Abstract
Background
Emerging evidence shows that cognitive dysfunction may occur following coronavirus disease 19 (COVID-19) infection which is one of the most common symptoms reported in researches of “Long COVID”. Several inflammatory markers are known to be elevated in COVID-19 survivors and the relationship between long-term inflammation changes and cognitive function remains unknown.
Methods
We assessed cognitive function and neuropsychiatric symptoms of 66 COVID-19 survivors and 79 healthy controls (HCs) matched with sex, age, and education level using a digital, gamified cognitive function evaluation tool and questionnaires at 15 months after discharge. Venous blood samples were collected to measure cytokine levels. We performed correlation analyses and multiple linear regression analysis to identify the factors potentially related to cognitive function.
Results
The COVID-19 survivors performed less well on the Trails (p = 0.047) than the HCs, but most of them did not report subjective neuropsychiatric symptoms. Intensive care unit experience (β = −2.247, p < 0.0001) and self-perceived disease severity (β = −1.522, p = 0.007) were positively correlated, whereas years of education (β = 0.098, p = 0.013) was negatively associated with the performance on the Trails. Moreover, the abnormally elevated TNF-α levels (r = −0.19, p = 0.040) were negatively correlated with performance on the Trails in COVID-19 group.
Conclusion
Our findings suggest that COVID-19 survivors show long-term cognitive impairment in executive function, even at 15 months after discharge. Serum TNF-α levels may be an underlying mechanism of long-term cognitive impairment in patients recovering from COVID-19.
Keywords: COVID-19, Cognitive function, Systematic inflammation, Long-COVID
1. Background
Coronavirus disease 19 (COVID-19),2 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),3 primarily causes respiratory symptoms, with fever and cough with or without sputum being the most common manifestations in symptomatic patients. However, the finding that the virus has the potential to cause central nervous system (CNS)4 damage suggests a possible role for the virus in the development of long-term neuropsychiatric sequelae (Kumar et al., 2021, Tandon, 2022).
Growing evidence suggests that there is a high frequency of persistent neuropsychiatric symptoms after the initial illness including fatigue, cognitive dysfunction, sleep disorders, mood disorder, and anxiety disorders that often refers to as ‘Long-COVID’(Badenoch et al., 2022, Hampshire et al., 2021). There are several mechanisms through which SARS-CoV-2 could damage the CNS, including direct invasion and indirect infection (Alvarez et al., 2022, Du et al., 2021, Iodice et al., 2021, Kumar et al., 2021). SARS-CoV-2 can invade the nervous system through the recognition of the virus’s spike proteins by cell membrane receptors (Amruta et al., 2021; Du et al., 2021). Another possible mechanism that the virus can indirectly infect the nervous system is the cytokine storm which refers to an uncontrolled excessive inflammatory reaction. It starts locally and spreads further in the body through systemic circulation. (Jose and Manuel, 2020). However, it is unclear which is the dominant mechanism that underlies the neuropsychiatric sequelae.
Several inflammatory markers, including interleukin (IL)5 -1β, IL-6, interferon (IFN)6 -γ, tumor necrosis factor (TNF)-α,7 chemokine interferon-γ inducible protein-10, Monocyte chemoattractant protein-1, and macrophage inflammatory protein 1-α, and T helper 2 cytokines, including the IL-4, IL-10, and IL-1 receptors, are elevated in the serum of patients with COVID-19 during the cytokine storm particularly related to the illness (Coperchini et al., 2020, Mehta et al., 2020). The persistence of the SARS-CoV2 and extension and amplification of the inherent immune mechanism will cause the dysfunction of immune response once the human immune system could not produce an appropriate immune response to the virus. This will lead a high inflammatory state of cytokine storm. (Pelaia et al., 2020) Previously, systemic inflammation has been shown to lead to cognitive decline, suggesting that COVID-19 survivors could experience cognitive deficits in the following years (Gorelick, 2010, Poletti et al., 2022).
It is known from previous pandemics that there are not only acute effects of the viral infection but also long-term sequelae such as depressed mood, anxiety symptoms, insomnia, loss of smell, ageusia, fatigue, and headache due to the virus infection itself as well as social effects due to quarantine, social distancing, and lockdown (Han et al., 2022, Kumar et al., 2021, Méndez et al., 2022). The COVID-19 pandemic has prompted many countries to adopt restraining measures to mitigate the spread of the disease (Nogueira et al., 2021). As the disease has a high level of transmissibility, the patients with COVID-19 are required to stay in isolated units. In Wuhan, patients with mild symptoms received treatment in temporary quarantined hospital facilities, whereas those with more severe symptoms received treatment from a designated hospital for more aggressive therapy (Hu et al., 2020). Isolation has been shown to influence cognitive function (Wang et al., 2022).
Some earlier studies found significant differences between patients with COVID-19 and matched control groups via self-report questionnaires on various measures, indicating short-term memory loss, trouble focusing attention, and concentration impairment/disorder in the patients (Amin-Chowdhury et al., 2021, González-Hermosillo et al., 2021, Klein et al., 2021). However, most of the existing studies have focused on assessing patients at 5 days to one year of follow-up after discharge from the hospital (Ceban et al., 2022, Miskowiak et al., 2022). Research focusing on neuropsychiatric consequences over one year is limited and should be investigated (Kawakami et al., 2022). Furthermore, changes in the levels of inflammatory factors in people who recovered from COVID-19 and their relationship with cognitive function remain unknown. Moreover, most of the existing studies rely on patients’ complaints in medical records or self-reported symptoms via telephone to evaluate and describe neuropsychiatric symptoms and neurocognitive function. Few studies have evaluated the neurocognitive function of COVID-19 survivors using integrated tools in which both self-reported questionnaires and neuropsychological tests were used.
In summary, SARS-CoV-2, which causes neuronal damage, can have a prolonged negative impact on cognitive function, daily functioning, and quality of life. It is important to understand the neuropsychiatric consequences as millions of individuals have been affected and more unfound. Similarly, evaluating cognitive consequences following COVID-19 is essential, particularly by evaluating the capacity of an individual to work effectively, participate in daily family activities, or make reasoned decisions (Kumar et al., 2021).
This study aimed to evaluate the cognitive function and neuropsychiatric symptoms in patients who recovered from COVID-19 at 15 months of follow-up after their discharge from hospital, and to investigate the potential associated factors, including systematic inflammation. We assessed the cognitive function of COVID-19 survivors using THINC-integrated tool (THINC-it),8 which is a digital, gamified cognitive function evaluation tool (Harrison et al., 2018, Zhang et al., 2020). Using this tool, we evaluated comprehensive neuropsychiatric/cognitive function in COVID-19 survivors after 15 months and in matched healthy controls (HC).
2. Methods
2.1. Study design and participants
This was an observational cross-sectional study. We recruited 66 patients hospitalized for SARS-CoV-2 infection who were willing to participate in this study. Voluntary COVID-19 survivors who visited the second outpatient clinic, West China Hospital, Sichuan University, about fifteenth-month after their discharge from April 2021 to August 2021 were enrolled in the study. Healthy volunteers were recruited from nearby communities between August 2021 and December 2021. All subjects gave written informed consent.
2.2. The patients with COVID-19 group
The criteria for enrollment of patients with COVID-19 were as follows: (1) patients who were hospitalized and willing to participate in the follow-up process, and (2) participants who signed an informed consent form and were able to understand and complete the tests. The exclusion criteria were as follows: (1) Patients have some neurological diseases such as cerebrovascular disease, neurodegenerative diseases, encephalitis, and traumatic brain injury which may cause cognitive impairment based on MRI examination (3-T MRI system Philips Achieva) (2) patients who could not understand or read the informed consent form, and (3) patients who is taking psychotropic medicines. Finally, we recruited 66 COVID-19 survivors aged 13–66 years old into the study.
2.3. HC group
In total, 79 healthy participants matched with sex, gender, and education level were recruited voluntarily. Both male and female participants aged between 17 and 61 years were included. Clinical examination eliminated cognitive impairment caused by physical illness, as well as family history of psychiatric disorders. We excluded participants who were pregnant or lactating women and those who had hearing or visual impairments.
3. Assessment
3.1. Demographic data and basic clinical information
Demographic data, including age, sex, marital status, educational background, smoking history, occupation, and religion were collected. Meanwhile, routine clinical data, including length of hospital stay, isolation time, duration of nucleic acid positivity, and self-perceived disease severity were collected. The self-perception of disease severity was registered on an ordinal scale.
3.2. Neuropsychiatric interview
The Mini International Neuropsychiatric Interview (MINI version 5.0.0)9 (Si et al., 2009) was conducted to determine the mental health diagnoses of all participants. The results were evaluated by a trained physician. In addition, the severity of anxiety, depression symptoms, and post-traumatic stress disorder (PTSD)10 symptoms was self-reported using the Generalized Anxiety Disorder Assessment (GAD-7)11 (He et al., 2010) Patient Health Questionnaire-9 (PHQ-9)12 (Sun et al., 2017) and a 4-item Post-traumatic Stress Disorder Checklist for Diagnostic and Statistical manual of Mental disorders-5 scale (PCL-5)13 (Fung et al., 2019) for the COVID-19 group.
3.3. Cognitive assessment
Cognitive assessment was performed using a digital, simplified Chinese version of the THINC-it tool, which has been proven to have good reliability and validity in the Chinese adult population (Liu et al., 2021). It includes a 5-item Perceived Deficits Questionnaire for Depression (PHQ-5D),14 Spotter, Symbol Check, Codebreaker, and Trails (Hou et al., 2020, Zhang et al., 2020). The validity of the four objective test sections of the THINC-it tool (Spotter, Symbol Check, Codebreaker, and Trails) was used to evaluate the subjunctive’s attention/concentration, executive function, working memory, and processing speed. The five consistent criterion tests were the Deficits Questionnaire for Depression (PDQ-5D), Reaction time paradigm (RTI),15 Digit Symbol Substitution Test (DSST),16 Trail Making Test-Part-B (TMT-B),17 and One-back Task (1-back)18 (Zhang et al., 2020). RTI was selected as a measure of attention and executive function; 1-back paradigm was selected to test the attention, memory, and reaction speed of the participants; DSST was chosen to identify the executive function, processing speed, and attention/concentration; and TMT-B was selected to assess the executive function of the participants.
In addition to these four objective measures of cognition, the PHQ-5D questionnaire was included as a subjective measure of general cognitive function, including attention/concentration, retrospective and prospective memory, and planning/organization, using a 5-points ordinal categorical response scale to reflect the frequency of experiencing a specific cognitive problem in the past 7 days (Harrison et al., 2018, Lam et al., 2018).
3.4. Social support scale and discrimination evaluation
Xiao revised a Chinese version of the social support scale based on the relevant information (Xiao, 1994). Its validity and reliability have been previously confirmed (Liu et al., 2008). The social support scale contains 10 items, including 3 objective support, 4 subjective support, and 3 support utilizations. The score varies from 12 to 65 points, with a higher score representing greater social support and diversity of social networks (Su et al., 2012). We also gathered information regarding discrimination factors, including being ostracized by neighbors, family members, relatives, and friends, and being insulted by others. All the above factors were registered on an ordinal scale assigning degrees of “none,” “somewhat,” “moderate,” “severe,” and “extraordinarily severe.”
3.5. Serum sample collection and analysis
Venous blood samples (5 ml) were collected from each patient. The blood samples were centrifuged at 3500 r/min for 15 min, and serum was extracted for analysis. Cytokines in the serum/plasma were measured by a multiplexed flow cytometric assay using a human cytokine (kit catalog number: HSTCMAG-28SK) on a Luminex ® system (MAGPX ® WITH xPONENT, version 4.2, Merck Millipore, USA). In serum/plasma, measurements of interferon-inducible T cell alpha chemoattractant (I-TAC), granulocyte-macrophage colony-stimulating factor (GM-CSF),19 fractalkine, IFN-γ, IL-10, macrophage inflammatory protein-3α (MIP-3α),20 IL-2(p70), IL-13, IL-17A, IL-β, IL-2, IL-4, IL-23, IL-5, IL-6, IL-7, IL-8, MIP-1, MIP-β, and TNF-α were performed. The analysis was performed according to the manufacturer’s instructions (MILLIPLEX Analyst 5.1, Merck Millipore, USA). The samples were measured in duplicates. The range of the standard curves for all measured cytokines was pg/ml.
4. Statistical analysis
In this study, the COVID-19 survivors and HC group were matched by propensity score matching to control for covariates including age, sex, and years of education. All the collected data were analyzed using the R (version 4.1.1) package with an alpha set for significance at p < 0.05, which represents an acceptable probability of Type I error in a statistical test. The Shapiro–Wilk method was used to assess the normality of continuous variables. T-tests were used for normally distributed data, and non-parametric Mann–Whitney U tests were applied for continuously skewed data. Categorical data were analyzed using the chi-square test. An association test was conducted using the Spearman method if the data were not normally distributed. Multiple linear regression analysis was used to explore factors related to cognitive function. Outlier and influential points were detected before all analyses. The sample size with adequate power was calculated by GPower (version 3.1.9.7). For non-normally distributed data, effect size r were estimated (Fritz et al., 2012).
Demographic data and basic clinical information were displayed as frequencies and expressed as percentages (n, %). In the cognitive test, Symbol Check and Codebreaker were negatively associated with the severity of cognitive impairment; however, the scores of Spotter and Trails showed the opposite trend. To maintain the direction of each test result consistently, the results of PDQ-5D, Spotter, and Trails were converted into standard z-scores and multiplied by − 1, so that the results of each sub-part of THINC-it would follow a trend where the higher the score, the better the cognitive function (McIntyre et al., 2017, Zhang et al., 2020).
A multiple linear regression model was used to assess the independent predictors of the test scores for significantly different cognitive functions. We included 13 independent variables, including age, sex, years of education, length of hospital stay, nucleic acid test positive time, isolation time, self-perception of disease severity, being ostracized by family, neighbors, and community, being verbally abused, social support score, PHQ-9 score, and GAD-7 score. All categorical independent variables were recoded into dummy variables, which were used in the regression analysis to represent subgroups of the sample in the model.
5. Result
5.1. Demographic information and clinical characteristics
The demographic information, cognitive performance, and neuropsychiatric data of the participants are presented in Table 1. In total, 66 patients hospitalized for COVID-19 and 79 HC volunteers were included in the present study after adjusting for age, sex, and educational background. There were no significant differences in age, sex, or education. Only 4 COVID-19 survivors had a MINI diagnosis consistent with depression and anxiety and most COVID-19 survivors did not report subjective neuropsychiatric symptoms. The COVID-19 group performed less well in the Trails with a small effect size (r = 0.21, p = 0.047) than the HC group.
Table 1.
Demographics and cognitive performance of the study population.
| No. (%) | ||||
|---|---|---|---|---|
| Characteristic | COVID-19 (n = 66) | HC (n = 79) | 95% CI | P-value |
| Age (median [IQR]), y | 35.50 [26.25, 46.00] | 29.00 [25.00, 39.00] | / | 0.104a |
| Gender | 0.496b | |||
| Female | 23 (34.8) | 33 (41.8) | ||
| Male | 43(65.3) | 46(58.2) | ||
| Marriage Status | / | 0.013b | ||
| Single | 21 (31.8) | 43 (54.4) | ||
| Divorced | 2 (3.0) | 4 (5.1) | ||
| Married | 43 (65.2) | 32 (40.5) | ||
| Education Background | ||||
| Years of education (median [IQR]), y | 15.00 [9.75, 16.00] | 15.00 [15.00, 16.00] | / | 0.088a |
| Primary school | 4 (6.1) | 3 (3.8) | ||
| Junior high school | 13 (19.7) | 11 (13.9) | ||
| Senior high school | 7 (10.6) | 1 (1.3) | ||
| College | 15 (22.7) | 25 (31.6) | ||
| Undergraduate | 22 (33.3) | 26 (32.9) | ||
| Graduate | 5 (7.6) | 13 (16.5) | ||
| Smoking History | / | 0.334b | ||
| Non-smoker | 49 (74.2) | 55 (69.6) | ||
| Occasionally smoking | 3 (4.5) | 7 (8.9) | ||
| Smoker | 7 (10.6) | 13 (16.5) | ||
| Smoking cessation | 7 (10.6) | 4 (5.1) | ||
| Occupation | / | 0.079b | ||
| Full-time or part-time job | 53 (80.3) | 56 (70.9) | ||
| Retired or Jobless | 4 (6.1) | 7 (8.9) | ||
| Students | 6 (9.1) | 16 (20.3) | ||
| Others | 3 (4.5) | 0(0.0) | ||
| Religion | / | 0.982b | ||
| Have religion | 6 (9.1) | 6(7.5) | ||
| THINC-it test | ||||
| Response time of Nback (median [IQR])c | 50.98 [44.38, 59.00] | 49.83 [42.53, 59.05] | / | 0.435a |
| Response time of DSST (median [IQR]) | 118.88[117.64,119.53] | 119.10[118.23,119.51] | / | 0.362a |
| The composite z-score of PDQ-5D (median [IQR]) | 0.07 [− 0.54, 0.68] | 0.10 [− 0.51, 1.02] | / | 0.767a |
| Correct number of Symbol Check (median [IQR]) | 21.50 [13.00, 29.00] | 24.00 [13.50, 33.00] | / | 0.236a |
| Correct number of Codebreaker (mean (SD)) | 40.41 ± 14.44 | 41.41 ± 17.86 | -6.30–4.31 | 0.711d |
| The composite z-score of Spotter (mean (SD)) | 0.03 ± 0.98 | 0.00 ± 1.01 | -0.29–0.36 | 0.843d |
| The composite z-score of Trail (median [IQR]) | 0.23 [0.04, 0.33] | 0.33 [0.01, 0.47] | / | 0.047a |
| Neuropsychiatric test | ||||
| PHQ-9 score (median [IQR]) | 0.00 [0.00, 0.00] | / | / | / |
| GAD-9 score(median [IQR]) | 0.00 [0.00, 0.00] | / | / | / |
| PCL-5 score (median [IQR]) | 0.00 [0.00, 0.00] | / | / | / |
| M.I.N.I teste | ||||
| Major depression disorder (n) | 4 | 0 | / | / |
| General anxiety disorder (n) | 3 | 0 | / | / |
The analysis was conducted by Mann-Whitney U test.
The analysis was conducted by χ2 test.
IQR: Interquartile range
The analysis was conducted by Two sample T test.
Only 4 patients were diagnosed with depression or anxiety disorders (3 MDD patients are comorbid with anxiety disorders)
The clinical characteristics of the COVID-19 survivors are displayed in Table 2. The median length of hospital stays, isolation time of hospitalized survivors, and duration of nucleic acid positivity were 20.50 (14.25–30.00), 45.00 (30.00–60.00), and 20.00 (10.00–30.00) days, respectively. The majority of the survivors’ self-perceived disease severity was general and mild. Furthermore, participants rarely had the experience of intensive care unit (ICU) stay, and none of them had extraordinarily severe ostracization by neighbors, community, family members, relatives, or friends.
Table 2.
Clinical characteristics of the study COVID-19 survivors.
| No. (%) | |
|---|---|
| Clinical characteristics | COVID-19 (n = 66) |
| Length of hospital stays (median [IQR]) | 20.50 [14.25, 30.00] |
| Isolation time (median [IQR]) | 45.00 [30.00, 60.00] |
| Duration of nucleic acid positivity (median [IQR]) | 20.00 [10.00, 30.00] |
| Social support score (mean (SD)) | 40.21 (7.25) |
| Self-perceived disease severity | |
| Particularly severity | 0 (0.0) |
| Severe | 5 (7.6) |
| Somewhat severe | 4 (6.1) |
| General | 18 (27.3) |
| Mild | 39 (59.1) |
| Experience of ICU stay | |
| Yes | 5 (7.6) |
| Ostracized by neighbors and community | |
| None | 39 (59.1) |
| Somewhat | 19 (28.8) |
| Moderate | 5 (7.6) |
| Severe | 3 (4.5) |
| Extraordinary severe | 0 (0.0) |
| Ostracized by family members | |
| None | 0 |
| Somewhat | 1 (1.5) |
| Moderate | 0 |
| Severe | 0 |
| Extraordinary severe | 0 |
| Insulted by others | |
| None | 47 (71.2) |
| Somewhat | 14 (21.2) |
| Moderate | 5 (7.6) |
| Severe | 0 (0.0) |
| Extraordinary severe | 0 (0.0) |
5.2. Multiple linear regression for the prediction of cognitive function
To streamline the presentation of the results, Table 3 reports the findings from the linear multiple regressions. The associations between the basic clinical data, discrimination data, and cognitive function are summarized in Table 3. In the model, the results of multiple linear regression indicated that the four predictors explained 32.76 % of the variance (R2 = 0.328, F (20,38) = 2.584, p = 0.004). Multiple linear regression analysis revealed a significant association between ICU experience (β = −2.247, p < 0.0001), years of education (β = 0.098, p = 0.013), and self-perception of disease severity (somewhat severe) (β = −1.522, p = 0.007). The factors of ICU stay and self-perceived disease severity were negatively associated with cognitive function. In contrast, years of education was positively associated with executive function.
Table 3.
Variables to predict the composite z-score of Trail.
| Variables | β | Std. error | t-value | P-value |
|---|---|---|---|---|
| (Intercept) | -0.546 | 0.628 | -0.531 | 0.598 |
| Experience of ICU stay (Yes)a | -2.247 | 0.262 | -3.579 | P < 0.0001 * ** |
| Gender (Female) | -0.110 | 0.047 | -0.422 | 0.675 |
| Age | 0.017 | 0.018 | 0.355 | 0.724 |
| Social support score | -0.028 | 0.038 | -1.596 | 0.117 |
| Years of education | 0.098 | 0.004 | 2.580 | 0.013* |
| Isolation time | 0.000 | 0.013 | 0.056 | 0.956 |
| Length of hospital stay | -0.006 | 0.015 | -0.465 | 0.644 |
| Duration of nucleic acid positivity | 0.014 | 0.279 | 0.920 | 0.362 |
| Self-perceived disease severity (general) | 0.149 | 0.542 | 0.534 | 0.596 |
| Self-perceived disease severity (Somewhat severe)b | -1.522 | 0.632 | -2.809 | 0.007 * * |
| Self-perceived disease severity (severe) | 1.197 | 1.099 | 1.893 | 0.065 |
| Ostracized by family members (somewhat) | -0.809 | 0.379 | -0.736 | 0.466 |
| Insulted by others (somewhat) | 0.241 | 0.469 | 0.636 | 0.528 |
| Insulted by others (moderate) | 0.225 | 0.293 | 0.479 | 0.634 |
| Ostracized by neighbors and community (somewhat) | 0.181 | 0.585 | 0.617 | 0.541 |
| Ostracized by neighbors and community (moderate) | 0.453 | 0.651 | 0.774 | 0.443 |
| Ostracized by neighbors and community (severe) | -0.343 | 0.529 | -0.526 | 0.601 |
| PHQ-9 score | 0.397 | 0.110 | 0.749 | 0.458 |
| GAD-7 score | -0.092 | 0.628 | -0.836 | 0.407 |
Signif. codes: 0 ‘* ** ’ 0.001 ‘* *’ 0.01 ‘* ’ 0.05 ‘.’ 0.1 ‘ ’ 1
Residual standard error: 0.8504 on 45 degrees of freedom
Multiple R-squared: 0.5345, Adjusted R-squared: 0.3276
F-statistic: 2.584 on 20 and 38 DF, p-value: 0.004166
: The reference variable is experience of ICU stays (NO)
: Self-perceived disease severity (mild)
5.3. Associations between neuropsychiatric symptoms, inflammatory factors, and the cognitive test
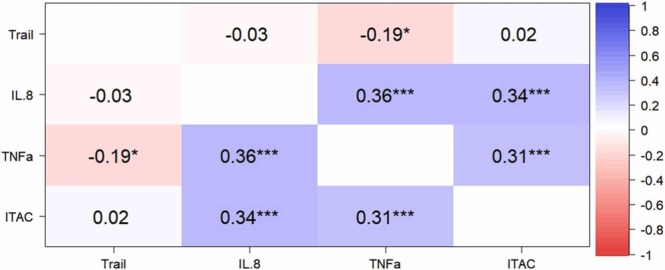
There is no statistically significance between scores for depression/anxiety/PTSD and cytokine levels and cognition. Comparison tests revealed significant differences after false discovery rate (FDR)21 correction between the COVID-19 (n = 55) and HC groups (n = 79) with regard to the I-TAC (adjusted p = 0.021), IL-8 (adjusted p < 0.0001), and TNF-α (adjusted p < 0.0001). The HC groups show higher I-TAC level (87.68 ± 32.56) compared with COVID-19 group (33.95 ± 9.59) in follow-up. Similarly, the IL.8 level is 18.58 ± 11.54 for HC groups which is higher than COVID-19 group (6.55 ± 6.51). However, the TNF-α is 8.39 ± 1.76 which is elevated in COVID-19 group than in HC group (6.58 ± 1.76). Eleven COVID-19 survivors did not participate in blood sample collection. Moreover, the correlation analyses indicated that TNF-α levels were negatively correlated with the composite z-score of the Trails after FDR correlation (Spearman’s Rho; r = −0.19, p = 0.040) ( Fig. 1).
Fig. 1.
Association analysis between the composite z-score of Trail and inflammatory factors. The matrix diagram shows the correlation coefficient and significance level after FDR correction. The TNF-α level is statistically significant correlated with the composite z-score of Trail. (r = −0.19, p = 0.040). The “* ” represents significance level, and the more of it, the more significance.
6. Discussion
To our knowledge, this is the first study on neurocognitive evaluation using the THINC-it tool for COVID-19 survivors in a 15-month follow-up time. Our analysis exhibited differences in the performance on the Trails, which evaluates executive function, compared with the HC group at 15-months of follow-up. A potential correlation between serum TNF-α levels and executive function has also been reported. In hospitalized COVID-19 survivors, executive function was negatively correlated with the experience of ICU stay and self-perceived disease severity. These associations were independent of age, sex, and years of education.
Cognitive deficit is one of the most common symptoms reported in research into Long COVID. Those who experienced “post-COVID-19 syndrome”/ “post-acute sequalae SARS-CoV-2”/ “Long COVID” following the COVID-19 infection may have cognitive dysfunction (Guo et al., 2022). Based on our understanding of the mechanism of the virus in the CNS and the emerging evidences available, one can expect to have a variety of cognitive consequences of COVID-19 infection including attention, dysexecutive symptoms and hypoperfusion (Kumar et al., 2021). In this study, COVID-19 survivors were admitted for a clinical physical examination to rule out cognitive impairment due to physical illness. We examined the objectively cognitive function of the 66 hospitalized COVID-19 survivors 15 months after hospital discharge, as well as in 79 recurred healthy participants based on age, sex, and educational background. We reported the differences in executive function between the two groups. Our results are in line with the results of a previous study that reported a significant difference in executive function between patients with COVID-19 and a healthy control group (Johnsen et al., 2021). Similarly, another study showed that the most pronounced impairments in patients with COVID-19 were seen in verbal learning and executive function evaluated using TMT-B, based on age, sex, and education, compared to a matched HC group (Miskowiak et al., 2021).
We did not find any group differences in attention, memory, reaction speed, or processing speed, which is contrary to earlier research (Ferrucci et al., 2021). A previous study using the Montreal Cognitive Assessment indicated an improvement in cognitive function from discharge time to 6 months of follow-up, while another study showed a greater increase in duration to complete the TMT-B at follow-up time in patients with COVID-19 compared to healthy controls (Douaud et al., 2022, Nersesjan et al., 2022). We can only speculate that the possible reason for the difference in results from previous studies might be that the cognitive function of COVID-19 survivors improved over time, and most of the earlier evaluations were performed using objective tools.
Further, we found that the experience of ICU stay and self-perceived disease severity were negatively associated with cognitive function. Patients with COVID-19 who had experienced ICU stay showed worse executive function at 15 months of follow-up. This result was in line with that from an earlier study which indicated that new or worse cognitive impairment commonly occurs and persists in survivors of ICU stay (McLoughlin et al., 2020). The COVID-19 survivors suffered some degree of isolation and discrimination; however, no significant correlation between discrimination or other social isolation factors and cognitive function have been identified. One possible reason might be that most of the discrimination faced by the patients with COVID-19 was mild, and none of them had experienced extraordinarily severe discrimination. As shown in Table 2, they received adequate social support from family members, friends, and society. Another possible reason is that the privacy of patients in Sichuan is well-protected and a certain degree of psychological education is provided to them, making them less vulnerable to social isolation and discrimination.
SARS-CoV-2 can damage the nervous system through indirect infection such as cytokine storm which refers to an uncontrolled excessive inflammatory reaction (Du et al., 2021). In current study, we demonstrated that there were differences in I-TAC, IL-8, and TNF-α levels after FDR correction between the two groups. Our finding is consistent with the higher levels of inflammatory factors reported in other studies (Heneka et al., 2020, Huang et al., 2020). In contrast, we did not find any differences in the levels of IL-13, IL-1b, IL-6, IL-23, fractalkine, MIP-3a, IL-17A, IL-5, IFN-γ, GM-CSF, IL-7, IL-4, IL-21, MIP-1a, IL-2 between the COVID-19 and HC groups. Furthermore, there was a significant correlation between the serum TNF-α levels and executive function at 15 months of follow-up. A pervious study showed the systemic inflammation was a predict factor to the neurocognitive performance (Mazza et al., 2021). Inflammatory markers including TNF-α, TNF-β, IL-1β, IL-4, IL-6, IL-8, and IL-13 was found to be correlated with post-acute sequelae of COVID-19 (Schultheiss et al., 2022).
Pro-inflammatory mediators can compromise the permeability of BBB via upregulation of cyclooxygenase-2 and activation of matrix metalloprotease (Dehghani et al., 2022). This enables cytokines to enter the CNS, causing microglial activation and oxidative stress, leading to the development of synergistic cognitive impairment (Baker et al., 2021). TNF-α is a prototypic proinflammatory cytokine that is crucial in initiating and sustaining the inflammatory response (Belarbi et al., 2012). Large amount of evidences show that TNF is a main mediator of secondary CNS damage after acute injury and under conditions of chronic inflammation (Probert, 2015). It exerts both homeostatic and pathophysiological effects in the CNS (Montgomery and Bowers, 2012). In healthy adults, TNF is constitutively expressed at low level, and has regulatory functions on crucial physiological processes while in pathological ones, astrocytes and mainly microglia release large amounts of TNF-α (Olmos and Lladó, 2014, Probert, 2015). Over release of TNF-α pathologically can be involved in the process of increased apoptosis and decreased neuroplasticity of nerve cells through neurotoxicity and lead to cognitive impairment(Olmos and Lladó, 2014).
In an earlier study, compared with wild-type mice, TNF-α deficient mice demonstrate a decreased latency in finding the underwater platform in the Morris water maze testing. This suggests enhanced hippocampal memory function in TNF-α knock-out mice (Golan et al., 2004). Moreover, Fiore et al. conducted the same assessments to invest whether endogenous brain TNF-α elevation in transgenic mice was associated with changes in learning capabilities; overexpression of TNF-α impaired hippocampal learning/memory function, indicating a suppressive role for high-level TNF-α in cognition (Fiore et al., 2000).
However, we did not find any correlation between depression/anxiety/PTSD scores and cytokine levels. This is in line with an earlier study which evaluate the relationship between psychiatric symptoms and hematological inflammatory markers. This may because that most COVID-19 survivors recovered from psychiatric symptoms, thereby inflammation is not a significant contributor to psychiatric morbidity in patients with COVID-19 in the long term. (Swami et al., 2022).
The current study was an observational cross-sectional study that evaluated the cognitive functions of hospitalized patients with COVID-19 at 15 months of follow-up, and its related risk factors. However, this study has some limitations. First, we could not gather the information which is related to the cognitive impairment, including prolonged hypoxia, requirement for ventilatory support, and steroid therapy from the hospitals. Second, although existing studies have shown that systemic or CNS inflammation can cause psychiatric symptoms and cognitive deficits, we did not find any correlation between depression/anxiety/PTSD symptoms and cognition. This may be because only 4 COVID-19 survivors in present study had symptoms of depression or anxiety. Third, the blood sample was collected only once at follow-up visiting so that we could not determine whether inflammatory levels remain correlated with cognitive decline over time. A further study on longitudinal changes in neuropsychiatric symptoms, cognitive function and inflammatory levels would be necessarily.
7. Conclusions
In conclusion, we observed that COVID-19 survivors remain subtle cognitive impairment compared with healthy ones, especially on executive function at 15-month from hospital discharge. Additionally, the experience of ICU stay and self-perceived illness severity were associated with executive function. The serum TNF-α level continued to be abnormal compared with matched HC and was correlated with executive function in COVID-19 survivors even at 15 months after recovery. This study provides evidence of long-term cognitive function impairments in patients with COVID-19, and its relationship with inflammatory profiles.
Funding
This work was supported by China National Key Research and Development Program (2020AAA0105005), Department of Science and Technology of Sichuan Province (Grant numbers 2020YFS0582, 2020YFS0231), 1·3·5 Project for Disciplines of excellence, West China Hospital, Sichuan University (Grant number ZYJC21004), the National Natural Science Foundation of China (Grant numbers 81871061), and by Postdoctoral Foundation of West China Hospital (Grant number 2020HXBH041 to M.Y.).
Declaration of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The authors would like to thank all the participants, as well as all facilitators that contributed to this research and especially Minlan Yuan.
Ethics Approval and Consent to Participate
The research processes adhered to the Declaration of Helsinki (2008) Ethical Principles for Medical Research Involving Human Subjects and were approved by the Medical Ethics Committee of West China Hospital, Sichuan University. Besides, all the voluntary subjects gave written consents and were informed they were at liberty to withdraw from the study at any time, with an additional examination.
Consent for Publication
Not applicable.
Authors’ contributions
DMH collected data on patients who came to the hospital for follow-up visits, analyzed data collected, and wrote the manuscript. RLY, LB, JYY, XL, BL, SYL, and SMZ collected patients’ follow-up data as well. MLY designed this research and the revised the draft critically for important intellectual content. WZ is the peer reviewer and designed this research. All authors read and gave the final approval of the version to be published.
Footnotes
COVID-19: The Coronavirus disease 19
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2
CNS: Central Nervous System
IL-: Interleukin-
IFN-: Interferon-
TNF-α: Tumor Necrosis Factor alpha
THINC-it: THINC-Integrated Tool
MINI: Mini International Neuropsychiatric Interview
PTSD: Post-traumatic stress disorder
GAD-7: Generalized Anxiety Disorder Assessment
PHQ-9: Patient Health Questionnaire-9
PCL-5: Post-traumatic Stress Disorder Checklist for Diagnostic and Statistical manual of Mental disorders-5 scale
PHQ-5D: 5-item Perceived Deficits Questionnaire for Depression
RTI: Reaction time paradigm
DSST:Digit Symbol Substitution Test
TMT-B: Trail Making Test-Part-B
1-back: One-back Task
GM-CSF: Granulocyte-macrophage Colony-stimulating Factor
MIP: Macrophage Inflammatory Protein
FDR: false discovery rate
References
- Alvarez M., Trent E., Goncalves B.S., Pereira D.G., Puri R., Frazier N.A., Sodhi K., Pillai S.S. Cognitive dysfunction associated with COVID-19: Prognostic role of circulating biomarkers and microRNAs. Front. Aging Neurosci. 2022;14:1020092. doi: 10.3389/fnagi.2022.1020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin-Chowdhury Z., Harris R.J., Aiano F., Zavala M., Bertran M., Borrow R., Linley E., Ahmad S., Parker B., Horsley A., Hallis B., Flood J., Brown K.E., Amirthalingam G., Ramsay M.E., Andrews N., Ladhani S.N. Characterising post-COVID syndrome more than 6 months after acute infection in adults; prospective longitudinal cohort study. medRxiv. 2021 2021.2003.2018.21253633. [Google Scholar]
- Badenoch J.B., Rengasamy E.R., Watson C., Jansen K., Chakraborty S., Sundaram R.D., Hafeez D., Burchill E., Saini A., Thomas L., Cross B., Hunt C.K., Conti I., Ralovska S., Hussain Z., Butler M., Pollak T.A., Koychev I., Michael B.D., Holling H., Nicholson T.R., Rogers J.P., Rooney A.G. Persistent neuropsychiatric symptoms after COVID-19: a systematic review and meta-analysis. Brain Commun. 2022;4 doi: 10.1093/braincomms/fcab297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H.A., Safavynia S.A., Evered L.A. The 'third wave': impending cognitive and functional decline in COVID-19 survivors. Br. J. Anaesth. 2021;126:44–47. doi: 10.1016/j.bja.2020.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belarbi K., Jopson T., Tweedie D., Arellano C., Luo W., Greig N.H., Rosi S. TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J. Neuroinflamm. 2012;9:23. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceban F., Ling S., Lui L.M.W., Lee Y., Gill H., Teopiz K.M., Rodrigues N.B., Subramaniapillai M., Di Vincenzo J.D., Cao B., Lin K., Mansur R.B., Ho R.C., Rosenblat J.D., Miskowiak K.W., Vinberg M., Maletic V., McIntyre R.S. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani A., Zokaei E., Kahani S.M., Alavinejad E., Dehghani M., Meftahi G.H., Afarinesh M.R. The potential impact of Covid-19 on CNS and psychiatric sequels. Asian J. Psychiatr. 2022;72 doi: 10.1016/j.ajp.2022.103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., Jbabdi S., Taschler B., Keating P., Winkler A.M., Collins R., Matthews P.M., Allen N., Miller K.L., Nichols T.E., Smith S.M. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Zhao W., Du L., Liu J. Neuropsychiatric symptoms associated with the COVID-19 and its potential nervous system infection mechanism: the role of imaging in the study. Psychoradiology. 2021;1:199–211. doi: 10.1093/psyrad/kkab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci R., Dini M., Groppo E., Rosci C., Reitano M.R., Bai F., Poletti B., Brugnera A., Silani V., D'Arminio Monforte A., Priori A. Long-lasting cognitive abnormalities after COVID-19. Brain Sci. 2021:11. doi: 10.3390/brainsci11020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M., Angelucci F., Alleva E., Branchi I., Probert L., Aloe L. Learning performances, brain NGF distribution and NPY levels in transgenic mice expressing TNF-alpha. Behav. Brain Res. 2000;112:165–175. doi: 10.1016/s0166-4328(00)00180-7. [DOI] [PubMed] [Google Scholar]
- Fritz C.O., Morris P.E., Richler J.J. Effect size estimates: current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012;141:2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- Fung H.W., Chan C., Lee C.Y., Ross C.A. Using the post-traumatic stress disorder (PTSD) checklist for DSM-5 to screen for PTSD in the Chinese context: a pilot study in a psychiatric sample. J. Evid. -Based Soc. Work. 2019;2019(16):643–651. doi: 10.1080/26408066.2019.1676858. [DOI] [PubMed] [Google Scholar]
- Golan, H., Levav, T., Mendelsohn, A., Huleihel, M., 2004. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cerebral cortex (New York, N.Y.: 1991) 14, 97–105. [DOI] [PubMed]
- González-Hermosillo J.A., Martínez-López J.P., Carrillo-Lampón S.A., Ruiz-Ojeda D., Herrera-Ramírez S., Amezcua-Guerra L.M., Martínez-Alvarado M.D.R. Post-Acute COVID-19 symptoms, a potential link with myalgic encephalomyelitis/chronic fatigue syndrome: a 6-month survey in a mexican cohort. Brain Sci. 2021:11. doi: 10.3390/brainsci11060760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick P.B. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann. N. Y. Acad. Sci. 2010;1207:155–162. doi: 10.1111/j.1749-6632.2010.05726.x. [DOI] [PubMed] [Google Scholar]
- Guo P., Benito Ballesteros A., Yeung S.P., Liu R., Saha A., Curtis L., Kaser M., Haggard M.P., Cheke L.G. COVCOG 2: cognitive and memory deficits in long COVID: a second publication from the COVID and cognition study. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.804937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Trender W., Chamberlain S.R., Jolly A.E., Grant J.E., Patrick F., Mazibuko N., Williams S.C., Barnby J.M., Hellyer P., Mehta M.A. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q., Zheng B., Daines L., Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022:11. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J.E., Barry H., Baune B.T., Best M.W., Bowie C.R., Cha D.S., Culpepper L., Fossati P., Greer T.L., Harmer C., Klag E., Lam R.W., Lee Y., Mansur R.B., Wittchen H.U., McIntyre R.S. Stability, reliability, and validity of the THINC-it screening tool for cognitive impairment in depression: a psychometric exploration in healthy volunteers. Int. J. Methods Psychiatr. Res. 2018;27 doi: 10.1002/mpr.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.Y., Li C.B., Qian J., Cui H.S., Wu W.Y. Reliability and validity of generalized anxiety disorder scale in general hospital outpatients. Shanghai Arch. Psychiatry. 2010;22:200–203. [Google Scholar]
- Heneka M.T., Golenbock D., Latz E., Morgan D., Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimer's Res. Ther. 2020;12:69. doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Yao S., Hu S., Zhou Q., Han H., Yu X., McIntyre R.S., Shi C. PSYCHOMETRIC properties of the Chinese version of the THINC-it tool for cognitive symptoms in patients with major depressive disorder. J. Affect. Disord. 2020;273:586–591. doi: 10.1016/j.jad.2020.03.146. [DOI] [PubMed] [Google Scholar]
- Hu Y., Chen Y., Zheng Y., You C., Tan J., Hu L., Zhang Z., Ding L. Factors related to mental health of inpatients with COVID-19 in Wuhan, China. Brain Behav. Immun. 2020;89:587–593. doi: 10.1016/j.bbi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iodice F., Cassano V., Rossini P.M. Direct and indirect neurological, cognitive, and behavioral effects of COVID-19 on the healthy elderly, mild-cognitive-impairment, and Alzheimer's disease populations. Neurol. Sci.: Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021;42:455–465. doi: 10.1007/s10072-020-04902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen S., Sattler S.M., Miskowiak K.W., Kunalan K., Victor A., Pedersen L., Andreassen H.F., Jørgensen B.J., Heebøll H., Andersen M.B., Marner L., Hædersdal C., Hansen H., Ditlev S.B., Porsbjerg C., Lapperre T.S. Descriptive analysis of long COVID sequelae identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Res. 2021:7. doi: 10.1183/23120541.00205-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N., Kim Y., Saito M., Fujishiro S. People's worry about long-term impact of COVID-19 pandemic on mental health. Asian J. Psychiatr. 2022;75 doi: 10.1016/j.ajp.2022.103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H., Asseo K., Karni N., Benjamini Y., Nir-Paz R., Muszkat M., Israel S., Niv M.Y. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin. Microbiol. Infect.: Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021;27:769–774. doi: 10.1016/j.cmi.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Veldhuis A., Malhotra T. Neuropsychiatric and cognitive sequelae of COVID-19. Front. Psychol. 2021;12 doi: 10.3389/fpsyg.2021.577529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam R.W., Lamy F.X., Danchenko N., Yarlas A., White M.K., Rive B., Saragoussi D. Psychometric validation of the perceived deficits questionnaire-depression (PDQ-D) instrument in US and UK respondents with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018;14:2861–2877. doi: 10.2147/NDT.S175188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.W., Li F.Y., Lian Y.L. Investigation of reliability and validity of the social support scale. J. Xinjiang Med. Univ. 2008:31. [Google Scholar]
- Liu W.Y., Zhang F.X., Guo Q., XU H., Liu C.P., Chen H.Y., Wang X.L., Hu Y., Zhang T.H., Li G.J., Liu X.H. Research on the cognitive function in patients with major depressive disorder based on Thinc-integrated. J. Clin. Psychiatry. 2021;31:434–437. [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., group C.-B.O.C.S., Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre R.S., Best M.W., Bowie C.R., Carmona N.E., Cha D.S., Lee Y., Subramaniapillai M., Mansur R.B., Barry H., Baune B.T., Culpepper L., Fossati P., Greer T.L., Harmer C., Klag E., Lam R.W., Wittchen H.U., Harrison J. The THINC-integrated tool (THINC-it) screening assessment for cognitive dysfunction: validation in patients with major depressive disorder. J. Clin. Psychiatry. 2017;78:873–881. doi: 10.4088/JCP.16m11329. [DOI] [PubMed] [Google Scholar]
- McLoughlin B.C., Miles A., Webb T.E., Knopp P., Eyres C., Fabbri A., Humphries F., Davis D. Functional and cognitive outcomes after COVID-19 delirium. Eur. Geriatr. Med. 2020;11:857–862. doi: 10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez R., Balanzá-Martínez V., Luperdi S.C., Estrada I., Latorre A., González-Jiménez P., Bouzas L., Yépez K., Ferrando A., Reyes S., Menéndez R. Long-term neuropsychiatric outcomes in COVID-19 survivors: a 1-year longitudinal study. J. Intern. Med. 2022;291:247–251. doi: 10.1111/joim.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K.W., Johnsen S., Sattler S.M., Nielsen S., Kunalan K., Rungby J., Lapperre T., Porsberg C.M. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K.W., Fugledalen L., Jespersen A.E., Sattler S.M., Podlekareva D., Rungby J., Porsberg C.M., Johnsen S. Trajectory of cognitive impairments over 1 year after COVID-19 hospitalisation: pattern, severity, and functional implications. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 2022;59:82–92. doi: 10.1016/j.euroneuro.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.L., Bowers W.J. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J. NeuroImmune Pharmacol.: Off. J. Soc. NeuroImmune Pharmacol. 2012;7:42–59. doi: 10.1007/s11481-011-9287-2. [DOI] [PubMed] [Google Scholar]
- Nersesjan V., Fonsmark L., Christensen R.H.B., Amiri M., Merie C., Lebech A.M., Katzenstein T., Bang L.E., Kjærgaard J., Kondziella D., Benros M.E. Neuropsychiatric and cognitive outcomes in patients 6 months after COVID-19 requiring hospitalization compared with matched control patients hospitalized for non-COVID-19 illness. JAMA Psychiatry. 2022;79:486–497. doi: 10.1001/jamapsychiatry.2022.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira J., Gerardo B., Silva A.R., Pinto P., Barbosa R., Soares S., Baptista B., Paquete C., Cabral-Pinto M., Vilar M.M., Simões M.R., Freitas S. Effects of restraining measures due to COVID-19: Pre- and post-lockdown cognitive status and mental health. Curr. Psychol. 2021:1–10. doi: 10.1007/s12144-021-01747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos G., Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti S., Palladini M., Mazza M.G., De Lorenzo R., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: role of depression and impact on quality of life. Eur. Arch. Psychiatry Clin. Neurosci. 2022;272:773–782. doi: 10.1007/s00406-021-01346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience. 2015;302:2–22. doi: 10.1016/j.neuroscience.2015.06.038. [DOI] [PubMed] [Google Scholar]
- Schultheiss C., Willscher E., Paschold L., Gottschick C., Klee B., Henkes S.S., Bosurgi L., Dutzmann J., Sedding D., Frese T., Girndt M., Holl J.I., Gekle M., Mikolajczyk R., Binder M. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022;3 doi: 10.1016/j.xcrm.2022.100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si T.M., Shu L., Dang W.M., Se Y.A., Chen J.X., Dong W.T., Kong Q.M., zhang W.H. Evaluation of the reliability and validity of Chinese version of the mini-international neuropsychiatric interview in patients with mental disorders. Chin. Ment. Health J. 2009;23:493–503. [Google Scholar]
- Su D., Wu X.N., Zhang Y.X., Li H.P., Wang W.L., Zhang J.P., Zhou L.S. Depression and social support between China' rural and urban empty-nest elderly. Arch. Gerontol. Geriatr. 2012;55:564–569. doi: 10.1016/j.archger.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Sun X.Y., Li Y.X., Yu C.Q., Li L.M. [Reliability and validity of depression scales of Chinese version: a systematic review]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2017;38:110–116. doi: 10.3760/cma.j.issn.0254-6450.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Swami M.K., Mahal P., Arora I.K., Mishra V.C., Panda T.K., Nebhinani N., Kumar D., Banerjee M., Garg M.K. Psychiatric morbidity among patients attending the post-COVID clinic and its association with hematological inflammatory markers. Asian J. Psychiatr. 2022;78 doi: 10.1016/j.ajp.2022.103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R. Moving beyond COVID. Asian J. Psychiatr. 2022;73 doi: 10.1016/j.ajp.2022.103178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.J., Shwani T., Liu J., Zhong P., Yang F., Schatz K., Zhang F., Pralle A., Yan Z. Molecular and cellular mechanisms for differential effects of chronic social isolation stress in males and females. Mol. Psychiatry. 2022;27:3056–3068. doi: 10.1038/s41380-022-01574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S.Y. Theoretical foundations and research applications of the social support scale. J. Clin. Psychiatry. 1994;4:98–100. [Google Scholar]
- Zhang W., Zhu N., Lai J., Liu J., Ng C.H., Chen J., Qian C., Du Y., Hu C., Chen J., Hu J., Wang Z., Zhou H., Xu Y., Fang Y., Shi C., Hu S. Reliability and validity of THINC-it in evaluating cognitive function of patients with bipolar depression. Neuropsychiatr. Dis. Treat. 2020;16:2419–2428. doi: 10.2147/NDT.S266642. [DOI] [PMC free article] [PubMed] [Google Scholar]