Abstract
Previous studies in humans and in animals have shown dramatic effects of cocaine on measures of brain function that persist into abstinence. The purpose of this study was to examine the neurobiological consequences of abstinence from cocaine, using a model that removes the potential confound of cocaine cues. Adult male rhesus monkeys self-administered cocaine (0.3 mg/kg/injection; N = 8) during daily sessions or served as food-reinforcement controls (N = 4). Two times per week, monkeys were placed in a neutral environment and presented with a cartoon video for ~30 min, sometimes pre- and sometimes post-operant session, but no reinforcement was presented during the video. After ~100 sessions and when the cocaine groups had self-administered 900 mg/kg cocaine, the final experimental condition was a terminal 2-[14C]-deoxyglucose procedure, which occurred in the neutral (cartoon video) environment; for half of the monkeys in each group, this occurred after 1 day of abstinence and for the others after 30 days of abstinence. Rates of local cerebral glucose metabolism were measured in 57 brain regions. Global rates of cerebral metabolism were significantly lower in animals 1 day and 30 days post-cocaine self-administration when compared to those of food-reinforced controls. Effects were larger in 30- vs. 1-day cocaine abstinence, especially in prefrontal, parietal and cingulate cortex, as well as dorsal striatum and thalamus. Because these measures were obtained from monkeys while in a neutral environment, the deficits in glucose utilization can be attributed to the consequences of cocaine exposure and not to effects of conditioned stimuli associated with cocaine.
Subject terms: Addiction, Behavioural methods
Introduction
Chronic cocaine use has been associated with a wide spectrum of emotional, social, and cognitive problems that are likely to contribute to patterns of continued drug use and relapse. Active cocaine users often display substantial deficits in cognitive processing including attention, working memory, decision making, and response inhibition [1–7]. These widespread cognitive deficits are considered to be the result of disruptions of both brain structure and function. Imaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have documented instances of decreased cerebral blood flow and glucose metabolism (see, for example [8–13]), as well as structural abnormalities [14–16] that correlate with impairments in cognitive processing.
Given the widespread impact of cocaine exposure throughout the brain, an important question is whether the functional and structural alterations associated with chronic cocaine use are reversed as a result of cessation of drug use, or whether these alterations persist despite abstinence. Some corollary questions include whether recovery occurs broadly in all systems, if it occurs at a similar rate throughout the brain, and whether impairments in some regions are more long-lasting, or even permanent. These are key issues for the development of efficacious treatment strategies and for determining the nature and duration of support needed at various stages of abstinence to prevent relapse.
These questions have, for the most part, received far less attention than questions about the direct effects of cocaine use and exposure [17]. Some recent studies, however, have begun to address the issue of recovery more specifically, and evidence for both structural and functional recovery following the cessation of cocaine use has begun to emerge. For example, when compared to current users, abstainers have denser cortical gray matter than current users [18, 19], along with improved white matter integrity [20]. In these studies, however, it was evident that significant differences between healthy controls and abstainers remained, suggesting persistent deficits. Functionally, abstinent users show fewer performance deficits [21, 22] as well as greater cortical activation in a variety of paradigms [23–25]. Although these investigations provide evidence suggesting recovery following the cessation of cocaine use, clinical studies can be challenging to interpret, especially when trying to identify effects attributable to cocaine. Most cocaine users also abuse a wide range of other licit and illicit drugs, many of which also have deleterious effects on behavior and brain structure and function, potentially confounding their interpretation. In addition, brain and behavioral differences may have pre-dated cocaine use, again making it difficult to ascribe persistent deficits to the effects of cocaine exposure rather than to pre-existing impairments.
One potential way to avoid these confounds is the use of an animal model where circumstances can be tightly controlled, and specific conditions systematically manipulated. Studies from this lab evaluating the functional consequences of cocaine self-administration in a nonhuman primate model have focused largely on the effects of cocaine after varying periods of self-administration experience. These studies have shown a progressive shift in the pattern of alterations in functional activity associated with the effects of cocaine that is dependent upon the degree of cocaine experience [26]. This shift suggests that adaptations have occurred in basal brain activity that account for the differences in the response to cocaine, but it remains to be demonstrated whether these functional alterations are evident in the absence of drug, or whether they are only manifest in the presence of cocaine. Of particular relevance, in previous studies the final session occurred in the environment in which monkeys had been self-administering cocaine. In order to control for the possibility that conditioned stimuli were influencing outcomes associated with functional brain changes, here the final experimental session was in a neutral environment in which cocaine was never available.
The primary goal of the current study was to determine, in a nonhuman primate model of chronic cocaine self-administration, the anatomical distribution of alterations in functional brain activity in the absence of cocaine, and to evaluate the persistence of any such changes in baseline functional brain activity following a period of forced abstinence. Rates of local cerebral glucose utilization (LCGU) of monkeys who self-administered cocaine for 100 days were measured with the 2-[14C]-deoxyglucose (2-DG) method in a neutral setting (never associated with cocaine or other reinforcement) 1 or 30 days after their last cocaine self-administration session, and compared to rates of LCGU in monkeys who responded for food reinforcement for similar durations under identical schedules of reinforcement. A second goal of the present study was to evaluate a novel behavioral procedure that could model “craving” for cocaine.
Methods
Subjects
Twelve experimentally naïve adult male rhesus monkeys (Macaca mulatta), ages 6–13 years, weighing between 8.59 and 13.4 kg at the start of the study, were individually housed in stainless steel cages with water ad libitum. While in their home cages, all monkeys had physical and visual contact with each other at all times. Body weight was maintained at ~98% of free-feeding weight with Lab Diet Monkey Chow, provided no sooner than 30 min post-session, and supplemented by fresh fruits and vegetables. All procedures were performed in accordance with established practices as described in the National Institutes of Health Guide for Care and Use of Laboratory Animals. All research protocols were reviewed and approved by the Animal Care and Use Committee of Wake Forest University. Each animal was prepared with indwelling intravenous catheters and vascular access ports (VAP; Model GPV, Access Technologies, Skokie, IL) according to previously described procedures [27, 28].
Self-administration procedures
Cocaine self-administration and food-reinforced responding occurred in ventilated and sound-attenuated operant chambers (1.5 × 0.74 × 0.76 m, Med Associates, East Fairfield, VT) that accommodated a primate chair (Model R001, Primate Products, Redwood City, CA), as described previously [29–31]. Monkeys were trained to respond on one of two levers under a fixed-interval (FI) schedule of 1.0-g banana-flavored pellet presentation; the FI was increased until 3-min was obtained. Each session began with illumination of a white light above the lever (the discriminative stimulus). Completion of the FI 3-min resulted in extinguishing of the white lever light and delivery of a pellet; sessions ended after 30 food presentations. All monkeys responded for at least 20 sessions and until stable performance was obtained (±20% of the mean for three consecutive sessions, with no trends in response rates).
Next, the pellet dispenser was disconnected and FI 3-min responding only resulted in stimulus changes (i.e., extinction of the white lever light) in the chamber, but no delivery of the food pellet. This occurred until responding declined by at least 80% for three consecutive sessions (typically within five sessions). After baseline performance had been re-established after extinction (5 sessions), monkeys were randomly assigned to one of three groups. One group served as controls (N = 4) and resumed responding under the FI 3-min schedule of food presentation for a total of 100 sessions followed by 1 (N = 2) or 30 (N = 2) days, during which time monkeys remained in their home cages with no operant sessions conducted.
Two groups of monkeys were assigned to chronic cocaine self-administration, consisting of 100 sessions in which responding was maintained by 0.3 mg/kg/injection cocaine (30 injections per session, for a total of 9.0 mg/kg/session), followed by cessation of operant sessions for 1 (N = 4) or 30 (N = 4) days. To assess “craving”, approximately every 2 weeks, an abstinence period of 1–3 days preceded an extinction session in which responding was recorded, but had no scheduled consequence and no stimulus changes throughout the session. Extinction sessions ended after 120 min and without a cocaine injection or food presentation (in control monkeys).
Neutral condition
In addition to operant sessions, all monkeys were presented with videos (nature or cartoons, alternated randomly) for 30 min, two times per week, in chambers distinct from those in which drug or food was available (non-drug environment). These chambers were Med Associate sound-attenuating cubicles that could accommodate a primate restraint chair; the front panel was cut out and a TV monitor was secured to the opening in the front wall. Video sessions took place at random times in relation to the operant session (before or after) to avoid any unintended conditioning with drug sessions. No responses were required or recorded at any time during these passive viewing sessions. One or 30 days following the cessation of food or cocaine self-administration, the 2DG experiment was conducted in the non-drug (video) chambers. In all, 2–4 days prior to the 2DG study, monkeys were surgically implanted with an arterial catheter in the opposite leg of the venous catheter, as previously described [31]. Food-control monkeys had two catheters implanted, one in the femoral vein and one in the femoral artery of the same leg.
Measurement of local cerebral glucose utilization
The 2DG procedure occurred 1 or 30 days after the last food or cocaine session in the non-drug (neutral condition) chamber. After the monkey was placed in the chamber, the video was started and the procedure initiated via infusion of an intravenous pulse of 2.76 MBq/kg [14C]2DG (PerkinElmer, Waltham, MA; specific activity 1850-2035 MBq/mmol) followed by a flush of heparinized saline through catheters that exited through the back of the chamber. Timed arterial blood samples were collected to measure plasma [14C] concentrations, determined by liquid scintillation spectrophotometry (Beckman Instruments, Fullerton, CA) and plasma glucose concentrations, assessed using a glucose analyzer (Analox Instruments, London, UK). Approximately 45 min after tracer injection, and while still in the chamber in which a video was playing, monkeys were euthanized by an intravenous overdose of sodium pentobarbital (100 mg/kg). Brains were removed rapidly, blocked, frozen in isopentane (−45 °C), and stored at −80 °C. Processing for autoradiography and quantitative densitometry of autoradiograms was carried out according to procedures previously reported [28].
Statistical analysis
Behavior
After establishing cocaine self-administration and food-reinforced responding, baseline responding under the FI 3-min schedule was operationally defined as the 3-day mean responding prior to the first extinction (“craving”) session. The two cocaine groups were combined (N = 8). One monkey from the cocaine group was initially exposed to extinction sessions on food-maintained responding, and his data are also included with the food group (N = 5). Responding during the FI was recorded in bins of 1/10th of the interval (i.e., 18 s) and an index of FI responding, quarter-life (QL) value was also calculated for baseline responding [32]. Note that the time in the 10th bin could be >18 s, but only one additional response would be recorded, leading to the reinforcer presentation. QL values represent the proportion of the FI that has elapsed when 25% of the responses in that interval had been emitted. Responses in each bin were summed across all FIs. To assess baseline responding, two-tailed t-tests were conducted comparing the food and cocaine groups, with p < 0.05 considered significant. To assess “craving”, responding during the extinction condition was compared between cocaine and food groups and abstinence days using a two-way repeated measures ANOVA with Group (Food, Cocaine) and Abstinence Day (1, 2, 3) as factors; p < 0.05 was considered significant. Statistical analyses were performed using GraphPad Prism v9.0.
Glucose metabolism
Rates of glucose utilization (µmol/100 g/min) were measured in 57 discrete brain regions. Values of rates of LCGU in individual brain regions were analyzed in six neuroanatomical groups (striatum, basal ganglia, frontal cortex, posterior cortex, thalamus, and limbic regions) (Table 2) by means of a two-way analysis of variance (treatment group X brain region, with brain region considered a repeated measure). Data from the food controls (1 vs 30 days abstinent) were combined for the purposes of this analysis. Comparisons of values in these two groups via t-test did not reveal any significant differences. In those neuroanatomical groups in which significant interactions and significant treatment effects (controls vs 1 day; controls vs 30 day; or 1 vs 30 day) were observed, individual brain regions were further assessed with Bonferroni tests for multiple comparisons within each brain region. Comparisons were limited to significant treatment effects. All statistical analyses were carried out using SPSS Statistical Software (version 18; IBM SPSS Software, Armonk, NY).
Table 2.
Effects of abstinence from chronic cocaine self-administration on rates of local cerebral glucose utilization in rhesus monkey braina.
| Brain region | Food control (N = 4) | 1 Day abstinence (N = 4) | 30 Days abstinence (N = 4) |
|---|---|---|---|
| Cocaine self-administration | |||
| Striatum | |||
| Rostral precommissural striatum | |||
| Caudate-dorsomedial | 56 ± 4.8 | 47 ± 1.5 | 42 ± 2.4* |
| Caudate-dorsolateral | 58 ± 4.1 | 49 ± 2.0 | 43 ± 1.8* |
| Caudate-ventromedial | 50 ± 4.0 | 42 ± 1.8 | 39 ± 1.8* |
| Putamen-dorsal | 59 ± 5.7 | 51 ± 2.1 | 46 ± 4.5 |
| Putamen-ventral | 57 ± 5.3 | 48 ± 2.1 | 45 ± 4.2 |
| Rostral nucleus accumbens | 41 ± 4.6 | 37 ± 1.9 | 33 ± 1.7 |
| Caudal precommissural striatum | |||
| Caudate-dorsomedial | 62 ± 4.6 | 54 ± 3.5 | 44 ± 3.0* |
| Caudate-dorsolateral | 60 ± 4.0 | 54 ± 2.4 | 43 ± 3.2* |
| Caudate-ventromedial | 49 ± 0.7 | 44 ± 3.4 | 39 ± 2.4 |
| Putamen-dorsal | 63 ± 3.8 | 57 ± 2.4 | 47 ± 4.1* |
| Putamen-ventral | 58 ± 5.5 | 51 ± 1.8 | 42 ± 3.3* |
| Nucleus accumbens-core | 42 ± 4.5 | 39 ± 1.7 | 34 ± 1.8 |
| Nucleus accumbens-shell | 38 ± 3.2 | 35 ± 2.2 | 31 ± 2.3 |
| Post-commissural striatum | |||
| Caudate | 50 ± 1.8 | 49 ± 5.7 | 49 ± 5.7 |
| Putamen | 54 ± 1.4 | 51 ± 5.8 | 51 ± 5.8 |
| Basal ganglia | |||
| Ventral tegmental area | 26 ± 0.7 | 24 ± 1.8 | 20 ± 3.3* |
| Globus pallidus-internal | 37 ± 0.9 | 33 ± 1.2 | 30 ± 1.0* |
| Globus pallidus-external | 36 ± 1.3 | 32 ± 1.0 | 29 ± 1.0* |
| Subthalamus | 63 ± 1.9 | 50 ± 2.5* | 51 ± 0.9* |
| Substantia nigra pars compacta | 50 ± 2.3 | 41 ± 2.7 | 38 ± 2.0* |
| Substantia nigra pars reticulata | 43 ± 1.0 | 38 ± 2.0 | 35 ± 1.5* |
| Frontal cortex | |||
| Frontal pole (area 10) | 45.± 1.9 | 37 ± 3.2 | 37 ± 3.2 |
| Medial prefrontal (areas 25,14,32) | 42 ± 3.0 | 40 ± 1.9 | 37 ± 2.5 |
| Orbital prefrontal (areas 11,12,13) | 53 ± 2.1 | 46 ± 2.3 | 42 ± 1.8* |
| Dorsolateral prefrontal (areas 45,46) | 54 ± 1.9 | 49 ± 1.4 | 45 ± 2.3* |
| Anterior cingulate (area 24) | 49 ± 3.0 | 45 ± 2.4 | 42 ± 2.8 |
| Motor (area 4) | 43 ± 1.9 | 48 ± 1.4 | 39 ± 2.7 |
| Temporal and parietal cortex | |||
| Temporal pole | 41 ± 4.4 | 36 ± 2.4 | 35 ± 5.3 |
| Insula | 53 ± 3.6 | 46 ± 1.3 | 43 ± 5.1 |
| Temporal gyrus (areas STG,TE,TF) | 54 ± 3.8 | 46 ± 1.3 | 47 ± 3.4 |
| Entorhinal | 43 ± 1.6 | 36 ± 2.1 | 36 ± 1.5* |
| Posterior cingulate (area 23, 31) | 58 ± 3.5 | 49 ± 1.9 | 44 ± 1.5* |
| Inferior parietal (area PE, PO) | 58 ± 2.1 | 53 ± 3.9 | 48 ± 1.1* |
| Medial parietal (area PG) | 52 ± 1.9 | 56 ± 3.3 | 47 ± 2.2 |
| Thalamus | |||
| Midline | 64 ± 1.1 | 52 ± 3.8* | 48 ± 2.9* |
| Mediodorsal | 60 ± 0.8 | 48 ± 2.0* | 46 ± 2.8* |
| Ventral anterior | 47 ± 2.0 | 40 ± 1.4 | 36 ± 3.0* |
| Parafascicular/centromedian | 50 ± 2.3 | 40 ± 2.1* | 39 ± 2.4* |
| Ventrolateral | 37 ± 1.2 | 39 ± 1.2 | 36 ± 4.3 |
| Lateral geniculate | 43 ± 1.6 | 39 ± 2.4 | 39 ± 2.2 |
| Medial geniculate | 72 ± 3.1 | 69 ± 6.3 | 66 ± 2.3 |
| Habenula | 43 ± 1.8 | 48 ± 7.9 | 38 ± 1.8 |
| Amygdala | |||
| Lateral | 39 ± 4.4 | 34 ± 1.9 | 36 ± 1.2 |
| Central | 25 ± 2.7 | 23 ± 1.2 | 25 ± 0.2 |
| Medial | 29 ± 2.7 | 27 ± 2.0 | 28 ± 0.7 |
| Basolateral | 38 ± 4.3 | 36 ± 2.3 | 37 ± 1.3 |
| Hypothalamus | |||
| Preoptic area | 32 ± 3.2 | 31 ± 2.7 | 28 ± 2.5 |
| Lateral | 33 ± 3.5 | 30 ± 0.9 | 30 ± 1.2 |
| Medial | 33 ± 3.6 | 31 ± 1.1 | 31 ± 1.3 |
| Paraventricular | 32 ± 5.7 | 31 ± 0.8 | 32 ± 3.4 |
| Limbic-associated | |||
| Bed nucleus of stria terminalis | 28 ± 2.6 | 28 ± 2.6 | 26 ± 1.5 |
| Lateral septum | 27 ± 3.5 | 26 ± 1.5 | 26 ± 2.2 |
| Extended amygdala | 29 ± 1.9 | 28 ± 2.2 | 26 ± 1.6 |
| Hippocampus (CA1) | 43 ± 3.3 | 37 ± 2.7 | 34 ± 0.9 |
| Hippocampus (CA3) | 40 ± 3.1 | 33 ± 0.2 | 30 ± 0.9* |
| Hippocampus (dentate gyrus) | 47 ± 3.0 | 42 ± 2.5 | 38 ± 1.1 |
| Subiculum | 50 ± 3.8 | 41 ± 2.1 | 39 ± 0.4* |
*p < 0.05, Bonferroni t-tests for multiple comparisons following two-way analysis of variance (treatment group X brain region, with brain region considered a repeated measure). Multiple comparisons were carried out within individual neuroanatomical groupings and testing only where there were significant group effects, e.g controls vs. 30 day abstinence.
aData represent rates of local cerebral glucose utilization (μmol/100 g/min) expressed as means ± S.E.M.
Results
Self-administration behavior
Under baseline conditions, total responses were significantly different among food-maintained controls and cocaine self-administration groups (t(11) = 2.772, p = 0.0182), as were response rates (t(11) = 3.328, p = 0.0067) and session length (t(11) = 4.745, p = 0.0006); QL values did not differ significantly between the groups (t(11) = 0.9268,p = 0.3739; see Table 1). QL values in both groups were >0.25, indicating schedule-appropriate responding under the FI schedule of reinforcement. For extinction session responding, a two-way repeated measures ANOVA found that there was not a significant effect of Abstinence Day on responding. However, there was a significant Group effect (F11,22 = 7.452, p < 0.0001), with significantly higher responding by monkeys in the cocaine group compared with food-reinforced control monkeys (Fig. 1). Post-hoc analyses for the cocaine group found that 3-days off from cocaine resulted in significantly higher response rates compared with baseline.
Table 1.
Mean fixed-interval 3-min responding during the last three sessions prior to the start of probe sessions food- and cocaine-reinforced groupsa.
| Group | Session Length (min)* | Response rate (resp/min)b | QL value |
|---|---|---|---|
| Food SA controls | 94.1 ± 1.3 | 26.28 ± 5.12 | 0.62 ± 0.05 |
| Cocaine SA | 313.7 ± 38.4 | 1.98 ± 1.61 | 0.52 ± 0.06 |
aData are expressed as means ± SEM; N = 5 (Food) and 8 (Cocaine) per group.
bSignificant differences between groups.
Fig. 1. Rates of responding (responses/s) during baseline (BL) and 1-, 2-, or 3-days off from access to food (open bars) or cocaine (filled bars).
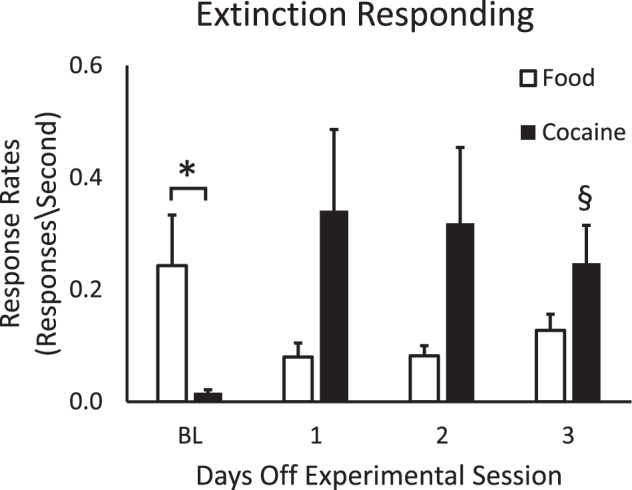
Baseline responding was the mean from sessions preceding all “days off” sessions. Data shown are means ± SEM for control monkeys (N = 5) and cocaine self-administration monkeys (N = 8). Asterisk indicates signficant differences between baseline food- and cocaine-group response rates; section sign indicates significant differences in response rates between baseline and extinction session responding.
Local cerebral glucose utilization
Plasma glucose levels measured just prior to the initiation of the 2DG procedure did not differ significantly between or within groups (data not shown). Rates of local cerebral glucose utilization expressed as µmol/100 g/min are shown in Table 2. Data from the two food-control groups were combined for the purpose of analysis since there were no significant differences in any brain region. For clarity of presentation data from specific brain regions were analyzed in six neuroanatomical clusters: striatum, basal ganglia, frontal cortex, temporal and parietal cortex, thalamus and limbic and hypothalamus (see Table 2).
Striatum
In the striatum (dorsal and ventral), there was a main effect of group (F2,9 = 5.5477, p < 0.05) and of brain region (F14,9 = 46.761, p < 0.001), but not a group X region interaction (F28,9 = 1.503, p = 0.109). Across striatal regions LCGU measured 30 days post-cocaine group was significantly lower than that of controls. Within the entire striatum, there were no other significant differences between groups including between 1- and 30-day post cocaine groups. Post-hoc analysis within individual brain regions revealed that rates of LCGU in monkeys measured 30 days after cocaine self-administration were significantly lower than those of food-reinforced controls in the dorsomedial and dorsolateral portions of both rostral and caudal precommissural striatum, as well as in the dorsal and ventral putamen in the caudal precommissural striatum (Figs. 2 and 3 and Table 2). LCGU did not differ between groups in any portion of the ventral striatum nor in areas of the post-commissural striatum (Figs. 2 and 3).
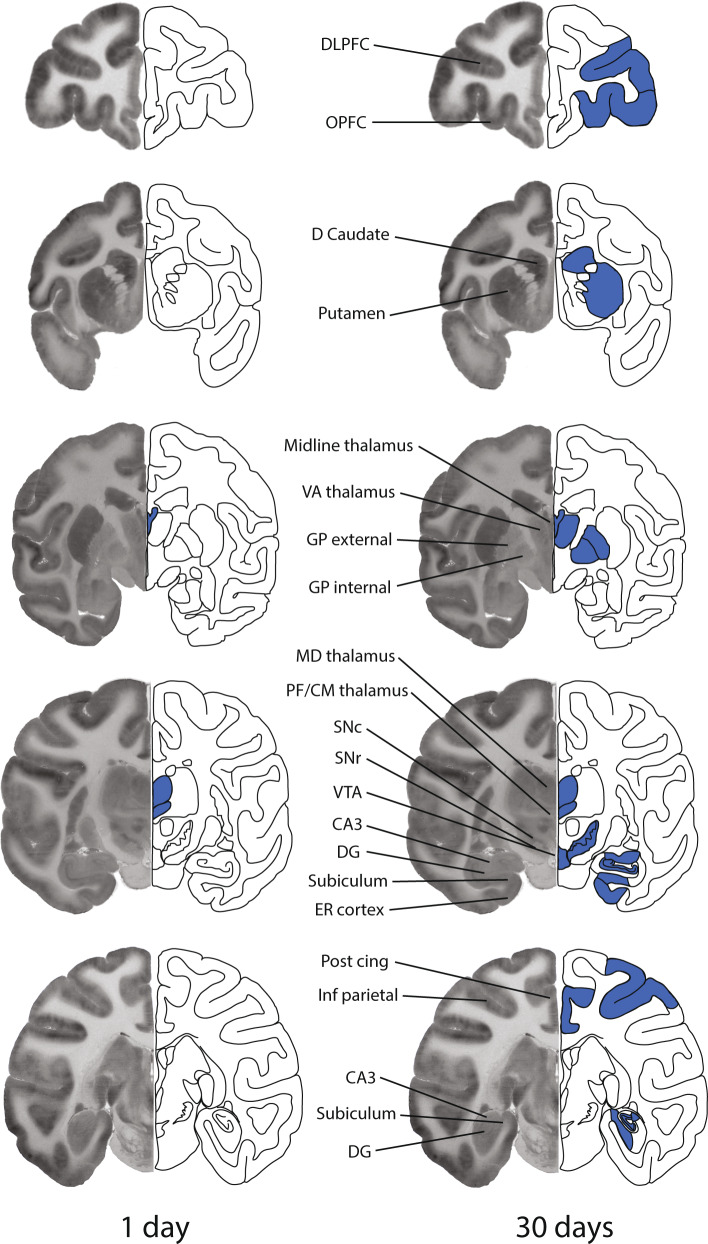
Fig. 2. Areas of significant alterations in local cerebral glucose utilization in the rhesus monkey brain 1 day (left panel) or 30 days (right panel) following the final session of 100 days of cocaine self-administration when accessed in a neutral environment.
Each brain image is a composite of a representative autoradiogram of 2-[14C]deoxyglucose uptake (left side) and a schematic (right side) of a coronal section of the monkey brain at one of five rostral to caudal levels corresponding to approximately +12.15, −00.45, −07.65, −13.05, and −18.00 from Bregma. Blue coloring superimposed on the schematics represents the location of significant decreases in rates of glucose utilization. Effects were bilateral, however for illustrative purposes the effects are depicted on the schematic side only. CA3, CA3 field of the hippocampus; D Caudate, dorsal caudate; DG dentate gyrus; DLPFC, dorsolateral prefrontal cortex; ER cortex, entorhinal cortex; GP external, globus pallidus external segment; GP internal, globus pallidus internal segment; Inf parietal, inferior parietal cortex; MD thalamus, mediodorsal thalamic nucleus; Med parietal, medial parietal cortex; OPFC, orbital prefrontal cortex; PF/CM thalamus, parafascicular/centromedian thalamic nuclei; Post cing, posterior cingulate; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; VA thalamus, ventral anterior thalamic nucleus; VTA, ventral tegmental area.
Fig. 3. Mean percent difference of rates of local cerebral glucose utilization (µmol/100 g/min) of selected brain regions in the striatum and basal ganglia of monkeys 1 day (N = 4) or 30 days (N = 4) after cocaine self-administration from rates of food controls (N = 4) when assessed in a neutral environment.
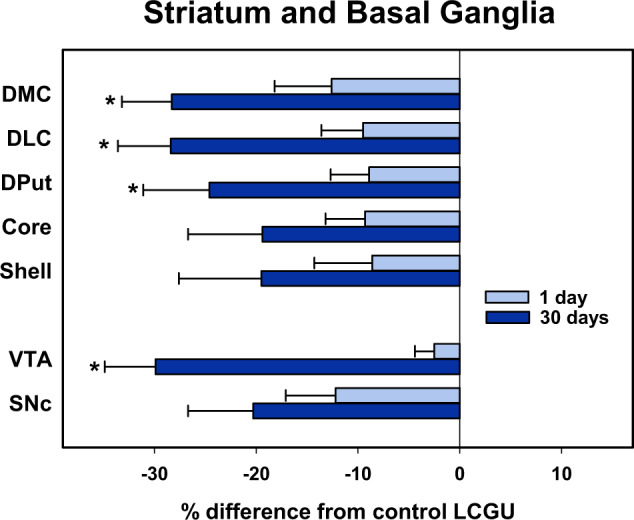
Percentages were calculated by comparing individual rates in the 1 and 30 days groups to the mean of food controls. Data shown are the mean ± SEM. *p < 0.05, Student’s t test between 1 and 30 day abstinence groups. DMC, caudal precommissural dorsomedial caudate; DLC, caudal precommissural dorsolateral caudate; DPut, caudal precommissural dorsal putamen; Core, nucleus accumbens core at the level of the caudal precommissural striatum; Shell, shell of the nucleus accumbens at the level of the caudal precommissural striatum; VTA, ventral tegmental area; SNc, substantia nigra compacta.
Basal ganglia
There was a significant main effect of group (F2,9 = 11.197, p < 0.005) and brain region (F5,9 = 59.531, p < 0.001). Post-hoc tests showed significant reductions both 1- and 30-days post-cocaine self-administration. There was also a trend to a group X region interaction (F10,9 = 1.985, p = 0.064). Further analyses of individual brain regions revealed that rates of LCGU of monkeys 30 days after cocaine self-administration were significantly reduced in the ventral tegmental area, substantia nigra compacta, substantia nigra reticulata, globus pallidus, and subthalamic nucleus when compared to food-reinforced controls. (Table 2 and Figs. 2 and 3).
Frontal cortex
Statistical analysis revealed a significant main effect of group (F2,9 = 6.911, p < 0.05) and of brain region (F5,9 = 11.085, p < 0.001) as well as a significant group X region interaction (F10,9 = 2.225, p = 0.033). Within the frontal cortex LCGU measured 30 days post-cocaine was significantly lower than that of controls. There were no other significant differences including between 1- and 30-day post cocaine groups. Post hoc analysis within individual cortical regions revealed that LCGU in monkeys 30 days after cocaine self-administration was significantly lower than that of food-reinforced controls in orbitofrontal (areas 11, 12, and 13) and dorsolateral (areas 45 and 46) prefrontal cortex (Table 2 and Figs. 2 and 4).
Fig. 4. Mean percent difference of rates of local cerebral glucose utilization (µmol/100 g/min) of selected brain regions in the neocortex and thalamus of monkeys 1 day (N = 4) or 30 days (N = 4) after cocaine self-administration from rates of food controls (N = 4) when assessed in a neutral environment.
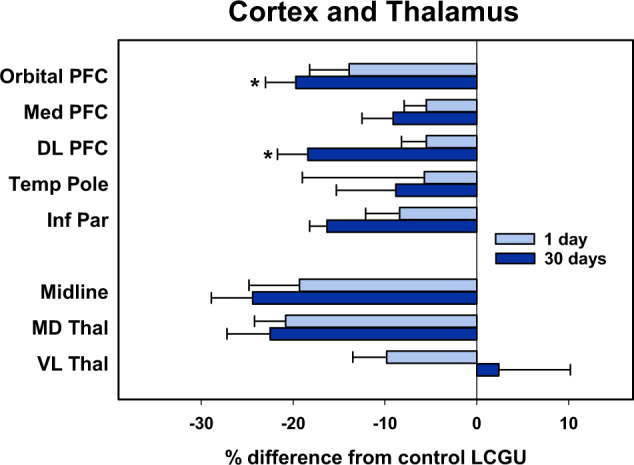
Percentages were calculated by comparing individual rates in the 1 and 30 days groups to the mean of food controls. Data shown are the mean ± SEM. *p < 0.05, Student’s t test between 1 and 30 day abstinence groups. Orbital PFC, orbital prefrontal cortex; Med PFC, medial prefrontal cortex; DL PFC, dorsolateral prefrontal cortex; Temp Pole, temporal pole; Inf Par, inferior parietal cortex; Midline, midline thalamus; MD Thal, mediodorsal thalamus; VL Thal, ventrolateral thalamus.
Temporal and parietal cortex
Statistical analysis revealed a significant main effect of group (F2,9 = 4.608, p < 0.05) and of brain region (F6,9 = 19.542, p < 0.001), but not a group X region interaction (F12,9 = 1.670, p < 0.109). LCGU measured 30 days post-cocaine was significantly lower than that of controls. There were no other significant differences including between 1- and 30-day post cocaine groups. Post hoc analysis within individual cortical regions revealed that LCGU in monkeys 30 days after cocaine self-administration was significantly lower than that of food-reinforced controls in the posterior cingulate and inferior parietal cortex. (Table 2 and Figs. 2 and 4).
Thalamus
Statistical analysis revealed a significant main effect of group (F2,9 = 7.841, p < 0.05) and of brain region (F7,9 = 20.232, p < 0.001), as well as a significant group X brain region interaction (F10,9 = 2.325, p = 0.029). Within the thalamus LCGU in the 30 day post-cocaine group was significantly lower than that of controls. There were no other significant differences between groups including between 1- and 30-day post cocaine groups. Post hoc analysis within individual thalamic regions revealed that rates of LCGU in monkeys measured 1 day after cocaine self-administration were significantly lower than those of food-reinforced controls in midline thalamic areas and the mediodorsal thalamus. Rates in monkeys measured 30 days after the cessation of cocaine self-administration were reduced in the midline areas, mediodorsal thalamus, ventral anterior, and parafasicular/centromedian nuclei (Table 2 and Figs. 2 and 4).
Limbic system
Statistical analysis revealed no significant main effect of group (F2,9 = 1.012, NS), but a significant effect of brain region (F14,9 = 37.743 p < 0.001).
Discussion
The goal of the current study was to identify the residual functional adaptations that accompany chronic cocaine exposure, in the absence of conditioned stimuli associated with cocaine reinforcement, one or 30 days after the last cocaine self-administration experience. The present results demonstrate that cocaine self-administration resulted in persistent reductions in functional brain activity, as reflected by the measurement of rates of glucose utilization, in cognitive processing regions of the prefrontal and parietal cortex, dorsal striatum, and thalamus when compared to non-drug, food-reinforced, and controls (Table 2 and Fig. 2). These differences were greater after more prolonged periods of withdrawal than after acute withdrawal from chronic cocaine exposure, suggesting that these deficits intensify over time (Figs. 3 and 4). Importantly, because these studies were carried out in a neutral environment, the deficits in glucose utilization were due almost exclusively to the consequences of cocaine exposure and not to effects of conditioned stimuli present during the terminal procedure.
The regions in which deficits in functional activity identified here as persisting after cessation of cocaine exposure are a subset of those that have been shown in our prior studies to exhibit altered metabolic activity following cocaine self-administration either after continuous self-administration or after a hiatus of 30 days [26, 29, 30]. This substantiates the idea that cocaine-induced altered activity is directly responsible for these deficits. When comparing the results of the current study to those in which functional activity was assessed in the presence of stimuli that predicted the availability of cocaine, there are, however, a number of significant differences. Anticipation of cocaine resulted in widespread increases in metabolic activity, as opposed to the decrements noted here [28]. In addition, although there was overlap in the brain regions in which metabolic activity was affected, there were important differences. These were most evident in cortex where anticipation altered regions associated with motivation and emotion (ventromedial prefrontal cortex, insula), while in the neutral environment cortical areas associated with more cognitive processing were involved (dorsolateral and orbital PFC, posterior cingulate, and inferior parietal). These data are consistent with the deficits in cognitive performance in nonhuman primates [33–36] and humans (see, for example, refs. [24, 37–41]) that persist with abstinence. Furthermore, the decreases in functional activity in brain regions particularly associated with decision making may underlie the increased risk of relapse that is characteristic of cocaine use disorder, especially in the earlier phases of abstinence that were modeled in the current study.
Behaviorally, the use of fixed-interval schedules of reinforcement, in which only one response is necessary after the passage of 3-min, made possible the self-administration of high cocaine doses (9.0 mg/kg/session), which can be considered modeling excessive cocaine use. Even under these conditions, responding was under schedule control, as represented by quarter-life values of 0.52. A major advantage of FI schedules is that there can be a large range of response rates without substantial differences in reinforcement frequency [42]. This was important because it was clear that 0.3 mg/kg/injection cocaine decreased response rates, which accounts for the significantly higher rates of food-maintained responding. Approximately every 2 weeks, the schedule of reinforcement was changed to a 2-h extinction session in which responding was recorded, but no reinforcer was delivered, in order to model “craving”. Responding was significantly higher in monkeys from the cocaine group compared to food controls, despite the fact that baseline responding under the FI 3-min was significantly higher in food-maintained compared to cocaine self-administration monkeys. Importantly, intermittent (i.e., every 2 weeks) extinction responding appeared stable, which could allow the use of this paradigm in pharmacological or behavioral intervention studies.
In previous studies, LCGU was determined in the operant chamber in which monkeys had a history of self-administering cocaine [26, 30, 31]. As a result, the contribution of conditioned reinforcers in the environment vs. neuropharmacological consequences of chronic cocaine exposure is unclear. To control for conditioned stimuli, monkeys were placed in a neutral environment (with videos presented) in which reinforcers were never available. Importantly, half the time this neutral condition occurred before and half the time after the operant session. This manipulation controlled for the possibility that the neutral environment would become a negative conditioned stimulus (CS-). In fact, according to Pavlovian conditioning theory, being placed in this environment 50% of the time before and 50% of the time after operant sessions assured that the environment was neutral, a condition termed “zero-order contingency” [43]. It was this environment in which LCGU was measured and, as a result, most functional effects noted below can be attributed to long-term cocaine self-administration.
Functionally, significant reductions were prominent in a number of brain regions including the thalamus (Table 2 and Fig. 2). The involvement of the thalamus is consistent with a growing literature on the role of the thalamus in cocaine self-administration behavior. In rodents, for example, lesions of the midline thalamic nucleus attenuated cocaine cue-induced reinstatement [44, 45], while activation accompanied cocaine reinstatement [46, 47]. In humans, neuroimaging studies of both current and abstinent cocaine users, recently reviewed by Huang and colleagues [48], have shown decreased activation as compared to healthy controls in the anticipation of monetary rewards [23, 49] and the presentation of cues [50], as well as performance of other tasks [51, 52]. Structural abnormalities [14, 18, 53], reduced thalamic connectivity [8, 54–56], and disruption of thalamic GABA function [57] have been reported, any or all of which could alter thalamic function.
Although the mechanisms responsible for the decrements in functional activity observed in this study are beyond the scope of the current investigation, there are many potential underlying sources that may have contributed to the alterations. Previous studies using this model, for example, have shown that alterations in the densities of dopamine D1 receptors, dopamine transporters, and group II metabotropic glutamate receptors were more prominent in dorsal rather than ventral striatal regions [58, 59], which may have contributed to this pattern of decreased functional activity. Cocaine use disorder has been associated with decreases in gray matter volume, when compared to healthy controls. Such decreases are particularly prominent in portions of the prefrontal cortex [15], which coincides with the deficits observed here. Similar gray matter deficits have been noted in insula, temporal and parietal cortex, and striatum [14, 15], regions where functional activity reductions were seen currently. Similarly, persistent alterations in functional activity in areas such as the prefrontal cortex, striatum, temporal, and parietal cortex, as measured with PET and fMRI, have been identified in numerous studies of cocaine users [60–62]. Many of these deficits were present in abstinent users, especially when testing occurred in the early phases of abstinence modeled in the current study [21, 23, 24, 63, 64]. Thus, the present findings are consistent with those in human cocaine users, and imply that those observed in cocaine users are indeed a result of the drug exposure itself, rather than confounding or pre-existing factors.
This study has some limitations. First, only two time points early in the course of abstinence were evaluated, therefore it is not clear whether any of these alterations in functional activity would persist for longer times or abate over time. Our previous work, examining the effects of cue exposure after 90 days of abstinence, would suggest that there is some abatement over time, however many of the effects persisted with the longer interval between final cocaine exposure and testing. A further limitation is that although the majority of functional deficits were found in brain regions involved in cognitive processing, no measures of cognition were examined over the course of the study. Finally, the study was, by design, cross-sectional with no repeated testing at different time points. Ideally, repeated testing over time would allow direct comparisons, both within and between monkeys exposed to cocaine over the course of exposure and after the cessation of drug use.
In summary, residual disruptions of functional brain activity were found throughout the dorsal striatum, thalamus, and prefrontal, temporal and parietal cortices following the cessation of chronic cocaine use. Since the studies were conducted in a neutral environment, our findings suggest that chronic cocaine exposure has profound effects on brain activity independent of the presence of conditioned stimuli. This pattern of decreased activity, largely in cognitive and decision making networks, supports the idea of an imbalance between cognitive control and motivational and emotional circuits central to many models of addiction [13, 65–67]. Finally, these data suggest that a focus on treatment strategies such as cognitive training and/or neuromodulation of cortical regions may help to enhance the likelihood of recovery.
Author contributions
LJP and MAN designed the study. HRS, MAN, TJRB, MDM, and SHN acquired and analyzed the data. LJP wrote the manuscript with significant input and revisions from HRS, MAN, and TJRB. All authors approved the final version of the manuscript.
Funding and disclosure
Funding for this study was provided by the National Institute of Drug Abuse grants DA09085 and DA06634.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363:3257–66. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology. 2008;197:421–31. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–73. doi: 10.1016/S0376-8716(01)00206-X. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–58. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Hanlon CA, Wesley MJ, Roth AJ, Miller MD, Porrino LJ. Loss of laterality in chronic cocaine users: an fMRI investigation of sensorimotor control. Psychiatry Res. 2010;181:15–23. doi: 10.1016/j.pscychresns.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–71. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 7.Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: a comprehensive review. Neurosci Biobehav Rev. 2013;37:1838–59. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanlon CA, Wesley MJ, Porrino LJ. Loss of functional specificity in the dorsal striatum of chronic cocaine users. Drug Alcohol Depend. 2009;102:88–94. doi: 10.1016/j.drugalcdep.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM, et al. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1993;5:419–27. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–6. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, et al. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–90. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 13.Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron. 2018;98:886–903. doi: 10.1016/j.neuron.2018.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23:615–24. doi: 10.1016/j.conb.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–42. doi: 10.1016/S0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 16.Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, et al. Mega-analysis of gray matter volume in substance dependence: general and substance-specific regional effects. Am J Psychiatry. 2019;176:119–28. doi: 10.1176/appi.ajp.2018.17040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humphreys K, Bickel WK. Toward a neuroscience of long-term recovery from addiction. JAMA Psychiatry. 2018;75:875–76. doi: 10.1001/jamapsychiatry.2018.0956. [DOI] [PubMed] [Google Scholar]
- 18.Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology. 2011;218:681–92. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parvaz MA, Moeller SJ, d’Oleire Uquillas F, Pflumm A, Maloney T, Alia-Klein N, et al. Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: a longitudinal study. Addict Biol. 2017;22:1391–401. doi: 10.1111/adb.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 2011;114:159–68. doi: 10.1016/j.drugalcdep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell RP, Foxe JJ, Ross LA, Garavan H. Intact inhibitory control processes in abstinent drug abusers (I): a functional neuroimaging study in former cocaine addicts. Neuropharmacology. 2014;82:143–50. doi: 10.1016/j.neuropharm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morie KP, Garavan H, Bell RP, De Sanctis P, Krakowski MI, Foxe JJ. Intact inhibitory control processes in abstinent drug abusers (II): a high-density electrical mapping study in former cocaine and heroin addicts. Neuropharmacology. 2014;82:151–60. doi: 10.1016/j.neuropharm.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Carroll KM, et al. Neurofunctional reward processing changes in cocaine dependence during recovery. Neuropsychopharmacology. 2016;41:2112–21. doi: 10.1038/npp.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend. 2012;121:45–53. doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169:406–14. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–62. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nader MA, Morgan D. Effects of negative punishment contingencies on cocaine self-administration by rhesus monkeys. Behav Pharmacol. 2001;12:91–9. doi: 10.1097/00008877-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Porrino LJ, Beveridge TJ, Smith HR, Nader MA. Functional consequences of cocaine expectation: findings in a non-human primate model of cocaine self-administration. Addict Biol. 2016;21:519–29. doi: 10.1111/adb.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–18. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- 30.Beveridge TJ, Smith HR, Nader SH, Nader MA, Porrino LJ. Functional consequences of cocaine re-exposure after discontinuation of cocaine availability. Neuropharmacology. 2014;85:528–37. doi: 10.1016/j.neuropharm.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- 32.Catania AC, Reynolds GS. A quantitative analysis of the responding maintained by interval schedules of reinforcement. J Exp Anal Behav. 1968;11:327–83. doi: 10.1901/jeab.1968.11-s327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould RW, Garg PK, Garg S, Nader MA. Effects of nicotinic acetylcholine receptor agonists on cognition in rhesus monkeys with a chronic cocaine self-administration history. Neuropharmacology. 2013;64:479–88. doi: 10.1016/j.neuropharm.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porrino LJ, Hampson RE, Opris I, Deadwyler SA. Acute cocaine induced deficits in cognitive performance in rhesus macaque monkeys treated with baclofen. Psychopharmacology. 2013;225:105–14. doi: 10.1007/s00213-012-2798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jedema HP, Song X, Aizenstein HJ, Bonner AR, Stein EA, Yang Y, et al. Long-term cocaine self-administration produces structural brain changes that correlate with altered cognition. Biol Psychiatry. 2020;89:376–385. doi: 10.1016/j.biopsych.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–34. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–94. doi: 10.1016/S1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolla KI, Funderburk FR, Cadet JL. Differential effects of cocaine and cocaine alcohol on neurocognitive performance. Neurology. 2000;54:2285–92. doi: 10.1212/WNL.54.12.2285. [DOI] [PubMed] [Google Scholar]
- 39.Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–71. doi: 10.1016/S0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hester R, Bell RP, Foxe JJ, Garavan H. The influence of monetary punishment on cognitive control in abstinent cocaine-users. Drug Alcohol Depend. 2013;133:86–93. doi: 10.1016/j.drugalcdep.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel KT, Stevens MC, Meda SA, Muska C, Thomas AD, Potenza MN, et al. Robust changes in reward circuitry during reward loss in current and former cocaine users during performance of a monetary incentive delay task. Biol Psychiatry. 2013;74:529–37. doi: 10.1016/j.biopsych.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeiler MD. Schedules of reinforcement: the controlling variables. In: Honig WK, Staddon JER, editors. Handbook of operant behavior. Prentice-Hall: Englewood Cliffs, N. J.; 1977.
- 43.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Varations in the efffectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: current research and theory. New York: Appleton-Century-Crofts; 1972.
- 44.Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–12. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- 45.James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, et al. Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS ONE. 2010;5:e12980. doi: 10.1371/journal.pone.0012980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matzeu A, Weiss F, Martin-Fardon R. Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking behavior. Neurosci Lett. 2015;608:34–9. doi: 10.1016/j.neulet.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelloux Y, Hoots JK, Cifani C, Adhikary S, Martin J, Minier-Toribio A, et al. Context-induced relapse to cocaine seeking after punishment-imposed abstinence is associated with activation of cortical and subcortical brain regions. Addict Biol. 2018;23:699–712. doi: 10.1111/adb.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang AS, Mitchell JA, Haber SN, Alia-Klein N, Goldstein RZ. The thalamus in drug addiction: from rodents to humans. Philos Trans R Soc Lond B Biol Sci. 2018;373:20170028. [DOI] [PMC free article] [PubMed]
- 49.Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, et al. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70:553–60. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, et al. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict Biol. 2010;15:504–16. doi: 10.1111/j.1369-1600.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell MR, Balodis IM, Devito EE, Lacadie CM, Yeston J, Scheinost D, et al. A preliminary investigation of Stroop-related intrinsic connectivity in cocaine dependence: associations with treatment outcomes. Am J Drug Alcohol Abuse. 2013;39:392–402. doi: 10.3109/00952990.2013.841711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, et al. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li CR. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol Depend. 2014;134:51–62. doi: 10.1016/j.drugalcdep.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS ONE. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W, Worhunsky PD, Zhang S, Le TM, Potenza MN, Li CR. Response inhibition and fronto-striatal-thalamic circuit dysfunction in cocaine addiction. Drug Alcohol Depend. 2018;192:137–45. doi: 10.1016/j.drugalcdep.2018.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang S, Hu S, Sinha R, Potenza MN, Malison RT, Li CS. Cocaine dependence and thalamic functional connectivity: a multivariate pattern analysis. Neuroimage Clin. 2016;12:348–58. doi: 10.1016/j.nicl.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Gatley SJ, Dewey SS, et al. Enhanced sensitivity to benzodiazepines in active cocaine-abusing subjects: a PET study. Am J Psychiatry. 1998;155:200–6. doi: 10.1176/ajp.155.2.200. [DOI] [PubMed] [Google Scholar]
- 58.Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34:1162–71. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Group II metabotropic glutamate receptors in the striatum of non-human primates: dysregulation following chronic cocaine self-administration. Neurosci Lett. 2011;496:15–9. doi: 10.1016/j.neulet.2011.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma L, Steinberg JL, Moeller FG, Johns SE, Narayana PA. Effect of cocaine dependence on brain connections: clinical implications. Expert Rev Neurother. 2015;15:1307–19. doi: 10.1586/14737175.2015.1103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart JL, May AC, Paulus MP. Bouncing back: brain rehabilitation amid opioid and stimulant epidemics. Neuroimage Clin. 2019;24:102068. doi: 10.1016/j.nicl.2019.102068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costumero V, Bustamante JC, Rosell-Negre P, Fuentes P, Llopis JJ, Avila C, et al. Reduced activity in functional networks during reward processing is modulated by abstinence in cocaine addicts. Addict Biol. 2017;22:479–89. doi: 10.1111/adb.12329. [DOI] [PubMed] [Google Scholar]
- 64.Hanlon CA, Beveridge TJ, Porrino LJ. Recovering from cocaine: insights from clinical and preclinical investigations. Neurosci Biobehav Rev. 2013;37:2037–46. doi: 10.1016/j.neubiorev.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 66.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]