Abstract
Central nervous system (CNS) infections caused by nontuberculous mycobacteria have been described previously, especially in patients with AIDS. To investigate specific aspects of the pathogenesis of this entity, C57BL bg+/bg− mice were infected intravenously with Mycobacterium avium, and cultures of blood and brain as well as histopathology examination of brain tissue were carried out at several time points up to 6 months after infection. Low-grade inflammatory changes with small aggregates of lymphocytes and macrophages as well as perivascular cuffing were seen early in the infection. A small number of bacteria could be observed in the parenchyma of the choroid plexus. Six months after infection, numerous bacteria were present within the foamy macrophage of the granulomatous lesions along the ventricle and meninges. None of the mice developed clinical signs of meningitis or encephalitis or even died spontaneously during the period of observation. Use of CD18−/− knockout mice indicated that transport of the bacterium within neutrophils or monocytes into the brain is unlikely. Mild chronic CNS infection developed in the mice during sustained systemic M. avium infection, similar to what has been reported in most human cases.
Central nervous system (CNS) infections due to nontuberculous mycobacteria (NTBM) usually manifest as meningitis or meningoencephalitis, but occasionally pure encephalitis or abscesses along neuraxes are found (5, 9). NTBM brain diseases are most often described in patients with AIDS or other immune-compromised patients, but it had also been reported in individuals without obvious underlying disease (5, 26, 28). CNS infections with NTBM can sometimes be misdiagnosed as Mycobacterium tuberculosis infection, resulting in incorrect treatment and death (11, 28). As the epidemic of human immunodeficiency virus infection and the application of immunosuppressive treatment expand, it has become more likely that doctors might come in contact with these usually infrequent infections. A suitable animal model may therefore help us to understand the immunopathophysiology of this disease, as well as to develop an effective therapy.
The Mycobacterium avium complex is the most common organism causing systemic opportunistic bacterial infections in patients with AIDS (13, 14, 29). It is also the leading cause of the NTBM brain disease in this population (5). Patients with M. avium infection usually have disseminated disease with bacteremia and frequently liver, spleen, bone marrow, and lymph node involvement. In addition, a variable percentage of these patients can have invasive infection in the brain (6, 11, 12, 15, 17, 27).
Murine models of disseminated M. avium infection have been developed since 1983 (2, 3, 10) and used extensively in the investigation of host response and in the screening of active therapeutic agents (1). In these models, bacterial burden and histopathology changes in the abdominal organs and lymph nodes had been thoroughly studied, but the neuraxes have not been evaluated.
The CNS is confined to an anatomic closed space, separated physically and immunologically from the rest of the body by the rigid bony cage of the skull as well as the blood barrier. Therefore, the clinical presentation, immune response, and drug treatment may be quite different for CNS infection and peripheral systemic infection with the same pathogen.
In an attempt to improve our knowledge about the pathogenesis of CNS infections caused by M. avium, we developed a murine model that shows brain pathology following bacteremia.
Four-month-old female C57BL bg+/bg− mice and C57BL/6J-Itgb2 (CD18−/− knockout [KO] mice) weighing 20 g (Jackson Laboratory, Bar Harbor, Maine) were used for the experiments. Mice were briefly anesthetized by halothane inhalation before inoculation of bacteria. M. avium strain 101 (serovar 1) was isolated from the blood of a patient with AIDS (3). M. avium organisms were cultured for 10 days at 37°C on Middlebrook agar 7H11 medium (Difco Laboratories, Detroit, Mich.) supplemented with oleic acid, albumin, dextrose, and catalase (OADC; Difco). Transparent colonies were suspended in Hanks' buffered salt solution (HBSS) and adjusted to the desired concentration by comparison with a McFarland turbidity standard. Samples obtained from the bacterial suspension were plated onto Middlebrook 7H11 agar to confirm the inoculum size. One hundred microliters of bacterial suspension was used for intravenous (i.v.) injection, and 40 μl of bacterial suspension was used for intracerebral (i.c.) injection.
In a preliminary protocol, we attempted to define the minimal doses required to obtain brain infection when bacteria were administered by i.c. inoculation. Mice were transiently anesthetized by halothane inhalation and challenged i.c. with 1.2 × 103, 1.2 × 104, or 1.2 × 106 organisms of M. avium. According to the method described by others (18), 40 μl of bacterial suspension was delivered through a 27-gauge needle by direct puncture into the cranium at the midline, 4 mm posterior to the orbit. At 1, 4, 8, and 78 days, the brain was obtained and homogenized, and the bacteria were quantitated after plating. In an alternative protocol that resembles the human infection, we infected mice with M. avium i.v. Three groups of mice were given suspensions of 1.2 × 107, 1.2 × 108, or 1.2 × 109 organisms in 100 μl of HBSS by tail vein injection. Blood and brain were obtained from 2 h to 2 months after M. avium infection to determine the bacterial load and histopathologic changes. Mice infected by the i.v. route were compared with mice receiving 3 × 104 organisms by i.c. infection.
For blood culture, 0.05 ml of blood was drawn from the tail vein and inoculated into BACTEC 12B radiometric medium (Johnson Laboratories, Towson, Md.). The number of bacteria present in the blood was determined by using the T100 method, as previously reported (3). For brain culture, mice were killed by intramuscular injection of ketamine (400 mg/kg) and xylazine (80 mg/kg), and the brain was removed and homogenized in 2 ml of Middlebrook 7H9 broth as previously described (18). Serial 10-fold dilutions of the homogenate were plated onto Middlebrook 7H11 agar supplemented with OADC. After incubation for 7 to 10 days at 37°C, the number of colonies was quantitated. For the histopathological tissue preparation, immediately after being killed, the mice were intracardially perfused with 30 ml of heparin solution (10 U/ml) followed by 60 ml of 4% paraformaldehyde. After subsequent fixation with 4% paraformaldehyde, the brain was embedded in paraffin, sectioned at 4 μm, and stained either with hematoxylin-eosin (HE) or by the Ziehl-Neelsen method.
Immunohistochemistry was carried out using paraffin preparations as described before (15, 16) with anti-CD4 and anti-CD8 antibodies (Pharmingen, San Diego, Calif.). Briefly, samples were deparaffinized and rehydrated, and the endogenous peroxidase was blocked with 3% H2O2 for 5 min. After washing, antigens were unmasked by treatment with 1 mM EDTA (pH 8) with heat until boiling. After washing, the sample was blocked with serum and then exposed to anti-CD8 (clone 53-5.8) or anti-CD4 (clone H129.19) antibodies followed by the biotinylated second antibody and the ABC reagent (Vector, Burlingame, Calif.). The number of positive cells was counted under the microscope by two individuals. As a control for staining, spleen tissues from infected mice were run in parallel.
In subsequent experiments, mice were infected with 6 × 109 bacteria by the i.v. route. From 4 to 11 mice were used for blood culture and 7 mice were used for brain culture at each time point between 2 h and 6 months. Histopathologic examination was performed during this period as well. In the experiments with CD18 KO mice, 3 × 108 bacteria were used to infect mice i.v. Ten mice were used per experimental group.
The results represent the mean ± the standard error of the mean (SEM). The significance of the results was determined by Student's t test and Fisher's exact test.
Mice tolerated M. avium infection very well when infected by either the i.v. or i.c. route. Except for a slight body weight loss observed at the end of the experiment, no mice died. No prominent movement impairment, convulsions, seizures, or gait disturbance was noted during 2 months of observation in the i.c.-infected mice or up to 6 months of observation in the i.v.-infected mice.
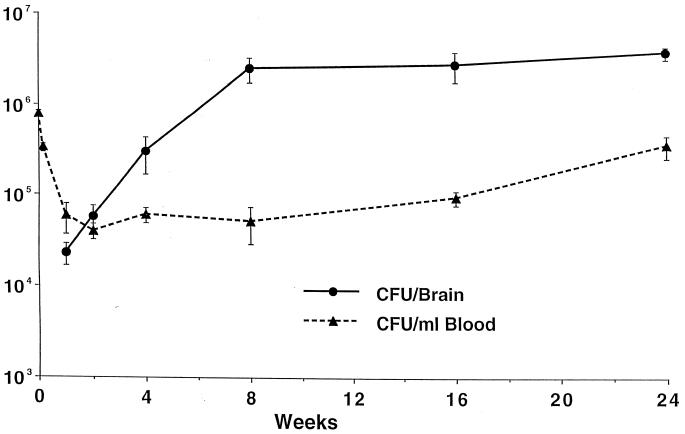
In mice given 1.2 × 107, 1.2 × 108, or 1.2 × 109 M. avium by the i.v. route, an early peak of bacteremia with magnitude related to the inoculum size could be detected 2 h after inoculation (Table 1). Sustained circulating levels of M. avium (104 CFU/ml) persisting for 2 months were observed in mice receiving 1.2 × 108 or more bacteria (Table 1). Similar results were seen in mice receiving 6 × 109 organisms and observed for up to 6 months (Fig. 1). The number of bacteria in the brain did not change significantly from 8 weeks to 6 months after infection.
TABLE 1.
Bacterial load in blood and brain after infection by different routes
| Time postinfection | Sample | Mean no. of bacteriaa (log CFU) (n = 3)
|
|||
|---|---|---|---|---|---|
| 107 CFU i.v. | 108 CFU i.v. | 109 CFU i.v. | 104 CFU i.c. | ||
| 2 h | Blood | 4.88 | 5.47 | >6.24 | |
| Brain | 0 | 0 | 0 | 2.13 | |
| 1 day | Blood | 3.80 | 4.78 | 4.58 | |
| Brain | 2.88 | 3.27 | 2.95 | 2.78 | |
| 7 days | Blood | 4.62 | 3.38 | 4.32 | |
| Brain | 3.151 | 3.88 | 4.01 | 3.381 | |
| 14 days | Blood | 2.56 | 2.65 | 4.88 | |
| Brain | 4.301,2 | 3.951 | 4.181 | 4.011,2 | |
| 28 days | Blood | <1.8 | 4.34 | 5.30 | |
| Brain | 3.651 | 4.561,2,3 | 4.691,2 | 4.511,2 | |
| 56 days | Blood | 2.36 | 4.04 | 3.71 | |
| Brain | 2.87 | 4.681,2,3 | 4.691,2 | 3.40 | |
1, P < 0.05 compared with the number of bacteria in the brain at day 1; 2, P < 0.05 compared with the number of bacteria in the brain at day 7; 3, P < 0.05 compared with the number of bacteria in the brain at day 14.
FIG. 1.
Mice were given 6 × 109 CFU of M. avium 101 i.v. Four to 11 mice were used for blood culture and 3 to 7 mice were used for brain culture at each timepoint. Data are the mean CFU ± SEM. Mice had a significant increase in bacterial load in the brain within the first 2 months (P = 0.0058).
In the i.c.-infected mice, bacteria could only be recovered from the brain of mice that received >103 organisms. The number of bacteria recovered from the brain 1 day after infection was much smaller than the inoculum used (Table 2). However, the number of organisms increased progressively afterwards (Table 2) and reached a load similar to the number of bacteria injected at about 1 month postinfection, when marked meningoencephalitis could be observed microscopically (Fig. 2). In the mice given 1.2 × 108 organisms as well as in mice receiving greater numbers of bacteria i.v., the bacterial burden in the brain increased progressively during the first 2 months after infection (Table 1) and then remained constant until the termination of the study (Fig. 1).
TABLE 2.
Number of bacteria in the brain of mice after i.c. inoculation
| Day after infection | No. of bacteria/braina
|
||
|---|---|---|---|
| 1.2 × 103 CFU | 1.2 × 104 CFU | 1.2 × 106 CFU | |
| 1 | 0 | 1.75 × 103 | 3.45 × 103 |
| 4 | 4.2 × 102 a | 1.95 × 103 | 3.3 × 104 a |
| 8 | 0 | 4.95 × 103 | 6.38 × 104 a |
| 78 | 0 | NDb | 1.78 × 103 |
P < 0.05 compared with the number of bacteria in the brain at day 1.
ND, not done.
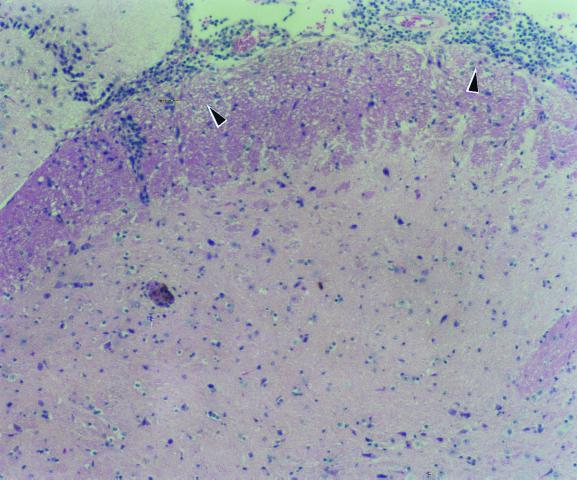
FIG. 2.
Brain histopathology, showing intense infiltration of lymphocytes and macrophages in the optical chiasm 2 weeks after i.c. infection.
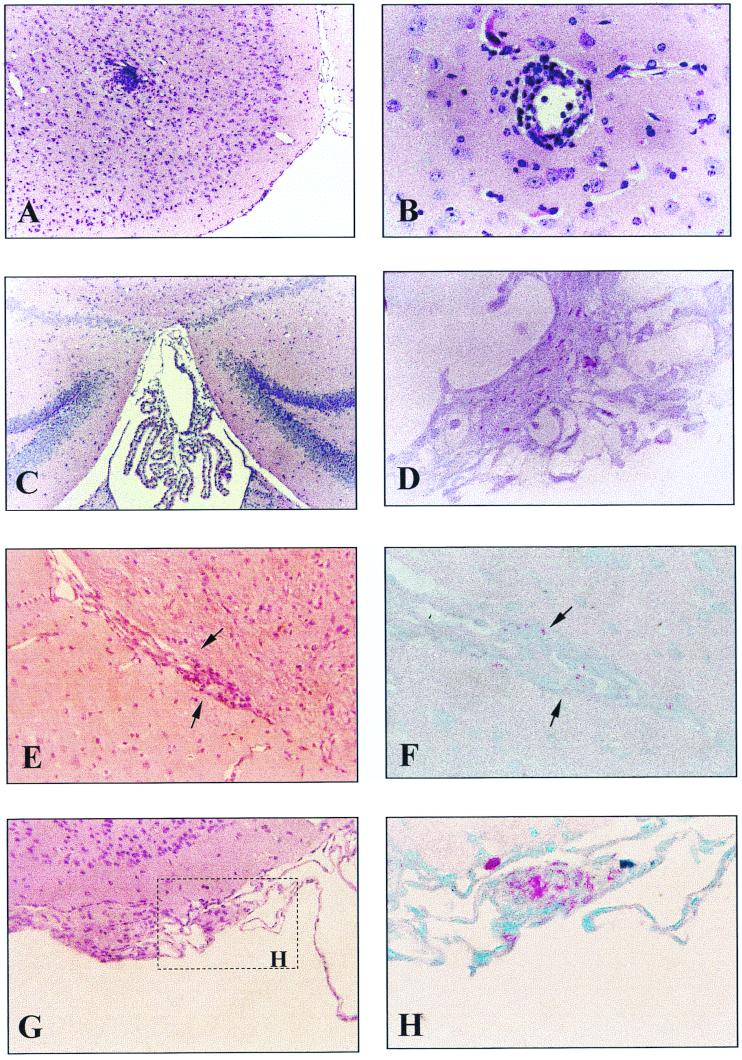
In both mice receiving 3 × 108 CFU of M. avium i.v. or 3 × 104 CFU of M. avium i.c., inflammatory changes in the brain were not observed until 1 month after infection. Nonetheless, bacteria were recovered from the brain early after infection. In the i.c.-infected mice, marked meningoencephalitis with prominent granulomatous infiltration (especially around the basal cistern), perivascular lymphocytes, and macrophages cuffing were observed 1 month after infection (Fig. 2), and frank periventricular and choroid plexus infiltration were detected 2 months later. However, there were only a few acid-fast bacilli in these lesions, and no necrosis or giant cells were seen. In the i.v.-infected mice, low-grade inflammation with macrophages and T lymphocytes was the only change observed. Immunohistochemical analysis showed that 80 ± 6% (mean ± SEM) of the lymphocytes were CD8+ T cells and 9 ± 3% stained for CD4+ T cells. Focal meningeal infiltration as well as vasculitis and small aggregates of lymphocytes and macrophages in the parenchyma could be observed 1 month after inoculation (Fig. 3). Mild choroid plexus infiltration accompanied by numerous acid-fast bacilli was seen in all the brains studied 2 months later (Fig. 3). Four months after infection, acid-fast bacilli could be easily found in the subarachnoid space. Aggregates of foamy histiocytes containing numerous acid-fast organisms were further identified 6 months after infection along the ventricle and meninges (Fig. 3).
FIG. 3.
Brain histopathology of mice receiving 3 × 108 CFU i.v. (A) At 1 month after inoculation, one nonnecrotizing granuloma was seen; HE strain. (B) Perivascular cuffing seen 2 months after inoculation. HE stain. (C) mild choroid plexus infiltration. HE stain. (D) acid-fast bacilli in the parenchyma of the choroid plexus. AFB stain. (E and F) At 4 months after infection there was cellular infiltration along the ventricle (E) (HE stain) and acid-fast bacilli were found in the lesion (F) (AFB stain). (G and H) At 6 months after inoculation, meningeal granuloma with foamy histiocytes was found (G) (HE stain) containing numerous acid-fast bacilli (H) (AFB stain). Magnifications: A and C, ×100; B, ×400; D, ×630; E and G, ×250; F and H, ×600.
To investigate whether transport within monocytes or neutrophils represented a mechanism of M. avium invasion of the CNS, we infected both C57BL and C57BL CD18−/− KO mice i.v. and determined the number of bacteria in the brain at several timepoints after infection. As shown in Table 3, CD18−/− KO mice, in which monocytes and neutrophils are significantly impaired in their ability to travel across the blood vessel wall, showed the same number of organisms in the brain as the C57BL wild-type mice.
TABLE 3.
Comparison of the number of M. avium in the brains of wild-type and CD18 KO mice after i.v. infectiona
| Day postinfection | 105 CFU/g of brain (mean ± SEM, n = 10)
|
|
|---|---|---|
| Wild type | CD18 KO | |
| 1 | 1.2 ± 0.4 | 1.6 ± 0.2 |
| 7 | 2.4 ± 0.3 | 2.7 ± 0.3 |
| 14 | 3.5 ± 0.4 | 3.9 ± 0.1 |
| 28 | 3.3 ± 0.6 | 4.0 ± 0.5 |
P > 0.05 for all the comparisons between number of bacteria in wild type and CD18 KO at each timepoint.
We have analyzed the kinetics of CNS invasion by M. avium after i.v. infection. Our results suggest that M. avium infection of the CNS during AIDS is an underrecognized entity. We used strain 101, which is a well-characterized virulent strain of M. avium (3). Other strains, such as the virulent strain 724 (22), however, may prove to be more invasive of the CNS or trigger a greater inflammatory response than strain 101 and should be investigated in this model in the future.
In our system, the number of bacteria in the brain increases with the duration and level of bacteremia, which suggests that the duration and magnitude of bacteremia play an important role in the pathogenesis of M. avium brain disease, as has been recognized for acute bacterial meningitis caused by other organisms, such as Streptococcus pneumoniae and Listeria monocytogenes (4).
In contrast to the marked inflammatory response observed in i.c.-infected mice, i.v.-infected mice with the same bacterial burden in the brain developed only a mild inflammatory response. Why the intensity of the immune response is different when bacteria enter the CNS via different routes is not understood, but the trauma caused by i.c. administration (bringing defense cells to the site) in association with the infectious process may be responsible for the inflammation. In fact, patients with M. avium brain infection, often associated with bacteremia, can show heavy cellular infiltration and bacterial burden in the wall of the small bowel and lymph nodes, although the inflammatory changes in the CNS are often mild (11, 15).
Histopathologic changes with M. avium CNS infection have not been well defined, but in the reported cases poorly formed granulomas without central necrosis, perivascular cuffing, and mild focal cellular infiltration in the meninges were mentioned as well as the existence of the characteristic aggregates of foamy histiocytes containing an abundance of intracellular bacilli (9, 11, 15). Likewise, our histopathologic findings in the brains of mice infected systemically with M. avium showed similar features, indicating that this murine model appears to be suitable for further pathophysiological study of M. avium brain infection.
Microscopically, acid-fast bacilli were present, especially in the parenchyma of the choroid plexus of i.v.-infected mice, suggesting that the choroid plexus may be an important route by which M. avium enters the neuraxes. The choroid plexuses are derived by invagination of the neuroepithelium and are therefore located within brain substance, although it shows continuity with the meninges (19). Despite this deviation and topography, the exceptionally high blood flow and lack of intercellular tight junctions in its thin fenestrated endothelium may serve as the preferable site for entry of pathogens into CNS. For instance, meningococcal meningitis is widely believed to begin in the choroid plexus, and a similar pathogenic sequence has been suggested for tuberculous meningitis, Haemophilus influenzae meningitis, Escherichia coli meningitis, and listerial meningoencephalitis (7, 20, 23, 24). Our data suggest that M. avium may penetrate the CNS by the choroid plexus with subsequent dissemination to the ventricular system and from there to the spinal fluid, meninges, and subarchoidal space. Furthermore, the results obtained with CD18 KO mice make it very unlikely that M. avium would enter the CNS within cells such as monocytes and neutrophils. In our model, the whole process takes approximately 4 to 6 months, and although a large number of bacteria can be seen in those above-mentioned areas of the brain, no apparent symptoms or signs can be observed in infected mice. It is possible that the mild inflammatory response associated with M. avium infection (16) with induction of low levels of tumor necrosis factor alpha and suppression of the release of chemokines such as interleukin-8 (21) might explain the near absence of symptoms. In fact, such a clinical course may explain the potentially underdiagnosed clinical entity in AIDS patients. Further studies addressing the cells and cytokines involved in the host response to CNS M. avium infection are under way.
Although nothing is known about the molecular mechanisms allowing M. avium to invade the CNS, our results suggest that bacteria might cross the blood-brain barrier by invading the epithelial cells in the choroidal plexus and not by crossing tight-junction endothelial cells. Other groups using SCID mice have noticed similar degrees of infection in the brain and absence of symptoms and signs (8).
Compared with the many well-developed acute bacterial meningitis models (25), chronic CNS infection models are not well established. In this study, we describe invasion of the CNS by M. avium via i.v. inoculation instead of direct i.c. injection, which resembles the natural course of human disease. This model may be useful in the investigation of the immunohistopathology cascade of NTBM brain infections.
Acknowledgments
We thank Karen Allen for preparing the manuscript.
This work was supported by funds of the Kuzell Institute. Dr. Wu was supported by a fellowship from Kaohsiung Chang Gung Memorial Hospital, Kaohsiung County, Taiwan.
REFERENCES
- 1.Bermudez L E, Inderlied C B, Kolonoski P, Wu M, Barbara-Burnham L, Young L S. Activities of bay Y 3118, levofloxacin, and ofloxacin alone or in combination with ethambutol against Mycobacterium avium complex in vitro, in human macrophages, and in beige mice. Antimicrob Agents Chemother. 1996;40:546–551. doi: 10.1128/aac.40.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez L E, Petrofsky M, Kolonoski P, Young L S. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J Infect Dis. 1992;165:75–79. doi: 10.1093/infdis/165.1.75. [DOI] [PubMed] [Google Scholar]
- 3.Bertram M A, Inderlied C B, Yadegar S, Kolonoski P, Yamada J K, Young L S. Confirmation of the beige mouse model for study of disseminated infection with Mycobacterium avium complex. J Infect Dis. 1986;154:194–195. doi: 10.1093/infdis/154.1.194. [DOI] [PubMed] [Google Scholar]
- 4.Besche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog. 1995;18:323–336. doi: 10.1006/mpat.1995.0029. [DOI] [PubMed] [Google Scholar]
- 5.Cegielski J P, Wallace R J., Jr Central nervous system infections with nontuberculous mycobacteria. Clin Infect Dis. 1997;25:1496–1497. doi: 10.1086/517015. [DOI] [PubMed] [Google Scholar]
- 6.Cegielski J P, Wallace R J, Durack D T. Infections due to nontuberculous mycobacteria. In: Scheld W M, Whitley R J, Durack E T, editors. Infections of the central nervous system. 2nd ed. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 445–461. [Google Scholar]
- 7.Daum A L S R, Scheifele D, Syriopolou V, Averill D R, Roberts M C, Stull T L. Pathogenesis of Haemophilus influenzae meningitis. In: Sell S H, Wright P F, editors. Haemophilus influenzae: epidemiology, immunology, and prevention of disease. New York, N.Y: Elsevier Science Publishing Co.; 1982. pp. 89–109. [Google Scholar]
- 8.Fattorini L, Mattei M, Placido R, Li B, Iona E, Agrimi U, Colizzi V, Orefici G. Mycobacterium avium infection in BALB/c and SCID mice. J Med Microbiol. 1999;48:577–583. doi: 10.1099/00222615-48-6-577. [DOI] [PubMed] [Google Scholar]
- 9.Flor A, Capdevila J A, Martin N, Gavalda J, Pahissa A. Nontuberculous mycobacterial meningitis: report of two cases and review. Clin Infect Dis. 1996;23:1266–1273. doi: 10.1093/clinids/23.6.1266. [DOI] [PubMed] [Google Scholar]
- 10.Gangadharam P R, Edwards C K D, Murthy P S, Pratt P F. An acute infection model for Mycobacterium intracellulare disease using beige mice: preliminary results. Am Rev Respir Dis. 1983;127:648–649. doi: 10.1164/arrd.1983.127.5.648. [DOI] [PubMed] [Google Scholar]
- 11.Gyure K A, Prayson R A, Estes M L, Hall G S. Symptomatic Mycobacterium avium complex infection of the central nervous system: a case report and review of the literature. Arch Pathol Lab Med. 1995;119:836–839. [PubMed] [Google Scholar]
- 12.Hawkins C C, Gold J W, Whimbey E, Kiehn T E, Brannon P, Cammarata R, Brown A E, Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986;105:184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- 13.Horsburgh C R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 14.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob C N, Henein S S, Heurich A E, Kamholz S. Nontuberculous mycobacterial infection of the central nervous system in patients with AIDS. South Med J. 1995;86:638–640. doi: 10.1097/00007611-199306000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kim S Y, Goodman J R, Petrofsky M, Bermudez L E. Mycobacterium avium infection of gut mucosa in mice associated with late inflammatory response and intestinal cell necrosis. J Med Microbiol. 1998;47:725–731. doi: 10.1099/00222615-47-8-725. [DOI] [PubMed] [Google Scholar]
- 17.Klatt C E, Nichols L, Noguchi T T. Evolving trends revealed by autopsies of patients with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1994;118:884–890. [PubMed] [Google Scholar]
- 18.Larsen R A, Bauer M, Weiner J M, Diamond D M, Leal M E, Ding J C, Rinaldi M G, Graybill J R. Effect of fluconazole on fungicidal activity of flucytosine in murine cryptococcal meningitis. Antimicrob Agents Chemother. 1996;40:2178–2182. doi: 10.1128/aac.40.9.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine S. Choroid plexus: target for systemic disease and pathway to the brain. Lab Invest. 1987;56:231–233. [PubMed] [Google Scholar]
- 20.Parkkinen J, Korhonen T K, Pere A, Hacker J, Soinila S. Binding sites in the rat brain for Escherichia coli S fimbriae associated with neonatal meningitis. J Clin Invest. 1988;81:860–865. doi: 10.1172/JCI113395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sangari F J, Petrofsky M, Bermudez L E. Mycobacterium avium infection of epithelial cells results in inhibition or delay in the release of interleukin-8 and RANTES. Infect Immun. 1999;67:5069–5075. doi: 10.1128/iai.67.10.5069-5075.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders B M, Frank A A, Cooper A M, Orme I M. Role of gamma delta T cells in immunopathology of pulmonary Mycobacterium avium infection in mice. Infect Immun. 1998;66:5508–5514. doi: 10.1128/iai.66.11.5508-5514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheld W M, Park T S, Dacey R G, Winn H R, Jane J A, Sande M A. Clearance of bacteria from cerebrospinal fluid to blood in experimental meningitis. Infect Immun. 1979;24:102–105. doi: 10.1128/iai.24.1.102-105.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seebach J, Bartholdi D, Frei K, Spanaus K S, Ferrero E, Widmer U, Isenmann S, Strieter R M, Schwab M, Pfister H, et al. Experimental Listeria meningoencephalitis: macrophage inflammatory protein-1 alpha and -2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J Immunol. 1995;155:4367–4375. [PubMed] [Google Scholar]
- 25.Tauber M G, Zwahlen A. Animal models for meningitis. Methods Enzymol. 1994;235:93–106. doi: 10.1016/0076-6879(94)35134-1. [DOI] [PubMed] [Google Scholar]
- 26.Uldry P A, Bogousslavsky J, Regli F, Chave J P, Beer V. Chronic Mycobacterium avium complex infection of the central nervous system in a nonimmunosuppressed woman. Eur Neurol. 1992;32:285–288. doi: 10.1159/000116843. [DOI] [PubMed] [Google Scholar]
- 27.Wallace J M, Hannah J B. Mycobacterium avium complex infection in patients with the acquired immunodeficiency syndrome: a clinicopathologic study. Chest. 1988;93:926–932. doi: 10.1378/chest.93.5.926. [DOI] [PubMed] [Google Scholar]
- 28.Weiss I K, Krogstad P A, Botero C, Von Seidlein L, Nash K. Fatal Mycobacterium avium meningitis after mis-identification of M. tuberculosis. Lancet. 1995;345:991–992. doi: 10.1016/s0140-6736(95)90741-6. [DOI] [PubMed] [Google Scholar]
- 29.Young L S. Mycobacterium avium complex infection. J Infect Dis. 1988;157:863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]