Abstract
This study examined the incidence trends of new-onset type 1 and type 2 diabetes in children and adolescents in Florida before and during the coronavirus disease 2019 (COVID-19) pandemic. In this observational descriptive cohort study, we used a validated computable phenotype to identify incident diabetes cases among individuals <18 years of age in the OneFlorida+ network of the national Patient-Centered Clinical Research Network between January 2017 and June 2021. We conducted an interrupted time series analysis based on the autoregressive integrated moving average model to compare changes in age-adjusted incidence rates of type 1 and type 2 diabetes before and after March 2020, when COVID-19 was declared a national health emergency in the U.S. The age-adjusted incidence rates of both type 1 and type 2 diabetes increased post–COVID-19 for children and adolescents. These results highlight the need for longitudinal cohort studies to examine how the pandemic might influence subsequent diabetes onset in young individuals.
Introduction
It is well-documented that individuals with diabetes have elevated risk for severe coronavirus disease 2019 (COVID-19) symptoms, hospital admission, and death (1,2). With respect to diabetes type and age, these increased risks appear primarily limited to adults with obesity and type 2 diabetes or established type 1 diabetes, with a suggestion of adverse events in younger persons with type 2 diabetes, but not children with type 1 diabetes (3). Additionally, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can lead to hyperglycemia in people without a history of diabetes potentially through decreased insulin secretion, enhanced release of counterregulatory hormones, increased insulin resistance, and/or impaired glucose disposal (4,5). However, controversies exist as to whether diabetes-related comorbidities are responsible for these observations, if a SARS-CoV-2–related diagnosis of diabetes is transient or durable, and whether direct infection of insulin-producing islet β-cells occurs with this virus (6). In an effort to address some of these knowledge voids, we examined the incidence trends of new-onset type 1 and type 2 diabetes in children and adolescents (i.e., those <18 years) in the state of Florida before and after March 2020, when the COVID-19 pandemic was declared a national health emergency in the U.S. (7), using real-world data from a large clinical research network in the national Patient-Centered Clinical Research Network. With this background, we hypothesized that the incidence of type 1 and type 2 diabetes increased during the COVID-19 pandemic.
Research Design and Methods
Study Design and Population
We obtained de-identified data between January 2017 and June 2021 from the OneFlorida+ network, one of the nine Patient-Centered Clinical Research Networks in the U.S. that contains robust longitudinal and linked patient records from various sources, including electronic health records and claims (8). Hogan et al. (8) provides a summary of sociodemographic characteristics of patients in the OneFlorida+ network, which contains electronic health record data for >4.2 million children from a mixture of insured and uninsured households. For our particular effort using OneFlorida+, we identified patients <18 years with type 1 or type 2 diabetes based on a newly developed computable phenotype algorithm adapted from methods described in Zhong et al. (9). First, patients were included if any of the following criteria were satisfied: 1) hemoglobin A1c level ≥6.5%, 2) fasting glucose level ≥126 mg/dL, 3) random plasma glucose level ≥200 mg/dL, 4) at least one diabetes diagnosis (excluding gestational diabetes) based on ICD-9-CM and ICD-10-CM codes, and/or 5) at least one prescription of a diabetes-related medication (e.g., insulin, metformin, or sulfonylureas). The index date of diabetes was defined as that of the earliest satisfied criterion. Next, patients who had no type 1 or type 2 diabetes diagnosis codes were removed. Patients with only type 1 diabetes diagnosis codes were classified as patients with type 1 diabetes. Individuals for whom the number of type 1 diabetes diagnosis codes was more than the type 2 diabetes diagnosis codes were also classified as patients with type 1 diabetes. The remainder were categorized as patients with type 2 diabetes. Demographic information is summarized in Table 1 for the children and adolescents who met the study’s inclusion criteria.
Table 1.
Race and ethnicity in the cohort evaluated from the OneFlorida+ Data Trust
| Type 1 diabetes | Type 2 diabetes | |
|---|---|---|
| Sample size (N) | 5,795 | 2,705 |
| Mean age (years) | 11.0 (4.1) | 12.5 (4.0) |
| Sex, n (%) | ||
| Female | 2,820 (48.7) | 1,631 (60.3) |
| Male | 2,975 (51.3) | 1,074 (39.7) |
| Race, n (%) | ||
| White | 3,692 (63.7) | 1,156 (42.7) |
| Black | 1,040 (17.9) | 1,133 (41.9) |
| Other | 703 (12.1) | 295 (10.9) |
| Unknown | 360 (6.2) | 121 (4.5) |
| Ethnicity, n (%) | ||
| Hispanic | 1,925 (33.2) | 789 (29.2) |
| Non-Hispanic | 3,413 (58.9) | 1,804 (66.7) |
| Other | 170 (2.9) | 47 (1.8) |
| Unknown | 287 (5.0) | 65 (2.3) |
Measures and Outcomes
We identified incident diabetes cases and calculated diabetes incidence rates following recommendations of Rassen et al. (10). Patients with diabetes were required to have at least 1 year of an observable period in OneFlorida+ prior to the index date to qualify as an incident case. Type 1 and type 2 diabetes incidence rates, with the corresponding CIs, were reported by quarter from January 2017 to June 2021 and calculated as the number of incident patients with diabetes in a quarter divided by the number of patients without diabetes in OneFlorida+ in the prior year. Each time point represents a cross-section of the OneFlorida+ population, accounting for individuals moving in and out of the cohort. All incidence rates were age-adjusted to the 2010 U.S. standard population and expressed as per 100,000 individuals (10). To quantify the effect of the COVID-19 pandemic on diabetes incidence rates, separate autoregressive integrated moving average (ARIMA) models were fit to test whether the quarterly type 1 and type 2 diabetes incidence increased during the COVID-19 pandemic. Fit indexes including the Akaike information criterion, Schwarz Bayesian criterion, R2, and absolute model error were used to choose the best model. Data analysis was performed using the Python and R programming languages.
Data and Resource Availability
The data generated and analyzed during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
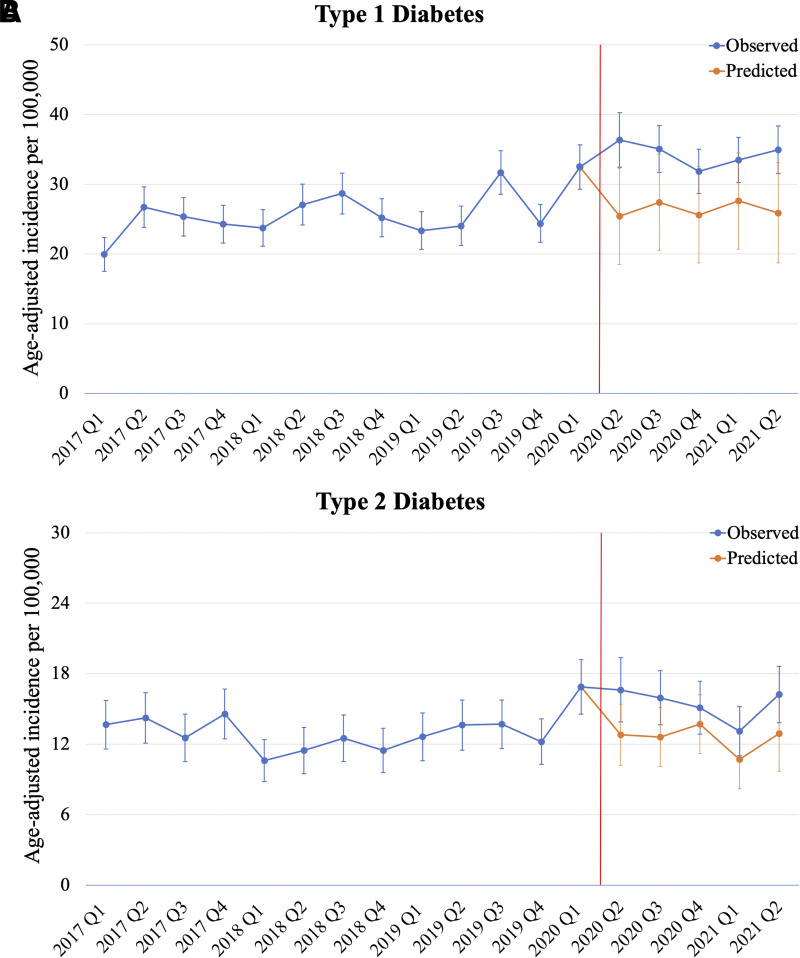
The quarterly counts and incidence rates of type 1 and type 2 diabetes in children and adolescents in OneFlorida+ between January 2017 and June 2021 are summarized in Table 2. A total of 5,795 and 2,705 children and adolescents were determined to have type 1 and type 2 diabetes, respectively, in this time span. The quarterly type 1 diabetes incidence rates ranged between 19.9 and 32.5/100,000 prior to the COVID-19 pandemic but increased to between 31.8 and 36.3/100,000 after March 2020. In contrast, the quarterly type 2 diabetes incidence rates ranged between 10.6 and 14.6/100,000 prior to the COVID-19 pandemic, then increased substantially to 16.9/100,000 in the first quarter of 2020, and plateaued thereafter, ranging from 13.1 to 16.6/100,000 after March 2020. However, results from interrupted ARIMA models (Fig. 1) show that the observed incidence rates (blue line) of both type 1 and type 2 diabetes were significantly higher than the corresponding ARIMA model–predicted incidence rates (orange line) after March 2020. Indeed, the change in incidence trends was significant for type 1 (coefficient 9.45; P = 0.0014) and type 2 diabetes (coefficient 4.30; P = 0.0088) before and after COVID-19 was declared a national health emergency in the U.S. (7).
Table 2.
Quarterly diabetes counts and incidence rates in children between January 2017 and June 2021 in the OneFlorida+ network
| Year and quarter | Individuals <18 years of age in the OneFlorida+ Network (N) | Type 1 diabetes | Type 2 diabetes | ||
|---|---|---|---|---|---|
| Count | Age-adjusted Incidence | Count | Age-adjusted Incidence | ||
| 2017 Q1 | 1,397,990 | 260 | 19.9 | 168 | 13.7 |
| 2017 Q2 | 1,351,036 | 330 | 26.7 | 168 | 14.2 |
| 2017 Q3 | 1,366,412 | 320 | 25.3 | 148 | 12.5 |
| 2017 Q4 | 1,407,369 | 310 | 24.3 | 182 | 14.6 |
| 2018 Q1 | 1,427,654 | 312 | 23.7 | 135 | 10.6 |
| 2018 Q2 | 1,320,581 | 332 | 27.1 | 131 | 11.5 |
| 2018 Q3 | 1,383,578 | 368 | 28.7 | 154 | 12.5 |
| 2018 Q4 | 1,400,667 | 328 | 25.2 | 141 | 11.5 |
| 2019 Q1 | 1,319,126 | 286 | 23.3 | 148 | 12.6 |
| 2019 Q2 | 1,293,809 | 283 | 24.0 | 155 | 13.6 |
| 2019 Q3 | 1,372,238 | 400 | 31.7 | 170 | 13.7 |
| 2019 Q4 | 1,381,352 | 314 | 24.4 | 152 | 12.2 |
| 2020 Q1 | 1,303,383 | 400 | 32.5 | 203 | 16.9 |
| 2020 Q2 | 946,004 | 330 | 36.3 | 142 | 16.6 |
| 2020 Q3 | 1,248,037 | 420 | 35.0 | 182 | 16.0 |
| 2020 Q4 | 1,271,211 | 391 | 31.8 | 174 | 15.1 |
| 2021 Q1 | 1,265,351 | 411 | 33.5 | 152 | 13.1 |
| 2021 Q2 | 1,192,435 | 402 | 34.9 | 176 | 16.2 |
| Total | 5,795 | 19.9 | 2,705 | 13.7 | |
Q, quarter.
Figure 1.
Interrupted time series plots of quarterly age-adjusted diabetes incidence rates in Florida. Type 1 (A) and type 2 diabetes (B) age-adjusted incidence rates before and during the COVID-19 pandemic, which was declared a U.S. national health emergency in March 2020 (7). The trends in blue are incidence rates observed in OneFlorida+, and the trends in orange are ARIMA model–predicted incidence rates. The change in incidence rates before and after March 2020 was significant for both type 1 (coefficient 9.45; P = 0.0014) and type 2 diabetes (coefficient 4.30; P = 0.0088). Q, quarter.
Discussion
To the best of our knowledge, the current study is among the first population-based studies to report a significant increase in the incidence of both type 1 and type 2 diabetes in U.S. children and adolescents during the COVID-19 pandemic. In the U.S., only a limited number of studies have focused on new-onset diabetes in the general pediatric population during COVID-19, reporting a significant increase in the number of cases (11–13), in agreement with our findings. While each of these efforts are key to understanding the impact of SARS-CoV-2 on diabetes, these studies were of small sample sizes and emphasized a population denominator (i.e., cases per 100,000). Further, our findings are in line with studies emanating from Romania and Germany (14,15), each of which reported a marked increase in the incidence of type 1 diabetes in children during the COVID-19 pandemic.
In settings of type 1 diabetes, it has been proposed by ourselves and others that SARS-CoV-2 infection can either act as a direct “trigger” for anti–β-cell immunity or, perhaps more likely in our view, in those at increased disease risk, a possible enhancement of preexisting islet autoimmunity, inflammation, and potentially stress-induced hyperglycemia, as seen with other infections, including the flu (6,14). In line with this latter notion, recently published data emanating from the U.S. and Germany do not support a link between SARS-CoV-2 infection and the development of islet autoimmunity that precedes type 1 diabetes (16). With respect to the observed increases in type 2 diabetes in children and adolescents, in this study, too, our presumption is that SARS-CoV-2 infection would impact the aforementioned metabolic processes and, particularly when combined with additional pandemic-related factors (e.g., school closings and increased sedentary lifestyle) (17), might precipitate disease in at-risk individuals. Due to unavailability of data on prior COVID-19 in our study population, it remains plausible that nonviral factors associated with the pandemic, such as stress, changes in diet, and reduced activity levels, could potentially have contributed toward the observed increase in diabetes onset in youth. Importantly, the incidence and prevalence of both forms of diabetes have been continually rising in children and adolescents for a number of decades (18,19); hence, factors related to the COVID-19 pandemic may be merely amplifying these existing trends.
Our study does possess some limitations, including the notion of several additional confounders that could have impacted the diabetes incidence trends. For example, we were unable to examine the history of SARS-CoV-2 infection among our study population. In Florida, recorded new cases of COVID-19 peaked in July 2020 and January 2021 (20). We suspect that the decline in type 2 diabetes incidence rates observed in 2021 quarter 1 may reflect individuals’ decisions to delay seeking health care during the COVID-19 surge. With this, our data show a rise in both forms of diabetes in the second quarter of 2021, which is generally in line with a recent report from the German Diabetes Prospective Follow-up Registry that demonstrated peaks in type 1 diabetes incidence ∼3 months after COVID-19 surges in a similar study time frame (15). In addition, we are aware of the potential for inaccuracies that can occur with ICD coding. However, we believe that overall, using standard disease-defining metrics across the years of this study, the potential impact of this latter facet on data analysis is reduced. Finally, there are open questions related to the impact, over time, of vaccination status. COVID-19 vaccines were not authorized by the U.S. Food and Drug Administration for use in children <12 years of age at any point during the time window examined. Vaccine emergency use authorization was granted first for individuals ≥16 years of age in December 2020 and then extended to those aged ≥12 years in May 2021 (21); thus, vaccination status is not expected to have influenced our analysis through 2021 quarter 2. In sum, results from our study highlight the need for longitudinal cohort studies that examine how the pandemic might influence subsequent diabetes onset.
Article Information
Funding. Y.G. and J.B. were funded in part by National Institutes of Health (NIH) National Cancer Institute grants 5R01CA246418, 3R01CA246418-02S1, 1R21CA245858-01A1, 3R21CA245858-01A1S1, and 1R21CA253394-01A1, NIH National Institute on Aging grant 5R21AG068717-02, and Centers for Disease Control and Prevention grant U18DP006512. This study was also supported by NIH grants to E.A.S. (RI-CRN-2020-005) and M.A.A. (U54 AI142766-03S1 and P01 AI042288).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.G., J.B., and M.A.A. conceived the study. Y.G. and A.C. performed all data analyses. Y.G. drafted the first version of the manuscript. All authors reviewed and edited the manuscript. J.B. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article is part of a special article collection available at diabetesjournals.org/journals/collection/43/Diabetes-and-COVID-19-Articles.
References
- 1. Hartmann-Boyce J, Rees K, Perring JC, et al. Risks of and from SARS-CoV-2 infection and COVID-19 in people with diabetes: a systematic review of reviews. Diabetes Care 2021;44:2790–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care 2021;44:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes 2021;22:202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reiterer M, Rajan M, Gómez-Banoy N, et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab 2021;33:2174–2188.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wan L, Gao Q, Deng Y, et al. GP73 is a glucogenic hormone contributing to SARS-CoV-2-induced hyperglycemia. Nat Metab 2022;4:29–43 [DOI] [PubMed] [Google Scholar]
- 6. Atkinson MA, Powers AC. Distinguishing the real from the hyperglycaemia: does COVID-19 induce diabetes? Lancet Diabetes Endocrinol 2021;9:328–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID-19) Outbreak . Federal Register, 18 March 2020. Accessed 26 August 2022. Available from https://www.federalregister.gov/documents/2020/03/18/2020-05794/declaring-a-national-emergency-concerning-the-novel-coronavirus-disease-covid-19-outbreak
- 8. Hogan WR, Shenkman EA, Robinson T, et al. The OneFlorida Data Trust: a centralized, translational research data infrastructure of statewide scope. J Am Med Inform Assoc 2022;29:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhong VW, Pfaff ER, Beavers DP, et al.; Search for Diabetes in Youth Study Group . Use of administrative and electronic health record data for development of automated algorithms for childhood diabetes case ascertainment and type classification: the SEARCH for Diabetes in Youth Study. Pediatr Diabetes 2014;15:573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rassen JA, Bartels DB, Schneeweiss S, Patrick AR, Murk W. Measuring prevalence and incidence of chronic conditions in claims and electronic health record databases. Clin Epidemiol 2018;11:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gottesman BL, Yu J, Tanaka C, Longhurst CA, Kim JJ. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr 2022;176:414–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolf RM, Noor N, Izquierdo R, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes 2022;23:433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chambers MA, Mecham C, Arreola EV, Sinha M. Increase in the number of pediatric new-onset diabetes and diabetic ketoacidosis cases during the COVID-19 pandemic. Endocr Pract 2022;28:479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vlad A, Serban V, Timar R, et al. Increased incidence of type 1 diabetes during the COVID-19 pandemic in Romanian children. Medicina (Kaunas) 2021;57:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamrath C, Rosenbauer J, Eckert AJ, et al. Incidence of type 1 diabetes in children and adolescents during the COVID-19 pandemic in Germany: results from the DPV Registry. Diabetes Care 2022;45:1762–1771 [DOI] [PubMed] [Google Scholar]
- 16. Rewers M, Bonifacio E, Ewald D, et al.; ASK Study Group and Fr1da Study Group . SARS-CoV-2 infections and presymptomatic type 1 diabetes autoimmunity in children and adolescents from Colorado, USA, and Bavaria, Germany. JAMA 2022;328:1252–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. An R. Projecting the impact of the coronavirus disease-2019 pandemic on childhood obesity in the United States: a microsimulation model. J Sport Health Sci 2020;9:302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths - selected counties and Indian reservations, United States, 2002-2015. MMWR Morb Mortal Wkly Rep 2020;69:161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawrence JM, Divers J, Isom S, et al.; SEARCH for Diabetes in Youth Study Group . Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA 2021;326:717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Florida Coronavirus Map and Case Count . The New York Times, 1 April 2020. Accessed 26 August 2022. Available from https://www.nytimes.com/interactive/2021/us/florida-covid-cases.html
- 21. U.S. Food and Drug Administration . FDA Approves First COVID-19 Vaccine, 23 August 2021. Accessed 28 May 2022. Available from https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine