supporting neurodevelopment, and preventing metabolic disorders in pre-clinical and clinical studies. Most human studies focused on infancy outcomes, whereas pre-clinical studies also examined outcomes at adolescence and young adulthood. While still understudied, both pre-clinical and clinical studies propose epigenetic modifications as an underlying mechanism. Optimal timing of intervention remains unclear.
Summary
Administration of selected probiotics to mothers has programming potential for sustaining life-long health of offspring. Administration protocols, specific windows of susceptibility, and individual-specific responses need to be further studied.
Keywords: Developmental Origins of Health and Disease, Probiotics, Nutritional programming, Gut microbiota, Pregnancy, Infant, Sex, Epigenetics, Human, Mouse, Rabbit, Pig, Rat
Introduction
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [1]. Probiotics can be delivered in various modalities such as dietary supplements, food products, or drugs, targeting individuals across the entire life spectrum. Probiotics stem from various taxa including both prokaryotic and eukaryotic microorganisms and display common and/or strain-specific benefits such as regulation of intestinal transit, competitive exclusion of pathogens, vitamin and specific bioactive synthesis, intestinal barrier enhancement, or immunomodulation [1]. The gut ecosystem is a determinant of health and a main target of probiotics administration. Established in early life, altered dynamics in this process are associated with disease later in life, such as asthma [2], allergy [3], eczema [3], obesity [4, 5], and susceptibility to infection [6]. Several probiotics have been studied for their role in early stages of life and ability to sustain health in mothers and children. Substantiated benefits in the context of eczema for lactobacilli alone or in combination with Bifidobacterium species administered to mothers or infants [7] have been translated into guidelines by the World Allergy Organization in 2015 [8]. These guidelines indicate that probiotic consumption during pregnancy might be beneficial for pregnant or breastfeeding women at high risk for having a child who develops allergies. Additional benefits of maternally administered probiotics include improvement of metabolic parameters (insulin levels and insulin resistance, very low density lipoprotein and total cholesterol concentration) in gestational diabetes mellitus (GDM) [9], reduced rectal and vaginal Group B Streptococci colonization before parturition (important to prevent offspring mortality caused by Early Onset Group B Streptococcus disease) [10], and reduced incidence of mastitis [11]. These studies are sparse and heterogeneous in terms of probiotics usage, vehicle, and dosage. Because maternal exposures such as metabolic syndrome and infection are associated with offspring outcomes [12, 13], it is likely that offspring can also be affected by maternal use of probiotics. In fact, maternal probiotics administration was shown to result in temporary colonization of the offspring and/or modulation of the offspring microbiota [14, 15].
In a recent meta-analysis, probiotic consumption during pregnancy and/or lactation was shown to be generally safe for pregnant mothers in terms of gastrointestinal symptoms, tachycardia, vaginal discharge, eczema, and headache [16]. Only one probiotic mix containing Lacticaseibacillus rhamnosus GR-1 and Limosilactobacillus reuteri RC-14 was associated with an increased risk of vaginal discharge and changes in stool consistency after consumption during the first and second trimesters of pregnancy [17]. Direct probiotic administration to infants between birth and 2 years of age has also been reported as safe. This was shown through a systematic review that stratified the incidence of an adverse effect according to the infant’s health condition (healthy, low-birth weight, dermatitis, diarrhea, or formula-fed) [18]. The utilization of probiotics during these sensitive periods of life could therefore be considered to support infant health. A survey conducted in Canada suggests that the public already utilizes probiotics as 50.8% of women with a child aged 2 years or younger reported giving a probiotic product to their infant. Responses came from 413 mothers enrolled in the Alberta Pregnancy Outcomes and Nutrition (APrON) study in 2012 [19].
It is known that an altered maternal microbiota during pregnancy can affect offspring microbiota establishment, immune development, and metabolic health throughout life [20–22]. Thus, interventions targeting the maternal microbiota, such as probiotics, may have the potential to program offspring health. The purpose of this review was to examine recent literature from pre-clinical and clinical studies on the effects of probiotic administration started during the pre-conception period or pregnancy on offspring outcomes.
Search Strategy and Selection Criteria
PubMed, Embase, and Cochrane Library were searched for original research articles including pre-clinical (animal) and clinical studies and meta-analyses over the last 5 years (January 2017–March 2022). The search terms used were as follows: “probiotics”, “gestation”, “pregnancy”, “lactation”, “pre-conception” or “preconception”, “pre-mating” or “premating”, “pre-conceptional”, combined or not with “offspring”, “infant”, “baby” and complemented with manual inspection of reference lists of the selected articles. Articles were selected if they included a probiotic intervention starting at pre-conception and/or during pregnancy and assessed offspring outcomes. Studies of probiotics administered in combination with other ingredients were included. We included studies using substantiated probiotics strains, according to the Food and Agriculture Organization of the United Nations (FAO) guidelines [23], as well as strains for which research is ongoing, especially at the clinical stage.
Articles were screened to retrieve information about the probiotic strains, dose, vehicle, and supplementation period and this information is reported in tabulated form according to the Population, Interventions, Comparisons, Outcomes and Study designs (PICOS) elements [24] and grouped as pre-clinical (Table 1) or clinical (Table 2) studies. Findings are discussed according to 6 offspring outcomes identified across the studies: (1) growth and anthropometric indices at birth (19 articles); (2) intestinal barrier and gut health (14 articles); (3) neurodevelopment and anxiety-like behavior (8 articles); (4) allergic diseases (12 articles); (5) metabolic disorders (14 articles); and (6) intestinal microbiota (18 articles).
Table 1.
Pre-clinical studies evaluating probiotic administration starting before or during gestation on offspring outcomes
| Condition, animal model | Probiotic, dose, vehicle, time | Offspring outcomes (compared to control) | Effect on gut microbiota in dams and offspring | Ref | |
|---|---|---|---|---|---|
| Before pregnancy | |||||
| Metabolic disorders | |||||
|
SPF C57BL/6 J mice receiving a HFD or not since preconception ± probiotics Dams n = 9–10/group Offspring n = 27–30/group (male and female) |
Bifidobacterium breve DM8310, Lactobacillus acidophilus DM8302, Lacticaseibacillus casei DM8121, Streptococcus thermophilus DM8309 2 × 109 CFU/day by gavage 6 weeks before mating–PND21 |
↓ body weight until PND42 PND21: ↓ total cholesterol, LDL Male: ↓ HDL PND42: ↓ total cholesterol, LDL Female: ↓ glucose and insulin. ↑ HDL compared to control diet |
Dams ↓ Bacteroidetes, ↑ Bacteroidetes S24-7, Allobaculum, Sutterella Offspring PND21: Ameliorate HFD-induced dysbiosis (Prevotella, Bacteroides, Bacteroidales S24-7). Female: ↑ ⍺-diversity PND42: No effect on β-diversity. Female: Ameliorate dysbiosis (↑ Bacteroidaceae, Lachnospiraceae, Sutterella; ↓ Lactobacillus, Prevotella, Helicobacter, Rikenellaceae, Parabacteroides) |
[93] | |
| Parasite infection | |||||
|
Swiss mice ± probiotics, inoculated with Toxocara canis on GD14 Dams n = 8/group Offspring n = 62–65/group (male and female) |
Saccharomyces boulardii CNCM I-745 1 × 107 CFU/day, added in feed 2 weeks before mating–PND21 |
PND21: ↓ 42% in the number of larvae transmitted, ↓ 50% larvae found in the brain |
Not assessed | [110] | |
| During pregnancy | |||||
|
Wistar rats ± probiotics Dams n = 6/group Offspring n = 48/group (male and female) |
Limosilactobacillus fermentum CECT5716 1 × 1010 CFU/day by gavage GD1–PND14 |
No effect on body weight or BMI PND14: Intestine: ↑ IgA Plasma: ↑ IgG2a, ↓ IgG2c, ↑ Th2-type Ig, no change cytokines; ↓ in palmitic acid and total saturated fatty acids, ↑ plasma linoleic acid, eicosadienoic acid, 17:0 and 22:0 carbons fatty acids |
Dams & Offspring PND14: No effect on cecal microbiota composition, diversity, or richness PND21: Strain detected in all the dams cecal content |
[58] | |
|
SPF C57BL/6 mice ± probiotics Dams n = 10/group Offspring n = 8–10/group/time point (male and female) |
Akkermansia muciniphila MucT (ATCC BAA-835) 1 × 108–109 CFU/day by gavage GD1–3 days before birth; PND3–PND21 |
Until PND42: No effect on body weight or intestinal tissue development |
Dams No effect during pregnancy PND21: ↑ A. muciniphila, Ruminococcus_1, ↓ Coriobacteriaceae UCG-002, Lachnospiraceae UCG-001, Ruminiclostridium Offspring No effect on A. muciniphila abundance or ⍺-diversity PND42: Change in β-diversity, ↓ Dubosiella, Lachnospiraceae_UCG_001, Parasutterella, ↑ Gordonibacter, Lachnospiraceae_UCG_006, Prevotellaceae_UCG_001; ↑ the D-glutamine and D-glutamate microbial metabolism pathway |
[98] | |
|
Bama mini-sows ± probiotics or synbiotics Dams n = 16/group Offspring n = 8/group (male and female) |
Mix: Lactiplantibacillus plantarum B90, Saccharomyces cerevisiae P11 Dose undisclosed, added in feed Synbiotic: probiotic mix + xylo-oligosaccharides (500 g/t feed) GD1–PND28 |
PND58 Probiotics: ↑ CAT, GPx and SOD activities in plasma, jejunum, and colon; ↓ malondialdehyde and H2O2 concentrations in plasma Synbiotics: ↑ CAT and SOD activities in plasma; ↑ glutathione, GPx activities and mRNA levels of antioxidant and mitochoindrial-related genes in jejunum; ↑ total antioxidant capacities in colon; ↓ colonic malondialdehyde |
Offspring PND58 Probiotics: ↑ Bacteroidetes relative abundance in the jejunum and Bifidobacterium in the jejunum and colon. No change of total bacteria, Bacteroides, Clostridium cluster IV, Lactobacillus in jejunum or colon Synbiotics: ↑ Firmicutes, Bacteroidetes, Bifidobacterium, Lactobacillus (jejunum) |
[69] | |
|
Bama mini-sows ± probiotics or synbiotics Dams n = 16/group Offspring n = 8/group (male and female) |
Mix: Lactiplantibacillus plantarum B90, Saccharomyces cerevisiae P11 Dose undisclosed, added in feed Synbiotic: probiotic mix + xylo-oligosaccharides (500 g/t feed) GD1–PND28 |
PND65 Probiotics: Improve immune response (Plasma: ↓ IL-2 and LPS concentrations, ↑ IgA; Jejunum: ↑ IL-10, interferon-α, sIgA; ileum: ↑ sIgA) ↑ jejunal villus height, villus height/crypt depth ratio Synbiotics: ↑ plasma IgA, ↑ jejunal IL-10, interferon-α, sIgA, ↑ ileal villus height |
Offspring PND65: Effect on β-diversity, no effect on ⍺-diversity Probiotics: ↑ relative abundance of Psychrobacter, Dialister, Oleomonas, and Facklamia (ileum) Synbiotics: ↑ Turicibacter, SMB53, Clostridium, Paracoccus, Thalassobius, Vibrio, Psychrobacter, and Blautia (jejunum); ↑Corynebacterium and Agrobacterium (ileum) |
[70] | |
|
White Rex rabbit ± low /middle/high dose probiotics Dams n = 20/group Offspring n = 30/group (male and female) |
Clostridium butyricum CCTCC AB: 2017089 1 × 103 CFU/g (low dose), 1 × 104 CFU/g (middle dose), 1 × 105 CFU/g (high dose) in feed GD1–PND35, PND35–PND63 (offspring) |
PND28: ↑ average daily weight gain PND63: ↑ α-amylase and chymotrypsin activity in SI; ↑ SI villi length and ↓ crypt depth; modulate SOD, GPx and CAT activity ↑ ZO-1, claudin, and occludin mRNA in SI High dose: ↓ IL-6, TNF-α and IFN-γ in ileum and colon; ↑ sIgA in duodenum. ↑ MyD88, TLR2, and TLR4 relative expression in SI |
Offspring: PND63 Dose dependent effects High and middle dose: ↑ total bacteria abundance, ↑ Firmicutes; ↑ Lactobacillus & Bifidobacterium; ↑ Clostridium cluster IV, Clostridium cluster XIVa, and Butyrivibrio fibrisolvens in small intestine ↑ Clostridium cluster XIVa in colon Low dose: ↑ Clostridium cluster IV, Clostridium cluster XIVa, Butyrivibrio fibrisolvens, and Lactobacillus in duodenum |
[99] | |
|
C57BL/6 J mice ± probiotics Dams Undisclosed Offspring n = 9–10/group (male) n = 10–12/group (female) |
Lactococcus lactis 5 × 105 CFU/ml in drinking water GD10.5–PND1 |
PND1: ↑ the density of cortical neurons (Tbr1-expressing neurons, Satb2-expressing neurons in male), ↑ the density of blood vessels in the cortical plate Females: ↑ mitotic neural progenitor cell numbers 10 weeks: ↓ Anxiety-like behaviors Females: ↓ Freezing time in cue associated learning |
Not assessed | [77] | |
|
SPF Sprague Dawley rats ± probiotics Dams n = 4–5/group Offspring n = 12–14/group/time point (male) n = 11–14/group/time point (female) |
Lactobacillus helveticus NS8 1 × 108 CFU/ml in drinking water GD13–GD22 |
↓ body weight from PND51 until PND100-105 Antianxiety effect: PND35-40: Elevated plus maze: ↑ proportion of time in the open arms; PND35-40 & PND100-105: Open field test: ↑ proportion of the time/entries spent in the center and the distance travelled in the center, ↓ proportion of the latency to reach the center from the peripheral zone |
Not assessed | [63] | |
|
C57BL/6 mice + probiotic or fixed probiotic Dams n = 9/group Offspring n = 3–4/group/time point (male) n = 3–4/group/time point (female) |
Lacticaseibacillus rhamnosus GG 1 × 108 CFU/day (dams) by gavage, 1 × 107 CFU/day (offspring) in feed GD18–birth, PND1–PND5 (offspring) |
No impact on body weight PND21: ↑ crypt depth, villus height (jejunum, ileum, colon); 8 months: ↑ goblet cells, ZO-1, Claudin-1, and Occludin mRNA expression; ↑ ZO-1 and Claudin-3 protein expression ↑ SOD 1 & 2 mRNA expression, GPx expression |
Offspring PND21: Strain detected in feces; no impact on ⍺-diversity, impact β-diversity; ↑ Akkermansia muciniphila and SCFA-producing bacteria (Ruminococcus, Coprococcus, Odoribacter, Faecalibaculum, and Lachnospiraceae bacterium A4) 8 months: Effect on β-diversity maintained ↑ Lactobacillus, Parasutterella, Bifidobacterium, Akkermansia muciniphila, ↓ Oscillibacter, Escherichia, Ruminococcus, Helicobacter, Alistipes timonensis |
[59] | |
|
C57BL/6 J mice + probiotic or fixed probiotic Dams Undisclosed Offspring n = 11–16/group (male) n = 10–13/group (female) |
Lacticaseibacillus rhamnosus GG 1 × 108 CFU/day (dams) by gavage, 1 × 107 CFU/day (offspring) in feed GD18–birth; PND1–PND5 (offspring) |
↑ BW at PND7, 14, 21, 28 PND21: ↑ Intestinal villus length, colonic crypt depth, cell proliferation and differentiation, tight junction proteins expression (ZO-1, claudin-3) 14 weeks (males): ↑ Fecal SCFA concentrations, ↓ anxiety-like behavior, ↑ colon epithelial growth factor receptor activation, serotonin transporter mRNA and protein levels; ↓ colon and serum 5-HT levels, ↑ brain-derived neurotrophic factor and ɣ-aminobutyric receptor |
Offspring PND21: No effect on ⍺-diversity, impact β-diversity, ↑Akkermansia, ↓ Helicobacter, Flavonifractor, Oscillibacter, Alistipes ↓ bacterial pathways (motility proteins, flagellar assembly and porphyrin and chlorophyll metabolism) 14 weeks (male): No effect on ⍺-diversity, impact β-diversity, ↑ Clostridium XIVa, Bifidobacterium ↓ Prevotella, Akkermansia, Bifidobacteria |
[62] | |
|
Landrace × Yorkshire sows ± probiotics; offspring ± synbiotics (split-plot design) Dams 14–15/group Offspring n = 4–5/pen, 18–19 pens/group (male and female) |
Bacillus subtilis C-3102 5 × 105 (gestation), 1 × 106 (lactation), 5 × 105 (offspring) CFU/g of feed Prebiotics (MOS, offspring) GD30–PND19; PND19–PND42 (offspring) |
No effect of nursery treatment on overall growth PND42: ↓ Weight in piglets born from probiotic supplemented sows |
Dams GD30, GD113, PND18: ↑ Fecal Bacillus subtilis C-3102 & total Bacillus sp. Offspring PND18: ↑ Fecal Bacillus subtilis C-3102 & total Bacillus sp. |
[111] | |
|
Great Dane Bitches ± synbiotics (started at GD35 or GD56) Dams n = 5/group Offspring n = 30–32/group (male and female) |
Mix: Enterococcus faecium DSM 10663, Lactobacillus acidophilus CECT 4529 3.36 × 108 and 1.03 × 1010 CFU/10 kg of BW, respectively, in tablets Prebiotics (FOS: 480 mg/10 kg, MOS: 48.6 mg/10 kg of BW) GD35 or 56–birth |
No effect on birth weight or litter parameters 9 weeks: ↓ Incidence of gastroenteritis 4-week supplementation had better results on first presentation of gastroenteritis |
Not assessed | [112] | |
|
Large White sows ± probiotics Dams n = 20/group Offspring n = undisclosed (male and female) |
Mixes: Bacillus subtilis A + Bacillus subtilis B (BS-A + B); Bacillus subtilis A + Bacillus licheniformis (BS-A + BL); or Bacillus subtilis B + Bacillus licheniformis (BS-B + BL) Total of 4 × 109 CFU/kg of feed GD85–PND21 |
PND21: ↑ Average weight and average daily gain at weaning BS-A + BL: ↑ number of weaned piglets |
Dams GD100-112: No effect on Bifidobacterium spp., Escherichia coli or total bacteria BS-B + BL: GD112: ↑ Clostridium cluster IV PND7: ↓ Firmicutes |
[60] | |
|
Landrace x Yorkshire sows ± probiotics Dams n = 16/group Offspring n = 12/dams (male and female) |
Bacillus subtilis PB6 4 × 108 CFU/kg of feed GD90–PND21 |
↑ litter size, ↓ BW PND14: ↓ Serum cortisol concentration PND21: ↑ Litter weights and litter weight gains |
Dams GD110: ↓ ⍺-diversity, ↑ Gemmatimonadetes, Acidobacteria, Ruminococcaceae_UCG-013 cc, ↓ Proteobacteria, Actinobacteria, Streptococcus relative abundance |
[61] | |
|
Pietrain sows ± probiotics Dams n = 10/group Offspring n = undisclosed (male and female, n = 5/group for hematological profile) |
Lactiplantibacillus plantarum CAM6 1 × 1010 CFU/day, in fermented juice (pineapple, banana, and papaya peel) GD90–PND28 |
↓ Number of deaths before weaning ↑ body weight from PND7 until PND28 PND28: ↓ Diarrhea incidence, ↑ serum concentration of Na + , pCO2, and D-β-hydroxybutyrate, leukocytes, lymphocytes, and platelets in the piglets |
Not assessed | [72] | |
|
Large White × Landrace sows ± probiotics; offspring ± probiotics (split-plot design) Dams n = 12/group Offspring n = 10–6/group (male and female) |
Bacillus altitudinis WIT588 4 × 109 CFU/day (gestation), 1.2 × 1010 CFU/day (lactation), 1 × 109 CFU/day (offspring) in feed GD100–PND26, PND26–PND54 (offspring) |
PND21: No effect on body weight PND105, PND126: ↑ body weight PND34: Duodenum: ↑ villus length and area, crypt depth; Jejunum: ↑ crypt depth and area |
Dams Strain detected in feces Offspring PND13: Strain detected in the feces of 12/20 piglets PND26: Strain detected in 16/20 piglets |
[68] | |
|
Landrace x Yorkshire sows ± low/high-dose probiotics Dams n = 5/group Offspring n = 55/group (male and female, n = 5/group for microbiota) |
Enterococcus faecium DSM 7134 2.7 × 107 CFU/kg (low-dose group), 5.4 × 108 CFU/kg of feed (high-dose) GD101–PND21 |
↓ piglet pre-weaning mortality PND7: ↓ Diarrhea score PND21: ↑ Weaning weight, average daily gain and gain:feed ratio |
Offspring PND21: ↑ Fecal Lactobacillus and Enterococci, ↓ Escherichia coli counts PND35: ↑ Fecal Lactobacillus and Enterococci |
[71] | |
| Atopic diseases | |||||
|
BALB/c mice exposed to ROFA or PBS on GD14, 16, 18 (air pollution exposure) Neonatal asthma model (ovalbumin sensitization) ± probiotics Dams Undisclosed Offspring n = 6–21/group (male and female) |
Bifidobacterium breve M-16 1 × 109 CFU/day in feed GD14–PND21 |
PND30: ↓ Eosinophils in the bronchoalveolar lavage fluid; ↓ allergic lung inflammation; ↓ mucus production; ↓ IL-5, IL-13, and Muc5ac gene expression |
Offspring PND30: ↓ Firmicutes proportion ↑ Gemella, Streptococcus, Dehalobacterium & ↓ Lactobacillus |
[81] | |
|
BALB/c mice ± probiotics AD induced with MC903 or not (offspring, PND21) Dams n = 6/group Offspring n = 8/group (male and female) |
Limosilactobacillus reuteri Fn041 or Lacticaseibacillus rhamnosus GG 1 × 109 CFU/day by gavage GD14–PND21; PND21–PND28 (offspring) |
PND28: Both strains: ↓ AD symptoms (skin swelling, mast cell, eosinophil infiltration), ↓ IL-4 and IL-12; L. reuteri Fn041: ↓ IgE, ↑ proportion CD4 + CD25 + Foxp3 + Tregs cells in mesenteric lymph nodes |
Offspring PND28: L. reuteri Fn041: ↑ Actinobacteria, Lactobacillus and Akkermansia; LGG: ↓ ⍺-diversity; ↑ Escherichia_Shigella, Enterococcus; Both strains: ↓ Alloprevotella, Klebsiella, Helicobacter |
[82] | |
| Inflammation | |||||
|
C57BL/6 mice ± probiotics, DSS induced inflammation at PND70 Dams n = 2/group Offspring n = 4/group (male, microbiota) n = 15–16/group (female, weight loss–sample size microbiota undisclosed) |
Mixes: Limosilactobacillus reuteri (strains 6798‐1, 6798‐jm, and 6798‐cm) or Lactobacillus johnsonii (strains 4901, 4903, 4931) 1 × 109 CFU/ml by gavage GD12–birth; PND7–PND21 (every other day) |
PND76: L. reuteri: ↓ post-DSS weight loss in females |
Offspring Females: Both strains, PND21: Altered Muribaculum and Ruminiclostridium abundance PND30: Significant effect on β-diversity, altered Lachnoclostridium, Muribaculum, and Tyzzerella abundances L. reuteri, PND70: ↓ Lachnoclostridium, Akkermansia, Parasutterella, Ruminiclostridium, Bacteroides, ↑ Lactobacillus, Muribaculum, and Bifidobacterium Males: L. reuteri, PND70: ↓ Alistipes abundance, no effect on female altered genus |
[73••] | |
|
SPF C57BL/6 J mice ± probiotics, IL-1β induced postnatal systemic inflammation (PND14 and 28) Dams n = 5–6/group Offspring n = 3–9/group/time point (male and female) |
Mix: Lactobacillus acidophilus ATCC 53544, Bifidobacterium infantis ATCC 15697 1 × 109 CFU/day each GD16–PND21 Undisclosed |
PND14: ↓ IL-1β-induced systemic levels of IL-6, KC, MCP-1, and IL-1β; normalize blood–brain-barrier permeability and occludin expression; regulate recruitment and extracellular matrix damage Promote neuronal development (↑ NeuN, NFL, and Syn1 expression) and oligodendrocyte progenitor cell development (↑ NG2 expression) PND28: No effect on IL-1β-induced ↑ of MCP-1 levels |
Not assessed | [113] | |
| Obesity | |||||
|
CD-1 IGS mice receiving a HFD or not started 6 weeks before mating until PND21, ± probiotics Dams n = 4/group Offspring n = 10–15/group/time point (male) n = 9–14/group/time point (female) |
Mix: Bacillus subtilis PXN®21®, Bifidobacterium bifidum PXN® 23™, Bifidobacterium breve PXN® 25™, Bifidobacterium infantis PXN® 27™, Bifidobacterium longum PXN® 30™, Lactobacillus acidophilus PXN® 35™, Lactobacillus delbrueckii ssp. bulgaricus PXN® 39™, Lactococcus casei PXN® 37™, Lactiplantibacillus plantarum PXN® 47™, Lacticaseibacillus rhamnosus PXN® 54™, Lactobacillus helveticus PXN® 45™, Ligilactobacillus salivarius PXN® 57™, Lactococcus lactis ssp. lactis PXN® 63™, Streptococcus thermophilus PXN® 66™ Total of 4 × 107 CFU/ml in drinking water GD0.5–PND21 |
PND21: ↑ Female and male body weight; altered expression of gene involved in synaptic plasticity: restored HFD-induced ↑ of GLUN2C in female and modulate genes expression in control and HFD groups (GLUN2A, PFKB3, female GLUN2B, male SYP and CREB1) ↑ IL-6 prefrontal cortex expression in control and HFD, ↓ liver TLR4, and liver IL-1B in males PND21 & PND112: Prevent HFD-induced anxiety-like behavior ↑ gut propionate, butyrate, and brain lactate in control and HFD PND112: ↑ Brain-derived neurotrophic factor expression (BDNF, PFKB3, ΔFOSB and male cFOS and ZIF-268), ↑ female liver IL-6 in control and HFD |
Dams PND21: ↑ Fecal Lactobacillus spp. in control and HFD, and ↑ Bifidobacterium spp. in control diet group |
[79••] | |
|
Sprague Dawley rats receiving HF diet or not from GD1 until PND21, ± probiotics Dams n = 3/group Offspring n = 7–8/group (male) |
Lacticaseibacillus casei 2 × 108 CFU/day by gavage GD1–PND21 |
12 weeks: No effect on body weight, Prevents HF-induced hypertension: ↓ systolic blood pressure from 6 to 12 weeks. ↓ acetate plasma levels; ↓ renal mRNA expression of Olfr78 |
Offspring 12 weeks Compared to control: ↑ Parabacteroides and Akkermansia muciniphila, ↓ Actinobacteria, Bacteroides, Bacteroides/Prevotella, Actinobacteria/Firmicutes ratio Compared to HF group: ↑ Lactobacillus, Acholeplasma, Bacteroides acidifaciens, ↓ Alkaliphilus, Leptolyngbya, Prevotella albensis, Ruminococcus albus |
[95] | |
|
Sprague Dawley rats receiving HFD or not from GD1 until PND21 ± probiotics Dams n = 3/group Offspring n = 8/group (male) |
Lacticaseibacillus casei 2 × 108 CFU/day by gavage GD1–PND21 |
16 weeks: Compared to HFD: No effect on BW, ↓ systolic blood pressure from 12 to 16 weeks, ↓ fecal propionate level, ↓ plasma TMAO levels and TMAO-to-TMA, ↑ DMA-to-TMAO conversion ratio, ↓ renal mRNA Ace expression |
Offspring 3 weeks: Compared to HFD: No effect on ⍺- or β-diversity; Prevent the HF-diet induced ↓ Alkaliphilus, Akkermansia abundances and several Lactobacillus species 16 weeks: Prevents the ↓ Lactobacillus |
[96] | |
|
Wistar rats receiving HFHC diet or not from GD1 until PND21 ± probiotics Dams n = 5/group Offspring n = 5–10/group (male) |
Lactiplantibacillus plantarum WJL 1 × 109 CFU/day by gavage GD1–birth; PND2-21 |
↓ body weight and length from PND30 to PND90 ↓ blood pressure and recovered vascular function in later life |
Dams Restore ⍺-diversity but not β-diversity No effect on dyslipidemia induced dysbiosis ↑ Lachnospiraceae (Ruminococcus, Blautia, Dorea, and Coprococcus) |
[94] | |
| Neurodegenerative disease | |||||
|
Wistar Albino rats receiving intraperitoneal injection of LPS (100 μg/kg) on GD17 (neuroinflammation) ± probiotics Dams n = 5/group Offspring n = 18/group (male and female) |
Ligilactobacillus salivarius ATCC 11741, or B. bifidum ATCC 29521, or both Total of 4 × 109 CFU/ml by gavage GD1–birth |
PND1: Improved brain APP levels and mRNA, gamma-secretase and beta-secretase levels, improved brain BDNF mRNA expression ↓ brain damage in the cortical area |
Not assessed | [114] | |
|
Sprague Dawley rats exposed to lead one week after mating until offspring sacrifice (memory dysfunction model) ± probiotics Dams n = 3/group Offspring n = 6–10/group (female) |
Mix: Bifidobacterium longum BL986, Lactobacillus acidophilus LA1063, Limosilactobacillus fermentum LF26, Lactobacillus helveticus LH43, Lacticaseibacillus paracasei LPC12, Lacticaseibacillus rhamnosus LRH10, Streptococcus thermophilus ST30 Total of 1 × 1010 CFU/day in drinking water GD7–PND21, PDN21–PND68 (offspring) |
PND22 & PND68: Reversed lead-led memory impairment and loss of hippocampal spines PND68: Restore IL-6 levels and the epigenetic event H3K27me3 level in the hippocampus |
Offspring PND68: Restore ⍺-diversity, Firmicutes/Bacteroidetes ratio, Proteobacteria, Actinobacteria. ↑ Helicobacter, Bifidobacterium, Bacteroides, ↓ Anaerovibrio, Ruminococcaceae_UCG-008, Lactobacillus |
[100] | |
AD atopic dermatitis, BMI body mass index, BW birth weight, CAT catalase, CFU colony forming units, FOS fructo-oligosaccharides, GD gestational day, GPx glutathione peroxidase, HDL high-density lipoprotein, HF high-fructose, HFD high-fat diet, HFHC high-fat and high-cholesterol, Ig immunoglobulin, IL interleukin, LDL low-density lipoprotein, LPS lipopolysaccharides, MOS mannan-oligosaccharides, MUC2 mucin 2, PND postnatal day, ROFA residual oil fly ash, SCFA short-chain fatty acids, SI small intestine, SOD superoxide dismutase, SPF specific pathogen-free, ZO-1 zonula occludens 1
Table 2.
Clinical studies evaluating probiotic administration starting before and during gestation on offspring outcomes
| Time of administration | Population characteristics | Probiotic, dose, vehicle | Infant outcomes | Effect on maternal and infant gut microbiota | Ref |
|---|---|---|---|---|---|
| Before pregnancy | |||||
| Healthy population | |||||
| 30.53 ± 3.40 years old women, Singapore, New Zealand, UK, n = 585 |
Mix: Lacticaseibacillus rhamnosus NCC 4007, Bifidobacterium animalis NCC 2812 1 × 1010 CFU/day each, in powder Myo-inositol (4 g/day), Vitamin D (10 ug/day), Riboflavin (1.8 mg/day), Vitamin B6 (2.6 mg/day), Zinc (10 mg/day) From preconception–birth |
No effect on BW ↓ number of preterm births |
Not assessed | [115] | |
| During pregnancy | |||||
| Healthy population | |||||
| 24.8 ± 5.3 years old women, Philippines, n = 208 |
Mix: Bifidobacterium lactis CNCC I-3446, Lactobacillus rhamnosus CGMCC 1.3724 1.4 × 109 CFU/day each, in powder Nutritional supplement (proteins, carbohydrates, fats, vitamins and minerals) 24–28 weeks of gestation–2 months after birth |
No effect on diarrhea incidence 12 months: ↑ weight, height, and weight-for-age z-score |
Not assessed | [116] | |
| 29.97 ± 3.84 years old women, Norway, n = 278 |
Mix: Lacticaseibacillus rhamnosus GG, Bifidobacterium animalis subsp. lactis Bb-12, Lactobacillus acidophilus La-5 5 × 1010, 5 × 1010, 5 × 109 CFU/day, respectively, in fermented milk 36 weeks of gestation–3 months after birth |
40% reduction in AD among 2-year-old children (ProPACT) Association of probiotic effect on AD with intrinsic infant gut microbiota |
Infants No effect on ⍺-diversity. Effect on β-diversity at 10 days between probiotic-treated infant developing AD or not. ↑ Bifidobacterium dentium in children developing AD in the probiotic group |
[83] | |
| 31 ± 3 years old women, Norway, n = 140 |
Mix: Lacticaseibacillus rhamnosus GG, Bifidobacterium animalis subsp. lactis Bb-12, Lactobacillus acidophilus La-5 5 × 1010, 5 × 1010, 5 × 109 CFU/day, respectively, in fermented milk 36 weeks of gestation–3 months after birth |
↓ risk of AD following probiotic supplementation (ProPACT) Peripheral blood regulatory T cells at 3 months: ↓ proportion of Th22 cells; No effect on Th1, Th2, Th9, Th17 Probiotic effect partially mediated through the reduction in Th22 cells |
Not assessed | [84] | |
| 29.6 ± 3.9 years old women, Norway, n = 298 |
Mix: Lacticaseibacillus rhamnosus GG, Bifidobacterium animalis subsp. lactis Bb-12, Lactobacillus acidophilus La-5 5 × 1010, 5 × 1010, 5 × 109 CFU/day, respectively, in fermented milk 36 weeks of gestation–3 months after birth |
Prevention of allergy (ProPACT)–early life gut mycobiota and maternal-offspring transfer No effect on offspring gut mycobiota |
Not assessed | [117] | |
| 27.2 ± 3.2 years old women, China, n = 30 |
Mix: Bifidobacterium longum, Lactobacillus delbrueckii bulgaricus, Streptococcus thermophilus 2 × 107, 2 × 106, 2 × 106 CFU/day, respectively, in tablets 32 weeks of gestation–birth |
No effect on birth anthropometrics |
Mothers No effect on ⍺- or β-diversity; 48 altered OTUs compared to control; ↓ Clostridiales, Clostridium_sensu_stricto, and Holdemanella; ↑ Porphyromonadaceae, Holdemanella, and Lachnospiraceae |
[52] | |
| 27.2 ± 1.16 years old women, China, n = 25 |
Mix: Bifidobacterium, Lactobacillus and Streptococcus (species undisclosed) 1 × 107, 1 × 106, 1 × 106 CFU/day, respectively, in tablets 32 weeks of gestation–birth |
No effect on birth anthropometrics | Not assessed | [53] | |
| 20–29 years old women, Indonesia, n = 70 |
Bifidobacterium animalis subsp. lactis HNO 19 1 × 109 CFU/day in capsules 36 weeks of gestation–3 months after birth |
No effect on infant gut mucosal integrity at birth and 3 months (urine IFABP, fecal ⍺-1-antitrypsin and calprotectin levels) | Not assessed | [118] | |
| Women aged 16 years old or older (29.0 ± 5.6), United Kingdom, n = 422 |
Mix: Lactobacillus salivarius CUL61, Lactobacillus paracasei CUL08, Bifidobacterium animalis subsp. lactis CUL34, Bifidobacterium bifidum CUL20 1 × 1010 CFU/day in capsules 36 weeks of gestation–birth; birth–6 months (infants) |
5 years: No protection against asthma outcomes or eczema | Not assessed | [86] | |
| 18–49 (28.59 ± 5.3) years old women, Iran, n = 175 |
Limosilactobacillus reuteri LR92 DSM 26866 1 × 108 CFU/day in oil droplets 36 weeks of gestation–birth |
↓ colic frequency and severity until 5 months No impact on BMI; No effect on feeding pattern |
Not assessed | [54•] | |
| At risk for allergic diseases | |||||
| 29.5 (26.2–32.7) years old women, with allergy in the family; Finland, n = 15 |
Mix: Lacticaseibacillus rhamnosus GG, Bifidobacterium animalis subsp. lactis Bb12 1 × 109 CFU/day in capsules 4 weeks of gestation–Birth |
No effect on birth weight and height, and weight and height at 1 and 6 months | Not assessed | [56•] | |
| 34 (30–36) years old women, history of treated asthma, eczema, or hay fever; New-Zealand, n = 423 |
Lacticaseibacillus rhamnosus HN001 6 × 109 CFU/day in capsules 14–16 weeks of gestation–6 months after birth |
No effect on eczema, wheeze, or atopic sensitization at 12 months | Not assessed | [90•] | |
| 34 (30–36) years old women, history of treated asthma, eczema, or hay fever; New-Zealand, n = 373 |
Lacticaseibacillus rhamnosus HN001 6 × 109 CFU/day in capsules 14–16 weeks of gestation–6 months after birth |
No effect on birth anthropometrics (BW, BL, BMI, head circumference) | Not assessed | [55] | |
| 18–45 (29) years old women, family history of allergic diseases, Sweden, n = 88 |
Limosilactobacillus reuteri DSM 17938 2 × 109 CFU per day, in oil droplets ω-3 PUFA (3.84 g/day) 20 weeks–birth |
No effect on BW | Not assessed | [119] | |
| 32.0 (29.0–33.0) years old women, family history of allergic diseases, Sweden, n = 63 |
Limosilactobacillus reuteri DSM 17938 2 × 109 (mothers), 1 × 108 CFU/day (infants), in oil droplets ω-3 PUFA (3.84 g/day) 20 weeks–birth; probiotics: birth–12 months (infants); ω-3: birth–6 months (mothers) |
All treatments: ↑ hypermethylation of cord blood CD4 + T cells Probiotics + ω-3: ↑ number of differentially methylated CpG sites (immune-related pathways) |
Not assessed | [109•] | |
| Women of undisclosed age; mother or father diagnosed with allergy disease; Finland, n = 422 |
Mix: Bifidobacterium breve Bb99, Propionibacterium freundenreichii subsp. shermanii JS, Lactobacillus rhamnosus Lc705, Lactobacillus rhamnosus GG 2 × 108, 2 × 109, 5 × 109, 5 × 109 CFU/day, respectively, in capsules GOS (infant, 0.8 g/day) 36 weeks–birth; birth–6 months (infants) |
Not assessed |
Infants: 3 months: ↑ Bifidobacterium breve, Lacticaseibacillus rhamnosus ↓ antibiotic & caesarian-associated changes Probiotic effect dependent on infant diet: Breastfed: ↑ Bifidobacteria, ↓ Proteobacteria and Clostridia |
[101] | |
| Women of undisclosed age; mother or father diagnosed with allergy disease; Finland, n = 807 |
Mix: Lacticaseibacillus rhamnosus GG, Lacticaseibacillus rhamnosus LC705, Bifidobacterium breve Bb99, Propionibacterium freudenreichii ssp. shermanii JS 1 × 1010, 1 × 1010, 4 × 108, 4 × 109 CFU/day, respectively, in capsules GOS (infant, 0.8 g/day) 36 weeks of gestation–birth; birth–6 months (infant) |
10 years: No effect on lifetime prevalence of any allergic disease. ↓ Lifetime prevalence of eczema and food allergy Between 5 and 10 years: ↑ allergic rhino-conjunctivitis in vaginally delivered children; ↓ upper respiratory tract infections in caesarean-delivered children |
Not assessed | [88] | |
| Women of undisclosed age, mother or father diagnosed with allergy disease; Finland, n = 642 |
Mix: Lacticaseibacillus rhamnosus GG, Lacticaseibacillus rhamnosus LC705, Bifidobacterium breve Bb99, Propionibacterium freudenreichii ssp. shermanii JS 1 × 1010, 1 × 1010, 4 × 108, 4 × 109 CFU/day, respectively, in capsules GOS (infant, 0.8 g/day) 36 weeks of gestation–birth; birth–6 months (infant) |
13 years: no differences in the prevalence rate of doctor-diagnosed allergic disease or allergic disease with IgE sensitization; ↑ inhalant-specific IgE sensitization Caesarean-delivered subgroup: ↓ incidence of allergy and eczema |
Not assessed | [89] | |
| Women with family history of eczema, asthma, gastrointestinal allergy, allergic urticaria or allergic rhino conjunctivitis; Sweden, n = 188 |
Limosilactobacillus reuteri ATCC 55730 1 × 108 CFU/day in oil droplets 36 weeks of gestation–birth (mother) & 1–3 days–12 months (infants) |
↓ DNA methylation of CD4 + T cells genes related to immune maturation and allergy development at birth | Not assessed | [85] | |
| Women of undisclosed age; mother or father diagnosed with allergy disease; New-Zealand, n = 342 |
Either Lacticaseibacillus rhamnosus HN001 or Bifidobacterium animalis subsp. lactis HN019 6 × 109 and 9 × 109 CFU/day, respectively, in capsules 35 weeks of gestation–birth; birth–2 years (infant) |
11 years HN001: ↓ Lifetime prevalence of eczema, atopic sensitization, wheeze; ↓ in 12-month prevalence of eczema HN019: no effect |
Not assessed | [87] | |
| Women of undisclosed age; mother or father diagnosed with allergy disease; New-Zealand, n = 342 |
Either Lacticaseibacillus rhamnosus HN001 or Bifidobacterium animalis subsp. lactis HN019 6 × 109 and 9 × 109 CFU/day, respectively, in capsules 35 weeks of gestation–birth; birth–2 years (infant) |
11 years Both strains: No effect on cognitive, behavioral and mood outcomes |
Not assessed | [78] | |
| Obesity | |||||
| 28.8 ± 5.7 years old women, BMI ≥ 30, New Zealand, n = 230 |
Mix: Lacticaseibacillus rhamnosus GG, Bifidobacterium subsp. lactis BB12 6.5 × 109 CFU/day in capsules 12 weeks–17 weeks of gestation |
No effect on BW No effect on infant anthropometrics, admission to NICU, and composite neonatal morbidity |
Not assessed | [47] | |
| 30.8 ± 4.8 years old women, BMI ≥ 25, Finland, n = 439 |
Mix: Lacticaseibacillus rhamnosus HN001, Bifidobacterium animalis ssp. lactis 420 1 × 1010 CFU/day each in capsules Fish oil capsules (2.4 g of n-3 fatty acids, with 1.9 g docosahexaenoic acid, 0.22 g eicosapentaenoic acid) 13 weeks of gestation–6 months |
No effects on birth anthropometrics | Not assessed | [120] | |
| > 18 years old women (30.7 ± 4.5), BMI ≥ 30 and < 35, Denmark, n = 49 |
Mix: Streptococcus thermophilus DSM 24731, Bifidobacterium breve DSM 24732, Bifidobacterium longum DSM 24736, Bifidobacterium infantis DSM 24737, Lactobacillus acidophilus DSM 24735, Lactiplantibacillus plantarum DSM 24730, Lacticaseibacillus paracasei DSM 24733, Lactobacillus delbrueckii subsp. bulgaricus DSM 24734 Total of 4.5 × 1010 CFU/day in capsules 14–20 weeks of gestation–birth |
No effect on BW and BL, LGA or SGA |
Mothers ↑ in ⍺-diversity and β-diversity from baseline to birth; ↑ abundance of Bifidobacterium, Lactobacillus, Streptococcus salivarius with time |
[48] | |
| > 18 years old women (31.3 ± 4.7), BMI ≥ 25, Australia, n = 411 |
Mix: Lacticaseibacillus rhamnosus GG, Bifidobacterium animalis spp. lactis BB-12 1 × 109 CFU/day in capsules 20 weeks of gestation–birth |
↓ SGA incidence, no effect on other birth outcomes (preterm, hypoglycemia, macrosomia, LGA, BW, % fat) | Not assessed | [92] | |
| > 18 years (29.5 ± 6.2) years old women, BMI ≥ 25, Iran, n = 126 |
Mix: Lactobacillus acidophilus La5, Bifidobacterium animalis spp. lactis lactis Bb12 1 × 1010 and 1 × 109 CFU/day, respectively, in yoghurt 24 weeks of gestation–birth |
↓ bilirubin levels at 3–5 days, ↓ treatments rate No effect on infant anthropometrics |
Not assessed | [49] | |
| GDM | |||||
| 18–40 years old (32.03 ± 5.53) women, at high-risk of GDM, Iran, n = 507 |
Mix: Lactobacillus acidophilus LA1, Bifidobacterium longum sp54, Bifidobacterium bifidum sp9 7.5 × 109, 1.5 × 109, 6 × 109 CFU/day, respectively, in capsules 14 weeks of gestation–24 weeks |
No effect on BW and macrosomia | Not assessed | [50] | |
| 18–45 years old (32.50–5.02) women with the diagnosis of GDM, Thailand, n = 57 |
Mix: Lactobacillus acidophilus, Bifidobacterium bifidum (strain undisclosed) 1 × 109 CFU/day, respectively, in capsules 24–27 weeks to 28–31 weeks of gestation |
No effect on BW or neonatal hypoglycemia | Not assessed | [51] | |
| 31.64 ± 5.97 years old women with the diagnosis of GDM, Iran, n = 84 |
Mix: Lactobacillus acidophilus, Bifidobacterium lactis (strain undisclosed) Total of 1 × 106 CFU/day in yoghurt For 8 weeks during the last trimester |
↓ BW; ↓ macrosome neonates; No change of BL, head circumference or GA |
Not assessed | [121] | |
AD atopic dermatitis, BL birth length, BMI body mass index, BW birth weight, CFU colony forming units, GA gestational age, GDM gestational diabetes mellitus, GOS galacto-oligosaccharides, IFABP intestinal fatty-acid binding protein, LGA large for gestational age, OTU operational taxonomic units, PUFA polyunsaturated fatty acids, SGA small for gestational age
Probiotics in the Context of the Developmental Origins of Health and Disease
The Developmental Origins of Health and Disease (DOHaD) paradigm is based on the principle of developmental plasticity, which refers to the phenomenon by which “a given genotype can give rise to a range of different physiological or morphological states in response to various environmental exposures throughout development.” The term programming refers to a stimulus introduced at a “critical” or “sensitive” period, which causes long-term consequences for an organism [25]. A key principle of DOHaD is the existence of “windows of opportunity”, i.e., the prenatal stages of life (from pre-conception to embryonic and fetal stages and birth), infancy, and adolescence [25]. These time-sensitive life stages of exposure lead to tissue-specific effects thought to optimize or alter one’s biological potentials, and promote long-term health or a disease state [26, 27]. For example, undernutrition during pregnancy induces structure and functional remodeling in the fetus preserving brain development and prioritizing survival, negatively impacting development of other functions such as glucose metabolism and insulin sensitivity [26]. With time, as the evolutionary advantage of developmental plasticity begins to decrease, an individual’s ability to adapt to positive or negative environmental challenges becomes more limited [26]. Historically, the area of DOHaD research has primarily focused on overnutrition or undernutrition, linked with the manifestation of non-communicable diseases in later life, such as obesity or diabetes mellitus. Prenatal and early infancy exposures have been specifically studied at the macro- and micro-nutrient levels, with offspring outcomes ranging from glucose homeostasis to blood pressure [28]. In utero epigenetic modifications may be underlying mechanisms [29]. The gut microbiota has been proposed to regulate host gene expression epigenetically [30, 31], for example, via DNA methylation [32] with implications for offspring disease susceptibility [33]. The intestinal microbiota is a dynamic and interactive across-kingdom ecosystem composed of characteristic microbial communities co-evolving with their host [34]. While what constitutes a health-compatible microbiota remains elusive [35], many taxa have been identified whose altered representation is associated with diseases. Specifically, in infancy, microbiota variation is a predictor of overweight [5], asthma, and allergy [3]. The microbiota is seeded by the maternal microbiota [36] and continues to develop during infancy [37] thus going through sequential stages of plasticity that encompass pregnancy and are susceptible to programming, while being a determinant of life-long health [38]. In this context, maternally administered probiotics can be used as a dietary intervention that targets the offspring intestinal ecosystem. In utero and early life exposure to probiotics may affect growth and gut health [39–41], suggesting that specific windows of opportunities may exist for probiotic administration.
Growth and Anthropometric Indices at Birth
Probiotics have a long history of use to support growth of farm animals [42, 43]. Investigation into the underlying mechanisms suggests that they play a role in hormone metabolism. For example, certain Lactiplantibacillus plantarum strains support Drosophila melanogaster larval growth via target of rapamycin (TOR)-dependent mechanisms and hormonal growth signaling [44]. Lactiplantibacillus plantarum strains also support systemic growth in undernourished mice via growth hormone sensitivity enhancement and increased tissue insulin-like growth factor 1 activity [45]. Previous studies investigating the impact of maternal probiotics intake on offspring growth have largely focused on metabolic or allergic diseases as main outcomes [39, 46]. In recent studies in overweight women and women diagnosed with GDM, mid- or late-pregnancy supplementation with several mixtures of Lactobacillus, Bifidobacterium, and Streptococcus species had no effect on infant anthropometrics at birth [47–51]. Recently, several studies were also completed in healthy participants. As shown in Table 2, supplementation with various probiotic mixes starting at the beginning of pregnancy, at the second trimester, or at the end of pregnancy had no impact on neonatal birth weight, body mass index, birth length, femur length, or head circumference [52, 53, 54•, 55, 56•]. These findings align with a recent meta-analysis investigating infant birth weight following exposure to Lacticaseibacillus rhamnosus strains alone or in mixtures with Streptococcus or Bifidobacterium strains started during mid- or late-pregnancy [57]. Sex-specific effects have not been comprehensively studied.
Birth anthropometrics are important prognostic markers of healthy growth. Long-term effects of maternal probiotics on offspring growth during infancy and until adulthood have not been investigated in clinical studies; however, some studies have been conducted in animals. In healthy rodent models, the probiotic strains Limosilactobacillus fermentum CECT5716 and Lacticaseibacillus rhamnosus GG supplemented during pregnancy and lactation did not impact offspring body weight at weaning [58, 59]. Though supplementation with Bacillus subtilis PB6 or A and B from late pregnancy until weaning led to increased body weight in healthy piglet models (male and female combined) [60, 61]. Similarly, maternal intake of Lacticaseibacillus rhamnosus GG from late pregnancy until birth, combined with neonatal administration during the first five days of life, increased offspring weight during the four weeks after birth compared to the control mice that received the inactivated strain [62]. Findings were combined from male and female piglets. Interestingly, supplementation with Lactobacillus helveticus NS8 at the end of gestation in a healthy rat model led to a decreased body weight from late adolescence (51 days of life) until adulthood (76 and 86 days of life), without influencing the weight difference between males and females [63]. These findings suggest that the duration of the follow-up measurements is an important consideration to fully determine the effect of maternal probiotics exposure on offspring growth.
Intestinal Barrier and Gut Health
The intestinal barrier is critical to host health and a recognized target for preventative and therapeutic strategies [64]. The gut microbiota contributes to the protection of the epithelium from luminal pathogens and antigens, both physically and chemically [65], and supports the development of the infant immune system [3]. The intestinal barrier and the microbiota co-evolve in early life and reciprocally influence each other, resulting in the establishment of a mature intestinal ecosystem [66]. Probiotics have long been recognized to sustain the intestinal barrier, including in early life [40, 41]. Additionally, studies have proposed that the intestinal barrier, including the tight junction and the toll-like receptor associated pathways, is under epigenetic regulation [33]. Interestingly, in piglets, a recognized relevant model for pediatric nutritional studies assessing intestinal outcomes [67], maternal probiotics supplementation with Bacillus altitudinis WIT588 starting during late pregnancy increases intestinal crypt depth and villus length at postnatal day 34 [68]. In mice, supplementation with Lacticaseibacillus rhamnosus GG increases cell proliferation and differentiation and tight junction protein expression at postnatal day 21 [59, 62]. These findings suggest that maternal probiotic supplementation supports offspring intestinal digestive and absorptive functions in infancy [69]. Sex effects were not examined in these studies.
The long-term impact of this Lacticaseibacillus rhamnosus GG supplementation was investigated at 8 months of age when mice displayed increased goblet cell numbers and tight junction gene expression [59]. This was accompanied by increased antioxidant enzyme activities [59], and this increase was also demonstrated in the adult progeny of mini-sows that had received a Lactiplantibacillus plantarum B90 and Saccharomyces cerevisiae P11 mix, alone or with xylooligosaccharides, since the beginning of gestation [69]. This enhanced antioxidant capacity may help reduce ageing-associated oxidative stress and was associated with an increased jejunal villus height at 65 days [70]. Overall, findings from these pre-clinical studies suggest that maternal probiotic supplementation may help maintain gut barrier integrity in early life and adulthood and may improve antioxidant status. Indeed, recent studies support preventative effects of maternally administered probiotics in offspring with impaired intestinal barrier. In sows, maternal intake of Enterococcus faecium DSM7134 and Lactiplantibacillus plantarum CAM6 was shown to reduce diarrhea incidence in offspring [71, 72]. In a clinical trial, Limosilactobacillus reuteri LR92 supplementation to healthy mothers starting at late pregnancy and until birth led to a decreased colic frequency and severity in infants until 5 months of age, although the influence of sex on this effect was not reported [54•]. Interestingly, a recent study in mice showed that maternal administration of a mix of three Limosilactobacillus reuteri strains protected female offspring from dextran sodium sulfate-induced colitis [73••], although no protective effects were seen in male offspring. Taken together, these findings suggest that administration of selected probiotics during pregnancy may beneficially prevent intestinal inflammation in the offspring. More studies are required to understand sex-differences in responses and underlying mechanisms.
Neurodevelopment and Anxiety-Like Behavior
The gut ecosystem, including the microbiota, and the peripheral and central nervous system entertain a continuous bi-directional communication that is typically referred to as the gut-brain axis. Underlying mechanisms include endocrine pathways through cortisol and the hypothalamic–pituitary–adrenal axis, immunomodulation through cytokines, and interaction with the vagus nerve and the enteric nervous system [74]. The effects of probiotics on the gut-brain axis have been recently reviewed; Mörkl et al. demonstrated that probiotics may be therapeutically beneficial in the context of depression, but not schizophrenia, while data for anxiety are lacking [74]. Interestingly, these conditions may be rooted into neurodevelopment in early life and environmental exposures during stages of plasticity [75]. Moreover, the gut microbiota has been shown to foster fetal thalamocortical axonogenesis [76]. Thus, probiotic exposure during early stages of life, including in utero, may have preventative potential. In fact, one study in mice found that maternal supplementation of Lactococcus lactis (strain not disclosed) from 10.5 days of gestation increased blood vessel numbers and size in the cortical plate and cortical neurons density in offspring of both sexes at postnatal day 1, while the numbers of mitotic neural progenitor cells were increased in females only [77]. Interestingly, effects were investigated in the context of anxiety, which is known to appear early among psychiatric disorders [75]. At 10 weeks of age, females but not males from the above study had a higher activity level in bright zones and reduced fearful behavior [77]. Similarly, adolescent offspring, especially females, of rats that received Lactobacillus helveticus NS8 in late pregnancy spent more time in open spaces compared to controls [63]. No effects on cognition and behavior were seen in 11 years old children born at risk for allergic disease and exposed to Lacticaseibacillus rhamnosus HN001 or Bifidobacterium animalis subsp. lactis HN019 since late gestation and until 2 years of age [78]. Interestingly, these benefits of probiotics are also seen in the context of obesity. Administration of a multi-strain probiotic to mouse dams since conception prevented obesity-induced anxiety-like behavior in offspring at weaning and at adulthood in both sexes [79••]. Thus, findings from these recent pre-clinical studies suggest that maternal probiotic intake may reduce anxiety-like behavior in the next generation, potentially through modulation of neurodevelopment processes in utero and early life. Moreover, these studies suggest that effects may be sex-specific. Since psychiatric disorders manifest differently between sexes and are more prevalent and damaging in females compared to males [80], more studies should be conducted to understand if maternally administered probiotics hold potential for sex-targeted clinical applications.
Allergic Diseases
The effect of probiotic supplementation during prenatal and early life on prevention of allergic diseases has been extensively studied, leading to the release of recommendations for populations at risk [7]. These guidelines are the same for both sexes, and previous studies largely focused on Lactobacillus species, alone or in combination with Bifidobacterium. Recent literature has continued investigating the mechanisms behind probiotic preventative effects. Studies in mice show that probiotics, typically administered starting at mid gestation, act through immune-mediated mechanisms (details in Table 1), in both models of asthma [81] and atopic dermatitis [82], and these effects may be strain-specific [82].
Interestingly, studies in healthy women receiving probiotics from 36 weeks of gestation to 3 months of lactation showed a reduction in infant atopic dermatitis incidence, which was associated with decreased proportion of Th22 but independent from Th1/Th2 balance [83, 84]. A clinical study of women with a family history of treated allergic diseases receiving Limosilactobacillus reuteri ATCC 55730 during late pregnancy reported a modulation in DNA methylation of CD4 + T cells genes related to immune maturation and allergy development in infants at birth, suggesting that epigenetic modifications may mediate the preventative effects of probiotics in this context [85]. Further studies are needed to decipher the exact mechanisms. Long-term effects were also investigated in clinical studies. While combined maternal and infant supplementation with a probiotic mix (Lactobacillus salivarius CUL61, Lactobacillus paracasei CUL08, Bifidobacterium animalis subsp. lactis CUL34, Bifidobacterium bifidum CUL20) had no effects on asthma or eczema prevalence in 5-year-old children [86], the administration of another probiotic mix (Lacticaseibacillus rhamnosus GG and LC705, Bifidobacterium breve Bb99, Propionibacterium freudenreichii ssp. shermanii JS) or of Lacticaseibacillus rhamnosus HN001 to the mother and infant decreased the lifetime prevalence of eczema and atopic sensitization [87, 88] and food allergy [88] in 10- and 11-year-old children, respectively. Probiotic preventive effects on upper respiratory tract infections [88] and eczema [89] were also reported in populations subgroups of caesarean-delivered infants, after 10 and 13 years, respectively. Of importance, Lacticaseibacillus rhamnosus HN001 was found not to have the same effects on infant eczema, wheeze, and atopic sensitization when given to the mother alone without infant supplementation [87, 90•], thus calling for more studies investigating the timing of probiotic administration.
Metabolic Disorders
Maternal nutritional status is a determinant of offspring metabolic health and both pre-clinical and clinical studies demonstrate that various components of the metabolic syndrome are susceptible to fetal programming [91]. The study of maternal probiotics administration and effects on offspring metabolic health is still in its infancy, with most human studies focused solely on maternal outcomes [47–49, 92]. In pre-clinical studies, offspring have been evaluated at weaning and adulthood, with hypertension and risk for cardiovascular disease being the most studied conditions [93–96]. Interestingly, the only pre-clinical study that we found administering probiotic since pre-conception identified sex- and time-specific (weaning versus young adults) benefits in offspring and stronger evidence for reduced cardiovascular dysfunction in female offspring [93]. Most other pre-clinical studies have been conducted in males only (Table 1).
A study in normal-weight women supplemented with Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis Bb-12 from the 4th week of pregnancy until birth showed a reduction in DNA methylation of obesity and weight gain-related genes in offspring, suggesting again that epigenetics might be a mechanism underlying probiotics programming effects [56•]. As pre-clinical studies revealed that significant weight differences are observable at weaning and adulthood, although males and females were not studied separately, it would be interesting to assess whether maternal probiotics effects on offspring growth are also observable during infancy and puberty, and in a sex-specific manner.
Intestinal Microbiota
Modulation of the microbiota is not a prerequisite for probiotics effects. In addition, resilience of an established gut microbiota makes it difficult to modify using exogenous microorganisms, though microbiota susceptibility is likely higher during early stages of plasticity. A recent meta-analysis found that maternal exposures including body mass index, drug-induced alteration of the microbiota, and probiotics administration influence the offspring intestinal microbiota [97]. In animal models, maternal probiotic supplementation had different effects on the offspring gut microbiota composition and structure, depending on the species administered and the time of administration [58, 59, 62, 69–71, 73••, 81, 82, 93, 95, 96, 98–100]. Different effects were also observed in various regions of the intestinal tract [69]. Interestingly, co-administration of probiotic Lactiplantibacillus plantarum B90 and Saccharomyces cerevisiae P11 with prebiotic xylooligosaccharides modified the probiotic effect on microbiota outcomes of piglets [69]. Probiotics with and without prebiotic increased Bacteroidetes and Bifidobacterium jejunal relative abundance, while only co-administration also increased Firmicutes and Lactobacillus [69]. Most human studies of maternally administered probiotics did not assess infant microbiota. For those that did, maternal probiotic intake was found to transiently increase the relative abundance of administered probiotic species or strain in the offspring [83, 101], regardless of the participants being healthy or at risk for allergic diseases (Table 2). Pre-clinical studies are also the only studies assessing microbial outcomes long-term, including effects on microbial diversity and/or composition at puberty in healthy mice [98] and mice born to dams exposed to an obesogenic diet [93], as well as at young adulthood [93, 95, 96]. Interestingly, some of these studies investigated microbiota outcomes in offspring of both sexes. This is relevant because there is evidence for sex-specific microbiota taxa as early as 2 weeks after birth [102], which likely become more pronounced during puberty [103]. Some of the probiotics studied showed sex-dependent effects on microbiota structure and taxa relative abundance [73••, 93]. This may be a result of hormonal interactions or of the offspring microbiota sex-driven effects. The latter is aligned with the finding that the infant baseline microbiota may determine the success of a maternal probiotic intervention aimed at preventing atopic dermatitis [83].
Notably, none of these studies used metagenomic approaches and thus it remains unknown if probiotics modulated microbial function. This is important because some pre-clinical studies found that microbial metabolites were altered in response to probiotics, including increased fecal short chain fatty acids in response to Lacticaseibacillus rhamnosus [62, 96] and decreased plasma trimethylamine N-oxide (TMAO) in response to Lacticaseibacillus casei [96]. Short-chain fatty acids such as acetate, propionate, and, in particular, butyrate are bacterial metabolites produced by fermentation of dietary fibers, and help maintain intestinal homeostasis [104]. TMAO is produced by liver oxidation of the intestinal microbial metabolite trimethylamine and is a marker of cardiovascular diseases [105]. It will be important for future studies to expand on these analyses to determine if programming effects of probiotics manifest at the functional level and to determine the quality and quantity of the microbial metabolites to which the host becomes exposed.
Conclusions
Here, we have reviewed recent pre-clinical and clinical studies investigating programming effects of maternally administered probiotics on offspring health. Studies published during the past 5 years expand on previous knowledge in the context of growth and gut health and additionally describe effects in the context of the metabolic syndrome and behavior (Fig. 1). Underlying mechanisms are also starting to be investigated. The study of probiotics in the context of DOHaD is in its infancy and it is not currently possible to make recommendations for clinical practice beyond allergic diseases. Pre-clinical studies are essential to investigate maternal programming effects of multiple outcomes in both male and female offspring in strictly controlled conditions and to elucidate molecular mechanisms. To encourage transparency and reproducibility of studies among research groups, it is important that these studies systematically report study details, including probiotic strain and dose along with timing and duration of probiotic exposure; the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines offer comprehensive recommendations for reporting [106].
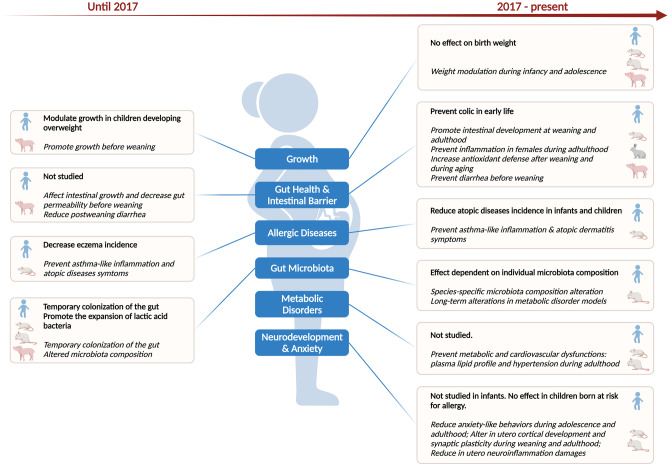
Fig. 1.
Offspring health outcomes programmed by maternal probiotic intake started prenatally. Previous knowledge (until 2017) included growth, gut health and intestinal barrier, allergic diseases, and gut microbiota findings. In addition to these outcomes, recent studies described effects on metabolic disorders, and neurodevelopment and anxiety. Clinical and pre-clinical findings are indicated in bold and italics, respectively. Icons depict findings assessed in infants or animal models (mice, rats, rabbits, or pigs). Created with BioRender.com
While most clinical studies initiated the administration of probiotics towards the end of pregnancy and continued until 3 months of lactation, pre-clinical studies started at the beginning of pregnancy and continued until weaning. There are no studies that isolated the pregnancy period, and only one mouse and one clinical study began supplementation at pre-conception; it would be important to understand which are the windows of susceptibility for probiotics to positively affect the offspring. Offspring characteristics, including sex, can also play a role. The male sex is more susceptible to in utero programming [107] and different placental DNA methylation patterns have been observed for male and female infants [108]. Interestingly, four studies reviewed here proposed DNA methylation as an underlying mechanism for programming effects of probiotics [56•, 84, 100, 109•]. Effects could also be mediated by the microbiota, with preventative benefits in inflammation [73••]. Interestingly, a clinical study of probiotics administered during late pregnancy and lactation for the prevention of atopic dermatitis found that individual microbiota characteristics, in this case, representation of Bifidobacterium dentium, could determine the effects of the probiotic intervention [83]. It would be important to study the programming effects of probiotics in infants at risk for altered microbial maturation patterns, for example very low birth weight or malnourished infants. Microbial maturation continues throughout adolescence and studies have in fact found that probiotic effects might appear during puberty and adulthood [62, 87–89, 94–96]. Beyond allergy-related outcomes, current human studies report findings at birth or in infants up to 2 years of age; longer trials will provide a more comprehensive understanding of the programming potential of probiotics. Finally, the studies reviewed were performed in Northern Europe, Asia, and Oceania calling for more trials to be conducted in America and Africa and to encompass ethnically diverse populations.
Funding
Work in the laboratory of EMC is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) (Grant # RGPIN-2019–06100) and the Canadian Institutes of Health Research (CIHR). EMC was the recipient of the Lawson Family Chair in Microbiome Nutrition Research. SES was funded by NSERC Graduate Scholarships-Master’s (NSERC CGS M) and Ontario Graduate Scholarship (OGS).
Compliance with Ethical Standards
Conflict of Interest
EMC has received research support from Lallemand Health Solutions and Ocean Spray and has received consultant fees or speaker or travel support from Danone and Lallemand Health Solutions.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Functional Foods
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 2.Arrieta M-C, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 3.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22:713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 4.Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes. 2014;38:1290–1298. doi: 10.1038/ijo.2014.119. [DOI] [PubMed] [Google Scholar]
- 5.Kalliomäki M, Carmen Collado M, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 6.Stewart C, Marrs E, Magorrian S, Nelson A, Lanyon C, Perry J, Embleton N, Cummings S, Berrington J. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr. 2012;101:1121–1127. doi: 10.1111/j.1651-2227.2012.02801.x. [DOI] [PubMed] [Google Scholar]
- 7.Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70:1356–1371. doi: 10.1111/all.12700. [DOI] [PubMed] [Google Scholar]
- 8.Fiocchi A, Pawankar R, Cuello-Garcia C, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ J. 2015;8:1–13. doi: 10.1186/s40413-015-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L, Ding C, Wu J, Chen X, Ng DM, Wang H, Zhang Y, Shi N. Probiotics and synbiotics show clinical efficacy in treating gestational diabetes mellitus: a meta-analysis. Prim Care Diabetes. 2021;15:937–947. doi: 10.1016/j.pcd.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Hanson L, VandeVusse L, Malloy E, Garnier-Villarreal M, Watson L, Fial A, Forgie M, Nardini K, Safdar N. Probiotic interventions to reduce antepartum Group B streptococcus colonization: a systematic review and meta-analysis. Midwifery. 2022;105:103208. doi: 10.1016/j.midw.2021.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiménez E, Manzano S, Schlembach D, Arciszewski K, Martin R, Ben Amor K, Roelofs M, Knol J, Rodríguez JM, Abou-Dakn M. Ligilactobacillus salivarius PS2 supplementation during pregnancy and lactation prevents mastitis: a randomised controlled trial. Microorganisms. 2021;9:1933. doi: 10.3390/microorganisms9091933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen JJ, Retterstøl K, Godang K, Roland MCP, Qvigstad E, Bollerslev J, Ueland T, Henriksen T, Holven KB. LDL cholesterol in early pregnancy and offspring cardiovascular disease risk factors. J Clin Lipidol. 2016;10:1369–1378. doi: 10.1016/j.jacl.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Schuchat A. Group B streptococcus. The Lancet. 1999;353:51–56. doi: 10.1016/S0140-6736(98)07128-1. [DOI] [PubMed] [Google Scholar]
- 14.Schultz M, Göttl C, Young RJ, Iwen P, Vanderhoof JA. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38:293–297. doi: 10.1097/00005176-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Gueimonde M, Sakata S, Kalliomäki M, Isolauri E, Benno Y, Salminen S. Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J Pediatr Gastroenterol Nutr. 2006;42:166–170. doi: 10.1097/01.mpg.0000189346.25172.fd. [DOI] [PubMed] [Google Scholar]
- 16.Sheyholislami H, Connor KL. Are probiotics and prebiotics safe for use during pregnancy and lactation? A systematic review and meta-analysis Nutrients. 2021;13:2382. doi: 10.3390/nu13072382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gille C, Böer B, Marschal M, et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am J Obstet Gynecol. 2016;215:608. doi: 10.1016/j.ajog.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 18.van den Nieuwboer M, Claassen E, Morelli L, Guarner F, Brummer RJ. Probiotic and synbiotic safety in infants under two years of age. Benef Microbes. 2014;5:45–60. doi: 10.3920/BM2013.0046. [DOI] [PubMed] [Google Scholar]
- 19.Bridgman SL, Azad MB, Field CJ, Letourneau N, Johnston DW, Kaplan BJ, Kozyrskyj AL. Maternal perspectives on the use of probiotics in infants: a cross-sectional survey. BMC Complement Altern Med. 2014;14:366. doi: 10.1186/1472-6882-14-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyangahu DD, Lennard KS, Brown BP, et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome. 2018;6:124. doi: 10.1186/s40168-018-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Perez G, Hicks AL, Tekieli TM, Radens CM, Williams BL, Lamousé-Smith ESN. Maternal antibiotic treatment impacts development of the neonatal intestinal microbiome and antiviral immunity. J Immunol. 2016;196:3768–3779. doi: 10.4049/jimmunol.1502322. [DOI] [PubMed] [Google Scholar]
- 22.Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, Hassoun A, Perera F, Rundle A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond) 2015;39:665–670. doi: 10.1038/ijo.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Agricultural Organization of the United Nations, World Health Organization. Probiotics in food: Health and nutritional properties and guidelines for evaluation - Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. Rome, Italy: FAO/WHO; 2006.
- 24.The Center for Reviews and Dissemination (CRD) Systematic Reviews: CRD’s guidance for undertaking reviews in health care. York: York Associates International; 2008. [Google Scholar]
- 25.Lucas A. Programming by early nutrition in man. Ciba Found Symp. 1991;156:38–50. [PubMed] [Google Scholar]
- 26.Calkins K, Devaskar SU. Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care. 2011;41:158–176. doi: 10.1016/j.cppeds.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas A. Role of nutritional programming in determining adult morbidity. Arch Dis Child. 1994;71:288–290. doi: 10.1136/adc.71.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langley-Evans SC. Nutrition in early life and the programming of adult disease: a review. J Hum Nutr Diet. 2015;28:1–14. doi: 10.1111/jhn.12212. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Hernández D, Poon AN, Kubant R, et al. High vitamin A intake during pregnancy modifies dopaminergic reward system and decreases preference for sucrose in Wistar rat offspring. J Nutr Biochem. 2016;27:104–111. doi: 10.1016/j.jnutbio.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Woo V, Alenghat T. Epigenetic regulation by gut microbiota. Gut Microbes. 2022;14:2022407. doi: 10.1080/19490976.2021.2022407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miro-Blanch J, Yanes O. Epigenetic regulation at the interplay between gut microbiota and host metabolism. Front Genet. 2019;10:638. doi: 10.3389/fgene.2019.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi K, Sugi Y, Nakano K, Tsuda M, Kurihara K, Hosono A, Kaminogawa S. Epigenetic control of the host gene by commensal bacteria in large intestinal epithelial cells. J Biol Chem. 2011;286:35755–35762. doi: 10.1074/jbc.M111.271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortese R, Lu L, Yu Y, Ruden D, Claud EC. Epigenome-Microbiome crosstalk: a potential new paradigm influencing neonatal susceptibility to disease. Epigenetics. 2016;11:205–215. doi: 10.1080/15592294.2016.1155011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg G, Rybakova D, Fischer D, et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8:103. doi: 10.1186/s40168-020-00875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McBurney MI, Davis C, Fraser CM, Schneeman BO, Huttenhower C, Verbeke K, Walter J, Latulippe ME. Establishing what constitutes a healthy human gut microbiome: state of the science, regulatory considerations, and future directions. J Nutr. 2019;149:1882–1895. doi: 10.1093/jn/nxz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferretti P, Pasolli E, Tett A, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 2018;24:133–145.e5. doi: 10.1016/j.chom.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derrien M, Alvarez A-S, de Vos WM. The gut microbiota in the first decade of life. Trends Microbiol. 2019;27:997–1010. doi: 10.1016/j.tim.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar A, Yoo JY, Valeria Ozorio Dutra S, Morgan KH, Groer M. The association between early-life gut microbiota and long-term health and diseases. J Clin Med. 2021 doi: 10.3390/jcm10030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luoto R, Kalliomäki M, Laitinen K, Isolauri E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int J Obes. 2010;34:1531–1537. doi: 10.1038/ijo.2010.50. [DOI] [PubMed] [Google Scholar]
- 40.Gareau MG, Wine E, Reardon C, Sherman PM. Probiotics prevent death caused by Citrobacter rodentium infection in neonatal mice. J Infect Dis. 2010;201:81–91. doi: 10.1086/648614. [DOI] [PubMed] [Google Scholar]
- 41.Fåk F, Ahrné S, Molin G, Jeppsson B, Weström B. Maternal consumption of Lactobacillus plantarum 299v affects gastrointestinal growth and function in the suckling rat. Br J Nutr. 2008;100:332–338. doi: 10.1017/S0007114507883036. [DOI] [PubMed] [Google Scholar]
- 42.Bernardeau M, Vernoux J-P. Overview of differences between microbial feed additives and probiotics for food regarding regulation, growth promotion effects and health properties and consequences for extrapolation of farm animal results to humans. Clin Microbiol Infect. 2013;19:321–330. doi: 10.1111/1469-0691.12130. [DOI] [PubMed] [Google Scholar]
- 43.Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb Pathog. 2017;106:162–170. doi: 10.1016/j.micpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Schwarzer M, Makki K, Storelli G, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 46.Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103:1792–1799. doi: 10.1017/S0007114509993898. [DOI] [PubMed] [Google Scholar]
- 47.Okesene-Gafa KAM, Li M, McKinlay CJD, et al. Effect of antenatal dietary interventions in maternal obesity on pregnancy weight-gain and birthweight: Healthy Mums and Babies (HUMBA) randomized trial. Am J Obstet Gynecol. 2019;221:152.e1–152.e13. doi: 10.1016/j.ajog.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Halkjær SI, de Knegt VE, Lo B, et al. Multistrain probiotic increases the gut microbiota diversity in obese pregnant women: results from a randomized, double-blind placebo-controlled study. Curr Dev Nutr. 2020;4:nzaa095. doi: 10.1093/cdn/nzaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asgharian H, Homayouni-Rad A, Mirghafourvand M, Mohammad-Alizadeh-Charandabi S. Effect of probiotic yoghurt on plasma glucose in overweight and obese pregnant women: a randomized controlled clinical trial. Eur J Nutr. 2020;59:205–215. doi: 10.1007/s00394-019-01900-1. [DOI] [PubMed] [Google Scholar]
- 50.Shahriari A, Karimi E, Shahriari M, Aslani N, Khooshideh M, Arab A. The effect of probiotic supplementation on the risk of gestational diabetes mellitus among high-risk pregnant women: a parallel double-blind, randomized, placebo-controlled clinical trial. Biomed Pharmacother. 2021;141:111915. doi: 10.1016/j.biopha.2021.111915. [DOI] [PubMed] [Google Scholar]
- 51.Kijmanawat A, Panburana P, Reutrakul S, Tangshewinsirikul C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J Diabetes Investig. 2019;10:163–170. doi: 10.1111/jdi.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, Li Z, Tye KD, et al. Probiotic supplementation during human pregnancy affects the gut microbiota and immune status. Front Cell Infect Microbiol. 2019;9:254. doi: 10.3389/fcimb.2019.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang P, Li Z, Tye KD, Chen Y, Lu T, He Z, Zhou J, Xiao X. Effects of an orally supplemented probiotic on the autophagy protein LC3 and Beclin1 in placentas undergoing spontaneous delivery during normal pregnancy. BMC Pregnancy Childbirth. 2020;20:216. doi: 10.1186/s12884-020-02905-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.• Pourmirzaiee MA, Famouri F, Moazeni W, Hassanzadeh A, Hajihashemi M. The efficacy of the prenatal administration of Lactobacillus reuteri LR92 DSM 26866 on the prevention of infantile colic: a randomized control trial. Eur J Pediatr. 2020;179:1619–26. This study adds to current knowledge on postnatal administration of probiotics to mitigate colic and suggests that maternal prenatal probiotic intake may also have potential. [DOI] [PubMed]
- 55.Wickens KL, Barthow CA, Murphy R, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br J Nutr. 2017;117:804–813. doi: 10.1017/S0007114517000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.• Vähämiko S, Laiho A, Lund R, Isolauri E, Salminen S, Laitinen K. The impact of probiotic supplementation during pregnancy on DNA methylation of obesity-related genes in mothers and their children. Eur J Nutr. 2019;58:367–77. This paper provides clinical mechanistic evidence underlying the programming effects by probiotics in the context of obesity. [DOI] [PubMed]
- 57.Pérez-Castillo ÍM, Fernández-Castillo R, Lasserrot-Cuadrado A, Gallo-Vallejo JL, Rojas-Carvajal AM, Aguilar-Cordero MJ. Reporting of perinatal outcomes in probiotic randomized controlled trials. A systematic review and meta-analysis Nutrients. 2021;13:256. doi: 10.3390/nu13010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azagra-Boronat I, Tres A, Massot-Cladera M, Franch À, Castell M, Guardiola F, Pérez-Cano FJ, Rodríguez-Lagunas MJ. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation impacts maternal and offspring lipid profile, immune system and microbiota. Cells. 2020;9:575. doi: 10.3390/cells9030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu T, Song X, An Y, et al. Lactobacillus rhamnosus GG colonization in early life ameliorates inflammaging of offspring by activating SIRT1/AMPK/PGC-1α pathway. Oxid Med Cell Longev. 2021;2021:3328505. doi: 10.1155/2021/3328505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han L, Azad MdAK, Huang P, Wang W, Zhang W, Blachier F, Kong X. Maternal supplementation with different probiotic mixture from late pregnancy to day 21 postpartum: consequences for litter size, plasma and colostrum parameters, and fecal microbiota and metabolites in sows. Front Vet Sci. 2022;9:726276. doi: 10.3389/fvets.2022.726276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Q, Li J, Cao M, et al. Dietary supplementation of Bacillus subtilis PB6 improves sow reproductive performance and reduces piglet birth intervals. Anim Nutr. 2020;6:278–287. doi: 10.1016/j.aninu.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou B, Jin G, Pang X, et al. Lactobacillus rhamnosus GG colonization in early life regulates gut-brain axis and relieves anxiety-like behavior in adulthood. Pharmacol Res. 2022;177:106090. doi: 10.1016/j.phrs.2022.106090. [DOI] [PubMed] [Google Scholar]
- 63.Niu Y, Liang S, Wang T, Hu X, Li W, Wu X, Jin F. Pre-Gestational intake of Lactobacillus helveticus NS8 has anxiolytic effects in adolescent Sprague Dawley offspring. Brain Behav. 2020 doi: 10.1002/brb3.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke J-D, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16:457–470. doi: 10.1038/s41579-018-0036-x. [DOI] [PubMed] [Google Scholar]
- 66.Rokhsefat S, Lin A, Comelli EM. Mucin–microbiota interaction during postnatal maturation of the intestinal ecosystem: clinical implications. Dig Dis Sci. 2016;61:1473–1486. doi: 10.1007/s10620-016-4032-6. [DOI] [PubMed] [Google Scholar]
- 67.Sciascia Q, Daş G, Metges CC. REVIEW: The pig as a model for humans: effects of nutritional factors on intestinal function and health1. J Anim Sci. 2016;94:441–452. doi: 10.2527/jas.2015-9788. [DOI] [Google Scholar]
- 68.Crespo-Piazuelo D, Gardiner GE, Ranjitkar S, Bouwhuis MA, Ham R, Phelan JP, Marsh A, Lawlor PG. Maternal supplementation with Bacillus altitudinis spores improves porcine offspring growth performance and carcass weight. Br J Nutr. 2022;127:403–420. doi: 10.1017/S0007114521001203. [DOI] [PubMed] [Google Scholar]
- 69.Wang K, Kong X, Azad MAK, Zhu Q, Xiong L, Zheng Y, Hu Z, Yin Y, He Q. Maternal probiotic or synbiotic supplementation modulates jejunal and colonic antioxidant capacity, mitochondrial function, and microbial abundance in Bama mini-piglets. Oxid Med Cell Longev. 2021;2021:6618874. doi: 10.1155/2021/6618874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang K, Hu C, Tang W, Azad MAK, Zhu Q, He Q, Kong X. The enhancement of intestinal immunity in offspring piglets by maternal probiotic or synbiotic supplementation is associated with the alteration of gut microbiota. Front Nutr. 2021;8:686053. doi: 10.3389/fnut.2021.686053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lan R, Kim I. Enterococcus faecium supplementation in sows during gestation and lactation improves the performance of sucking piglets. Vet Med Sci. 2019;6:92–99. doi: 10.1002/vms3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Betancur C, Martínez Y, Tellez-Isaias G, Castillo R, Ding X. Effect of oral administration with Lactobacillus plantarum CAM6 strain on sows during gestation-lactation and the derived impact on their progeny performance. Mediators Inflamm. 2021;2021:6615960. doi: 10.1155/2021/6615960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.•• Krishna M, Engevik M, Queliza K, Britto S, Shah R, Ruan W, Wang H, Versalovic J, Kellermayer R. Maternal Lactobacillus reuteri supplementation shifts the intestinal microbiome in mice and provides protection from experimental colitis in female offspring. FASEB Bioadv. 2021;4:109–20. This study considers individual susceptibility to colitis and shows that maternal probiotics may mitigate its effects in adult offspring. It is also one of the few studies assessing sex-specific responses. [DOI] [PMC free article] [PubMed]
- 74.Mörkl S, Butler MI, Holl A, Cryan JF, Dinan TG. Probiotics and the microbiota-gut-brain axis: focus on psychiatry. Curr Nutr Rep. 2020;9:171–182. doi: 10.1007/s13668-020-00313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leonardo ED, Hen R. Anxiety as a developmental disorder. Neuropsychopharmacology. 2008;33:134–140. doi: 10.1038/sj.npp.1301569. [DOI] [PubMed] [Google Scholar]
- 76.Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, Kazantsev M, Wilson CJ, Rendon T, Hsiao EY. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586:281–286. doi: 10.1038/s41586-020-2745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Surzenko N, Pjetri E, Munson CA, Friday WB, Hauser J, Mitchell ES. Prenatal exposure to the probiotic Lactococcus lactis decreases anxiety-like behavior and modulates cortical cytoarchitecture in a sex specific manner. PLoS ONE. 2020;15:e0223395. doi: 10.1371/journal.pone.0223395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slykerman RF, Kang J, Van Zyl N, et al. Effect of early probiotic supplementation on childhood cognition, behaviour and mood a randomised, placebo-controlled trial. Acta Paediatr. 2018;107:2172–2178. doi: 10.1111/apa.14590. [DOI] [PubMed] [Google Scholar]
- 79.•• Radford-Smith DE, Probert F, Burnet PWJ, Anthony DC. Modifying the maternal microbiota alters the gut–brain metabolome and prevents emotional dysfunction in the adult offspring of obese dams. Proc Natl Acad Sci USA. 2022;119:e2108581119. One of the few papers connecting gut microbiota, metabolic syndrome, and brain function and the first to target this tripartite interaction in the context of DOHaD and the use probiotics as preventative therapeutics. [DOI] [PMC free article] [PubMed]
- 80.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terada-Ikeda C, Kitabatake M, Hiraku A, Kato K, Yasui S, Imakita N, Ouji-Sageshima N, Iwabuchi N, Hamada K, Ito T. Maternal supplementation with Bifidobacterium breve M-16V prevents their offspring from allergic airway inflammation accelerated by the prenatal exposure to an air pollutant aerosol. PLoS ONE. 2020;15:e0238923. doi: 10.1371/journal.pone.0238923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Y, Qi C, Li X, et al. Prevention of atopic dermatitis in mice by Lactobacillus reuteri Fn041 through induction of regulatory T cells and modulation of the gut microbiota. Mol Nutr Food Res. 2022;66:2100699. doi: 10.1002/mnfr.202100699. [DOI] [PubMed] [Google Scholar]
- 83.Avershina E, Cabrera Rubio R, Lundgård K, Perez Martinez G, Collado MC, Storrø O, Øien T, Dotterud CK, Johnsen R, Rudi K. Effect of probiotics in prevention of atopic dermatitis is dependent on the intrinsic microbiota at early infancy. J Allergy Clin Immunol. 2017;139:1399–1402. doi: 10.1016/j.jaci.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 84.Rø ADB, Simpson MR, Rø TB, Storrø O, Johnsen R, Videm V, Øien T. Reduced Th22 cell proportion and prevention of atopic dermatitis in infants following maternal probiotic supplementation. Clin Exp Allergy. 2017;47:1014–1021. doi: 10.1111/cea.12930. [DOI] [PubMed] [Google Scholar]
- 85.Forsberg A, Huoman J, Söderholm S, Bhai Mehta R, Nilsson L, Abrahamsson TR, Ernerudh J, Gustafsson M, Jenmalm MC. Pre- and postnatal Lactobacillus reuteri treatment alters DNA methylation of infant T helper cells. Pediatr Allergy Immunol. 2020;31:544–553. doi: 10.1111/pai.13240. [DOI] [PubMed] [Google Scholar]
- 86.Davies G, Jordan S, Brooks CJ, Thayer D, Storey M, Morgan G, Allen S, Garaiova I, Plummer S, Gravenor M. Long term extension of a randomised controlled trial of probiotics using electronic health records. Sci Rep. 2018;8:7668. doi: 10.1038/s41598-018-25954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wickens K, Barthow C, Mitchell EA, Kang J, van Zyl N, Purdie G, Stanley T, Fitzharris P, Murphy R, Crane J. Effects of Lactobacillus rhamnosus HN001 in early life on the cumulative prevalence of allergic disease to 11 years. Pediatr Allergy Immunol. 2018;29:808–814. doi: 10.1111/pai.12982. [DOI] [PubMed] [Google Scholar]
- 88.Peldan P, Kukkonen AK, Savilahti E, Kuitunen M. Perinatal probiotics decreased eczema up to 10 years of age, but at 5–10 years, allergic rhino-conjunctivitis was increased. Clin Exp Allergy. 2017;47:975–979. doi: 10.1111/cea.12924. [DOI] [PubMed] [Google Scholar]
- 89.Kallio S, Kukkonen AK, Savilahti E, Kuitunen M. Perinatal probiotic intervention prevented allergic disease in a Caesarean-delivered subgroup at 13-year follow-up. Clin Exp Allergy. 2019;49:506–515. doi: 10.1111/cea.13321. [DOI] [PubMed] [Google Scholar]
- 90.• Wickens K, Barthow C, Mitchell EA, et al. Maternal supplementation alone with Lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatr Allergy Immunol. 2018;29:296–302. This paper contrasts administration of probiotics during pregnancy and breastfeeding to mother only versus mothers and their offspring, thus contributing essential knowledge to determine optimal probiotic administration protocols to program specific offspring outcomes. [DOI] [PubMed]
- 91.de Gusmão Correia ML, Volpato AM, Águila MB, Mandarim-de-Lacerda CA. Developmental origins of health and disease: experimental and human evidence of fetal programming for metabolic syndrome. J Hum Hypertens. 2012;26:405–419. doi: 10.1038/jhh.2011.61. [DOI] [PubMed] [Google Scholar]
- 92.Callaway LK, McIntyre HD, Barrett HL, et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care. 2019;42:364–371. doi: 10.2337/dc18-2248. [DOI] [PubMed] [Google Scholar]
- 93.Guo Y, Wang Z, Chen L, Tang L, Wen S, Liu Y, Yuan J. Diet induced maternal obesity affects offspring gut microbiota and persists into young adulthood. Food Funct. 2018;9:4317–4327. doi: 10.1039/C8FO00444G. [DOI] [PubMed] [Google Scholar]
- 94.Guimarães KSDL, Braga VDA, Noronha SISRD, et al. Lactiplantibacillus plantarum WJL administration during pregnancy and lactation improves lipid profile, insulin sensitivity and gut microbiota diversity in dyslipidemic dams and protects male offspring against cardiovascular dysfunction in later life. Food Funct. 2020;11:8939–8950. doi: 10.1039/D0FO01718C. [DOI] [PubMed] [Google Scholar]
- 95.Hsu C-N, Lin Y-J, Hou C-Y, Tain Y-L. Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients. 2018;10:E1229. doi: 10.3390/nu10091229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hsu C-N, Hou C-Y, Chan JYH, Lee C-T, Tain Y-L. hypertension programmed by perinatal high-fat diet: effect of maternal gut microbiota-targeted therapy. Nutrients. 2019;11:2908. doi: 10.3390/nu11122908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grech A, Collins CE, Holmes A, Lal R, Duncanson K, Taylor R, Gordon A. Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis. Gut Microbes. 2021;13:1897210. doi: 10.1080/19490976.2021.1897210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qi Y, Yu L, Tian F, Zhao J, Zhang H, Chen W, Zhai Q. A. muciniphila supplementation in mice during pregnancy and lactation affects the maternal intestinal microenvironment. Nutrients. 2022;14:390. doi: 10.3390/nu14020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu L, Zeng D, Yang M, et al. Probiotic Clostridium butyricum improves the growth performance, immune function, and gut microbiota of weaning rex rabbits. Probiotics & Antimicro Prot. 2019;11:1278–1292. doi: 10.1007/s12602-018-9476-x. [DOI] [PubMed] [Google Scholar]
- 100.Xiao J, Wang T, Xu Y, Gu X, Li D, Niu K, Wang T, Zhao J, Zhou R, Wang H-L. Long-term probiotic intervention mitigates memory dysfunction through a novel H3K27me3-based mechanism in lead-exposed rats. Transl Psychiatry. 2020;10:25. doi: 10.1038/s41398-020-0719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Korpela K, Salonen A, Vepsäläinen O, et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome. 2018;6:182. doi: 10.1186/s40168-018-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen J, Li H, Hird SM, Chen M-H, Xu W, Maas K, Cong X. Sex differences in gut microbial development of preterm infant twins in early life: a longitudinal analysis. Front Cell Infect Microbiol. 2021;11:671074. doi: 10.3389/fcimb.2021.671074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, Lusis AJ. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7:313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong JMW, de Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 105.Yang S, Li X, Yang F, et al. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol. 2019;10:1360. doi: 10.3389/fphar.2019.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.The ARRIVE guidelines 2.0. In: ARRIVE Guidelines. https://arriveguidelines.org/arrive-guidelines. Accessed 14 Oct 2021
- 107.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJP. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–335. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martin E, Smeester L, Bommarito PA, Grace MR, Boggess K, Kuban K, Karagas MR, Marsit CJ, O’Shea TM, Fry RC. Sexual epigenetic dimorphism in the human placenta: implications for susceptibility during the prenatal period. Epigenomics. 2017;9:267–278. doi: 10.2217/epi-2016-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.• Huoman J, Martínez-Enguita D, Olsson E, Ernerudh J, Nilsson L, Duchén K, Gustafsson M, Jenmalm MC. Combined prenatal Lactobacillus reuteri and ω-3 supplementation synergistically modulates DNA methylation in neonatal T helper cells. Clin Epigenetics. 2021;13:135. This paper shows that omega-3 fatty acids and L. reuteri have a synergetic effect on DNA methylation in neonates, suggesting that co-administration of probiotics with other supplements may be a strategy to amplify effects. [DOI] [PMC free article] [PubMed]
- 110.Cruz LX, Hirsch CD, de Moura MQ, de Avila LFC, Martins LHR, Klafke GB, Conceição FR, Berne MEA, Scaini CJ. Saccharomyces boulardii reduces the vertical transmission of Toxocara canis larvae in mice. J Helminthol. 2021;95:e11. doi: 10.1017/S0022149X20001030. [DOI] [PubMed] [Google Scholar]
- 111.Menegat MB, DeRouchey JM, Woodworth JC, Dritz SS, Tokach MD, Goodband RD. Effects of Bacillus subtilis C-3102 on sow and progeny performance, fecal consistency, and fecal microbes during gestation, lactation, and nursery periods. J Anim Sci. 2019;97:3920–3937. doi: 10.1093/jas/skz236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Melandri M, Aiudi GG, Caira M, Alonge S. A Biotic Support During Pregnancy to Strengthen the Gastrointestinal Performance in Puppies. Front Vet Sci. 2020;7:417. doi: 10.3389/fvets.2020.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu J, Lu L, Yu Y, Baranowski J, Claud EC. Maternal administration of probiotics promotes brain development and protects offspring’s brain from postnatal inflammatory insults in C57/BL6J mice. Sci Rep. 2020;10:8178. doi: 10.1038/s41598-020-65180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kar F, Hacioglu C, Kar E, Donmez DB, Kanbak G. Probiotics ameliorates LPS induced neuroinflammation injury on Aβ 1–42, APP, γ-β secretase and BDNF levels in maternal gut microbiota and fetal neurodevelopment processes. Metab Brain Dis. 2022 doi: 10.1007/s11011-022-00964-z. [DOI] [PubMed] [Google Scholar]
- 115.Godfrey KM, Barton SJ, El-Heis S, Kenealy T, Nield H, Baker PN, Chong YS, Cutfield W, Chan S-Y. Myo-inositol, probiotics, and micronutrient supplementation from preconception for glycemia in pregnancy: NiPPeR international multicenter double-blind randomized controlled trial. Diabetes Care. 2021;44:1091–1099. doi: 10.2337/dc20-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mantaring J, Benyacoub J, Destura R, Pecquet S, Vidal K, Volger S, Guinto V. Effect of maternal supplement beverage with and without probiotics during pregnancy and lactation on maternal and infant health: a randomized controlled trial in the Philippines. BMC Pregnancy Childbirth. 2018;18:193. doi: 10.1186/s12884-018-1828-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schei K, Avershina E, Øien T, Rudi K, Follestad T, Salamati S, Ødegård RA. Early gut mycobiota and mother-offspring transfer. Microbiome. 2017;5:107. doi: 10.1186/s40168-017-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dewanto NEF, Firmansyah A, Sungkar A, Dharmasetiawani N, Sastroasmoro S, Kresno SB, Suradi R, Bardosono S, Prasetyo D. The effect of Bifidobacterium animalis lactis HNO19 supplementation among pregnant and lactating women on interleukin-8 level in breast milk and infant’s gut mucosal integrity. Medical Journal of Indonesia. 2017;26:204–211. doi: 10.13181/mji.v26i3.1481. [DOI] [Google Scholar]
- 119.Forsberg A, Abrahamsson TR, Nilsson L, Ernerudh J, Duchén K, Jenmalm MC. Changes in peripheral immune populations during pregnancy and modulation by probiotics and ω-3 fatty acids. Sci Rep. 2020;10:18723. doi: 10.1038/s41598-020-75312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pellonperä O, Mokkala K, Houttu N, Vahlberg T, Koivuniemi E, Tertti K, Rönnemaa T, Laitinen K. Efficacy of fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: a randomized, placebo-controlled, double-blind clinical trial. Diabetes Care. 2019;42:1009–1017. doi: 10.2337/dc18-2591. [DOI] [PubMed] [Google Scholar]
- 121.Sahhaf Ebrahimi F, Homayouni Rad A, Mosen M, Abbasalizadeh F, Tabrizi A, Khalili L. Effect of L. acidophilus and B. lactis on blood glucose in women with gestational diabetes mellitus: a randomized placebo-controlled trial. Diabetol Metab Syndr. 2019;11:75. doi: 10.1186/s13098-019-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]