Abstract
The malaria parasite Plasmodium falciparum utilizes molecules present on the surface of uninfected red blood cells (RBC) for rosette formation, and a dependency on ABO antigens has been previously shown. In this study, the antirosetting effect of immune sera was related to the blood group of the infected human host. Sera from malaria-immune blood group A (or B) individuals were less prone to disrupt rosettes from clinical isolates of blood group A (or B) patients than to disrupt rosettes from isolates of blood group O patients. All fresh clinical isolates and laboratory strains exhibited distinct ABO blood group preferences, indicating that utilization of blood group antigens is a general feature of P. falciparum rosetting. Soluble A antigen strongly inhibited rosette formation when the parasite was cultivated in A RBC, while inhibition by glycosaminoglycans decreased. Furthermore, a soluble A antigen conjugate bound to the cell surface of parasitized RBC. Selective enzymatic digestion of blood group A antigen from the uninfected RBC surfaces totally abolished the preference of the parasite to form rosettes with these RBC, but rosettes could still form. Altogether, present data suggest an important role for A and B antigens as coreceptors in P. falciparum rosetting.
Although erythrocytes have traditionally been considered relatively inert containers of hemoglobin, mounting evidence suggests that they in fact bear numerous surface molecules that are active in microbial attachment processes (22). The asexual stages of the malaria parasite Plasmodium falciparum utilize molecules on the surface of uninfected red blood cells (RBC) for rosette formation, i.e., binding of parasite-infected RBC (pRBC) to uninfected RBC. Rosetting has been associated with the occurrence of severe malaria, i.e., cerebral malaria and anemia (5, 14, 17, 18). The P. falciparum erythrocyte membrane protein 1 (PfEMP1), a member of a family of high-molecular-weight polypeptides encoded by the var genes (4, 20, 21), has been identified as a rosetting ligand (8, 19). Various host receptors on the surface of uninfected RBC have been proposed, including ABO blood group antigens (7), CD36 (11), CD35 (19), and heparan sulfate (HS) or HS-like molecules (3, 8). Still, little is known about the relative contribution of different receptors in rosetting and their prevalence in nature. Previous studies have implicated the ABO blood group type in rosetting (1, 18, 24) and strain-specific blood group preferences for rosette formation have been described previously (7).
In this study, we examine the utilization of ABO blood group antigens for rosette formation in laboratory strains and fresh clinical isolates. We assess the antirosetting effect of hyperimmune sera in relation to the ABO blood group type and examine the relationship of the A antigen to the rosetting phenotype.
MATERIALS AND METHODS
Patients, parasites, and sera.
Two P. falciparum strains, FCR3S1 (a blood group A-preferring line [7] cloned by limiting dilution from FCR3S, which originated from the FCR strain isolated in The Gambia, West Africa [9]) and TM 284 (a blood group B-preferring strain isolated from a malaria patient in Thailand [7]), were cultivated according to standard procedures (25). Fresh P. falciparum rosetting clinical isolates were obtained from patients attending the Albert Schweitzer Hospital, Lambaréné, Gabon. Assessment for parasite growth and rosetting was done as described previously (3). Immune sera were obtained from healthy volunteers living in areas of endemicity (2). Nonimmune sera were from healthy Swedish volunteers.
Reagents.
Heparin and HS from porcine intestine (Løvens Kemiske Fabrik, Ballerup, Denmark), chondroitin sulfate A (Sigma), H-disaccharide (Fucα1-2Galβ), A-trisaccharide (GalNAcα1-3[Fucα1-2]Galβ), and B-trisaccharide (Galα1-3[Fucα1-2]Galβ) from Calbiochem-Novabiochem Corp. (La Jolla, Calif.) and Accurate Chemical & Scientific Corp. (Westbury, N.Y.) were used in the assays. A-tri-PAA-Fluorescein, a complex based on a synthetic polyacrylamide with 20% A trisaccharide-carbohydrate content, was from Syntesome Glycosystems (Munich, Germany). Treatments of blood group O RBC, blood group A RBC, and parasite cultures were performed with heparinase III (0.033 IU/ml, 25°C, pH 7.5; Sigma and Seikagaku Corp. [Tokyo, Japan]), Clostridium perfringens neuraminidase (0.1 IU/ml, 37°C, pH 6.0; Sigma), trypsin (37°C, pH 7.5; Sigma), chymotrypsin (37°C, pH 7.5; Sigma), and sodium periodate (10 mM, 15 min at room temperature [RT]) as described previously (3) and with α-N-acetyl-galactosaminidase, α-galactosaminidase, or α-galactosidase (all from Sigma) as described below. ABO blood group was determined by a standard hemagglutination technique using monoclonal antibodies (MAbs) (Biogenesis, Poole, England).
Rosette inhibition assays.
For assessment of rosette disruption, aliquots (25 μl) of parasite cultures were mixed with heat-inactivated serum diluted in RPMI medium in a 96-well microtiter plate, at a final dilution of 1:10. After incubation for 30 min at 37°C, the rosetting rate was assessed and compared to that of culture mixed with nonimmune serum (2). For assessment of rosette reformation, glycoconjugates were added to the cultures at a concentration of 100 μg/ml (heparin, HS, and chondroitin sulfate A) or 125 mM (A and B trisaccharides and H disaccharide) as described previously (3). Disruption of rosettes was subsequently done mechanically by drawing the cells through an injection needle (diameter = 0.60 mm) 8 to 10 times. The rosettes were then allowed to re-form. Inhibition was estimated by comparing the rosetting rate of each sample with that of a sample without additives (7).
Serum-induced agglutination assay.
The infected erythrocyte agglutination assay was performed at a serum end dilution of 1:5 and scored as described previously (2). Sera from nonimmune Swedish donors were used as controls.
Assessment of the blood group preference of fresh P. falciparum isolates.
A competition assay using RBC labeled with carboxy-fluorescein diacetate (C-FDA) (Sigma) was performed as described previously (7), with some modifications (3). Briefly, fresh RBC of blood group A Rh+, B Rh+, or O Rh+ from nonimmune donors were incubated with C-FDA (250 μg/ml) for 15 min at RT. The cells were washed three times with RPMI medium and finally resuspended at a 5% hematocrit in malaria culture medium (RPMI 1640-HEPES, 25 mM sodium bicarbonate, 10 μg of gentamicin per ml) containing 10% AB+ serum. Three separate aliquots (100 μl) of the same parasite culture were each mixed with an equal volume of a C-FDA-labeled RBC suspension (blood groups A, B, and O, respectively). The rosettes were disrupted mechanically as indicated above. After a 30-min incubation at 37°C, rosette re-formation was assessed and the proportion of labeled to unlabeled RBC participating in rosettes was calculated. The blood group preference was defined by comparing the rates obtained for A, B, and O RBC, respectively.
Enzymatic conversion of blood group A RBC into blood group O RBC.
The treatment of RBC was performed essentially as described previously (13, 16). Briefly, RBC were washed three times in sodium citrate buffer, pH 4.5. Twenty microliters of packed erythrocytes was subjected to treatment with 2 IU of α-N-acetyl-galactosaminidase or α-galactosaminidase at 37°C for 120 min. Degradation of blood group A antigen was monitored by using fluorescein isothiocyanate (FITC)-labeled lectin from Bandeiraea simplicifolia (Sigma) and blood group A MAb (Biogenesis).
Appearance of blood group O antigen was assessed by using FITC-labeled lectin from Tetragonolobus purpureas (Sigma). The relative capacity of enzymatically treated RBC to form rosettes was determined as follows: α-N-acetyl-galactosaminidase- or α-galactosaminidase-treated blood group O Rh+ or blood group A Rh+ RBC were labeled with C-FDA as indicated above. Controls were mock treated and labeled in parallel.
Labeled enzyme-treated RBC were mixed with an equal volume of fresh rosetting culture (5% hematocrit) grown in blood group O or blood group A RBC. The rosettes were disrupted mechanically and incubated for 30 min at 37°C to allow re-formation. The proportion of labeled to unlabeled RBC in rosettes was then assessed by epifluorescence microscopy (Nikon). Enzymatic treatment of blood group B RBC with α-galactosidase was performed as described above.
Binding of fluorescent A-trisaccharide to pRBC.
A culture with late-stage parasites was washed three times in phosphate-buffered saline (PBS) and incubated for 30 min at RT in the presence of the A-trisaccharide conjugate (100 μg/ml). After three washes in PBS, an aliquot was mixed with ethidium bromide to counterstain the pRBC. Surface fluorescence was assessed by epifluorescence microscopy (Nikon). The fluorescence rate was expressed as the number of fluorescent pRBC relative to the total number of pRBC (n = 300).
Competition of FITC-conjugate binding by soluble A-trisaccharide and H-disaccharide was assayed by simultaneously adding a fixed concentration of FITC conjugate (100 μg/ml) and a variable concentration of competitor to a parasite culture previously washed three times in PBS. After 30 min of incubation at RT, the culture was washed three times in PBS and the fluorescence rate was measured as indicated above.
Cultures washed three times in PBS were subjected to trypsin treatment at indicated concentrations for 10 min at 37°C. Immediately after trypsin addition, rosettes were disrupted mechanically. The reaction was stopped after 10 min with an excess of soybean trypsin inhibitor (Sigma). After two washes in PBS the sample was divided in two portions. One portion was resuspended in malaria culture medium containing 10% AB+ serum, and the rosetting rate was assessed after 30 min as indicated above. The second portion was subjected to labeling with A-trisaccharide–FITC, after which the fluorescence rate was measured.
Data analysis.
Statistical analyses were done with the StatView program (Abacus Concepts Inc.). Data were analyzed by Student's t test and Mann-Whitney U test as indicated below.
RESULTS
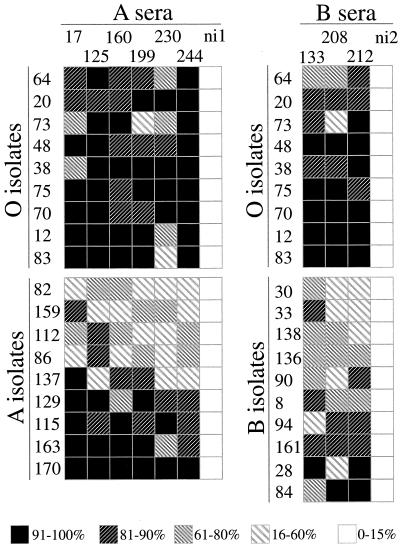
Twenty-eight clinical isolates were tested for rosette disruption and agglutination by using ABO blood group-compatible immune sera. When the effect of 9 different immune sera on different fresh clinical isolates (9 from blood group O hosts, 9 from blood group A hosts, and 10 from blood group B hosts) was tested, a significantly better antirosetting response to the sera was exhibited by isolates from blood group O individuals than was exhibited by those from either blood group A or blood group B (P < 0.0005 for O versus A and P < 0.0026 for O versus B; Mann-Whitney U test) (Fig. 1). In contrast, no significant difference in the agglutination pattern was found between the different groups (data not shown).
FIG. 1.
Rosette disruption by immune sera in relation to ABO blood group. Nine samples of immune serum from healthy individuals, six of blood group A and three of blood group B, were tested for antirosetting activity on isolates from hosts of blood group A, B, or O. Antirosetting activity (percent inhibition of rosetting) is depicted in a five-level grey scale as indicated. Two serum samples from nonimmune Swedes (ni1 and ni2) were used as controls.
When 24 clinical isolates were assayed for their blood group preferences as described above, a distinct preference for either blood group A or blood group B or a preference for both blood group A and blood group B appeared. Blood group A was the most preferred blood group (n = 10); a simultaneous preference for blood groups A and B was observed to be the second most common (n = 9). Still, in most of the cases where a simultaneous preference for groups A and B was exhibited, there was a predominant preference for either group A or group B. Five isolates were distinctly blood group B preferring, and, interestingly, none of the wild isolates exhibited a preference for blood group O. In this material, the specific blood group preference of each isolate was not related to the blood group of the human host from which the parasitized blood was drawn; i.e., the parasites cultivated in hosts of blood group A, B, or O did not significantly differ in their blood group preferences. However, there were distinct differences in the mean rosette size, with 4.9 (standard deviation [SD], 0.47), 5.0 (SD, 0.82), and 3.2 (SD, 0.53) RBC per rosette, respectively, for cultures in blood groups A, B, and O (P = 0.0002 for O versus A, P = 0.0003 for O versus B, and P = 0.85 for A versus B; Student's t test). Also, isolates from patients with different blood groups did not exhibit significant differences in parasitemia (mean, 4.8%; range, 1.2 to 8%) and rosetting rates (mean, 48.6%; range, 23.8 to 86.2%).
In order to test whether the blood group of the host would affect the sensitivity of rosettes for glycosaminoglycans (GAGs), the same parasite strain was cultivated in RBC of a different blood group. When cultivated in their preferred blood group, laboratory strains presented decreased sensitivity to the GAGs heparin, HS, and chondroitin sulfate A. Regardless of blood group preference (A or B), the sensitivity was highest when the parasite was cultivated in blood group O and lowest when cultivated in the preferred blood group (Table 1). Inversely, the highest inhibition of rosette reformation by appropriate blood group trisaccharides (A-trisaccharide for FCR3S1 and B-trisaccharide for TM284) appeared when the parasite was cultivated in the preferred blood group (Table 1).
TABLE 1.
Rosette inhibition by GAGs and blood group sugars on FCR3S1 and TM 284 strains cultivated in blood group A, B, or O RBC
| Strain (blood group) | % Inhibition by:
|
|||||
|---|---|---|---|---|---|---|
| Heparin | HS | Chondroitin sulfate A | A-trisacch. | B-trisacch. | H-disacch. | |
| FCR3S1 (O) | 96 (9.1)a | 87 (6.8) | 11 (3.1) | 23 (4.5) | 11 (3.5) | 15 (7.1) |
| FCR3S1 (A) | 52 (7.1) | 39 (5.3) | 8 (2.9) | 76 (7.3) | 25 (5.1) | 21 (4.8) |
| FCR3S1 (B) | 57 (5.9) | 44 (6.3) | 9 (3.4) | 34 (4.3) | 35 (6.7) | 18 (3.9) |
| TM 284 (O) | 35 (7.2) | 19 (4.8) | 62 (5.7) | 22 (5.7) | 24 (6.4) | 20 (5.4) |
| TM 284 (B) | 27 (6.3) | 21 (6.2) | 32 (6.9) | 33 (3.9) | 58 (8.9) | 18 (3.8) |
Inhibition of rosette formation was performed as described in Materials and Methods. Results are means (SDs are in parentheses) from at least three separate experiments. A-trisacch., A-trisaccharide; B-trisacch., B-trisaccharide; H-disacch., H-disaccharide.
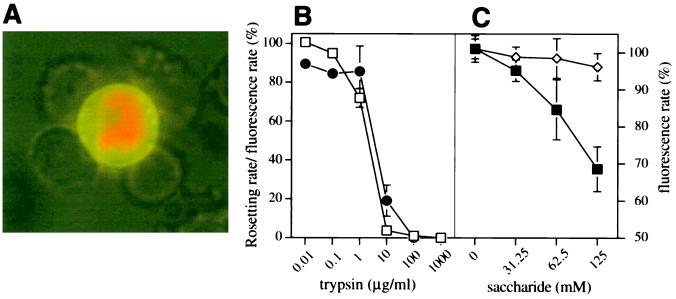
The specific inhibitory effect of A-trisaccharide was further investigated. In order to identify the target cells involved in A-trisaccharide-mediated rosette inhibition, an FITC-labeled A-trisaccharide conjugate was incubated with parasite cultures. Mature-stage pRBC, but not uninfected RBC, bound the A-trisaccharide conjugate (Fig. 2A). The binding of the conjugate was trypsin sensitive and closely related to the rosetting rate of a trypsin-treated culture (Fig. 2B). Neuraminidase treatment of pRBC did not inhibit binding (data not shown). The binding of the A-trisaccharide conjugate was dose-dependently inhibited by soluble A-trisaccharide, while the H-disaccharide had no effect (Fig. 2C).
FIG. 2.
Binding of A antigen to surfaces of parasite-infected erythrocytes. (A) A antigen conjugate was allowed to bind to a living culture. The pRBC were counterstained with ethidium bromide and visualized by UV microscopy. (B) Sensitivity of rosetting (□) and A antigen conjugate binding by pRBC (●) of FCR3S1 cultures to trypsin treatment as indicated in Materials and Methods. (C) Competition of A antigen conjugate binding to living cultures by A-trisaccharide (■) and H-disaccharide (◊). All results shown are means ± SDs (error bars) from three separate experiments.
In order to investigate what host receptors or combinations of receptors were acting in different blood groups, we performed chemical and specific enzymatic treatments of cultures (pRBC) and uninfected RBC. Sodium periodate treatment of uninfected RBC had a similar inhibitory effect on the rosette-forming capacity of treated RBC (mean inhibition for group A RBC, 71.2% [SD, 4.9]; mean inhibition for group O RBC, 74.8% [SD, 5.6]), indicating that receptors of glycan nature are important in both cell types. Trypsin and chymotrypsin treatment totally abolished rosetting (pRBC) in blood groups A and O (Fig. 2B and data not shown). When the blood group A-preferring FCR3S1 strain was cultivated in blood group A RBC, the antirosetting effect of heparinase III was significantly reduced. When added to cultures in blood group O, the inhibition ranged from 80 to 100% (reference 3 and data not shown), while the mean inhibition in blood group A was 23.5% (SD, 7.3), indicating that a heparinase-sensitive molecule was not the only receptor involved.
These observations led us to investigate the possible presence of simultaneous receptors. Blood group A RBC suspensions were treated with α-N-acetyl-galactosaminidase, depleting the erythrocytic blood group A antigen from its terminal α-N-acetyl-galactosamine. The efficacy of the treatment was monitored by using FITC-conjugated lectins. B. simplicifolia (A antigen specific)-mediated agglutination and fluorescence of RBC disappeared after treatment, whereas T. purpureas (H antigen specific)-mediated fluorescence and agglutination increased. Reactivity with blood group A MAb was not totally abolished but was significantly reduced, as described previously (16).
Treatment of blood group A RBC with α-N-acetyl-galactosaminidase drastically decreased their rosette-forming capacity, whereas no effect on group O RBC was observed; i.e., the FCR3S1 strain exhibited equal preference for group O RBC and α-N-acetyl-galactosaminidase-treated group A RBC (O), whereas group O RBC were unaffected by the same treatment (Table 2). Thus, α-N-acetyl-galactosaminidase-treated A RBC could still form rosettes but the preference of the parasite for these RBC was totally abolished. Treatment with α-galactosaminidase had no effect (data not shown). When FCR3S1 was cultivated in blood group B RBC, treatment with α-galactosidase (depleting the terminal galactose from the B antigen) also had an effect on the preference of the parasite. The rosetting rate for α-galactosidase-treated B cells versus untreated B cells was 0.78 (SD, 0.09), and the rate was 1.01 (SD, 0.10) for untreated B cells versus untreated B cells. This indicates that a relative preference for the group B RBC compared to the O RBC also exists.
TABLE 2.
Relative rosette-forming capacities of blood group A and blood group O RBCa
| Blood group | Treatment | Relative rosette-forming capacityb
|
|
|---|---|---|---|
| A | O | ||
| A | Mock | 1.02 (0.13) | 2.75 (0.19) |
| Enzyme | 0.32 (0.05) | 0.91 (0.08) | |
| O | Mock | 0.41 (0.07) | 0.93 (0.05) |
| Enzyme | 0.46 (0.11) | 0.91 (0.13) | |
Blood group A RBC either treated with enzyme (α-N-acetyl-galactosaminidase) or mock treated were allowed to compete for rosette formation with A or O RBC, as described in Materials and Methods. The same experiments were performed in parallel with O RBC.
The relative rosette-forming capacity was calculated as the ratio of the number of mock- or enzyme-treated RBC participating in rosetting/the number of untreated RBC from the parasite culture participating in rosetting (see Materials and Methods). Values are means from at least three experiments (standard deviations are shown in parentheses).
DISCUSSION
Present and earlier data speak in favor of glycans as common rosetting receptors in many P. falciparum strains (3, 7, 8), but other important receptors (in blood group O-cultivated parasites) such as complement receptor 1 or CD35 and CD36 (11, 19) have been described. Parallel to usage of these receptors, our data suggest that all P. falciparum strains have the potential to use another receptor confined to the ABO blood group system.
In rosetting P. falciparum parasites, specific blood group preferences have been seen in all laboratory strains and so far in all wild isolates tested. In clinical isolates the preference was sometimes less clear-cut, i.e., simultaneous preference for blood group A and B was seen. This may be due to clonal diversity in isolates (23), although simultaneous preference for A and B in the same strain cannot be excluded. Importantly, in no case was preference for blood group O observed. Experimental findings support the interpretation that the receptor site is contained within the distal sugars of the ABO antigens and coexists with another receptor(s), e.g., the observation that the sensitivity of the cell-cell binding to HS or heparin (or to other GAGs) drastically decreases when parasites are cultivated in their preferred blood group (A or B) compared to when they are cultivated in blood group O. Soluble A-trisaccharide (for FCR3S1) and B-trisaccharide (for TM 284) strongly inhibited formation of rosettes when the parasites were grown in the preferred blood group, but the rosettes were never abolished. In addition, blood group preference (for A) could be altered by enzymatic digestion of blood group A antigen, without abolishing rosetting.
Disruption of rosettes by heparin and HS is strongly connected to the presence of N-sulfate groups in heparin and HS chains, whereas 2-O- and 6-O-sulfation has minor importance. Further, mono- and disaccharides derived from heparin and HS, which contain N-sulfated glucosamine, inhibit rosetting, whereas other sulfated mono- and disaccharides have little or no effect (3). Likewise, the H-disaccharide (Fucα1-2Galβ) did not significantly inhibit rosette reformation, whereas the A-trisaccharide (GalNAcα1-3[Fucα1-2]Galβ) did, indicating that the terminal α-N-acetyl-galactosamine is important for competition. Although more specific components are determinant (e.g., N-sulfation), polyvalency given by a larger glycan is also an important molecular feature for binding (A. Barragan et al., submitted for publication). This may explain why larger glycans or a polyvalent A-trisaccharide construct is a better competitor than smaller monovalent units.
A number of observations argue against a role for the group O determinant (and the H antigen) in rosetting. Blood group O preference was never seen in laboratory or wild isolates, and group O RBC exhibit rosetting abilities to the same degree as RBC from the Bombay phenotype (lacking the terminal fucose of the H-disaccharide) (7). In addition, a soluble H-disaccharide did not have any inhibitory effect on rosetting nor did it compete out A-trisaccharide conjugate binding to pRBC when tested on parasites cultivated in blood group O RBC.
Blood group A-preferring (FCR3S1) and blood group B-preferring (TM 284) rosetting parasites retain their blood group preferences after being propagated for more than 100 cycles in blood group O RBC. This indicates that a single ligand or genetically linked ligands are expressed on the surface of the pRBC, accounting for binding to the blood group A antigen or B antigen, respectively, and to one or more other receptors on the RBC. One such parasite-derived ligand is PfEMP1. Indeed, we have recently shown in the FCR3S1.2 strain that the rosetting ligand PfEMP1 has affinity for multiple receptors and that the affinities for HS or heparin and blood group antigens reside mainly within the DBL-1 (Duffy binding-like) domain and do not overlap (Q. Chen, A. Heddini, A. Barragan, V. Fernandez, F. Pearce, and M. Wahlgren, submitted for publication; A. Barragan et al., submitted). The simultaneous existence of two rosetting receptors, one of which is associated with the ABO blood group system, may well explain why rosettes are larger and stronger (6) when the same parasites are cultivated in their preferred blood groups. These observations are also in line with the fact that a parasite ligand with multiple binding affinities, like PfEMP1 var. 1 from the FCR3S1.2 strain, may bind to more than one host receptor depending on availability (Chen et al., submitted). Hypothetically, the existence of more than one ligand (with the same sensitivity to trypsin as PfEMP1) still cannot be ruled out.
There are solid field data showing the influence of ABO blood group type on rosetting (1, 18, 24), and RBC polymorphisms also affect rosette formation (6, 19). Interestingly, blood group A has been reported to be a risk factor for the development of severe malaria (10, 15) and blood group O seems to confer a relative protection against cerebral malaria compared to blood groups A and B (12). Why the blood group O gene, although common in Africa, has not attained a high fixation rate may be explained by diversity in the rosetting receptor preferences and/or the simultaneous importance of other ABO blood group-independent mechanisms of protection against severe malaria, e.g., complement receptor 1 polymorphisms (19), GAG-related polymorphisms (3, 8); Chen et al., submitted, and CD36 (11). In relation to the pathology of severe malaria, it is tempting to speculate whether the present findings could relate to excessive sequestration due to enhanced rosetting. Could the blood group of the host in combination with a parasitic preference for that same blood group (A, B, or A and B) constitute an enhanced risk factor?
The differences in antirosetting effect of immune sera between isolates from group O hosts and group A and group B hosts, respectively, most likely reflect differences in the strength of the rosetting binding mechanism between the isolates. The fact that the agglutination pattern was very similar for the different groups argues against differences in antigen recognition by the hyperimmune sera. Whether the observed differences in antirosetting potency may have importance in a situation of preimmunity and/or buildup of immunity is a matter for further investigation.
In summary, we have here shown that A and B antigens are determinants for rosetting in clinical P. falciparum isolates. We have also provided evidence for the presence of at least two different simultaneously acting receptors for PfEMP1-mediated rosetting (8;) Chen et al., submitted. P. falciparum appears to have chosen a strategy of diverse glycan interactions with the RBC surface for rosetting. A therapeutic approach aimed at rosetting ligands would probably implicate multiple targets where blood group preference of the parasite in combination with blood group of the patient may be determinant factors. Development of tools that will permit in vitro identification of potential virulence factors in P. falciparum infection will be helpful when approaching therapeutic targeting of virulence factors.
ACKNOWLEDGMENTS
We thank D. Spillmann for helpful comments on the manuscript.
This study was financed by the Swedish Medical Research Council, the Karolinska Institutet, INCO-DC contracts (IC-18-CT98-0362 and CT98-0359), and the Fortüne Program, Medical Faculty, University of Tübingen.
REFERENCES
- 1.Al-Yaman F, Genton B, Mokela D, Raiko A, Kati S, Rogerson S, Reeder J, Alpers M. Human cerebral malaria: lack of significant association between erythrocyte rosetting and disease severity. Trans R Soc Trop Med Hyg. 1995;89:55–58. doi: 10.1016/0035-9203(95)90658-4. [DOI] [PubMed] [Google Scholar]
- 2.Barragan A, Kremsner P G, Weiss W, Wahlgren M, Carlson J. Age-related buildup of humoral immunity against rosetting and agglutination epitopes in African areas of malaria endemicity. Infect Immun. 1998;66:4783–4787. doi: 10.1128/iai.66.10.4783-4787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barragan A, Spillmann D, Wahlgren M, Carlson J. Plasmodium falciparum: molecular background of strain specific rosette disruption by glycosaminoglycans and sulfated glycoconjugates. Exp Parasitol. 1999;91:133–143. doi: 10.1006/expr.1998.4349. [DOI] [PubMed] [Google Scholar]
- 4.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 5.Carlson J, Helmby H, Hill A V S, Brewster D, Greenwood B M, Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 6.Carlson J, Nash G B, Gabutti V, Al-Yaman F, Wahlgren M. Natural protection against severe Plasmodium falciparum malaria due to impaired rosette formation. Blood. 1994;84:3909–3914. [PubMed] [Google Scholar]
- 7.Carlson J, Wahlgren M. Plasmodium falciparum erythrocyte rosetting is mediated by promiscuous lectin-like interactions. J Exp Med. 1992;176:1311–1317. doi: 10.1084/jem.176.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Barragan A, Fernandez V, Sundström A, Schichtherle M, Sahlén A, Carlson J, Datta S, Wahlgren M. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J Exp Med. 1998;187:15–23. doi: 10.1084/jem.187.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez V, Treutiger C J, Nash G B, Wahlgren M. Multiple adhesive phenotypes linked to rosetting-binding of erythrocytes in Plasmodium falciparum malaria. Infect Immun. 1998;66:2969–2975. doi: 10.1128/iai.66.6.2969-2975.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer P, Boone P. Severe malaria associated with blood group. Am J Trop Med Hyg. 1998;58:122–123. doi: 10.4269/ajtmh.1998.58.122. [DOI] [PubMed] [Google Scholar]
- 11.Handunnetti S M, van Schravendijk M R, Hasler T, Barnwell J W, Greenwalt D E, Howard R J. Involvement of CD36 on erythrocytes as a rosetting receptor for Plasmodium falciparum-infected erythrocytes. Blood. 1992;80:2097–2104. [PubMed] [Google Scholar]
- 12.Hill A V S. Malarial resistance genes: a natural selection. Trans R Soc Trop Med Hyg. 1992;86:225–226. doi: 10.1016/0035-9203(92)90282-h. , 232. [DOI] [PubMed] [Google Scholar]
- 13.Hoskins L, Larson G, Naff G. Blood group A immunodeterminants on human red cells differ in biologic activity and sensitivity to α-N-acetyl-galactosaminidase. Transfusion. 1995;35:813–821. doi: 10.1046/j.1537-2995.1995.351096026361.x. [DOI] [PubMed] [Google Scholar]
- 14.Kun J F J, Schmidt-Ott R J, Lehman L G, Lell B, Luckner D, Greve B, Matousek P, Kremsner P G. Meozoite surface antigen 1 and 2 genotypes and rosetting Plasmodium falciparum in severe and mild malaria in Lambaréné, Gabon. Trans R Soc Trop Med Hyg. 1998;92:110–114. doi: 10.1016/s0035-9203(98)90979-8. [DOI] [PubMed] [Google Scholar]
- 15.Lell B, May J, Schmidt-Ott R, Lehman L, Luckner D, Greve B, Matousek P, Schmid D. The role of red blood cell polymorphisms in resistance and susceptibility to malaria. Clin Infect Dis. 1999;28:794–799. doi: 10.1086/515193. [DOI] [PubMed] [Google Scholar]
- 16.Lenny L, Goldstein J. The production of group O cells. Bio/Technology. 1991;19:25–100. doi: 10.1016/b978-0-7506-9120-8.50009-3. [DOI] [PubMed] [Google Scholar]
- 17.Newbold C, Warn P, Black G, Berendt A, Craig A, Snow B, Msobo M, Peshu N, Marsh K. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am J Trop Med Hyg. 1997;57:389–398. doi: 10.4269/ajtmh.1997.57.389. [DOI] [PubMed] [Google Scholar]
- 18.Rowe A, Obeiro J, Newbold C I, Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe A J, Moulds J M, Newbold C I, Miller L H. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 20.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L H. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su X-Z, Heatwole V M, Wertheimer S P, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 22.Telen M. Erythrocyte blood group antigens: not so simple after all. Blood. 1995;85:299–306. [PubMed] [Google Scholar]
- 23.Thaitong S, Beale G H, Fenton B, McBride J, Rosario V, Walker A, Walliker D. Clonal diversity in a single isolate of the malaria parasite Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1984;78:242–245. doi: 10.1016/0035-9203(84)90287-6. [DOI] [PubMed] [Google Scholar]
- 24.Udomsangpetch R, Todd J, Carlson J, Greenwood B M. The effects of haemoglobin genotype and of ABO blood group on the formation of rosettes by Plasmodium falciparum infected red blood cells. Am J Trop Med Hyg. 1993;48:149–153. doi: 10.4269/ajtmh.1993.48.149. [DOI] [PubMed] [Google Scholar]
- 25.Udomsangpetch R, Wåhlin B, Carlson J, Berzins K, Torii M, Aikawa M, Perlmann P, Wahlgren M. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med. 1989;169:1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]