Abstract
Intrauterine exposure to metabolic dysfunction leads to offspring metabolic dysfunction in human and rodent models, but underlying mechanisms are unclear. The mediobasal hypothalamus (MBH) is involved in energy homeostasis and weight regulation, and MBH gliosis is associated with obesity and insulin resistance. We tested the hypothesis that offspring exposed to gestational diabetes mellitus (GDM) in utero versus those unexposed would show evidence of MBH gliosis. Participants in the BrainChild Study (age 7–11 years with confirmed GDM exposure or no GDM exposure) underwent brain MRI to acquire T2-weighted images. By using the amygdala (AMY) and white matter (WM) as reference regions, MBH:AMY and MBH:WM T2 signal ratios were calculated as a radiologic measure of MBH gliosis. Linear regressions were used to examine associations between GDM exposure (GDM overall) and by timing of GDM exposure (≤26 weeks or >26 weeks) and MBH gliosis. Associations between prepregnancy BMI and child MBH gliosis were examined in secondary analyses. There were no differences in T2 signal ratios in children exposed versus not exposed to GDM overall, but children exposed to early GDM (≤26 weeks of gestation) had higher MBH:WM signal ratios than those not exposed (β = 0.147; SE 0.06; P = 0.03), adjusting for child’s age, sex, and BMI z score and maternal prepregnancy BMI, whereas no associations were seen for the control ratio (AMY:WM). Prepregnancy BMI was not associated with evidence of MBH gliosis. Early exposure to GDM was associated with radiologic evidence of MBH gliosis in children. These data provide mechanistic insight into brain pathways by which exposure to GDM may increase risk for metabolic dysfunction.
Introduction
Exposure to gestational diabetes (GDM) in utero increases the risk for childhood obesity (1). Animal models have demonstrated that maternal GDM increases offspring adiposity, metabolic dysfunction, and disordered feeding behaviors (2,3). Human studies have established associations between maternal GDM in utero and reduced insulin sensitivity at birth (4). Preclinical studies have suggested neural mechanisms whereby maternal GDM might negatively impact offspring health. In rodents, intrauterine exposure to maternal GDM or obesity causes abnormal development of hypothalamic circuitry and function, leading to obesity later in life (5). Offspring of diabetic dams display abnormally organized hypothalamic feeding pathways (6), and offspring of dams with obesity demonstrate upregulation of endoplasmic stress regulation genes and disruption of the melanocortin circuits, resulting in neonatal leptin resistance (7). In nonhuman primates, offspring of mothers fed a high-fat diet while pregnant exhibit developmental alterations in hypothalamic control of energy homeostasis, as well as an increase in activated hypothalamic microglia (8,9). In humans, children exposed to maternal GDM by 26 weeks of gestation demonstrate greater orbitofrontal cortex food cue reactivity, increased energy intake, and increased waist-to-height ratios (10) as well as altered hypothalamic response to glucose, predicting greater future weight gain (11). These findings suggest that maternal GDM alters tissue structure or composition in fetal brain, specifically in the hypothalamus, with consequences for brain function and risk for metabolic disorders.
The mediobasal hypothalamus (MBH) is a key region in of the brain for regulation of energy balance. In animal models and humans, gliosis, or reactive inflammatory changes in the glia, occur in the MBH and are associated with obesity and insulin resistance (12–17). Hypothalamic gliosis can be assessed by MRI (18,19) and is observed in children and adults with obesity and type 2 diabetes (16,19,20). We examined associations between exposure to GDM diagnosed at ≤26 or >26 weeks of gestation or no GDM exposure on MBH gliosis in children aged 7–11 years. We hypothesized that children exposed to GDM in utero would show evidence of MBH gliosis compared with unexposed children. We also examined whether maternal BMI is associated with MBH gliosis.
Research Design and Methods
Participants
The BrainChild Study is a prospective cohort study recruiting healthy children between 7 and 11 years of age born at Kaiser Permanente Southern California hospital with documented exposure to maternal GDM or no GDM exposure during the index pregnancy (11). Children who were born to mothers with pre-DM, used medications known to alter metabolism, or did not have a brain scan were excluded (10).
Exposure
GDM diagnosis was confirmed from the electronic medical record (EMR) on the basis of 1) plasma glucose ≥200 mg/dL from a 50-g glucose challenge test or 2) two or more elevated values on a 100-g or 75-g oral glucose tolerance test (fasting ≥95 mg/dL, 1 h ≥180 mg/dL, 2 h ≥155 mg/dL, 3 h ≥140 mg/dL) (21). Gestational age at GDM diagnosis was calculated using the date of the glucose test result that met GDM diagnosis criteria, date of delivery, and gestational age at delivery per the EMR. Mothers’ prepregnancy BMI was calculated from documented maternal height and weight measurements in the EMR obtained closest to the date of their last menstrual period.
Neuroimaging Outcomes
After a 12-h overnight fast, T2-weighted, high-resolution axial images were acquired (slice thickness 3.47 mm) on a Siemens MAGNETOM Prisma Fit 3T MRI scanner with a 20-channel head coil. The slice of interest containing the MBH was identified visually by both raters (S.C. and S.M.) who were blinded to exposure of GDM versus no GDM. Regions of interest (ROIs) were drawn and matched in volume to our previous ROIs using OsiriX MD 12.0.1 image processing software. Once established, the same ROIs were placed for all participants. Reference regions were placed in the center of the amygdala (AMY) and in a homogenous white matter (WM) area lateral and slightly posterior to the AMY on the same slice. The MBH ROI was placed adjacent to the third ventricle (medially) and anteriorly at the optic chiasm split to encompass the arcuate nucleus. All ROIs were placed manually by two raters, a subset (n = 40) was used to assess interrater reliability (interclass correlations were 98% for left and right AMY, 97% left WM, 87% right WM, and 90% and 93% for left and right MBH, respectively; all P < 0.0001).
T2 signal ratios were calculated as the bilateral mean from left and right T2 signal ratios and included MBH:AMY and MBH:WM (ratios of interest) and AMY:WM (control ratio). The MBH T2 signal was measured through two T2 signal ratios: MBH:AMY, a gray matter (AMY) reference, and MBH:WM, a white matter (WM) reference, which would be informative if gray matter changes were widespread (19). Higher MBH:AMY or MBH:WM T2 signal ratios indicate higher MBH T2 signal, correlating with the extent of gliosis on histology (13,19,22). Previously, we have evaluated MBH T2 signal through T2 signal ratios (19).
Statistical Analysis
Linear regression models were used to examine relationships between exposure to GDM and evidence of MBH gliosis on the basis of the T2 signal ratio. The initial model was unadjusted (model 0), then adjusted for the child’s age and sex (model 1), followed by maternal prepregnancy BMI (model 2), and finally by the child’s BMI z score (model 3). As >90% of children were prepubertal, we did not control for puberty status in the models. GDM exposure was modeled in two ways: 1) yes or no exposure to GDM and 2) GDM diagnosed at ≤26 weeks of gestation (GDM ≤26 group), GDM diagnosed at >26 weeks of gestation (GDM >26 group), and no GDM (no GDM group). These temporal cutoff values for GDM were based on previous studies from our group showing associations between GDM ≤26 and altered hypothalamic function and eating behavior (10,11).
We also explored associations between maternal prepregnancy BMI and childhood MBH gliosis, adjusting for covariates as noted above for models 0 and 1 and adjusting for GDM exposure (model 2) and then GDM and the child’s BMI z score (model 3). Maternal prepregnancy BMI was modeled as a continuous variable (scaled in units of 5). All analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC).
Data and Resource Availability
Data and resources are available upon request.
Results
Participant Characteristics
A total of 159 children were enrolled, of whom 122 had usable MRI data and were included in the analyses. Demographic data by group are presented in Table 1, and mean MBH:AMY, MBH:WM, and AMY:WM T2 signal ratios are shown in Table 2.
Table 1.
Demographic data
| No GDM group (n = 51) | GDM >26 group (n = 47) | GDM ≤26 group (n = 24) | Total (N = 122) | |
|---|---|---|---|---|
| Maternal prepregnancy BMI, n (%) | ||||
| Normal (<25 kg/m2) | 11 (21.6) | 14 (29.8) | 2 (8.3) | 27 (22.1) |
| Overweight (≥25 and <30 kg/m2) | 14 (27.5) | 20 (42.6) | 5 (20.8) | 39 (32) |
| Obesity (≥30 kg/m2) | 26 (51) | 13 (27.7) | 17 (70.8) | 56 (45.9) |
| Age (years), mean (SD) | 9.0 (1.14) | 8.7 (1.15) | 8.6 (1.31) | 8.8 (1.17) |
| Sex, n (%) | ||||
| Male | 24 (47.1) | 18 (38.3) | 10 (41.7) | 52 (42.6) |
| Female | 27 (52.9) | 29 (61.7) | 14 (58.3) | 70 (57.4) |
| Tanner staging, n (%) | ||||
| 1 | 44 (86.3) | 38 (80.9) | 20 (83.3) | 102 (83.6) |
| 2 | 4 (7.8) | 6 (12.8) | 4 (16.7) | 14 (11.5) |
| 3 | 2 (3.9) | 2 (4.3) | 0 (0) | 4 (3.3) |
| 4 | 1 (2) | 1 (2.1) | 0 (0) | 2 (1.6) |
| BMI (kg/m2), mean (SD) | 19.3 (3.70) | 20.2 (5.52) | 19.4 (4.12) | 19.7 (4.55) |
| BMI z score, mean (SD) | 0.8 (1.02) | 0.9 (1.31) | 0.9 (0.96) | 0.9 (1.12) |
| Body fat (%), mean (SD) | 25.0 (8.07) | 27.1 (11.05) | 25.9 (8.13) | 26.0 (9.32) |
| Waist-to-hip ratio, mean (SD) | 0.9 (0.06) | 0.9 (0.06) | 0.9 (0.06) | 0.9 (0.06) |
Table 2.
T2 signal ratios
| Mean (95%CI) | |||
|---|---|---|---|
| T2 signal ratio | GDM ≤26 group (n = 24) | GDM >26 group (n = 47) | No GDM group (n = 51) |
| MBH:AMY | 1.25 (1.18–1.32) | 1.18 (1.14–1.23) | 1.19 (1.15–1.22) |
| MBH:WM | 2.25 (2.11–2.38) | 2.13 (2.06–2.20) | 2.11 (2.05–2.18) |
| AMY:WM | 1.80 (1.75–1.86) | 1.80 (1.77–1.84) | 1.78 (1.75–1.81) |
Higher MBH:AMY or MBH:WM T2 signal ratios signify evidence of gliosis.
In Utero Exposure to GDM and Childhood MBH Gliosis
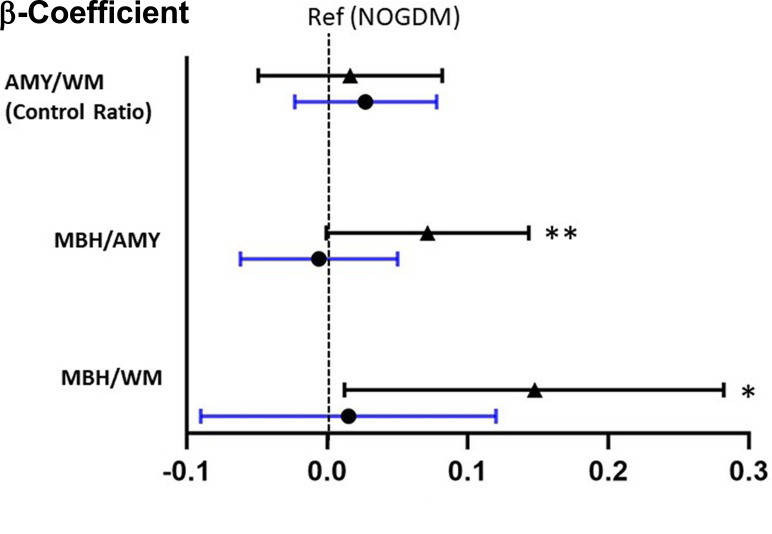
There were no significant differences in mean MBH:AMY or MBH:WM T2 signal ratios between the GDM and no GDM groups (Supplementary Table 1). Considering the GDM ≤26 and GDM >26 groups versus the no GDM group, all models demonstrated significantly higher MBH:WM T2 signal ratios in the GDM ≤26 group compared with the no GDM group, and strong trends were seen for the MBH:AMY T2 signal ratio measure (Table 3). Figure 1 depicts the magnitudes, 95% CIs, and significance of differences in adjusted β-coefficients (model 3) among the three groups, demonstrating higher MBH:AMY and MBH:WM T2 signal ratios only in the GDM ≤26 group.
Table 3.
Comparisons in T2 signal ratios between the GDM ≤26 and GDM >26 groups versus the no GDM group
| GDM ≤26 group | GDM >26 group | |||
|---|---|---|---|---|
| Ratio and model | β-Coefficient (SE) | P | β-Coefficient (SE) | P |
| MBH:AMY | ||||
| 0 | 0.0626 (0.0338) | 0.07*** | −0.0044 (0.0276) | 0.89 |
| 1 | 0.0641 (0.0342) | 0.06*** | −0.0042 (0.0279) | 0.88 |
| 2 | 0.0707 (0.036) | 0.05*** | −0.0055 (0.0281) | 0.84 |
| 3 | 0.0715 (0.0364) | 0.05*** | −0.0058 (0.0288) | 0.84 |
| MBH:WM | ||||
| 0 | 0.1337 (0.0634) | 0.04 | 0.014 (0.0518) | 0.79 |
| 1 | 0.1384 (0.0642) | 0.03 | 0.0167 (0.0525) | 0.75 |
| 2 | 0.1508 (0.0679) | 0.03 | 0.141 (0.0528) | 0.79 |
| 3 | 0.1477 (0.0683) | 0.03 | 0.0153 (0.053) | 0.77 |
| AMY:WM | ||||
| 0 | 0.0187 (0.0311) | 0.55 | 0.0227 (0.0254) | 0.37 |
| 1 | 0.021 (0.0313) | 0.50 | 0.0258 (0.0256) | 0.32 |
| 2 | 0.02 (0.0332) | 0.55 | 0.026 (0.0258) | 0.32 |
| 3 | 0.0164 (0.0332) | 0.62 | 0.0273 (0.0257) | 0.29 |
Model 0, no adjustment; model 1, adjusted for child’s age and sex; model 2: adjusted for child’s age and sex and maternal prepregnancy BMI; model 3, adjusted for child’s age and sex, maternal prepregnancy BMI, and child’s BMI z score.
Reference: no GDM group.
P < 0.05; value shows a trend toward significance.
Figure 1.
β-Coefficients between the GDM ≤26 (▲) and GDM >26 (•) groups compared with the no GDM group (NOGDM). All models were adjusted for child age, child BMI z score, child sex, and maternal prepregnancy BMI. *P < 0.05, **P = 0.05. Ref, reference.
In Utero Exposure to Prepregnancy BMI and Childhood MBH Gliosis
Exploratory analyses between maternal prepregnancy BMI in 5-unit increments and offspring MBH gliosis showed that prepregnancy BMI was not associated with higher T2 signal ratios for MBH:AMY or MBH:WM in unadjusted or adjusted models (Supplementary Table 2).
Discussion
With the use of MRI, we demonstrated that early intrauterine exposure to GDM was associated with higher MBH T2 signal ratios in 7–11-year-old offspring, a finding that was maintained even when controlling for potential confounders. Higher T2 signal intensity is a radiologic marker of gliosis (18,19), and findings were limited to the MBH region. We found statistically significant associations using the MBH:WM T2 signal ratio and strong positive trends using the MBH:AMY ratio, whereas control AMY:WM ratios did not differ by GDM exposure in any model. Thus, early exposure to GDM may promote MBH gliosis in childhood.
Gliosis is the pathognomonic cellular response of central nervous system tissue to injury and involves infiltration and activation of microglia and astrocytes. T2 signal changes in the MBH were initially correlated with histologically verified measures of reactive gliosis in mice exposed to a high-fat diet (18) and subsequently verified in humans through correlating histopathologic presence of MBH gliosis in postmortem human brains with MRI measures of T2 signal in the MBH (19). Using MRI, MBH gliosis has been found to be associated with obesity and insulin resistance in nonpregnant adults and children (13,15,20). In children, radiologic evidence of MBH gliosis is positively associated with BMI z score and predicts adiposity gain in overweight children at risk for obesity (23). Moreover, MBH gliosis predicts worsening insulin sensitivity in adults (15). These previous studies imply that the current findings of MBH gliosis in children exposed to GDM in utero could be related to a subsequent increased risk for metabolic disease.
The current findings were limited to children exposed to GDM early in pregnancy, suggesting that early exposure to GDM may be a critical factor driving the observed structural changes in the MBH of offspring. Clinically speaking, GDM diagnosis typically occurs between 24 and 28 weeks of gestation based on American College of Obstetricians and Gynecologists guidelines. That differences were seen between groups segregated at 26 weeks of gestation suggests that earlier exposure to glucose dysregulation has a different downstream impact on offspring versus a later exposure. We cannot distinguish whether a greater severity and/or timing of hyperglycemia in the early exposure group contributed to the offspring outcomes. Moreover, other gestational age cutoff values should be investigated to understand underlying implications that timing and severity of maternal glucose dysregulation poses upon offspring.
We recently reported findings from the BrainChild Study showing that children exposed to GDM before 26 weeks of gestation had increased hypothalamic blood flow (a marker of hypothalamic activation) in response to glucose that predicted greater future weight gain (11). Our current data demonstrate that children exposed to GDM in early gestation also have structural changes in tissue composition of the hypothalamus. Therefore, structural changes in the hypothalamus may underlie functional changes in the hypothalamic response to oral glucose observed in these same children; longitudinal studies are necessary to determine temporal relationships. Collectively, these findings provide evidence that both structural and functional components of the hypothalamus are altered after early maternal GDM exposure, strengthening the argument that exposure to glucose dysregulation during early gestation may affect long-term offspring metabolic health through effects on the development of neural circuits regulating energy balance.
Maternal obesity is also associated with metabolic abnormalities in offspring (2). In the current study, no relationships were found between maternal prepregnancy BMI and childhood MBH gliosis. These findings suggest that maternal obesity alone may not be the driver of subsequent MBH gliosis but may be acting in concert with glucose dysregulation and/or postnatal environmental factors.
Our study strengths include the ability to associate a quantifiable measure (MRI T2 signal ratios) in children with maternal pregnancy data documented in the EMR. As all women received prenatal care in the same system, testing for GDM was uniform for the group, strengthening exposure quality. However, other factors that could contribute to our findings include genetics, maternal gestational diet, and/or the child’s diet and lifestyle. Dietary exposures (both maternal and child) deserve further study, as data in nonhuman primates have demonstrated an association with maternal high-fat diet and offspring brain response to glucose (9). However, since we demonstrated MBH gliosis in offspring aged 7–11 years only among those with early intrauterine exposure to glucose dysregulation, this suggests that glucose effects supersede prenatal and early-life dietary factors. Finally, while previous data supporting increased T2 signal is associated with gliosis (18,19), T2 signal ratios are an indirect measure of gliosis.
In conclusion, our findings suggest that exposure to in utero GDM ≤26 weeks of gestation, is associated with higher T2 signal ratios in the MBH in childhood. Our study provides mechanistic insight into potential pathways by which exposure to GDM in utero may increase risks for long-term metabolic disease in offspring through an effect on the brain. Mechanisms whereby gliosis is stimulated in the setting of GDM and alternative pathways (e.g., insulin signaling, effects on gut microbiome) deserve further investigation, likely in preclinical studies. Finally, future human studies should include detailed maternal pregnancy dietary data, early childhood lifestyle data, and longitudinal follow-up to further elucidate the effects of the intrauterine environment on offspring hypothalamic gliosis and its role in metabolic disease development in childhood and beyond.
Article Information
Funding. This study was supported by National Institutes of Diabetes and Digestive and Kidney Diseases grants R01DK116858 (to K.A.P.), K24HL144917 (to E.A.S.), and P30DK035816 (to University of Washington Nutrition and Obesity Research Center).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.A.S. and K.A.P. were responsible for the study concept. S.M., E.A.S., and K.A.P. interpreted the data analysis. T.C., A.X., and K.A.P. conducted the statistical analyses. B.A. and K.A.P. assisted with data acquisition and analysis of images. S.C., S.M., E.A.S., L.W.O., and K.A.P. prepared the tables and figures and wrote portions of the manuscript.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21066211.
E.A.S. and K.A.P. were co-principal investigators for this study.
References
- 1. Kawasaki M, Arata N, Miyazaki C, et al. Obesity and abnormal glucose tolerance in offspring of diabetic mothers: a systematic review and meta-analysis. PLoS One 2018;13:e0190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun Y, Shen Z, Zhan Y, et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth 2020;20:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tam WH, Ma RC, Yang X, et al. Glucose intolerance and cardiometabolic risk in adolescents exposed to maternal gestational diabetes: a 15-year follow-up study. Diabetes Care 2010;33:1382–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo ZC, Delvin E, Fraser WD, et al. Maternal glucose tolerance in pregnancy affects fetal insulin sensitivity. Diabetes Care 2010;33:2055–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breton C. The hypothalamus-adipose axis is a key target of developmental programming by maternal nutritional manipulation. J Endocrinol 2013;216:R19–R31 [DOI] [PubMed] [Google Scholar]
- 6. Steculorum SM, Bouret SG. Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology 2011;152:4171–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park S, Jang A, Bouret SG. Maternal obesity-induced endoplasmic reticulum stress causes metabolic alterations and abnormal hypothalamic development in the offspring. PLoS Biol 2020;18:e3000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 2010;151:1622–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sullivan EL, Rivera HM, True CA, et al. Maternal and postnatal high-fat diet consumption programs energy balance and hypothalamic melanocortin signaling in nonhuman primate offspring. Am J Physiol Regul Integr Comp Physiol 2017;313:R169–R179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo S, Angelo BC, Chow T, et al. Associations between exposure to gestational diabetes mellitus in utero and daily energy intake, brain responses to food cues, and adiposity in children. Diabetes Care 2021;44:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Page KA, Luo S, Wang X, et al. Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain. Diabetes Care 2019;42:1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dorfman MD, Thaler JP. Hypothalamic inflammation and gliosis in obesity. Curr Opin Endocrinol Diabetes Obes 2015;22:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valdearcos M, Douglass JD, Robblee MM, et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab 2018;27:1356. [DOI] [PubMed] [Google Scholar]
- 15. Rosenbaum JL, Melhorn SJ, Schoen S, et al. Evidence that hypothalamic gliosis is related to impaired glucose homeostasis in adults with obesity. Diabetes Care 2022;45:416–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kreutzer C, Peters S, Schulte DM, et al. Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes 2017;66:2407–2415 [DOI] [PubMed] [Google Scholar]
- 17. Buckman LB, Thompson MM, Moreno HN, Ellacott KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol 2013;521:1322–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee D, Thaler JP, Berkseth KE, Melhorn SJ, Schwartz MW, Schur EA. Longer T(2) relaxation time is a marker of hypothalamic gliosis in mice with diet-induced obesity. Am J Physiol Endocrinol Metab 2013;304:E1245–E1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schur EA, Melhorn SJ, Oh SK, et al. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity (Silver Spring) 2015;23:2142–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sewaybricker LE, Schur EA, Melhorn SJ, et al. Initial evidence for hypothalamic gliosis in children with obesity by quantitative T2 MRI and implications for blood oxygen-level dependent response to glucose ingestion. Pediatr Obes 2019;14:e12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goyal A, Gupta Y, Singla R, Kalra S, Tandon N. American Diabetes Association “Standards of Medical Care-2020 for Gestational Diabetes Mellitus”: a critical appraisal. Diabetes Ther 2020;11:1639–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Valdearcos M, Myers MG Jr, Koliwad SK. Hypothalamic microglia as potential regulators of metabolic physiology. Nat Metab 2019;1:314–320 [DOI] [PubMed] [Google Scholar]
- 23. Sewaybricker LE, Kee S, Melhorn SJ, Schur EA. Greater radiologic evidence of hypothalamic gliosis predicts adiposity gain in children at risk for obesity. Obesity (Silver Spring) 2021;29:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]