Abstract
In our previous data-driven analysis of evolving patterns of islet autoantibodies (IAb) against insulin (IAA), GAD (GADA), and islet antigen 2 (IA-2A), we discovered three trajectories, characterized according to multiple IAb (TR1), IAA (TR2), or GADA (TR3) as the first appearing autoantibodies. Here we examined the evolution of IAb levels within these trajectories in 2,145 IAb-positive participants followed from early life and compared those who progressed to type 1 diabetes (n = 643) with those remaining undiagnosed (n = 1,502). With use of thresholds determined by 5-year diabetes risk, four levels were defined for each IAb and overlaid onto each visit. In diagnosed participants, high IAA levels were seen in TR1 and TR2 at ages <3 years, whereas IAA remained at lower levels in the undiagnosed. Proportions of dwell times (total duration of follow-up at a given level) at the four IAb levels differed between the diagnosed and undiagnosed for GADA and IA-2A in all three trajectories (P < 0.001), but for IAA dwell times differed only within TR2 (P < 0.05). Overall, undiagnosed participants more frequently had low IAb levels and later appearance of IAb than diagnosed participants. In conclusion, while it has long been appreciated that the number of autoantibodies is an important predictor of type 1 diabetes, consideration of autoantibody levels within the three autoimmune trajectories improved differentiation of IAb-positive children who progressed to type 1 diabetes from those who did not.
Introduction
Development of islet autoantibodies (IAb) precedes the clinical diagnosis of type 1 diabetes. The presence or absence (positivity/negativity) of IAb, age at appearance, and number of IAb are known to predict the risk of clinical disease (1,2). The longitudinal IAb patterns are, however, heterogeneous, and these patterns may reflect distinct disease subtypes and different pathways to clinical diagnosis (3–7).
Previous prospective studies following participants with increased genetic risk for type 1 diabetes have identified different initiation patterns of islet autoimmunity: insulin autoantibodies (IAA), antibodies against GAD65 (GADA), and antibodies against islet antigen 2 (IA-2A) as the first appearing IAb (8–10). IAA first or GADA first are two main patterns at initiation of islet autoimmunity and have been associated with DR4 and DR3 HLA haplotypes, respectively, and with different ages at first positivity. A third pattern is multiple IAb, most often both IAA and GADA, appearing simultaneously at seroconversion.
The Type 1 Diabetes Intelligence (T1DI) cohort has combined IAb data from five prospectively followed study cohorts, following a total of 24,662 unique participants (2). In our recent data-driven analyses using a Continuous-Time Hidden Markov Model (CT-HMM) and the presence or absence of IAA, GADA and/or IA-2A as well as age of observation, we discovered three main autoimmune trajectories: predominantly multiple IAb (TR1), IAA (TR2), or GADA (TR3) as the first appearing autoantibodies (11,12). Of note, each trajectory consisted of multiple component states that are manifested by distinct IAb probabilities and ages at event. For each trajectory the initial state is essentially autoantibody negative (e.g., TR2-0) and the following states are numbered sequentially and describe the evolution of autoantibody profile in that trajectory. For example, TR2-1 represents component state 1 of trajectory 2 (TR2) which predominantly includes children with a high probability of IAA as the first appearing IAb. Further, the trajectories were associated with varying ages at first IAb appearance as well as timing and overall risk of progression to type 1 diabetes.
Several studies have shown that beyond the presence or absence of the various IAb, the level of IAb plays an important role in prediction of type 1 diabetes (13–21). Further, there is heterogeneity among the IAb with regard to the association of antibody level and progression risk (15). To examine the role of IAb levels in the combined T1DI cohort, we previously harmonized IAb levels originally measured in the five T1DI studies. We used these harmonized IAb levels to effectively stratify 5 year progression to type 1 diabetes in this large multinational cohort (22).
Here, we sought to expand on previous observations to visualize and determine how autoantibody levels differ within the three trajectories and between those who have progressed to diabetes and those who have not. To refine the trajectories and their component states, we categorized the intensity of the antibody response of IAA, GADA, and IA-2A into four IAb-level groups (negative for IAb, L0; low positive IAb level, L1; medium positive IAb level, L2; and highest positive IAb level, L3) and analyzed the evolution of these IAb levels in each trajectory. Since most participants who develop autoimmunity follow one of the three trajectories, we specifically compared participants who were diagnosed with type 1 diabetes during the follow-up with those who remained undiagnosed at the end of their follow-up.
Research Design and Methods
Study Population and Trajectories
T1DI has combined data from 24,662 unique individuals who participated in five prospectively followed study cohorts, from Finland (Type 1 Diabetes Prevention and Prediction Study [DIPP]), Germany (BABYDIAB), Sweden (Diabetes Prediction in Skåne [DiPiS]), and the U.S. (Diabetes Autoimmunity Study in the Young [DAISY], Diabetes Evaluation in Washington [DEW-IT]) (2). Of the five original studies, DAISY, DEW-IT, DiPiS, and DIPP included HLA genotype as inclusion criterion in considering children with high-risk, moderate-risk, or specific lower-risk HLA genotype eligible for follow-up, as described in detail by Anand et al. (2). In addition, BABYDIAB and DAISY recruited newborns with first-degree relatives with type 1 diabetes for follow-up. From the T1DI cohort, we analyzed 2,145 participants (42,209 visits) who had two or more visits and any IAb positivity at least once (11,12,23). Supplementary Table 1 shows the number of samples by participants’ age, and Supplementary Table 2 presents the sampling intervals in the five prospective studies. In our previous analysis, we discovered three islet autoimmunity progression trajectories and their component states in a data-driven way using a CT-HMM. In that work, each trajectory was characterized according to the predominant autoantibody pattern observed in the first positive serum sample of the study participants as follows: multiple IAb first (TR1), IAA first (TR2), or GADA first (TR3), each including specific states of transition (11,12). Each individual may enter the trajectory in any state at any age but can only stay at the same state or proceed to the next state in transition. In this cohort, 643 (30%) participants (11,566 visits) were diagnosed with type 1 diabetes by the end of their observation period, hereafter referred to as diagnosed, and 1,502 participants (30,643 visits) remained undiagnosed at the end of their observation period, hereafter referred to as undiagnosed. The development of clinical onset of type 1 diabetes was ascertained according to the American Diabetes Association criteria (24). Median age of the diagnosed participants at the last observation, which represents age at diagnosis, was 7.62 years (interquartile range 4.19–11.22), while median age of the undiagnosed participants at the last observation was 12.87 years (9.29 to 15.42). The median follow-up time of all participants was 11.6 years (6.64–14.47). The model assigned each participant exclusively to one of the three trajectories as defined above. Table 1 includes description of the study cohort.
Table 1.
Distribution of undiagnosed and diagnosed participants in three trajectories over sex, seroconversion age, and diagnosis age
| Diagnosed | Undiagnosed | |||||
|---|---|---|---|---|---|---|
| TR1 (n = 256) | TR2 (n = 273) | TR3 (n = 114) | TR1 (n = 483) | TR2 (n = 257) | TR3 (n = 762) | |
| Sex, n (%) | ||||||
| Male | 155 (61) | 146 (53) | 52 (46) | 283 (59) | 145 (56) | 409 (54) |
| Female | 101 (39) | 127 (47) | 62 (54) | 200 (41) | 112 (44) | 353 (46) |
| Age of seroconversion | 2.51 (1.51–4.2) | 1.79 (1.04–3.13) | 4.05 (2.3–6.01) | 4.98 (2.02–8.03) | 6.0 (2.42–9.18) | 6.5 (3.99–9.62) |
| Age of diagnosis | 4.07 (1.88–7.06) | 3.85 (1.74–6.75) | 5.68 (2.92–9.08) | — | — | — |
Data are median (25th percentile–75th percentile) unless otherwise indicated.
IAb Levels
Previous work harmonized IAb levels as multiples of upper limit of normal (mULN) to facilitate combined analysis (22). We converted autoantibody level measurements into mULN by dividing the measurement by the positivity threshold level for the corresponding assay. Positive autoantibody test results will have a value ≥1.0, and negative autoantibody test results will have a value <1.0. The continuous values (mULN) were then categorized into four level groups (Table 2).
Table 2.
Four IAb levels of mULN for the three IAb: GADA, IAA, IA-2A
| GADA | IAA | IA-2A | |
|---|---|---|---|
| L0 | <1.0 | <1.0 | <1.0 |
| L1 | 1.0–5.3 | 1.0–3.5 | 1.0–2.4 |
| L2 | 5.4–20.7 | 3.6–5.4 | 2.5–235.1 |
| L3 | ≥20.8 | ≥5.5 | ≥235.2 |
The threshold values between L1 and L2 were the autoantibody type–specific thresholds that effectively stratified 5 year progression to type 1 diabetes at the confirmatory visit (22). The threshold values between L2 and L3 were specified as the levels corresponding to the 75th percentile of the respective autoantibody-positive cohort.
Data Visualization and Statistical Analysis Methods
We used an interactive data visualization method called DPVis (25) to characterize the IAb levels in the three trajectories. Using this method, we visualized each participant visit having an autoantibody level by overlaying a color-labeled dot corresponding to IAb level onto the three trajectories. We also visualized the proportion of the four IAb levels (L0, L1, L2, L3) that the participants belonged to over their observation periods using stacked bar charts. Then, we visualized the IAb levels of individual participants within their observations as parallel bar charts. These charts depict the major trends and differences among the four levels of IAb of individual participants within the three trajectories. We then computed “dwell time,” the proportion of the total duration of follow-up spent at a given level, by the four IAb levels per trajectory and analyzed differences between the diagnosed and undiagnosed participants within each trajectory using χ2 tests. We further sorted participants by the maximum IAb level each participant achieved over their observation period. In particular, we stratified individuals by the maximum levels (L0, L1, L2, L3) of IA-2A that each participant achieved, because high IA-2A levels have been associated with rapid progression from autoimmunity to overt type 1 diabetes. Then, we analyzed differences in dwell times in different GADA and IAA levels between the diagnosed and undiagnosed participants within each trajectory by χ2 tests. Finally, we compared the diabetes-free survival rates of young children with single IAb positivity and different IAb levels (L1, L2, and L3) following the screening protocols recommended in prior studies (26–28).
Data and Resource Availability
The data that support the findings of this study are not publicly available because they were used under license for the current study only. Data are, however, available on reasonable request with permission from the originating sites, whose representatives are William Hagopian (DEW-IT), Markus Lundgren (DiPiS), Marian Rewers (DAISY), Riitta Veijola (DIPP), and Anette Ziegler (BABYDIAB).
Results
Overall Differences in IAb Levels Between the Diagnosed and the Undiagnosed Participants
To investigate the differences between individuals who were diagnosed or not diagnosed during the study period, we separated the two groups of participants into different panels in each figure. Further, for both groups, each individual was categorized into one of three trajectories (TR1, TR2, and TR3), where the individual could appear in one or more states (e.g., TR1-0, TR1-1, and TR1-2). Here we first describe the layout of the visualized data and then present detailed descriptions for each trajectory and analysis.
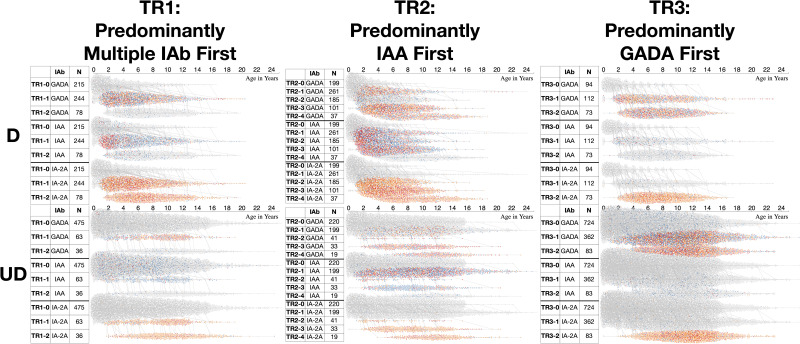
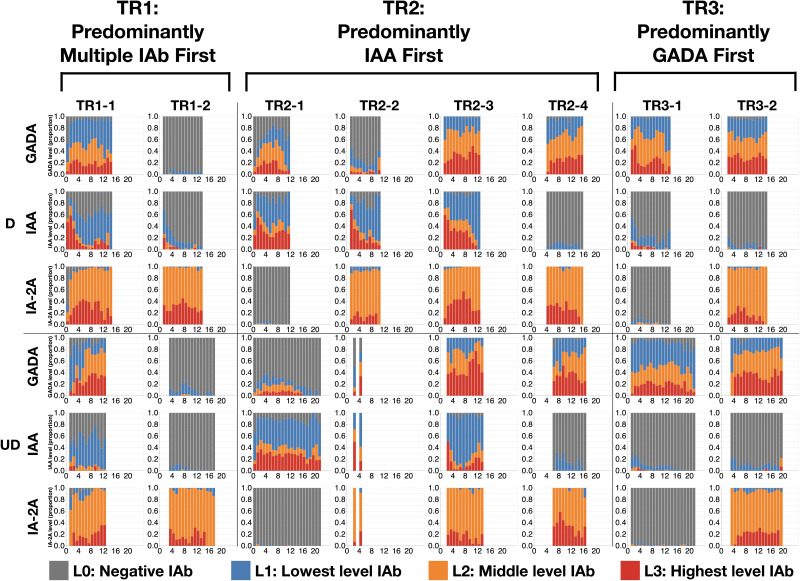
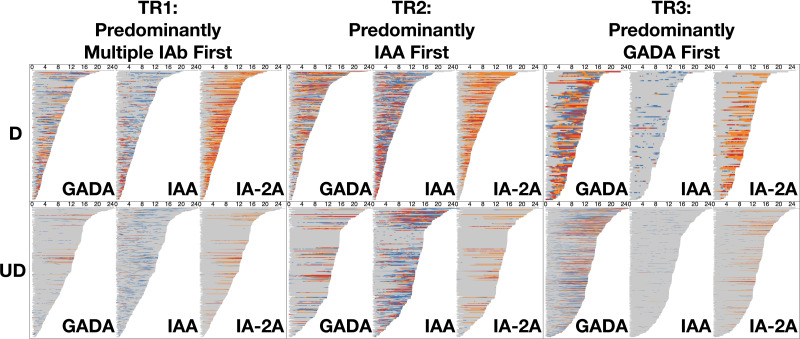
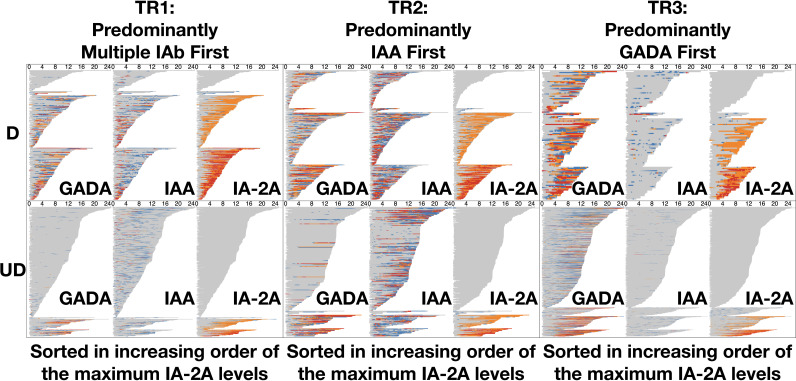
With use of the DPVis method (Fig. 1), the three autoimmune trajectories and their component states were visualized for the diagnosed and undiagnosed participants. In the visualization, each individual visit was color-coded to denote the four levels of GADA, IAA, or IA-2A. Figure 2 illustrates the normalized proportions of the four autoantibody levels for each IAb at all visits categorized by age. Supplementary Fig. 1 shows the distribution of the four IAb levels for all visits at all ages. Figure 3 visualizes the length of time that each individual participant spent at one of the four IAb levels (“dwell time”) as marked with the four colors across their observation period. Table 3 quantitatively compares the proportion of these dwell times at the four autoantibody levels between the diagnosed and undiagnosed participants. Since IA-2A positivity is known to predict relatively rapid progression to type 1 diabetes (20,22,29–31), we further stratified participants by the maximum IA-2A levels they reached during the observation period and compared the dwell times at the four levels of IAA and GADA between the diagnosed and undiagnosed participants (Fig. 4, Table 4, and Supplementary Fig. 2). Similarly, Supplementary Fig. 3 illustrates the dwell times at various IAb levels in individuals with stratification by maximum IAA or GADA level. Supplementary Figs. 4–6 show the cumulative diabetes-free survival rates of children with single IAb positivity at the age of 2 and 6 years with different IAb levels (L1, L2, and L3).
Figure 1.
Visualization of the entire data set by trajectory and IAb level comparing diagnosed and undiagnosed individuals and illustrating the differences. Three autoimmune trajectories and their component states overlaid with autoantibody levels toward type 1 diabetes. The diagram includes three subfigures summarizing the three respective trajectories and their component states overlaid with the IAb levels: TR1, TR2, and TR3. Each subfigure consists of two plots (top, bottom); the top plot shows trajectories for the diagnosed (D), and the bottom shows those for the undiagnosed (UD). The table on the left includes three columns: 1) component state label, 2) IAb type (GADA, IAA, or IA-2A), and 3) the total number of participants per state (row). The waterfall chart on the right shows visits (dots) colored according to the IAb level (gray, L0; blue, L1; orange, L2; red, L3). y-axis represents component states, and x-axis represents age of participants in years. In TR1, most diagnosed children advance from TR1-0 (IAb negative) to TR1-1 (positive for multiple IAb) and TR1-2 (IA-2A positive). The distributions of autoantibody levels over age show higher proportion of IAA L3 (red) in early age of the diagnosed participants compared with the undiagnosed participants. In TR2, the diagnosed participants frequently have IAA L3 (red) at early ages across all positive states, whereas the undiagnosed participants have fewer IAA positive visits and those with L3 are spread across ages. In TR3, both the diagnosed and the undiagnosed participants advance to IAb-positive states, TR3-1 and TR3-2, but the timing is later for the undiagnosed.
Figure 2.
Summary of IAb levels at each visit by age comparing diagnosed and undiagnosed individuals. Normalized proportions of autoantibody levels over age are depicted. The diagram shows 48 panels (6 rows, 8 columns) summarizing the normalized proportion of autoantibody levels over participants’ age. Component panels represent the diagnosed and undiagnosed groups for each of the eight IAb-positive states (TR1-1, TR1-2, TR2-1, TR2-2, TR2-3, TR2-4, TR3-1, TR3-2) and three IAb types (GADA, IAA, IA-2A). For example, TR1-1 indicates the first positive component state of trajectory TR1, predominantly multiple IAb first. Each panel includes a stacked bar chart that shows the proportion of visits in percentage (y-axis), which are broken down into stacks of four IAb levels, over ages of participants in years (x-axis). We excluded visits with no autoantibody measurement and age ranges with <10 observations. In TR1-1, TR2-1, and TR2-2, the proportion of the highest IAA level (L3) at early ages (<2 years) tends to be higher for the diagnosed participants than for the undiagnosed. In TR3-1, the proportion of the highest GADA level (L3) at early ages (<2 years) appears higher for the diagnosed compared with the undiagnosed participants. D, diagnosed; UD, undiagnosed.
Figure 3.
Development of autoantibody levels and dwell times for individual participants sorted by duration of follow-up. The diagram includes six panels (two rows and three columns) summarizing the dwell time of individual participants at each autoantibody level (gray, L0; blue, L1; orange, L2; red, L3) for three IAb (GADA, IAA, IA-2A) over their ages in years (x-axis) per trajectory (column) and per diagnosis (row). In each panel, we sorted participants (horizontal bars) by their age at last observation with increasing order from top to bottom. Overall, the undiagnosed participants have longer follow-up time as seen in the horizontal length of bars across the board. Most of the diagnosed participants tend to show dynamic changes of autoantibody levels and longer dwell times at higher levels over the follow-up period compared with the undiagnosed participants. In all trajectories an evolution to high levels of IA-2A frequently precedes diagnosis. D, diagnosed; UD, undiagnosed.
Table 3.
The proportion of dwell times (total duration of follow-up at a given level) in percentages by the four IAb levels per trajectory and diagnosis for each autoantibody
| IAb, trajectory | Diagnosis | N | L0 | L1 | L2 | L3 |
|---|---|---|---|---|---|---|
| GADA | ||||||
| TR1*** | D | 256 | 71.5 | 13.9 | 9.0 | 5.6 |
| UD | 483 | 95.5 | 2.4 | 1.0 | 1.1 | |
| TR2*** | D | 273 | 53.7 | 16.1 | 18.3 | 11.9 |
| UD | 257 | 84.9 | 5.6 | 4.7 | 4.7 | |
| TR3*** | D | 114 | 43.1 | 19.9 | 21.9 | 15.1 |
| UD | 762 | 74.3 | 13.9 | 6.9 | 4.9 | |
| IAA | ||||||
| TR1 | D | 256 | 76.4 | 14.4 | 3.1 | 6 |
| UD | 483 | 86.9 | 10.9 | 1.2 | 1 | |
| TR2* | D | 273 | 45.1 | 27.3 | 8.8 | 18.9 |
| UD | 257 | 64.3 | 21 | 4.5 | 10.3 | |
| TR3 | D | 114 | 91.5 | 7.2 | 0.5 | 0.7 |
| UD | 762 | 98 | 1.8 | 0.1 | 0.1 | |
| IA-2A | ||||||
| TR1*** | D | 256 | 56.7 | 1.3 | 29.4 | 12.5 |
| UD | 483 | 93.9 | 0.4 | 4.7 | 0.9 | |
| TR2*** | D | 273 | 53.8 | 2.2 | 33.5 | 10.5 |
| UD | 257 | 91 | 0.5 | 6.9 | 1.6 | |
| TR3*** | D | 114 | 69.7 | 1.6 | 23.2 | 5.5 |
| UD | 762 | 94.3 | 0.8 | 3.9 | 1 |
D, diagnosed; UD, undiagnosed. χ2 tests show significant differences in the proportions between diagnosis within each trajectory:
P < 0.05;
P < 0.001.
Figure 4.
Development of autoantibody levels and dwell times for individual participants sorted by their maximum IA-2A level. The diagram includes six panels (two rows: diagnosed, undiagnosed; three columns: TR1, TR2, TR3) summarizing the dwell time of individual participants at each autoantibody level (gray, L0; blue, L1; orange, L2; red, L3) for three IAb (GADA, IAA, IA-2A) over their ages per trajectory per diagnosis. In each panel, participants in each trajectory (column) are sorted by the maximum level of IA-2A with increasing order from top to bottom. More than one-half of diagnosed participants across the three trajectories reach high IA-2A levels (L2, L3) during follow-up. On the other hand, a majority of undiagnosed participants across the three trajectories stay IA-2A negative (L0) during follow-up. D, diagnosed; UD, undiagnosed.
Table 4.
Proportion of dwell times for GADA and IAA by IAb, diagnosis, and maximum IA-2A level each participant achieved during observation
| Trajectory | IA-2A level | Type 1 diabetes | N | GADA levels | P | IAA levels | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L0 | L1 | L2 | L3 | L0 | L1 | L2 | L3 | ||||||
| TR1 | L0 | D | 13 | 96.5 | 3.5 | 0 | 0 | n.s. | 83.2 | 12.8 | 0.4 | 3.6 | n.s. |
| UD | 411 | 98.6 | 1.2 | 0.1 | 0.1 | 86.2 | 11.3 | 1.4 | 1.1 | ||||
| L1 | D | 8 | 92.7 | 5.1 | 0.4 | 1.8 | n.s. | 95.5 | 2.2 | 0.4 | 1.9 | * | |
| UD | 6 | 93.9 | 4.7 | 1.4 | 0 | 87.6 | 11.7 | 0 | 0.7 | ||||
| L2 | D | 105 | 66.5 | 18.3 | 9.8 | 5.4 | n.s. | 72.2 | 14.5 | 4.8 | 8.5 | * | |
| UD | 40 | 82 | 6.6 | 7.7 | 3.6 | 86.8 | 11.4 | 1.3 | 0.4 | ||||
| L3 | D | 103 | 64.7 | 15.3 | 10.4 | 9.5 | n.s. | 72.6 | 19.4 | 2.4 | 5.6 | n.s. | |
| UD | 25 | 69.4 | 13.5 | 4.5 | 12.7 | 82.9 | 14.3 | 2.2 | 0.6 | ||||
| TR2 | L0 | D | 69 | 57.8 | 14.8 | 16.5 | 11 | *** | 43.9 | 28.9 | 5.7 | 21.6 | ** |
| UD | 206 | 94.6 | 2.6 | 1.8 | 1 | 67.3 | 18.1 | 4.3 | 10.4 | ||||
| L1 | D | 9 | 52.5 | 3.4 | 26.7 | 17.4 | *** | 35 | 29.6 | 16.2 | 19.1 | *** | |
| UD | 8 | 86 | 14 | 0 | 0 | 64.4 | 29.2 | 0 | 6.4 | ||||
| L2 | D | 111 | 53.5 | 19.8 | 16.4 | 10.4 | * | 36.2 | 29.7 | 12.9 | 21.2 | n.s. | |
| UD | 27 | 34.6 | 18.7 | 22.4 | 24.3 | 42 | 35 | 8.6 | 14.3 | ||||
| L3 | D | 74 | 40.5 | 17.9 | 25.3 | 16.3 | n.s. | 46.4 | 28.3 | 8.8 | 16.5 | * | |
| UD | 16 | 36.9 | 19.7 | 18.6 | 24.8 | 50.3 | 39.9 | 4.2 | 5.6 | ||||
| TR3 | L0 | D | 34 | 43.9 | 23.7 | 23.4 | 9.1 | *** | 90.2 | 9.2 | 0.1 | 0.5 | n.s. |
| UD | 590 | 77.4 | 15.6 | 4.9 | 2.2 | 98.7 | 1.1 | 0.1 | 0.1 | ||||
| L1 | D | 5 | 43.2 | 20.9 | 12 | 23.8 | *** | 69 | 17.2 | 2.6 | 11.2 | *** | |
| UD | 52 | 85.3 | 5.1 | 3.2 | 6.4 | 98.5 | 1.3 | 0.2 | 0 | ||||
| L2 | D | 43 | 37.6 | 18.5 | 28 | 15.8 | ** | 92.3 | 6.5 | 0.8 | 0.4 | n.s. | |
| UD | 80 | 60.4 | 9.4 | 16.4 | 13.8 | 95.3 | 4.4 | 0.1 | 0.2 | ||||
| L3 | D | 29 | 31 | 23.9 | 22.5 | 22.6 | n.s. | 94.1 | 5.7 | 0.1 | 0.1 | n.s. | |
| UD | 39 | 38.5 | 14.9 | 23.6 | 23 | 94 | 5.5 | 0.1 | 0.4 | ||||
D, diagnosed; UD, undiagnosed. Proportions (%) of dwell times at different GADA and IAA levels between the diagnosed and undiagnosed were analyzed with χ2 tests:
P < 0.05;
P < 0.01;
P < 0.001; n.s., not significant (P > 0.05).
Overall, the evolution of IAb levels in each of the three trajectories appears different between the diagnosed and undiagnosed participants. The IAb levels detected at the age of 2 or 6 years among those who had single IAb positivity can be used to stratify type 1 diabetes risk. Details of these differences are presented below.
IAb Levels in TR1 (Predominantly Multiple IAb First)
In TR1, high levels of IAA appeared more prevalent in the diagnosed participants than the undiagnosed participants (Figs. 1 and 2). The most prominent pattern was that high IAA levels were seen among the diagnosed participants at early ages, younger than 3 years of age, whereas among the undiagnosed IAA remained mostly at low levels regardless of age. Among the diagnosed, in 59% of visits that were categorized to TR1-1 and occurred between 1 and 2 years of age participants reached the highest level (L3) for IAA. Among the undiagnosed, participants in the same age range reached L3 for IAA at only 8% of TR1-1 visits. In TR1, the proportion of visits with L3 of IA-2A or GADA appeared similar between the diagnosed and undiagnosed participants across their ages (Fig. 2).
In TR1, there were differences in the distribution of dwell times in the four different autoantibody levels between the diagnosed and undiagnosed participants for GADA and IA-2A but not for IAA (Fig. 3 and Table 3). For GADA, the diagnosed participants spent significantly more time with GADA positivity across the three positive levels combined compared with the undiagnosed (29% vs. 5%, respectively; P < 0.001) (Table 3). However, the proportions of dwell times among the three positive GADA levels were similar. For IAA, both the overall time of antibody positivity and the proportions of dwell times among the three positive levels were similar between the diagnosed and the undiagnosed. For IA-2A, the diagnosed participants stayed positive significantly longer than the undiagnosed participants (43% vs. 6%; P < 0.001) (Table 3), but as with GADA, the proportions of dwell times among the three positive IA-2A levels were similar.
To investigate the interplay of IA-2A and other autoantibodies in TR1, we compared the diagnosed and the undiagnosed who reached four different levels of IA-2A. We found weakly significant differences in the proportion of dwell times in the four IAA levels (Table 4) (P < 0.05), with the diagnosed spending more time in higher levels. There were, however, no significant differences in the proportion of dwell times between the diagnosed and the undiagnosed in any of the four GADA levels.
IAb Levels in TR2 (Predominantly IAA First)
Similar to TR1, in the diagnosed participants in TR2, high levels of IAA were more prevalent than in the undiagnosed, particularly at early ages. Figure 2 and Supplementary Fig. 1 show that among the diagnosed, 55% of observations that were categorized to TR2-1 and occurred between 1 and 2 years of age reached the highest level (L3) for IAA. Among the undiagnosed, only 31% of observations that were categorized to TR2-1 for the same age range reached L3 for IAA.
Table 3 shows that in TR2 there were significant differences in the distribution of dwell times in the four different autoantibody levels between the diagnosed and undiagnosed for GADA (P < 0.001), IAA (P < 0.05), and IA-2A (P < 0.001). For GADA, the diagnosed participants spent significantly more time with GADA positivity across the three positive levels combined, compared with the undiagnosed (46% vs. 15%, respectively; P < 0.001) (Table 3), but the distribution across positive levels was similar. For IAA, unlike IAA levels in TR1, the diagnosed participants spent significantly more time with IAA positivity than the undiagnosed (55% vs. 36%; P < 0.05) (Table 3), but again the distribution across positive levels was similar. For IA-2A, the diagnosed participants stayed at positive levels significantly longer than the undiagnosed participants (46% vs. 9%; P < 0.001) (Table 3), with no noticeable difference in the distribution of the three positive levels.
In TR2, there were significant differences in dwell times in the four GADA levels between the diagnosed and undiagnosed for those who remained negative for IA-2A (L0) or reached L1 or L2 (Table 4) (P < 0.001, P < 0.001, and P < 0.05, respectively). However, no noticeable difference was found in the distribution of dwell times across positive GADA levels among those at L3 of IA-2A. Significant differences were observed in dwell times of the four IAA levels between the diagnosed and undiagnosed who remained negative for IA-2A (L0) or reached L1 or L3 (P < 0.01, P < 0.001, P < 0.05), with the diagnosed spending more time in higher IAA levels.
IAb Levels in TR3 (Predominantly GADA First)
Similar to the other two trajectories, in TR3, high levels of GADA were more prevalent among the diagnosed compared with the undiagnosed, particularly at early ages. Figure 2 and Supplementary Fig. 1 show that among the diagnosed participants categorized to TR3-1, the proportions of observations at high GADA levels (L3) at age 1–2 years and 2–3 years (43% and 50%, respectively) were higher compared with those for the undiagnosed (3% and 17%).
In TR3, there were significant differences in the distribution of dwell times in the four different autoantibody levels between the diagnosed and the undiagnosed participants for GADA and IA-2A (Table 3) (P < 0.001 and P < 0.001, respectively) but not for IAA. For GADA, the diagnosed participants spent significantly more time with GADA positivity (57%), compared with 26% of the undiagnosed (Table 3) (P < 0.001), but the distribution among positive levels was similar. For IA-2A, the diagnosed participants stayed at positive levels significantly longer than the undiagnosed (30% vs. 6%; P < 0.001), again, with no noticeable difference in distribution among positive levels.
In TR3, there were significant differences in dwell times in the four GADA levels between the diagnosed and undiagnosed for those who remained negative for IA-2A (L0) or reached L1 or L2 (Table 4) (P < 0.001, P < 0.001, P < 0.01, respectively), with the diagnosed spending more time with higher GADA levels. There were also significant differences in dwell times in the four IAA levels between the diagnosed and the undiagnosed participants who reached IA-2A level of L1 (P < 0.001), but the distribution in positive IAA levels was similar between the diagnosed and undiagnosed.
Survival Analyses
Survival analyses showed differences in progression to type 1 diabetes compared between IAb levels, among participants who had single IAb positivity at the age of 2 years. Altogether 206 participants had single GADA positivity at age 2 years, and those with GADA L2 or L3 progressed faster to diabetes than those with GADA L1 (P < 0.001) (Supplementary Fig. 4). There were no statistically significant differences in progression rate between participants with GADA L2 and L3. A total of 327 participants had single IAA positivity at age 2 years, and those with IAA L3 progressed faster to diabetes than those with IAA L1 (P < 0.001) (Supplementary Fig. 5). Participants with IAA L2 progressed only marginally faster to diabetes than those with IAA L1 (P = 0.056). There were no statistically significant differences in progression rate between participants with IAA L2 and L3. Positivity for single IA-2A was observed in 50 participants at the age of 2 years. The participants with IA-2A L2 and L3 progressed faster to diabetes than those with IA-2A L1 (P < 0.01) (Supplementary Fig. 6). No statistically significant differences in progression rate were observed between participants with IA-2A L2 and L3.
At the age of 6 years, 253 participants had single GADA positivity. Participants with GADA L2 progressed faster to diabetes than those with GADA L1 (P = 0.012) (Supplementary Fig. 4) but no differences in progression rate were observed between participants with GADA L2 and L3 or with GADA L1 and L3. A total of 148 participants had single IAA positivity at the age of 6 years, but no differences in progression rates among participants with IAA L1, L2, and L3 were observed (Supplementary Fig. 5). Single IA-2A positivity was present in 92 participants at age 6 years, and participants with IA-2A L3 progressed faster to diabetes than those with IA-2A L1 (P = 0.014) (Supplementary Fig. 6). There were no statistically significant differences in progression rates compared between participants with IA-2A L2 and L3 and participants with IA-2A L1 and L2.
We also conducted survival analyses for participants who had multiple IAb positivity at the ages of 2 and 6 years and compared diabetes-free survival rates between different IAb levels. However, no statistically significant differences in progression rates were found for comparisons between the IAb levels.
Discussion
In the current study we refined previously described IAb trajectories by adding information about autoantibody levels to explore differential patterns between individuals who do or do not progress to type 1 diabetes within the observation time. Overall, each trajectory showed unique IAb level transition patterns. In each trajectory the undiagnosed participants showed patterns generally similar to those of the diagnosed with two notable differences: 1) their age at transition from negativity to positivity was delayed, and 2) they had a higher proportion of participants who only reached L1. In sum, the undiagnosed participants had generally lower IAb levels and later appearance of IAb than the diagnosed participants.
In particular, when participants at risk had a single positivity at the age of 2 years, the higher level (L2/3) of GADA, IAA, or IA-2A was associated with faster progression to diabetes in comparison with the lower level of positivity (L1). The participants with single IAA or single GADA positivity at the age of 2 years were likely to belong to TR2 (predominantly IAA only first) or TR3 (predominantly GADA only first), based on the previous findings (12). Therefore, IAb levels at an early age can be informative with respect to the main IAb trajectories and associated risks of progression to type 1 diabetes.
The major strength of this study is the visualization of the IAb trajectories enriched with autoantibody levels. This approach provided unique data-driven insights into IAb levels within the main autoimmune trajectories. Data visualizations can help for both identification and understanding of patterns that cannot be easily summarized statistically, thereby facilitating the process of generating new hypotheses. For example, in the predominantly GADA-initiated trajectory (TR3), the distribution of positive GADA levels was conspicuously similar between the diagnosed and undiagnosed (Fig. 2), but the undiagnosed had initiation of the positive level later and it persisted longer before transition to the higher levels (Fig. 3). Another strength is the very large multinational cohort of children who were at increased risk for type 1 diabetes, either with positive family history or having HLA-conferred risk for the disease.
Differences in the original cohort studies were, however, a clear limitation. For overcoming this, special attention was paid to harmonization of the autoantibody levels and HLA risk groups (2,22). Another limitation is the small number of participants in some of the trajectory-related states (Fig. 2). In addition, information on ZnT8A was not available for our analyses and should be added in analyses of future studies because ZnT8A may be the first autoantibody to appear and the analysis could be further refined (32,33). Moreover, autoantibodies to tetraspanin-7 may further contribute to our understanding of the implications of varying autoimmune trajectories of type 1 diabetes (34). Since the population in the cohort is young, the findings need to be validated with use of data from older individuals in order to be generalizable. In addition to immunophenotyping with autoantibody patterns, metabolic assessment can be especially useful to identify approaching stage 3 type 1 diabetes. Moreover, combining metabolic data with IAb trajectory information might further improve individual risk assessment and thereby help to refine selection criteria for intervention trials.
Multiple studies have reported the autoantibody-specific initiation of islet autoimmunity (8,9) and evolution of IAb pattern based on positivity or negativity (35–38). In our recent data-driven analysis, also based on binary autoantibody categories, we demonstrated the longitudinal profile of the three main patterns of islet autoimmunity (12). Here we have enhanced this approach by including IAA, GADA, and IA-2A levels, which helps to further distinguish future progressors from those who remain healthy. Endotypes of islet autoimmunity have thus far been characterized by the first-appearing autoantibodies (4,39,40). However, it is apparent that the longitudinal evolution of IAb together with their levels can better define the putative endotypes. This article provides a novel approach of analyzing the dynamic patterns of autoantibody levels and comparing the dwell times of the three autoantibodies at a given level between the diagnosed and undiagnosed participants.
In this analysis we used data from prospective studies with IAb information from frequently sampled longitudinal visits beginning from early ages. In contrast, IAb screening programs may identify autoantibody-positive children at any age without knowledge of prior IAb history. Thus, it is important to have refined understanding of trajectories and the significance of dynamic IAb levels for prediction of individual risk of progression. Better understanding of risk may have important implications for future research and interventions.
In conclusion, visualization of islet autoimmunity progression using IAb levels, order of appearance, and trajectories can enhance insights into type 1 diabetes pathogenesis. It has long been appreciated that the number of autoantibodies is an important predictor of type 1 diabetes; in this study we further refine the main trajectories using the dynamic patterns of autoantibody levels. Furthermore, these data show that not only positivity for a single IAb observed at an early age but also the IAb level can be used for risk stratification for type 1 diabetes. In the future, artificial intelligence approaches to analyzing these trends in the complex data sets may allow these patterns to be better translated to prediction of progression to diabetes in children. The findings in this study need to be validated in an independent cohort. Future work is also needed to correlate the observed trajectories with changes in β-cell function and glycemia in order to test whether longitudinal IAb patterns should influence individual risk assessment and selection criteria for intervention trials.
Article Information
Acknowledgments. The authors thank the T1DI Study Group for their help in this work. The T1DI Study Group consists of the following members: JDRF, Jessica Dunne, Olivia Lou, and Frank Martin; IBM, Vibha Anand, Mohamed Ghalwash, Eileen Koski, Bum Chul Kwon, Ying Li, Zhiguo Li, Bin Liu, Ashwani Malhotra, Shelley Moore, and Kenney Ng; DiPiS, Helena Elding Larsson, Josefine Jönsson, Åke Lernmark, Markus Lundgren, Marlena Maziarz, and Lampros Spiliopoulos; BABYDIAB, Peter Achenbach, Christiane Winkler, and Anette Ziegler; DIPP, Heikki Hyöty, Jorma Ilonen, Mikael Knip, Jorma Toppari, and Riitta Veijola; DEW-IT, William Hagopian, Michael Killian, and Darius Schneider; and DAISY, Brigitte Frohnert, Jill Norris, Marian Rewers, Andrea Steck, Kathleen Waugh, and Liping Yu.
Funding. This work was supported by funding from JDRF (IBM, 1-RSC-2017-368-I-X, 1-IND-2019-717-I-X, and 2-RSC-2020-980-I-X; DAISY, 1-SRA-2019-722-I-X, 1-RSC-2017-517-I-X, and 5-ECR-2017-388-A-N; DiPiS, 1-SRA-2019-720-I-X and 1-RSC-2017-526-I-X; DIPP, 1-RSC-2018-555-I-X and 1-SRA-2019-721-I-X; DEW-IT, 1-SRA-2019-719-I-X and 1-RSC-2017-516-I-X); and BABYDIAB, 1-SRA-2019-723-I-X) as well as the National Institutes of Health (DAISY, DK032493, DK032083, DK104351, and DK116073, and DiPiS, DK26190) and the Centers for Disease Control and Prevention (DEW-IT, UR6/CCU017247). The DIPP study was funded by JDRF (grants 1-SRA-2016-342-M-R, 1-SRA-2019-732-M-B), the European Union (grant BMH4-CT98-3314), Novo Nordisk Foundation, Academy of Finland (decision no. 292538 and Centre of Excellence in Molecular Systems Immunology and Physiology Research 2012–2017 decision no. 250114), Special Research Funds for University Hospitals in Finland, Diabetes Research Foundation, Finland, and Sigrid Juselius Foundation, Finland. The BABYDIAB study was funded by the German Federal Ministry of Education and Research to the German Center for Diabetes Research. The DiPiS study was funded by Swedish Research Council (grant no. 14064), Swedish Childhood Diabetes Foundation, Swedish Diabetes Association, Nordisk Insulin Fund, Skåne University Hospital (SUS) funds, Lion Club International, district 101-S, the Royal Physiographic Society, Skåne County Council Foundation for Research and Development, and LUDC-IRC/EXODIAB funding from the Swedish Foundation for Strategic Research (Dnr IRC15-0067) and Swedish Research Council (Dnr 2009-1039). Additional funding for DEW-IT was provided by the Hussman Foundation and by the Washington State Life Science Discovery Fund.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.C.K. took responsibility for all analyses presented. All authors made substantial contributions to conception and design of the manuscript, participated in drafting the manuscript or revising it critically for important intellectual content, and gave final approval of the version to be submitted. B.C.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 82nd Scientific Sessions of the American Diabetes Association, New Orleans, LA, 3–7 June 2022.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20736742.
B.C.K., V.A., E.K., and K.N. are current employees of IBM.
References
- 1. Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anand V, Li Y, Liu B, et al.; T1DI Study Group . Islet autoimmunity and HLA markers of presymptomatic and clinical type 1 diabetes: joint analyses of prospective cohort studies in Finland, Germany, Sweden, and the U.S. Diabetes Care 2021;44:2269–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Powers AC. Type 1 diabetes mellitus: much progress, many opportunities. J Clin Invest 2021;131:e142242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leete P, Mallone R, Richardson SJ, Sosenko JM, Redondo MJ, Evans-Molina C. The effect of age on the progression and severity of type 1 diabetes: potential effects on disease mechanisms. Curr Diab Rep 2018;18:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol 2019;15:635–650 [DOI] [PubMed] [Google Scholar]
- 6. So M, O’Rourke C, Ylescupidez A, et al. Characterising the age-dependent effects of risk factors on type 1 diabetes progression. Diabetologia 2022;65:684–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Endesfelder D, Zu Castell W, Bonifacio E, et al.; TEDDY Study Group . Time-resolved autoantibody profiling facilitates stratification of preclinical type 1 diabetes in children. Diabetes 2019;68:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ilonen J, Hammais A, Laine AP, et al. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes 2013;62:3636–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krischer JP, Lynch KF, Schatz DA, et al.; TEDDY Study Group . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015;58:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giannopoulou EZ, Winkler C, Chmiel R, et al. Islet autoantibody phenotypes and incidence in children at increased risk for type 1 diabetes. Diabetologia 2015;58:2317–2323 [DOI] [PubMed] [Google Scholar]
- 11. Kwon BC, Achenbach P, Dunne JL, et al.; T1DI Study Group . Modeling disease progression trajectories from longitudinal observational data. AMIA Annu Symp Proc 2021;2020:668–676 [PMC free article] [PubMed] [Google Scholar]
- 12. Kwon BC, Anand V, Achenbach P, et al.; T1DI Study Group . Progression of type 1 diabetes from latency to symptomatic disease is predicted by distinct autoimmune trajectories. Nat Commun 2022;13:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steck AK, Dong F, Waugh K, et al. Predictors of slow progression to diabetes in children with multiple islet autoantibodies. J Autoimmun 2016;72:113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kulmala P, Savola K, Petersen JS, et al.; The Childhood Diabetes in Finland Study Group . Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes. A population-based study. J Clin Invest 1998;101:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steck AK, Johnson K, Barriga KJ, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care 2011;34:1397–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barker JM, Barriga KJ, Yu L, et al.; Diabetes Autoimmunity Study in the Young . Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004;89:3896–3902 [DOI] [PubMed] [Google Scholar]
- 17. So M, Speake C, Steck AK, et al. Advances in type 1 diabetes prediction using islet autoantibodies: beyond a simple count. Endocr Rev 2021;42:584–604 [DOI] [PubMed] [Google Scholar]
- 18. Sosenko JM, Skyler JS, Palmer JP, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial-Type 1 Study Group . The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care 2013;36:2615–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ziegler AG, Ziegler R, Vardi P, Jackson RA, Soeldner JS, Eisenbarth GS. Life-table analysis of progression to diabetes of anti-insulin autoantibody-positive relatives of individuals with type I diabetes. Diabetes 1989;38:1320–1325 [DOI] [PubMed] [Google Scholar]
- 20. Achenbach P, Warncke K, Reiter J, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 2004;53:384–392 [DOI] [PubMed] [Google Scholar]
- 21. Bonifacio E, Bingley PJ, Shattock M, et al. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet 1990;335:147–149 [DOI] [PubMed] [Google Scholar]
- 22. Ng K, Stavropoulos H, Anand V, et al. Islet autoantibody type-specific titer thresholds improve stratification of risk of progression to type 1 diabetes in children. Diabetes Care 2022;45:160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lange JM, Minin VN. Fitting and interpreting continuous-time latent Markov models for panel data. Stat Med 2013;32:4581–4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 25. Kwon BC, Anand V, Severson KA, et al. DPVis: visual analytics with hidden Markov models for disease progression pathways. IEEE Trans Vis Comput Graph 2021;27:3685–3700 [DOI] [PubMed] [Google Scholar]
- 26. Chmiel R, Giannopoulou EZ, Winkler C, Achenbach P, Ziegler AG, Bonifacio E. Progression from single to multiple islet autoantibodies often occurs soon after seroconversion: implications for early screening. Diabetologia 2015;58:411–413 [DOI] [PubMed] [Google Scholar]
- 27. Bonifacio E, Weiß A, Winkler C, et al. An age-related exponential decline in the risk of multiple islet autoantibody seroconversion during childhood. Diabetes Care 2021;44:2260–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghalwash M, Dunne JL, Lundgren M, et al.; Type 1 Diabetes Intelligence Study Group . Two-age islet-autoantibody screening for childhood type 1 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol 2022;10:589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacobsen LM, Larsson HE, Tamura RN, et al.; TEDDY Study Group . Predicting progression to type 1 diabetes from ages 3 to 6 in islet autoantibody positive TEDDY children. Pediatr Diabetes 2019;20:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Decochez K, De Leeuw IH, Keymeulen B, et al.; Belgian Diabetes Registry . IA-2 autoantibodies predict impending type I diabetes in siblings of patients. Diabetologia 2002;45:1658–1666 [DOI] [PubMed] [Google Scholar]
- 31. Gorus FK, Balti EV, Vermeulen I, et al.; Belgian Diabetes Registry . Screening for insulinoma antigen 2 and zinc transporter 8 autoantibodies: a cost-effective and age-independent strategy to identify rapid progressors to clinical onset among relatives of type 1 diabetic patients. Clin Exp Immunol 2013;171:82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pöllänen PM, Ryhänen SJ, Toppari J, et al. Dynamics of islet autoantibodies during prospective follow-up from birth to age 15 years. J Clin Endocrinol Metab 2020;105:e4638–e4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McLaughlin KA, Richardson CC, Ravishankar A, et al. Identification of tetraspanin-7 as a target of autoantibodies in type 1 diabetes. Diabetes 2016;65:1690–1698 [DOI] [PubMed] [Google Scholar]
- 35. Köhler M, Beyerlein A, Vehik K, et al.; TEDDY study group . Joint modeling of longitudinal autoantibody patterns and progression to type 1 diabetes: results from the TEDDY study. Acta Diabetol 2017;54:1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Endesfelder D, Hagen M, Winkler C, et al. A novel approach for the analysis of longitudinal profiles reveals delayed progression to type 1 diabetes in a subgroup of multiple-islet-autoantibody-positive children. Diabetologia 2016;59:2172–2180 [DOI] [PubMed] [Google Scholar]
- 37. Bauer W, Veijola R, Lempainen J, et al. Age at seroconversion, HLA genotype, and specificity of autoantibodies in progression of islet autoimmunity in childhood. J Clin Endocrinol Metab 2019;104:4521–4530 [DOI] [PubMed] [Google Scholar]
- 38. Vehik K, Lynch KF, Schatz DA, et al.; TEDDY Study Group . Reversion of β-cell autoimmunity changes risk of type 1 diabetes: TEDDY study. Diabetes Care 2016;39:1535–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 2020;43:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krischer JP, Lynch KF, Lernmark Å, et al.; TEDDY Study Group . Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY study. Diabetes Care 2017;40:1194–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]