Abstract
A profound eosinophil infiltration of granulomas is observed in the lungs of Mycobacterium bovis bacillus Calmette Guérin-infected gamma interferon receptor-deficient mice. Blockade of eosinophil proliferation and recruitment into the lung by treatment with anti-interleukin-5 monoclonal antibody marginally reduced mycobacterial growth within the lung but did not affect dissemination of the infection to other tissues.
Mice deficient in gamma interferon (IFN-γ−/−) or the IFN-γ receptor (IFN-γR−/−) are extremely susceptible to infection with tuberculosis-causing organisms (3, 6). The unrestricted growth of mycobacteria observed in these mice is accompanied by a striking increase in the number of eosinophilic aggregates at the site of infection (3, 4). As blood and tissue eosinophilia induced by helminth infection is dependent on the production of the Th2 cytokine interleukin-5 (IL-5) (2, 8), the presence of eosinophils in the cellular infiltrate at the site of mycobacterial infection strongly suggests that increased levels of IL-5 are produced in vivo during Mycobacterium bovis bacillus Calmette Guérin (BCG) infection in the absence of IFN-γ signaling. Indeed, several studies have shown that elevated levels of Th2 cytokines are produced by lymphocytes from mycobacterium-infected IFN-γ−/− and IFN-γR−/− mice (4, 9).
Eosinophil infiltration of the lungs and peripheral eosinophilia have been associated with tuberculosis disease in Mycobacterium tuberculosis-infected patients (5, 10). In our study, we considered the possibility that the lung eosinophilia observed in intranasally BCG-infected IFN-γR−/− mice may play a contributory role in their susceptible phenotype. We hypothesised that the eosinophil influx may exaggerate the disease severity because eosinophils, which have been shown to phagocytose mycobacteria (1), may provide an intracellular habitat in which BCG could proliferate in an unrestricted manner. BCG-infected eosinophils might also have contributed to hematogenous dissemination of BCG, leading to an increased bacterial load in other tissues.
To study the effect of eosinophilia on the progression of M. bovis BCG infection in IFN-γR−/− mice, we inhibited infection-induced blood and lung eosinophilia by treatment with anti-IL-5 monoclonal antibody (MAb). IFN-γR−/− mice were a gift from M. Aguet (University of Zürich, Zürich, Switzerland), and mice were maintained on a 129/Sv/Ev genetic background. Wild-type 129/Sv/Ev mice were used as controls. All experiments were approved by the Wellington School of Medicine Animal Ethics Committee.
To establish a lung infection, mice were infected intranasally with BCG strain Pasteur (a gift from AgResearch Wallaceville, Upper Hutt, New Zealand) which had been grown, stored, and enumerated as previously described (7). To facilitate the intranasal infection, mice were anesthetised by intraperitoneal injection of ketamine and xylazine, and then 50 μl of BCG diluted in phosphate-buffered saline with 0.05% Tween 80 (Sigma) was slowly pipetted onto the external nares to be inhaled by the mice. At 4 weeks postinfection, mice were sacrificed by lethal anesthesia, and organs and tissues were harvested for analysis.
Whole organs were homogenized in sterile 1% Tween 80 in distilled water using an Ultra Turrax T25 Disperser (IKA Works, Kuala Lumpur, Malaysia). Tenfold serial dilutions of homogenates were made in sterile water with 1% Tween 80. Samples were plated onto Middlebrook 7H11 agar (Difco, Detroit, Mich.) using autoclaved glass bacterial cell spreaders (Biolab Scientific, Auckland, New Zealand) and incubated for 21 days at 37°C. Colonies counts are expressed as CFU per organ. The efficacy of anti-IL-5 MAb treatment was calculated by use of the Kruskal-Wallis test for two groups.
Tissue samples for histological analysis were fixed in 10% phosphate-buffered formyl saline for 24 h and embedded in paraffin wax. Sections of 3 μm in width were cut and stained with hematoxylin and eosin and then examined by light microscopy.
To determine the number of circulating eosinophils during infection, mice were placed in a restraining device, and a small incision was made at the end of the tail. A 10-μl volume of blood was added to 90 μl of Turks solution. Blood smears were prepared and stained with Diff-Quik (Dade International Inc., Miami, Fla.) according to the manufacturer's instructions. Percentages of macrophages, lymphocytes, neutrophils, and eosinophils were determined microscopically using standard histological criteria.
Anti-mIL-5 MAb (TRFK4 or TRFK5; rat immunoglobulin G [IgG]) was purified from hybridoma supernatant using protein G affinity columns (Pharmacia Biotech, Uppsala, Sweden). Mice were injected intraperitoneally with 2 mg of anti-mIL-5 MAb twice weekly, as previously described (2), commencing on day 0 of the BCG infection. Control mice were injected twice weekly with 2 mg of control rat Ig, purified from serum using protein G columns.
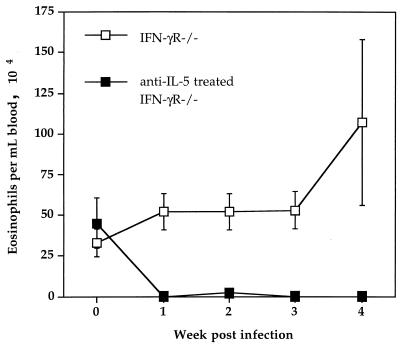
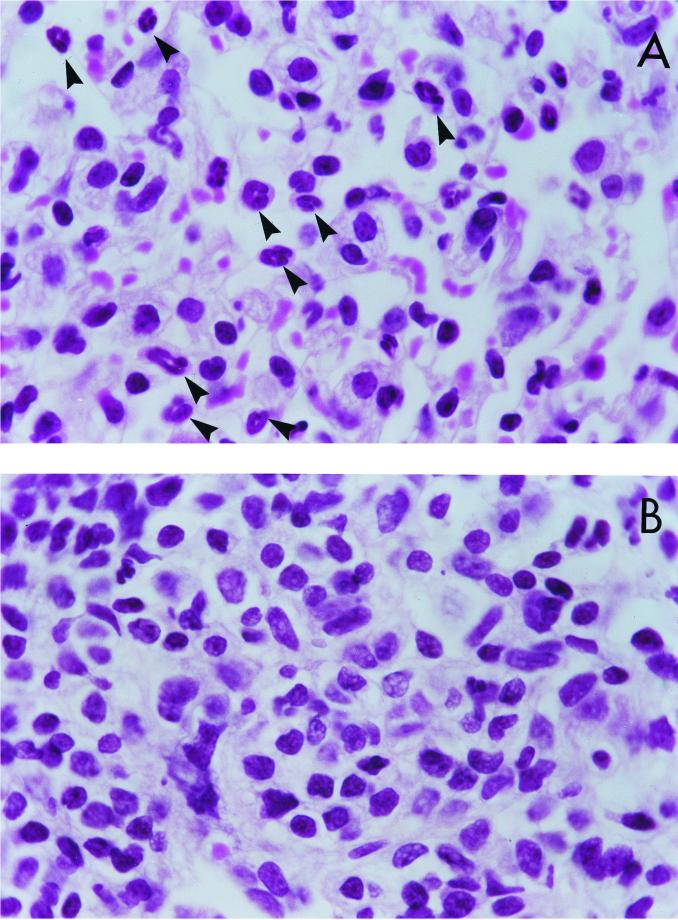
As expected, following intranasal BCG infection control IFN-γR−/− mice had an increase in blood eosinophils (Fig. 1) as well as an accumulation of eosinophils in the lung (Fig. 2A). In contrast, IFN-γR−/− mice treated with anti-IL-5 had no detectable eosinophils in the blood (Fig. 1). Very few eosinophils were detectable in the lungs of anti-IL-5-treated IFN-γR−/− mice, and the cellular infiltrate consisted primarily of mononuclear cells (Fig. 2B).
FIG. 1.
Anti-IL-5 MAb treatment of IFN-γR−/− mice eliminates BCG infection-induced blood eosinophilia. IFN-γR−/− age- and sex-matched mice were infected intranasally with 5 × 105 BCG strain Pasteur organisms and were treated with anti-IL-5 MAb or with control rat IgG. Blood samples were taken at the time points indicated after BCG infection. Differential counts were made by staining blood smears with Diff-Quik. Data points represent the mean number (± standard error) of eosinophils per milliliter of blood from five mice.
FIG. 2.
Anti-IL-5 MAb treatment of IFN-γR−/− mice eliminates BCG infection-induced accumulation of eosinophils in the lung. High-power micrographs of lung sections from IFN-γR−/− mice, taken 4 weeks after intranasal BCG infection, are shown. Mice were infected with 5 × 105 BCG strain Pasteur organisms and were treated with control rat IgG (A) or with anti-IL-5 MAb (B). Lungs were fixed in formalin, and 1- to 2-μm sections were stained with hematoxylin and eosin. The sections shown are representative of samples taken from five mice per treatment group. Eosinophils are marked by arrowheads.
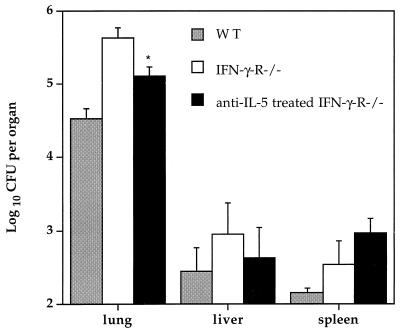
We compared viable bacterial counts in control IFN-γR−/− mice with those in IFN-γR−/− mice treated with anti-IL-5 MAb at 4 weeks postinfection. As is typically observed following intranasal BCG infection, the bulk of viable bacteria were found within the lungs of all infected mice, and the bacterial counts in tissues from control IFN-γR−/− mice were notably higher than those in wild-type control mice (Fig. 3). Significantly, IFN-γR−/− mice treated with anti-IL-5 MAb had a threefold decrease in the number of viable BCG organisms present in the lung compared with control IFN-γR−/− mice (Fig. 3). No significant difference was observed in viable bacterial counts in the liver and spleen compared with control IFN-γR−/− mice (Fig. 3).
FIG. 3.
Effect of anti-IL-5 MAb treatment on tissue bacterial counts in IFN-γR−/− deficient mice. Wild-type (WT) or IFN-γR−/− age- and sex-matched mice were infected intranasally with 5 × 105 BCG strain Pasteur organisms. One group of IFN-γR−/− mice was treated with anti-IL-5 MAb, and the other was treated with control rat IgG. Mice were sacrificed at 4 weeks after infection. Viable bacterial counts in the lung, liver, and spleen were measured by plating serial dilutions of blood or tissue homogenates on Middlebrook 7H11 agar. Data points represent the geometric mean (± standard error) of viable bacterial counts per organ from five mice. ∗, statistically significant compared with untreated IFN-γR−/− mice (P < 0.05), as calculated by the Kruskel-Wallis test for two groups.
Based on other studies, the effect of IL-5 deficiency appears to be restricted to defects in the development of CD5+ B cells and the absence of eosinophilia (2, 8). Therefore, although not tested in this study, it is unlikely that anti-IL-5 MAb treatment would have affected other aspects of the immune response that could have altered the course of mycobacterial infection.
In conclusion, the modest beneficial effect of anti-IL-5 MAb treatment on the ability of IFN-γR−/− mice to control the intranasal BCG infection within the lung suggests that the heightened susceptibility of IFN-γR−/− mice to mycobacterial infection can be attributed in part to the elevated levels of IL-5 and influx of eosinophils to the site of infection. The eosinophils may exacerbate the course of mycobacterial infection by interfering with macrophage activation and function or by providing an intracellular environment which promotes mycobacterial growth. In light of these results, further investigation into the impact of Th2 immune responses on clinical mycobacterial disease is warranted.
REFERENCES
- 1.Castro A G, Esaguy N, Macedo P M, Aguas A P, Silva M T. Live but not heat-killed mycobacteria cause rapid chemotaxis of large numbers of eosinophils in vivo and are ingested by the attracted granulocytes. Infect Immun. 1991;59:3009–3014. doi: 10.1128/iai.59.9.3009-3014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffman R L, Seymour B W, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 3.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erb K, Kirman J, Delahunt B, Le Gros G. Infection of mice with M. bovis BCG induces both Th1 and Th2 immune responses in the absence of interferon-γ signaling. Eur Cytokine Networks. 1999;10:147–153. [PubMed] [Google Scholar]
- 5.Flores M, Merino-Angulo J, Tanago J G, Aguirre C. Late generalized tuberculosis and eosinophilia. Arch Int Med. 1983;143:182. [PubMed] [Google Scholar]
- 6.Flynn J L, Chan J C, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirman J, McCoy K, Hook S, Prout M, Delahunt B, Orme I, Frank A, Le Gros G. CTLA-4 blockade enhances the immune response induced by mycobacterial infection but does not lead to increased protection. Infect Immun. 1999;67:3786–3792. doi: 10.1128/iai.67.8.3786-3792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopf M, Brombacher F, Hodgkin P D, Ramsay A J, Milbourne E A, Dai W J, Ovington K S, Behm C A, Köhler G, Young I G, Matthaei K I. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 9.Murray P J, Young R A, Daley G Q. Hematopoietic remodeling in interferon-γ-deficient mice infected with mycobacteria. Blood. 1998;91:2914–2924. [PubMed] [Google Scholar]
- 10.Vijayan V-K, Reetha A-M, Jawahar M S, Sankaran K, Prabhakar R. Pulmonary eosinophilia in pulmonary tuberculosis. Chest. 1992;101:1708–1709. doi: 10.1378/chest.101.6.1708. [DOI] [PubMed] [Google Scholar]