Abstract
Dendritic cells (DCs) play a central role in the generation of acquired immunity to infections by pathogenic microorganisms. Salmonella enterica serotype Typhimurium is known to survive and proliferate intracellularly within macrophages and nonphagocytic cells, but no data exist on how this pathogen interacts with DCs. In this report, we show the capacity of serotype Typhimurium to survive within the established mouse DC line CB1. In contrast to the case for the macrophage model, the compartments of DCs containing serotype Typhimurium are devoid of lysosomal membrane glycoproteins and the PhoPQ two-component regulatory system is not essential for pathogen intracellular survival.
Salmonellae are facultative intracellular pathogens causing self-limiting gastroenteritis (food poisoning) and systemic infections (5). Penetration of the intestinal epithelium by these pathogens occurs by preferential bacterial invasion of M cells located in Peyer's patches (16, 35). Control of infection depends on the subsequent interaction of Salmonella with underlying immune cells such as polymorphonuclear leukocytes and macrophages (17).
In vitro analysis has provided information on how Salmonella avoids killing by immune cells (17). Studies have been mostly focused on the interaction of Salmonella with macrophages (1–4, 6, 12, 20, 24, 25, 27, 31, 33, 37). Hallmarks of this interaction are the rapid targeting of the pathogen to vacuoles containing lysosomal membrane glycoproteins (LGPs) (27, 31), induction of apoptosis (3, 20, 25, 33), and the central role of the two-component response regulator PhoP-PhoQ in controlling survival of intracellular bacteria (12, 24).
Numerous studies have demonstrated that dendritic cells (DCs) play a crucial role in generating acquired immunity (reviewed in references 32 and 36). DCs are the most efficient antigen-presenting cells that are able to activate resting T cells, initiating primary immune responses in vivo (29, 32, 36). Furthermore, DCs are located in T-cell-dependent areas of central lymphoid organs, which are known to be targets for Salmonella during systemic infections (17). DCs are also present in intestine-associated lymphoid tissues such as Peyer's patches, and so they eventually might interact with Salmonella during the early steps of the infection. Despite the putative relevance of DCs, information on how DCs interact with Salmonella is scarce. In fact, only one recent study has addressed this interaction, using Salmonella enterica serotype Dublin and DCs expanded from peripheral lymphoid organs (21). The study showed that serotype Dublin is able to survive within DCs (21).
To define in better detail the interaction of Salmonella with DCs, we have analyzed the fate of another S. enterica serotype, serotype Typhimurium, upon infection of the fully competent established murine spleen DC line CB1 (29). These cells display the morphologic, immunophenotypic, and functional attributes of fresh DCs, including the capacity to prime T cells in vivo (29). We also tested the potential role of the PhoP-PhoQ regulatory system in the interaction of bacteria with these DCs. In contrast to previous observations in macrophages and nonphagocytic cells (7–9, 11, 23, 27, 31), trafficking of serotype Typhimurium within DCs is not associated with targeting of the pathogen to host vacuoles containing LGPs. Moreover, a serotype Typhimurium phoP mutant did not show relevant differences in survival compared to the parental wild-type (wt) strain. These results suggest the existence of unique interactions between DCs and serotype Typhimurium that are absent in other host cell types.
CB1 DCs were grown in Iscove's modified Dulbecco's medium (Biowhittaker, Verviers, Belgium) supplemented with 5% fetal calf serum and 5 mM glutamine (GIBCO Laboratories). These cells were infected with the virulent S. enterica serotype Typhimurium strain SL1344 (15) or the isogenic derivate SV4056 (phoP7953::Tn10) (10). Bacteria were grown overnight in Luria-Bertani (LB) medium at 37°C without shaking and then added to CB1 cells seeded in 24-well plates (ca. 5 × 104 cells/well) at a bacterium/DC ratio of 10:1. After 10 min of infection, extracellular bacteria were removed by gentle washing with phosphate-buffered saline (PBS) (pH 7.4), and fresh culture medium containing 100 μg of gentamicin ml−1 was added. At 2 h postinfection, the gentamicin concentration was lowered to 10 μg ml−1. At different time intervals, infected DCs were washed with PBS (pH 7.4) and lysed with 1% Triton X-100. The number of viable intracellular bacteria was estimated by plating serial dilutions in Luria-Bertani agar as described previously (22).
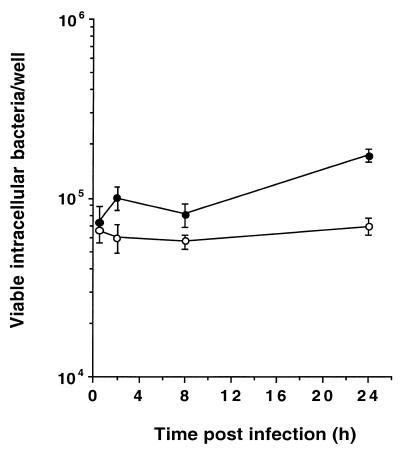
Figure 1 shows the numbers of viable intracellular serotype Typhimurium SL1344 (wt) and SV4056 (phoP) bacteria at different times after infection of CB1 cells. The two strains were equally able to survive, but were not able to proliferate, within these cells. Thus, ratios of viable intracellular bacteria at 24 versus 0.5 h were 2.36 ± 0.19 (wt, SL1344) and 1.07 ± 0.12 (phoP, SV4056). These phenotypes have similarities to the survival kinetics described for serotype Typhimurium within in vitro-activated or mouse-isolated macrophages (1, 2). However, and in contrast to the macrophage infection model (12, 24), the phoP mutation seems not to have an effect on the survival of intracellular serotype Typhimurium within DCs. Our results also partly differ from those of the recent study performed with serotype Dublin and DCs (21), since no reduction in the number of viable intracellular bacteria was detected at long infection times. We explain this discrepancy by the fact that our incubation time for bacteria with DCs was only 10 min, whereas in the study of Marriott et al. (21) an infection time of 90 min was used. The prolonged incubation of bacteria with DCs might produce overinfection and host cell death, an effect reported in the serotype Dublin study (21) and not observed in our serotype Typhimurium-DC model (data not shown). Globally, our results support that serotype Typhimurium has the capacity to efficiently invade and survive within DCs by a PhoPQ-independent mechanism(s).
FIG. 1.
Intracellular survival of S. enterica serotype Typhimurium within the mouse DC line CB1. Cells were infected for 10 min with serotype Typhimurium SL1344 (wt) (●) or SV4056 (phoP) (○), and the viability of intracellular bacteria was monitored for the times indicated. Results are mean values and standard deviations from a representative experiment of a total of four repetitions.
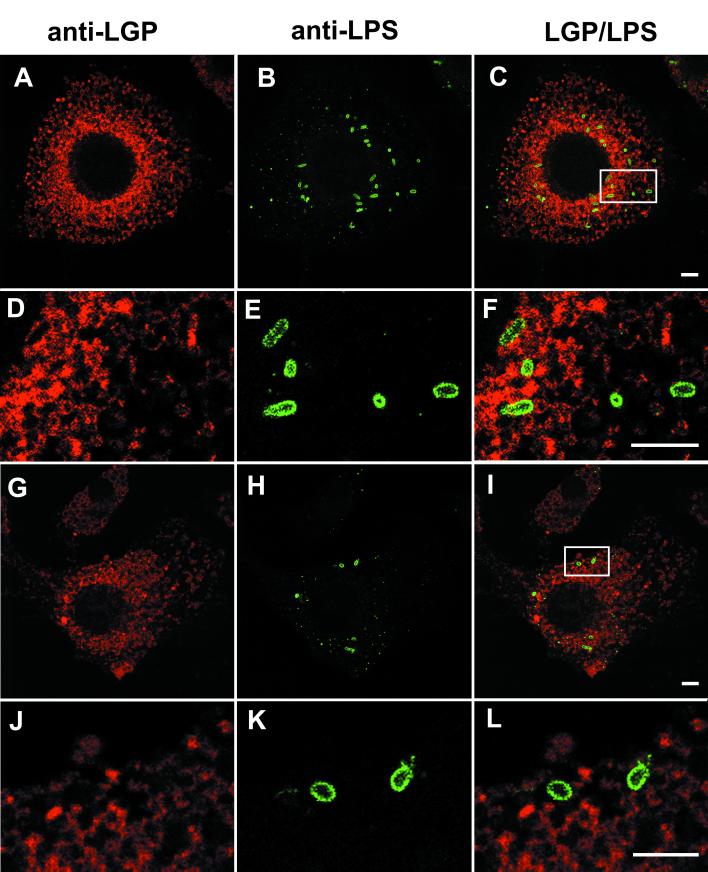
We next evaluated whether the unique behavior of intracellular S. enterica serotype Typhimurium within DCs could be related to alterations in the pathogen intracellular trafficking route. It is known that in macrophages and nonphagocytic cells, serotype Typhimurium is targeted to vacuolar compartments containing large amounts of LGPs (7–9, 11, 23, 27, 31). In nonphagocytic cells such as HeLa epithelial cells, this vacuolar fusion process is dependent on Rab7 GTPase activity and is followed by the formation of filamentous structures containing LGPs (11, 23). Considering these results, we analyzed by indirect immunofluorescence microscopy the distribution of LGPs in serotype Typhimurium SL1344-infected CB1 cells. At different time intervals, infected cells were washed with PBS (pH 7.4), fixed with 3% paraformaldehyde for 10 min at room temperature, and double labeled with rat monoclonal antibody 1D4B, recognizing mouse LGP lamp-1 (Developmental Studies Hydridoma Bank, Iowa State University) (dilution, 1:10), and rabbit polyclonal anti-serotype Typhimurium lipopolysaccharide (LPS) antibody (Difco Laboratories, Detroit, Mich.; catalog no. 2948-47-6) (dilution, 1:200). Secondary antibodies used included Texas Red-goat antirat IgG and fluorescein isothiocyanate (FITC)-goat antirabbit IgG (Jackson ImmunoResearch Laboratories Inc., Bio/Can Scientific, Mississagua, Ontario, Canada) (dilution, 1:100). Labeling was performed as previously described (8). Confocal laser fluorescence microscopy was used to analyze the samples with a Bio-Rad Radiance 2000 system. Targeting of the serotype Typhimurium-containing vacuoles (SCV) to LGP-containing compartments of DCs was not observed at any of the postinfection times tested, up to 24 h. Figure 2A to F shows a focal plane image indicating lack of colocalization of LGP with wt intracellular bacteria at 24 h postinfection. Analogous results were obtained when DCs were infected with the serotype Typhimurium phoP derivative SV4056 (Fig. 2G to L). By direct quantitative analysis performed with the microscope, it was estimated that only 5% of intracellular wt and phoP bacteria were present in LGP-containing compartments at all postinfection times tested from 2 h postinfection (data not shown). At these times, more than 70% of intracellular organisms are enclosed in LGP-containing compartments of macrophages or epithelial cells (8, 11, 27, 31). Bacterium-host cell marker colocalization was not seen in either SL1344 (wt)- or SV4056 (phoP)-infected CB1 cells labeled for other host markers, such as lamp-2 (another type of LGP), cathepsin-D and lysosomal acid phosphatase (lysosomal enzymes), and Rab6 GTPase (involved in late endosome trafficking) (data not shown). These results suggest that biogenesis of SCV in DCs follows a previously uncharacterized route for serotype Typhimurium that does not involve fusion with lysosomes or late endosomes. The unique biogenesis process proposed for SCV in DCs contrasts with that reported for Chlamydia species or Mycobacterium tuberculosis phagosomes in this cell type (19, 28). In the latter cases, the pathogens are targeted to lysosomal compartments (19, 28). In summary, in contrast to what has been demonstrated for serotype Typhimurium in macrophages and nonphagocytic cells and for other intracellular pathogens in DCs, serotype Typhimurium is not targeted to LGP-containing compartments of DCs.
FIG. 2.
The SCV in DCs is devoid of LGPs. CB1 cells were infected for 10 min with S. enterica serotype Typhimurium SL1344 (wt) (A to C) or SV4056 (phoP) (G to I) and processed at 24 h postinfection for confocal laser fluorescence microscopy. Primary antibodies were rat monoclonal anti-LGP (A, D, G, and J) and rabbit polyclonal anti-serotype Typhimurium LPS (B, E, H, and K). Secondary antibodies were Texas Red-conjugated antirat IgG and FITC-conjugated antirabbit IgG. All images shown correspond to the same focal plane (0.3-μm width). (C, F, I, and L) Merged images. (D to F and J to L) Images are enlargements of the areas marked in panels C and I, respectively. Images were processed with the Adobe Photoshop 5.5 software for Macintosh. Bars, 5 μm.
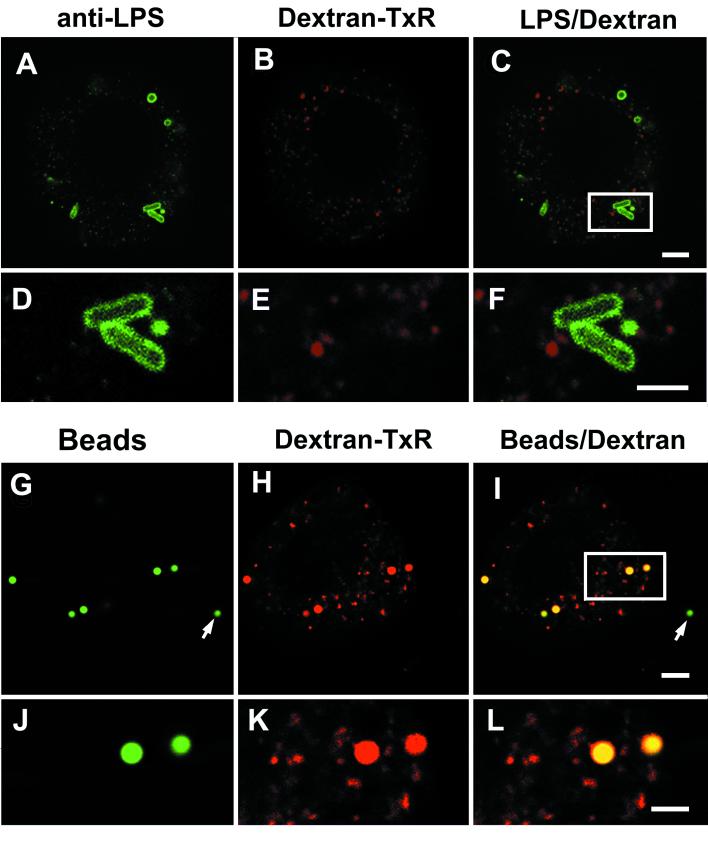
Another important hallmark of the trafficking route of serotype Typhimurium within macrophages and nonphagocytic cells is the functional separation between the endocytic route and the route followed by the SCV (8, 23, 31, 37). Thus, when serotype Typhimurium-infected cells are incubated in tissue culture medium containing fluid endocytic tracers, these compounds do not label the SCV (8). It has also been shown that when serotype Typhimurium infects cells preloaded with fluid endocytic tracers, intracellular bacteria are not targeted to endocytic compartments containing the tracer (8). Although this phenomenon seems to occur in all nonphagocytic cells tested, independent studies have shown contradictory results for macrophages. One study claimed extensive fusion of SCV with tracer-preloaded lysosomes (27), while others have demonstrated limited delivery of fluid endocytic tracers to the SCV (31, 37). When similar experiments were performed with serotype Typhimurium-infected CB1 cells, we observed that the SCV could not be labeled with fluid endocytic fluorescent markers. Fixable dextran conjugated with Texas Red (DX-TxR) (1 mg ml−1; molecular weight, 70,000) (Molecular Probes, Eugene, Oreg.) was used as fluid endocytic tracer. DX-TxR was added to DCs at 4 h prior to bacterial infection, and the cells were washed extensively. These cells were then incubated for 30 min in the absence of tracer and further infected for 10 min with serotype Typhimurium SL1344 (wt). Infected cells were fixed at 2 h postinfection and labeled with rabbit polyclonal anti-serotype Typhimurium LPS and FITC-goat antirabbit IgG antibodies. Figure 3A to F shows that no colocalization exists between DX-TxR-loaded compartments and SCV. Similar results were obtained with DCs infected with the strain SV4056 (phoP) (data not shown). As a positive control for endocytic fusion, inert latex Fluospheres beads (1.0-μm diameter, green-yellow fluorescence) (reference no. L-5281; Molecular Probes) were used. CB1 cells were preloaded with DX-TxR as indicated above and incubated in the presence of Fluospheres beads for 30 min. The beads were removed by extensive washing, and cells were fixed 2 h later. Confocal laser microscopy confirmed that, in contrast to intracellular bacteria, the beads phagocytized by CB1 cells were located in endocytic compartments containing DX-TxR (Fig. 3G to L). These results demonstrate that SCV are impaired for fusion with compartments of the highly active endocytic route reported for DCs (34).
FIG. 3.
Fluid endocytic tracers do not reach the S. enterica serotype Typhimurium compartment in CB1 cells. (A to F) Cells were loaded with DX-TxR for 4 h prior to bacterial infection. DX-TxR was removed, and after 30 min, CB1 cells were infected with the wt strain SL1344 (see text for details). Infected cells were fixed at 2 h postinfection. (G to L) As a positive control for fusion with endocytic compartments, latex Fluospheres beads were used. All images shown correspond to the same focal plane (0.3-μm width). (C, F, I, and L) Merged images. (D to F and J to L) Images are enlargements of the areas marked in panels C and I, respectively. The arrows in panels G and I show beads located outside the cell. Images were processed with the Adobe Photoshop 5.5 software for Macintosh. Bars, 2 μm.
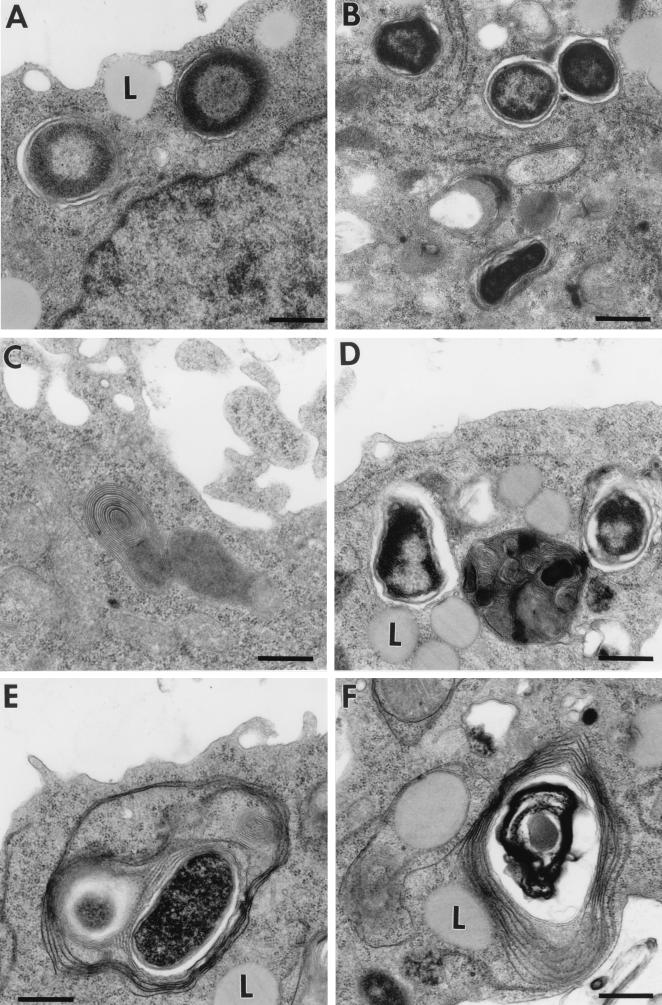
To further characterize the compartment containing S. enterica serotype Typhimurium within DCs, we performed an ultrastructural analysis of this compartment by transmission electron microscopy (TEM). Infected cells were processed for TEM as described previously (13). The TEM analysis demonstrated two important aspects. First, intracellular serotype Typhimurium cells appeared at all time intervals in membrane-bound compartments (Fig. 4A and B). This evidence supports the concept that serotype Typhimurium inhabits a membrane-bound vacuole lacking LGPs. Second, clear differences in the types of vacuolar membrane surrounding serotype Typhimurium SL1344 (wt) and SV4056 (phoP) were observed. Thus, while the wt strain is mainly surrounded by a single membrane (Fig. 3A and B), the phoP mutant is enclosed within multilaminar structures, reminiscent of major histocompatibility complex (MHC) class II compartments described for human and mouse DCs (18, 26, 30). At a late infection time (24 h), 90 to 95% of the SCV containing the phoP bacteria display these multilaminar structures (Fig. 4D and F). Unlike the case for the phoP mutant, it was estimated that only 5% of the SCV containing wt intracellular bacteria displayed the multilaminar structure (Fig. 4B and C). These differences are striking considering that neither SL1344 (wt) nor SV4056 (phoP) is enclosed in LGP-positive compartments (see above [Fig. 2]). Together, these results indicate that wt serotype Typhimurium residing within DCs is located in a vacuole unrelated to the typical MHC class II multilaminar compartment, which has been shown to play a direct role in antigen presentation (18, 26). The PhoPQ system could be involved in blocking fusion of SCV with this specialized compartment. This assumption is in concordance with the role assigned to the PhoPQ system in preventing the processing and presentation of antigens by activated macrophages (38).
FIG. 4.
Ultrastructure of SCV in the mouse DC line CB1 at different times postinfection as observed by TEM. (A) SL1344 (wt), 4 h; (B) SL1344 (wt), 24 h; (C) typical MHC class II multilaminar compartment present in an SL1344 (wt)-infected CB1 cell; (D and E) SV4056 (phoP), 4 h; (F) SV4056 (phoP), 24 h. Note the presence of multilaminar structures surrounding the phoP mutant. L, lysosomal compartment (see reference 13). Bars, 1 μm.
The results described in this report show a new type of interaction of S. typhimurium with a host cell type that has a central role in the immune response. The hallmark of this interaction is the intracellular survival, but not proliferation, of the pathogen in a specialized compartment devoid of LGPs and not related to the MHC class II multilaminar compartment of DCs. Interestingly, other intracellular pathogens, such as Listeria monocytogenes or Bordetella bronchiseptica, proliferate within the same mouse DC line, CB1, used in the present study (13, 14). However, no data exist on how the intracellular trafficking routes of these pathogens are modulated. Unlike the case for other cells previously studied, S. enterica serotype Typhimurium resides within DCs in a compartment lacking LGPs and not resembling MHC class II compartments, which might indicate that DCs are host cells with specialized functions during Salmonella infection. Further investigations are required to identify host markers present in the SCV of DCs and the exact role of PhoPQ in modulating the host immune response by altering the biological function of DCs.
Acknowledgments
This work was supported by grants from “Acción Integrada España-Alemania” (HA1996-0049) and the “DAAD-Acciones Hispano- Alemanas” program (314-Al-e-dr).
REFERENCES
- 1.Alpuche-Aranda C M, Racoosin E L, Swanson J A, Miller S I. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchmeier N A, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L M, Kaniga K, Galán J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 4.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlay B B. Molecular and cellular mechanisms of Salmonella pathogenesis. Curr Top Microbiol Immunol. 1994;192:163–185. doi: 10.1007/978-3-642-78624-2_8. [DOI] [PubMed] [Google Scholar]
- 6.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-del Portillo F. Interaction of Salmonella with lysosomes of eukaryotic cells. Microbiología SEM. 1996;12:259–266. [PubMed] [Google Scholar]
- 8.García-del Portillo F, Finlay B B. Targeting of Salmonella typhimurium to vesicles containing lysosomal membrane glycoproteins bypasses compartments with mannose-6-phosphate receptors. J Cell Biol. 1995;129:81–97. doi: 10.1083/jcb.129.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-del Portillo F, Finlay B B. The varied lifestyles of intracellular pathogens within eucaryotic vacuolar compartments. Trends Microbiol. 1995;3:373–380. doi: 10.1016/s0966-842x(00)88982-9. [DOI] [PubMed] [Google Scholar]
- 10.García-del Portillo F, Pucciarelli M G, Casadesús J. DNA methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc Natl Acad Sci USA. 1999;96:11578–11583. doi: 10.1073/pnas.96.20.11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-del Portillo F, Zwick M B, Leung K Y, Finlay B B. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc Natl Acad Sci USA. 1993;90:10544–10548. doi: 10.1073/pnas.90.22.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groisman E A, Saier M H., Jr Salmonella virulence: new clues to intramacrophage survival. Trends Biochem Sci. 1990;15:30–33. doi: 10.1016/0968-0004(90)90128-x. [DOI] [PubMed] [Google Scholar]
- 13.Guzmán C A, Rohde M, Bock M, Timmis K N. Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells. Infect Immun. 1994;62:5528–5537. doi: 10.1128/iai.62.12.5528-5537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzmán C A, Rohde M, Chakraborty T, Domman E, Hudel M, Wehland J, Timmis K N. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect Immun. 1995;63:3665–3673. doi: 10.1128/iai.63.9.3665-3673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoiseth S K, Stocker B A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 16.Jones B D, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones B D, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 18.Kleijmeer M J, Ossevoort M A, van Veen C J H, van Hellemond J J, Neefjes J J, Kast W M, Melief C J M, Geuze J H. MHC class II compartments and the kinetics of antigen presentation in activated mouse spleen dendritic cells. J Immunol. 1995;154:5715–5724. [PubMed] [Google Scholar]
- 19.Larsson M, Majeed M, Ernst J D, Magnusson K E, Stendahl O, Forsum O. Role of annexins in endocytosis of antigens in immature human dendritic cells. Immunology. 1997;92:501–511. doi: 10.1046/j.1365-2567.1997.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindgren S W, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marriott I, Hammond T G, Thomas E K, Bost K L. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokine expression. Eur J Immunol. 1999;29:1107–1115. doi: 10.1002/(SICI)1521-4141(199904)29:04<1107::AID-IMMU1107>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Martínez-Moya M, de Pedro M A, Schwarz H, García-del Portillo F. Inhibition of Salmonella intracellular proliferation by non-phagocytic eucaryotic cells. Res Microbiol. 1998;149:309–318. doi: 10.1016/s0923-2508(98)80436-1. [DOI] [PubMed] [Google Scholar]
- 23.Méresse S, Steele-Mortimer O, Finlay B B, Gorvel J-P. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 1999;18:4394–4403. doi: 10.1093/emboj/18.16.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller S I. PhoP/PhoQ: macrophage-specific modulators of Salmonella virulence? Mol Microbiol. 1991;5:2073–2078. doi: 10.1111/j.1365-2958.1991.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 25.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nijman H W, Kleijmeer M J, Osservoot M A, Oorschot V M J, Vierboom M P N, van de Keur M, Kenemans P, Geuze H J, Melief C J M. Antigen capture and major histocompatibility class II compartments of freshly isolated and cultured human blood dendritic cells. J Exp Med. 1995;182:163–174. doi: 10.1084/jem.182.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh Y-K, Alpuche-Aranda C, Berthiaume E, Jinks T, Miller S I, Swanson J A. Rapid and complete fusion of macrophage lysosomes with phagosomes containing Salmonella typhimurium. Infect Immun. 1996;64:3877–3883. doi: 10.1128/iai.64.9.3877-3883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ojcius D M, Bravo de Alba Y, Kanellopoulos J M, Hawkins R A, Kelly K A, Rank R G, Dautry-Varsat A. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol. 1998;160:1297–1303. [PubMed] [Google Scholar]
- 29.Paglia P, Girolomoni G, Robbiati F, Granucci F, Ricciardi-Castagnoli P. Immortalized dendritic cell line fully competent in antigen presentation initiates primary T cell responses in vivo. J Exp Med. 1993;178:1893–1901. doi: 10.1084/jem.178.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierre P, Mellman I. Exploring the mechanisms of antigen presentation by cell fractionation. Curr Opin Immunol. 1998;10:145–153. doi: 10.1016/s0952-7915(98)80242-2. [DOI] [PubMed] [Google Scholar]
- 31.Rathman M, Barker L P, Falkow S. The unique trafficking pattern of Salmonella typhimurium-containing phagosomes in murine macrophages is independent of the mechanism of bacterial entry. Infect Immun. 1997;65:1475–1485. doi: 10.1128/iai.65.4.1475-1485.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis e Sousa C, Sher A, Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr Opin Immunol. 1999;11:392–399. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- 33.Richter-Dahlfors A, Buchan A M J, Finlay B B. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly within macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocomptability complex class II compartments: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siebers A, Finlay B B. M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 1996;4:22–29. doi: 10.1016/0966-842x(96)81501-0. [DOI] [PubMed] [Google Scholar]
- 36.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:217–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 37.Uchiya K, Barbieri M A, Funato K, Shah A H, Stahl P D, Groisman E A. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wick M J, Harding C V, Twesten N J, Normark S J, Pfeifer J D. The phoP locus influences processing and presentation of Salmonella typhimurium antigens by activated macrophages. Mol Microbiol. 1995;16:465–476. doi: 10.1111/j.1365-2958.1995.tb02411.x. [DOI] [PubMed] [Google Scholar]