Abstract
Although probiotics are often indiscriminately prescribed, they are not equal and their effects on the host may profoundly differ. In vitro determination of the attributes of probiotics should be a primary concern and be performed even before clinical studies are designed. In fact, knowledge on the biological properties a microbe possesses is crucial for selecting the most suitable bacteriotherapy for each individual. Herein, nine strains (Bacillus clausii NR, OC, SIN, T, Bacillus coagulans ATCC 7050, Bifidobacterium breve DSM 16604, Limosilactobacillus reuteri DSM 17938, Lacticaseibacillus rhamnosus ATCC 53103, and Saccharomyces boulardii CNCM I-745) declared to be contained in six commercial formulations were tested for their ability to tolerate simulated intestinal conditions, adhere to mucins, and produce β-galactosidase, antioxidant enzymes, riboflavin, and d-lactate. With the exception of B. breve, all microbes survived in simulated intestinal fluid. L. rhamnosus was unable to adhere to mucins and differences in mucin adhesion were evidenced for L. reuteri and S. boulardii depending on oxygen levels. All microorganisms produced antioxidant enzymes, but only B. clausii, B. coagulans, B. breve, and L. reuteri synthesize β-galactosidase. Riboflavin secretion was observed for Bacillus species and L. rhamnosus, while d-lactate production was restricted to L. reuteri and L. rhamnosus. Our findings indicate that the analyzed strains possess different in vitro biological properties, thus highlighting the usefulness of in vitro tests as prelude for clinical research.
Subject terms: Microbiology, Medical research
Introduction
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”1. Several lactic acid bacteria belonging to the genera Lactobacillus, Lacticaseibacillus, Lactiplantibacillus, Lentilactobacillus, Levilactobacillus, Ligilactobacillus, and Limosilactobacillus (a.k.a. Lactobacillus), Streptococcus, and Enterococcus, as well as some Bifidobacterium and spore-forming Bacillus species, have been shown to possess a plethora of in vitro and in vivo beneficial attributes, being progressively included in a great number of probiotic formulations commercialized worldwide2,3. In addition, the yeast Saccharomyces cerevisiae has increasingly taken on as probiotic microorganism in the last decades4.
Orally administered probiotics are commonly used for preventing and/or treating some gastrointestinal conditions linked to an unbalanced gut flora or gut barrier alteration, including antibiotic-associated, traveler, and acute-infectious diarrhea, Clostridium difficile infection, inflammatory bowel diseases, and irritable bowel syndrome3,5. In addition, the use of probiotics for the treatment of some other conditions (e.g. allergy, osteoporosis, lactose intolerance, metabolic syndromes, as well as neurological, cardiovascular, respiratory, and liver diseases) has progressively been documented3.
Probiotics can transit through and eventually colonize the gastrointestinal environment to exert their beneficial effects on host health6,7. Several reports highlighted the ability of probiotics to enhance gut barrier function and host immune response, and to reduce the oxidative stress due to accumulation of oxygen reactive species (ROS)5,8,9. Probiotic microorganisms can compete with pathogens for nutrients and mucosal binding sites, as well as produce antimicrobial molecules, thus counteracting infections by pathogenic organisms5. Probiotics can also produce vitamins and food-degrading enzymes that can be helpful during digestion and in compensating vitamin deficiencies3,10. The production of riboflavin by probiotics is an attractive issue since riboflavin deficiency (i.e. ariboflavinosis), derived from diets lacking riboflavin-rich products, currently represents the most common vitamin deficiency in developing countries11. Besides their beneficial properties, probiotic strains can also synthesize molecules (e.g. d-lactate) potentially exerting negative effects in some individuals12–14.
Since probiotic properties are often species- or strain- specific15, knowledge on the peculiar properties of strains is crucial for choosing probiotic therapies based on specific individual needs.
The present study aimed at evaluating and comparing some in vitro properties of nine microbial strains isolated from commercial formulations sold as containing probiotics. In particular, the ability of these microbes to survive in simulated intestinal fluid, adhere to mucins, produce antioxidant enzymes (i.e. catalase and superoxide dismutase), riboflavin (i.e. vitamin B2), lactase (i.e. β-galactosidase), and d-lactate were investigated. The in vitro analysis of probiotic attributes of microbes isolated from preparations present on the market can be helpful in elucidating the potential beneficial effects provided by these formulations in vivo.
Results
Survival of probiotic microorganisms in simulated intestinal fluid
Bacillus clausii strains NR, OC, SIN, and T, Bacillus coagulans ATCC 7050, Bifidobacterium breve DSM 16604, Limosilactobacillus reuteri DSM 17938, Lacticaseibacillus rhamnosus ATCC 53103, and Saccharomyces boulardii CNCM I-745, declared to be contained in six commercial formulations were isolated and used throughout this study.
The analyzed microbial strains exhibited a very different behavior in simulated intestinal fluid (Fig. 1). The total colony forming units (CFU) number of B. clausii NR and T started to decrease at 4 h (P < 0.01 compared to 2 h) and 2 h (P < 0.001 compared to time 0), respectively. For both strains, the number of CFU recorded after these time points was stable. After 8 h of incubation, a reduction in the total amount of cells compared to the inoculum was registered for both strains. The total CFU number of B. clausii OC decreased after 2 h of incubation in the juice (P < 0.01 compared to 0 min), while started to increase at 6 h (P < 0.01 compared to 4 h). Following an initial decrease at 2 h (P < 0.05 compared to time 0), B. clausii SIN was able to multiply in the fluid starting from 8 h of incubation (P < 0.05 compared to 4 h; Fig. 1) and only a slight reduction of 0.240-Log was observed at this time compared to the inoculum.
Figure 1.
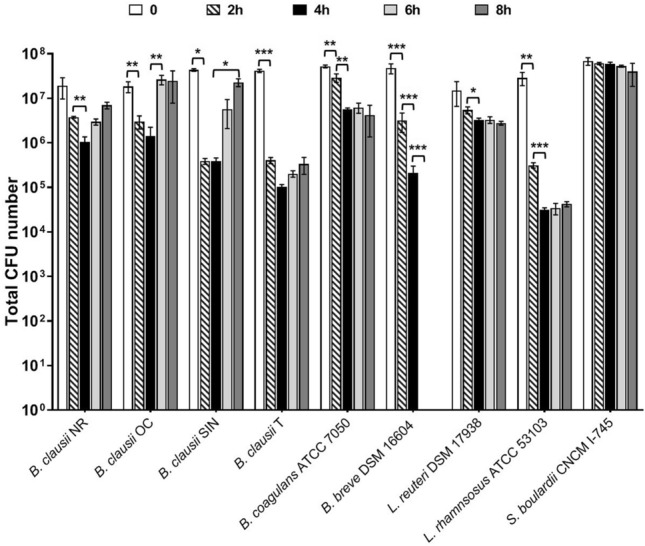
Survival of probiotic strains in simulated intestinal juice. Microbial counts (total CFU number) were performed at the inoculum (0, open bars) and after 2 (diagonally hatched bars), 4 (closed bars), 6 (light grey bars), and 8 (dark grey bars) hours of incubation at 37 °C. Three independent biological replicates with two technical replicates each were performed. Data are expressed as the mean ± standard deviation. For each strain, ANOVA for repeated measures followed by Tukey HSD test was applied to compare the total CFU numbers obtained at each time point. *P < 0.05, **P < 0.01, ***P < 0.001.
A decrease in the total number of B. coagulans and L. reuteri was registered at different times post inoculation (P < 0.01 for B. coagulans at 2 h and 4 h compared to 0 and 2 h, respectively; P < 0.05 for L. reuteri at 4 h compared to 2 h; Fig. 1). Interestingly, the total CFU number of B. breve progressively decreased in the simulated intestinal fluid (decrease of about 1 − Log, P < 0.001 at 2 h compared to 0 min and at 4 h compared to 2 h) and no residual living cells were obtained starting from 4 h of incubation (P < 0.001 at 6 h compared to 4 h). The total number of L. rhamnosus decreased after 2 h and 4 h (P < 0.01 at 2 h compared to 0 min and P < 0.001 at 4 h compared to 2 h). Afterwards, the amount of L. rhamnosus cells was stable over time with a reduction in the total amount of cells of 2.816 − Log at 8 h compared to the inoculum. Lastly, S. boulardii cells persisted in the juice for up to 8 h.
Overall, these results indicate that vegetative cells can behave differently in simulated intestinal fluid, depending on the tested microbial strain. Additionally, our findings highlight the ability of B. clausii NR and T, B. coagulans, L. reuteri, L. rhamnosus, and S. cerevisiae strains to survive in simulated intestinal conditions for up to 480 min. Interestingly, only B. clausii OC and SIN demonstrated the peculiar ability to grow even in the absence of external nutrient sources following an initial decrease in their number.
In vitro adhesion of probiotic microbes to mucins
Adhesion of probiotic microorganisms to the gastrointestinal mucus is required for the interaction with host cells, thus allowing probiotics to exert their beneficial activities6. Therefore, we investigated the ability of B. clausii, B. coagulans, B. breve, L. reuteri, L. rhamnosus, and S. boulardii strains to adhere to mucins by using a microplate assay16.
For all B. clausii strains, the CFU/well obtained after incubation on mucins in aerobic conditions was significantly higher than that of the negative controls (P < 0.001 for strain NR; P < 0.01 for B. clausii OC and SIN; P < 0.05 for B. clausii T; Fig. 2a). As shown in Fig. 2b, the B. clausii strains were also able to adhere to mucins following incubation in anaerobic atmosphere (P < 0.01 for B. clausii NR, SIN, and T; P < 0.001 for B. clausii OC compared to negative control). Different behaviors were observed with the other microbial strains. B. coagulans and B. breve were able to adhere to mucins in both aerobic (P < 0.001; Fig. 2a) and anaerobic atmosphere (P < 0.001; Fig. 2b). On the other hand, L. rhamnosus was unable to bind mucins in both conditions, since the total CFU/well recovered from the negative controls was greater than that obtained from mucins (P < 0.01 in aerobic atmosphere and P < 0.001 in anaerobic atmosphere; Fig. 2a,b). While L. reuteri did not adhere to mucins in aerobiosis (Fig. 2a), its adhesion to mucins was substantially increased in anaerobic atmosphere (P < 0.001; Fig. 2b). Conversely, S. boulardii was found to adhere to mucins in aerobic atmosphere (P < 0.01; Fig. 2a) but not in anaerobic conditions (Fig. 2b).
Figure 2.

Adhesion of probiotic microbes to porcine mucins. (a) Amount of cells (CFU/well) mechanically extracted from agar containing mucins (open bars) and from agar without mucins (i.e. negative control, grey bars) after incubation at 37 °C in aerobiosis. (b) Amount of cells (CFU/well) mechanically extracted from agar containing porcine mucins (open bars) and from agar without mucins (i.e. negative control, grey bars) after incubation at 37 °C in anaerobiosis. Three independent biological replicates with two technical replicates each were performed. Data are expressed as the mean ± standard deviation. For each strain, the two-tailed Student’s t-test was used to compare the CFU/well obtained on mucins and negative control wells. *P < 0.05, **P < 0.01, ***P < 0.001.
Production of β-galactosidase by probiotics
Administration of β-galactosidase-producing probiotics can be advantageous in people affected by lactose intolerance for reducing symptoms caused by lactose malabsorption10. Therefore, we wondered whether B. clausii, B. coagulans, B. breve, L. reuteri, L. rhamnosus, and S. boulardii could produce β-galactosidase.
All B. clausii strains, B. coagulans, B. breve, and L. reuteri strains were able to grow on M63 minimal medium and to degrade X-Gal on M63 and LB agar plates, producing blue colonies (data not shown). In contrast, L. rhamnosus and S. boulardii strains were unable to grow on the minimal medium and formed white colonies on LB agar plates, thus resulting negative for β-galactosidase. The enzymatic activity produced by positive strains was quantified by using the Miller’s method17. As shown in Fig. 3, the amount of β-galactosidase produced by B. clausii, B. coagulans, B. breve, and L. reuteri strains was significantly higher than that of the negative control (P < 0.01 for B. clausii SIN; P < 0.001 for B. clausii NR, OC, and T, B. coagulans, B. breve, and L. reuteri), thus confirming the ability of these strains to synthetize β-galactosidase. Interestingly, L. reuteri produced the highest amount of enzyme compared to the other β-galactosidase producing strains (P < 0.05 compared to B. breve; P < 0.001 compared to the other strains; Fig. 3). B. coagulans and B. breve synthetized higher enzyme levels than the B. clausii strains (P < 0.001). The enzymatic activity detected for B. clausii SIN was significantly lower compared to the other B. clausii strains (P < 0.05 compared to B. clausii NR and OC; P < 0.001 compared to B. clausii T).
Figure 3.
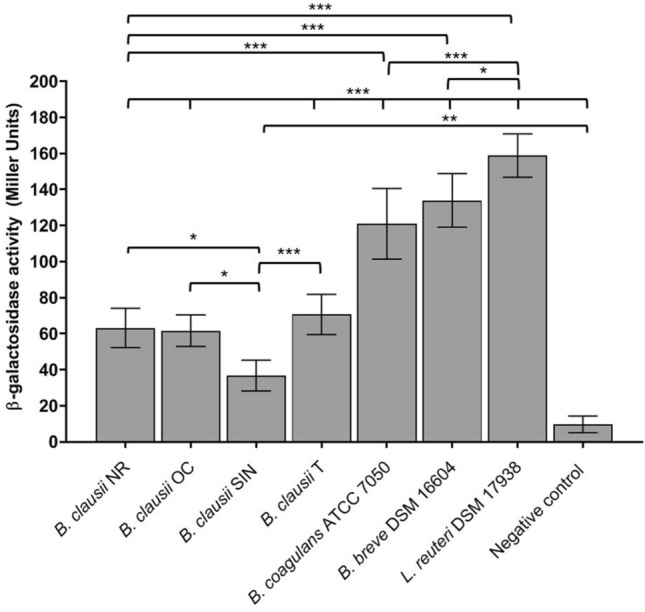
Quantification of the β-galactosidase activity (Miller Units) produced by probiotic microbes that resulted able to synthesize the enzyme on solid media. P. mirabilis ATCC 12453 was used as negative control in the assay (i.e. negative control). Three independent biological replicates with two technical replicates each were performed. Data are expressed as the mean ± standard deviation. The ANOVA for independent data followed by Tukey HSD test was applied. *P < 0.05, ***P < 0.001.
Overall, these findings highlight the production of β-galactosidase by the analyzed probiotic B. clausii, B. coagulans, B. breve, and L. reuteri strains.
Production of catalase and superoxide dismutase by probiotic microbes
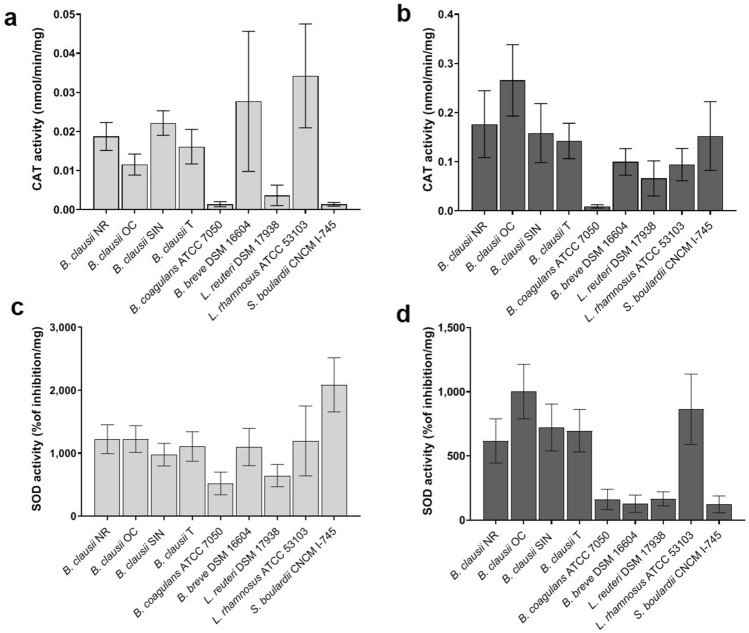
The production of catalase (CAT) and superoxide dismutase (SOD) by probiotic microorganisms is thought to help the host in reducing oxidative stress8. The ability of B. clausii, B. coagulans, B. breve, L. reuteri, L. rhamnosus, and S. boulardii to produce CAT and SOD was evaluated by quantifying these enzymatic activities in cell lysates and culture supernatants collected from actively replicating cells grown in BHIG.
For all the strains, CAT and SOD activities were detected in both cellular compartments. The CAT levels were found to be higher in culture supernatants than in cell lysates (Fig. 4a,b).
Figure 4.
Quantification of catalase (CAT) and superoxide dismutase (SOD) activities produced by probiotic strains. (a) CAT activity (nmol/min/mg) determined in cell lysates (light grey bars). (b) CAT activity (nmol/min/mg) determined in culture supernatants (dark grey bars). (c) SOD activity (% of inhibition/mg) determined in cell lysates (light grey bars). (d) SOD activity (% of inhibition/mg) determined in culture supernatants (dark grey bars). Three independent biological replicates with two technical replicates each were performed. Data are expressed as the mean ± standard deviation. The ANOVA for independent data followed by Tukey HSD test was applied.
When CAT activity was quantified in cell lysates (Fig. 4a), L. rhamnosus showed the highest levels of intracellular CAT (P < 0.05 compared to B. clausii NR, P < 0.01 compared to B. clausii T, P < 0.001 compared to B. clausii OC, B. coagulans, L. reuteri, and S. boulardii).
In culture supernatants (Fig. 4b), the highest CAT levels were detected for B. clausii OC (P < 0.05 compared to B. clausii SIN, P < 0.01 compared to S. boulardii and B. clausii T, and P < 0.001 compared to B. breve L. reuteri, and L. rhamnosus).
As shown in Fig. 4c,d, the SOD activity was generally higher in cell lysates than in culture supernatants. When SOD was quantified in cell lysates (Fig. 4c), the highest activity was obtained for S. boulardii (P < 0.001 compared to the other strains). By quantifying the SOD activity in culture supernatants (Fig. 4d), the highest SOD activity was measured for B. clausii strains and L. rhamnosus (P < 0.001 compared to the other strains). Significant differences were found in the extracellular SOD levels among the B. clausii strains. In fact, B. clausii OC showed higher SOD activity compared to NR and T (P < 0.01 and P < 0.05, respectively).
Taken together, our results indicate that all the tested strains are sufficiently equipped of antioxidant enzymes that can help in reducing oxidative stress in the host.
Production of riboflavin and D-lactate by probiotic microbes
Probiotic microorganisms able to secrete riboflavin are attractive resources that can be useful to compensate host deficiency for this vitamin. Only B. clausii, B. coagulans, and L. rhamnosus strains were found able to produce riboflavin (P < 0.001 compared to the negative control, Table 1). B. coagulans was found to produce the highest level of riboflavin compared to the other strains (P < 0.001). These results highlight the ability of B. clausii NR, OC, SIN, and T, as well as of B. coagulans and L. rhamnosus strains to actively secrete vitamin B2 during growth.
Table 1.
Amount of riboflavin produced by probiotic strains.
| Strains | Riboflavin (ng/ml) |
|---|---|
| Bacillus clausii NR | 22.26 ± 2.71 |
| Bacillus clausii OC | 25.50 ± 1.98 |
| Bacillus clausii SIN | 25.99 ± 4.83 |
| Bacillus clausii T | 25.33 ± 5.44 |
| Bacillus coagulans ATCC 7050 | 324.63 ± 78.20 |
| Lacticaseibacillus rhamnosus ATCC 53103 | 99.80 ± 18.73 |
It has been suggested that administration of d-lactate producing strains should be carefully considered in patients at risk of developing d-lactic acidosis12,14.
As shown in Fig. 5, only L. reuteri and L. rhamnosus were found able to secrete d-lactate (P < 0.001 compared to the negative control), with L. reuteri producing significantly more d-lactate than L. rhamnosus (P < 0.001).
Figure 5.
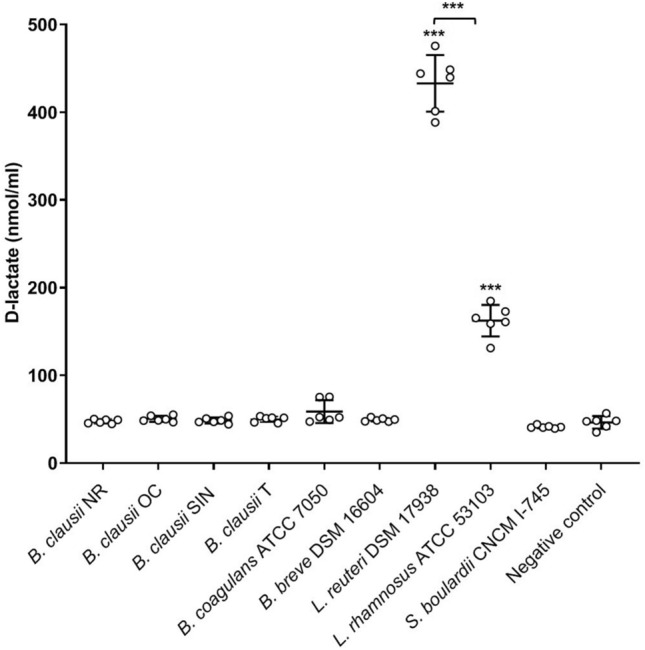
Production of d-lactate by probiotic microbes. Scatter plot of d-lactate concentration (nmol/ml) values determined in deproteinized culture supernatants of each microbe. Deproteinized BHIG was used as negative control in the assay (i.e. negative control). Three independent biological replicates with two technical replicates each were performed. The mean ± standard deviation was also shown. The ANOVA for independent data followed by Tukey HSD test was applied. ***P < 0.001.
Discussion
The ability to survive in the presence of bile and adhere to the intestinal mucus are considered key properties that orally administered probiotics should widely possess6,7. Alkaline artificial fluids containing bile are commonly adopted as models for evaluating the potential behavior of probiotic microorganisms in the intestinal environment18–22. Since the gastrointestinal tract is covered by mucus, mainly formed by mucins6,23, incubation of microbes on agar containing mucins in microplates represents a simple and reliable method for investigating their mucin-binding ability and has been adopted in several studies15,24–26.
In accordance with its documented bile salt-tolerance and ability to resist to alkaline pH27, S. boulardii CNCM I-745 well survived in the artificial intestinal fluid for up to 8 h. In the artificial intestinal fluid, our results for B. breve DSM 17938, L. reuteri DSM 17938, and L. rhamnosus ATCC 53103 are in line with evidences showing that Bifidobacterium and Lactobacillus species exhibit variable resistance to simulated intestinal conditions depending on the species and strain tested19. For this reason, bile-salt adapted strains and several microencapsulation techniques have been developed to increase the ability of these bacteria to tolerate harsh conditions and survive in the small intestine28.
The finding that B. clausii OC and SIN were able to replicate in the artificial intestinal juice without nutrients lead us to hypothesize that these strains had used the nutrients released by dead cells for multiplication. This behavior agrees with previous studies in which we showed that the spore mixture of strains NR, OC, SIN, and T can germinate and vegetative cells multiply in both the simulated intestinal fluid and human intestine22,29. It is important to underline that the tolerance of B. clausii and B. coagulans to simulated intestinal conditions can be improved by administering these strains as spores, which are generally contained in commercial products.
Since oxygen levels progressively decrease along the intestine30, we tested the ability of the selected microorganisms to interact with mucins in both aerobic and anaerobic atmosphere. The results obtained for B. clausii NR, OC, SIN, and T and B. coagulans ATCC 7050 could be expected, since genomic analysis of these B. clausii strains and of the B. coagulans S-lac strain revealed the presence of three mucus binding proteins and type-IV filamentous adhesins, respectively31,32. In addition, a role of flagellin, the main flagellar filament-component, in mucin adhesion was demonstrated for a probiotic Bacillus cereus strain33. In line with studies highlighting the adhesive properties of different B. breve strains34,35, B. breve DSM 16604 was found to adhere to mucins. Although the factors involved in B. breve mucin-adhesion are not fully clarified, type IVb tight adherence pili required for glycoprotein interaction have been shown to be essential for the in vivo murine gut colonization34.
Among Lactobacillus species, adhesive properties are extremely strain-specific and depend on the presence of specific adhesion factors6,7,23,36–39. The finding that L. rhamnosus ATCC 53103 was unable to bind mucins correlates with a previous study indicating that some L. rhamnosus strains isolated from different formulations were severely compromised in mucin-binding ability40. L. reuteri DSM 17938 and S. boulardii CNCM I-745 adhesion to mucins appeared to be influenced by oxygen levels, in line with their oxygen requirement.
Lactose intolerance is a clinical condition caused by β-galactosidase deficiency that results in the inability to degrade lactose contained in milk and derivatives10,41.
The ability to produce β-galactosidase is widely distributed among the Bifidobacterium and Lactobacillus genera and some strains were shown to bring clinical benefits in people affected by lactose intolerance and malabsorption41–44. However, the production of β-galactosidase by bacteria of these genera appeared strain-specific43. The production of this enzyme was also documented for some B. coagulans strains45,46.
In line with the evidence that many L. reuteri strains are able to synthesize considerable amounts of β-galactosidase47,48, the highest level of this enzyme was evidenced for L. reuteri ATCC DSM 17938. Herein we demonstrate that B. clausii can produce β-galactosidase. Among the tested B. clausii strains, we found that strain SIN produced lower levels of the enzyme. This result agrees with previous studies indicating that the analyzed B. clausii strains exhibit some quantitative differences in their proteome49,50. Overall, our results suggest that the tested B. clausii, B. coagulans, B. breve, and L. reuteri strains can potentially be useful in reducing the symptomatology associated with lactose malabsorption, thus providing beneficial effects in individuals affected by lactose intolerance.
Oxidative stress is caused by massive ROS accumulation and is associated with nucleic acid, protein, and lipid modifications, thus leading to cell apoptosis, cellular aging and to the development of several chronic diseases9,51. CAT and SOD play a crucial role as antioxidant enzymes, being involved in hydrogen-peroxide and superoxide radical scavenging, respectively9. Due to the production of antioxidant enzymes, a role of probiotics in the prevention of oxidative stress and several ROS-linked diseases has been proposed in the last decades8,9,52. The finding that the tested Lactobacillus strains showed catalase activity was unexpected, since lactobacilli are generally catalase negative. We can only hypothesize that the adopted assay detects a reduction in the amount of hydrogen peroxide due to the activity of non-catalase enzymes. Other studies will be required to address whether these strains possess an alternative enzymatic activity able to degrade hydrogen peroxide. The results obtained with B. clausii, B. coagulans, B. breve, L. reuteri, L. rhamnosus, and S. boulardii strains indicate that their administration could be helpful in reducing or alleviating ROS accumulation, thus preventing oxidative stress. In particular, the finding that the levels of secreted CAT were higher than those found in the cytoplasm suggests that the analyzed microbes could contribute to counteract extracellular hydrogen peroxide accumulation in vivo, thus exerting a cytoprotective effect.
The B-group and water-soluble vitamin riboflavin is the precursor of flavin adenine mononucleotide and flavin adenine dinucleotide, which act as coenzymes in several redox reactions and are involved in the metabolism of carbohydrates, proteins, lipids, other vitamins, and ketone bodies53–55. Ariboflavinosis due to dietary inadequacy or gut microbiota dysbiosis can be responsible for a variety of conditions, including cataract, childhood neuropathy, anemia, hypertension, certain types of cancer, and diabetes mellitus53,54. For this reason, the use of riboflavin-producing strains and/or administration of probiotic formulations supplemented with exogenous riboflavin can represent a promising source to prevent or ameliorate host ariboflavinosis.
The ability of B. clausii NR, OC, SIN, and T to produce riboflavin confirms a previous study in which these strains were tested for vitamin B2 production using an auxotrophic B. cereus mutant56. In accordance with the presence of the riboflavin-metabolic pathway in the B. coagulans genome57,58, we found that B. coagulans ATCC 7050 was a very good producer of riboflavin. The detection of riboflavin in L. rhamnosus ATCC 53103 supernatants agrees with previous data reported for this strain59.
d-lactic acidosis is a neurological disorder typical of patients affected by short bowel syndrome (SBS) and due to massive d-lactate accumulation in the bloodstream15,60,61. The administration of d-lactate producing probiotics in the development of d-lactic acidosis has been widely debated and currently appears to be a controversial issue1,62–65. However, some authors proposed that d-lactate producing strains should not be administered to patients at risk of developing d-lactic acidosis, including those with SBS and neonates12,14. In accordance with the presence of d-lactate dehydrogenase in species belonging to the Lactobacillus genus15,59,66,67, we only detected d-lactate in cell culture supernatants of L. reuteri and L. rhamnosus strains.
In conclusion, our findings demonstrate that the investigated probiotic strains are characterized by distinct biological properties in vitro. On the other hand, the only use of in vitro analyses cannot be conclusive to explain the behavior probiotic strains exhibit in vivo, due to the complexity of the gastrointestinal tract. However, we believe that studies investigating the in vitro properties of microbes isolated from commercial products can be of translational relevance, since supporting clinical research in the comprehension of the beneficial effects these formulations may provide in vivo.
Methods
Bacterial strains and culture conditions
Strains used in this study are listed in Table 2. Microbes were isolated from commercial formulations purchased at the local pharmacy. The isolated species were identified by Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (MALDI-TOF MS) and the strain names as declared by manufactures on the product labels adopted throughout the study. The multi-strain formulation Enterogermina (100 μl) was seeded on Brain Heart Infusion (BHI; Thermo Fisher Scientific, USA) agar plates supplemented with 50 μg/ml rifampicin, 50 μg/ml chloramphenicol, 200 μg/ml streptomycin, or 25 μg/ml tetracycline for selective isolation of B. clausii NR, OC, SIN, and T, respectively. The B. clausii strains used in this study are characterized by a low level of intra-specific genome diversity but showed strain-specific proteomic signaturers31,48,49. For mono-strain formulations, microbial isolation was performed as follows. B. coagulans was isolated by seeding 100 μl of Lactò Più on Trypticase Soy agar containing 5% horse blood and plates were incubated at 37 °C for 48–72 h. Neovaxitiol (100 μl) was streaked on Bifidus selective agar (Merck KGaA, Germany) containing 0.116 g/l of BSM Supplement (Merck KGaA) and grown for 48–72 h in anaerobic atmosphere by using Thermo Scientific™ AnaeroGen™ Compact (Thermo Fisher Scientific) for isolating B. breve. For L. reuteri and L. rhamnosus isolation, 5 drops of Reuflor and Dicoflor were streaked on De Man, Rogosa, Sharpe agar (Thermo Fisher Scientific) and plates were incubated at 37 °C for 48 h in 5% CO2 by using Thermo Scientific™ CO2Gen™ Compact (Thermo Fisher Scientific). For S. cerevisiae isolation, one capsule of CODEX was dissolved in 5 ml of sterile phosphate buffered saline (PBS, 1 M KH2PO4, 1 M K2HPO4, 5 M NaCl, pH 7.2) and 100 μl were seeded on SABOURAUD-2% dextrose agar (Merck KGaA). Plates were incubated at 30 °C for 48–72 h. Microorganisms were maintained as stocks at − 80 °C until use. When required, liquid cultures were performed in BHI supplemented with 1% (w/v) glucose (BHIG) or Luria Bertani (LB, Carlo Erba, Italy) at 37 °C.
Table 2.
Probiotic strains used in this study and commercial formulations from which they were isolated.
| Strain declared on the product label | Product | Manufacturer |
|---|---|---|
| Bacillus clausii NR | Enterogermina | Sanofi, France |
| Bacillus clausii OC | Enterogermina | Sanofi, France |
| Bacillus clausii SIN | Enterogermina | Sanofi, France |
| Bacillus clausii T | Enterogermina | Sanofi, France |
| Bacillus coagulans ATCC 7050 | Lactò Più | RECORDATI S.p.A., Italy |
| Bifidobacterium breve DSM 16604 | Neovaxitiol | IBSA Farmaceutici, Italy |
| Limosilactobacillus reuteri DSM 17938* | Reuflor | Italchimici S.p.A, Italy |
| Lacticaseibacillus rhamnosus ATCC 53103* | Dicoflor | AG Pharma S.r.l, Italy |
| Saccharomyces boulardii CNCM I-745 | CODEX | Zambon Italia, Italy |
*The nomenclature of these strains, originally reported as Lactobacillus reuteri DSM 17938 and Lactobacillus rhamnosus ATCC 53103 on the product labels, was updated according to the current nomenclature69.
MALDI-TOF MS
For each microbe, well-isolated colonies were spotted on MALDI plates and overlaid with 1 μl of 70% ethanol, 1 μl of formic acid, and 1 μl of acetonitrile. After the addition of 1 μl of a-cyano-4-hydroxycinnamic acid matrix to each spot, plates were air-dried. The identification was performed by using the MALDI-TOF Microflex LT Mass Spectrometer (Bruker, USA). Spectra were acquired at a laser frequency of 60 Hz with an acquisition range from 1.960 to 20.000 Da, imported into the integrated MALDI Biotyper software (version 3.1, Bruker), and compared with reference spectra collected in the database. A score ≥ 2.00 indicated identification at the species level.
Microbial behavior in simulated intestinal fluid
To prepare simulated intestinal fluid, 0.3% Oxgall bile salts (Merck KGaA) and 0.1% pancreatin (Merck KGaA) were dissolved in sterile 0.85% NaCl solution and adjusted to pH 8.020,21. For each microbe, 100 µl of overnight cultures were inoculated in 5 ml of fresh BHIG and grown at 37 °C to OD600 of ~ 1.5. Cultures were centrifuged at 4500 rpm for 15 min at 4 °C and washed twice with sterile PBS. Supernatants were removed and microbial pellets suspended in 5 ml of simulated intestinal juice. Cultures were incubated at 37 °C for 0, 2 h, 4 h, 6 h, and 8 h in microaerophilic atmosphere by using a candle jar. At each time point, aliquots (100 μl) of the suspensions were serially diluted and seeded on solid media. The number of CFU was determined and the total CFU number contained in the juice calculated.
Mucin adhesion in aerobic and anaerobic conditions
Microbial adhesion to mucins was assessed as described by Tsilia et al.16 with some modifications. Briefly, 100 µl of overnight cultures were inoculated in 25 ml of fresh BHIG and grown to an OD600 of ~ 1.5. Cultures were centrifuged at 4500 rpm for 10 min at 4 °C and pellets washed two times with sterile PBS. 500 µl of the suspensions were added to 48 well plates (Merck KGaA) containing 600 µl of mucin agar (pH 6.8), constituted by 5% (w/v) mucins from porcine stomach type II (Merck KGaA) and 1% (w/v) bacteriological agar. As negative controls, 500 µl of the same suspensions were inoculated on wells containing 600 µl of 1% (w/v) bacteriological agar. Plates were incubated at 37 °C for 90 min at 50 rpm in aerobic and anaerobic conditions. After incubation, the liquid phase was discarded and wells washed two times with 500 µl of PBS to remove loosely adhered cells. Microbial extraction was performed by mechanical method. The whole solid layers were aseptically transferred in 5 ml of physiological peptone solution and homogenized for 5 min. Aliquots were seeded on agar plates for quantification of adhered bacteria by the plate count method.
Preparation of cell lysates and culture supernatants
Protein samples were prepared by inoculating microbial cells in 25 ml of BHIG. Cultures were grown at 37 °C to OD600 of ~ 1.8. Supernatants were separated from pellets by centrifugation at 10,000×g, collected, and filtered through 0.22 μm filters to completely remove cells. Microbial pellets were washed two times with sterile cold PBS and suspended in 1 ml of PBS. Cell suspensions were added with an equal amount of zirconia beads (0.1 mm of diameter) and lysed with a Bead Beater homogenizer by alternating 4 cycles of 1.0 min of lyses and 10 min of refrigeration in ice bath. Cell debris were removed by centrifugation at 10,000 rpm for 15 min at 4 °C and the soluble fractions collected. The protein content of cell lysates and culture supernatants was determined by using the BCA Protein Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Protein samples were stored at − 80 °C until use.
Quantification of enzymatic activities
The activity of CAT and SOD were quantified in cell lysates and culture supernatants. CAT and SOD activities were measured by using the Catalase Assay kit (abcam, UK) and the Superoxide Dismutase Activity Assay kit (Colorimetric, abcam), respectively, following the manufacturer’s instructions.
Qualitative evaluation of β-galactosidase production was performed by inoculating microbial cells in 5 ml of LB broth containing 0.5 mM IPTG (Thermo Fisher Scientific) for 16 h at 37 °C. Microbial cultures (10 μl) were dropped on M63 synthetic medium (2 g/l (NH4)2SO4, 13.6 g/l KH2PO4, 0.5 mg/l FeSO4 × 7H2O, 1 mM MgSO4, 0.001 g/l thiamin, 10 g/l lactose, pH 7.0) and LB agar plates, both containing 40 µg/ml X-Gal (Merck KGaA) and 0.5 mM IPTG. Plates were incubated at 37 °C for 48–72 h. Quantification of the β-galactosidase activity was performed as described by Miller17. Briefly, microbial cells were inoculated in 5 ml of LB broth supplemented with 0.5 mM IPTG and grown at 37 °C to OD600 of ~ 0.5. Cells were collected by centrifuging 1 ml of each culture at 8000×g at 4 °C for 5 min and suspended in 1 ml of cold Z buffer (60 mM Na2HPO4 × 7H2O, 40 mM NaH2PO4 × H2O, 10 mM KCl, 1 mM MgSO4 × 7H2O, and 50 mM β-mercaptoethanol, pH 7.0). Cells permeabilization was performed by adding 20 μl of 0.1% (w/v) sodium dodecyl sulfate (Merck KGaA) and 40 µl of chloroform and by vortexing the tubes for 10 s. Samples (100 µl) were diluted in 900 μl of Z buffer and supplemented with 200 µl of 4 mg/ml orto-nitrofenil-β-galactopyranoside (ONPG, Merck KGaA). The reaction was conducted at 28 °C by monitoring the yellow color development and was stopped by adding 250 µl of 1 M Na2CO3. β-galactosidase activity was calculated by the equation (Eq. (1)):
| 1 |
where OD420 is the optical density measured at 420 nm, OD550 the optical density measured at 550 nm, OD600 the optical density measured at 600 nm, T the reaction time (expressed in min), and V the volume of culture assayed (expressed in ml). Proteus mirabilis ATCC 12453 and Escherichia coli K12 were used as negative and positive controls in the assays, respectively.
Quantification of riboflavin
The amount of riboflavin in culture supernatants was determined by using the Enzyme-linked immunosorbent assay kit for vitamin B2 (Cloud-clone Corp., USA) according to manufacturer’s instructions. Sterile BHIG was used as negative control of the assay.
Evaluation of D-lactate production
Before performing d-lactate quantification, 1 ml of culture supernatants was deproteinized by precipitation with 10% (v/v) TCA 6N68. The presence of d-lactate in supernatants was evaluated by using the colorimetric d-lactate Assay Kit (abcam) according to manufacturer’s instructions. Deproteinized BHIG was used as negative control.
Statistical analysis
For all the experiments, three independent biological replicates with two technical replicates each were performed. Data were expressed as the mean ± standard deviation (S.D). Both statistical analyses and graphs were realized on GraphPad Prism version 8.0.2 (GraphPad Software Inc., USA, https://www.graphpad.com/scientific-software/prism/). For assessing the viability of each microbe in simulated intestinal juice, the one-way analysis of variance (ANOVA) for repeated measure followed by Tukey HSD test was applied to compare the total CFU numbers obtained at each time point. For mucin adhesion experiments, the two-tailed Student’s t-test was used to compare the CFU/well obtained for each strain on mucins and negative control wells. For the other assays, ANOVA for independent data followed by Tukey HSD test was used to separately compare the amount of molecules produced by each strain. A two-sided P-value (P) < 0.05 was considered significant.
Acknowledgements
This work was sponsored and funded by Sanofi S.p.A (SER CHC 86/19).
Author contributions
D.M., M.C., F.C., A.L., and E.G. conceived and planned the experiments. D.M., M.C., and F.C. performed the experiments. All the authors analyzed the data, wrote the manuscript, and approve submission.
Competing interests
D.M., M.C., F.C., and A.L. have no conflict of interest to declare. E.G. has been a lecturer for Sanofi S.p.A.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Diletta Mazzantini and Marco Calvigioni.
References
- 1.Hill C, et al. Expert consensus document The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 2.Jeżewska-Frąckowiak J, et al. The promises and risks of probiotic Bacillus species. Acta Biochim. Pol. 2018;65:509–519. doi: 10.18388/abp.2018_2652. [DOI] [PubMed] [Google Scholar]
- 3.Stavropoulou E, Bezirtzoglou E. Probiotics in medicine: A long debate. Front. Immunol. 2020;11:2192. doi: 10.3389/fimmu.2020.02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen S, Mansell TJ. Yeasts as probiotics: Mechanisms, outcomes, and future potential. Fungal Genet. Biol. 2020;137:103333. doi: 10.1016/j.fgb.2020.103333. [DOI] [PubMed] [Google Scholar]
- 5.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019;25:716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 6.Celebioglu HU, Svensson B. Dietary nutrients, proteomes, and adhesion of probiotic lactobacilli to mucin and host epithelial cells. Microorganisms. 2018;6:90. doi: 10.3390/microorganisms6030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019;103:6463–6472. doi: 10.1007/s00253-019-09978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9:521. doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali SS, Ahsan H, Zia MK, Siddiqui T, Khan FH. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020;44:e13145. doi: 10.1111/jfbc.13145. [DOI] [PubMed] [Google Scholar]
- 10.Oak SJ, Jha R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019;59:1675–1683. doi: 10.1080/10408398.2018.1425977. [DOI] [PubMed] [Google Scholar]
- 11.Mosegaard S, Dipace G, Bross P, Carlsen J, Gregersen N, Olsen RKJ. Riboflavin deficiency-implications for general human health and inborn errors of metabolism. Int. J. Mol. Sci. 2020;21:3847. doi: 10.3390/ijms21113847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders ME, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164–185. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao SSC, Rehman A, Yu S, Andino NM. Brain fogginess, gas and bloating: A link between SIBO, probiotics and metabolic acidosis. Clin. Transl. Gastroenterol. 2018;9:162. doi: 10.1038/s41424-018-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Akker CHP, et al. Probiotics and preterm infants: A position paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020;70:664–680. doi: 10.1097/MPG.0000000000002655. [DOI] [PubMed] [Google Scholar]
- 15.Vitetta L, Coulson S, Thomsen M, Nguyen T, Hall S. Probiotics, D-lactic acidosis, oxidative stress and strain specificity. Gut Microbes. 2017;8:311–322. doi: 10.1080/19490976.2017.1279379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsilia V, Van den Abbeele P, Van de Wiele T. Improved in vitro assay for determining the mucin adherence of bacteria sensitive to Triton X-100 treatment. Folia Microbiol. (Praha) 2015;60:435–442. doi: 10.1007/s12223-015-0376-0. [DOI] [PubMed] [Google Scholar]
- 17.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 18.Augustijns P, et al. A review of drug solubility in human intestinal fluids: Implications for the prediction of oral absorption. Eur. J. Pharm. Sci. 2014;57:322–332. doi: 10.1016/j.ejps.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Grimoud J, et al. In vitro screening of probiotic lactic acid bacteria and prebiotic glucooligosaccharides to select effective synbiotics. Anaerobe. 2010;16:493–500. doi: 10.1016/j.anaerobe.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Jensen H, Grimmer S, Naterstadm K, Axelsson L. In vitro testing of commercial and potential probiotic lactic acid bacteria. Int. J. Food Microbiol. 2012;153:216–222. doi: 10.1016/j.ijfoodmicro.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Tokatli M, Gülgör G, Elmacı SB, İşleyen NA, Özçelik F. In vitro properties of potential probiotic indigenous lactic acid bacteria originating from traditional pickles. Biomed. Res. Int. 2015;2015:315819. doi: 10.1155/2015/315819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vecchione A, et al. Compositional quality and potential gastrointestinal behavior of probiotic products commercialized in Italy. Front. Med. (Lausanne) 2018;5:59. doi: 10.3389/fmed.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Tassell ML, Miller MJ. Lactobacillus adhesion to mucus. Nutrients. 2011;3:613–636. doi: 10.3390/nu3050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsilia V, et al. Bacillus cereus adhesion to simulated intestinal mucus is determined by its growth on mucin, rather than intestinal environmental parameters. Foodborne Pathog. Dis. 2015;12:904–913. doi: 10.1089/fpd.2014.1926. [DOI] [PubMed] [Google Scholar]
- 25.Tsilia V, Kerckhof FM, Rajkovic A, Heyndrickx M, Van de Wiele T. Bacillus cereus NVH 0500/00 can adhere to mucin but cannot produce enterotoxins during gastrointestinal simulation. Appl. Environ. Microbiol. 2015;82:289–296. doi: 10.1128/AEM.02940-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roussel C, et al. Anti-infectious properties of the probiotic Saccharomyces cerevisiae CNCM I-3856 on enterotoxigenic E. coli (ETEC) strain H10407. Appl. Microbiol. Biotechnol. 2018;102:6175–6189. doi: 10.1007/s00253-018-9053-y. [DOI] [PubMed] [Google Scholar]
- 27.Hossain MN, Afrin S, Humayun S, Ahmed MM, Saha BK. Identification and growth characterization of a novel strain of Saccharomyces boulardii isolated from soya paste. Front. Nutr. 2020;7:27. doi: 10.3389/fnut.2020.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao M, et al. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020;19:857–874. doi: 10.1111/1541-4337.12532. [DOI] [PubMed] [Google Scholar]
- 29.Ghelardi E, et al. Survival and persistence of Bacillus clausii in the human gastrointestinal tract following oral administration as spore-based probiotic formulation. J. Appl. Microbiol. 2015;119:552–559. doi: 10.1111/jam.12848. [DOI] [PubMed] [Google Scholar]
- 30.Espey MG. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013;55:130–140. doi: 10.1016/j.freeradbiomed.2012.10.554. [DOI] [PubMed] [Google Scholar]
- 31.Khatri I, Sharma S, Ramya TN, Subramanian S. Complete genomes of Bacillus coagulans S-lac and Bacillus subtilis TO-A JPC, two phylogenetically distinct probiotics. PLoS ONE. 2016;11:e0156745. doi: 10.1371/journal.pone.0156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatri I, Sharma G, Subramanian S. Composite genome sequence of Bacillus clausii, a probiotic commercially available as Enterogermina®, and insights into its probiotic properties. BMC Microbiol. 2019;19:307. doi: 10.1186/s12866-019-1680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez B, et al. Identification of surface proteins involved in the adhesion of a probiotic Bacillus cereus strain to mucin and fibronectin. Microbiology (Reading) 2009;155:1708–1716. doi: 10.1099/mic.0.025288-0. [DOI] [PubMed] [Google Scholar]
- 34.O'Connell Motherway M, et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc. Natl. Acad. Sci. U.S.A. 2011;108:11217–11222. doi: 10.1073/pnas.1105380108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engevik MA, et al. Bifidobacterium dentium fortifies the intestinal mucus layer via autophagy and calcium signaling pathways. MBio. 2019;10:e01087–e1119. doi: 10.1128/mBio.01087-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackenzie DA, et al. Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology. 2010;156:3368–3378. doi: 10.1099/mic.0.043265-0. [DOI] [PubMed] [Google Scholar]
- 37.Nishiyama K, et al. Adhesion properties of Lactobacillus rhamnosus mucus-binding factor to mucin and extracellular matrix proteins. Biosci. Biotechnol. Biochem. 2015;79:271–279. doi: 10.1080/09168451.2014.972325. [DOI] [PubMed] [Google Scholar]
- 38.Arena MP, Capozzi V, Spano G, Fiocco D. The potential of lactic acid bacteria to colonize bionic and abiotic surfaces and the investigation of their interactions. Appl. Microbiol. Biotechnol. 2017;101:2641–2657. doi: 10.1007/s00253-017-8182-z. [DOI] [PubMed] [Google Scholar]
- 39.Deng Z, et al. Glyceraldehyde-3-phosphate dehydrogenase increases the adhesion of Lactobacillus reuteri to host mucin to enhance probiotic effects. Int. J. Mol. Sci. 2020;21:9756. doi: 10.3390/ijms21249756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sybesma W, Molenaar D, van Ijcken W, Venema K, Kort R. Genome instability in Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2013;79:2233–2239. doi: 10.1128/AEM.03566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fassio F, Facioni MS, Guagnini F. Lactose maldigestion, malabsorption, and intolerance: A comprehensive review with a focus on current management and future perspectives. Nutrients. 2018;10:1599. doi: 10.3390/nu10111599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida CC, Lorena SL, Pavan CR, Akasaka HM, Mesquita MA. Beneficial effects of long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr. Clin. Pract. 2012;27:247–251. doi: 10.1177/0884533612440289. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, et al. Analysis of β-galactosidase production and their genes of two strains of Lactobacillus bulgaricus. Biotechnol. Lett. 2012;34:1067–1071. doi: 10.1007/s10529-012-0870-2. [DOI] [PubMed] [Google Scholar]
- 44.Mandal H, Bagchi T. In vitro screening of indigenous Lactobacillus isolates for selecting organisms with better health-promoting attributes. Appl. Biochem. Biotechnol. 2018;185:1060–1074. doi: 10.1007/s12010-018-2709-3. [DOI] [PubMed] [Google Scholar]
- 45.Liu P, Xie J, Liu J, Ouyang J. A novel thermostable β-galactosidase from Bacillus coagulans with excellent hydrolysis ability for lactose in whey. J. Dairy Sci. 2019;102:9740–9748. doi: 10.3168/jds.2019-16654. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, et al. Cloning, expression, and bioinformatics analysis and characterization of a β-galactosidase from Bacillus coagulans T242. J. Dairy Sci. 2021;14:2735. doi: 10.3168/jds.2020-18942. [DOI] [PubMed] [Google Scholar]
- 47.Hidalgo-Morales M, Robles-Olvera V, García HS. Lactobacillus reuteri beta-galactosidase activity and low milk acidification ability. Can. J. Microbiol. 2005;51:261. doi: 10.1139/w04-134. [DOI] [PubMed] [Google Scholar]
- 48.Ibrahim SA, et al. Enhancement of alpha- and beta-galactosidase activity in Lactobacillus reuteri by different metal ions. Biol. Trace Elem. Res. 2010;136:106–116. doi: 10.1007/s12011-009-8519-2. [DOI] [PubMed] [Google Scholar]
- 49.Lippolis R, et al. Comparative proteomic analysis of four Bacillus clausii strains: Proteomic expression signature distinguishes protein profile of the strains. J. Proteom. 2011;74:2846–2855. doi: 10.1016/j.jprot.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 50.Lippolis R, et al. Comparative secretome analysis of four isogenic Bacillus clausii probiotic strains. Proteome Sci. 2013;11:28. doi: 10.1186/1477-5956-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gill JG, Piskounova E, Morrison SJ. Cancer, oxidative stress, and metastasis. Cold Spring Harb. Symp. Quant. Biol. 2016;81:163–175. doi: 10.1101/sqb.2016.81.030791. [DOI] [PubMed] [Google Scholar]
- 52.Tomusiak-Plebanek A, et al. Lactobacilli with superoxide dismutase-like or catalase activity are more effective in alleviating inflammation in an inflammatory bowel disease mouse model. Drug Des. Dev. Ther. 2018;12:3221–3233. doi: 10.2147/DDDT.S164559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thakur K, Tomar SK, Singh AK, Mandal S, Arora S. Riboflavin and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2017;57:3650–3660. doi: 10.1080/10408398.2016.1145104. [DOI] [PubMed] [Google Scholar]
- 54.Suwannasom N, Kao I, Pruß A, Georgieva R, Bäumler H. Riboflavin: The health benefits of a forgotten natural vitamin. Int. J. Mol. Sci. 2020;21:950. doi: 10.3390/ijms21030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olfat N, Ashoori M, Saedisomeolia A. Riboflavin is an antioxidant: A review update. Br. J. Nutr. 2022;4:1–9. doi: 10.1017/S0007114521005031. [DOI] [PubMed] [Google Scholar]
- 56.Salvetti S, Celandroni F, Ghelardi E, Baggiani A, Senesi S. Rapid determination of vitamin B2 secretion by bacteria growing on solid media. J. Appl. Microbiol. 2003;95:1255–1260. doi: 10.1046/j.1365-2672.2003.02095.x. [DOI] [PubMed] [Google Scholar]
- 57.Su F, Xu P. Genomic analysis of thermophilic Bacillus coagulans strains: Efficient producers for platform bio-chemicals. Sci. Rep. 2014;4:3926. doi: 10.1038/srep03926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapse NG, Engineer AS, Gowdaman V, Wagh S, Dhakephalkar PK. Functional annotation of the genome unravels probiotic potential of Bacillus coagulans HS243. Genomics. 2019;111:921–929. doi: 10.1016/j.ygeno.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 59.LeBlanc JG, et al. B-group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2017;111:1297–1309. doi: 10.1111/j.1365-2672.2011.05157.x. [DOI] [PubMed] [Google Scholar]
- 60.Kang KP, Lee S, Kang SK. D-lactic acidosis in humans: Review of update. Electrol. Blood Press. 2006;4:53–56. doi: 10.5049/EBP.2006.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kowlgi NG, Chhabra L. D-lactic acidosis: An underrecognized complication of short bowel syndrome. Gastroenterol. Res. Pract. 2015;2015:476215. doi: 10.1155/2015/476215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mack DR. D(−)-lactic acid-producing probiotics, D(−)-lactic acidosis and infants. Can. J. Gastroenterol. 2004;18:671–675. doi: 10.1155/2004/342583. [DOI] [PubMed] [Google Scholar]
- 63.Uchida H, et al. D-lactic acidosis in short-bowel syndrome managed with antibiotics and probiotics. J. Pediatr. Surg. 2004;39:634–636. doi: 10.1016/j.jpedsurg.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 64.Munakata S, et al. A case of D-lactic acid encephalopathy associated with use of probiotics. Brain Dev. 2010;32:691–694. doi: 10.1016/j.braindev.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 65.Łukasik J, Salminen S, Szajewska H. Rapid review shows that probiotics and fermented infant formulas do not cause D-lactic acidosis in healthy children. Acta Paediatr. 2018;107:1322–1326. doi: 10.1111/apa.14338. [DOI] [PubMed] [Google Scholar]
- 66.Huang Y, You C, Liu Z. Cloning of D-lactate dehydrogenase genes of Lactobacillus delbrueckii subsp. bulgaricus and their roles in D-lactic acid production. 3 Biotech. 2017;7:194. doi: 10.1007/s13205-017-0822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chai LJ, et al. Deciphering the D-/L-lactate-producing microbiota and manipulating their accumulation during solid-state fermentation of cereal vinegar. Food Microbiol. 2020;92:103559. doi: 10.1016/j.fm.2020.103559. [DOI] [PubMed] [Google Scholar]
- 68.Mayeur C, et al. Faecal D/L lactate ratio is a metabolic signature of microbiota imbalance in patients with short bowel syndrome. PLoS ONE. 2013;8:e54335. doi: 10.1371/journal.pone.0054335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng J, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]